Abstract
Human corneal transplantation (keratoplasty) is typically considered to have superior short- and long-term outcomes and lower requirement for immunosuppression compared to solid organ transplants because of the inherent immune privilege and tolerogenic mechanisms associated with the anterior segment of the eye. However, in a substantial proportion of corneal transplants, the rates of acute rejection and/or graft failure are comparable to or greater than those of the commonly transplanted solid organs. Critically, while registry data and observational studies have helped to identify factors that are associated with increased risk of corneal transplant failure, the extent to which these risk factors operate through enhancing immune-mediated rejection is less clear. In this overview, we summarize a range of important recent clinical and basic insights related to high-risk corneal transplantation, the factors associated with graft failure, and the immunological basis of corneal allograft rejection. We highlight critical research areas from which continued progress is likely to drive improvements in the long-term survival of high-risk corneal transplants. These include further development and clinical testing of predictive risk scores and assays; greater use of multicenter clinical trials to optimize immunosuppressive therapy in high-risk recipients and robust clinical translation of novel, mechanistically-targeted immunomodulatory and regenerative therapies that are emerging from basic science laboratories. We also emphasize the relative lack of knowledge regarding transplant outcomes for infection-related corneal diseases that are common in the developing world and the potential for greater cross-pollination and synergy between corneal and solid organ transplant research communities.
HISTORICAL AND GLOBAL SIGNIFICANCE OF CORNEAL TRANSPLANTATION AND FACTORS ASSOCIATED WITH HIGH IMMUNOLOGICAL RISK
The landmark report by Eduard Zirm in 1905 of a successful full-thickness corneal transplant in a 45-year-old farm laborer with lime burn preceded, by several decades, the subsequent successes of vascularized organ transplants.1,2 Following the introduction of topical corticosteroid therapies in the 1950s, corneal transplantation (keratoplasty) has become established as the primary sight-restoring procedure for corneal blindness in developed and developing countries.3 Furthermore, while partial-thickness (lamellar) keratoplasty has now become the preferred transplant procedure for many corneal disorders,4 full-thickness allograft remains the most frequently utilized treatment worldwide for corneal conditions associated with significant stromal opacity or vascularization such as bacterial, fungal, or viral infections; severe atopic disorders; ocular trauma and prior graft loss. Corneal opacity is reported to be between the second and fourth most common cause of blindness globally, but its prevalence in different geographical regions is poorly understood and is probably underestimated.3,5 In India alone, the number of individuals with unilateral corneal blindness is projected to increase to >10 million by 2020.3,6 In contrast to other causes of blindness, a relatively high proportion of those affected are young, with approximately 20% of childhood blindness attributed to corneal disorders.5 Bilateral corneal disease resulting in total loss of vision is especially common in the developing world.3 Thus, the potential societal impact of global progress in preventing corneal disease and restoring sight for individuals suffering from corneal blindness is substantial.
In contrast to other forms of allogeneic transplantation, corneal allografts are often perceived as having high long-term success rates and little requirement for systemic or lifelong immunosuppression. Notably, however, the successful keratoplasty performed by Zirm in the absence of immunosuppression was carried out on the same day as other corneal transplants, which failed to achieve lasting clarity (including a graft to the contralateral eye of same recipient)—leading the pioneering surgeon to contemplate the risk factors responsible for graft acceptance or failure.1 Since then, outcomes analyses for tens of thousands of full-thickness and lamellar corneal transplants have consistently demonstrated that long-term functional graft survival rates are high for recipients of first transplants with noninflammatory corneal disease such as keratoconus and other corneal dystophies.7 However, other recipient subgroups experience substantially poorer long-term outcomes.7
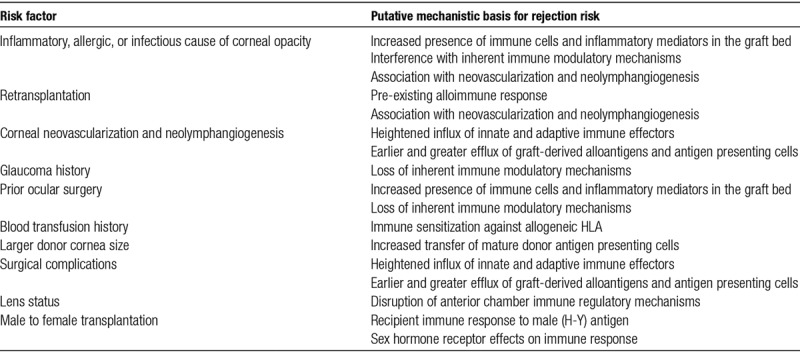
Immunological rejection and its prevention or avoidance lies at the center of corneal transplant prognosis. Specific risk factors for corneal allograft rejection have been well recognized for decades and are generally used to place potential transplant recipients into low- or high-risk categories to decide whether or not to proceed with transplantation and which immunosuppressive regimen to employ.8 In “high-risk” corneal transplant recipients, rejection episodes occur in 30%–60% of grafts and up to 70% fail within 10 years despite local or systemic immunosuppressive therapy.7-9 Common mechanistic features among these factors that may specifically increase the risk of rejection are heightened alloimmune response and/or increased access of the recipient immune system to the corneal tissue and cornea-derived antigens (Table 1). Nonetheless, the extent to which these factors represent independent risks for rejection is not well documented and it seems likely that some mediate adverse effects on corneal transplant survival through nonimmunological mechanisms. Furthermore, as is clear from Table 1, some of the commonly-reported risk factors for rejection and/or graft failure may be interlinked—for example, inflammatory diseases (including rejection of a prior transplant) may promote the formation of new blood and lymph vessels, which subsequently amplify alloantigen presentation and trafficking of immune cells into the graft to trigger rejection.
TABLE 1.
Frequently cited factors contributing to high risk for immunological rejection of corneal transplants and their putative mechanisms
With the accumulation of new knowledge from clinical follow-up analyses and basic science investigations, it is pertinent to ask to what extent these insights have been incorporated into the clinical management of high-risk corneal transplants and to what extent new knowledge has the potential to impact care globally. For example, apart from the assignment of risk level based on the number of corneal quadrants with neovascularization, the clinical use of rejection risk scores or calculators is uncommon. In a recent retrospective study of full-thickness corneal transplant outcomes across a broad risk spectrum, Tourkmani et al determined the predictive value of each component of a 7-factor risk score system for graft failure at 1 year posttransplant. Although limited by cohort size and only focused on host risk factors, the study demonstrated independent predictive value of only 3 of 7 factors: ocular hypertension, ocular inflammation, and corneal neovascularization.10 Given the persistently poor outcomes for high-risk corneal allografts and the critical need for expansion of corneal transplantation in the developing world, it is clear that additional research to improve risk prediction, link it to specific immunological or nonimmunological mechanisms and evaluate targeted posttransplant management protocols is an important priority. To be consistently accurate among diverse patient populations, risk scores may need to take into consideration the underlying cause of corneal disease. It is noteworthy that, while a large proportion of the global burden of sight-threatening corneal disease stems from infection and other highly inflammatory conditions,3,6 much of the detailed risk and outcomes research in corneal transplantation has come from developed countries in which noninflammatory corneal diseases are proportionately more common.
In the remaining sections of this review, we summarize recent advances in knowledge and technology that may be of relevance to the future development of approaches to improve outcomes for corneal transplants at high risk for immunological adverse events linked to poor long-term graft survival.
RECENT REGISTRY AND MULTICENTER OUTCOMES DATA FOR HIGH-RISK CORNEAL TRANSPLANTS
Registry-based data document corneal transplant outcomes on a large scale that is free from single surgeon or center bias. This is particularly important for understanding the effects of practice trends over time on the frequency and outcomes of high-risk transplants. Three important graft registries that provide public outcome data are the Australian Corneal Graft Registry (ACGR), the Swedish Corneal Transplant Register (SCTR), and the UK Transplant Registry (UKTR). The ACGR issues intermittent comprehensive reports with a level of detail beyond that otherwise published and compares risk factors for graft survival via multivariate regression modelling. The most recent ACGR report (2018) included data from 33 920 transplants carried out between 2000 and 2017 and, along with recent outputs from the SCTR,11 UKTR,9 and other data repositories,12,13 has provided several important insights into the current rates of corneal transplant success for specific high-risk categories.
Recipient Cornea Neovascularization
The ACGR report confirms that presence and extent of recipient cornea neovascularization is associated with reduced graft survival. Survival of full-thickness transplants at 4 years in the absence of neovascularization was 83% compared to 73%, 66%, 63%, and 50% for 1-, 2-, 3-, and 4-quadrant neovascularization, respectively.7 In contrast, a 2015 report from the UKTR did not find neovascularization to be a risk factor for rejection of transplant for Fuchs endothelial dystrophy (FED) or pseudophakic bullous keratopathy (PBK), although full-thickness and lamellar transplants were combined in this analysis.9 These results suggest that, even for a well-established risk factor such as neovascularization, there is more to be learned about the underlying pathophysiology and independence from other risk factors for individual transplant indications. For example, in a prevascularized mouse model of high-risk corneal transplantation, it was demonstrated that selective inhibition of (clinically invisible) new lymphatic vessels was sufficient to abrogate the increased allograft rejection associated with neovascularization.14 From a global perspective, it is likely that the pathophysiological basis and risk-modifying effects of neovascularization following infectious and traumatic causes of corneal disease (which are highly prevalent in the developing world) differ from those associated with dystrophic and noninfectious inflammatory conditions, which constitute the major indications for transplantation in the developing countries that generate the majority of registry data.
Repeat Transplantation
Outcome statistics from the past 5 years confirm that retransplantation following failure of one or more corneal allografts to the same eye is associated with high-risk for graft loss regardless of the primary corneal disease.7,11,15 Within the ACGR, average survival at 4 years for a first ipsilateral full-thickness transplant for the low immunological risk condition keratoconus was 95%. This reduced sequentially with subsequent grafts (second, 86%; third, 71%; fourth or subsequent, 56%).7 Recent UKTR data are closely consistent, with 5-year survival of full-thickness transplants for keratoconus being 92% for the first graft compared to 79% for second; 54% for third and 42% for fourth or more.15 Similarly, SCTR data reported failure rates in retransplants to be 17% at 2 years compared to 6% for first transplants.11 Of interest, the ACGR reports that 2 or more preceding grafts in the contralateral eye is an independent risk factor for failure.7
Other Host Factors
Recent data from the ACGR and the USA Cornea Disease Study highlight the significance of inflammation/steroid use and raised intraocular pressure (IOP)/glaucoma before transplantation as important risk factors for reduced graft survival.7,13 For example, average survival of full-thickness corneal transplants at 4 years was 85% compared to 58% for presence or absence of pretransplant inflammation/steroid use and 81% compared to 55% for normal or raised IOP, respectively.7 Similarly, the USA Cornea Disease Study reported 10-year failure rate among 1090 full-thickness transplants for FED or PBK to be 58% if there was a history of glaucoma at the time of transplantation compared to 22% without.13 As noted above, these 2 risk factors were also shown to be independent of corneal neovascularization for prediction of 1-year graft failure by Tourkmeni et al.10 A recent study from the UK involving 5-year outcome analysis of over 18 000 full- and partial-thickness corneal transplants confirmed that donor-recipient gender matching was associated with superior graft survival and, for some indications, with reduced rejection.16 Although the effect of gender matching was attenuated for some high-risk indications, the finding supports the longstanding immunological concept that indirect presentation of minor histocompatibility antigens (such as the male H-Y antigen) plays a dominant role in corneal transplant rejection.16
Perhaps surprisingly, the efficacy of HLA matching in high-risk corneal transplantation remains undetermined.17 In the 1990s, the US based Collaborative Corneal Transplantation Studies failed to demonstrate the efficacy of HLA matching but it was subsequently recognized that the serology-based tissue typing used in the study differed from molecular technique typing in 55% of the cases. Nonrandomized studies typically show a beneficial effect of HLA typing, and HLA matching is still performed in high-risk corneal transplantation at some European centers. In the UK, a prospective, longitudinal clinical trial (CTFS-II) aimed at determining the influence of HLA class II matching (by DNA-based technique) on time to first rejection of high-risk full-thickness corneal transplants has recently completed its enrolment and follow-up and will undoubtedly shed more light on this question.17 Recent studies also support a role for recipient polymorphisms of genes including thrombospondin-1, which are involved in the immune response and regulation of angiogenesis in the anterior eye, in determining rejection risk.18
Surgical Factors
Increased surgeon volume, higher rate of surgeon follow-up, and graft diameter between 7.75 and 8.5 mm were all shown to have independent positive effects on corneal transplant survival in the ACGR.7 In contrast, peripheral iridectomy or anterior vitrectomy at the time of transplantation were not found to be independent risk factors for graft failure.7 The effect of lens status remains less clear. In the ACGR, absence of the lens (aphakia) before or after full-thickness transplantation was associated with reduced graft survival, while lens status was not found to be a significant risk factor for failure in the Cornea Donor Study.7,13 These recent results suggest that, similar to solid organ transplantation, surgeon experience and ongoing specialized follow-up play an important role in the long-term success of corneal allografts. The relative success rates for full-thickness and partial-thickness/lamellar corneal transplants for specific indications and risk categories remain a topic of intense interest among ophthalmic surgeons worldwide and is discussed in more detail below. It should be noted, however, that recent registry reports also provide an important source of noncenter-specific data regarding outcomes for the different transplant procedures.7,15,19
Despite the clear value of registry studies for understanding transplant outcome trends and risk factors for graft loss, it must be acknowledged that data reporting to large national or international registries may be inconsistent in regard to key indices such as diagnosis of rejection and may also become less informative over time due to loss of follow-up.20 Strikingly, the most recent ACGR report provides long-term graft survival data for relatively small numbers of transplants performed for the indications of trauma (638; 2.5%), corneal ulcer (495; 2.0%), and nonherpetic infection (445; 1.8%), rendering the risk factor analyses for these globally common indications less clinically useful.7 Thus, there is also a need for more single- and multicenter prospective studies in which large numbers of corneal transplant recipients are longitudinally followed with a high degree of accuracy and clinical detail.
DEVELOPMENTS IN SURGICAL PROCEDURES AND THEIR IMPLICATIONS FOR HIGH-RISK CORNEAL TRANSPLANTS
Until the late 1990s, full-thickness corneal transplantation was by far the dominant procedure regardless of the type and extent of corneal disease. Since then, several new surgical approaches have been developed to minimize the transplanted volume of corneal donor tissue and, although small, the number of transferred donor antigen presenting cells (APCs).4 These partial-thickness/lamellar corneal transplant procedures have gained widespread acceptance for low-risk conditions. However, their advantages and disadvantages under high-risk conditions and in regard to the impact of immunological rejection are less well established. Figure 1 illustrates the basic anatomy of the cornea and the configurations of the most commonly used transplant procedures. In the following sections, we review some of the most recent clinical research studies involving lamellar corneal transplant procedures. While these studies have primarily involved corneal diseases with relatively low levels of inflammation and rejection risk, the as-yet-unproven potential for lamellar grafts (and other innovations derived from them) to reduce the frequency or severity of acute rejection under high immunological risk conditions represents a critical area for knowledge growth in the coming years.
FIGURE 1.
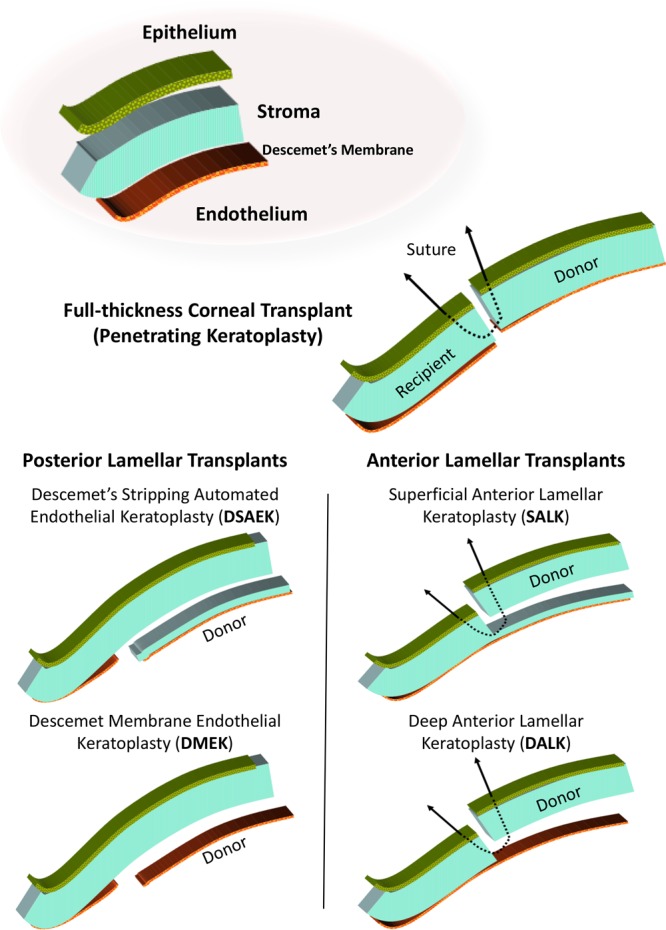
Illustration of the structure of the healthy cornea and the configurations currently in use for full thickness (penetrating keratoplasy) and posterior and anterior partial thickness (lamellar keratoplasty) corneal allo-transplantation.
Treatment of Endothelial Failure
The integrity of the corneal endothelium is essential for maintenance of corneal transparency as it regulates fluid balance between the aqueous humor and corneal stroma. Human corneal endothelial cells have no or very limited regenerative capacity, rendering the endothelial layer particularly vulnerable to dysfunction. Corneal endothelial failure may occur as a result of FED—a bilateral, hereditary, progressive disease of the posterior cornea21—or as a result of secondary bullous keratopathy following intraocular surgery and inflammation. As an alternative to full-thickness transplantation for corneal endothelial failure, partial-thickness/lamellar transplantation (also called endothelial keratoplasty), performed as Descemet stripping automated endothelial keratoplasty (DSAEK) or Descemet membrane endothelial keratoplasty (DMEK), has gained widespread popularity over the past 10 years.4 In these procedures (as illustrated in Figure 1), the recipient corneal endothelium and subendothelial (Descemet’s) membrane are removed in an 8–9 mm diameter and a donor graft consisting of endothelial cells on Descemet’s membrane (in DMEK) or supported by 50–200 µm corneal donor stroma (in DSAEK) is transplanted to the anterior chamber, unfolded and attached to the posterior side of the recipient cornea.4 Although these procedures were mainly developed to accelerate visual rehabilitation, reduce corneal astigmatism, and preserve the integrity of the eye, both techniques also significantly reduce the amount of transplanted allogeneic tissue. In DMEK, theoretically, no donor APCs and only a single layer of donor endothelial cells are transferred to the recipient.
Recent retrospective studies provide evidence that DSAEK or DMEK may have lower rejection rates than full-thickness allografts. For example, among 200 transplants performed for FED, graft rejection episodes were reported in 16% of full-thickness and in 5% of DSAEK recipients up to 2 years after surgery.22 Similarly, in 168 corneal allografts for secondary bullous keratopathy, the 4-year rejection rates for full-thickness and DSAEK recipients were 15% and 4%, respectively.19 In a single-center study of 905 DMEK recipients, rejection episodes were rare with estimated probabilities at 1, 2, and 4 years of 0.9%, 2.3%, and 2.3%.23 Importantly, however, there have been no randomized, controlled clinical trials to definitively compare the short- and long-term outcomes of DSAEK/DMEK and full-thickness corneal allografts for endothelial failure under low- or high-risk conditions and such definitive studies are clearly needed. A further refinement in the treatment of corneal endothelial failure could be the injection of ex vivo-expanded corneal endothelial cells into the anterior chamber. In a potentially ground-breaking study, it has recently been shown that injection of allogeneic corneal endothelial cells that had been culture-expanded in the presence of a rho-associated protein kinase inhibitor safely restored corneal clarity and endothelial density in 11 patients with bullous keratopathy.24
Treatment of Stromal Disease
In corneal stromal diseases with a presumed normal endothelium (eg, keratoconus, stromal dystrophies, corneal scars after trauma, or infection), anterior lamellar (stroma only) transplantation preserves the recipient endothelium and has the potential to reduce rejection and graft failure.4 Although there are no prospective randomized trials to support this approach, retrospective analyses comparing outcomes to full-thickness corneal transplants provide encouraging results.25 Furthermore, a technique for surgical baring of the recipient Descemet’s membrane has recently been developed (Figure 1) and, in a randomized clinical trial of this technique, the visual outcomes for full-thickness and anterior lamellar transplants were similar.26 Using synthetic stromal tissue could potentially eliminate immunological failures and the need for immune-suppressive medication after corneal transplantation. Although not yet introduced into routine clinical practice, biosynthetic implants have been administered in subjects with corneal stromal disease and they have been shown to remain stably integrated and avascular for 24 months after surgery in the absence of corticosteroid therapy.27 Other approaches to manufacturing “biocornea” substitutes for human corneal tissue such as fish scale-derived collagen matrix are in preclinical testing and may eventually provide a nonimmunogenic alternative to corneal transplantation for high-risk diseases of the stroma.28,29
Lamellar Transplantation for High-risk Corneal Transplantation
A key question in regard to lamellar transplants is whether the potential immunological benefits of reduced graft immunogenicity—specifically whether partial-thickness transplants may be less susceptible to rejection than full-thickness transplants—are applicable to high-risk conditions in which a lamellar graft is technically feasible. The circumstance in which this has been most clearly evaluated is retransplantation following failure of one or more full-thickness transplants.30-33 In a case series from 6 tertiary referral centers from Europe, the US, and Asia comprising 246 DSAEK lamellar transplants carried out following failure of a full-thickness transplant, Mitry et al observed a failure rate of 19% with a median follow-up time of 17 months. In this series, a history of acute rejection in the prior transplant was common (63%) and increased the risk for subsequent acute rejection post-DSAEK. In total, acute rejection of the lamellar graft occurred in 17% of cases and was the single strongest risk factor for graft failure.31 This important report indicates that acceptable results can be achieved with lamellar corneal transplantation in a high immunological risk setting but emphasizes the fact that, even with a reduced burden of new alloantigens, acute rejection will remain a significant limiting factor. A further note of caution can be found in the results reported by Keane et al in a study from the ACGR, which compared clinical characteristics of subjects retransplanted after failure of a first full-thickness graft, who received either a second full-thickness transplant (n = 335) or a DSEK/DSAEK lamellar graft (n = 65). In this report, retransplantation with a lamellar graft was associated with significantly poorer graft survival in an adjusted multivariable analysis. Graft survival was also negatively impacted by occurrence of one or more rejection episodes.30 A key message from these retrospective studies in which the influence of unmeasured and uncontrolled clinical decision-making cannot be accounted for is that randomised clinical trials will be necessary to fully understand whether lamellar transplants are associated with reduced immunological risk.
Other Corneal Replacement Approaches
In patients with repeated failed corneal transplants or deficiency of the corneal epithelium-regenerating limbal stem cells (LSCs), implantation of a fully artificial cornea (keratoprosthesis) may overcome immunological barriers and the need for a viable corneal recipient epithelium. The Boston type I keratoprosthesis, which is assembled around a corneal donor graft and the Osteo-Odonto-Keratoprosthesis, which consists of a prevascularized tooth and bone cylinder implanted into the anterior eye, have both been successfully applied as transplant alternatives in no-option patients with corneal blindness.4,34 In severe unilateral corneal injury with total LSC deficiency and conjunctivalization of the cornea, a 2-step procedure involving replacement of the LSC population by grafting of ex vivo-cultured autologous LSCs to restore the epithelial surface followed by full thickness corneal allotransplantation has been recently reported by Figueiredo et al in 23 recipients with encouraging outcomes.35 This strategy builds upon the pioneering work of Pelligrini and others between the 1990s and the present to develop successful ex vivo-cultured LSC transplant procedures for severe corneal burns.36
CURRENT PRACTICE AND RECENT CLINICAL TRIAL EVIDENCE FOR TOPICAL AND SYSTEMIC IMMUNOSUPPRESSION IN HIGH-RISK CORNEAL TRANSPLANTATION
Strategies to prevent and treat corneal transplant rejection vary widely, particularly in high-risk recipients. The universal use of topical corticosteroids for the first 6 postoperative months is likely to be the principal reason for the low frequency of rejection during this period. In high-risk transplant recipients, topical corticosteroids are typically continued indefinitely at a low dose to mitigate the risk of rejection, but the use of systemic immunosuppression remains more variable. A 2011 survey conducted by The Cornea Society found that 30% of surgeons routinely use systemic corticosteroids to prevent rejection in high-risk recipients and 48% use topical cyclosporine A (CsA).37 For the treatment of rejection, 69% of survey respondents favored systemic corticosteroids and 56% prescribed topical CsA, whereas only 5% used oral noncorticosteroid immunosuppression.37 Such variations in clinical practice are reflective of the lack of consensus on the optimum approach to the prevention and treatment of rejection in high-risk corneal transplantation.
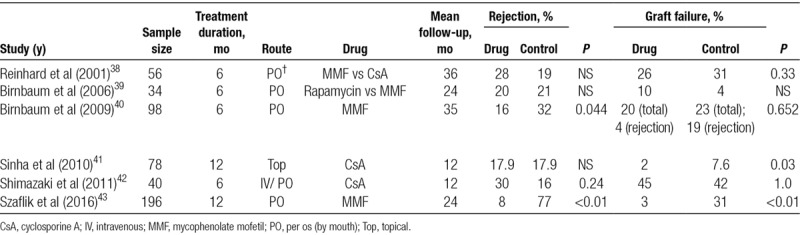
The efficacy of mycophenolate mofetil (MMF), CsA, and rapamycin (sirolimus) in the prevention of high-risk corneal transplant rejection has been evaluated in a small number of clinical trials (Table 2). In a prospective, randomized, multicenter trial comparing MMF (1 g twice daily for 6 mo) to standard treatment with topical corticosteroids and 3 weeks of postoperative oral steroids in 98 high-risk corneal transplant recipients, MMF was associated with superior rejection-free graft survival over approximately 3 years of follow-up (83% versus 64.5%; P = 0.044).40 The overall graft survival did not differ but rejection-related failure was considerably higher in the control group. In keeping with this, a systematic review concluded that MMF might reduce the risk of rejection by approximately 50%.44 More recently, Szaflik et al in a prospective 2-year study of MMF therapy, compared with an equal number of non-MMF-treated retrospective controls, reported that MMF decreased the risk of rejection in high-risk corneal transplantation from 31% to 3%.43 MMF has also been compared with rapamycin for high-risk penetrating keratoplasty in a small clinical trial. The 2 immunosuppressive drugs were found to be equally effective at preventing graft rejection but rapamycin was associated with a higher frequency of adverse effects.39 Overall, a persuasive argument exists for the use of MMF in high-risk corneal transplant patients based on these studies. In contrast, a randomized, controlled trial of oral CsA compared to topical corticosteroid alone showed a nonsignificant trend toward a higher rejection rate in CsA-treated high-risk recipients (30% versus 16%) with similar graft survival (45% versus 42%).42 Available clinical trial evidence does not support the use of topical CsA either in the prevention of graft rejection in high-risk corneal transplantation.41
TABLE 2.
Summary of recent clinical trials of MMF and CsA for the prevention of rejection and graft failure in high-risk corneal transplantation recipients
Other systemic immunosuppressants, most notably tacrolimus, have been used for the prevention of rejection in high-risk corneal transplantation with some evidence of efficacy but have not been subjected to randomized, controlled trials in this setting.8 Reversing rejection in high-risk grafts is a significant challenge and there remains a paucity of evidence to support treatment regimens other than the widely adopted combination of intensive topical corticosteroids and systemic prednisolone. In a prospective, randomized clinical trial, Javadi et al reported that no benefit accrued from the addition of topical CsA to topical corticosteroids in the management of acute corneal transplant rejection with regard to duration of treatment, time to resolution of the episode, and number of subsequent rejection episodes.45
To conclude, the reported clinical trials provide evidence of moderate quality that supports the use of MMF in preventing immune reactions in high-risk corneal transplantation but the evidence does not provide enough certainty to support a consensus on optimum therapy.44 Perhaps surprisingly, definitive evidence to guide systemic corticosteroid therapy in acute allograft rejection is lacking whereas systemic noncorticosteroid immunosuppression has no proven role to play in treating rejection. Thus, even as new immunomodulatory strategies are emerging, there is a striking need for well-designed randomized clinical trials to more clearly define the benefits and limitations of conventional topical and systemic immunosuppressive therapies.
DEVELOPMENTS IN BASIC SCIENCE AND ANIMAL MODELS
High-risk models of corneal transplantation most often focus on interfering with the avascular status of the cornea. In both the mouse and rat, this involves placing intrastromal sutures into the central cornea of the recipient 7–14 days before keratoplasty.46,47 These intrastromal sutures stimulate the ingress of blood and lymphatic vessels, which allow immune cells to enter the graft and cause accelerated rejection. Alternatively, neovessel formation can be induced by a chemical burn to the recipient cornea.48 Other high-risk corneal transplantation models include preinfection with herpes simplex virus,49 induction of allergy,50 and presensitization of the host immune system with a graft-specific antigen.51 In all cases, the tempo of graft rejection is accelerated in the high-risk setting compared to animals in which no “risk-modifying” method has been employed.
A logical treatment strategy in prevascularized high-risk corneal transplant models is to target the blood and lymphatic vessels, which are trafficking potentially alloreactive immune cells into the graft. Dohlman et al targeted different vascular endothelial growth factor (VEGF) isoforms and found that posttransplant neutralization of VEGF-A to limit hemangiogenesis in a prevascularized mouse model resulted in greater improvement in graft survival than targeting VEGF isoforms involved in lymphangiogenesis. In addition to reducing neovascularization, this VEGF-trap method targeting VEGF-A resulted in reduced graft infiltration by macrophage and T cells.52 In contrast, as previously mentioned, Dietrich et al observed, in a similar prevascularized model, that selective inhibition of neolymphangiogenesis before transplantation abrogated rejection of subsequent allografts despite the ingrowth of new blood vessels.14 More recently, Salabarria et al showed that locally-restricted VEGF depletion increases transplantation success in a mouse high-risk model by modulating the recipient corneal microenvironment and inducing tolerogenic mechanisms.53 Similarly, subconjunctival injection of the tyrosine kinase inhibitor sorafenib, which inhibits VEGF receptor signaling, reduced neovascularization, lymphangiogenesis, and graft failure in a prevascularized mouse model of high-risk corneal transplantation.54 In keeping with these preclinical studies, a case series of human high-risk transplants treated with localized delivery of anti-VEGF-A antibody has also reported promising results.55 Using a different strategy, Hou et al administered a photodynamic therapy after verteporfin injection to reduce the neovascularized area of the cornea before transplantation and observed a significant reduction in blood and lymphatic vessels 1 week after treatment with a subsequent improvement in graft survival.56 Subsequently, the same group demonstrated in the mouse model of suture-induced corneal neovascularization that “cross-linking” of corneal collagen fibers by topical riboflavin and UV A irradiation—a protocol used clinically to reduce the rate of corneal distortion in keratoconus—regressed mature blood and lymph vessel invasion and increased the survival of fully MHC-mismatched allografts.57 Compared to control conditions, this potentially clinically applicable protocol was associated with apoptosis of intrastromal vascular endothelial cells and reduced CD45+ immune cell infiltration in the recipient corneas before transplantation.57 Interestingly, in a recent pilot study, fine-needle vessel coagulation combined with bevacizumab treatment has shown promising results to improve graft survival in high-risk transplant patients.58
Corneal tissue contains a resident population of APCs. In the prevascularized setting, these APCs may utilize new vessels infiltrating the graft bed to migrate to draining lymphoid tissue and activate host allo-reactive T cells, beginning a cascade leading to rejection. Recently, Tahvildari et al demonstrated that subconjunctival injection of interleukin 10 and transforming growth factor β to the donor resulted in a more tolerogenic phenotype of donor cornea-resident APCs associated with prolonged allograft survival following transplantation onto a prevascularized graft bed.59 The cornea is also a potential target for gene therapy as it is accessible and is routinely stored for several days in vitro before transplantation. Qin et al devised a strategy whereby CD25 siRNA was administered using a nonviral vector perioperatively. This treatment resulted in reduced immune cell infiltrate and significantly prolonged allograft survival in a high-risk rat model.60 More recently, the safety and efficacy of an ex-vivo gene therapy approach targeting neovascularization has been successfully tested in a rabbit high-risk transplant model.61
Despite the promising nature of recent animal model-based studies of high-risk corneal transplantation, it should be highlighted that few, if any, experimental studies replicate the disease environments of severe bacterial and fungal infection or traumatic and chemical damage to the cornea that drive neovascularization in large numbers of adults and children in developing countries.3,5,6
Other novel approaches and mechanistic insights to high-risk corneal transplant rejection have been recently described in animal models. For example, the future potential for corneal xenotransplantation is supported by the work of Coi et al, which demonstrated significant prolongation of porcine cornea survival in rhesus monkey recipients following anti-CD154 (CD40L) therapy.62 Also in a pig-to-nonhuman primate corneal xenograft model, Vabres et al demonstrated that anterior lamellar grafts from hCTLA-Ig-transgenic pigs had prolonged time to final rejection.63 Fascinatingly, in a mouse corneal allograft model, Paunicka et al demonstrated that the increased rejection rate of second transplants was independent of graft MHC type and laterality but was attributable to substance P release in both eyes that occurred as a result of corneal nerve transection during trephining of the native cornea and inhibited regulatory T cells.64 This work, therefore, reveals a previously unappreciated role for corneal nerves in dictating the immunological outcome of repeat corneal transplants or transplants carried out following previous ocular surgeries.
Cellular therapies may offer the potential to target both neovascularization and immune rejection. As with solid organ transplantation and other immune-mediated conditions, preclinical corneal transplantation has also been a focus for mesenchymal stem/stromal cell (MSC) therapy.65-67 Promising results have been reported by a number of groups in conventional immunological risk animal models.65-69 Recently, we have also demonstrated that pretransplant infusions of third-party allogeneic MSC results in substantially improved graft survival in a rat high immunological risk corneal allograft model in which recipients were presensitized by inoculation with cornea donor-derived splenocytes.70 The immunomodulatory effect of third-party allogeneic MSC was shown to be compatible with coadministration of MMF70 and these animal model results have provided the preclinical evidence base for a Phase 1b clinical trial of healthy donor bone marrow-derived allogeneic MSC in high-risk human corneal retransplant recipients (www.visicort.eu). Finally, immunotherapy designed to expand regulatory T-cell populations may also hold promise for reducing rejection of high-risk corneal transplants, as demonstrated by Tahvildari et al in preinflamed mouse model in which low-dose interleukin 2 was administered from 3 days before to 6 weeks posttransplant.47
OPPORTUNITIES FOR CROSS-COLLABORATION WITH OTHER FIELDS OF Transplantation
Many of the challenges and emerging areas of research progress described in the preceding sections in relation to high-risk corneal transplantation have parallels within other fields of transplantation. With increased interdisciplinary collaboration, recent advances in clinical organ transplantation and transplant immunology may well be applicable to recipients of corneal allografts. For example, the risks and benefits of biological agents directed against specific T and B cell targets (eg, anti-thymocyte globulin; anti-CD25, anti-CD20 and anti-CD52 monoclonal antibodies; belatacept and other costimulatory blockers), complement-targeting agents (eg, eculizumab), and small-molecule inhibitors of immunological signaling pathways (eg, JAK-STAT inhibitors and sphingosine 1 phosphate receptor modulators) have been elucidated in clinical trials of organ transplant recipients,71-73 while comparable data are lacking for high-risk corneal transplants. Similarly, protocols for induction of donor-specific tolerance and for use of immunomodulatory cell therapies have advanced to the clinical arena in organ transplantation in the past decade74 and may be adaptable to specific high-risk indications for corneal transplantation. Finally, taking another example from the field of organ transplantation, further development and application of clinical risk stratification systems, biomarker tests of accessible tissues, and biostatistical models to predict outcomes and guide therapy on a longitudinal basis could be of significant value for the personalized care of high-risk corneal transplants in the future.75,76
KEY KNOWLEDGE GAPS AND FUTURE NEEDS
Corneal transplantation has an important historical significance in the field of organ and tissue transplantation both from technical and immunological perspectives.1,4,77,78 However, the persistent poor outcomes for high-risk corneal transplants worldwide highlight the need for new initiatives and collaborations within and beyond the field of ophthalmology. As summarized in the sections above and in Figure 2, there have been many recent interesting and promising scientific developments with the potential to improve the outcomes of high-risk corneal transplantation. However, apart from the vital insights gained from global epidemiological surveys and regional registries, much of the clinical, translational, and basic research effort remains institutionalized and the output of multicenter clinical trials to address fundamental or novel therapeutic strategies continues to be low. Among the key gaps and needs that we have identified as being critical for realizing the potential to achieve higher long-term graft survival for high-risk corneal transplant recipients, we would highlight the following:
FIGURE 2.
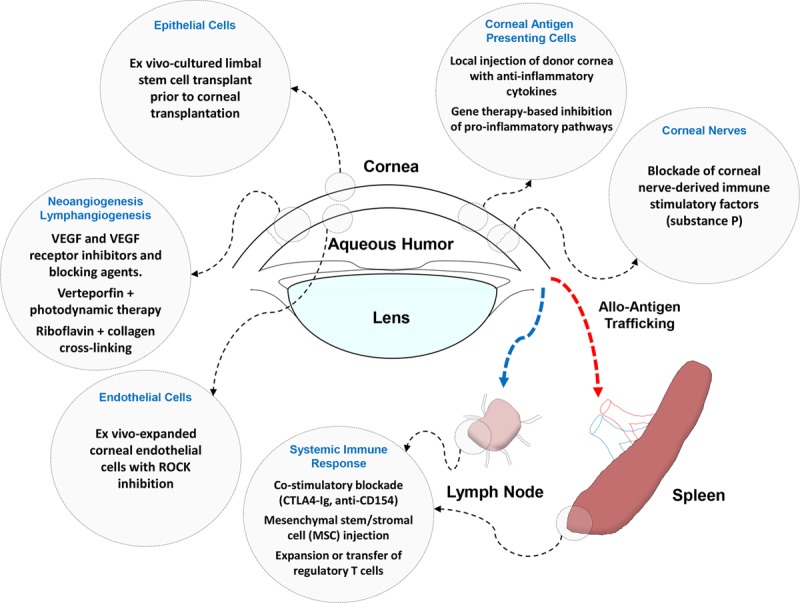
Representative examples of recently reported, mechanistically informed preclinical and early-phase clinical strategies for improving the outcomes for high-risk corneal allotransplants. VEGF, vascular endothelial growth factor.
Large-scale research projects aimed at developing and validating specific tools to define rejection risk, monitor for rejection, and inform the use of and response to immunosuppressive therapies. Individualization to specific causes of corneal disease and increased attention to transplant indications that are more prevalent in the developing world will greatly increase the global impact of such risk-prediction studies.
Robust, multicenter clinical trial initiatives to address unanswered questions regarding donor selection, procedural choice, and optimal immunosuppressive regimens among high-risk corneal transplant recipients. Although it appears clear that currently available systemic immunosuppressive agents have the potential to increase the success rate for corneal transplants with recognized high immunological risk, initiation of such clinical trials and further training of corneal surgeons in the optimal use of potent antirejection drugs will be necessary to consistently and safely achieve this benefit. Inclusion of centers in the developing world may be of critical importance and value in this regard.
Multidisciplinary, translational pipelines to more closely tailor preclinical modelling to clinical transplant scenarios and to effectively move promising, novel therapies and management strategies through the clinical trial phases. Additional collaborations between ophthalmologists and relevant experts in translational medicine and biomedical industries are needed to address the current gap between basic and clinical research.
Further understanding of the pathophysiology of neovascularization, immunological rejection, and chronic endothelial deterioration of full- and partial-thickness corneal allografts under specific high-risk conditions. Although animal models will undoubtedly continue to identify novel mechanistic insights and targetable pathogenic pathways, innovative clinical research to study local and systemic pathophysiology in human high-risk corneal transplant recipients has great potential to accelerate the pace of exploitable discovery.
Footnotes
The authors are supported by funding from the European Commission (EU FP7 Collaborative Health Project VISICORT [grant number 602470]) and by the European Regional Development Fund. M.D.G. and T.R. are also supported by grants from Science Foundation Ireland (Principal Investigator Award to T.R. [grant number 12/IA/1624]; REMEDI Strategic Research Cluster [grant number 09/SRC-B1794]; CÚRAM Research Centre [grant number 13/RC/2073]).
The authors declare no conflicts of interest.
W.J.A., C.G., M.D.G., D.J.G., J.H., P.L., C.C.M., U.P., T.R., D.M.T., and B.V. participated in the writing of the article and approved the final article.
ORCIDs: Matthew D Griffin (0000-0002-8701-8056), David J Gunn (0000-0001-8056-927X), Thomas Ritter 0000-0001-7709-8489.
REFERENCES
- 1.Armitage WJ, Tullo AB, Larkin DF. The first successful full-thickness corneal transplant: a commentary on Eduard Zirm’s landmark paper of 1906. Br J Ophthalmol 2006901222–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zirm EK. Eine erfolgreiche totale keratoplastik (a successful total keratoplasty). 1906. Refract Corneal Surg 19895258–261 [PubMed] [Google Scholar]
- 3.Oliva MS, Schottman T, Gulati M. Turning the tide of corneal blindness. Indian J Ophthalmol 201260423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan DTH, Dart JKG, Holland EJ, et al. Corneal transplantation. Lancet 20123791749–1761 [DOI] [PubMed] [Google Scholar]
- 5.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 201296614–618 [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Vashist P, Tandon R, et al. Prevalence of corneal diseases in the rural Indian population: the Corneal Opacity Rural Epidemiological (CORE) study. Br J Ophthalmol 201599147–152 [DOI] [PubMed] [Google Scholar]
- 7.Williams KA, Keane MC, Coffey NE, et al. Flinders Unviersity; The Australian corneal graft registry 2018 report. Available at https://dspace.flinders.edu.au/xmlui/handle/2328/37917. Published 2018. [Google Scholar]
- 8.Abud TB, Di Zazzo A, Kheirkhah A, et al. Systemic immunomodulatory strategies in high-risk corneal transplantation. J Ophthalmic Vis Res 20171281–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueiredo GS, Jones MN, Krishna Y, et al. ; National Health Service Blood and Transplant Ocular Tissue Advisory Group (OTAG); (OTAG Audit Study 17) Transplant rejection following endothelial keratoplasty and penetrating keratoplasty in the United Kingdom: incidence and survival. Am J Ophthalmol 2015160416–421 [DOI] [PubMed] [Google Scholar]
- 10.Tourkmani AK, Sánchez-Huerta V, De Wit G, et al. Weighing of risk factors for penetrating keratoplasty graft failure: application of risk score system. Int J Ophthalmol 201710372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claesson M, Armitage WJ. Clinical outcome of repeat penetrating keratoplasty. Cornea 2013321026–1030 [DOI] [PubMed] [Google Scholar]
- 12.Flockerzi E, Maier P, Böhringer D, et al. ; all German Keratoplasty Registry Contributors Trends in corneal transplantation from 2001 to 2016 in Germany: a report of the DOG-section cornea and its keratoplasty registry. Am J Ophthalmol 201818891–98 [DOI] [PubMed] [Google Scholar]
- 13.Sugar A, Gal RL, et al. ; Writing Committee for the Cornea Donor Study Research Group Factors associated with corneal graft survival in the cornea donor study. JAMA Ophthalmol 2015133246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol 2010184535–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aboshiha J, Jones MNA, Hopkinson CL, et al. Differential survival of penetrating and lamellar transplants in management of failed corneal grafts. JAMA Ophthalmol 2018136859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkinson CL, Romano V, Kaye RA, et al. ; National Health Service Blood Transplant Ocular Tissue Advisory Group and Contributing Ophthalmologists (OTAG Study 20) The influence of donor and recipient gender incompatibility on corneal transplant rejection and failure. Am J Transplant 201717210–217 [DOI] [PubMed] [Google Scholar]
- 17.Armitage WJ, Winton HL, Jones MNA, et al. Corneal transplant follow-up study II (CTFS II): a prospective clinical trial to determine the influence of HLA class II matching on corneal transplant rejection: baseline donor and recipient characteristics. Br J Ophthalmol 2019103132–136 [DOI] [PubMed] [Google Scholar]
- 18.Winton HL, Bidwell JL, Armitage WJ. Thrombospondin-1 polymorphisms influence risk of corneal allograft rejection. Invest Ophthalmol Vis Sci 2014552115–2120 [DOI] [PubMed] [Google Scholar]
- 19.Pedersen IB, Ivarsen A, Hjortdal J. Graft rejection and failure following endothelial keratoplasty (DSAEK) and penetrating keratoplasty for secondary endothelial failure. Acta Ophthalmol 201593172–177 [DOI] [PubMed] [Google Scholar]
- 20.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014141723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eghrari AO, Riazuddin SA, Gottsch JD. Fuchs corneal dystrophy. Prog Mol Biol Transl Sci 201513479–97 [DOI] [PubMed] [Google Scholar]
- 22.Hjortdal J, Pedersen IB, Bak-Nielsen S, et al. Graft rejection and graft failure after penetrating keratoplasty or posterior lamellar keratoplasty for Fuchs endothelial dystrophy. Cornea 201332e60–e63 [DOI] [PubMed] [Google Scholar]
- 23.Hos D, Tuac O, Schaub F, et al. Incidence and clinical course of immune reactions after descemet membrane endothelial keratoplasty: retrospective analysis of 1000 consecutive eyes. Ophthalmology 2017124512–518 [DOI] [PubMed] [Google Scholar]
- 24.Kinoshita S, Koizumi N, Ueno M, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med 2018378995–1003 [DOI] [PubMed] [Google Scholar]
- 25.Borderie VM, Guilbert E, Touzeau O, et al. Graft rejection and graft failure after anterior lamellar versus penetrating keratoplasty. Am J Ophthalmol 20111511024–1029.e1 [DOI] [PubMed] [Google Scholar]
- 26.Sogutlu Sari E, Kubaloglu A, Unal M, et al. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for macular corneal dystrophy: a randomized trial. Am J Ophthalmol 2013156267–274.e1 [DOI] [PubMed] [Google Scholar]
- 27.Fagerholm P, Lagali NS, Merrett K, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2:46ra61. doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 28.van Essen TH, Lin CC, Hussain AK, et al. A fish scale-derived collagen matrix as artificial cornea in rats: properties and potential. Invest Ophthalmol Vis Sci 2013543224–3233 [DOI] [PubMed] [Google Scholar]
- 29.Chen SC, Telinius N, Lin HT, et al. Use of fish scale-derived biocornea to seal full-thickness corneal perforations in pig models. Plos One. 2015;10:e0143511. doi: 10.1371/journal.pone.0143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keane MC, Galettis RA, Mills RA, et al. ; for Contributors to the Australian Corneal Graft Registry A comparison of endothelial and penetrating keratoplasty outcomes following failed penetrating keratoplasty: a registry study. Br J Ophthalmol 20161001569–1575 [DOI] [PubMed] [Google Scholar]
- 31.Mitry D, Bhogal M, Patel AK, et al. Descemet stripping automated endothelial keratoplasty after failed penetrating keratoplasty: survival, rejection risk, and visual outcome. JAMA Ophthalmol 2014132742–749 [DOI] [PubMed] [Google Scholar]
- 32.Tarantino-Scherrer JN, Kaufmann C, Bochmann F, et al. Visual recovery and endothelial cell survival after descemet stripping automated endothelial keratoplasty for failed penetrating keratoplasty grafts—a cohort study. Cornea 2015341024–1029 [DOI] [PubMed] [Google Scholar]
- 33.Wang F, Zhang T, Kang YW, et al. Endothelial keratoplasty versus repeat penetrating keratoplasty after failed penetrating keratoplasty: a systematic review and meta-analysis. Plos One. 2017;12:e0180468. doi: 10.1371/journal.pone.0180468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avadhanam VS, Smith HE, Liu C. Keratoprostheses for corneal blindness: a review of contemporary devices. Clin Ophthalmol 20159697–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figueiredo GS, Salvador-Culla B, Baylis OJ, et al. Outcomes of penetrating keratoplasty following autologous cultivated limbal epithelial stem cell transplantation. Stem Cells 201836925–931 [DOI] [PubMed] [Google Scholar]
- 36.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010363147–155 [DOI] [PubMed] [Google Scholar]
- 37.Kharod-Dholakia B, Randleman JB, Bromley JG, et al. Prevention and treatment of corneal graft rejection: current practice patterns of the cornea society (2011). Cornea 201534609–614 [DOI] [PubMed] [Google Scholar]
- 38.Reinhard T, Reis A, Böhringer D, et al. Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high-risk keratoplasty patients: 3 years’ results of a randomized prospective clinical trial. Graefes Arch Clin Exp Ophthalmol 2001239367–372 [DOI] [PubMed] [Google Scholar]
- 39.Birnbaum F, Reis A, Böhringer D, et al. An open prospective pilot study on the use of rapamycin after penetrating high-risk keratoplasty. Transplantation 200681767–772 [DOI] [PubMed] [Google Scholar]
- 40.Birnbaum F, Mayweg S, Reis A, et al. Mycophenolate mofetil (MMF) following penetrating high-risk keratoplasty: long-term results of a prospective, randomised, multicentre study. Eye (Lond) 2009232063–2070 [DOI] [PubMed] [Google Scholar]
- 41.Sinha R, Jhanji V, Verma K, et al. Efficacy of topical cyclosporine A 2% in prevention of graft rejection in high-risk keratoplasty: a randomized controlled trial. Graefes Arch Clin Exp Ophthalmol 20102481167–1172 [DOI] [PubMed] [Google Scholar]
- 42.Shimazaki J, Den S, Omoto M, et al. Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am J Ophthalmol 201115233–39.e1 [DOI] [PubMed] [Google Scholar]
- 43.Szaflik JP, Major J, Izdebska J, et al. Systemic immunosuppression with mycophenolate mofetil to prevent corneal graft rejection after high-risk penetrating keratoplasty: a 2-year follow-up study. Graefes Arch Clin Exp Ophthalmol 2016254307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abudou M, Wu T, Evans JR, et al. Immunosuppressants for the prophylaxis of corneal graft rejection after penetrating keratoplasty. Cochrane Database Syst Rev. 2015:Cd007603. doi: 10.1002/14651858.CD007603.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Javadi MA, Feizi S, Karbasian A, et al. Efficacy of topical ciclosporin A for treatment and prevention of graft rejection in corneal grafts with previous rejection episodes. Br J Ophthalmol 2010941464–1467 [DOI] [PubMed] [Google Scholar]
- 46.Jie Y, Pan Z, Chen Y, et al. Non-specific tolerance induced by staphylococcal enterotoxin B in treating high risk corneal transplantation in rats. Br J Ophthalmol 200589364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahvildari M, Omoto M, Chen Y, et al. In vivo expansion of regulatory T cells by low-dose interleukin-2 treatment increases allograft survival in corneal transplantation. Transplantation 2016100525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao XW, Fu Y, Li WJ, et al. Mechanism of immune tolerance induced by donor derived immature dendritic cells in rat high-risk corneal transplantation. Int J Ophthalmol 20136269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuffova L, Knickelbein JE, Yu T, et al. High-risk corneal graft rejection in the setting of previous corneal herpes simplex virus (HSV)-1 infection. Invest Ophthalmol Vis Sci 2016571578–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes NJ, Chen PW, Niederkorn JY. Allergic conjunctivitis renders CD4(+) T cells resistant to T regulatory cells and exacerbates corneal allograft rejection. Am J Transplant 2013131181–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vitova A, Kuffová L, Klaska IP, et al. The high-risk corneal regraft model: a justification for tissue matching in humans. Transpl Int 201326453–461 [DOI] [PubMed] [Google Scholar]
- 52.Dohlman TH, Omoto M, Hua J, et al. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 201599678–686 [DOI] [PubMed] [Google Scholar]
- 53.Salabarria AC, Braun G, Heykants M, et al. Local VEGF-A blockade modulates the microenvironment of the corneal graft bed. Am J Transplant 20191–11 [DOI] [PubMed] [Google Scholar]
- 54.Cho YK, Shin EY, Uehara H, et al. Effect of sorafenib in a murine high risk penetrating keratoplasty model. Int J Ophthalmol 201710834–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dekaris I, Gabrić N, Drača N, et al. Three-year corneal graft survival rate in high-risk cases treated with subconjunctival and topical bevacizumab. Graefes Arch Clin Exp Ophthalmol 2015253287–294 [DOI] [PubMed] [Google Scholar]
- 56.Hou Y, Le VNH, Clahsen T, et al. Photodynamic therapy leads to time-dependent regression of pathologic corneal (lymph) angiogenesis and promotes high-risk corneal allograft survival. Invest Ophthalmol Vis Sci 2017585862–5869 [DOI] [PubMed] [Google Scholar]
- 57.Hou Y, Le VNH, Tóth G, et al. UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high-risk corneal transplant survival. Am J Transplant 2018182873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hos D, Le VNH, Hellmich M, et al. Risk of corneal graft rejection after high-risk keratoplasty following fine-needle vessel coagulation of corneal neovascularization combined with bevacizumab: a pilot study. Transplant Direct. 2019;5:e452. doi: 10.1097/TXD.0000000000000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tahvildari M, Emami-Naeini P, Omoto M, et al. Treatment of donor corneal tissue with immunomodulatory cytokines: a novel strategy to promote graft survival in high-risk corneal transplantation. Sci Rep. 2017;7:971. doi: 10.1038/s41598-017-01065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin Q, Shi Y, Zhao Q, et al. Effects of CD25siRNA gene transfer on high-risk rat corneal graft rejection. Graefes Arch Clin Exp Ophthalmol 20152531765–1776 [DOI] [PubMed] [Google Scholar]
- 61.Fouladi N, Parker M, Kennedy V, et al. Safety and efficacy of OXB-202, a genetically engineered tissue therapy for the prevention of rejection in high-risk corneal transplant patients. Hum Gene Ther 201829687–698 [DOI] [PubMed] [Google Scholar]
- 62.Choi HJ, Lee JJ, Kim DH, et al. Blockade of CD40-CD154 costimulatory pathway promotes long-term survival of full-thickness porcine corneal grafts in nonhuman primates: clinically applicable xenocorneal transplantation. Am J Transplant 201515628–641 [DOI] [PubMed] [Google Scholar]
- 63.Vabres B, Le Bas-Bernardet S, Riochet D, et al. Hctla4-ig transgene expression in keratocytes modulates rejection of corneal xenografts in a pig to non-human primate anterior lamellar keratoplasty model. Xenotransplantation 201421431–443 [DOI] [PubMed] [Google Scholar]
- 64.Paunicka KJ, Mellon J, Robertson D, et al. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am J Transplant 2015151490–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Treacy O, O’Flynn L, Ryan AE, et al. Mesenchymal stem cell therapy promotes corneal allograft survival in rats by local and systemic immunomodulation. Am J Transplant 2014142023–2036 [DOI] [PubMed] [Google Scholar]
- 66.Ko JH, Lee HJ, Jeong HJ, et al. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci U S A 2016113158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy N, Lynch K, Lohan P, et al. Mesenchymal stem cell therapy to promote corneal allograft survival: current status and pathway to clinical translation. Curr Opin Organ Transplant 201621559–567 [DOI] [PubMed] [Google Scholar]
- 68.Oh JY, Lee RH, Yu JM, et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther 2012202143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy N, Treacy O, Lynch K, et al. TNF-α/IL-1β-licensed mesenchymal stromal cells promote corneal allograft survival via myeloid cell-mediated induction of foxp3+ regulatory T cells in the lung. Faseb J 2019339404–9421 [DOI] [PubMed] [Google Scholar]
- 70.Lohan P, Murphy N, Treacy O, et al. Third-party allogeneic mesenchymal stromal cells prevent rejection in a pre-sensitized high-risk model of corneal transplantation. Front Immunol. 2018;9:2666. doi: 10.3389/fimmu.2018.02666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grenda R. Biologics in renal transplantation. Pediatr Nephrol 2015301087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vincenti F, Silva HT, Busque S, et al. Evaluation of the effect of tofacitinib exposure on outcomes in kidney transplant patients. Am J Transplant 2015151644–1653 [DOI] [PubMed] [Google Scholar]
- 73.Horwitz JK, Chun NH, Heeger PS. Complement and transplantation: from new mechanisms to potential biomarkers and novel treatment strategies. Clin Lab Med 20193931–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scalea JR, Tomita Y, Lindholm CR, et al. Transplantation tolerance induction: cell therapies and their mechanisms. Front Immunol. 2016;7:87. doi: 10.3389/fimmu.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapal M, Le Borgne F, Legendre C, et al. A useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney Int 2014861130–1139 [DOI] [PubMed] [Google Scholar]
- 76.Kurian SM, Williams AN, Gelbart T, et al. Molecular classifiers for acute kidney transplant rejection in peripheral blood by whole genome gene expression profiling. Am J Transplant 2014141164–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pleyer U, Schlickeiser S. The taming of the shrew? The immunology of corneal transplantation. Acta Ophthalmol 200987488–497 [DOI] [PubMed] [Google Scholar]
- 78.George AJ, Larkin DF. Corneal transplantation: the forgotten graft. Am J Transplant 20044678–685 [DOI] [PubMed] [Google Scholar]


