Abstract
Purpose of review:
The goal of this manuscript is to discuss the new diagnostic and potential treatment options for gut disease in systemic sclerosis (SSc). The concepts of quantification of gut perfusion and motility is reviewed. The risks of empiric therapeutics and challenges of studying the microbiome in SSc is discussed.
Recent findings:
There are diagnostics that can provide information on gut perfusion and function that are of value in SSc. Easily implemented diagnostic tests are critical to avoid complications of empiric therapy. The role of the microbiome and drugs that target dysmotility are areas of active research.
Summary:
SSc-related gastrointestinal tract involvement can be heterogeneous in clinical presentation and disease course. Noninvasive gastrointestinal measurement techniques that quantify neural communications with microvasculature in SSc can potentially guide the proper addition and discontinuation of therapeutics. The role of the microbiome and the role of nitric oxide on gut function are important areas of research for understanding gut dysfunction in SSc.
Keywords: Systemic sclerosis, scleroderma, gastrointestinal tract dysmotility, microbiome, ischemia-reperfusion
Introduction:
The importance of understanding gastrointestinal tract (GIT) involvement in systemic sclerosis (SSc) is underscored by the fact that it is the most commonly involved internal organ in this disease. Symptoms of GIT involvement can be the presenting feature in 10% of SSc patients, and occurs during disease course in up to 95% of SSc patients (1). In addition to the enormous morbidity associated with SSc-related GIT involvement, there is a 6–12% mortality attributed to end-stage GIT manifestations in SSc patients (2, 3)
While there are some recent advances in certain aspects of SSc management, predominantly with a focus on tailoring treatment to the individual patient phenotype, there are challenges to studying GIT involvement in SSc (4). In particular, GIT involvement can be heterogeneous in clinical presentation and disease course. Symptoms often precede laboratory or anatomical abnormalities, and absence of symptoms does not exclude dysfunction (5). Furthermore, GIT involvement is not adequately differentiated in sub-groups of SSc patients, so comparative analyses of intervention outcomes are challenging. For example, gastroesophageal reflux is common in SSc, but the correlation of severity to use of empiric therapeutics, disease duration, autoantibodies and skin subsets is not well characterized. The role of endogenous and exogenous influences on SSc-related GIT dysfunction highlights the importance clinical and research tools that characterize this patient population.
The natural history of SSc pathogenesis, which includes a fibro-proliferative vasculopathy, immunological abnormalities and progressive fibrosis, provides structure for an approved understanding of SSc-related GIT disease. In particular, if the dysfunctional endothelial cell and disturbed endothelial-mesenchymal transition is important, diagnostics that are able to delineate perfusion abnormalities, immune dysregulation, and irreversible fibrosis have the potential to provide the treating physician with a meaningful tool for understanding interventions. In this review, we will discuss new GIT diagnostic tools that may be applied for a better understanding of this common problem. We will discuss the unique challenges of empiric therapeutics targeting symptoms.
Diagnostics Testing Incorporating Systemic Sclerosis Pathogenesis
The two most common and early problems affecting systemic sclerosis (SSc) patients are Raynaud’s phenomenon and gastrointestinal symptoms (6, 7). Raynaud’s phenomenon is the term used to describe episodic excessive vasoconstriction of the digital microvasculature in response to the environment, including cold exposure from the peripheral sensory neurons and/or emotional stress from central nervous system afferents. It is associated with digital color changes reflecting the disruption of perfusion and oxygenation of affected tissues. While initially RP is a functional issue, over time structural changes in the vessels include intimal hyperplasia and adventitial fibrosis (8). An intense or prolonged Raynaud’s phenomenon episode can result in a digital ulcer, which in the SSc patient population is correlated with more severe gastrointestinal involvement, suggesting that perhaps these two manifestations of SSc share a pathogenesis link of progressive ischemia-reperfusion (9). Specifically, if communication between the nervous and vascular systems is important for tissue homeostasis and repair (10). As such, understanding the role of the nervous system and prolonged inadequate oxygenated blood flow on gastrointestinal tissue vitality is of importance. Since upper gastrointestinal disorders, such as gastroparesis, chronic unexplained nausea and vomiting, functional dyspepsia can have similar symptom complexes, the implementation of therapeutics can best be augmented by an improved understanding of the effects of neural communications on the gastrointestinal microvasculature in SSc.
While the use of nailfold videocapillaroscopy is the standard of care for bedside clinical vasculopathy monitoring (11), our group has recently shown that sublingual videomicroscopy for the assessment of the microvascular function in SSc may have greater relevance to GIT dysfunction (12). Sublingual videomicroscopy provides detailed and objective information about the status of the microcirculation and has been successfully used for the assessment of other diseases characterized by microcirculatory dysfunction (13, 14).
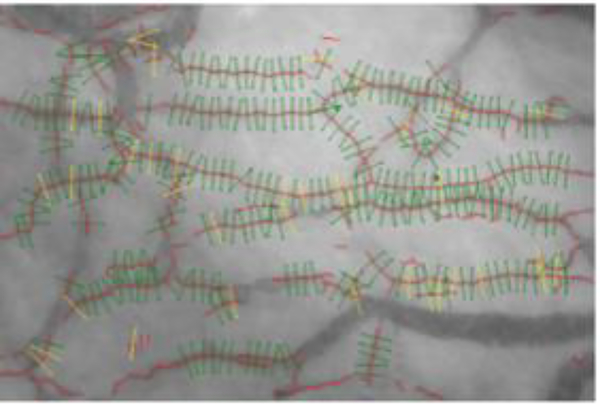
Sublingual videomicroscopy (Figure 1) can be performed with an intravital microscope equipped with sidestream dark field (SDF) imaging (15–17) and an automated capture and analysis system that determines functional outcomes in thousands of 10 μm microvessel segments, such as markers of microvascular perfusion (i.e., microvascular density and longitudinal red blood cell fraction) and glycocalyx penetrability. We have used this technique to assess glycocalyx penetrability and microvascular function in patients with systemic sclerosis (12). A typical test may involve three trials, taking 5–10 minutes and producing immediate objective analysis and output from ~3,000 microvessel segments per trial. Much like nailfold videocapillaroscopy, this vascular data can be used longitudinally to correlate findings to both symptoms and treatment response.
Figure 1:
Automated capture and analysis of sublingual microvasculature. Sidestream dark field imagery captures video recordings of the sublingual microvasculature. In each recording, the automated analysis system divides microvessels (red) into 10 μm perfused (green) or non-perfused (yellow) microvessel segments. Subsequently, markers of microvascular perfusion and glycocalyx penetrability are assessed in perfused microvessel segments. Adapted from Figure 1 in Machin DR, Gates PE, Vink H, Frech TM, Donato AJ. Automated Measurement of Microvascular Function Reveals Dysfunction in Systemic Sclerosis: A Cross-sectional Study. J Rheumatol. 2017 with permission from The Journal of Rheumatology.
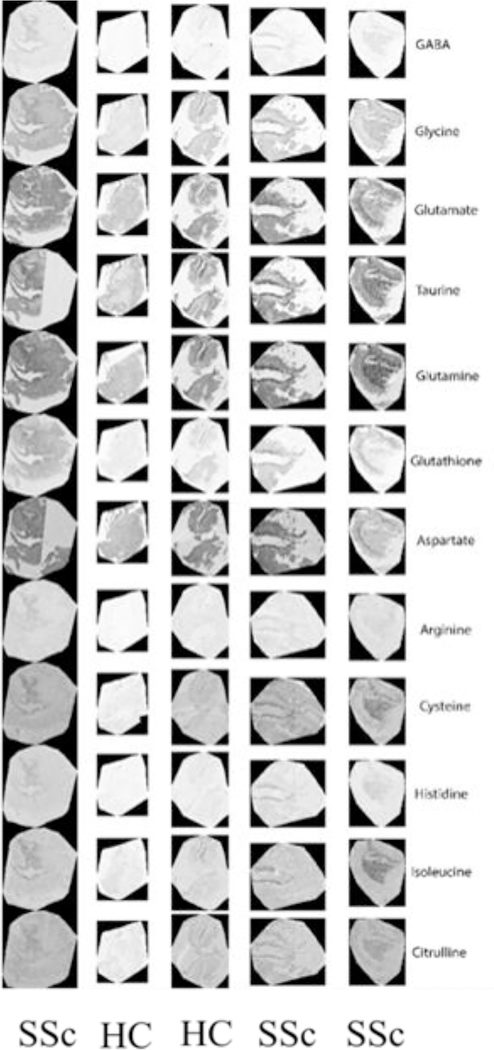
Direct GIT tissue analysis of vasculopathy in SSc is challenging because conventional endoscopic resection techniques are restricted to superficial layers of the gastrointestinal wall. Endoscopic full-thickness resection (EFTR) is an evolving technique endoscopic full-thickness biopsy, which allows histopathological examination of the myenteric ganglia (18). This technique applied to SSc biopsies has the potential to clarify whether treatments targeting the myenteric ganglia for motility will be effective in an some patients, especially if intermittent ischemia is responsible for ischemic/hypoxic neuropathy in the enteric nervous system. Another research technique that can be applied to submucosal biopsies is Computational Molecular Phenotyping (CMP), which is a novel method developed to establish the identity and metabolic status of tissues at the cellular or sub-cellular level (19). CMP differentiates the small molecular components of cells, revealing unique metabolic signatures that allow for the identification and exploration of normal and pathologic tissues. Essentially, CMP maps micro-molecules of the metabolome as cellular imagery, thus enabling the study of cell physiology under stresses such as genetic disease, oxidative challenge or toxin exposure. When we applied this technique to five esophageal biopsy tissues of age-matched patients (three SSc and two healthy controls) using an array of small molecule signatures (Figure 2) (20), we found abnormalities of arginine and glutamate in the SSc specimens. Arginine, a rate-limiting substrate for nitric oxide synthase, is a critical mediator of vasodilation, and glutamate, is involved in the regulation of the brain-gut axis and conveys information, via afferent fibers, from the gut to the brain and via efferent fibers, from the brain to control gut secretion and motility (21).
Figure 2:
Computational Molecular Phenotyping of Esophageal Biopsies: Three systemic sclerosis (SSc) and two Healthy Control (HC) were age-matched and analyzed for molecular signatures.
Ischemia and reperfusion injury can induce motility issues of the GIT (22). The SmartPill is an FDA approved device that measures regional, multiregional, and global gastrointestinal motility in a non-invasive manner (23), and may be of particular value to the SSc where information about the entire GIT is of value. The SmartPill provides information on gastric emptying time (GET), small bowel transit time (SBTT), colon transit time (CTT), and whole gut transit time (WGTT), and can be administered within the office setting without the need for sedation or endoscopy. To date, antroduodenaljejunal and colonic manometry are considered the gold standards for assessment of gastrointestinal motility disorders. However, despite earlier claims that these manometric techniques could differentiate between a neuropathic and myopathic disorder, there is in fact no correlation in manometric patterns that can differentiate these disorders (24). Previously it has been reported that SSc results in a myopathic pattern on manometric testing suggesting an underlying myopathic pathology, however, this may no longer be the case based on a study that evaluated manometric patterns with histologic analysis from full thickness gastrointestinal biopsy specimens (25). Therefore, the SmartPill wireless motility capsule represents a reasonable alternative to these invasive tests that are only available at specialty centers, as the SmartPill can also provide intraluminal pressure measurements that can implicate the presence of underlying pathology such as a neuropathic or myopathic disorder even when gastrointestinal transit times are within normal range. Abnormalities on any of the above testing modalities can be suggestive of an underlying autonomic nervous system disorder, and can help guide the initiation of therapeutics.
Treatment Considerations for Systemic Sclerosis Gastrointestinal Tract
One particular challenge in SSc is differentiating primary and secondary abnormalities in gut flora that might impact on the clinical manifestations within the GIT. Dysbiotic states may be secondary to altered autonomic nervous system outflow to the gut (26). A recent study in two different cohorts of patients with SSc and healthy controls reported different microbial compositions (27). Firmicutes showed a significantly increased abundance in SSc patients (63.5 and 42.8%) compared to healthy controls (33%). Bacteroidetes was decreased in one of the cohorts (21.3%) as compared to healthy controls (63.2%). In both SSc cohorts, Clostridium was more abundant in patients with low gastrointestinal symptom severity, Lactobacillus was more abundant in patients with mild constipation and Prevotella was more abundant in patients with moderate to severe gastrointestinal symptom severity. While these genera are potential targets to avert or treat gastrointestinal dysfunction in SSc, more research is need to understand the role of dietary modification, pro/pre-biotic supplementation, or fecal transplantation as therapy to selectively promote the growth of desired microbiota (28). Microbiota are fundamentally communal organisms and exist in mutualistic, interdependent networks that are crucial to their survival. Thus, the role of empiric antibiotics for small bacterial overgrowth and high dose acid suppression that can possibly disrupt the function of biologically beneficial microbiome populations needs clarification in SSc.
Specific bacterial populations in the mammalian mouth and gut play a critical role in regulating nitric oxide (NO) under conditions of hypoxia and stress (29), which influences gastrointestinal motility. Enteric neurons are either excitatory (releasing acetylcholine) or inhibitory (releasing nitric oxide and vasoactive intestinal peptide). The principal non-adrenergic non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract is NO and is produced by nNOS, expressed in inhibitory enteric neurons. NO activates soluble guanylate cyclase (sGC), producing an increase in the intracellular cyclic guanosine-3’,5’-monophosphate (cGMP), leading to muscle relaxation. In SSc patients, it is proposed that gastrointestinal tract neural dysfunction is caused by ischemia-induced neuronal damage and that collagen tissue accumulation results in nerve compression (30). While apoptosis and remodeling of endothelial cells triggered ROS-mediated oxidative stress is suggested in skin pathogenesis (31) and impaired NO-mediated vascular function is suggested on extremity vascular function measurements (32), the role of the endothelial cell and NO in neurovascular dysfunction in the GIT is more challenging to study. Studies in SSc have shown that mucosal blood flow to the stomach and duodenum are reduced and that vascular insufficiency occurs before smooth muscle atrophy develops, supporting the role of progressive altered neurovascular communication (33). Importantly, the underlying pathological changes are similar in all parts of the GIT, from the esophagus to the rectum, suggesting that generalized altered neurovascular communication is a critical feature of gastrointestinal tract pathogenesis. The effect on motility of commonly prescribed vasodilators prescribed in SSc needs clarification (34).
An important aspect to dysmotility management in SSc is reduction or elimination of medications that may be contributing to symptoms, such as opioids used for pain management or aluminum-containing antacids used for dyspepsia. The role of medications that influence perfusion may be more difficult to eliminate, such as calcium channel blockers and phosphodiesterase inhibitors (PDE5I). These medications are commonly used as empiric therapeutics for Raynaud’s phenomenon. Calcium channel blockers, such as nifedipine and amlodipine are associated with worsening reflux disease (35) and PDE5I drugs, such as sildenafil, can impair interdigestive motor activity of the esophagus, antrum and duodenum (36, 37).
Pharmacological management options for GIT dysmotility in SSc is limited, and there are still many unmet needs for treating gastroparesis and constipation. Although erythromycin is currently used “off-label” as a gastric prokinetic, in addition to a potential effect on the microbiome, it is associated with tachyphylaxis and may inhibit cytochrome P-450 CYP3A4, leading to unwanted drug interactions increasing the risk of ventricular arrhythmias and sudden death (38). In the United States, metoclopramide is the only Federal Drug Administration (FDA)-approved medication for the treatment of gastroparesis, but the recommendation is that treatment should be for no longer than 12 weeks. Domperidone can only be prescribed in the United States through the FDA expanded access to investigational drugs, but is approved for prescription in most other countries including European countries. The current concern of all promotility agents is cardiac arrhythmia, thus many drugs are in development, which improves the selectivity and specificity for intrinsic and gut-brain pathways (39).
Summary
It is well recognized that the gastrointestinal manifestations of SSc are complex and difficult to manage, however there are newer diagnostic techniques that are available, which enable an improved understanding of pathogenesis. These include biopsy techniques, such as EFTR and CMP; sublingual videomicroscopy; and Smartpill motility tests that can help investigate the interface of nervous system with progressive vasculopathy and fibrosis. Importantly, bedside and noninvasive gastrointestinal measurement techniques can help move clinicians away from empiric initiation of therapeutics and more properly guide the addition and discontinuation of therapeutics. Specifically, the role of the microbiome, promotility drugs that target intrinsic and gut-brain pathways, and the role of NO on gut function are important research areas for understanding gut dysfunction in SSc.
Acknowledgment:
The authors would like to recognize the University of Utah CCTS/PPH Translational Science Grant, which funded Dr. Bryan Jones and Dr. Erinn Downs-Kelly who performed the CMP analyzes described in this article.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
Recently published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Gyger G, Baron M. Gastrointestinal manifestations of scleroderma: recent progress in evaluation, pathogenesis, and management. Curr Rheumatol Rep. 2012;14(1):22–9. [DOI] [PubMed] [Google Scholar]
- 2.Poudel DR, Jayakumar D, Danve A, Sehra ST, Derk CT. Determinants of mortality in systemic sclerosis: a focused review. Rheumatol Int. 2018;38(10):1847–58.• The review discusses the newer focus of gastrointestinal tract disease on mortality in the international systemic sclerosis research community
- 3.Poudel DR, Derk CT. Mortality and survival in systemic sclerosis: a review of recent literature. Curr Opin Rheumatol. 2018;30(6):588–93. [DOI] [PubMed] [Google Scholar]
- 4.Eldoma M, Pope J. The contemporary management of systemic sclerosis. Expert Rev Clin Immunol. 2018;14(7):573–82.• This contemporary management article highlights the importance of the individual patient phenotype.
- 5.Bae S, Allanore Y, Furst DE, Bodukam V, Coustet B, Morgaceva O, et al. Associations between a scleroderma-specific gastrointestinal instrument and objective tests of upper gastrointestinal involvements in systemic sclerosis. Clin Exp Rheumatol. 2013;31(2 Suppl 76):57–63. [PubMed] [Google Scholar]
- 6.Lepri G, Guiducci S, Bellando-Randone S, Giani I, Bruni C, Blagojevic J, et al. Evidence for oesophageal and anorectal involvement in very early systemic sclerosis (VEDOSS): report from a single VEDOSS/EUSTAR centre. Ann Rheum Dis. 2015;74(1):124–8. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca C, Abraham D, Ponticos M. Neuronal regulators and vascular dysfunction in Raynaud’s phenomenon and systemic sclerosis. Curr Vasc Pharmacol. 2009;7(1):34–9. [DOI] [PubMed] [Google Scholar]
- 8.Rodnan GP, Myerowitz RL, Justh GO. Morphologic changes in the digital arteries of patients with progressive systemic sclerosis (scleroderma) and Raynaud phenomenon. Medicine (Baltimore). 1980;59(6):393–408. [DOI] [PubMed] [Google Scholar]
- 9.Galluccio F, Allanore Y, Czirjak L, Furst DE, Khanna D, Matucci-Cerinic M. Points to consider for skin ulcers in systemic sclerosis. Rheumatology (Oxford). 2017;56(suppl_5):v67–v71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71(3):406–24. [DOI] [PubMed] [Google Scholar]
- 11.Rossi D, Russo A, Manna E, Binello G, Baldovino S, Sciascia S, et al. The role of nail-videocapillaroscopy in early diagnosis of scleroderma. Autoimmun Rev. 2013;12(8):821–5. [DOI] [PubMed] [Google Scholar]
- 12.Machin DR, Gates PE, Vink H, Frech TM, Donato AJ. Automated Measurement of Microvascular Function Reveals Dysfunction in Systemic Sclerosis: A Cross-sectional Study. J Rheumatol. 2017.• This study introduces the use of sublingual vascular measurement and glycocalyx in systemic sclerosis.
- 13.Nussbaum C, Cavalcanti Fernandes Heringa A, Mormanova Z, Puchwein-Schwepcke AF, Bechtold-Dalla Pozza S, Genzel-Boroviczeny O. Early microvascular changes with loss of the glycocalyx in children with type 1 diabetes. J Pediatr. 2014;164(3):584–9 e1. [DOI] [PubMed] [Google Scholar]
- 14.Tachon G, Harrois A, Tanaka S, Kato H, Huet O, Pottecher J, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. 2014;42(6):1433–41. [DOI] [PubMed] [Google Scholar]
- 15.Cabrales P, Vazquez BY, Tsai AG, Intaglietta M. Microvascular and capillary perfusion following glycocalyx degradation. J Appl Physiol. 2007;102(6):2251–9. [DOI] [PubMed] [Google Scholar]
- 16.Eskens BJ, Mooij HL, Cleutjens JP, Roos JM, Cobelens JE, Vink H, et al. Rapid insulin-mediated increase in microvascular glycocalyx accessibility in skeletal muscle may contribute to insulinmediated glucose disposal in rats. PLoS One. 2013;8(1):e55399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broekhuizen L, Lemkes B, Mooij HL, Meuwese MC, Verberne H, Holleman F, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(12):2646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt A, Meier B, Caca K. Endoscopic full-thickness resection: Current status. World J Gastroenterol. 2015;21(31):9273–85.• The study highlihts the value of endoscopic full-thickness biopsy interpretation,
- 19.Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Tucker J, Marc RE. Retinal Remodeling and Metabolic Alterations in Human AMD. Front Cell Neurosci. 2016;10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marc RE, Jones BW. Molecular phenotyping of retinal ganglion cells. J Neurosci. 2002;22(2):413–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filpa V, Moro E, Protasoni M, Crema F, Frigo G, Giaroni C. Role of glutamatergic neurotransmission in the enteric nervous system and brain-gut axis in health and disease. Neuropharmacology. 2016;111:14–33. [DOI] [PubMed] [Google Scholar]
- 22.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20(4):214–23.•• This report introduces the concept of changes to the gut with ischemia-reperfusion injury.
- 23.Sarosiek I, Selover KH, Katz LA, Semler JR, Wilding GE, Lackner JM, et al. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther. 2010;31(2):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malagelada C, Karunaratne TB, Accarino A, Cogliandro RF, Landolfi S, Gori A, et al. Comparison between small bowel manometric patterns and full-thickness biopsy histopathology in severe intestinal dysmotility. Neurogastroenterol Motil. 2018;30(3).•• This article highlights the value of combining study techniques to understand pathophysiology of dysmotility.
- 25.Camilleri M, Bharucha AE, di Lorenzo C, Hasler WL, Prather CM, Rao SS, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20(12):1269–82. [DOI] [PubMed] [Google Scholar]
- 26.Mayer EA, Hsiao EY. The Gut and Its Microbiome as Related to Central Nervous System Functioning and Psychological Well-being: Introduction to the Special Issue of Psychosomatic Medicine. Psychosom Med. 2017;79(8):844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkmann ER. Intestinal microbiome in scleroderma: recent progress. Curr Opin Rheumatol. 2017;29(6):553–60. [DOI] [PubMed] [Google Scholar]
- 28.Bellocchi C, Volkmann ER. Update on the Gastrointestinal Microbiome in Systemic Sclerosis. Curr Rheumatol Rep. 2018;20(8):49.•• This article discusses the microbiome in SSc and highlights challenges and opportunities for future studies.
- 29.Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2017;105:48–67.•• This article discusses the interaction between the microbiome and vascular health with a focus on the role of nitric oxide.
- 30.Tian XP, Zhang X. Gastrointestinal complications of systemic sclerosis. World J Gastroenterol. 2013;19(41):7062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frech TM, Revelo MP, Drakos SG, Murtaugh MA, Markewitz BA, Sawitzke AD, et al. Vascular leak is a central feature in the pathogenesis of systemic sclerosis. J Rheumatol. 2012;39(7):1385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clifton HL, Machin DR, Groot HJ, Frech TM, Donato AJ, Richardson RS, et al. Attenuated nitric oxide bioavailability in systemic sclerosis: Evidence from the novel assessment of passive leg movement. Exp Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiev KP, Cabane J. Digestive tract involvement in systemic sclerosis. Autoimmun Rev. 2011;11(1):68–73. [DOI] [PubMed] [Google Scholar]
- 34.Carbone F, Tack J. The effect of sildenafil on gastric motility and satiation in healthy controls. United European Gastroenterol J. 2018;6(6):846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakaji G, Fujihara M, Fukata M, Yasuda S, Odashiro K, Maruyama T, et al. Influence of common cardiac drugs on gastroesophageal reflux disease: multicenter questionnaire survey. Int J Clin Pharmacol Ther. 2011;49(9):555–62. [DOI] [PubMed] [Google Scholar]
- 36.Bortolotti M, Mari C, Lopilato C, La Rovere L, Miglioli M. Sildenafil inhibits gastroduodenal motility. Aliment Pharmacol Ther. 2001;15(2):157–61. [DOI] [PubMed] [Google Scholar]
- 37.Bortolotti M, Mari C, Giovannini M, Pinna S, Miglioli M, Pandolfo N. Effects of sildenafil on esophageal motility of normal subjects. Dig Dis Sci. 2001;46(11):2301–6. [DOI] [PubMed] [Google Scholar]
- 38.Thielemans L, Depoortere I, Perret J, Robberecht P, Liu Y, Thijs T, et al. Desensitization of the human motilin receptor by motilides. J Pharmacol Exp Ther. 2005;313(3):1397–405. [DOI] [PubMed] [Google Scholar]
- 39.Valentin N, Acosta A, Camilleri M. Early investigational therapeutics for gastrointestinal motility disorders: from animal studies to Phase II trials. Expert Opin Investig Drugs. 2015;24(6):769–79.•• This is a comprehensive review of gastrointestinal tract dysmotlity therapeutics and the rationale for their use.