Abstract
Sleeping sickness and malaria are parasitic diseases with overlapping geographical distributions in sub-Saharan Africa. We hypothesized that the immune response elicited by an infection with Trypanosoma brucei, the etiological agent of sleeping sickness, would inhibit a subsequent infection by Plasmodium, the malaria parasite, decreasing the severity of its associated pathology. To investigate this, we established a new co-infection model in which mice were initially infected with T. brucei, followed by administration of P. berghei sporozoites. We observed that a primary infection by T. brucei significantly attenuates a subsequent infection by the malaria parasite, protecting mice from experimental cerebral malaria and prolonging host survival. We further observed that an ongoing T. brucei infection leads to an accumulation of lymphocyte-derived IFN-γ in the liver, limiting the establishment of a subsequent hepatic infection by P. berghei sporozoites. Thus, we identified a novel host-mediated interaction between two parasitic infections, which may be epidemiologically relevant in regions of Trypanosoma/Plasmodium co-endemicity.
Author summary
Despite the geographical overlap between the parasites that cause sleeping sickness and malaria, the reciprocal impact of a co-infection by T. brucei and Plasmodium had hitherto not been assessed. We hypothesized that the strong immune response elicited by a T. brucei infection could potentially limit the ability of Plasmodium parasites to infect the same host. In this study, we showed that a primary infection by T. brucei significantly attenuates a subsequent infection by the malaria parasite. Importantly, a significant proportion of the co-infected mice do not develop Plasmodium parasitemia, and those few that do, do not display symptoms of severe malaria and survive longer than their singly infected counterparts. We further showed that the prevention or delay in appearance of malaria parasites in the blood results from a dramatic impairment of the preceding liver infection by Plasmodium, which is mediated by the strong immune response mounted against the primary T. brucei infection. Our study provides new insights for a novel inter-pathogen interaction that may bear great epidemiological significance in regions of Trypanosoma/Plasmodium co-endemicity.
Introduction
The term co-infection refers to a condition in which a host is concomitantly infected by two or more infectious agents [1]. It is estimated that co-infections occur in over one sixth of the world’s human population [2]. The outcome of the interaction between two or more co-infecting agents may be complex. It may result in either the enhancement or the suppression of the growth of one or both pathogen(s), and may lead to the aggravation or improvement of the pathology of either infection [1].
Co-infections between the malaria parasite and other pathogens are highly prevalent in Sub-Saharan Africa [3–6]. Malaria is a disease caused by intracellular Plasmodium parasites, which infect their mammalian hosts through the bite of infected female Anopheles mosquitoes during a blood meal. Inoculated sporozoites reach the blood circulation and home to the liver, where they cross the endothelium of sinusoids and traverse several hepatocytes before productively infecting a final one. Inside the hepatocyte, sporozoites differentiate into intrahepatic or exoerythrocytic forms (EEF) that replicate extensively, originating thousands of merozoites. At the end of the obligatory but asymptomatic liver stage of infection, merozoites are released into the bloodstream, infecting red blood cells (RBCs) and leading to pathology [7].
Sub-Saharan African populations are also affected by human African trypanosomiasis (HAT), or sleeping sickness, a neglected tropical disease caused by extracellular Trypanosoma brucei parasites. Upon a blood meal by the Glossina fly, trypanosomes are released into the skin of the mammalian host, rapidly reaching and replicating in the blood, and eventually colonizing other organs, such as the brain, skin and adipose tissue [8, 9]. During an infection by T. brucei, the host’s innate immune system encounters several parasite antigens, such as the variant surface glycoprotein (VSG) and the trypanosome-lymphocyte triggering factor (TLTF) [10]. The former is usually associated with the activation of both T and B lymphocytes, as well as macrophages, leading to a pro-inflammatory Th1 response, characterized by the production of several cytokines, including IFN-γ, TNF-α and IL-6, as well as nitric oxide. The latter further contributes to the production of IFN-γ through the early activation of natural killer cells [11].
In humans, concomitant infections by Trypanosoma and Plasmodium parasites have been reported in regions where malaria and HAT are co-endemic [12–19]. However, the impact of these co-infections on pathology has only been assessed in two clinical studies [16, 19]. A cross-sectional study in Southern Sudan showed that trypanosome-infected patients presented a reduced number of white blood cells, while that number was increased in co-infected patients [19]. In contrast, no major differences were observed in the clinical and treatment outcomes of Trypanosoma/Plasmodium co-infected patients in Tanzania and Uganda, relative to those infected by T. b. rhodesiense only [16]. To the best of our knowledge, only two studies have investigated Trypanosoma/Plasmodium co-infections experimentally, using mouse models of infection [20, 21]. The first study showed that when erythrocytic stages of P. chabaudi were inoculated in mice with an ongoing T. brucei infection, the onset of Plasmodium parasitemia was delayed but rapidly reached a peak equivalent to that observed in the absence of trypanosomes [20]. A more recent study reported that mice simultaneously infected with T. brucei and P. berghei display higher parasitemia for both pathogens, leading to a significant decrease in host survival relative to mice infected with either one of these parasites alone [21].
Given that T. brucei elicits the production of IFN-γ, a cytokine that has been previously shown to inhibit the development of Plasmodium parasites [22–24], we postulated that a T. brucei infection might influence the host’s susceptibility to the malaria parasite. To test this hypothesis, a co-infection protocol employing rodent T. brucei and P. berghei parasites, two well-established models for sleeping sickness and malaria, respectively [8, 25], was designed to investigate in vivo the impact of an ongoing T. brucei infection on a subsequent infection by P. berghei. We observed that a primary infection by T. brucei significantly attenuates a subsequent infection by the malaria parasite, protecting mice from experimental cerebral malaria and improving host survival, relative to mice infected only with Plasmodium. Our results further show that an ongoing infection by T. brucei leads to a significant increase of IFN-γ levels in the host’s liver, which dramatically inhibits a subsequent hepatic infection by P. berghei.
Results
T. brucei infection attenuates a subsequent infection by the malaria parasite
To assess whether the outcome of an infection with the malaria parasite is affected by an ongoing T. brucei infection, BALB/cByJ or C57BL/6J mice were initially infected with T. brucei and, five days later, exposed to the bites of five P. berghei-infected Anopheles mosquitoes, the lowest dose previously shown to warrant infection of 100% of the exposed mice [26]. Having observed a high intra- and inter-experimental variability in the number of mosquitoes that ingest a blood meal, as well as different biting preferences for T. brucei-infected and uninfected mice (S1 Fig), we subsequently infected animals by i.v. injection of 500 GFP-expressing P. berghei sporozoites, employed as a surrogate of the inoculum delivered by the bites of five infected mosquitoes [7]. Whereas 80% of the mice infected only with P. berghei developed an erythrocytic infection within 5 to 6 days after sporozoite inoculation, none of the co-infected BALB/cByJ mice presented P. berghei parasitemia for up to 15 days after sporozoite administration (Figs 1A and S2A). C57BL/6J mice are typically more susceptible to hepatic infection by P. berghei and constitute a good model for experimental cerebral malaria (ECM) [27, 28]. Strikingly, when a similar experimental setup was employed in the C57BL/6J mouse strain, 70% of the co-infected mice did not display a P. berghei erythrocytic infection for up to 15 days after sporozoite administration, whereas only 1 out of 10 mice infected with P. berghei alone did not develop parasitemia (Figs 1B and S2B). We further observed that the prepatent period in the few C57BL/6J mice that did develop an erythrocytic infection was at least two days longer in co-infected mice than in mice infected only with P. berghei. In a Plasmodium infection, the duration of the prepatent period has been described as a good predictor for the amount of infective merozoites that exit the liver, which directly depends on the total hepatic parasite load [29]. Therefore, our results suggest that an initial infection with T. brucei leads to a strong reduction in P. berghei hepatic burden in the co-infected mice, which either prevents the appearance of Plasmodium parasitemia or significantly delays its onset.
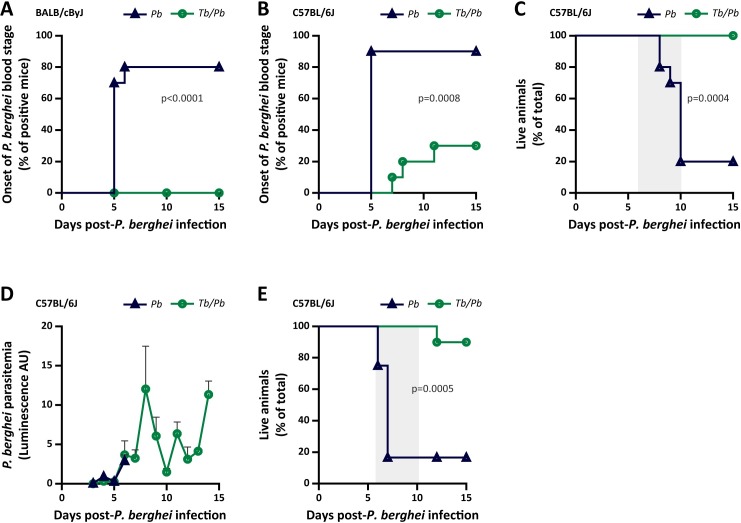
Fig 1. T. brucei infection protects mice from malaria.
(A) Assessment of P. berghei prepatency period following inoculation of 500 P. berghei sporozoites into naïve BALB/cByJ mice (Pb—blue line) or BALB/cByJ mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of mice displaying P. berghei parasitemia, as measured by flow cytometry. The pooled data of 10 mice from in two independent experiments is shown. (B) Assessment of P. berghei prepatency period following inoculation of 500 P. berghei sporozoites into naïve C57BL/6J mice (Pb—blue line) or C57BL/6J mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of mice displaying P. berghei parasitemia, as measured by flow cytometry. The pooled data of 10 mice from two independent experiments is shown. (C) Mouse survival following inoculation of 500 P. berghei sporozoites into naïve C57BL/6J mice (Pb—blue line) or C57BL/6J mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of live mice from a pool of 10 mice employed in two independent experiments. (D) Assessment of P. berghei parasitemia after inoculation of 1 x 106 iRBCs into naïve C57BL/6J mice (Pb—blue line) or C57BL/6J mice infected 5 days earlier with T. brucei (Tb/Pb—green line). The mean bioluminescence and SEM of the pooled data of 10 mice from two independent experiments is shown. (E) Mouse survival following inoculation of 1 x 106 iRBCs into naïve C57BL/6J mice (Pb—blue line) or C57BL/6J mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of live mice from a pool of 10 mice from two independent experiments. For A and B, the Mantel-Cox (log rank) test was employed to compare the onset of P. berghei parasitemia, indicating statistically significant differences for the Tb/Pb group compared to the Pb control group. For C and E, the Mantel-Cox (log rank) test was employed to compare survival curves, indicating statistically significant differences for Tb/Pb compared to the Pb control, and the time window for ECM development is depicted by the grey-shaded area.
The Trypanosoma/Plasmodium co-infection had a major impact on malaria severity. In fact, whereas ~90% of the blood stage-positive control C57BL/6J mice succumbed within 8 to 10 days after sporozoite administration with signs of ECM, none of the co-infected mice that developed parasitemia displayed ECM symptoms or died within that time frame (Fig 1C). It has been reported that a delay in the onset of P. berghei parasitemia in C57BL/6J mice is associated with protection against cerebral pathology and increased host survival [30–33]. Thus, the absence of ECM in co-infected C57BL/6J mice could be due to the delay in the onset of P. berghei parasitemia and/or to a direct impact of the T. brucei infection on Plasmodium erythrocytic development. In order to assess these possible scenarios experimentally, the liver stage of the Plasmodium life cycle was bypassed by inoculating P. berghei-iRBCs into T. brucei-infected or naïve mice, and both groups of animals were monitored for the ensuing parasitemia and disease symptoms. Our results show that although the replication of P. berghei blood-stages is not compromised by an ongoing trypanosome infection (Fig 1D), co-infected mice did not develop ECM symptoms and displayed a significant increase in survival, compared with P. berghei single-infected mice (Fig 1E), indicating that T. brucei can directly attenuate Plasmodium erythrocytic infection, in a liver-independent manner. Of note, T. brucei parasitemia was unaffected by the injection of P. berghei-iRBCs on day 5 of trypanosome infection, indicating that T. brucei replication in the blood is not affected by P. berghei (S3 Fig).
Altogether, our data show that a primary infection by T. brucei renders mice more resistant to a subsequent infection by P. berghei, strongly reducing malaria pathology, decreasing disease severity, and improving host survival relative to mice infected only with Plasmodium.
T. brucei infection reduces a subsequent liver infection by P. berghei
Having observed that a primary infection by T. brucei prevents or delays the appearance of P. berghei parasitemia in co-infected mice, we investigated the impact of an ongoing T. brucei infection on the liver stage of the Plasmodium life cycle. To this end, 3 x 104 P. berghei sporozoites, a dose that enables an accurate quantification of hepatic infection, were inoculated into C57BL/6J mice infected with T. brucei parasites 2 to 25 days earlier (Fig 2A). Forty-six hours after sporozoite injection, the P. berghei liver load was determined by quantitative real-time reverse transcriptase-PCR (qRT-PCR) and compared with that of mice infected with P. berghei only (Fig 2B). Our results show that if P. berghei sporozoites were inoculated 2 days after T. brucei infection, when trypanosomes are not yet detectable in the blood, co-infected mice displayed a P. berghei liver load similar to that observed in single-infected mice. However, when sporozoites were injected 5, 8, 12, 15 or 25 days after T. brucei infection, co-infected mice showed a striking ~82–96% reduction in P. berghei liver load compared to control mice (Fig 2B). A similar reduction (~85%) was detected when mice were co-infected with T. brucei and P. yoelli, which indicates that T. brucei impairs the hepatic infection by several Plasmodium species (S4 Fig).
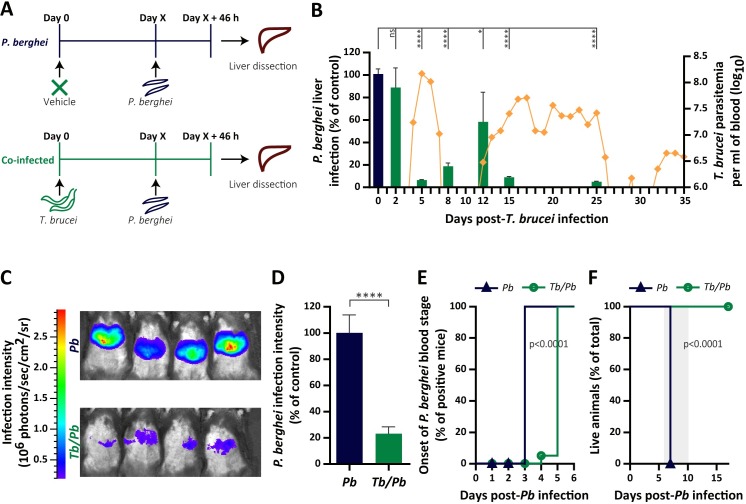
Fig 2. T. brucei attenuates hepatic infection by P. berghei.
(A) Schematic illustration of the co-infection experimental design. The arrows indicate the times of T. brucei and/or P. berghei inoculation, and of liver dissection for quantification of P. berghei liver load. (B) P. berghei liver infection load (bars–primary YY axis) determined by qRT-PCR 46 h after sporozoite injection into naïve mice (blue bar) or mice previously infected by T. brucei (green bars), and T. brucei parasitemia (dots–secondary YY axis) determined daily by microscopy. All P. berghei infections were performed on the same day, with prior T. brucei infections staggered to give a consistent day for subsequent P. berghei infection. The time points indicated on the XX-axis correspond to the number of days that elapsed between T. brucei inoculation and sporozoite injection. Bars represent the mean values of two independent experiments and error bars indicate the SEM. The one-way ANOVA with post-test Dunnett was employed to assess the statistical significance of differences between the experimental groups. ns, not significant, * P < 0.05 and **** P < 0.0001. (C) Representative bioluminescence images of mouse livers 46 h after inoculation of 3 x 104 P. berghei sporozoites into either naïve mice (Pb—top) or mice infected 5 days earlier with T. brucei (Tb/Pb—bottom). (D) Quantification of the P. berghei liver infection load measured by bioluminescence 46 h after sporozoite injection into naïve mice (blue bar) or mice previously infected by T. brucei (green bar). Bars represent the mean values of three independent experiments and error bars indicate the SEM. The Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups (**** P < 0.0001). (E) Assessment of P. berghei prepatency period following inoculation of 3 x 104 sporozoites into naïve mice (Pb—blue line) or mice infected 5 days beforehand with T. brucei (Tb/Pb—green line). Percentage of mice displaying P. berghei parasitemia, as measured by flow cytometry. The pooled data from 10 mice employed in two independent experiments is shown The Mantel-Cox (log rank) test was employed to compare the onset of P. berghei parasitemia curves, indicating statistically significant differences for Tb/Pb compared to the Pb control. (F) Mouse survival following inoculation of 3 x 104 P. berghei sporozoites into naïve C57BL/6J mice (Pb—blue line) or C57BL/6J mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of live mice from a pool of 10 mice employed in two independent experiments. The Mantel-Cox (log rank) test was employed to compare survival curves, indicating statistically significant differences for Tb/Pb compared to the Pb control, and the time window for ECM development is depicted by the grey-shaded area.
In view of these results, P. berghei sporozoites were inoculated 5 days after the initial T. brucei infection in all subsequent co-infection experiments. To further confirm the inhibition of P. berghei liver infection by a primary T. brucei infection, we administered luciferase-expressing P. berghei sporozoites into naïve mice and into mice infected 5 days earlier with T. brucei, and subsequently assessed the hepatic infection in both groups of mice 46 h later by bioluminescence. In agreement with our qRT-PCR results, co-infected mice displayed a ~80% reduction in P. berghei liver burden, as measured by luciferase activity, when compared to control mice (Fig 2C and 2D). Moreover, when P. berghei infection was allowed to proceed to the blood, although all co-infected mice eventually developed Plasmodium parasitemia, they displayed a 2-day increase in prepatency period relative to single-infected mice (Fig 2E). The delay in the onset of P. berghei parasitemia observed in co-infected mice confirms a marked decrease in the number of infective merozoites that exit the liver of these mice, relative to their counterparts infected only with Plasmodium. Importantly, all the co-infected mice survived for longer than 17 days and did not present ECM symptoms, whereas infected control mice succumbed within 7 days after sporozoite administration with signs of ECM (Fig 2F).
To assess whether the observed protection against Plasmodium erythrocytic infection and ECM is time-dependent, P. berghei sporozoites were injected into mice 15 days after T. brucei inoculation, employing P. berghei single-infected mice as controls, and infection was allowed to proceed to the blood. We observed that co-infected mice displayed a 2-day delay in the appearance of P. berghei parasitemia and did not develop ECM symptoms (S5 Fig). These results are similar to those obtained for mice infected with P. berghei sporozoites 5 days after the initial T. brucei infection (Fig 2E), indicating that T. brucei-mediated protection against blood stage Plasmodium infection and ECM is not time-restricted.
Altogether, our data show that a primary infection by T. brucei not only attenuates the severity of the blood stage of P. berghei infection but also significantly reduces its hepatic infection, the first stage of Plasmodium life cycle in the mammalian host.
T. brucei infection reduces the number of P. berghei-infected hepatocytes
The remarkable impairment of P. berghei liver infection by T. brucei, and the fact that the liver stage is a major bottleneck in Plasmodium infection prompted us to investigate the mechanisms behind this inhibitory effect. In rodents, Plasmodium parasites take around 50–60 h to invade, develop and egress from the liver. Thus, we started by investigating the time frame of inhibition of P. berghei liver infection by T. brucei. Using a co-infection protocol similar to that described above, we assessed Plasmodium hepatic infection 30 min, 2 h, 6 h, 12 h, 24 h and 46 h after sporozoite injection. We observed that the P. berghei liver load of co-infected mice was ~55% lower than that of single-infected control mice as early as 30 minutes after sporozoite injection, and gradually decreased down to a ~95% reduction relative to control mice at 46 h post-infection (hpi) (Fig 3A).
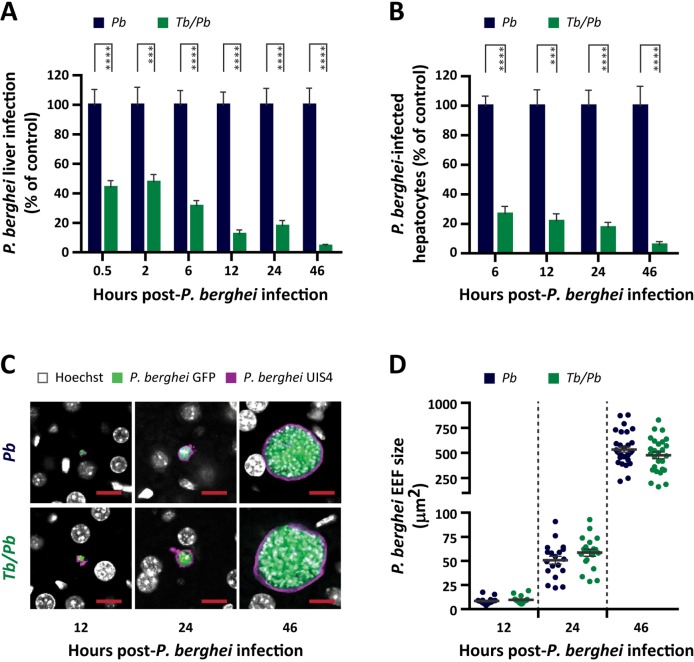
Fig 3. T. brucei impairs early liver stage of P. berghei infection.
(A) P. berghei liver infection load quantified by qRT-PCR 30 min, 2 h, 6 h, 12 h, 24 h and 46 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb–blue bars) or mice infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of three to five independent experiments and error bars indicate the SEM. The two-way ANOVA with post-test Bonferroni was employed to assess the statistical significance of differences between experimental groups. *** P < 0.001 and **** P < 0.0001. (B) Number of P. berghei-infected hepatocytes per square millimeter of liver section quantified by immunofluorescence microscopy 6 h, 12 h, 24 h and 46 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb–blue bars) or mice infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of one representative experiment out of two independent experiments and error bars indicate the SEM. The two-way ANOVA with post-test Bonferroni was employed to assess the statistical significance of differences between experimental groups. *** P < 0.001 and **** P < 0.0001. (C) Representative confocal microscopy images of EEFs at 12 h, 24 h and 46 h after injection of 3 x 104 P. berghei sporozoites into naïve mice or mice infected 5 days earlier with T. brucei. White: Hoechst—nuclear staining; green: P. berghei GFP labeling showing the parasite heat shock protein 70; purple: P. berghei UIS4 labeling showing the parasitophorous vacuole membrane. Scale bars, 10 μm. (D) EEF area at 12 h, 24 h and 46 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb—blue dots) or mice infected 5 days earlier with T. brucei (Tb/Pb—green dots), assessed by immunofluorescence microscopy. Results are expressed as the mean values of one representative experiment out of two independent experiments and error bars indicate the SEM.
A decrease in Plasmodium liver load may result from either a reduction in the number of infected hepatocytes or from a defect in the parasite’s capacity to replicate. To evaluate these two hypotheses, liver sections from single- or co-infected mice were analyzed by immunofluorescence microscopy at various time points of Plasmodium liver infection. We observed that the number of infected hepatocytes in co-infected mice was ~50% lower than in control mice at 6 hpi, and further decreased until 5% of that number at 46 hpi (Fig 3B), suggesting that most EEFs are eliminated during a co-infection. Of note, no significant differences were observed in the area of the Plasmodium EEF developing in the livers of control and co-infected mice at the selected time points (Fig 3C and 3D), indicating that an ongoing T. brucei infection does not inhibit the intrahepatic replication of the malaria parasite.
Collectively, our data show that the impairment caused by an ongoing T. brucei infection on a subsequent liver infection by P. berghei stems exclusively from a marked reduction in the number of P. berghei-infected hepatocytes, and is independent from the P. berghei’s ability to replicate inside these cells.
Lymphocytes activated by T. brucei prevent hepatocyte invasion by P. berghei
Given that the inhibitory effect of T. brucei on Plasmodium hepatic infection was only observed when P. berghei sporozoites were injected at least two days after trypanosome infection (Fig 2B), we hypothesized that this inhibitory effect could result from an immune response mounted against T. brucei infection. To test this hypothesis, we first assessed the liver immune landscape in P. berghei-only, T. brucei-only and co-infected mice by multi-parameter flow cytometry analysis of hepatic cells collected at a time point corresponding to 6 h after sporozoite injection. We found that whereas the relative proportions of liver immune cell subsets were not significantly different between the different groups of mice, the total number of leukocytes in the livers of T. brucei- and co-infected mice was increased relative to P. berghei-only or non-infected mice (S6 Fig). Microscopy analysis of histology liver sections revealed that, as early as three days after inoculation of T. brucei, there was minimal tissue damage, with apoptosis of few hepatocytes, and discrete foci of neutrophil infiltration (Fig 4A and 4B). At day 5 of T. brucei infection, hepatocellular apoptosis was marked and associated with multifocal infiltration by a population of mononuclear cells (Fig 4A and 4B), mostly composed of macrophages and lymphocytes (Fig 4A).
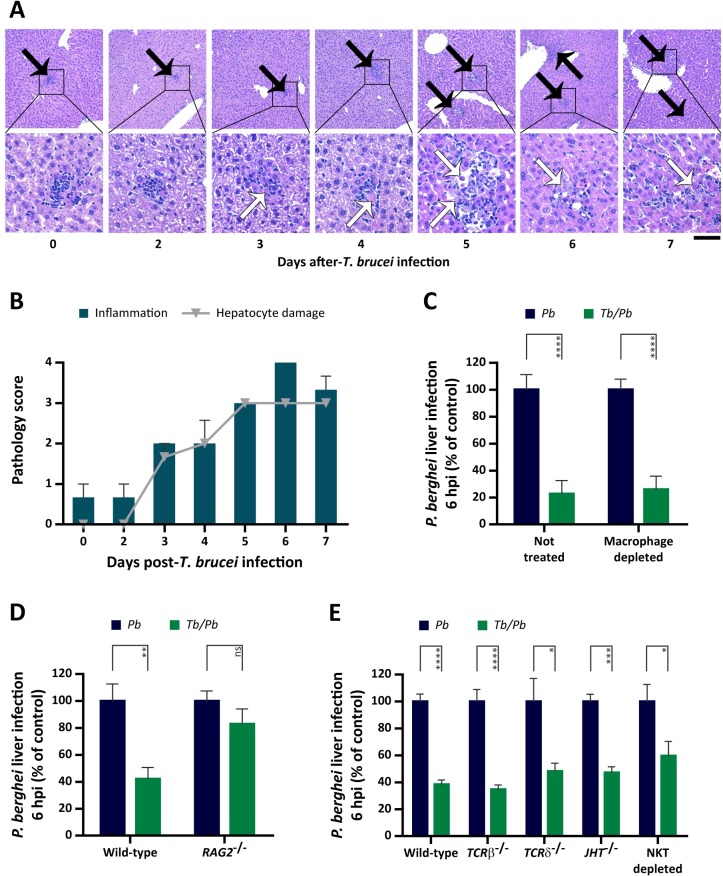
Fig 4. T. brucei-activated lymphocytes are required to inhibit P. berghei liver infection.
(A) Representative microphotographs of liver from non-infected and T. brucei-infected mice (3–4 mice per time-point); depicted are the inflammatory cell infiltrates (black arrow) and hepatocellular damage/apoptosis seen at different time-points (XX axis) of T. brucei infection. Hematoxylin and Eosin. Original magnification 10x (upper panel; scale bar, 200 μm) and 40x (lower panel; scale bar, 50 μm). (B) Graphic representation of the severity of inflammatory cell infiltration (bars) and hepatocellular damage (dots), both scored through histopathology using a 5-tier system with 0–4 grading scale (0, absent; 1, minimal; 2, mild; 3, moderate; 4, marked). Time points indicated on the XX-axis correspond to the days of T. brucei infection. (C) P. berghei liver infection load quantified by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb–blue bars) or mice infected 5 days earlier with T. brucei (Tb/Pb–green bars), non- or clodronate-treated 48 h prior to P. berghei infection. Bars represent the mean values of four independent experiments and error bars indicate the SEM. (D) P. berghei liver infection load quantification by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into wild-type and RAG2-/- mice, either naïve (Pb–blue bars) or infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of three independent experiments and error bars indicate the SEM. (E) P. berghei liver infection load quantification by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into wild-type, TCRβ-/-, TCRδ-/-, JHT-/- and NKT depleted mice, either naïve (Pb–blue bars) or infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of two to three independent experiments with error bars indicating the SEM. For C to E, the Mann-Whitney test was employed to assess the statistical significance of differences between the experimental groups. ns, not significant, * P < 0.05, *** P < 0.001 and **** P < 0.0001.
Since macrophages are able to eliminate sporozoites [34], we tested whether macrophages recruited to the liver during a T. brucei infection could be responsible for eliminating Plasmodium in this co-infection model. To this end, phagocytic cells were depleted from both naïve and T. brucei-infected mice by administration of clodronate-filled liposomes 48 h prior to sporozoite injection. The efficiency of phagocytic cell depletion was confirmed by quantification of mRNA levels of specific macrophage marker genes (Clec4f, F4/80 and CD68) in the liver [35], which showed a significant reduction in the number of macrophages in clodronate-treated mice (S7A–S7C Fig). We observed that phagocytic cell-depleted co-infected mice displayed a reduced P. berghei liver load, similar to that observed in non-treated co-infected mice (Figs 4C and S8), indicating that the impairment of P. berghei liver infection at 6 hpi by a primary T. brucei infection is independent of macrophages.
To assess the involvement of lymphocytes on the reduced ability of P. berghei sporozoites to establish a liver infection in the presence of T. brucei, mice genetically deficient for T and B cells (RAG2-/-) were co-infected and Plasmodium liver load was assessed 6 h after sporozoite injection. We observed that, in contrast to wild-type co-infected mice, which displayed a ~50% reduction in P. berghei liver load at this time point, RAG2-/- co-infected mice displayed a P. berghei liver burden similar to that observed in RAG2-/- single-infected mice (Figs 4D and S8). These results show that lymphocytes activated during a T. brucei infection inhibit a subsequent hepatocyte infection by P. berghei sporozoites. To identify the lymphocyte sub-population responsible for the observed phenotype, co-infections were performed in three distinct knock-out mouse strains, which lack either B, αβ or γδ T cells (JHT-/-, TCRβ-/- and TCRδ-/-, respectively), as well as in mice depleted of NKT cells by injection of the anti-NK1.1 antibody. The efficiency of NKT cell depletion was confirmed by flow cytometry analysis (S7D and S7E Fig). We observed that, in the absence of each of these cell types, an ongoing T. brucei infection still impaired a subsequent Plasmodium infection (Figs 4E and S8), indicating that none of these subsets of cells is, on its own, responsible for the phenotype under investigation.
Overall, our data show that a T. brucei infection results in a strong inflammatory response in the liver that includes mononucleated immune cells. Although lymphocytes are necessary for T. brucei’s inhibitory effect, our results suggest that different lymphocyte subsets may synergistically mediate the observed impairment of P. berghei liver infection.
Interferon-γ levels correlate positively with the impairment of P. berghei liver infection
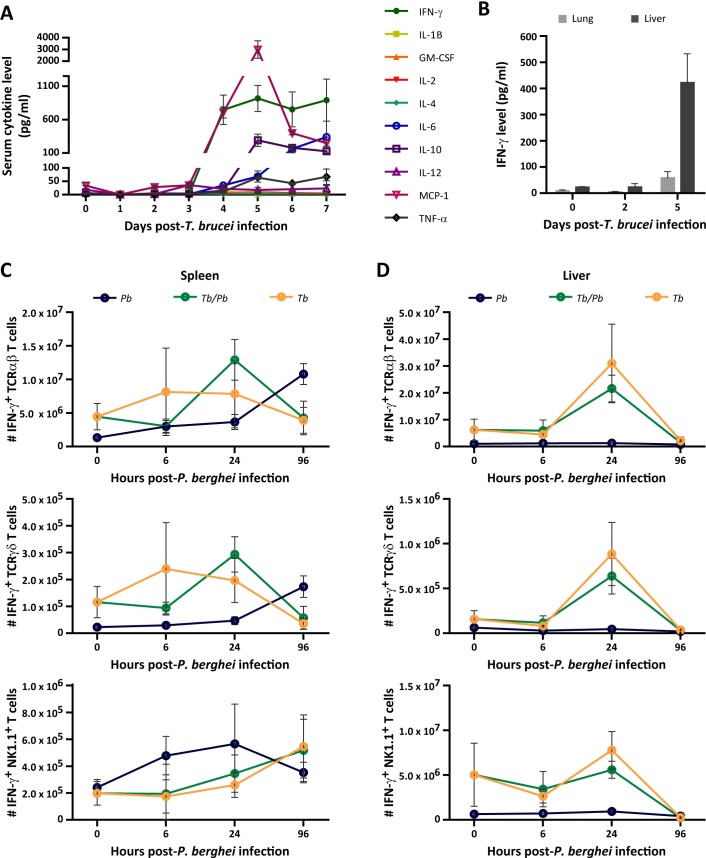
Having demonstrated that the impairment of P. berghei liver infection by T. brucei is mediated by lymphocytes, and that this effect does not depend on a single lymphocyte population, we hypothesized that it could be dependent on the release of a specific factor produced by more than one lymphocyte subset. To investigate this, we first determined the serum levels of a panel of cytokines/chemokines throughout the first week of T. brucei infection. Our results showed that from the fourth day of T. brucei infection onwards, mice displayed an increase in the levels of IFN-γ, IL-1β, IL-2, IL-6, MCP-1 and TNF-α (Fig 5A). MCP-1, a chemokine that promotes migration and infiltration of monocytes/macrophages [36], displayed the highest fold-change increase at day 5 of T. brucei infection. However, given that phagocytic cells are not involved in the inhibition of Plasmodium liver infection by T. brucei (Fig 4C), we suspect that MCP-1 may not be crucial for mediating this impairment.
Fig 5. T. brucei elicits a strong immune response systemically and in the liver.
(A) IFN-γ, IL-1B, GM-CSF, IL-2, IL-4, IL-6, IL-10, IL-12p70, MCP-1 and TNF-α quantification in serum by immunoassay from mice non-infected or infected only with T. brucei. The time points indicated on the XX-axis correspond to the days of T. brucei infection. Dots represent the mean values of three to four mice from one independent experiment with error bars indicating the SEM. (B) Quantification of IFN-γ by immunoassay in the lung (light grey bar) and liver (dark grey bar) of both non-infected mice and mice infected for two and five days with T. brucei. The time points indicated on the XX-axis correspond to the days of T. brucei infection. Bars represent the mean values of three to four mice from one independent experiment and error bars indicate the SEM. (C-D) Multi-parameter flow cytometry-based quantification of IFN-γ-producing TCRαβ, TCRγδ and NK1.1+ lymphocytes in the spleens (C) and livers (D) of mice infected only with P. berghei (Pb—blue dots), infected with P. berghei on the fifth day of T. brucei infection (Tb/Pb–green dots) and infected for 5 days only with T. brucei (Tb—yellow dots). The time points indicated on the XX-axis correspond to the time after injection of 3 x 104 P. berghei sporozoites, with 0 h corresponding to day 5 of T. brucei infection. Dots represent the mean values of four mice from one independent experiment with error bars indicating SD.
The second most abundant cytokine in the serum was IFN-γ, which is in agreement with previous reports [37, 38]. Next, we assessed IFN-γ levels in the liver, where the impairment of P. berghei infection is observed, and in the lungs, as a negative control. Our results showed that IFN-γ levels were 18-fold higher in the livers of T. brucei-infected mice than in those of control naïve mice, in contrast with the lung, where IFN-γ levels were similar in T. brucei-infected and in control mice (Fig 5B).
To confirm the importance of IFN-γ on the observed phenotype, we assessed the extent of protection against P. berghei hepatic infection and the corresponding levels of IFN-γ mRNA in the liver, following drug-mediated elimination of trypanosomes. To this end, mice infected with T. brucei for 4 days were treated with berenil and infected with P. berghei sporozoites 1 or 4 days post-treatment. We observed that the inhibitory effect of T. brucei on P. berghei hepatic infection 6 hpi is maintained when sporozoites are injected 1 day after the drug treatment but is significantly decreased when P. berghei is inoculated 4 days after berenil administration (S9A Fig), indicating that the protective effect of trypanosomes is progressively lost as they are eliminated from circulation. Crucially, the levels of IFN-γ mRNA in the livers of these mice correlated positively with the extent of protection against P. berghei hepatic infection by T. brucei (S9B Fig). Additionally, we quantified the levels of IFN-γ mRNA in the livers of RAG2-/-, as well as of TCRβ-/-, TCRδ-/-, JHT-/- or NKT cell-depleted co-infected mice. Our results showed that in co-infected mice lacking individual subsets of lymphocytes, the levels of IFN-γ transcripts were similar to those detected in co-infected wild-type mice (S10 Fig), consistent with the fact that, in these mice, the inhibitory effect of T. brucei on P. berghei liver infection was still observed (Fig 4E). In contrast, IFN-γ levels were ~7-fold lower in RAG2-/- than in wild-type co-infected mice (S10 Fig). Collectively, these results support a pivotal role for this cytokine in the phenotype under study.
We then sought to identify the systemic and hepatic cellular sources of IFN-γ. We quantified the number of IFN-γ-producing cells in spleen, as a proxy for systemic responses, and in the liver, at different times of infection of P. berghei-only, T. brucei-only and co-infected mice. We observed that IFN-γ production during the liver stage of P. berghei infection is substantially enhanced in all cell subsets from both organs, except for splenic NK1.1+ lymphocytes, from T. brucei- and co-infected mice relative to P. berghei-only or non-infected mice (Fig 5C and 5D). These observations confirm that T. brucei infection leads to the production of IFN-γ by several subsets of lymphocytes, and that IFN-γ production is not abrogated by the individual absence of any of these cell populations (S10A Fig).
Interferon-γ produced during T. brucei infection inhibits hepatocyte invasion by P. berghei
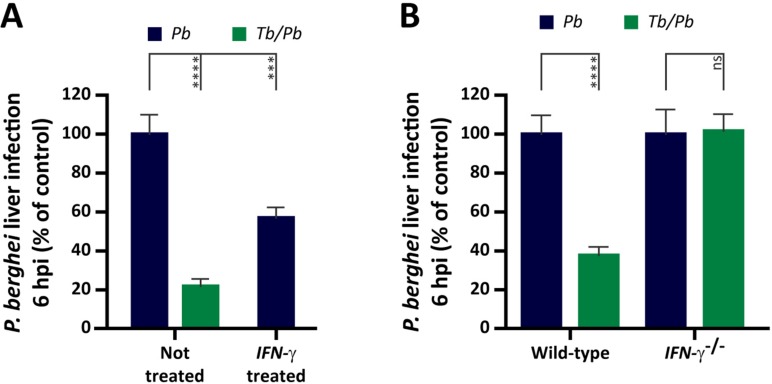
Mice treated with IFN-γ prior to Plasmodium sporozoite injection display a reduction in the ensuing liver infection [22–24, 39]. Since these studies have only assessed Plasmodium liver burden at 46 hpi, it remains unknown which stage of the Plasmodium liver infection was inhibited by IFN-γ. Thus, we wondered whether mice treated with IFN-γ would display a reduction in P. berghei hepatic load as early as 6 hpi. It has been described that only 0.2% of the IFN-γ administered i.p. reaches the serum, and that its half-life is ~2.16 h [40]. Therefore, to obtain IFN-γ serum levels in naïve mice equivalent to those present in the serum of mice infected with T. brucei for 5 days, 0.5 μg of recombinant IFN-γ was injected i.p. into mice 2 h prior to sporozoite injection, at the time of infection, and 2 h later. qRT-PCR analysis of liver samples collected 6 h after sporozoite injection revealed a reduction in the liver parasite load of mice pre-treated with IFN-γ similar to that observed in co-infected mice (Fig 6A).
Fig 6. IFN-γ mediates the inhibitory effect of T. brucei on P. berghei liver infection.
(A) P. berghei liver infection load quantification by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (blue bar), IFN-γ-treated mice (blue bar), or mice infected 5 days earlier with T. brucei (green bar). Bars represent the mean values of four independent experiments and error bars indicate the SEM. The one-way ANOVA with post-test Dunnett was employed to assess the statistical significance of differences between experimental groups. *** P < 0.001 and **** P < 0.0001. (B) P. berghei liver infection load quantification by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into wild-type and IFN-γ-/- mice, either naïve (Pb–blue bars) or infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of three independent experiments and error bars indicate the SEM. The Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups. ns, not significant and **** P < 0.0001.
To assess the function of IFN-γ in the T. brucei-mediated impairment of hepatocyte invasion by P. berghei, IFN-γ deficient mice (IFN-γ-/-) were subjected to the co-infection protocol described above. We found that, in contrast to wild-type mice, but similarly to RAG2-/- mice, the P. berghei liver load of IFN-γ-/- co-infected mice was comparable to that of single-infected IFN-γ-/- mice (Figs 6B and S8), indicating that IFN-γ is necessary for the phenotype under study. Importantly, administration of recombinant IFN-γ to RAG2-/- mice led to a partial reduction of Plasmodium liver load (S10B Fig), although less pronounced than that observed for wild-type mice (Fig 6A). This difference in magnitude is not surprising, as it is known that lymphocytes may respond to IFN-γ by further producing this cytokine [41], an effect that is absent in RAG2-/- mice, which lack T and B lymphocytes.
Collectively, our data show that a primary T. brucei infection leads to the production of an array of pro-inflammatory cytokines/chemokines, among which lymphocyte-derived IFN-γ. We conclude that IFN-γ produced during a T. brucei infection is required for the impairment of a subsequent hepatocyte infection by P. berghei. Importantly, our results show for the first time that IFN-γ exerts a marked inhibitory effect during the very early stages (up to 6 hpi) of Plasmodium liver infection.
Discussion
Several studies have demonstrated that the course of a Plasmodium infection can be influenced by the presence of a second pathogen in the same host, either by aggravating malaria-associated pathology [21, 42], or by conferring protection against disease severity [43, 44]. However, despite the geographical overlap between the etiological agents of sleeping sickness and malaria, whether an ongoing infection by T. brucei has an impact on a subsequent infection by Plasmodium had hitherto not been assessed. We hypothesized that the strong immune response elicited by a T. brucei infection [45] could limit the establishment and/or development of a subsequent infection by the malaria parasite. Our results showed that mice with a patent T. brucei infection, and detectable parasitemia, and subsequently infected with P. berghei sporozoites, display a reduction in the ensuing liver infection by the malaria parasite, relative to mice infected only with P. berghei. We further showed that the impairment of P. berghei liver infection is observed from very early stages of hepatic infection by the malaria parasite, and that the number of Plasmodium intrahepatic forms in co-infected mice continuously decreases until the end of the liver stage of infection.
Liver damage is a frequent sign of pathology during African Trypanosomiasis [46–49], and mononuclear cell infiltrates have been observed in the livers of T. brucei-infected animals, at a late stage of infection [47, 50, 51]. In fact, liver mononuclear immune cells, including resident Kupffer cells, have been suggested to play a critical role in trypanosome clearance from the bloodstream [52–54]. The histopathology analysis of liver sections collected from mice infected only by T. brucei revealed that hepatocyte damage can be observed as early as 3 days after inoculation with trypanosomes, and might result from the infiltration and multifocal distribution of inflammatory cells [55]. Of note, when sporozoites were administered to mice on the second day of T. brucei infection, a time when the livers of those mice were still undamaged, P. berghei liver infection in co-infected mice was similar to that observed in P. berghei single-infected mice. Conversely, we found that mice infected for 5 days with T. brucei, corresponding to the day of P. berghei sporozoite injection in our studies, displayed significant liver inflammation and hepatocellular damage. Therefore, we hypothesized that the hyperinflammatory state of mice infected with T. brucei could be responsible for the inability of P. berghei sporozoites to establish a liver infection. We showed that T. brucei-activated lymphocytes are required to impair a subsequent P. berghei infection, and showed that this impairment is mediated by IFN-γ. During a T. brucei infection, liver NK, NKT and CD8+ T cells are the early sources of IFN-γ, which is required to further recruit neutrophils and monocytes that are responsible for the direct elimination of trypanosomes [38, 54, 56]. Interestingly, it has also been shown that a primary infection by P. yoelii triggers a type-I interferon signaling cascade, leading to an enrichment of NKT cells in the liver, which produce IFN-γ and inhibit a subsequent infection by the same parasite [57]. We found that the levels of IFN-γ mRNA in the livers of T. brucei-infected mice lacking T and B cells were significantly lower than those observed in wild-type control mice. Interestingly, IFN-γ and IL-4 have been implicated in the protection conferred by Schistosoma against a subsequent P. yoelii hepatic infection [58]. Although T. brucei does not promote the production of IL-4, the load of IFN-γ appears to be sufficient to protect against a Plasmodium infection.
Treatment with IFN-γ, both in vitro and in vivo, leads to a reduction in the number of P. berghei intrahepatic forms present at the late stages of P. berghei liver infection [22–24, 39]. Our results reveal for the first time that IFN-γ reduces the number of P. berghei intrahepatic forms as early as 6 hours post-sporozoite injection. Both a general increase in the number of leukocytes recruited to the liver and an overall enhancement in IFN-γ production are observed in T. brucei-only- and co-infected mice, indicating that, during the co-infection, the immune response is mainly dictated by the primary infection. Since IFN-γ levels throughout infection by T. brucei have been shown to closely mirror its parasitemia curve [59], the impairment in P. berghei infection shown in Fig 2B follows a similar trend to those of both T. brucei parasitemia and IFN-γ serum levels. Additionally, the progressively smaller inhibitory effect of trypanosomes as they are eliminated from circulation by drug treatment is accompanied by a decrease in the levels of IFN-γ mRNA in the liver, which further supports the conclusion that IFN-γ is a critical factor in the T. brucei-mediated impairment of P. berghei liver infection. Also of note, co-infected mice display a marked increase in the number of hepatic cells capable of producing IFN-γ at 24 h after P. berghei sporozoite injection (Fig 5D), which may contribute to explaining the progressive decrease observed in the number of EEFs from 6 to 46 hpi (Fig 3B).
Despite IFN-γ‘s implication in the recruitment of macrophages to the liver and in their activation during the early phases of a T. brucei infection [54, 60–62], and the capacity of macrophages to eliminate sporozoites upon the establishment of a P. berghei liver infection [34], our results do not suggest an involvement of these cells in the impairment of P. berghei liver infection by T. brucei. Since the IFN-γ-mediated elimination of Plasmodium intrahepatic forms has been shown to either involve nitric oxide production [23] or the induction of noncanonical autophagy pathway [63], we propose that at least one of these processes may dictate the impairment of P. berghei hepatic infection by T. brucei. Further investigation is required to fully clarify the mechanism whereby IFN-γ mediates the impairment of the establishment of P. berghei hepatic infection in the context of a co-infection with T. brucei.
In a S. mansoni/P. chabaudi co-infection model, increased levels of IFN-γ render A/J co-infected mice less susceptible to infection by the malaria parasite, relative to that observed in schistosome-free A/J mice [64]. In contrast, mice co-infected with T. crassiceps and P. yoelli survived longer than mice infected with the malaria parasite only, which the authors correlated with a reduction in IFN-γ levels [65]. These studies point to a crucial role for IFN-γ in modulating a secondary Plasmodium infection. Interestingly, in mice infected only with Plasmodium, divergent roles for IFN-γ on the development of severe malaria have been described (reviewed in [66]). Whereas early production of IFN-γ has been shown to protect against ECM [67], IFN-γ production by CD4+ T cells can lead to CD8+ T cell accumulation in the brain and promotes the development of ECM [68]. We showed that T. brucei-mediated protection against erythrocytic Plasmodium infection and ECM is not time-restricted and still occurs when P. berghei sporozoites are injected 15 days after the primary infection, a time when T. brucei parasitemia is detectable, and serum IFN-γ levels are high [59]. Our data further reveal an early peak in the number of hepatocytes that are able to produce IFN-γ at 24 h after P. berghei sporozoite injection in co-infected mice (Fig 5D), consistent with that reported to confer protection against ECM [67]. Collectively, these observations suggest that, besides its inhibitory effect on P. berghei hepatic infection, IFN-γ may also have an impact on malaria-associated pathology.
During a T. cruzi infection, NK cells are the major source of IFN-γ which is required to limit parasite replication [69]. Interestingly, mice co-infected with T. cruzi and P. berghei do not develop ECM symptoms, presumably due to a reduction in the accumulation of CD8+ T cells in the brain of those animals [44]. Therefore, during a T. brucei/P. berghei co-infection, the accumulation of IFN-γ by T. brucei-activated lymphocytes prior to the onset of Plasmodium parasitemia may limit the accumulation of CD8+ T cells and reduce malaria severity in the co-infected mice.
Our results showed that the reduction of P. berghei hepatic infection by T. brucei prevented the appearance of Plasmodium parasitemia in 50% and in 100% of C57Bl/6J and BALB/cByJ co-infected mice, respectively. We further observed that co-infected mice that developed P. berghei parasitemia did not display symptoms of severe malaria and survived significantly longer than mice that had not been exposed to trypanosomes. In order to assess the impact of trypanosomiasis on the blood stage of Plasmodium infection, the liver stage of the malaria parasite's life cycle was bypassed by inoculating P. berghei-infected RBCs into T. brucei-infected or control mice. Although P. berghei parasitemia became detectable in all the mice with an ongoing T. brucei infection, co-infected mice never presented severe malaria symptoms and survived longer than mice that had not been infected with trypanosomes prior to P. berghei inoculation. Collectively, our results show for the first time that a primary T. brucei infection renders mice more resistant to malaria, which happens as a consequence not only of a T. brucei-dependent reduction in the P. berghei hepatic infection, but also of a direct effect of the T. brucei infection on a P. berghei blood infection.
To the best of our knowledge, this is the first report addressing whether the outcome of an infection with the malaria parasite is affected by an ongoing T. brucei infection. Our results show that a primary T. brucei infection leads to the production of an array of pro-inflammatory cytokines/chemokines, among which lymphocyte-derived IFN-γ, which significantly reduces a subsequent hepatic infection by P. berghei sporozoites. Since IFN-γ and T cells have been extensively implicated in vaccine-mediated protection against Plasmodium liver infection (reviewed in [70–72]), it would be interesting to assess the impact of an ongoing infection by a pathogen that elicits a high IFN-γ response, such as T. brucei, on the efficiency of malaria vaccination. Moreover, the ensuing P. berghei blood infection was absent in most of the co-infected mice and for those in which the malaria parasite could establish a blood infection, the co-infected mice never presented severe symptoms and survived longer. In view of the geographical overlap between infections by T. brucei and Plasmodium, it would be interesting to assess whether individuals suffering from sleeping sickness in these regions display some degree of protection against severe malaria.
Materials and methods
Mice
Male C57BL/6J and BALB/cByJ mice (6–8 weeks old) were purchased from Charles River Laboratories (Lyon, France). RAG2-/-, TCRβ-/- and JHT-/- mice (7–10 weeks old) were bred and obtained from the specific pathogen-free (SPF) facilities at Instituto Gulbenkian de Ciência (Oeiras, Portugal). TCRδ-/- and IFN-γ-/- mice (7–10 weeks old), kindly provided by Bruno Silva-Santos, were bred in SPF facilities at Instituto de Medicina Molecular João Lobo Antunes (iMM JLA) (Lisbon, Portugal). All the animals were housed and kept in the SPF rodent facility of the iMM JLA, a licensed establishment that complies with Directive 2010/63/EU.
Ethics statement
All the experimental animal work was performed in strict compliance to the guidelines of our institution’s animal ethics committee, who also approved this study (under authorization AWB_2015_09_MP_Malaria), and in accordance with the Federation of European Laboratory Animal Science Associations (FELASA) guidelines.
Parasites and infections
The transgenic 90–13 cell-line of the Trypanosoma brucei brucei AnTaT 1.1 pleomorphic strain [73] (hereafter referred to as T. brucei) was employed throughout this study. Mice were infected by intraperitoneal (i.p.) injection of 2 x 103 motile parasites, obtained from cryostabilates, as previously described [8, 50]. GFP [74]- or luciferase [75]-expressing Plasmodium berghei ANKA rodent malaria parasites (hereafter referred to as P. berghei) and Plasmodium yoelii yoelii 17XNL [76] (hereafter referred to as P. yoelii) were used throughout this study. For the assessment of mosquito feeding preferences, 6–10 P. berghei-infected mosquitoes were allowed to feed on uninfected or T. brucei-infected mice for 30 min in the dark, and the percentage of mosquitoes that ingested a blood meal was determined by visual inspection of their midguts. For intravenous (i.v.) sporozoite injections, P. berghei and P. yoelii sporozoites were obtained by dissection of salivary glands from infected female Anopheles stephensi mosquitoes, reared at iMM JLA. Mice were infected by retro-orbital i.v. injection of 500 or 3 x 104 sporozoites, under isoflurane anesthesia. P. berghei-infected RBCs (iRBCs) were obtained from an infected homologous donor mouse. Mice were infected by i.p. injection of 1 x 106 iRBCs.
Assessment of experimental cerebral malaria (ECM) symptoms
ECM development was monitored daily using the rapid murine coma and behavior scale (RMCBS) score, as described in [77]. Mice with RMCBS score equal or below 5/20 were classified as displaying ECM symptoms and euthanized immediately.
Assessment of parasitemia
T. brucei parasitemia was assessed by counting the number of trypanosomes in blood samples, collected from the tail vein, using an Olympus CKX31 inverted microscope (Olympus, Tokyo, Japan). P. berghei parasitemia was monitored daily by flow cytometry, using a drop of tail-blood diluted in phosphate-buffered saline (PBS), as previously described [78]. GFP-expressing parasites were detected in the green fluorescent channel, FL1-H, using a BD LSRFortessa X-20 (BD Biosciences, Franklin Lakes, NJ, USA) flow cytometer with the FACSDiva software (version 8.0, BD BIOSCIENCES, Franklin Lakes, NJ, USA). The collected data was after analyzed with FlowJo software (version 10.0, Ashland, OR, USA) and results are expressed as the percentage of iRBCs in the sample.
Quantification of in vivo P. berghei hepatic infection
Mouse hepatic infection was assessed at either 30 min, 2 h, 6 h, 12 h, 24 h or 46 h after P. berghei sporozoite inoculation and quantified either by real-time in vivo imaging employing the IVIS Lumina Imaging System (Caliper LifeSciences, Waltham, MA, USA), as previously described [75], or by quantitative real-time reverse transcriptase-PCR (qRT-PCR), also as previously described [79]. P. berghei bioluminescence was measured as total flux (photons/s) and analyzed with the Living Image software (version 3.0, PerkinElmer, Waltham, MA, USA). For qRT-PCR analyses, 0.7–0.9 mg of livers collected by dissection of infected mice were mechanically homogenized in TRIzol (BioRad, Hercules, CA, USA), RNA was extracted following the manufacturer’s instructions and converted into complementary DNA (cDNA) as described below. Liver P. berghei load was quantified by qRT-PCR, as previously described [80], using primers specific for P. berghei 18S rNA (Table 1). Expression of the endogenous mouse housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (Hprt) was used for normalization.
Table 1. List of primer sequences.
| Target gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| P. berghei 18S rRNA | AAGCATTAAATAAAGCGAATACATCCTTAC | GGAGATTGGTTTTGACGTTTATGTG |
| CLEC4f | TGAGTGGAATAAAGAGCCTCCC | TCATAGTCCCTAAGCCTCTGGA |
| CD68 | AGCTGCCTGACAAGGGACACT | AGGAGGACCAGGCCAATGAT |
| F4/80 | CCCAGCTTATGCCACCTGCA | TCCAGGCCCTGGAACATTGG |
| IFN-γ | CACACTGCATCTTGGCTTTG | TCTGGCTCTGCAGGATTTTC |
| Hprt | TTTGCTGACCTGCTG GATTAC | CAAGACATTCTTTCCAGTTAAAGTTG |
RNA extraction, complementary DNA synthesis and qRT-PCR
RNA was extracted following the manufacturer’s instructions and quantified using a NanoDrop DR 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA USA). cDNA was synthesized from 1 μg of RNA, using the NZYTech cDNA synthesis kit (NZYTech, Lisbon, Portugal), according to the manufacturer’s recommendations, and employing the following thermocycling parameters: 25°C for 10 min, 55°C for 30 min, and 85°C for 5 min. The qRT-PCR reaction was performed in a total volume of 10 μl reaction employing an Applied Biosystems StepOne Plus equipment (Applied Biosystems, Foster City, CA, USA), using the SYBR Green PCR Master Mix (Applied Biosystems) and the following thermocycling parameters: 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 15 s and 60°C for 1 min, melting stage was done at 95°C for 15 s, 60°C for 1 min, and 95°C for 30 s. Primer pairs used to detect target gene transcripts are listed in Table 1. Gene expression was analyzed by the comparative CT method (ΔΔCT) and the expression level of all target genes was normalized to that of Hprt.
Immunofluorescence microscopy analysis of in vivo P. berghei hepatic infection
Livers were collected at either 6, 12, 24 or 46 h after P. berghei sporozoite inoculation, and fixed in 4% (v/v) paraformaldehyde (PFA) (SantaCruz Biotechnology, Dallas, TX, USA) for at least 12 h at room temperature (RT). Liver sections of 50 μm thickness were stained and analyzed as previously described [79, 81]. Briefly, slices were incubated in permeabilization/blocking solution containing 1% (w/v) bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) and 0.5% (v/v) Triton-X100 in PBS (Sigma-Aldrich) at RT for 1 h, followed by a 2 h incubation at RT with an anti-UIS4 antibody (goat, homemade; dilution 1:500). Liver sections were further incubated for 1 hour in a 1:500 dilution of anti-GFP-Alexa 488 antibody (Invitrogen, Carlsbad, CA, USA) and anti-goat Alexa-Fluor 568 (Invitrogen, Carlsbad, CA, USA) in the presence of a 1:1,000 dilution of Hoechst 33342 (Invitrogen, Carlsbad, CA, USA). After washing, liver sections were mounted on microscope slides with Fluoromount (SouthernBiotech, Birmingham, AL, USA). Widefield images for P. berghei intrahepatic forms’ size determination were acquired employing a Zeiss Axiovert 200M microscope (Carl Zeiss, Oberkochen, Germany). Confocal images were acquired using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany). Images were processed with the ImageJ software (version 1.47, NIH, Bethesda, MD, USA).
Treatment of T. brucei infection
Trypanosomes were eliminated by i.p. injection of 250 ng of Berenil (Sigma-Aldrich), 4 days after T. brucei infection. P. berghei sporozoites were inoculated into treated mice 1 or 4 days after drug administration. Livers were collected 6 h after sporozoite injection and processed as described above for the quantification of P. berghei liver load by qRT-PCR.
Liver histopathology
Livers collected from mice infected for 2 to 7 days with T. brucei or from non-infected mice used as controls were formalin-fixed in neutral buffered formalin, paraffin-embedded, cut in 4 μm sections, and stained with hematoxylin (Bio-Optica, Milan, Italy) and eosin (Thermo Fisher Scientific). Tissue sections were analyzed by a pathologist blinded to experimental groups in a Leica DM2000 microscope coupled to a Leica MC170 HD microscope camera (Leica Microsystems, Wetzlar, Germany). Inflammatory cell infiltration and hepatocellular damage were scored using a 5-tier system with 0–4 grading scale (0, absent; 1, minimal; 2, mild; 3, moderate; 4, marked).
Isolation of liver and spleen leukocytes
Livers and spleens were collected at 0 h, 6 h, 24 h and 96 h after P. berghei sporozoite inoculation, mechanically homogenized in PBS containing 2 U/ml DNAse (Sigma-Aldrich), using a 100 μm cell strainer, and centrifuged at 410 x g for 8 min at room temperature (RT). Liver cell pellets were further fractionated using a 35% (v/v) Percoll gradient medium (Sigma-Aldrich) diluted in Roswell Park Memorial Institute 1640 medium (RPMI) (Gibco-Thermo Fisher Scientific, Waltham, MA USA), and centrifuged at 1,360 x g for 20 min without brake at 20°C. Liver and spleen cell pellets were washed with PBS and centrifuged at 410 x g for 8 min at RT. RBCs were depleted by a 3 min incubation at RT with ammonium-chloride-potassium solution, which was stopped by washing with PBS 2% FBS (Gibco-Thermo Fisher Scientific) (FACS buffer). The leukocyte suspension was centrifuged at 410 x g for 8 min at RT and resuspended in PBS for subsequent staining.
Analysis of liver and spleen leukocytes by flow cytometry
One million leukocytes from each mouse were plated in 96-well plates, centrifuged at 845 x g for 3 min at 4°C and incubated with α-CD16/CD32 (eBioscience/Thermo Fisher Scientific, Waltham, MA, USA) for 20 min at 4°C. Liver and/or spleen leukocytes were there surface-stained for 20 min at 4°C using appropriate combinations of the following fluorochrome-conjugated anti-mouse monoclonal antibodies: FITC or eFluor450anti-TCRγδ (clone GL3), PE anti-NKp46 (clone 29A1.4), PerCP/Cy5.5 anti-CD3ɛ (clone 145-2C11), PE or PE/Cy7 anti-NK1.1 (clone PK136), Alexa Fluor 647 anti-SiglecF (clone ES0-2440), PE/Cy7 anti-Ly6G (clone AE8), FITC or APC/Cy7 anti-CD11b (clone M1/70), Brilliant Violet (BV)421 or BV510 anti-CD4 (clone RM4-5), BV510 or Alexa Fluor 700 anti-CD45 (clone 30-F11), BV605 anti-CD19 (clone 6D5), BV605 anti-Ly6C (clone HK1.4), and BV711 anti-CD8α (clone 53–6.7), plus eFluor 506 or eFluor 780 Fixable Viability Dye (eBioscience, Thermo Fisher Scientific) for dead cell exclusion. All antibodies were from eBioscience (Thermo Fisher Scientific) or Biolegend. Cells were acquired on a BD LSRFortessa or LSRFortessa X-20 (BD Biosciences) and data acquisition and analysis were carried out using the FACSDiva (version 6.2) and FlowJo (version 10.5.3, FlowJo) software packages, respectively.
In vivo depletion of macrophages
Macrophages were depleted by i.v. injection of 1 mg of liposome-encapsulated clodronate (Liposoma B.V., Amsterdam, The Netherlands), 48 h prior to P. berghei sporozoite inoculation. An equivalent volume of liposome-encapsulated PBS was injected i.v. into control mice. Livers were collected 6 h after sporozoite injection and processed as described above for the quantification of P. berghei liver load by qRT-PCR.
In vivo depletion of Natural Killer (NK) and Natural Killer T cells (NKT)
NK and NKT cells were depleted by i.v. injection of 200 μg of anti-NK1.1 (clone PK136, Bio X Cell, West Lebanon, NH, USA), 24 hours prior to P. berghei sporozoite inoculation. Livers were collected 6 h after sporozoite injection and processed as described above for the quantification of the P. berghei liver load by qRT-PCR.
Quantification of serum, liver and lung cytokines/chemokines
Blood was collected by heart puncture of mice infected 2 to 7 days earlier with T. brucei. After clotting, samples were centrifuged at 1,000 x g for 10 min at 4°C, and serum was transferred to a fresh tube. Livers and lungs were obtained by dissecting mice infected 2 to 7 days earlier with T. brucei and homogenized in lysis buffer containing 20 mM Tris HCl, pH 7.5, 0.5% (v/v) Tween 20, 150 mM NaCl, and 1% (v/v) protease inhibitor cocktail in PBS. Tissue homogenates were centrifuged at 10,000 x g for 10 min at 4°C and the supernatants transferred to fresh tubes. Uninfected mice were used as controls. The serum levels of an array of pro-inflammatory cytokines/chemokines (IFN-γ, IL-1B, GM-CSF, IL-2, IL-4, IL-6, IL-10, IL-12p70, MCP-1 and TNF-α) were determined using the multiplexing addressable laser bead immunoassay (Eve Technologies, Calgary, Canada).
Quantification of IFN-γ-producing lymphocytes in the liver and spleen by flow cytometry
One million liver or spleen leukocytes were stimulated in a 96-well plate for 4 h in the presence of 50 ng/ml PMA, 500 ng/mL ionomycin and 10 μg/ml brefeldin (all from Sigma). After this period, cells were Fc-blocked (using α-CD16/CD32) and surface-stained, as described above, using the following monoclonal antibodies: FITC anti-CD11b, eFluor450 anti-TCRγδ, Alexa Fluor 700 anti-CD45 and PE/Cy5.5 anti-NK1.1, plus eFluor 780 fixable viability dye for dead cells discrimination. Cells were then washed with FACS buffer, followed by fixation and permeabilization using the Transcription Factor Staining Buffer Set, according to the manufacturer’s instructions (eBioscience, Thermo Fisher Scientific). Intracellular staining was performed for 30 min at 4°C using BV510 anti-CD4, BV711 anti-CD8α, PE anti-IFN-γ (clone XMG1.2; BD Pharmingen) and PE/Cy7 anti-CD3ɛ. Cells were washed, resuspended in FACS buffer and acquired on a BD LSRFortessa X-20 (BD Biosciences). Data was analyzed using FlowJo v10.5.3 (FlowJo, LLC).
In vivo treatment with IFN-γ
IFN-γ was administered to mice by i.p. injection of 0.5 μg of recombinant murine IFN-γ (PeproTech, Rocky Hill, NJ, USA), 2 hours prior to inoculation of P. berghei sporozoites, at the time of infection and 2 h later. Livers were collected 6 h after sporozoite injection and processed as described above for the quantification of the P. berghei liver load by qRT-PCR.
Statistical analyses
Data are expressed as mean ± standard deviation (SD) or standard error of the mean (SEM). Statistical analyses were performed using the GraphPad Prism 6 software (La Jolla, CA, USA). Significant differences were determined using One-way analysis of variance (ANOVA), Log-Rank (Mantel-Cox) test, Two-way ANOVA or Two-tailed Mann-Whitney test. Significances are represented as indicated in each figure: ns–not significant, * P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001.
Supporting information
Assessment of mosquito feeding on uninfected or T. brucei-infected BALB/cByJ (A) or C56BL/6J (B) mice. Percentage of mosquitoes that ingested a blood meal and SEM of the pooled data of 15 mice from three independent experiments for each mouse strain are shown. Each dot represents one mouse exposed to the bites of 6 (1 experiment per mouse strain) or 10 (2 experiments per mouse strain) P. berghei-infected mosquitoes.
(TIF)
(A) Assessment of P. berghei parasitemia by flow cytometry after inoculation of 500 sporozoites into naïve BALB/cByJ mice (Pb—blue line) or BALB/cByJ mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of iRBCs and SEM of the pooled data of 10 mice from two independent experiments is shown. (B) Assessment of P. berghei parasitemia by flow cytometry after inoculation of 500 sporozoites into naïve C57BL/6J mice (Pb—blue line) or C57BL/6J mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of iRBCs and SEM of the pooled data of 10 mice from two independent experiments are shown.
(TIF)
Optical microscopy-based assessment of T. brucei parasitemia in C57BL/6J mice infected only with trypanosomes (Tb–light green line) or inoculated with 1 x 106 P. berghei-iRBCs 5 days after infection with T. brucei (Tb/Pb–dark green line). The geometrical means of the number of trypanosomes per ml of blood and SD of the pooled data of 5 mice from one experiment are shown.
(TIF)
P. yoelii liver infection load determined by qRT-PCR 46 h after sporozoite injection into naïve mice (blue bar) or mice previously infected by T. brucei (green bar). Bars represent the mean values of two independent experiments and error bars indicate the SEM. The Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups (*** P < 0.001).
(TIF)
(A) Assessment of P. berghei prepatency period following inoculation of 3 x 104 P. berghei sporozoites into naïve C57BL/6J mice (Pb - blue line) or C57BL/6J mice infected 15 days earlier with T. brucei (Tb/Pb - green line). Percentage of mice displaying P. berghei parasitemia, as measured by flow cytometry. The pooled data of 4–6 mice from one independent experiment is shown. (B) Assessment of P. berghei parasitemia by flow cytometry after inoculation of 3 x 104 GFP-expressing P. berghei sporozoites into naïve C57BL/6J mice (Pb–blue line) or C57BL/6J mice infected 15 days earlier with T. brucei (Tb/Pb–green line). Percentage of iRBCs and SEM of the pooled data of 4–6 mice from one independent experiment are shown. (C) Mouse survival following inoculation of 3 x 104 P. berghei sporozoites into naïve C57BL/6J mice (Pb–blue line) or C57BL/6J mice infected 15 days earlier with T. brucei (Tb/Pb–green line). The pooled data of 4–6 mice from one independent experiment is shown. Time window for ECM development is depicted by the grey-shaded area.
(TIF)
Multi-parameter flow cytometry-based quantification of leukocytes in the liver of mice not infected, infected for 5 days with T. brucei (Tb), co-infected with P. berghei and T. brucei (Tb/Pb), or infected only with P. berghei (Pb). Livers were collected 6 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb) or mice infected 5 days earlier with T. brucei (Tb/Pb). Cell numbers are presented for the following populations (analyzed within live CD45+ cells; n = 4 per group): monocytes/macrophages (CD3neg NK1.1neg CD11b+ Ly6Gneg), neutrophils (CD3neg NK1.1neg CD11b+ Ly6G+), γδ T cells (CD3+ TCRγδ+), CD8+ or CD4+ T cells (CD3+ TCRγδneg NK1.1neg CD8+ or CD4+, respectively) and NK1.1+ cells (CD3neg/+ TCRγδneg NK1.1+).
(TIF)
(A-C) Clec4f, F4/80 and CD68 gene expression quantification by qRT-PCR in the liver 6 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb–blue bars) or mice infected 5 days earlier with T. brucei (Tb/Pb–green bars), non (solid)- or clodronate (patterned)-treated 48 h prior to P. berghei infection. Bars represent the mean values of four independent experiments and error bars indicate the SEM. Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups. ** P < 0.001 and **** P < 0.0001. (D) Representative plots of flow cytometry gating strategy to analyze NK/NKT cells. (E) Assessment of NK/NKT depletion efficiency by flow cytometry in the liver 6 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb–blue) or mice infected 5 days earlier with T. brucei (Tb/Pb–green), injected (open circles) or not (solid circles) with anti-NK1.1 antibody. Results represent the mean values of one representative experiment out of two independent experiments and error bars indicate the SEM. Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups. ns, not significant and ** P < 0.01.
(TIF)
P. berghei liver infection load quantification by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into wild-type, macrophage depleted, RAG2-/-, TCRβ-/-, TCRδ-/-, JHT-/-, NKT depleted and IFN-γ-/- mice, either naïve (Pb–blue bars) or infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of each experimental group normalized to P. berghei-infected wild-type controls, of two to three independent experiments, with error bars indicating the SEM. The Mann-Whitney test was employed to assess the statistical significance of differences between the experimental groups. ns, not significant, * P < 0.05, *** P < 0.001 and **** P < 0.0001.
(TIF)
(A) Left: P. berghei liver infection load quantification by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into untreated (solid bars) or berenil-treated (patterned bars) mice, either naïve (blue bars), or infected 5 or 8 days earlier with T. brucei (green bars). Right: treatment and infections schedule. Berenil was administered to mice 4 days after T. brucei inoculation and mice were subsequently infected with P. berghei sporozoites 1 or 4 days after berenil treatment. Bars represent the mean values of 5 mice from one independent experiment and error bars indicate the SEM. (B) Quantification of IFN-γ gene expression by qRT-PCR in the liver 6 h after injection of 3 x 104 P. berghei sporozoites into untreated (solid bars) or berenil-treated (patterned bars) mice, either naïve (blue bars), or infected 5 or 8 days earlier with T. brucei (green bars). Bars represent the mean values of 5 mice from one independent experiment and error bars indicate the SEM.
(TIF)
(A) Quantification of IFN-γ gene expression by qRT-PCR in the liver 6 h after injection of 3 x 104 P. berghei sporozoites into wild-type, RAG2-/-, TCRβ-/-, TCRδ-/-, JHT-/- and NKT depleted mice, either naïve (Pb–blue bars) or infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of two to three independent experiments and error bars indicate the SEM. (B) qRT-PCR- based quantification of P. berghei liver infection load 6 h after injection of 3 x 104 P. berghei sporozoites into naïve or IFN-γ-treated RAG2-/- mice. Bars represent the mean values of two independent experiments and error bars indicate the SEM. For A and B, the Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups. ns, not significant, * P < 0.05, ** P < 0.01.
(TIF)
Acknowledgments
We are very grateful to Karine Serre and Ângelo Chora for the intellectual input and critical discussion; to Ângelo Chora for kindly providing IFN-γ; to Bruno Silva-Santos for kindly providing TCRδ-/- and IFN-γ-/- mice; to Hiroshi Kubo for kindly providing NK1.1 antibody and the protocol of NK depletion; to Diana Fontinha and Diana Moita for the help in experiments involving flow cytometry; to Ana Parreira and Filipa Teixeira for producing Plasmodium-infected mosquitoes; and to the Bioimaging, Flow Cytometry, Comparative Pathology and Rodent facilities of iMM JLA for technical support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by Fundação para a Ciência e Tecnologia, Portugal (FCT) grants UID/BIM/50005/2019 (Ministério da Ciência, Tecnologia e Ensino Superior (MCTES) through Fundos do Orçamento de Estado) and PTDC-SAU-INF-29550-2017 to M.P. M.S.-V. was supported by a FCT fellowship PD/BD/105838/2014. A.T. was supported by a FCT fellowship PD/BD/138891/2018. A.M.M. was supported by a FCT fellowship SFRH/BPD/80693/2011. L.M.F. was supported by Howard Hughes Medical Institute (55007419) and FCT grant (PTDC/BIM-MET/4471/2014). M.P. was supported by FCT Investigador FCT 2013 and CEEC 2018 fellowships. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cox FE. Concomitant infections, parasites and immune responses. Parasitology. 2001;122 Suppl:S23–38. Epub 2001/07/10. 10.1017/s003118200001698x . [DOI] [PubMed] [Google Scholar]
- 2.Griffiths EC, Pedersen AB, Fenton A, Petchey OL. The nature and consequences of coinfection in humans. J Infect. 2011;63(3):200–6. Epub 2011/06/28. 10.1016/j.jinf.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14(11):1118–26. Epub 2013/10/23. 10.1038/ni.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salam N, Mustafa S, Hafiz A, Chaudhary AA, Deeba F, Parveen S. Global prevalence and distribution of coinfection of malaria, dengue and chikungunya: a systematic review. BMC Public Health. 2018;18(1):710 Epub 2018/06/09. 10.1186/s12889-018-5626-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwenti TE. Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies. Res Rep Trop Med. 2018;9:123–36. Epub 2018/08/14. 10.2147/RRTM.S154501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotez PJ, Molyneux DH. Tropical anemia: one of Africa's great killers and a rationale for linking malaria and neglected tropical disease control to achieve a common goal. PLoS Negl Trop Dis. 2008;2(7):e270 Epub 2008/07/31. 10.1371/journal.pntd.0000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prudencio M, Rodriguez A, Mota MM. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol. 2006;4(11):849–56. Epub 2006/10/17. 10.1038/nrmicro1529 . [DOI] [PubMed] [Google Scholar]
- 8.Trindade S, Rijo-Ferreira F, Carvalho T, Pinto-Neves D, Guegan F, Aresta-Branco F, et al. Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice. Cell Host Microbe. 2016;19(6):837–48. Epub 2016/05/31. 10.1016/j.chom.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capewell P, Cren-Travaille C, Marchesi F, Johnston P, Clucas C, Benson RA, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5 Epub 2016/09/23. 10.7554/eLife.17716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansfield JM, Paulnock DM. Regulation of innate and acquired immunity in African trypanosomiasis. Parasite Immunol. 2005;27(10–11):361–71. Epub 2005/09/24. 10.1111/j.1365-3024.2005.00791.x . [DOI] [PubMed] [Google Scholar]
- 11.Ponte-Sucre A. An Overview of Trypanosoma brucei Infections: An Intense Host-Parasite Interaction. Front Microbiol. 2016;7:2126 Epub 2017/01/14. 10.3389/fmicb.2016.02126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum J, Schmid C, Burri C. Clinical aspects of 2541 patients with second stage human African trypanosomiasis. Acta Trop. 2006;97(1):55–64. Epub 2005/09/15. 10.1016/j.actatropica.2005.08.001 . [DOI] [PubMed] [Google Scholar]
- 13.Priotto G, Pinoges L, Fursa IB, Burke B, Nicolay N, Grillet G, et al. Safety and effectiveness of first line eflornithine for Trypanosoma brucei gambiense sleeping sickness in Sudan: cohort study. BMJ. 2008;336(7646):705–8. Epub 2008/03/07. 10.1136/bmj.39485.592674.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLean LM, Odiit M, Chisi JE, Kennedy PG, Sternberg JM. Focus-specific clinical profiles in human African Trypanosomiasis caused by Trypanosoma brucei rhodesiense. PLoS Negl Trop Dis. 2010;4(12):e906 Epub 2010/12/15. 10.1371/journal.pntd.0000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagira JM, Maina N, Njenga J, Karanja SM, Karori SM, Ngotho JM. Prevalence and types of coinfections in sleeping sickness patients in kenya (2000/2009). J Trop Med. 2011;2011:248914 Epub 2011/09/15. 10.1155/2011/248914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuepfer I, Hhary EP, Allan M, Edielu A, Burri C, Blum JA. Clinical presentation of T.b. rhodesiense sleeping sickness in second stage patients from Tanzania and Uganda. PLoS Negl Trop Dis. 2011;5(3):e968 Epub 2011/03/17. 10.1371/journal.pntd.0000968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillet P, Mumba Ngoyi D, Lukuka A, Kande V, Atua B, van Griensven J, et al. False positivity of non-targeted infections in malaria rapid diagnostic tests: the case of human african trypanosomiasis. PLoS Negl Trop Dis. 2013;7(4):e2180 Epub 2013/05/03. 10.1371/journal.pntd.0002180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato CD, Nanteza A, Mugasa C, Edyelu A, Matovu E, Alibu VP. Clinical profiles, disease outcome and co-morbidities among T. b. rhodesiense sleeping sickness patients in Uganda. PLoS One. 2015;10(2):e0118370 Epub 2015/02/27. 10.1371/journal.pone.0118370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maina NW, Kagira JM, Oberle M, Ndung'u JJ, Brun R. Co-infection of sleeping sickness patients with malaria and loiasis in southern Sudan. Journal of Protozoology Research. 2010;20(2):12–9. [Google Scholar]
- 20.Millott SM, Cox FE. Interactions between Trypanosoma brucei and Babesia spp. and Plasmodium spp. in mice. Parasitology. 1985;90 (Pt 2):241–54. 10.1017/s0031182000050952 . [DOI] [PubMed] [Google Scholar]
- 21.Ademola IO, Odeniran PO. Co-infection with Plasmodium berghei and Trypanosoma brucei increases severity of malaria and trypanosomiasis in mice. Acta Trop. 2016;159:29–35. 10.1016/j.actatropica.2016.03.030 . [DOI] [PubMed] [Google Scholar]
- 22.Ferreira A, Schofield L, Enea V, Schellekens H, van der Meide P, Collins WE, et al. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986;232(4752):881–4. 10.1126/science.3085218 . [DOI] [PubMed] [Google Scholar]
- 23.Mellouk S, Green SJ, Nacy CA, Hoffman SL. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991;146(11):3971–6. . [PubMed] [Google Scholar]
- 24.Vergara U, Ferreira A, Schellekens H, Nussenzweig V. Mechanism of escape of exoerythrocytic forms (EEF) of malaria parasites from the inhibitory effects of interferon-gamma. J Immunol. 1987;138(12):4447–9. . [PubMed] [Google Scholar]
- 25.Prudencio M, Mota MM, Mendes AM. A toolbox to study liver stage malaria. Trends Parasitol. 2011;27(12):565–74. 10.1016/j.pt.2011.09.004 . [DOI] [PubMed] [Google Scholar]
- 26.Vaughan JA, Scheller LF, Wirtz RA, Azad AF. Infectivity of Plasmodium berghei sporozoites delivered by intravenous inoculation versus mosquito bite: implications for sporozoite vaccine trials. Infect Immun. 1999;67(8):4285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheller LF, Wirtz RA, Azad AF. Susceptibility of different strains of mice to hepatic infection with Plasmodium berghei. Infect Immun. 1994;62(11):4844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wykes MN, Good MF. What have we learnt from mouse models for the study of malaria? Eur J Immunol. 2009;39(8):2004–7. 10.1002/eji.200939552 . [DOI] [PubMed] [Google Scholar]
- 29.Zuzarte-Luis V, Sales-Dias J, Mota MM. Simple, sensitive and quantitative bioluminescence assay for determination of malaria pre-patent period. Malar J. 2014;13:15 10.1186/1475-2875-13-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sa ECC, Nyboer B, Heiss K, Sanches-Vaz M, Fontinha D, Wiedtke E, et al. Plasmodium berghei EXP-1 interacts with host Apolipoprotein H during Plasmodium liver-stage development. Proc Natl Acad Sci U S A. 2017;114(7):E1138–E47. 10.1073/pnas.1606419114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeil J, Sepp KJ, Heiss K, Meister M, Mueller AK, Borrmann S. Protection against malaria by immunization with non-attenuated sporozoites under single-dose piperaquine-tetraphosphate chemoprophylaxis. Vaccine. 2014;32(45):6005–11. 10.1016/j.vaccine.2014.07.112 . [DOI] [PubMed] [Google Scholar]
- 32.Lewis MD, Behrends J, Sa ECC, Mendes AM, Lasitschka F, Sattler JM, et al. Chemical attenuation of Plasmodium in the liver modulates severe malaria disease progression. J Immunol. 2015;194(10):4860–70. 10.4049/jimmunol.1400863 . [DOI] [PubMed] [Google Scholar]
- 33.Friesen J, Silvie O, Putrianti ED, Hafalla JC, Matuschewski K, Borrmann S. Natural immunization against malaria: causal prophylaxis with antibiotics. Sci Transl Med. 2010;2(40):40ra9 10.1126/scitranslmed.3001058 . [DOI] [PubMed] [Google Scholar]
- 34.Tavares J, Formaglio P, Thiberge S, Mordelet E, Van Rooijen N, Medvinsky A, et al. Role of host cell traversal by the malaria sporozoite during liver infection. J Exp Med. 2013;210(5):905–15. 10.1084/jem.20121130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51(2):511–22. 10.1002/hep.23337 . [DOI] [PubMed] [Google Scholar]
- 36.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26. 10.1089/jir.2008.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Namangala B, Noel W, De Baetselier P, Brys L, Beschin A. Relative contribution of interferon-gamma and interleukin-10 to resistance to murine African trypanosomosis. J Infect Dis. 2001;183(12):1794–800. 10.1086/320731 . [DOI] [PubMed] [Google Scholar]
- 38.Cnops J, De Trez C, Stijlemans B, Keirsse J, Kauffmann F, Barkhuizen M, et al. NK-, NKT- and CD8-Derived IFNgamma Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia. PLoS Pathog. 2015;11(6):e1004964 10.1371/journal.ppat.1004964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schofield L, Ferreira A, Altszuler R, Nussenzweig V, Nussenzweig RS. Interferon-gamma inhibits the intrahepatocytic development of malaria parasites in vitro. J Immunol. 1987;139(6):2020–5. . [PubMed] [Google Scholar]
- 40.Hofstra CL, Van Ark I, Hofman G, Nijkamp FP, Jardieu PM, Van Oosterhout AJ. Differential effects of endogenous and exogenous interferon-gamma on immunoglobulin E, cellular infiltration, and airway responsiveness in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1998;19(5):826–35. 10.1165/ajrcmb.19.5.3027 . [DOI] [PubMed] [Google Scholar]
- 41.Girdlestone J, Wing M. Autocrine activation by interferon-gamma of STAT factors following T cell activation. Eur J Immunol. 1996;26(3):704–9. 10.1002/eji.1830260329 . [DOI] [PubMed] [Google Scholar]
- 42.Normark J, Nelson M, Engstrom P, Andersson M, Bjork R, Moritz T, et al. Maladjusted host immune responses induce experimental cerebral malaria-like pathology in a murine Borrelia and Plasmodium co-infection model. PLoS One. 2014;9(7):e103295 10.1371/journal.pone.0103295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nacher M, Singhasivanon P, Traore B, Vannaphan S, Gay F, Chindanond D, et al. Helminth infections are associated with protection from cerebral malaria and increased nitrogen derivatives concentrations in Thailand. Am J Trop Med Hyg. 2002;66(3):304–9. 10.4269/ajtmh.2002.66.304 . [DOI] [PubMed] [Google Scholar]
- 44.Egima CM, Macedo SF, Sasso GR, Covarrubias C, Cortez M, Maeda FY, et al. Co-infection with Trypanosoma cruzi protects mice against early death by neurological or pulmonary disorders induced by Plasmodium berghei ANKA. Malar J. 2007;6:90 10.1186/1475-2875-6-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stijlemans B, Caljon G, Van Den Abbeele J, Van Ginderachter JA, Magez S, De Trez C. Immune Evasion Strategies of Trypanosoma brucei within the Mammalian Host: Progression to Pathogenicity. Front Immunol. 2016;7:233 10.3389/fimmu.2016.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amole BO, Clarkson AB Jr., Shear HL. Pathogenesis of anemia in Trypanosoma brucei-infected mice. Infect Immun. 1982;36(3):1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anosa VO, Kaneko JJ. Pathogenesis of Trypanosoma brucei infection in deer mice (Peromyscus maniculatus). Ultrastructural pathology of the spleen, liver, heart, and kidney. Vet Pathol. 1984;21(2):229–37. 10.1177/030098588402100216 . [DOI] [PubMed] [Google Scholar]
- 48.Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2010;375(9709):148–59. 10.1016/S0140-6736(09)60829-1 . [DOI] [PubMed] [Google Scholar]
- 49.Stijlemans B, De Baetselier P, Magez S, Van Ginderachter JA, De Trez C. African Trypanosomiasis-Associated Anemia: The Contribution of the Interplay between Parasites and the Mononuclear Phagocyte System. Front Immunol. 2018;9:218 10.3389/fimmu.2018.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rijo-Ferreira F, Carvalho T, Afonso C, Sanches-Vaz M, Costa RM, Figueiredo LM, et al. Sleeping sickness is a circadian disorder. Nat Commun. 2018;9(1):62 10.1038/s41467-017-02484-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Muylder G, Daulouede S, Lecordier L, Uzureau P, Morias Y, Van Den Abbeele J, et al. A Trypanosoma brucei kinesin heavy chain promotes parasite growth by triggering host arginase activity. PLoS Pathog. 2013;9(10):e1003731 10.1371/journal.ppat.1003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dempsey WL, Mansfield JM. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol. 1983;130(1):405–11. . [PubMed] [Google Scholar]
- 53.Albright JW, Long GW, Albright JF. The liver as a major site of immunological elimination of murine trypanosome infection, demonstrated with the liver perfusion model. Infect Immun. 1990;58(6):1965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosschaerts T, Guilliams M, Stijlemans B, De Baetselier P, Beschin A. Understanding the role of monocytic cells in liver inflammation using parasite infection as a model. Immunobiology. 2009;214(9–10):737–47. 10.1016/j.imbio.2009.06.010 . [DOI] [PubMed] [Google Scholar]
- 55.Guicciardi ME, Malhi H, Mott JL, Gores GJ. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3(2):977–1010. 10.1002/cphy.c120020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caljon G, Mabille D, Stijlemans B, De Trez C, Mazzone M, Tacchini-Cottier F, et al. Neutrophils enhance early Trypanosoma brucei infection onset. Sci Rep. 2018;8(1):11203 10.1038/s41598-018-29527-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SHI. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014;7(2):436–47. 10.1016/j.celrep.2014.03.018 . [DOI] [PubMed] [Google Scholar]
- 58.Moriyasu T, Nakamura R, Deloer S, Senba M, Kubo M, Inoue M, et al. Schistosoma mansoni infection suppresses the growth of Plasmodium yoelii parasites in the liver and reduces gametocyte infectivity to mosquitoes. PLoS Negl Trop Dis. 2018;12(1):e0006197 10.1371/journal.pntd.0006197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez R, Demick KP, Mansfield JM, Paulnock DM. Type I IFNs play a role in early resistance, but subsequent susceptibility, to the African trypanosomes. J Immunol. 2008;181(7):4908–17. 10.4049/jimmunol.181.7.4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murray PK, Jennings FW, Murray M, Urquhart GM. The nature of immunosuppression in Trypanosoma brucei infections in mice. I. The role of the macrophage. Immunology. 1974;27(5):815–24. [PMC free article] [PubMed] [Google Scholar]
- 61.Shi M, Pan W, Tabel H. Experimental African trypanosomiasis: IFN-gamma mediates early mortality. Eur J Immunol. 2003;33(1):108–18. 10.1002/immu.200390013 . [DOI] [PubMed] [Google Scholar]
- 62.Shi M, Wei G, Pan W, Tabel H. Trypanosoma congolense infections: antibody-mediated phagocytosis by Kupffer cells. J Leukoc Biol. 2004;76(2):399–405. 10.1189/jlb.1003500 . [DOI] [PubMed] [Google Scholar]
- 63.Boonhok R, Rachaphaew N, Duangmanee A, Chobson P, Pattaradilokrat S, Utaisincharoen P, et al. LAP-like process as an immune mechanism downstream of IFN-gamma in control of the human malaria Plasmodium vivax liver stage. Proc Natl Acad Sci U S A. 2016;113(25):E3519–28. 10.1073/pnas.1525606113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshida A, Maruyama H, Kumagai T, Amano T, Kobayashi F, Zhang M, et al. Schistosoma mansoni infection cancels the susceptibility to Plasmodium chabaudi through induction of type 1 immune responses in A/J mice. Int Immunol. 2000;12(8):1117–25. 10.1093/intimm/12.8.1117 . [DOI] [PubMed] [Google Scholar]
- 65.Salazar-Castanon VH, Juarez-Avelar I, Legorreta-Herrera M, Govezensky T, Rodriguez-Sosa M. Co-infection: the outcome of Plasmodium infection differs according to the time of pre-existing helminth infection. Parasitol Res. 2018;117(9):2767–84. 10.1007/s00436-018-5965-9 . [DOI] [PubMed] [Google Scholar]
- 66.King T, Lamb T. Interferon-gamma: The Jekyll and Hyde of Malaria. PLoS Pathog. 2015;11(10):e1005118 10.1371/journal.ppat.1005118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell AJ, Hansen AM, Hee L, Ball HJ, Potter SM, Walker JC, et al. Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun. 2005;73(9):5645–53. 10.1128/IAI.73.9.5645-5653.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villegas-Mendez A, Greig R, Shaw TN, de Souza JB, Gwyer Findlay E, Stumhofer JS, et al. IFN-gamma-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol. 2012;189(2):968–79. 10.4049/jimmunol.1200688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64(1):128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165(3):1453–62. 10.4049/jimmunol.165.3.1453 . [DOI] [PubMed] [Google Scholar]
- 71.Mendes AM, Scholzen A, Mueller A-K, Shahid M. Khan, Sauerwein RW, Prudêncio M. Whole-Sporozoite Malaria Vaccines In: Mota MM, Rodriguez A, editors. Malaria. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 72.Beeson JG, Kurtovic L, Dobano C, Opi DH, Chan JA, Feng G, et al. Challenges and strategies for developing efficacious and long-lasting malaria vaccines. Sci Transl Med. 2019;11(474). 10.1126/scitranslmed.aau1458 . [DOI] [PubMed] [Google Scholar]
- 73.Engstler M, Boshart M. Cold shock and regulation of surface protein trafficking convey sensitization to inducers of stage differentiation in Trypanosoma brucei. Genes Dev. 2004;18(22):2798–811. 10.1101/gad.323404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137(1):23–33. 10.1016/j.molbiopara.2004.04.007 . [DOI] [PubMed] [Google Scholar]
- 75.Ploemen IH, Prudencio M, Douradinha BG, Ramesar J, Fonager J, van Gemert GJ, et al. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One. 2009;4(11):e7881 10.1371/journal.pone.0007881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin JW, Annoura T, Sajid M, Chevalley-Maurel S, Ramesar J, Klop O, et al. A novel 'gene insertion/marker out' (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS One. 2011;6(12):e29289 10.1371/journal.pone.0029289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carroll RW, Wainwright MS, Kim KY, Kidambi T, Gomez ND, Taylor T, et al. A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One. 2010;5(10). 10.1371/journal.pone.0013124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liehl P, Zuzarte-Luis V, Chan J, Zillinger T, Baptista F, Carapau D, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med. 2014;20(1):47–53. 10.1038/nm.3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mendes AM, Albuquerque IS, Machado M, Pissarra J, Meireles P, Prudencio M. Inhibition of Plasmodium Liver Infection by Ivermectin. Antimicrob Agents Chemother. 2017;61(2). 10.1128/AAC.02005-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruna-Romero O, Hafalla JC, Gonzalez-Aseguinolaza G, Sano G, Tsuji M, Zavala F. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol. 2001;31(13):1499–502. 10.1016/s0020-7519(01)00265-x . [DOI] [PubMed] [Google Scholar]
- 81.Meireles P, Sales-Dias J, Andrade CM, Mello-Vieira J, Mancio-Silva L, Simas JP, et al. GLUT1-mediated glucose uptake plays a crucial role during Plasmodium hepatic infection. Cell Microbiol. 2017;19(2). 10.1111/cmi.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of mosquito feeding on uninfected or T. brucei-infected BALB/cByJ (A) or C56BL/6J (B) mice. Percentage of mosquitoes that ingested a blood meal and SEM of the pooled data of 15 mice from three independent experiments for each mouse strain are shown. Each dot represents one mouse exposed to the bites of 6 (1 experiment per mouse strain) or 10 (2 experiments per mouse strain) P. berghei-infected mosquitoes.
(TIF)
(A) Assessment of P. berghei parasitemia by flow cytometry after inoculation of 500 sporozoites into naïve BALB/cByJ mice (Pb—blue line) or BALB/cByJ mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of iRBCs and SEM of the pooled data of 10 mice from two independent experiments is shown. (B) Assessment of P. berghei parasitemia by flow cytometry after inoculation of 500 sporozoites into naïve C57BL/6J mice (Pb—blue line) or C57BL/6J mice infected 5 days earlier with T. brucei (Tb/Pb—green line). Percentage of iRBCs and SEM of the pooled data of 10 mice from two independent experiments are shown.
(TIF)
Optical microscopy-based assessment of T. brucei parasitemia in C57BL/6J mice infected only with trypanosomes (Tb–light green line) or inoculated with 1 x 106 P. berghei-iRBCs 5 days after infection with T. brucei (Tb/Pb–dark green line). The geometrical means of the number of trypanosomes per ml of blood and SD of the pooled data of 5 mice from one experiment are shown.
(TIF)
P. yoelii liver infection load determined by qRT-PCR 46 h after sporozoite injection into naïve mice (blue bar) or mice previously infected by T. brucei (green bar). Bars represent the mean values of two independent experiments and error bars indicate the SEM. The Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups (*** P < 0.001).
(TIF)
(A) Assessment of P. berghei prepatency period following inoculation of 3 x 104 P. berghei sporozoites into naïve C57BL/6J mice (Pb - blue line) or C57BL/6J mice infected 15 days earlier with T. brucei (Tb/Pb - green line). Percentage of mice displaying P. berghei parasitemia, as measured by flow cytometry. The pooled data of 4–6 mice from one independent experiment is shown. (B) Assessment of P. berghei parasitemia by flow cytometry after inoculation of 3 x 104 GFP-expressing P. berghei sporozoites into naïve C57BL/6J mice (Pb–blue line) or C57BL/6J mice infected 15 days earlier with T. brucei (Tb/Pb–green line). Percentage of iRBCs and SEM of the pooled data of 4–6 mice from one independent experiment are shown. (C) Mouse survival following inoculation of 3 x 104 P. berghei sporozoites into naïve C57BL/6J mice (Pb–blue line) or C57BL/6J mice infected 15 days earlier with T. brucei (Tb/Pb–green line). The pooled data of 4–6 mice from one independent experiment is shown. Time window for ECM development is depicted by the grey-shaded area.
(TIF)
Multi-parameter flow cytometry-based quantification of leukocytes in the liver of mice not infected, infected for 5 days with T. brucei (Tb), co-infected with P. berghei and T. brucei (Tb/Pb), or infected only with P. berghei (Pb). Livers were collected 6 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb) or mice infected 5 days earlier with T. brucei (Tb/Pb). Cell numbers are presented for the following populations (analyzed within live CD45+ cells; n = 4 per group): monocytes/macrophages (CD3neg NK1.1neg CD11b+ Ly6Gneg), neutrophils (CD3neg NK1.1neg CD11b+ Ly6G+), γδ T cells (CD3+ TCRγδ+), CD8+ or CD4+ T cells (CD3+ TCRγδneg NK1.1neg CD8+ or CD4+, respectively) and NK1.1+ cells (CD3neg/+ TCRγδneg NK1.1+).
(TIF)
(A-C) Clec4f, F4/80 and CD68 gene expression quantification by qRT-PCR in the liver 6 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb–blue bars) or mice infected 5 days earlier with T. brucei (Tb/Pb–green bars), non (solid)- or clodronate (patterned)-treated 48 h prior to P. berghei infection. Bars represent the mean values of four independent experiments and error bars indicate the SEM. Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups. ** P < 0.001 and **** P < 0.0001. (D) Representative plots of flow cytometry gating strategy to analyze NK/NKT cells. (E) Assessment of NK/NKT depletion efficiency by flow cytometry in the liver 6 h after injection of 3 x 104 P. berghei sporozoites into naïve mice (Pb–blue) or mice infected 5 days earlier with T. brucei (Tb/Pb–green), injected (open circles) or not (solid circles) with anti-NK1.1 antibody. Results represent the mean values of one representative experiment out of two independent experiments and error bars indicate the SEM. Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups. ns, not significant and ** P < 0.01.
(TIF)
P. berghei liver infection load quantification by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into wild-type, macrophage depleted, RAG2-/-, TCRβ-/-, TCRδ-/-, JHT-/-, NKT depleted and IFN-γ-/- mice, either naïve (Pb–blue bars) or infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of each experimental group normalized to P. berghei-infected wild-type controls, of two to three independent experiments, with error bars indicating the SEM. The Mann-Whitney test was employed to assess the statistical significance of differences between the experimental groups. ns, not significant, * P < 0.05, *** P < 0.001 and **** P < 0.0001.
(TIF)
(A) Left: P. berghei liver infection load quantification by qRT-PCR 6 h after injection of 3 x 104 P. berghei sporozoites into untreated (solid bars) or berenil-treated (patterned bars) mice, either naïve (blue bars), or infected 5 or 8 days earlier with T. brucei (green bars). Right: treatment and infections schedule. Berenil was administered to mice 4 days after T. brucei inoculation and mice were subsequently infected with P. berghei sporozoites 1 or 4 days after berenil treatment. Bars represent the mean values of 5 mice from one independent experiment and error bars indicate the SEM. (B) Quantification of IFN-γ gene expression by qRT-PCR in the liver 6 h after injection of 3 x 104 P. berghei sporozoites into untreated (solid bars) or berenil-treated (patterned bars) mice, either naïve (blue bars), or infected 5 or 8 days earlier with T. brucei (green bars). Bars represent the mean values of 5 mice from one independent experiment and error bars indicate the SEM.
(TIF)
(A) Quantification of IFN-γ gene expression by qRT-PCR in the liver 6 h after injection of 3 x 104 P. berghei sporozoites into wild-type, RAG2-/-, TCRβ-/-, TCRδ-/-, JHT-/- and NKT depleted mice, either naïve (Pb–blue bars) or infected 5 days earlier with T. brucei (Tb/Pb–green bars). Bars represent the mean values of two to three independent experiments and error bars indicate the SEM. (B) qRT-PCR- based quantification of P. berghei liver infection load 6 h after injection of 3 x 104 P. berghei sporozoites into naïve or IFN-γ-treated RAG2-/- mice. Bars represent the mean values of two independent experiments and error bars indicate the SEM. For A and B, the Mann-Whitney test was employed to assess the statistical significance of differences between experimental groups. ns, not significant, * P < 0.05, ** P < 0.01.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.