Purpose of review
In this review, we summarize the recently published literature that demonstrates the efficacy and safety of autologous haematopoietic stem cell therapy (AHSCT) in multiple sclerosis (MS) and highlight the importance of supportive care required for the safe and well-tolerated delivery of AHSCT.
Recent findings
MS is an autoimmune inflammatory and degenerative disorder of the central nervous system (CNS). In the majority of patients, the illness runs a relapsing remitting course (RRMS), culminating in a secondary progressive phase with gradual accumulation of fixed disabilities. Currently available disease-modifying therapies suppress CNS inflammation but have a limited effect on preventing disease progression for which there remains no effective therapy. Over the last two decades, there has been increasing evidence that AHSCT is a highly effective therapeutic strategy for treatment-resistant inflammatory types of MS, especially RRMS. Concerns about the safety of AHSCT in MS, usually a nonlife-threatening disease, have previously limited its use. However, AHSCT can now be delivered safely with major long-term benefits because of increasing transplant centre experience, judicious patient selection and good supportive care.
Summary
MS is currently the fastest growing indication for AHSCT in Europe. Supportive care before, during and after the transplant period is key to the successful delivery of AHSCT.
Keywords: autologous haematopoietic stem cell transplantation, multiple sclerosis
INTRODUCTION
Multiple sclerosis (MS) is an autoimmune disorder of the central nervous system (CNS) characterized by inflammatory demyelination and subsequently gliosis and axonal loss. It is the commonest cause of nontraumatic disability in young adults with a prevalence of around 1 in 1000 [1]. Typically, MS presents in adults at the age of 20–40 years and is twice as common in women as in men. In 85–90% of cases, the disease runs a relapsing remitting course (RRMS) characterized by defined episodes of new or worsening neurological symptoms and signs followed by partial or complete recovery [2]. The majority of those patients transit into a secondary progressive phase (SPMS) with gradual accumulation of fixed disabilities [3]. About 10–15% of affected patients develop a progressive disability from disease onset denoting a primary progressive course [2].
Over the last two decades, an increasing number of disease-modifying therapies (DMTs) have become available for the treatment of MS. These agents target the inflammatory component of the disease reducing relapse rate and the accumulation of lesions in the CNS and ultimately the accumulating disability. To date, 14 DMTs have been approved with varying efficacy and safety profiles [4]. Despite their use, many patients continue to have breakthrough relapses and disability progression [5]. This subgroup of patients represents a real therapeutic challenge.
Progression of neurological disability in MS has traditionally been measured by the Expanded Disability Status Scale (EDSS, Table 1) [1,6]. In addition, No Evidence of Disease Activity (NEDA) is now increasingly used as an outcome measure in routine practice and clinical trials [7]. NEDA is a composite measure of three components representing a state of absent clinical relapses, disability progression and MRI disease activity [7]. In recent phase III randomized clinical trials (RCTs) of modern DMTs, only 37–47.7% of patients maintained NEDA at 2–5 years after the treatment with high efficacy DMTs, such as natalizumab [8], ocrelizumab [9] and alemtuzumab [5,10,11].
Table 1.
Kurtzke's functional systems and Expanded Disability Status Scale in multiple sclerosis
| EDSS | Neurological disability |
| 0.0 | Normal neurological examination |
| 1.0 | Physical signs only with no disability |
| 1.5 | Physical signs only in more than one FS; no disability |
| 2.0 | Minor disability in one FS score |
| 2.5 | Minor disability in two FS |
| 3.0 | Fully ambulatory with moderate disability in one FS score or minor disability in three or four FS |
| 3.5 | Fully ambulatory with moderate disability in one FS and minor disability in one or two FS or moderate disability in two FS |
| 4.0 | Ambulatory for ≥500 m, severe disability in one FS |
| 4.5 | Ambulatory for ≥300 m; severe disability in one FS and minor or moderate disability in other FS |
| 5.0 | Ambulatory for ≥ 200 m |
| 5.5 | Ambulatory for ≥ 100 m |
| 6.0 | Requires unilateral assistance (one stick) to walk 100 m |
| 6.5 | Requires bilateral assistance (two sticks) to walk 20 m |
| 7.0 | Wheelchair bound; able to transfer without help |
| 7.5 | Wheelchair bound; needs help to transfer |
| 8.0 | Restricted to chair or bed; has effective arm function |
| 8.5 | Restricted to bed most of the day; retains some arm function |
| 9.0 | Bedbound; able to communicate and eat |
| 9.5 | Bedbound; unable to communicate or eat |
| 10.0 | Death due to MS |
FS, functional system score (MS-related disability); MS, multiple sclerosis. Adapted with permission from [1].
Autologous haematopoietic stem cell therapy (AHSCT), also known as autologous haematopoietic stem cell transplantation, is a well-established procedure used mainly to treat haematological malignancies [12]. In the last two decades, it has been increasingly used for the treatment of aggressive autoimmune diseases [13–18]. Based originally on preclinical experiments in animal models of MS, the first AHSCT procedures as a treatment for MS were performed in 1995. Overseen by the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT), the EBMT registry now includes over 3000 patients treated with various types of HSCT for autoimmune diseases, with over 1400 patients treated with AHSCT for MS. Other international registries and institutional databases include more than a thousand MS additional patients treated with AHSCT worldwide [12–18].
AHSCT aims to eradicate the aberrant immune system and reset immune tolerance to prevent ongoing and recurrent neuroinflammation in MS [13,18]. In distinction to DMTs, AHSCT is an intensive one-off treatment after which most patients will not require additional therapies. A common misconception is that autologous haematopoietic stem cells (AHSCs) differentiate into neuronal cells that repair damaged CNS tissue. Instead, AHSCs differentiate only into haematopoietic and other immune cells in vivo and are therefore used as a supportive product to speed haematopoietic recovery following the administration of high-dose systemic cytotoxic therapy. This immunochemotherapy – referred to as the ‘conditioning regimen’ [18] [usually a combination of high-dose chemotherapy and antithymocyte globulin (ATG)] removes autoreactive T cells and other immune effectors. The AHSC infusion not only enables recovery from chemotherapy-induced cytopenia, but is also associated with immune ‘re-booting’ [13,19]. The treatment is followed by rapid resolution of neuroinflammatory activity, whereas longer term alterations in immune reconstitution are associated with sustained clinical responses.
Concerns regarding the toxicity of AHSCT in MS, usually a nonlife-threatening disease, previously limited its use. However, with increasing transplant centre experience and judicious patient selection, AHSCT can be performed safely with minimal risk of treatment-related mortality. MS is currently the fastest growing indication for AHSCT in Europe [16] and supported as a standard-of-care in the EBMT indications practice guidelines [20,21▪].
Box 1.
no caption available
CLINICAL STUDIES OF AUTOLOGOUS HAEMATOPOIETIC STEM CELL THERAPY IN RELAPSING REMITTING MULTIPLE SCLEROSIS
Over the last 5 years, increasing studies of AHSCT in RRMS have been reported, reflecting both its safe delivery and efficacy in respect to relapse rates, MRI activity, disability progression, fatigue and quality of life [22–24]. Despite the differences in their designs and transplant technique, these studies showed remarkable consistency in clinical and radiological outcomes. For example, progression-free survival (with progression defined as confirmed increase in EDSS by 0.5–1 point from baseline), was reported as 70–91% [25] with 68–70% of patients maintained NEDA at 3–5 years after ASHCT [17,26].
The EBMT phase II ‘ASTIMS’ RCT compared AHSCT with mitoxantrone [27]. AHSCT was superior in suppressing neuroinflammation, reflected by MRI activity and relapse rate, although the study was too small to identify an impact on disability which is likely to be related to high prevalence of patients with SPMS in the accrual [27]. Recently, the interim results of ‘MIST’, the first phase III multicentre RCT, with 110 patients with RRMS randomized to either nonmyeloablative AHSCT or best available DMTs, have confirmed the superiority of AHSCT over most standard DMTs with sustained improvement in clinical and radiological outcomes in patients randomized to the AHSCT arm [28▪▪]. Further trials are required to compare the efficacy of AHSCT with modern highly effective DMTs (alemtuzumab, ocrelizumab and cladribine).
Improved safety and efficacy of AHSCT in MS is best attributed to patient selection, choice of conditioning regimen and centre experience [16,29]. The current consensus is that AHSCT is best used to treat younger patients (less than 45 years), with short disease duration (less than 10 years), who are not very disabled (EDSS >5.5) and who have highly active RRMS (at least one relapse in the previous 12 months with evidence of MRI disease activity) despite the use of DMTs [14,21▪]. The EBMT recommends the procedure to be performed in accredited centres, where there is evidence to support improved outcomes in well-selected patients [16,21▪,29,30▪]. In addition, the ADWP has written plainly worded guidance for patients and their carers to explain the nature of the procedure, its risks and who may benefit from it, especially if patients self-refer outside their own health services to units offering AHSCT or similar procedures abroad [30▪].
The autologous haematopoietic stem cell therapy procedure and the role of supportive care
AHSCT encompasses a multistep procedure: stem cell mobilization and harvesting; conditioning, stem cell reinfusion, cytopenia and engraftment; posttransplant recovery and immune reconstitution [31] (Fig. 1.). All phases require supportive care.
FIGURE 1.
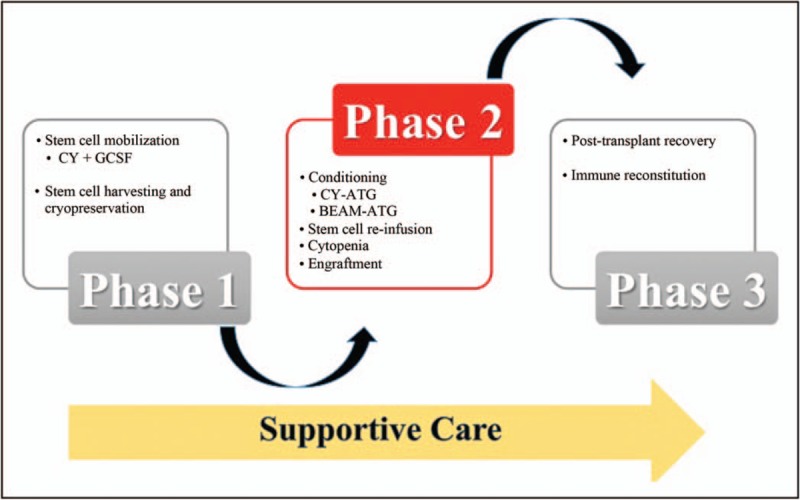
Autologous haematopoietic stem cell therapy (AHSCT). AHSCT encompasses a multistep procedure of three phases: stem cell mobilization and harvesting and cryopreservation; conditioning, stem cell reinfusion, cytopenia and engraftment; posttransplant recovery and immune reconstitution. All stages require supportive care. ATG, antithymocyte globulin; BEAM, carmustine, etoposide, cytarabine and melphalan; CY, cyclophosphamide; GCSF, granulocyte colony-stimulating factor.
MOBILIZATION AND HARVESTING
The first phase is mobilization of AHSCs from bone marrow into peripheral blood using granulocyte colony-stimulating factor (GCSF) and cyclophosphamide, which prevents further MS relapses while enhancing AHSC mobilization into the blood [32–34]. AHSCs are collected by leukapheresis followed by cryopreservation until the patient is ready for AHSCT. AHSCs may be purified by ex-vivo manipulation, but no added benefit on clinical outcomes has been demonstrated [21▪,31]. The quality of the graft, in terms of adequate number of collected and cryopreserved stem cells, contributes to the safety of the procedure [35].
CONDITIONING REGIMEN, STEM CELL INFUSION AND CYTOPENIA
The next phase is the administration of the conditioning regimen, which involves in-patient admission for ablation of the immune system with high-dose chemotherapy. Conditioning regimens vary according to the intensity of immunoablation, and have been classified as ‘low’, ‘intermediate’ and ‘high’ [21▪]. ‘High-intensity’ regimens have high efficacy, but also greater short-term and long-term toxicity [18,36], whereas ‘low’-intensity regimens have a higher rate of treatment failure [21▪]. Therefore, ‘intermediate-intensity’ myelo-ablative regimens, such as the ‘BEAM-ATG’ (a conditioning regimen originally adapted from the treatment of lymphoma) and the lympho-ablative regimen –‘cyclophosphamide-ATG’ (adopted from the treatment of aplastic anaemia), are the most frequently used in MS [18,21▪,37]. In line with the EBMT recommendations, most centres make a choice of either BEAM-ATG or cyclophosphamide-ATG depending on centre experience [21▪]. Rabbit-derived ATG is more commonly reported, but horse-derived ATG may also be used safely and effectively [38].
Once the conditioning regimen has been given, the previously harvested cells are thawed under controlled conditions and reinfused, usually with hydration, antiemetics and other supportive care measures covering the systemic effect of the dimethylsuphoxide and cytolysis debris, unless the product is washed to remove them after thawing. Pancytopenia follows for 10–14 days until the infused AHSCs reconstitute requiring support with irradiated red cell and platelet transfusions, and hormonal therapy to prevent menstrual bleeding. Prophylactic and therapeutic antiinfective agents (antibacterial, antiviral and antifungal) are necessary to cover immunosuppressed periods. Central line access is necessary for chemotherapy, fluids, transfusions and drugs, requiring scupulous care to minimize complications, especially infection.
SPECIFIC SUPPORTIVE CARE FOR AUTOLOGOUS HAEMATOPOIETIC STEM CELL THERAPY IN MULTIPLE SCLEROSIS
AHSCT in MS is a complex multidisciplinary procedure and patients are heterogeneous in relation to the level and nature of disability and comorbidities. Alongside the transplant specialists, the input of experienced MS neurologists, supportive care clinicians, including therapy teams, infectious diseases, dieticians and psychologists, is essential to ensure a good recovery and favourable long-term outcomes.
Supportive care management is essential in some specific areas, as follows:
-
(1)
There are special considerations for neurological toxicity (Table 2), which is reported in 17% of patients within 60 days of AHSCT [31]. MS patients may experience worsening of their neurological symptoms secondary to fever (pseudorelapse or Uhthoff's phenomenon) in the context of infections or due to ATG-induced fever [32,39]. The effect of fever is usually temporary [28▪▪,32]. Patients may also develop deterioration in mobility, worsening spasticity and fatigue because of the prolonged bed-bound treatment spell. Preexisting disability and dehydration may predispose to an increase risk of falls and platelet transfusion thresholds may be set higher to avoid complications. Although platelets levels tend to be reduced, patients are often bedbound and thromboembolic risks should be considered during the conditioning phase and upon platelet recovery until full mobilization.
-
(2)
Side-effects of ATG require special consideration in the context of MS. Acute febrile and allergic reactions are common. Slow infusion rate, high-dose steroid and antihistamine cover are vital [21▪,32], and consideration needs to be actively given to individualized rate of administration to ensure patient stability. As ATG persists beyond its administration, management of fever in MS patients undergoing AHSCT needs to include not only prompt administration of broad-spectrum antibiotics as per unit policy, but also consideration of the ongoing risk of allergic-type fever. A de-escalating regimen of oral or intravenous steroid may be used beyond the conditioning period, and additional pulse doses of steroids (methylprednisolone) may be necessary alongside routine workup for neutropenic sepsis. However, the use of steroids adds to the risks of fluid retention, impaired glycaemic control, infection and impaired early phase of immunological engraftment, mandating close attention to fluid balance and clinical biochemistry, as well as a low index of suspicion for infection markers, which may be suppressed by steroids.
-
(3)
The urinary tract may be more problematic in patients with MS, who may have difficulty voiding, detrusor-sphincter dyssynergia or permanent in-situ catheters [40]. A history of urinary tract infection presents a risk of sepsis during neutropenia and bacterial resistance. A urinary bacterial test before admission might help to identify multiresistant germs before the aplastic phase. If there is residual urine on admission, then placement of a catheter should be considered during the administration of cyclophosphamide, which comes with a risk of haemorrhagic cystitis, even with routine prophylaxis with Mesna and hyperhydration.
-
(4)
As with other AHSCT procedures, the gastrointestinal tract may be temporarily affected, with varying degrees of nausea, vomiting and anorexia, with the conditioning regimen, followed by oropharangeal mucositis, abdominal symptoms and bowel disturbances, which tend to last until the recovery from neutropenia [41,42]. With severe gastrointestinal symptoms, intravenous drug administration and total parenteral nutrition may be required. Other expected chemotherapy-related complications include transient alopecia, liver toxicity (from chemotherapy, antifungals and norethisterone), fertility impairment and cardiac toxicity [41].
-
(5)
All patients are at risk of deconditioning. On top of their preexisting disability, this represents a challenge in recovery. Ideally, patients should be assessed by neurologically experienced physiotherapists prior to AHSCT to consider the need for ‘prehabilitation’ to reduce the inevitable decline in function that will come with AHSCT [43]. At the very least, there should be some planning for rehabilitation requirements following discharge. In severely disabled patients, this may best be delivered away from the transplant facility and in specialized neurorehabilitation setting.
Table 2.
AHSCT-related complications that commonly affect multiple sclerosis patients
| MS-related risk factors | Supportive measures to prevent and/or treat complications | |
| Early adverse effects of AHSCT | ||
| ATG-fever | Cytokine release | Steroids, antipyretics, exclude sepsis |
| Worsening of neurological symptoms | Fever (infection/ATG-fever) | Treatment of infection with antimicrobials Treatment of ATG-fever |
| Urinary tract infections | Altered bladder function Urinary catheters to minimize the risk of haemorrhagic cystitis | Antimicrobials Good rehydration |
| Haemorrhagic cystitis | Altered bladder function | Urinary catheter Good rehydration |
| EBV reactivation | Previous exposure to EBV | Close blood monitoring for EBV DNA |
| CMV reactivation | Previous exposure to CMV | Close blood monitoring for CMV DNA |
| Pneumonia | Muscular weakness Immobility | Antimicrobials Early mobilization |
| Deep vein thrombosis risk | Immobility Limb weakness | Early mobilization Anticoagulants |
| Falls | Limb weakness/disability Dehydration | Physiotherapy Fluid monitoring |
| Late adverse effects | ||
| Secondary autoimmune diseases | Pretreatment with Alemtuzumab or ATG | Close follow up and monitoring Input from other medical specialities |
ATG, antithymocyte globulin; CMV, cytomegalovirus; EBV, Epstein-Barr virus; MS, multiple sclerosis.
EARLY FOLLOW UP
The whole AHSCT procedure usually requires in-patient hospital admission for about 4 weeks. Following stabilization and discharge, patients require close ongoing monitoring to ensure safe recovery. Prophylactic aciclovir and pneumocystis pneumonia prophylaxis should continue for 6–12 months, and antifungals for 3 months, in accordance with local policies. As with AHSCT for other procedures (such as myeloma), quality of life will take some months to completely recover usually because of profound lethargy, even though some RRMS patients may experience a rapid response in terms of their neurological recovery [21▪,28▪▪].
The additional immune suppression, particularly with the inclusion of ATG in the conditioning regimens, means that there is an ongoing risk of viral reactivations. Aciclovir prevents HSV-1/2 and VZV reactivation. Screening for cytomegalovirus (CMV) and Epstein-Barr virus (EBV) reactivation, similar to allogeneic haematopoietic stem cell transplantation protocols, for the first 3 months and sometimes beyond, is essential so that preemptive treatment can be instituted promptly, especially for CMV, which may be life-threatening. EBV reactivation is detected in up to 80% of MS patients with previous EBV exposure who receive ATG-conditioning regimens and may also be related to previous DMTs [44]. Symptomatic reactivation of EBV can be occasionally associated with de-novo monoclonal gammopathy and new neurological sequelea (not related to MS) as well as lymphoproliferative disorders [44].
LONG-TERM FOLLOW-UP: NEUROLOGICAL AND FOR ‘LATE EFFECTS’ OF HAEMATOPOIETIC STEM CELL THERAPY
Follow-up should involve the treating neurologist to assess MS status and the transplant haematologist should oversee routine posttransplant ‘late effects’ screening for immune, endocrine and reproductive function and late cancers.
Infection may be an ongoing risk and routine posttransplant vaccination schedules should be considered in all patients [21▪]. The risk of progressive multifocal leucoencephalopathy (PML) appears to be lower than with DMTs [45]. Indeed, no cases of PML have been reported so far, including patients previously treated with natalizumab with high titres of John Cunningham virus antibodies [45].
Endocrinopathy after AHSCT primarily affects thyroid and gonadal function [21▪,46]. Transient amenorrhea is commonly seen among treated women. Recovery of menstrual cycle was observed in all women less than 32 years old after approximately 5 months, regardless of the conditioning regimen used, restoration of menstruation following AHSCT was reported in 38% up to the age of 41 [47]. AHSCT confers a risk for permanent infertility, but reports of successful pregnancies with no congenital or developmental disease in the newborn are reassuring [48]. Counselling of patients on fertility risks and gamete or embryo cryopreservation should be an integral component of the consultation and consenting for AHSCT. Hypogonadism should be corrected with hormone replacement as appropriate.
Posttransplant cancers are rare in MS [49]. The excess incidence of normally expected rates is unknown, but in a long-term follow up study, nine out of 281 patients developed new neoplastic conditions following AHSCT, including myelodysplastic syndrome, breast cancer, glioblastoma multiforme, prostate and cervical cancer [49].
Another complication is the development of secondary autoimmune diseases [46], although these seem to be rare after AHSCT compared with some DMTs, such as alemtuzumab [32,50]. EBMT registry and other studies have shown that secondary autoimmune disease (most commonly thyroid disease) occurs with a long-term incidence of 4–6% [21▪,46,49].
CONCLUSION
AHSCT provides a one-off intensive therapeutic procedure for DMT-resistant poor prognosis inflammatory forms of MS, especially RRMS. Safety has significantly improved because of improved patient selection, the choice of conditioning regimen and the increasing experience and accreditation of transplant centres. The risk: benefit profile is increasingly acceptable to various neurology communities. Multidisciplinary team, including supportive care expertise, during the peritransplant and posttransplant period is key to the safe and successful delivery of AHSCT in MS.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
J.A.S. has received speaker's fees from Jazz, Janssen, Mallinckrodt and Gilead. There are no conflicts of interest for the remaining authors.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010; 9:520–532. [DOI] [PubMed] [Google Scholar]
- 2.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17:162–173. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler 2003; 9:260–274. [DOI] [PubMed] [Google Scholar]
- 4.Lucchetta RC, Tonin FS, Borba HHL, et al. Disease-modifying therapies for relapsing-remitting multiple sclerosis: a network meta-analysis. CNS Drugs 2018; 32:813–826. [DOI] [PubMed] [Google Scholar]
- 5.Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72:152–158. [DOI] [PubMed] [Google Scholar]
- 6.Kurtzke JF. Disability rating scales in multiple sclerosis. Ann N Y Acad Sci 1984; 436:347–360. [DOI] [PubMed] [Google Scholar]
- 7.Giovannoni G, Bermel R, Phillips T, et al. A brief history of NEDA. Mult Scler Relat Disord 2018; 20:228–230. [DOI] [PubMed] [Google Scholar]
- 8.Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009; 8:254–260. [DOI] [PubMed] [Google Scholar]
- 9.Havrdova E, Arnold DL, Bar-Or A, et al. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult Scler J Exp Transl Clin 2018; 4: 2055217318760642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 2017; 89:1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology 2017; 89:1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passweg JR, Baldomero H, Bader P, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant 2017; 52:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander T, Farge D, Badoglio M, et al. Hematopoietic stem cell therapy for autoimmune diseases: clinical experience and mechanisms. J Autoimmun 2018; 92:35–46. [DOI] [PubMed] [Google Scholar]
- 14.Snowden JA, Sharrack B, Akil M, et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases: a guide for the generalist. Clin Med (Lond) 2018; 18:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snowden JA, Badoglio M, Alexander T. The rise of autologous HCT for autoimmune diseases: what is behind it and what does it mean for the future of treatment? An update on behalf of the EBMT Autoimmune Diseases Working Party. Expert Rev Clin Immunol 2019; 16:1–5. [DOI] [PubMed] [Google Scholar]
- 16.Snowden JA, Badoglio M, Labopin M, et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv 2017; 1:2742–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sormani MP, Muraro PA, Schiavetti I, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology 2017; 88:2115–2122. [DOI] [PubMed] [Google Scholar]
- 18.Kelsey PJ, Oliveira MC, Badoglio M, et al. Haematopoietic stem cell transplantation in autoimmune diseases: from basic science to clinical practice. Curr Res Transl Med 2016; 64:71–82. [DOI] [PubMed] [Google Scholar]
- 19.Snowden JA. Rebooting autoimmunity with autologous HSCT. Blood 2016; 127:8–10. [DOI] [PubMed] [Google Scholar]
- 20.Duarte RF, Labopin M, Bader P, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant 2019; 54:1525–1552. [DOI] [PubMed] [Google Scholar]
- 21▪.Sharrack B, Saccardi R, Alexander T, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant 2019; doi: 10.1038/s41409-019-0684-0. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Updated EBMT guidelines for the application and future development of AHSCT for MS and other immune-mediated neurological diseases.
- 22.Bose G, Atkins HL, Bowman M, et al. Autologous hematopoietic stem cell transplantation improves fatigue in multiple sclerosis. Mult Scler 2018; 1352458518802544. doi: 10.1177/1352458518802544. [DOI] [PubMed] [Google Scholar]
- 23.Nash RA, Hutton GJ, Racke MK, et al. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology 2017; 88:842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burman J, Iacobaeus E, Svenningsson A, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry 2014; 85:1116–1121. [DOI] [PubMed] [Google Scholar]
- 25.Fangfang Ge HLL, Ting Chang. Efficacy and safety of autologous hematopoietic stem-cell transplantation in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci 2018; 40:479–487. [DOI] [PubMed] [Google Scholar]
- 26.Mancardi GL, Sormani MP, Di Gioia M, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multicentre experience. Mult Scler 2012; 18:835–842. [DOI] [PubMed] [Google Scholar]
- 27.Mancardi GL, Sormani MP, Gualandi F, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology 2015; 84:981–988. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Burt RK, Balabanov R, Burman J, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA 2019; 321:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first randomized trial comparing the efficacy of AHSCT with currently available DMTs. The results of the trial showed that AHSCT is superior to DMTs and prolonged the time to disease progression.
- 29.Das J, Sharrack B, Snowden JA. Autologous haematopoietic stem cell transplantation in multiple sclerosis: a review of current literature and future directions for transplant haematologists and oncologists. Curr Hematol Malig Rep 2019; 14:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30▪.Jessop H, Farge D, Saccardi R, et al. General information for patients and carers considering haematopoietic stem cell transplantation (HSCT) for severe autoimmune diseases (ADs): A position statement from the EBMT Autoimmune Diseases Working Party (ADWP), the EBMT Nurses Group, the EBMT Patient, Family and Donor Committee and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Bone Marrow Transplant 2019; 54:933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]; This position statement provides guidance for patients and nonspecialist clinicians considering AHSCT for autoimmune diseases and when considering treatment abroad.
- 31.Saccardi R, Kozak T, Bocelli-Tyndall C, et al. Autologous stem cell transplantation for progressive multiple sclerosis: Update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler J 2006; 12:814–823. [DOI] [PubMed] [Google Scholar]
- 32.Burt RK, Balabanov R, Han X, et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 2015; 313:275–284. [DOI] [PubMed] [Google Scholar]
- 33.Openshaw H, Stuve O, Antel JP, et al. Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor. Neurology 2000; 54:2147–2150. [DOI] [PubMed] [Google Scholar]
- 34.Burt RK, Fassas A, Snowden J, et al. Collection of hematopoietic stem cells from patients with autoimmune diseases. Bone Marrow Transplant 2001; 28:1–12. [DOI] [PubMed] [Google Scholar]
- 35.Jantunen E, Fruehauf S. Importance of blood graft characteristics in auto-SCT: implications for optimizing mobilization regimens. Bone Marrow Transplant 2011; 46:627–635. [DOI] [PubMed] [Google Scholar]
- 36.Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 2016; 388:576–585. [DOI] [PubMed] [Google Scholar]
- 37.Mancardi G, Sormani MP, Muraro PA, et al. Intense immunosuppression followed by autologous haematopoietic stem cell transplantation as a therapeutic strategy in aggressive forms of multiple sclerosis. Mult Scler 2018; 24:245–255. [DOI] [PubMed] [Google Scholar]
- 38.Moore JJ, Massey JC, Ford CD, et al. Prospective phase II clinical trial of autologous haematopoietic stem cell transplant for treatment refractory multiple sclerosis. J Neurol Neurosurg Psychiatry 2019; 90:514–521. [DOI] [PubMed] [Google Scholar]
- 39.Morris ES, Sharrack B, Dalley CD, Snowden JA. The Uhthoff phenomenon: a potential post transplant complication in advanced progressive multiple sclerosis. Bone Marrow Transplant 2007; 40:1003–1004. [DOI] [PubMed] [Google Scholar]
- 40.Motavasseli D, Chesnel C, Charlanes A, et al. Adherence to anticholinergic therapy and clean intermittent self-catheterization in patients with multiple sclerosis. Int Neurourol J 2018; 22:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snowden JAGD, Bird JM, Boland E, et al. Guidelines for screening and management of late and long-term consequences of myeloma and its treatment. A British Society for Haematology Guideline. Br J Haematol 2017; 176:888–907. [DOI] [PubMed] [Google Scholar]
- 42.Snowden JAAS, Ashcroft J, D'Sa S, et al. on behalf of BCSH and UK Myeloma Forum. Guidelines for supportive care in myeloma. Br J Haematol 2011; 154:76–103. [DOI] [PubMed] [Google Scholar]
- 43.Keen C, Skilbeck J, Ross H, et al. Is it feasible to conduct a randomised controlled trial of pretransplant exercise (prehabilitation) for patients with multiple myeloma awaiting autologous haematopoietic stem cell transplantation? Protocol for the PREeMPT study. BMJ Open 2018; 8:e021333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehra V, Rhone E, Widya S, et al. Epstein-Barr virus and monoclonal gammopathy of clinical significance in autologous stem cell transplantation for multiple sclerosis. Clin Infect Dis 2019; pii: ciz047. doi: 10.1093/cid/ciz047. [DOI] [PubMed] [Google Scholar]
- 45.Mariottini A, Innocenti C, Forci B, et al. Safety and efficacy of autologous hematopoietic stem-cell transplantation following natalizumab discontinuation in aggressive multiple sclerosis. Eur J Neurol 2019; 26:624–630. [DOI] [PubMed] [Google Scholar]
- 46.Daikeler T, Labopin M, Di Gioia M, et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood 2011; 118:1693–1698. [DOI] [PubMed] [Google Scholar]
- 47.Maciejewska M, Snarski E, Wiktor-Jedrzejczak W. A preliminary online study on menstruation recovery in women after autologous hematopoietic stem cell transplant for autoimmune diseases. Exp Clin Transplant 2016; 14:665–669. [DOI] [PubMed] [Google Scholar]
- 48.Snarski E, Snowden JA, Oliveira MC, et al. Onset and outcome of pregnancy after autologous haematopoietic SCT (AHSCT) for autoimmune diseases: a retrospective study of the EBMT autoimmune diseases working party (ADWP). Bone Marrow Transplant 2015; 50:216–220. [DOI] [PubMed] [Google Scholar]
- 49.Muraro PA, Pasquini M, Atkins HL, et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol 2017; 74:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costelloe L1 JJ, Coles A. Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev Neurotherap 2012; 12:335–341. [DOI] [PubMed] [Google Scholar]


