Objective:
The aim of this study was to assess safety and efficacy of pancreatic duct occlusion (PDO) with neoprene-based glue in selected patients undergoing pancreatoduodenectomy (PD) at high risk of postoperative pancreatic fistula (POPF).
Background Data:
PD is the reference standard approach for tumors of the pancreaticoduodenal region. POPF is the most relevant complication after PD. PDO has been proposed as an alternative to anastomosis to manage the pancreatic stump.
Methods:
A single-center, prospective, nonrandomized trial enrolled 100 consecutive PD for cancer. Patients at high risk for POPF according to Fistula Risk Score (FRS) >15% (≥6 points) were treated with PDO using neoprene glue (study cohort); patients with FRS ≤15% (≤5 points) received pancreaticojejunal anastomosis (PJA: control cohort). Primary endpoint was complication rate grade ≥3 according to Dindo–Clavien Classification (DCC). Other postoperative outcomes were monitored (ClinicalTrials.gov NCT03738787).
Results:
Fifty-one patients underwent PDO and 49 PJA. DCC ≥3, postoperative mortality, and POPF grade B-C were 25.5% versus 24.5% (P = 0.91), 5.9% versus 2% (P = 0.62), and 11.8% versus 16.3% (P = 0.51) in the study versus control cohort, respectively. At 1 and 3 years, new-onset diabetes was diagnosed in 13.7% and 36.7% of the study cohort versu 4.2% and 12.2% in controls (P = 0.007).
Conclusions:
PDO with neoprene-based glue is a safe technique that equalizes early outcome of selected patients at high risk of POPF to those at low risk undergoing PJA. Neoprene-based PDO, however, triples the risk of diabetes at 1 and 3 years.
Keywords: neoprene, pancreatic duct occlusion, pancreatic fistula, pancreatic stump, pancreatoduodenectomy
In 1935, Allen Whipple first described the pancreatoduodenectomy (PD) 1: an operation that remains burdened by significant morbidity, mainly related to postoperative pancreatic fistula (POPF). 2 The incidence of POPF is 5% to 30% 3 and pancreatic texture, diameter and position of Wirsung duct, blood loss, body mass index (BMI), and pancreatic disease etiology are the identified risk factors. 4 No technique has proven to be superior to others in avoiding this complication.5,6,7
In the 1980s, pancreatic duct occlusion (PDO) was explored as alternative to pancreaticojejunal anastomosis (PJA). Among other compounds, the neoprene latex – a polychloroprene homopolymer with a natural pH of 12, low viscosity and heat resistance – was tested in clinical trials.8,9 Peculiar of neoprene latex is the ability to depolymerize in contact with the basic pH of the pancreatic juice, hardening into a semisolid cast of the Wirsung duct. Despite these attractive features, the scientific evidence of neoprene-based PDO efficacy is lacking.8,10,11,12,13,14
In our practice, we have continued to consider PDO with neoprene in life-threatening post-Whipple reoperations, aiming to control severe pancreatic fistulas and avoid total pancreatectomy. The present prospective study was designed to investigate under standardized conditions safety and efficacy of neoprene-based PDO in a selected group of PD at high risk of POPF.
METHODS
Study Design
This is a single-center, prospective, nonrandomized, parallel cohort study, (NCT03738787) designed to assess safety and efficacy of stabilized neoprene-based glue (Pancreas-Lock, SALF Pharma, Cenate Sotto, BG, Italy) for intraoperative occlusion of the pancreatic remnant after PD.
The trial design is summarized in Figure 1.
FIGURE 1.

Study flowchart and main outcomes.
The study enrolled 100 patients undergoing radical PD for various cancers of the pancreaticoduodenal region from January 2015 to December 2017. After tumor and pancreatic head removal, patients with a fistula risk score (FRS) 4 >15% (≥6 points) were considered at high risk for POPF and thus treated with PDO using neoprene glue (study cohort). A parallel cohort of control patients who were operated on during the same period and considered at low risk of POPF (FRS ≤15%: ≤5 points) received conventional pancreatic-jejunal reconstructions (control cohort).
The primary endpoint of the study was to compare in the 2 cohorts the 30-day complication rate according to Dindo-Clavien Classification (DCC) 15. Secondary endpoints of the study were the evaluation of new-onset insulin-dependent diabetes along the follow-up and patients survival outcomes. For the evaluation of secondary endpoints, the study cohort also included those controls who required relaparotomy because of an unmanageable pancreatic fistula and were rescued from total pancreatectomy by means of PDO with neoprene-based glue (rescued patients).
Technical Aspects and Definitions
All patients underwent open pancreatoduodenectomy. Lymphadenectomy was performed according to the International Study Group on Pancreatic Surgery (ISGPS) recommendations. 16 The FRS was assessed intraoperatively through a calculator 4 taking into account gland texture, Wirsung diameter (measured with a ruler), blood loss, and histology. In the study cohort, a 16G-18G catheter was inserted as distal as possible into the pancreatic duct of the remnant pancreas and slowly retracted while injecting 5 to 15 mL of neoprene glue. About 10 minutes were allowed for glue hardening, before completing the stump closures with a continuous polypropylene suture. In patients undergoing conventional reconstruction, duct-to-mucosa (22 cases: 44.8%) or double-layer PJA (27 cases: 55.2%) was adopted according to intraoperative conditions, leaving an internal drainage catheter into the pancreatic duct. 17 At the end of surgery, 2 passive drains were placed. Amylase level from drainage fluid was assessed on day 1, 3, 5, and thereafter if clinically indicated. Drains were removed on postoperative day 7 unless clinically contraindicated.
ISGPS definition of pancreatic fistula was used, that is, any drain fluid on or after postoperative day 3 with amylase level >3 times the upper normal limit (100 U/L). Fistula diagnosis was applied only to B-C grade.2,18 Postoperative complications were assessed using Dindo-Clavien classification 17 and Comprehensive Complication Index. 19
Follow-Up Schedule and Metabolic evaluation
Before surgery and during follow-up at 1, 3, 6, and 12 months all enrolled patients underwent clinical and physical examination, standard laboratory tests, and assessment of antidiabetic drugs use. Metabolic status was assessed by fasting glycemia, glycosylated hemoglobin, C-peptide, fasting insulin, and Homeostasis Model Assessment (HOMA) insulin resistance index before surgery and at the end of follow-up. 20 Total cholesterol, triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol (HDL-C) were also measured. Patients were defined diabetics if they had history of type II diabetes mellitus or if they met standard criteria. 21
Statistical Methods
The study was conducted according to a Gehan design 22 in which the first part was replaced by a 2-stage Simon scheme, determining upfront the maximum number of patients suffering severe complications after PDO which would have suffice study interruption (Fig. 1).23,24 The null hypothesis would have been rejected whether the complication rate according to DCC ≥3 was ≤0.4 in the first 20 patients. The enrolment of 7 and 13 patients was required, being 4 and 9, respectively, the number of patients with DCC ≥3 justifying study interruption. This design has a type I error of 0.045 and a power of 0.81 when the true proportion of responses is 0.7. After the DCC ≥3 complication rate was deemed acceptable, the recruitment continued prospectively.
Continuous variables are presented as median (1st–3rd quartile) and compared using Pearson rank analysis. Categorical variables are presented as absolute numbers and percentages. Baseline characteristics are compared using the Student t test, chi-square test, or Fisher exact test, as appropriate. Follow-up time was calculated by means of reverse Kaplan–Meier method. Time-to-event curves were calculated with the Kaplan–Meier method and compared by the log-rank test. Overall survival was calculated from the date of surgery to the date of death from any cause or the date of last follow-up. Non–cancer-specific survival was calculated from the date of surgery to the date of death for nontumoral events, censoring for patients deceased for tumor recurrence or alive at last follow-up. Statistical analyses were conducted using SPSS (version 20.0). A 2-sided P < 0.05 was considered statistically significant.
RESULTS
The study cohort included 51 patients at high risk for POPF who underwent PDO with neoprene-based glue. During the same period, 49 patients at low risk for POPF underwent PD with PJ anastomosis (controls).
Baseline and intraoperative characteristics of the 2 populations are summarized in Table 1. The 2 cohorts were similar for most clinical characteristics, with the exception of baseline albumin levels (lower in the study cohort, P = 0.04) and tumor stage, more advanced in the study group receiving PDO with respect to controls; notably, these are factors that per-se are associated with higher risk of POPF regardless the FRS score. A significant higher number of patients with metastatic lymph nodes was in fact detected in the PDO group (P = 0.022). A median of 8.5 mL (7–11) of neoprene-based preparation was injected in the pancreatic stump of the study cohort. No significant differences in terms of operative time and transfusions were registered between cohorts. As per study design, fistula risk score was significantly higher in the study cohort (median 22 vs 6; P < 0.001).
TABLE 1.
Baseline Characteristics of 2 Cohorts of Patients Undergoing Pancreatoduodenectomy at Different Risk of POPF
| High Risk of POPF Neoprene PDO (n = 51) | Low Risk of POPF PJ Anastomosis (n = 49) | P | |
| Age, y | 68 (62–75) | 65 (57–71) | 0.24 |
| Sex (male) | 28 (54.9%) | 26 (53.1%) | 0.85 |
| BMI, kg/m2 | 24 (22–26) | 24 (22–26) | 0.83 |
| Preoperative diabetes | 13 (25.5%) | 11 (22.5%) | 0.72 |
| Liver cirrhosis | 3 (5.9%) | 0 | 0.24 |
| ASA score | 0.47 | ||
| 1 | 0 | 4 (8.2%) | |
| 2 | 40 (78.4%) | 35 (71.4%) | |
| 3 | 11 (21.6%) | 9 (18.4%) | |
| 4 | 0 | 1 (2.0%) | |
| Albumin level, g/dL | 4.0 (3.8–4.3) | 4.2 (3.9–4.5) | 0.04 |
| Blood glucose, mg/dL | 106 (91–126) | 106 (93–124) | 0.87 |
| Insulin level, μU/mL | 6.9 (4.8–9.5) | 8.4 (6.0–12.5) | 0.75 |
| C-peptide, ng/mL | 2.4 (1.7–3.3) | 2.5 (1.7–2.9) | 0.14 |
| Total cholesterol, mg/dL | 169 (149–193) | 183 (152–218) | 0.24 |
| HDL | 49 (40–61) | 50 (35–55) | 0.45 |
| LDL | 80 (56–104) | 124 (80–134) | 0.03 |
| Triglycerides, mg/dL | 90 (72–115) | 89 (73–149) | 0.52 |
| Preoperative HOMA score | 1.68 (1.35–3.08) | 1.88 (1.21–3.48) | 0.51 |
| Neoadjuvant * | 0.15 | ||
| Chemotherapy | 9 (15.8%) | 4 (8.2%) | |
| Radiotherapy | 1 (2%) | 0 | |
| Preoperative | 0.43 | ||
| ERCP stenting | 20 (39.2%) | 23 (46.9%) | |
| PTBD | 2 (3.9%) | 3 (6.1%) | |
| Tumor histology | 0.38 | ||
| PDAC | 33 (64.7%) | 32 (65.3%) | |
| BDAC | 8 (15.7%) | 6 (12.2%) | |
| PapAC | 5 (9.8%) | 4 (8.2%) | |
| DuodAC | 4 (7.8%) | 2 (4.1%) | |
| pNET | 1 (2.0%) | 5 (10.2%) | |
| Lymph node status | 0.016 | ||
| Negative | 14 (27.5%) | 26 (53.1%) | |
| Positive | 37 (72.5%) | 23 (46.9%) | |
| Intraoperative characteristics | |||
| Operative time, minutes | 480 (400–553) | 490 (438–540) | 0.78 |
| Neoprene injection, mL | 8.5 (7–11) | 0 | |
| Blood loss, mL | 200 (100–200) | 200 (100–200) | 0.77 |
| Fistula Risk Score | 22% (18–25%) | 6% (6–9%) | <0.001 |
| FRS class (points) | <0.001 | ||
| Negligible (0) | 0 (0%) | 0 (0%) | |
| Low (1–2) | 0 (0%) | 34 (69.4%) | |
| Intermediate (3–6) | 14 (27.4%) | 14 (28.6%) | |
| High (7–10) | 37 (72.6%) | 1 (2.0%) | |
| Vascular resection | 7 (13.7%) | 6 (12.2%) | 1 |
Numbers are presented as absolute numbers (%) or medians (interquartile range). ASA indicates American Society of Anesthesiologists; BDAC, biliary duct adenocarcinoma; BMI, Body Mass Index; DuodAC, adenocarcinoma of duodenum; ERCP, endoscopic retrograde cholangio-pancreatography; PapAC, adenocarcinoma of the papilla; pNET, pancreatic neuroendocrine tumor; PTBD, percutaneous transhepatic biliary drainage.
*Subgroup analysis did not show significant differences with respect to patients, tumor stage and operation characteristics among neoadjuvant-receiving vs naïve patients.
Primary Outcome Measures
The median follow-up time of the study versus control cohorts was 15 (9–26) versus 21 (11–39) months (P = 0.6); no patient was lost at follow-up.
Postoperative outcomes are shown in Table 2. Complications described as DCC ≥3 at 30 days (primary endpoint) occurred in 13 patients (25.5%) of the study cohort and in 12 (24.5%) controls (P = 0.91). Overall complication rate (DCC: 1–5) was 56.9% (29 patients) in the study cohort and 55.1% (27 patients) in controls (P = 0.86). The most common complication in both cohorts was delayed gastric emptying (15.7%–18.4%), followed by POPF grade B-C in the control cohort (16.3%) and pleural effusion in the study cohort (15.7%) (Fig. 2). No adverse event directly correlated with neoprene glue PDO was observed. In particular, the rate of chemical pancreatitis was 0%, even in those who underwent rescue PDO.
TABLE 2.
Perioperative Outcomes After Pancreatoduodenectomy in the 2 Patient cohorts Under Study
| High Risk of POPF Neoprene PDO (n = 51) | Low Risk of POPF PJ Anastomosis (n = 49) | P | |
| Patients receiving blood cell transfusion | 8 (15.7%) | 7 (14.3%) | 0.84 |
| Patients with uneventful course | 22 (43.1%) | 22 (44.9%) | 0.86 |
| 30 Days’ overall postoperative morbidity | 29 (56.9%) | 27 (55.1%) | 0.86 |
| 30 Days’ DCC ≥ 3 morbidity | 13 (25.5%) | 12 (24.5%) | 0.91 |
| Comprehensive Complication Index | 20.9 (0.0–34.2) | 20.9 (0.0–33.5) | 0.61 |
| POPF | 6 (11.8%) | 8 (16.3%) | 0.51 |
| Grade B | 5 (9.8%) | 3 (6.1%) | |
| Grade C | 1 (2.0%) | 5 (10.2%) | |
| Postoperative complications | 0.86 | ||
| Surgical site infection | 5 (9.8%) | 2 (4.1%) | |
| Pneumonia | 1 (2.0%) | 2 (4.1%) | |
| Pleural effusion | 8 (15.7%) | 7 (14.3%) | |
| Cardiological complications | 2 (3.9%) | 2 (4.1%) | |
| Neurological complications | 1 (2.0%) | 1 (2.0%) | |
| Bleeding | 7 (13.7%) | 5 (10.2%) | |
| Biliary fistula | 4 (7.8%) | 7 (14.3%) | |
| Intra-abdominal abscess | 4 (7.8%) | 2 (4.1%) | |
| Lymphatic fistula | 2 (3.9%) | 2 (4.1%) | |
| Delayed gastric empting | 8 (15.7%) | 9 (18.4%) | |
| Postoperative pancreatitis | 0 (0%) | 0 (0%) | |
| Maximum DCC grade of complication | 0.81 | ||
| 1 | 3 (5.9%) | 1 (2.0%) | |
| 2 | 12 (23.5%) | 14 (28.6%) | |
| 3 | 9 (17.6%) | 10 (20.4%) | |
| 4 | 2 (3.9%) | 1 (2.0%) | |
| 5 | 3 (5.9%) | 1 (2.0%) | |
| Reoperation | 7 (13.7%) | 8 (16.3%) | 0.72 |
| Cause of reoperation | |||
| Bleeding | 4 (7.8%) | 4 (8.2%) | |
| Biliary fistula | 2 (3.9%) | 3 (6.1%) | |
| Pancreatic fistula | 0 (0%) | 4 (8.2%) | |
| Bowel perforation/dehiscence | 3 (5.9%) | 0 (0%) | |
| Wound dehiscence | 0 (0%) | 1 (2.0%) | |
| Salvage Neoprene duct occlusion * | – | 7 (14.3%) | |
| Length of hospital stay | 16 (13–25) | 15 (13–24) | 0.61 |
| Readmission rate | 3 (6.0%) | 0 (0.0%) | 0.24 |
| Cause of readmission | |||
| Dehydration (ileostomy) | 1 (2.0%) | 0 (0%) | |
| Fistula drain reposition | 1 (2.0%) | 0 (0%) | |
| Wound infection | 1 (2.0%) | 0 (0%) | |
| 90 Days’ mortality | 3 (5.9%) | 1 (2.0%) | 0.62 |
| Cause of death | |||
| Intracranial haemorrhage | 1 (2.0%) | 0 (0%) | |
| Liver failure | 1 (2.0%) | 0 (0%) | |
| Sepsis | 1 (2.0%) | 0 (0%) | |
| Intra-abdominal haemorrhage | 0 (0%) | 1 (2.0%) | |
Numbers are presented as absolute numbers (%) or medians (interquartile range).
*Diabetic induced treatment was not different between groups when excluding from calculation the 7 nondiabetic patients who received rescued PDO. However, the rate of new-onset diabetes at 3 years was 23.8% vs 12.2% (P = 0.04) in the study vs control cohort, respectively.
FIGURE 2.
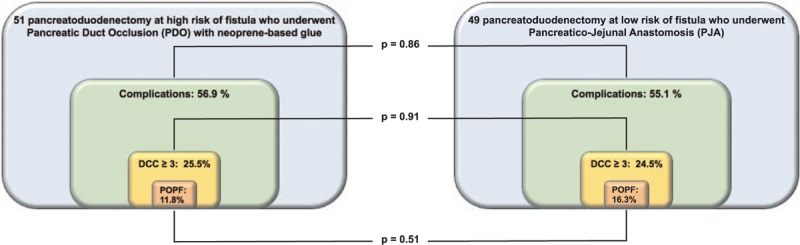
Postoperative complications. Rates of general complications, DCC ≥3 complications, and clinically relevant pancreatic fistula are summarized. No significant difference was observed between cohorts (primary endpoint).
No difference between cohorts was observed in terms of reoperations (13.7% vs 16.3%, P = 0.72), hospital stay (13 vs 15 days, P = 0.61) and readmission rate. No patients in both cohorts underwent total pancreatectomy, as 7 controls required reoperation (14.3%) and were rescued successfully with neoprene duct occlusion and takedown of the PJ anastomosis.
All postoperative-related deaths occurred within 30 days from surgery and no difference in 90 days’ mortality was observed between cohorts (P = 0.62). In details, 3 patients died in the study cohort (5.9%) because of intracranial bleeding, liver insufficiency, and sepsis, whereas 1 control died (2.0%) because of postoperative bleeding and multiorgan failure. As no worsening in morbidity and mortality was observed after duct occlusion in patients at high risk of POPF with respect to the parallel cohort at low risk of fistula receiving PJA, the efficacy of duct occlusion with neoprene was confirmed.
Secondary Outcome Measures
Non–cancer-specific survival at 1 and 3 years (Fig. 3A, continuous lines) did not differ among groups (98% and 98% in the study cohort vs 94.1% and 91.1% in controls: P = 0.7). Conversely, overall survival (Fig. 3 A, dotted lines) reflected the more advanced tumor stage observed in PDO (1- and 3-year survival: 73.9% and 34.1% in the study cohort vs 86.8% and 64.4% in the control cohort, P = 0.009).
FIGURE 3.
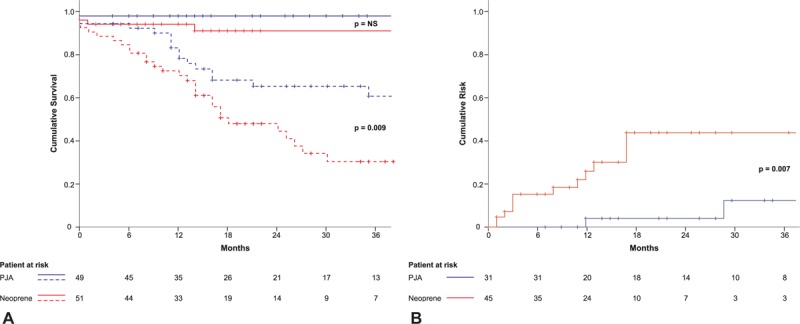
Postoperative outcomes. (A) Non–cancer-specific survival (continuous lines) and overall survival (dotted lines) in the 2 study cohorts. (B) Cumulative risk of developing new-onset diabetes in patients with no stigmata of diabetes at the time of surgery. The prognosis of pancreatoduodenectomy is mainly tumor-related. Duct occlusion with neoprene glue helps to overcome safely the early postoperative period in patients at high risk of pancreatic fistula, although does not protect against cancer-related outcome and is followed by increased occurrence of long-term diabetes.
At baseline no difference in terms of BMI (24 vs 24 kg/m2), preoperative diabetes (13 vs 11 patients), fasting blood glucose levels (106 vs 106 mg/dL), insulin level (6.9 vs 8.4 μU/mL) C-peptide (2.4 vs 2.5 ng/mL), HOMA (1.68 vs 1.88), triglycerides, and total cholesterol was found in the 2 cohorts. Only preoperative LDL cholesterol was slightly higher in the control cohort (80 vs 124 mg/dL, P = 0.03).
Figure 3B reports the risk curve for new-onset diabetes: at 1 and 3 years diabetes occurred in 13.7% and 36.7% in the study cohort and 4.2% and 12.2% in the control cohort (P = .007). Post-neoprene-induced diabetes required insulin treatment in the long-term in 58.3% of cases, whereas both patients with new onset post-PJ anastomosis become insulin-dependent. Also, a significant difference in median C-peptide and blood glucose level was detected in favor of controls [0.84 vs 1.3 μU/mL (P = 0.009) and 117 vs 95.5 mg/dL (P = 0.002) in study vs control cohort, respectively]. A trend toward higher blood glucose levels and more pronounced weight loss in the study cohort was observed along the follow-up (Supplementary Fig. 1), during which no significant difference was found in BMI, insulin level, cholesterol and triglycerides. In the surviving patients, median HOMA ratio variations from surgery to the end of 20 months follow-up were −67.3% (−75.3%; 55.1%) in the study cohort and −26.7% (−71.4%; 20.2%) in controls, with no statistical difference (P = 0.803) found (Supplementary Fig. 2).
DISCUSSION
Technical and perioperative advancements have reduced the mortality rate after pancreatoduodenectomy below 2% to 5%. 25 However, complications requiring surgical, endoscopic, or radiological intervention (Dindo-Clavien grade ≥3) remain as high as 30% to 40%, mainly attributable to the onset of pancreatic fistula.26,27,28,29,30 The results of the present prospective series of 100 consecutive PD are in line with those general figures (Table 2), whereas differ on offering pancreatic-duct occlusion with neoprene glue to patients at significant risk for POPF (FRS >15%: ≥6 points). Notably, about half of the collected patients were at high risk of fistula and 27% had a borderline risk (FRS = 6 points) considered eligible to the neoprene glue duct occlusion. These rates are higher than in previous studies4,31 and that might be partly explained by the selection of several unfavorable conditions for PJ-anastomosis (ie, nonadenocarcinoma tumors: 35%, previous major surgery: 18%, low-albumin and liver cirrhosis: 6%, chronic pancreatitis: 0%).
Management of the remnant pancreas after PD remains problematic, with no criterion standard available.5,6,7,32 Closure of the pancreatic remnant after PD has been repeatedly proposed and several studies compared PJ anastomosis with stump closure by various Wirsung-occluding materials.9,27,33,34,35,36,37 All these studies and 1 randomized controlled trial (RCT) failed to demonstrate a significant advantage of stump closure over conventional pancreatic-enteric anastomosis, turning the PDO into an abandoned practice. That appears unjustified considering the suboptimal patients selection of previous studies.
Above all, the practice of neoprene duct occlusion has persisted in case of severe fistula requiring relaparotomy, to rescue the pancreatic remnant and avoid total pancreatectomy associated with a mortality >40%.38,39,40 As described in Figure 1 and Table 2, in the presented study rescue PDO with neoprene glue occurred in 7 patients, without observing any associated mortality.
In rescue settings or in case of high-risk of fistula, total pancreatectomy with autologous islet transplantation can be a promising alternative to control post-surgical diabetes, although <20% of insulin independence is expected in the long term.41,42
The innovative characteristics of the present prospective study rely on the predetermined intraoperative identification of PD at high risk of fistula as the subgroup eligible to duct occlusion, without any attempt of PJ anastomosis. The risk of POPF was defined by factors widely recognized as weak points for restoring pancreatic-digestive continuity.4,43 Of note, in this study also patients with a FRS score of 6 – at the upper boundary of the intermediate risk class 4 – were considered at high risk of POPF and included in the occlusion cohort, anticipating a potential benefit from the technique also in intermediate-risk conditions. The study resulted in a nonsignificant difference in postoperative outcome among 2 parallel cohorts of patients at different risk of POPF. Therefore, the primary endpoint was met (Fig. 2), confirming the neoprene glue as an easy, safe, and reliable device able to replace PJA in patients at high risk for POPF.
Notably, the observed neoprene-associated complication rate was lower than in large series of patients with elevated FRS undergoing PJ anastomosis (ie, overall complication rate: 56.9% vs 77.4%; DCC ≥3: 25.5% vs 35.6%, clinically relevant fistulae: 11.8% vs 29%).44,45
As previously described, the incidence of postsurgical new-onset diabetes was higher in neoprene-PDOs with respect to the PJ-anastomosis cohort (Fig. 3B). Most likely, neoprene-induced fibrosis was responsible for a significant reduction of the preoperative versus postoperative levels of insulin and C-peptide in neoprene-occluded patients with respect to controls (P = 0.035 and <0.001, respectively), noting however that presurgical borderline glucose and C-peptide conditions were more frequent in nondiabetic neoprene-treated versus control patients (Table 1), in agreement with previous reports.
As expected, the long-term patients’ survival was mainly related to tumor histology and stage, which were significantly more advanced in the neoprene PDO patients (Table 1). As previously described, more advanced locoregional cancer stages in the pancreatoduodenal region are associated with complex PD procedures and high risk of fistula in case of pancreaticoenteric anastomosis. 46
When survival analysis was restricted to non–cancer-related death (Fig. 3A), no difference in survival was observed between neoprene-treated versus control patients. This is in favor of pancreatic-duct occlusion in patients with locally advanced cancer requiring complex procedures. In such a subgroup of risky PD the use of neoprene PDO may equalize the early outcome of such a population in need of adjuvant treatment to that of patients undergoing PJA under standardized conditions.
The present study has limitations related to the relatively small sample size and to the non-random, although prospective, fashion design. Should the use of neoprene glue be rejuvenated after the present demonstration of safety and efficacy, prospective RCT may be redefined, focusing on the specific subgroup of patients at high risk of POPF. Finally, the heterogeneity of indications to PD and lack of specific monitoring impede to draw any conclusions on long-term impact of PDO on pancreatic exocrine insufficiency.
In conclusion, PDO with neoprene-based glue is safe and potentially efficacious. This technique can replace anastomosis in patients at high risk of POPF and avoid total pancreatectomy in the emergency setting. On the downside, this technique triples the 1- and 3-year risk of new-onset diabetes.
Supplementary Material
Acknowledgment
The authors thank Michele Zaninelli, Giovanna Speranza, and Carla Angeletti for studying and preparing the device described in this investigation.
DISCUSSANTS
José M.R. Angel (Guadalajara, Spain):
First, I want to thank the ESA Scientific Committee for allowing me to comment on the work of Prof. Mazzaferro and his co-workers.
The article deals with a very interesting topic for pancreatic surgeons, which is an approach for avoiding a pancreatic fistula after a PD in high-risk fistula patients using neoprene glue occlusion.
The study has a nice methodology. First, they have demonstrated the facts and safety of the technique, before comparing duct occlusion in high-risk patients with PJA in non-low-risk patients.
My main concern is that they used a fistula rate score cut-off that differs from Callery et al. In my opinion, a pure RCT would be more powerful than a prospective study.
So, I have 2 questions:
First, could you please explain the reasons behind your decision to modify the cut-off of FRS?
Second, why did you not perform a pure RCT after checking the safety?
Response From Vincenzo Mazzaferro (Milan, Italy):
Thank you for your comments. With respect to the first question, we included point 6 of the fistula risk score – and declared that upfront – because we wanted to be more inclusive in the population at risk of a fistula, in case of a difficult Whipple operation. After all, the patients with 6 points consisted of about 27% of the whole series. This is slightly above the standard, but not significantly different from those patients with a score >8. In my opinion, this series presented a more evident bias in the selection, represented by a lack of general conditions favoring PJ-anastomosis, such as the absence of chronic pancreatitis and high rate of nonadenocarcinoma tumors, previous major surgery, and the presence of liver cirrhosis.
With respect to the randomized control trial, the aims of our study were as follows: to rejuvenate an almost abandoned technique; to find the best setting for a patient's condition, to offer duct occlusion; and to plan a randomized control study, after having demonstrated the safety and effectiveness of the technique. Looking at the results of the presented study, a randomized control trial can be proposed with a noninferiority design, which compares PDO with neoprene-based glue versus conventional PJ reconstruction in patients at a high risk of a post-duodenopancreatectomy fistula, and especially, in those with locoregional advanced cancer.
Antonio D. Pinna (Abu Dhabi, United Arab Emirates):
I have 2 very short questions. First of all, I would have liked you to compare the neoprene with the PJA in a comparable patient population. Second, neoprene has shown higher cancer-related mortality compared to the other group. One-third of the patients also had diabetes. Do you think that the neoprene-treated patients would actually have been better candidates for a total pancreatectomy?
Response From Vincenzo Mazzaferro (Milan, Italy):
The patients who were given neoprene duct occlusion were selected after an intraoperative determination of their high risk of postoperative fistula. Before starting this prospective study, we had the experience of using neoprene as a salvage procedure in patients with severe post-Whipple leaks who – thanks to the neoprene glue – were rescued and avoided a total pancreatectomy. Hence, we thought that the best comparator for neoprene duct occlusion were the patients at a high risk of fistula, rather than the general population or those with a good predicted result of PJ anastomosis. This was incorporated into the presented prospective study design.
With respect to the correlation between duct occlusion and cancer-related mortality, this was related to the fact that the number of patients with positive lymph nodes made up 75% of the patients in the neoprene-treated group versus 20% to 25% in the anastomosis group. This was a sort of inverse selection bias, which favored duct occlusion in patients with more advanced cancer and diabetes. I don’t know whether these were truly candidates for total pancreatectomies. For sure, the local advancement of cancer implied extensive dissection, blood loss, pancreatic gland manipulation, and ultimately, the inclusion of more of these patients in the duct occlusion group.
Christiane Bruns (Cologne, Germany):
I have 3 questions regarding the oncological outcome. As I understood, you decided on neoprene-based glue injection in the pancreatic duct intraoperatively. Did you also analyze the pancreatic resection margin through intraoperative frozen sections? Did you include patients in the study, in whom you had to perform a vascular resection? Third, which kind of anastomotic technique did you use for the pancreatojejunostomy?
Response From Vincenzo Mazzaferro (Milan, Italy):
No, we did not use any oncological marker or margin assessment to make a final decision on duct occlusion. The final decision was based on the pancreatic fistula score, which included blood loss, the texture of the pancreatic gland, the size of the Wirsung duct, and pancreatic/biliary versus ampullary/duodenal cancer.
The number of vascular reconstructions was similar in both groups. In fact, this did not influence the decision of whether we should use the glue.
With respect to the technique of conventional reconstruction, duct-to-mucosa or double-layer PJ anastomosis was adopted according to intraoperative conditions. According to the numbers, about half of the patients were included in each of these 2 alternatives.
Pierre-Alain Clavien (Zurich, Switzerland):
Thank you, Dr. Mazzaferro, for presenting this provocative study at the ESA, which expectedly triggers an animated discussion. I have 2 questions. First, this procedure consistently resulted in disasters not a long time ago. Am I correct? Consequently, any attempt at occluding the Wirsung duct was abandoned in favor of a total pancreatectomy, when a PJA was not suitable. So, what are you really doing differently? Please, tell us why this is now suddenly successful.
Next, you showed us 2 risk groups, and chose to perform the glue approach in the group with the highest risk and the standard anastomosis in the one with the lowest risk. Yet, you obtained quite similar results in the glue group, despite the presence of much higher risk factors. If we follow this logic, and challenge you a little bit, why should we continue performing anastomoses, rather than just simplifying the procedure by injecting glue into all patients? Are you ready to complete a RCT, which would include all patients irrespective of the degree of risk?
Response From Vincenzo Mazzaferro (Milan, Italy):
Thank you for your provocative questions. As expected, the group of patients with a low risk of fistula had a better outcome. They had a lower incidence of diabetes, reduced weight loss, better performance status, and so on. So, patients in the PJA were favored in the long run. This is clear from the data, confirming that conventional anastomosis should be pursued whenever possible. However, in particularly difficult procedures correlated with high-risk conditions, duct occlusion is an alternative that can be used safely and effectively. Because of neoprene glue duct injection, 2 very different postoperative outcomes may be equalized into a more predictable and lower-risk status, which allows better in-hospital performance after a pancreatoduodenectomy. I’m not saying that we have to replace PJA with duct occlusion. Conversely, our study demonstrates that a subgroup of least favorably placed patients can be rescued with a simple, inexpensive technique, which is able to return them to a standard postoperative period at a low risk of serious complications.
As mentioned above in my reply to Professor Ramia Angel, I do not think that a RCT, which includes all patients irrespective of the degree of fistula risk, is justified. Conversely, a noninferiority trial comparing neoprene duct occlusion vs. conventional PJ anastomosis in patients at a high risk of fistula is well supported by the result of the present prospective study.
Melina Kibbe (Chapel Hill, NC):
I actually do think that you need to perform this trial in the high-risk group. My question is very basic. Why are you using neoprene glue? Why aren’t you using more of a biologic? This is like rubber that you’re putting in there. So, why neoprene when there are many biologic options out there?
Response From Vincenzo Mazzaferro (Milan, Italy):
We work in the laboratory with our faculty of chemistry in modifying the neoprene glue with respect to previous formulations. The glue used in this study has a peculiar characteristic with respect to biological, expensive materials, in that the neoprene latex is a low-viscosity, milky fluid that depolymerizes in contact with the basic pH of the pancreatic juice, hardening into a semisolid cast of the Wirsung duct in front of the surgeon's eyes within a few minutes.
Although duct occlusion with this material is a pure surgical, hand-guided maneuver, the neoprene glue maintains sophisticated characteristics, which, with respect to biologic materials (data not shown), allows a predictable and permanent effect. It allows us to avoid a total pancreatectomy.
Footnotes
Authors contribution: conception and study design: V.M.; acquisition of data and protocol management: M.V., C.C., M.F., J.C.; analysis and interpretation: V.M., C.S., M.D.D.B., M.B.; Writing committee: M.V., C.S., N.P., M.B., V.M.
The authors report no conflicts of interest.
At the time of manuscript submission the first author has a pending submission for a European patent on the neoprene formulation used in this study.
REFERENCES
- 1. Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg 1935; 102:763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017; 161:584–591. [DOI] [PubMed] [Google Scholar]
- 3. Eshmuminov D, Schneider MA, Tschuor C, et al. Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 International Study Group Pancreatic Fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB 2018; 20:992–1003. [DOI] [PubMed] [Google Scholar]
- 4. Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 2013; 216:1–14. [DOI] [PubMed] [Google Scholar]
- 5. Shrikhande SV, Sivasanker M, Vollmer CM, et al. Pancreatic anastomosis after pancreatoduodenectomy: A position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2017; 161:1221–1234. [DOI] [PubMed] [Google Scholar]
- 6. Wang W, Zhang Z, Gu C, et al. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: a network meta-analysis of randomized control trials. Int J Surg 2018; 57:111–116. [DOI] [PubMed] [Google Scholar]
- 7. Testini M, Piccinni G, Lissidini G, et al. Surgical management of the pancreatic stump following pancreato-duodenectomy. J Visc Surg 2016; 153:193–202. [DOI] [PubMed] [Google Scholar]
- 8. Di Carlo V, Chiesa R, Pontiroli AE, et al. Pancreatoduodenectomy with occlusion of the residual stump by Neoprene injection. World J Surg 1989; 13:105–110. discussion 110-1. [DOI] [PubMed] [Google Scholar]
- 9. Tran K, Van Eijck C, Di Carlo V, et al. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg 2002; 236:422–428. discussion 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahrén B, Tranberg KG, Andrén-Sandberg A, et al. Subtotal pancreatectomy for cancer: closure of the pancreatic remnant with staplers. HPB Surg 1990; 2:29–35. discussion 35-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gebhardt C. Clinical studies on duct occlusion with prolamine. Horm Metab Res Suppl 1983. 56–58. [PubMed] [Google Scholar]
- 12. Marczell AP, Stierer M. Partial pancreaticoduodenectomy (Whipple procedure) for pancreatic malignancy: occlusion of a non-anastomosed pancreatic stump with fibrin sealant. HPB Surg 1992; 5:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorenz D, Wolff H, Waclawiczek H. Pancreatic duct occlusion in resection treatment of chronic pancreatitis and cancer of the head of the pancreas. A 3-year follow-up study. Chirurg 1988; 59:90–95. [PubMed] [Google Scholar]
- 14. Idezuki Y, Goetz FC, Lillehei RC. Late effect of pancreatic duct ligation on beta cell function. Am J Surg 1969; 117:33–39. [DOI] [PubMed] [Google Scholar]
- 15. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tol JAMG, Johanna AM, Gouma DJ, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014; 156:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang G-Q, Li X-H, Ye X-J, et al. Internal Versus external drainage with a pancreatic duct stent for pancreaticojejunostomy during pancreaticoduodenectomy for patients at high risk for pancreatic fistula: a comparative study. J Surg Res 2018; 232:247–256. [DOI] [PubMed] [Google Scholar]
- 18. Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005; 138:8–13. [DOI] [PubMed] [Google Scholar]
- 19. Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index. Ann Surg 2013; 258:1–7. [DOI] [PubMed] [Google Scholar]
- 20. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 21. Association AD American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care 2017; 40:S11–S24. [DOI] [PubMed] [Google Scholar]
- 22. Gehan EA. The determination of the number of patients required in a preliminary and follow-up trial of a new chemotherapeutic agent. J Chronic Dis 1961; 13:346–353. [DOI] [PubMed] [Google Scholar]
- 23. Jung S-H, Lee T, Kim K, et al. Admissible two-stage designs for phase II cancer clinical trials. Stat Med 2004; 23:561–569. [DOI] [PubMed] [Google Scholar]
- 24. Mander AP, Wason JMS, Sweeting MJ, et al. Admissible two-stage designs for phase II cancer clinical trials that incorporate the expected sample size under the alternative hypothesis. Pharm Stat 2012; 11:91–96. [DOI] [PubMed] [Google Scholar]
- 25. Beger HG, Mayer B. Early postoperative and late metabolic morbidity after pancreatic resections: an old and new challenge for surgeons—a review. Am J Surg 2018; 216:131–134. [DOI] [PubMed] [Google Scholar]
- 26. Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): a systematic review and analysis of the POPF-related mortality rate in Sixty thousands, seven hundreds and thirty-nine patients retrieved from the English literature published between 1990 and 2015. Medicine 2017; 96:e6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benzoni E, Zompicchiatti A, Saccomano E, et al. Postoperative complications linked to pancreaticoduodenectomy. An analysis of pancreatic stump management. J Gastrointestin Liver Dis 2008; 17:43–47. [DOI] [PubMed] [Google Scholar]
- 28. Bassi C, Buchler MW, Fingerhut A, et al. Predictive factors for postoperative pancreatic fistula. Ann Surg 2015; 261:e99. [DOI] [PubMed] [Google Scholar]
- 29. Sandini M, Bernasconi DP, Fior D, et al. A high visceral adipose tissue-to-skeletal muscle ratio as a determinant of major complications after pancreatoduodenectomy for cancer. Nutrition 2016; 32:1231–1237. [DOI] [PubMed] [Google Scholar]
- 30. Pulvirenti A, Marchegiani G, Pea A. Sun-Whe K, Hiroki Y, et al. Pancreatic Fistula. Pancreatic Cancer. Berlin: Springer; 2017. 317–327. [Google Scholar]
- 31. Miller BC, Christein JD, Behrman SW, et al. A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg 2014; 18:172–179. [DOI] [PubMed] [Google Scholar]
- 32. Yang SH, Dou KF, Sharma N, et al. The methods of reconstruction of pancreatic digestive continuity after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. World J Surg 2011; 35:2290–2297. [DOI] [PubMed] [Google Scholar]
- 33. Katsaragakis S, Antonakis P, Konstadoulakis MM, et al. Reconstruction of the pancreatic duct after pancreaticoduodenectomy: a modification of the Whipple procedure. J Surg Oncol 2001; 77:26–29. discussion 30. [DOI] [PubMed] [Google Scholar]
- 34. Fromm D, Schwarz K. Ligation of the pancreatic duct during difficult operative circumstances. J Am Coll Surg 2003; 197:943–948. [DOI] [PubMed] [Google Scholar]
- 35. Theodosopoulos T, Dellaportas D, Yiallourou AI, et al. Pancreatic remnant occlusion after Whipple's procedure: an alternative oncologically safe method. ISRN Surg 2013; 2013:960424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mauriello C, Polistena A, Gambardella C, et al. Pancreatic stump closure after pancreatoduodenectomy in elderly patients: a retrospective clinical study. Aging Clin Exp Res 2017; 29:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alfieri S, Quero G, Rosa F, et al. Indications and results of pancreatic stump duct occlusion after duodenopancreatectomy. Updates Surg 2016; 68:287–293. [DOI] [PubMed] [Google Scholar]
- 38. Bressan AK, Wahba M, Dixon E, et al. Completion pancreatectomy in the acute management of pancreatic fistula after pancreaticoduodenectomy: a systematic review and qualitative synthesis of the literature. HPB 2018; 20:20–27. [DOI] [PubMed] [Google Scholar]
- 39. Balzano G, Pecorelli N, Piemonti L, et al. Relaparotomy for a pancreatic fistula after a pancreaticoduodenectomy: a comparison of different surgical strategies. HPB 2014; 16:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nentwich MF, El Gammal AT, Lemcke T, et al. Salvage completion pancreatectomies as damage control for post-pancreatic surgery complications: a single-center retrospective analysis. World J Surg 2015; 39:1550–1556. [DOI] [PubMed] [Google Scholar]
- 41. Balzano G, Piemonti L. Autologous Islet transplantation in patients requiring pancreatectomy for neoplasm. Cur Diab Rep 2014; 14:512. [DOI] [PubMed] [Google Scholar]
- 42. Balzano G, Maffi P, Nano R, et al. Autologous Islet transplantation in patients requiring pancreatectomy: a broader spectrum of indications beyond chronic pancreatitis. Am J Tranplant 2016; 14:1812–1826. [DOI] [PubMed] [Google Scholar]
- 43. Mungroop TH, van Rijssen LB, van Klaveren D, et al. Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg 2019; 269:937–943. [DOI] [PubMed] [Google Scholar]
- 44. Ecker BL, McMillan MT, Asbun HJ, et al. Characterization and optimal management of high-risk pancreatic anastomoses during pancreatoduodenectomy. Ann Surg 2018; 267:608–616. [DOI] [PubMed] [Google Scholar]
- 45. McMillan MT, Ecker BL, Behrman SW, et al. Externalized stents for pancreatoduodenectomy provide value only in high-risk scenarios. J Gastrointest Surg 2016; 20:2052–2062. [DOI] [PubMed] [Google Scholar]
- 46. Marchegiani G, Andrianello S, Malleo G, et al. Does size matter in pancreatic cancer?: Reappraisal of tumour dimension as a predictor of outcome beyond the TNM. Ann Surg 2017; 266:142–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


