Abstract
What we know about cortical development during adolescence largely stems from analyses of cross‐sectional or cohort‐sequential samples, with few studies investigating brain development using a longitudinal design. Further, cortical volume is a product of two evolutionarily and genetically distinct features of the cortex ‐ thickness and surface area, and few studies have investigated development of these three characteristics within the same sample. The current study examined maturation of cortical thickness, surface area and volume during adolescence, as well as sex differences in development, using a mixed longitudinal design. 192 MRI scans were obtained from 90 healthy (i.e., free from lifetime psychopathology) adolescents (11‐20 years) at three time points (with different MRI scanners used at time 1 compared to 2 and 3). Developmental trajectories were estimated using linear mixed models. Non‐linear increases were present across most of the cortex for surface area. In comparison, thickness and volume were both characterised by a combination of non‐linear decreasing and increasing trajectories. While sex differences in volume and surface area were observed across time, no differences in thickness were identified. Furthermore, few regions exhibited sex differences in the cortical development. Our findings clearly illustrate that volume is a product of surface area and thickness, with each exhibiting differential patterns of development during adolescence, particularly in regions known to contribute to the development of social‐cognition and behavioral regulation. These findings suggest that thickness and surface area may be driven by different underlying mechanisms, with each measure potentially providing independent information about brain development. Hum Brain Mapp 37:2027–2038, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: brain structure, cortical development, FreeSurfer, neurodevelopment, sex differences
Abbreviations
- EATQR
Early Adolescent Temperament Questionnaire‐Revised
- LMM
Linear mixed models;
- MRI
Magnetic resonance imaging
- SES
Socioeconomic classification
INTRODUCTION
Adolescence is a period of marked brain development, second only to infancy in its extent [Jernigan et al., 2011]. Early neuroimaging research identified inverted‐U shaped developmental trajectories of cortical gray matter volume across the first two decades of life, with “peak” gray matter volumes being attained for different brain regions at differing points during adolescence, and females maturing earlier than males [Giedd et al., 1999; Lenroot et al., 2007]. However, some studies have identified earlier peaks during late childhood [Aubert‐Broche et al., 2013; Wierenga et al., 2014], and others have found different (i.e., linear or U‐shaped) trajectories of development (Tamnes et al., 2013).
Research focused on cortical thickness has also produced mixed findings. The majority of studies have shown widespread cortical thinning across childhood and adolescence, with most studies identifying linear reductions across development [Raznahan et al., 2011b; Tamnes et al., 2010; Wierenga et al., 2014]. However, some studies show thickening in language areas (Sowell et al., 2007) and in the temporal lobe [Raznahan et al., 2011a], while others have found no change or quadratic trajectories in similar regions [Mutlu et al., 2013].
A striking omission in the literature on adolescent structural brain development to date is the lack of studies utilizing longitudinal designs. This is unsurprising, given the immense time and resources required to carry out longitudinal brain imaging studies. However, there are a number of shortcomings of cross‐sectional and cross‐ or cohort‐sequential studies that limit interpretability of findings. Cross‐sectional studies can make statements only about interindividual variability (and not intraindividual change), and when comparisons are made across large age spans, it is likely that variance due to cohort exceeds that due to age [Schaie, 1994]. With cross‐ and cohort‐sequential designs, there has been much debate and disagreement about the ability of these designs, and analysis of resulting data, to truly separate cohort from age effects [Hertzog and Schaie, 1982]. A recent review [Mills and Tamnes, 2014] highlighted the challenges involved in drawing inferences about brain developmental processes from such studies, and argued for the necessity of within‐subjects longitudinal studies.
Further, despite the fact that cortical volume is a product of cortical thickness and surface area, there has been little attempt to integrate findings across these different measures of gray matter structure. The investigation of the development of all three properties of the cortical mantle is both logical and important to build a comprehensive picture of adolescent brain development. Few previous studies have investigated developmental patterns of all three measures, with all using cross‐sectional or cross/cohort‐sequential designs, and none investigating development at the whole brain vertex‐wise level. Raznahan et al. [2011b] found, within their sample of healthy 3 to 30 year olds, that all three cortical measure showed inverted‐U shaped trajectories at the whole brain level, with differences in the timing of curve peaks for each measure. Wierenga et al. [2014] found that the association between age and thickness was predominantly linear (decreasing), age and volume was quadratic, and age and surface area was cubic, across most regions in a sample of 7 to 23 year olds. Peaks in volume and surface area were identified at approximately the same age. Mills et al. [2014] also found similar trajectories for volume and surface area in a small number of regions of interest. While volume tended to peak during late childhood before decreasing into adulthood, surface area followed a similar but cubic trajectory, reaching a peak in early adolescence before decreasing into the early twenties. In comparison, cortical thickness in most regions decreased linearly across adolescence.
These studies also identified significant sex differences in cortical development. Raznahan et al. [2011b] found sex effects in relation to cortical volume and surface area, but not thickness, with females reaching “peaks” earlier than their male counterparts. While Mills et al. [2014] identified a similar effect, this was limited to surface area within the temporoparietal junction. Wierenga et al. [2014] also identified sex differences in volume and thickness development within a small number of regions, though much of the cortex did not exhibit such effects. Therefore, discrepancies exist in the current literature on sex differences in the developmental of thickness, volume, and surface area, which warrants further investigation to clarify potential sexual dimorphism in cortical development.
In sum, there are significant gaps in understanding patterns of structural brain development during adolescence. Studies are needed that longitudinally investigate the development of multiple properties of the cortex across the entire brain. The present study addresses these gaps by utilizing a mixed‐longitudinal design to examine the concurrent development of cortical volume, thickness and surface area in a sample of adolescents assessed from 11 to 20 years of age, as well as investigating potential sex differences in these developmental patterns.
MATERIALS AND METHODS
Participants
The sample described in the current study was derived from a larger longitudinal cohort enrolled in the Orygen Adolescent Development Study, conducted in Melbourne, Australia. Students (N = 2,453) in the final year of primary school were recruited from schools across metropolitan Melbourne to participate in an initial school‐screening phase, which involved completion of the Early Adolescent Temperament Questionnaire‐Revised [EATQR; Capaldi and Rothbart, 1992] Based on their scores on this measure, a smaller sample of 415 students was selected to be part of the study, which has previously been described in detail by Yap et al. [2011]. Adolescents at the extreme ends of the distribution for temperamental factors of Effortful Control and Neuroticism were oversampled to maximize inter‐individual differences in psychological well‐being. Specifically, an equal number of participants were recruited from the following range of SDs above and below mean: i) 0‐1 ii) 1‐2 iii) 2‐2.5 and iv) greater than 2.5, in order to emphasise distribution at the tails.
Of the selected adolescents, 245 agreed to participate in further research. These participants were screened for Axis 1 disorders using the Schedule for Affective Disorder and Schizophrenia for School‐Aged Children: Present and Lifetime Version (K‐SADS‐PL) [Kaufman and Schweder, 2004], and those who met the criteria for current or past MDD, substance‐use disorder or eating disorder were excluded due to the broader aims of the study. Remaining participants were invited to take part in brain magnetic resonance imaging (MRI) and psychopathology assessments (see below for details) at three time points, when they were aged approximately 12, 16 and 19, respectively. IQ was also assessed at baseline using a short form of the Wechsler Intelligence Scale for Children, Fourth Version [WISC‐IV; Wechsler, 2003] and socioeconomic classification (SES) was assessed based on the Australian National University Four (ANU4) Scale [Jones and McMillan, 2001]. A number of adolescents declined participation in the MRI assessments, resulting in 177 participants completing an MRI assessment at least one time point.
At each assessment wave, adolescents were assessed for current and past Axis I psychopathology using the K‐SADS. Following exclusions for MRI image and surface estimation quality (N = 11), and adolescents who were diagnosed with an Axis I mental illness (N = 76), 90 participants (n = 49 males) aged 11 to 20 years were available for analysis. Thirty‐five of these participants had three scans, 32 had two scans and 23 had one scan. Table 1 presents a breakdown of the number of participants available at each time point. Males and females did not differ on the demographic and cognitive variables listed in Table 2 (all P values > 0.05). In addition, refer to Supporting Information Figure S1 for a distribution of male and female participants across the three time points. The final sample exhibited normal distribution on all temperamental factors (i.e., Negative Affectivity, Effortful Control, Surgency, Affiliation; P > 0.05), suggesting that the sampling bias used for recruiting participants had “re‐normalized”. The final sample also did not differ from the initial school screening sample (N = 2453) on socioeconomic disadvantage [t (2439) = −1.292; P = 0.197] or sex (Pearson's χ 2 = 2.245; P = 0.691). Informed consent was obtained from the child and at least one parent/guardian at each time point, consistent with the guidelines of the Human Research Ethics Committee at the University of Melbourne, Australia.
Table 1.
Sample size
| Sex | |||
|---|---|---|---|
| Male | Female | Total | |
| Total | 49 | 41 | 90 |
| T1 | 40 | 29 | 69 |
| T2 | 37 | 35 | 72 |
| T3 | 25 | 26 | 51 |
T1 = Time 1, T2 = Time 2, T3 = Time 3.
Table 2.
Sample characteristics
| Sex | |||
|---|---|---|---|
| Male | Female | Total | |
| T1 age (years) | 12.77; 0.45 | 12.72; 0.44 | 12.75; 0.44 |
| T2 age (years) | 16.71; 0.64 | 16.67; 0.53 | 16.69; 0.58 |
| T3 age (years) | 19.09; 0.54 | 18.97; 0.43 | 19.03; 0.49 |
| Delay time 1‐2 (years) | 3.81; 0.17 | 3.79; 0.19 | 3.80; 0.18 |
| Delay time 2‐3 (years) | 2.36; 0.17 | 2.32; 0.24 | 2.34; 0.21 |
| Estimate Full Scale IQ | 108.39; 18.83 | 108.78; 17.94 | 108.57; 18.32 |
| SES | 61.75; 21.48 | 62.42; 20.42 | 62.05; 20.88 |
NB: Values represent mean; standard deviation.
T1 = Time 1, T2 = Time 2, T3 = Time 3, IQ = intelligence quotient, SES = socioeconomic status.
MRI Acquisition and Analysis
Image acquisition
At T1, MRI scans were performed on a 3 Tesla GE scanner at the Brain Research Institute, Austin and Repatriation Medical Centre, Melbourne, Australia, with the following parameters: repetition time = 36 msec; echo time = 9msec; flip angle = 35°, field of view = 20cm, 124 T1‐weighted contiguous slices (voxel dimensions = 0.4883 × 0.4883 × 1.5mm). MRI scans at T2 and T3 were performed on a 3 Tesla Siemens scanner at the Royal Children's Hospital, Melbourne, Australia, with the following parameters: repetition time = 1,900 msec; echo time = 2.24 msec; flip angle = 9°, field of view = 23cm; 176 T1‐weighted contiguous slices (voxel dimensions = 0.9mm3).
Image processing
Images were transferred to an SGI/Linux workstation for morphometric analysis. Cortical reconstruction was performed using the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/), which provides a set of tools to reconstruct topologically correct and geometrically accurate surface models of the inner and outer cortical boundaries, thus deriving multiple anatomical measures, including cortical volume, thickness, and surface area.
In order to address issues arising from longitudinal and/or multisite studies (such as geometric distortion and voxel dimension drift), images were processed through the longitudinal stream of FreeSurfer version 5.3 [Reuter et al., 2012], which creates a within‐unbiased subject template space and average image from all available time points using robust, inverse consistent registration [Reuter and Fischl, 2011]. The template is used as an estimate to initialize subsequent segmentation processes in the longitudinal stream for each time point, providing common information regarding anatomical structures. This process can deal with different intensity scales, guarantees inverse consistency (i.e., symmetry), and automatically estimates a sensitivity parameter to detect outlier regions in the image. It significantly improves the repeatability and power of cortical measurements, having superior robustness with respect to noise, intensity scaling and outliers when compared to alternate registration tools [Reuter et al., 2010]. Further, similar longitudinal analysis pipelines have been shown to result in different interpretations of developmental trajectories previously reported using cross‐sectional analyses from the same datasets, suggesting that it is better at modeling the unique nature of within‐subject measurements [Aubert‐Broche et al., 2013]. All images were also corrected for tissue signal inhomogeneity using FreeSurfer's N3 correction (optimized for 3 Tesla images), a non‐parametric non‐uniformity intensity normalization method, which reduces sensitivity to changes in scanner platform and improves accuracy and robustness during cortical segmentation [Boyes et al., 2008; Zheng et al., 2009].
Each individual's cortical reconstruction was visually inspected by a trained researcher to ensure that optimal grey/white and grey/cerebrospinal fluid classification had occurred based on differences in tissue intensity signals, and manual edits were made where necessary to ensure/improve quality of the output. However, as mentioned above, there were 11 scans that were deemed unusable due to poor quality of the image output. Following this process, measurements were obtained for 34 cortical structures per hemisphere, as labeled by the Desikan‐Killiany atlas [Desikan et al., 2006]. All FreeSurfer image processing was conducted on a high performance computing facility at the Melbourne Neuropsychiatry Centre, Melbourne, Australia.
Interscanner reliability
Although different scanners were used at Time 1 versus Times 2 and 3, our previous work has shown no inter‐scanner bias [Vijayakumar et al., 2013; Whittle et al., 2014]. However, to fully address any concerns that changes in cortical metrics over time may be due to measurement bias from the different scanner platforms and acquisition parameters, a reliability analysis was undertaken using four individuals who were scanned on both BRI and RCH scanners over a time interval of 1–2 weeks. Analyses were conducted on the same regions of interest (two from the medial surface and two from the lateral surface) as those graphically depicted in the results section (see Fig. 1). Table 3 reports the mean absolute difference, between scanners at BRI (Time 1) and RCH (Times 2 and 3), in thickness, surface area and volume for these four regions of interest. It also contains mean test–retest reproducibility errors across subjects, which is calculated as the absolute test–retest percent change relative to the mean test–retest value for each subject (i.e., mean of measurements from the two scanners). All regions exhibit less than 10% change in thickness, volume and surface area between the two scanners. Given the importance of this issue, the data from the inter‐scanner reliability analysis was applied to the current study sample using a descriptive procedure proposed by Lebel and Beaulieu [2011], in order to determine if the mean amount of change experienced by the study sample was likely to have occurred over and above that expected from scanner effects. We calculated standard deviations for each ROI within each reliability subject based on their scores from the different scanners. A group average standard deviation was then calculated for each ROI (mean SD across all subjects), which are listed in Table 3. These values provide estimates of the measurement variability in each ROI that can be expected from scanner differences alone. The average SD data was applied to the study sample in order to determine the proportion (i.e., percentage) of subjects that experienced greater change (either increases or decreases) than the average SD. For each subject, change for each ROI was calculated using a difference score (i.e., cortical thickness for time 2 – time 1). Those with difference scores within 1 SD (determined from the reliability study) were considered to not change, while those with difference scores greater than 1 SD were considered to experience true change (over and above scanner effects). When the majority of subjects (i.e., >50%) experienced longitudinal change over and above that expected from scanner effects, this is taken as evidence that changes in cortical metrics identified by the mixed models in the study sample was reliable. The results from our sample, presented in Figure 1, indicate that for each ROI the majority of individuals (>50%) experienced greater cortical change over time than could be attributed to inter‐scanner variance alone based on the reliability estimates.
Figure 1.
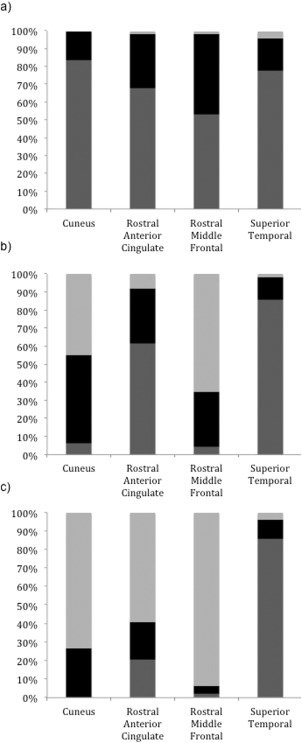
Proportion of participants for whom ROI increased (dark grey), decreased (light grey) or did not change (black) based on the inter‐scanner reliability analysis for (a) surface area, (b) volume, and (c) thickness.
Table 3.
Reliability statistics based on a separate sample of individuals scanned at both neuroimaging sites
| Thickness | Surface area | Volume | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure | Mean absolute difference (mm) | Reproducibility Error (%) | Average SD | Mean absolute difference (mm2) | Reproducibility Error (%) | Average SD | Mean absolute difference (mm3) | Reproducibility Error (%) | Average SD |
| Cuneus | 0.200 | 9.906 | 0.141 | 98.00 | 6.86 | 69.30 | 223.75 | 7.262 | 158.22 |
| Rostral anterior cingulate | 0.082 | 2.693 | 0.058 | 31.75 | 3.77 | 22.45 | 118.00 | 4.058 | 83.44 |
| Rostral medial frontal | 0.114 | 4.387 | 0.081 | 151.25 | 2.64 | 106.95 | 870.00 | 4.511 | 615.18 |
| Superior temporal | 0.078 | 2.624 | 0.054 | 65.00 | 1.58 | 45.96 | 592.00 | 4.089 | 418.61 |
NB: The group reproducibility error for each structure is derived by averaging the reproducibility errors across subjects, where for each subject the error is estimated as the absolute test–retest thickness percent change relative to the mean test–retest thickness.
Statistical Analysis
All statistical analyses were conducted in SPSS. Linear mixed models (LMM) were used to analyze the data on each of the regions estimated from FreeSurfer's automated cortical parcellation process, with an autoregressive covariance structure modeling the repeated measurements over time. LMMs provides a flexible and powerful statistical framework for the analysis of longitudinal data. It has been suggested as superior to commonly used statistical approaches, including repeated measures analysis of variance (or within‐subject ANOVA) and cross‐sectional (General Linear Model‐based) analysis of summary measurements (i.e., annualized percentage differences), as these methods are known to be sub‐optimal for longitudinal data since they do not model the covariance structure of serial measurements appropriately and cannot handle imperfect timing and/or subject dropout (i.e., unbalanced data), in particular those cases with only a single time point [Gibbons et al., 2010]. Furthermore, we wanted to ensure consistency with our methodology to that used by prior studies in the field [Aubert‐Broche et al., 2013; Mills et al., 2014; Mutlu et al., 2013; Raznahan et al., 2011a,b; Wierenga et al., 2014], given that inconsistencies can occur from the use of differing techniques (i.e., general vs mixed models, step‐down vs indices of fit for model selection).
We began by first identifying the best fitting developmental model by comparing linear (Y = Intercept + d ij + β 1 (age) + e ijk) and quadratic effects of age (Y = Intercept + d ij + β 1 (age) + β 2 (age2) + e ijk) and the null model (Y = Intercept + d ij + e ijk). Each cortical metric (i.e., thickness, volume, surface area) was modeled within each i th subject at each j th vertex. The d ij term represents the random effect of the intercept within each vertex in each subject. The e ijk represents the normally distributed residual error term. All models were run with mean‐centered continuous variables. A more complex model (i.e., linear over null, or quadratic over linear or null) was chosen if P < 0.05 and the BIC indicated better model fit (i.e., value smaller by two or more). Following the identification of the best fitting developmental model, we investigated whether the inclusion of sex effects improved model fit. We examined whether both a main effect of sex (e.g. for linear developmental models: Y = Intercept + d ij + β 1 (age) + β 2 (sex) + e ijk) and an interaction of sex and age (e.g. for linear models: Y = Intercept + d ij + β 1 (age) + β 2 (sex) + β 3 (age*sex) + e ijk) improved the developmental model. Again, more complex models (i.e., inclusion of more parameters) were chosen based on P < 0.05 and BIC values.
In order to examine the effect of potential outliers, the distribution of standardized residuals from the analyses was examined. Where the potential influence of outliers was of concern, the FreeSurfer generated surfaces were visually checked to ensure there were no issues with image quality or the cortical surface estimation.
RESULTS
Analyses revealed significant quadratic and linear effects of age across all three measures, albeit in different brain areas. Significant main effects of sex were also identified, but interactions between age and sex were limited in number. Refer to Supporting Information Tables S1–S6 for further details about the final best‐fit models for each region within the three cortical measures.
Figure 2 shows the quadratic and linear effects of age for cortical thickness, volume and surface area. There were significant negative quadratic effects (i.e., non‐linear increases) in surface area across much of the medial and lateral surface of the cortex, spanning the frontal, temporal and occipital lobes. In addition, linear increases were identified within parts of the left lateral frontal cortex, bilateral cingulate, and right motor cortices. The only areas showing (linear) reductions in surface area were parts of the left posterior temporal, caudal middle frontal and parahippocampal cortices, and the right insula. In comparison, there was more variation in cortical thickness. Much of the temporal and occipital lobes, and the primary motor and somatosensory cortices showed increases in thickness (primarily negative quadratic effects; i.e., non‐linear increases). However, reductions in thickness were also present across most of the medial surface (cuneus, precuneus, and medial prefrontal cortex), the lateral frontal and parietal lobes. While most of this change was quadratic within the left hemisphere, more variation (i.e., quadratic and linear change) was present in the right hemisphere. In terms of volumetric change, there were significant negative quadratic effects (i.e., non‐linear increases) across most of the lateral surface, apart from linear decreases within the parts of the prefrontal and parietal regions. There were other developmental changes observed within the medial surface, with significant (primarily quadratic) reductions present in the cuneus, precuneus, and left medial orbitofrontal regions, but increases present in the cingulate, the superior frontal gyrus and parts of the temporal lobe. Similar findings were identified across the left and right hemisphere in relation to volumetric change based on visual inspection. In order to further illustrate the nature of quadratic effects, we plotted the change in two medial and two lateral regions for surface area, volume and thickness (see Supporting Information Fig. S2). Furthermore, vertex‐wise analyses of the same data using Matlab (with FDR correction for multiple comparisons) revealed very similar results to that identified using the parcellated FreeSurfer output (see Supporting Information Fig. S3).
Figure 2.
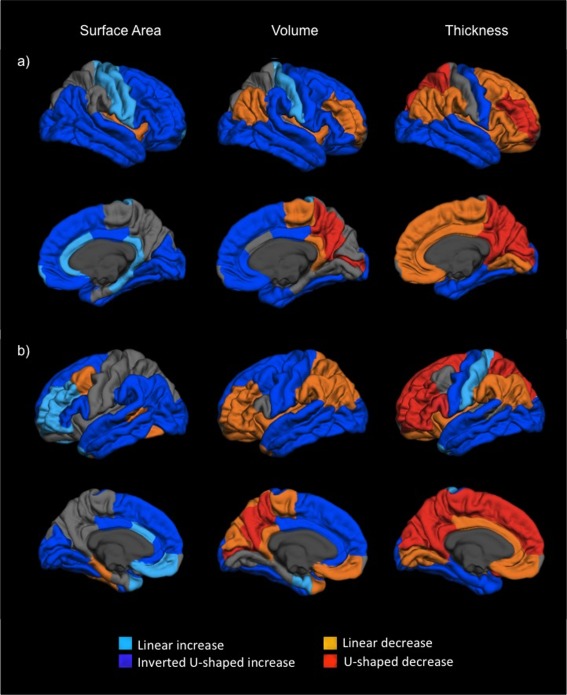
Effect of age on cortical surface area, thickness and volume estimates for, (a) right, and (b) left hemispheres. Dark blue and light blue indicate inverted U‐shaped (i.e., negative quadratic) and linear increases, respectively. Red and orange represent U‐shaped (i.e., positive quadratic) and linear decreases, respectively. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Significant main effects of sex were identified across much of the cortex in relation to surface area and volume, with males exhibiting larger structures than females (as shown in Fig. 3). In comparison, only a small poster region within the superior temporal sulcus was found to exhibit sex differences in cortical thickness. Regions exhibiting significant interactions between sex and age included the bilateral lingual cortex for thickness, right postcentral, left cuneus, left parahippocampal gyrus and left parstriangularis for volume, and the left superior temporal sulcus and frontal pole for surface area. Change within all these regions was characterised by either greater reductions or less increases (based in normative developmental patterns) in females compared to males. The only region exhibiting the opposite pattern of findings was left superior temporal sulcus in relation to surface area, where females exhibited greater increases than males.
Figure 3.
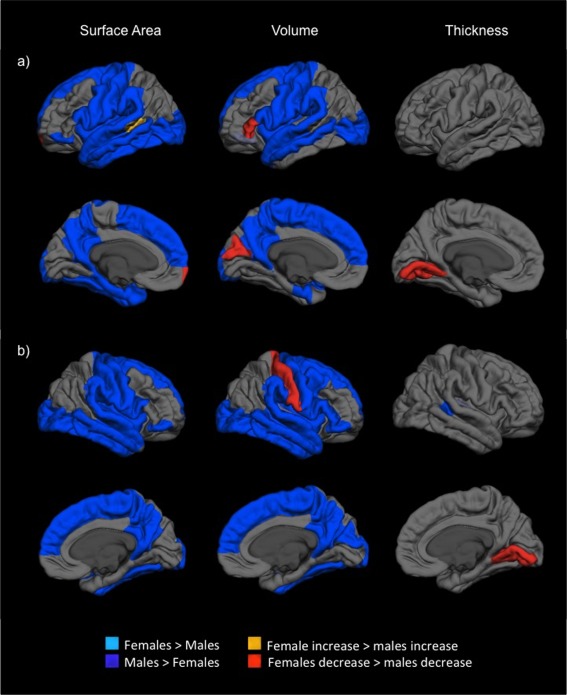
Sex differences in mean cortical surface area, thickness and volume estimates across age for, (a) left, and (b) right hemispheres. The blue color indicates main effects of sex, while red/yellow color indicates significant sex differences in development (i.e., age*sex interactions). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The current study has shown, using a mixed‐longitudinal design, distinct patterns of development of cortical volume, thickness and surface area during adolescence. We found a combination of linear and quadratic trajectories of change in the cortex. Specifically, surface area showed non‐linear increases across adolescence over most of the cortical mantle. Thickness was characterized by both linear and non‐linear decreasing trajectories across much of the frontal and parietal regions, as well as non‐linear increases in temporo‐occipital regions. In comparison, volume showed non‐linear increases in parts of the frontal and temporo‐occipital regions, as well as linear reductions within frontal and parietal regions. Sex differences in development were observed in a few regions, with females exhibiting greater reductions in males. In addition, males were found to have larger volumes and area than females across much of cortex over time.
Our finding of inverted U‐shaped trajectories for volume across much of the cortex is consistent with previous research [Giedd et al., 1999; Lenroot et al., 2007; Mills et al., 2014]. Notable exceptions to this pattern were found in the lateral and medial prefrontal cortex, and posterior cingulate/precuneus/cuneus region, where developmental trajectories were either U‐shaped or linearly decreasing. These patterns of change are consistent with previous research by Wierenga et al. [2014], who found U‐shaped trajectories of volume change in the cuneus, precuneus, and posterior cingulate. Similarly, Tamnes et al. [2013] also found a decreasing trajectory of volume change in the precuneus.
Our finding of widespread quadratic change in thickness across age is at odds with studies finding predominantly linear decreasing trajectories of cortical thickness across adolescence [Raznahan et al., 2011b; Tamnes et al., 2010; Wierenga et al., 2014]. However, our results are also consistent with some previous research. For example, regions where we found U‐shaped trajectories, including the medial and lateral prefrontal cortex and precuneus/cuneus are strikingly similar to those regions found by Mutlu et al. [2013] to show quadratic developmental effects. Wierenga et al. [2014] also identified a U‐shaped trajectory in the cuneus.
Regarding surface area, we could not investigate cubic developmental effects that have been reported previously [Mills et al., 2014; Wierenga et al., 2014], given that only three MRI scans were available to model within person change over time. However, our finding of an inverted‐U shaped trajectory of surface area across the cortex is consistent with patterns found in previous research for the age period sampled in the current study.
The mechanisms contributing to the distinct developmental patterns of volume, thickness and surface area are unclear. Early volumetric studies proposed that post‐peak reductions in gray matter volume during adolescence partly reflect synaptic pruning [Blakemore, 2008; Giedd et al., 1999]. Given that volume is, by geometric definition, the product of surface area and thickness, a fact that is clearly reflected in our results (see Fig. 3), an understanding of the mechanisms underlying the development of thickness and surface area is of particular interest. Cortical area and thickness result from well‐differentiated ontogenic stages during corticogenesis. The radial unit hypothesis, which proposes that neurons within the same ontogenetic column (running perpendicular to the brain surface) share a common origin and migrate to their location within the cortex during development [Rakic, 1995, 2007], argues that surface area is dependent on the number of columns, while thickness is driven by the number of cells within each column (Rakic, 1988). Further, animal studies suggest that surface area expansion, but not change in cortical thickness, is dependent on afferent input from subcortical structures during early development [Rakic, 1995]. Experimental manipulation of progenitor cells, which are thought to play a role in neurogenesis, has also been found to affect cortical thickness, but not cortical surface area [Pontious et al., 2008]. Thus, while development of cortical surface area may be dependent on input from subcortical structures, development of cortical thickness may be more dependent on factors that are local or intrinsic to the cortex.
During adolescence, there is evidence for at least two concurrent processes that are hypothesized to be related to changes in cortical thickness. The first process involves dynamic synaptic reorganization, with post‐mortem studies identifying a reduction in the number of synapses, neuropil, and/or cortical glial cells during adolescence [Huttenlocher and Dabholkar, 1997; Petanjek et al., 2011]. The second process involves white matter changes, including the encroachment of subcortical white matter, and continued intracortical myelination [Benes et al., 1994; Yakovlev and Lecours, 1967]. Regarding surface area, development has been suggested to be related to changes in gyrification, with increases related to surface area expansion. However, this direction of causality remains uncertain (i.e., does increase in surface area lead to increase in gyrification or vice versa), and some have failed to identify any changes in sulcal patterns with age [Hill et al., 2010]. Regardless of the specific underlying mechanisms, it is likely that thickness and surface area provide independent information about brain development, particularly given the evidence that they capture distinct evolutionary [Rakic, 1995], genetic [Panizzon et al., 2009], and cellular [Chenn and Walsh, 2002] processes, and are influenced differentially by common genetic variants [Joyner et al., 2009] and environmental factors [Park and Reuter‐Lorenz, 2009].
The functional implications of our finding of differential growth patterns for different brain regions are currently unclear. It is of interest that regions where thickness and volume exhibited a decreasing quadratic trajectory, namely, the dorsolateral prefrontal cortex, medial prefrontal cortex, cuneus, precuneus, and superior parietal cortex, correspond to brain regions responsible for higher order social‐cognitive and behavioral regulatory functions, as well as self‐referential and theory of mind processes [Amft et al., 2015]. Of note, there is evidence from studies using both functional and structural connectivity analysis, that these regions show ‘maturational coupling’ (i.e., correlated patterns of change over time) [Raznahan et al., 2011a; Uddin et al., 2011]. Thus, it is possible that the coordinated pattern of development of these regions underlies the development of social‐cognitive functioning and emotional/behavioral regulation during adolescence [Blakemore and Choudhury, 2012].
We also found sex differences across time for much of the cortex in relation to volume and surface area. Males were found to have larger structures than females across most of the medial surface, as well as within the fronto‐parietal junction, and temporal and occipital lobes on the lateral surface. In comparison, we failed to identify any such effects in relation to cortical thickness. These findings are consistent with Wierenga et al., [2014] and Mills et al. [2014] who found significant sex main effects in cortical volume and surface area, but minimal differences in cortical thickness across time. We also identified a few regions that exhibited sex differences in cortical development, with females generally exhibiting greater reductions in size compared to their male counterparts. However, it should be noted that most of the brain did not exhibit such effects for any of the three metrics, similar to Wierenga et al. [2014] findings. While some studies, particularly earlier ones conducted in this field, have identified earlier or accelerated maturation of the female brain [Lenroot et al., 2007; Mutlu et al., 2013; Sowell et al., 2004], numerous other studies have since failed to identify such effects, or only done so within a small number of regions [Aubert‐Broche et al., 2013; Mills et al., 2014; Raznahan et al., 2011b; Tamnes et al., 2013]. The most common explanation for potential sex differences in cortical development is the sexually dimorphic effects of puberty, with support coming from experimental studies on animal and more recently observational studies in humans [Bramen et al., 2011; Peper et al., 2009; Raznahan et al., 2010; Sisk and Foster, 2004]. However, the inconsistencies in this field highlight the need for further studies to examine the potential effect of puberty and sex‐hormones on cortical development.
Limitations
There are a number of limitations to this study that should be considered. We only investigated development during the second decade of life. Consequently, we have missed important changes occurring during childhood and towards the end of the adolescent period. The availability of three MRI scans per individual, as well as the limited variance in age at each time point, limited modeling of development of quadratic and linear patterns of change. Therefore, we were not able to comment on cubic patterns of development, or other non‐linear models of change (such as negative exponential models).
The main analyses were conducted using regions that were parcellated by FreeSurfer, which is consistent with much of the previous literature and permits the use of indices of fit for model selection. However, the vertex‐wise analyses conducted in Matlab indicate that, particularly for larger parcellated regions, different developmental patterns may be evident within each structure. Another limitation was the change in scanner between waves 1 and 2. We have attempted to address this issue within the interscanner reliability study, which found that most individuals experienced change between waves 1 and 2 greater than what would be expected from any scanner‐related differences. However, future studies would benefit from conducting all waves of assessment on the same scanner to remove this potential confound.
CONCLUSIONS
In this important investigation of the development of cortical volume, thickness and surface area during the adolescent period, we found evidence for predominantly quadratic patterns of growth across all measures, quadratic increases prominent for surface area, and quadratic decreases prominent for thickness (and to a lesser degree, volume) in a number of medial and lateral areas known to contribute to the development of social‐cognition and behavioral regulation during adolescence. Our findings clearly illustrate differential growth patterns for each measure, and are consistent with the known fact that volume is a product of thickness and surface area. Further research is required to understand both the mechanisms driving the different developmental patterns observed, as well as their functional and behavioral implications.
Supporting information
Supporting Information 1
Supporting Information 2
ACKNOWLEDGMENTS
Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory at the Melbourne Neuropsychiatry Centre. The authors thank the Brain Research Institute and Royal Children's Hospital for support in acquiring the neuroimaging data, and the families who participated in the study. Dr. Whittle is supported by a NHMRC Career Development Fellowship (ID: 1007716). Prof. Yücel is supported by a NHMRC Fellowship (ID: 1021973).
REFERENCES
- Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB (2015): Definition and characterization of an extended social‐affective default network. Brain Struct Funct 220:1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert‐Broche B, Fonov V, García‐Lorenzo D, Mouiha A, Guizard N, Coupé P, Eskildsen SF, Collins DL (2013): A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. NeuroImage 82:393–402. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P (1994): Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry 51:477–484. [DOI] [PubMed] [Google Scholar]
- Blakemore S‐J (2008): Development of the social brain during adolescence. Quarter J Exp Psychol 61:40–49. [DOI] [PubMed] [Google Scholar]
- Blakemore S‐J, Choudhury S (2006): Development of the adolescent brain: Implications for executive function and social cognition. J Child Psychol Psychiatry 47:296–312. [DOI] [PubMed] [Google Scholar]
- Boyes RG, Gunter JL, Frost C, Janke AL, Yeatman T, Hill DL, Bernstein MA, Thompson PM, Weiner MW, Schuff N (2008): Intensity non‐uniformity correction using N3 on 3‐T scanners with multichannel phased array coils. Neuroimage 39:1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER (2011): Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex 21:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, Rothbart MK (1992): Development and validation of an early adolescent temperament measure. J Early Adolesc 12:153–173. [Google Scholar]
- Chenn A, Walsh CA (2002): Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297:365–369. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, DuToit S (2010): Advances in analysis of longitudinal data. Annu Rev Clin Psychol 6:79–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2:861–863. [DOI] [PubMed] [Google Scholar]
- Hertzog CK, Schaie KW. On the analysis of sequential data in life‐span developmental research; 1982.
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D (2010): Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci USA 107:13135–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS (1997): Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387:167–178. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Baare WF, Stiles J, Madsen KS (2011): Postnatal brain development: Structural imaging of dynamic neurodevelopmental processes. Prog Brain Res 189:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones FL, McMillan J (2001): Scoring occupational categories for social research: A review of current practice, with Australian examples. Work, Employ Soc 15:539–563. [Google Scholar]
- Joyner AH, Bloss CS, Bakken TE, Rimol LM, Melle I, Agartz I, Djurovic S, Topol EJ, Schork NJ, Andreassen OA (2009): A common MECP2 haplotype associates with reduced cortical surface area in humans in two independent populations. Proc Natl Acad Sci USA 106:15483–15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Schweder A. 2004. Schedule for affective disorders and schizophrenia for school‐age children‐ present and lifetime version (K‐SADS‐PL) In: Hilsenroth MJ, Segal DL, editors. Comprehensive Handbook of Psychological Assessment, Personality Assessment. Hoboken, NJ: Wiley; pp 247–255. [Google Scholar]
- Lebel C, Beaulieu C (2011): Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC (2007): Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Tamnes CK (2014): Methods and considerations for longitudinal structural brain imaging analysis across development. Dev Cognit Neurosci 9:172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Lalonde F, Clasen LS, Giedd JN, Blakemore S‐J (2014): Developmental changes in the structure of the social brain in late childhood and adolescence. Soc Cognit Affect Neurosci 9:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu AK, Schneider M, Debbané M, Badoud D, Eliez S, Schaer M (2013): Sex differences in thickness, and folding developments throughout the cortex. Neuroimage 82:200–207. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema‐Notestine C, Eyler LT, Jernigan TL, Prom‐Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE (2009): Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter‐Lorenz P (2009): The adaptive brain: Aging and neurocognitive scaffolding. Ann Rev Psychol 60:173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre‐Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE (2009): Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology 34:332–342. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HB, Rakic P, Kostović I (2011): Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontious A, Kowalczyk T, Englund C, Hevner RF (2008): Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci 30:24–32. [DOI] [PubMed] [Google Scholar]
- Rakic P (1995): A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trend Neurosci 18:383–388. [DOI] [PubMed] [Google Scholar]
- Rakic P (1988): Specification of cerebral cortical areas. Science 241:170–176. [DOI] [PubMed] [Google Scholar]
- Rakic P (2007): The radial edifice of cortical architecture: From neuronal silhouettes to genetic engineering. Brain Res Rev 55:204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN (2010): Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci USA 107:16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lerch JP, Lee N, Greenstein D, Wallace GL, Stockman M, Clasen L, Shaw PW, Giedd JN (2011a): Patterns of coordinated anatomical change in human cortical development: A longitudinal neuroimaging study of maturational coupling. Neuron 72:873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN (2011b): How does your cortex grow? J Neurosci 31:7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Fischl B (2011): Avoiding asymmetry‐induced bias in longitudinal image processing. Neuroimage 57:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B (2010): Highly accurate inverse consistent registration: A robust approach. Neuroimage 53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012): Within‐subject template estimation for unbiased longitudinal image analysis. Neuroimage 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. (1994): Developmental designs revisited. In: Cohen SH, Reese HW, editors. Life-span Developmental Psychology. Hillsdale, NJ: Erlbaum. pp 45–64.
- Sisk CL, Foster DL (2004): The neural basis of puberty and adolescence. Nat Neurosci 7:1040–1047. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW (2004): Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, et al. (2007): Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 17:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due‐Tønnessen P, Walhovd KB (2010): Brain maturation in adolescence and young adulthood: Regional age‐related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex 20:534–548. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Dale AM, Østby Y, Grydeland H, Richardson G, Westlye LT, Roddey JC, Hagler JDJ, Due‐Tønnessen P (2013): Brain development and aging: Overlapping and unique patterns of change. Neuroimage 68:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V (2011): Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci 31:18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Whittle S, Yücel M, Dennison M, Simmons J, Allen NB. (2013): Prefrontal Structural Correlates of Cognitive Control during Adolescent Development: A 4‐Year Longitudinal Study. [DOI] [PubMed]
- Wechsler, D. (2003). Wechsler Intelligence Scale for Children‐Fourth Edition. San Antonio TX: Harcourt Assessment, Inc. [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yücel M, Pantelis C, McGorry P (2014): Structural brain development and depression onset during adolescence: A prospective longitudinal study. Am J Psychiatry 171:564–571. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S (2014): Unique developmental trajectories of cortical thickness and surface area. Neuroimage 87:120–126. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours A‐R (1967): The myelogenetic cycles of regional maturation of the brain. Regional Development of the Brain in Early Life 3–70. [Google Scholar]
- Yap MB, Allen NB, O'Shea M, Di Parsia P, Simmons JG, Sheeber L (2011): Early adolescents' temperament, emotion regulation during mother–child interactions, and depressive symptomatology. Dev Psychopathol 23:267–282. [DOI] [PubMed] [Google Scholar]
- Zheng W, Chee MW, Zagorodnov V (2009): Improvement of brain segmentation accuracy by optimizing non‐uniformity correction using N3. Neuroimage 48:73–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information 2


