Abstract
Age‐related behavioral declines may be the result of deterioration of white matter tracts, affecting brain structural (SC) and functional connectivity (FC) during resting state. To date, it is not clear if the combination of SC and FC data could better predict cognitive/motor performance than each measure separately. We probed these relationships in the cingulum bundle, a major white matter pathway of the default mode network. We aimed to attain deeper knowledge about: (a) the relationship between age and the cingulum's SC and FC strength, (b) the association between SC and FC, and particularly (c) how the cingulum's SC and FC are related to cognitive/motor performance separately and combined. We examined these associations in a healthy and well‐educated sample of 165 older participants (aged 64‐85). SC and FC were acquired using probabilistic tractography to derive measures to capture white matter integrity within the cingulum bundle (fractional anisotropy, mean, axial and radial diffusivity) and a seed‐based resting‐state functional MRI correlation approach, respectively. Participants performed cognitive tests measuring processing speed, memory and executive functions, and motor tests measuring motor speed and grip force. Our data revealed that only SC but not resting state FC was significantly associated with age. Further, the cingulum's SC and FC showed no relation. Different relationships between cognitive/motor performance and SC/FC separately were found, but no additive effect of the combined analysis of cingulum's SC and FC for predicting cognitive/motor performance was apparent. Hum Brain Mapp 37:855–867, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: cingulum, cognitive aging, functional connectivity, multimodal imaging, tractography
Abbreviations
- AD
axial diffusivity
- DMN
default mode network
- FA
fractional anisotropy
- FC
functional connectivity
- GLM
general linear models
- MD
mean diffusivity
- mPFC
medial prefrontal cortex
- PCC
posterior cingulate cortex
- RD
radial diffusivity
- rs‐fMRI
resting‐state functional Magnetic Resonance Imaging
- SC
structural connectivity
- ROI
regions of interest
- WM
white matter
INTRODUCTION
It is well established that healthy aging is associated with changes in behavior, neuroanatomical, and functional brain metrics [Bennett and Madden, 2013; Ferreira and Busatto, 2013; Fjell and Walhovd, 2010]. However, if or how the age‐related decline in behavioral measures can be explained by neuroanatomical and/or functional intra‐individual differences is still not fully understood. One underlying mechanism for behavioral deterioration as well as for loss of functional connectivity (FC) could be described by the disconnection hypothesis [O'Sullivan et al., 2001]. The hypothesis is based on Geschwind's proposal of disconnection syndromes [Geschwind, 1965], which suggests the importance of white matter (WM) networks for higher order function. Within this framework the decline in cognitive abilities could be related to deterioration of WM tract integrity because of its impact on communication between brain regions.
Indeed, there is evidence for microstructural deterioration of WM tracts in old age that seems to be more pronounced in frontal than in posterior brain regions [Bennett and Madden, 2013]. In addition, processing speed as well as higher‐order cognitive abilities such as, executive functions and episodic memory are reported to be associated with WM integrity in normal healthy aging [Madden et al., 2012], supporting the disconnection hypothesis.
With the fairly recent development of resting‐state functional Magnetic Resonance Imaging (rs‐fMRI), it has become possible to study intrinsic FC between different brain regions when participants are at rest, thus removing the confound of strategic task performance effects. Several networks, each consisting of multiple brain regions, have been identified reliably [Fox and Raichle, 2007]. The default mode network (DMN) is one of the intrinsic resting‐state networks that has been most studied with respect to aging. The DMN includes the medial prefrontal cortex, posterior cingulate/retrospinal cortex, inferior parietal lobule and the hippocampus [Buckner et al., 2008]. These regions are important for cognitive and motor functioning and therefore age‐related changes in DMN FC strength could potentially explain part of the variation in behavioral performance with age [Ferreira and Busatto, 2013]. Indeed, several studies have reported decreases in DMN connectivity strength in group comparisons between young and older participants [Dennis and Thompson, 2014] but also a cross‐sectional analysis over a larger age range depicted a linear decline of FC strength over the life span [Onoda et al., 2012]. However, a recent longitudinal study investigating participants with a mean age of 65.2 (range 49‐79 years) at baseline did not detect any changes in DMN FC strength after 6 years follow‐up [Persson et al., 2014], although they did show that a decrease in default mode connectivity strength was associated with a decline in memory performance.
Considering that WM tracts reflect, to a considerable degree, the anatomical connections for FC it can be argued that investigating solely structural connectivity (SC) or FC provides a limited view of the complexity of the human brain, and combined analysis could reveal more information about brain‐behavior relationships. Nonetheless, studies investigating the association between SC and FC are still scant in the field of healthy aging. So far, one study found that integrity of the WM tract connecting the posterior cingulate cortex (PCC) and hippocampus was associated with FC strength between the two regions [Teipel et al., 2010]. In addition, FC strength between the PCC and the medial prefrontal cortex (mPFC) was related with WM integrity in a larger region of interest (ROI) containing the cingulum bundle [Andrews‐Hanna et al., 2007]. Further, after controlling for age, FC strength was associated with performance in three domains: memory, executive functioning, and processing speed [Andrews‐Hanna et al., 2007]. Unfortunately, the association between the underlying WM integrity and cognitive performance was not analyzed.
To date, it is not clear if the combination of FC and SC can explain unique variance in cognitive performance in addition to that which is explained by SC alone. To answer this question, we analyzed SC and FC of the cingulum bundle. We chose this specific tract because: (a) it anatomically connects two major hubs of the DMN (i.e., PCC and mPFC) and is a DMN connection that is most robustly affected by age [Andrews‐Hanna et al., 2007]; (b) it is reliably traceable [Greicius et al., 2009; Khalsa et al., 2013; van den Heuvel et al., 2008]; and (c) WM integrity within the cingulum is associated with several cognitive outcome measures (psychomotor performance, inhibition, and semantic memory) [Metzler‐Baddeley et al., 2012]. To gain insight about SC differences based on WM integrity measures, we derived mean fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD), from diffusion tensor imaging (DTI) data, within a tractography based cingulum mask. To describe FC strength we conducted a seed‐based rs‐fMRI correlation approach between two major hubs of the DMN. All associations were investigated within a healthy, well‐educated sample of older participants. We hypothesize that there is a relationship between SC and FC strength since SC of the cingulum bundle describes the underlying anatomical connection between the PCC‐mPFC functional connectivity [Greicius et al., 2009]. Further, we hypothesize that cognitive and motor performance is associated with SC since tests employed in this study were previously related to WM integrity in healthy aging samples [Bartzokis et al., 2010; Fjell and Walhovd, 2010; Metzler‐Baddeley et al., 2012; Zahr et al., 2009]. Consequently, we suggest that FC strength is positively related to behavioral performance. Lastly, as WM tracts are the fibers that connect brain regions, thus, representing a basis for a brain‐behavior relationship, the overarching aim of this study was to examine if FC explains unique variance in behavior in addition to that explained by SC. We hypothesize that the combination of SC and FC data will serve as a better predictor for behavioral measures than each connectivity measure separately. By combining SC and FC measures from a large sample, we aim to gain further insight into the relationship between brain and cognition in healthy aging.
MATERIALS AND METHODS
Participants
We sampled 201 subjects from the first time point of the longitudinal healthy aging brain (LHAB) database project conducted at the International Normal Aging and Plasticity Imaging Center (INAPIC) at the University of Zurich, Switzerland [Zöllig et al., 2011] who had available data for DTI, fMRI, education, and blood pressure. Participants were all older than 64, had a Mini Mental State Examination [MMSE; Folstein et al., 1975] score higher than 26, had no history of neurological or psychiatric diseases, were right handed and passed MRI safety requirements. All participants gave voluntary informed consent, in accordance with guidelines from the Helsinki declaration, prior to participation.
MR Acquisition
All scans were performed using a 3.0 T Philips Ingenia scanner (Philips Medical Systems, Best, The Netherlands). For each participant, the following images were acquired:
Two high‐resolution T1‐weighted (T1w) anatomical images using a 3D Turbo‐Field‐Echo (TFE) sequence with echo time (TE) = 3.799 ms, repetition time (TR) = 8.18 ms, field of view (FOV) = 240 × 240 mm, acquisition matrix = 240 × 240, 160 slices, isotropic voxel size=1 mm3, flip angle=8°, number of signal average (NSA) = 1, duration ∼7:30 minutes.
Diffusion‐weighted single‐shot spin echo EPI sequence scans with the following specifications: TE = 55 ms, TR = 23,983 ms, flip‐angle = 90°, SENSE factor R = 2.0, FOV = 224 × 224 mm, voxel size 2 × 2 × 2 mm, 75 slices, no gap. Diffusion‐weighted scans were performed along 32 non‐collinear directions with a maximum b‐factor of 1000 s/mm2, complemented by a single b = 0 s/mm2 scan. Acquisition time was ∼15 minutes.
T2*‐weighted BOLD image parameters were: TR = 2 s, TE = 21 ms, flip angle=76°, FOV = 220 × 220 mm, voxel size 3.43 × 3.43 × 3.5 mm, 43 axial slices. For resting state fMRI 225 volumes were collected within ∼8 minutes. Prior to the functional scan, participants were instructed to lay still, relax and to look, but not stare at the fixation cross during data acquisition.
MRI Data Analysis
T1w image preprocessing
T1w images were averaged using the AnatomicalAverage script from the FMRIB Software Library (FSL) toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki). The averaged images were corrected for field inhomogeneity using N4ITK [Tustison et al., 2010] within an intracranial mask that was obtained using FSL's brain extraction tool (BET). The bias field corrected averaged image was skull stripped using FSL's BET. For further analysis we refer to this image as the averaged T1w image.
Analysis of DTI Data
DTI image preprocessing
FSL version 5.0.4 was used for eddy current correction and correction of head motion of the diffusion weighted images. FSL's diffusion toolbox was used for tensor fitting to obtain FA/MD/AD and RD maps [Smith et al., 2004].
Probabilistic tractography
We used a seed‐based probabilistic approach to track the cingulum bundle. The tractography was therefore conducted between a posterior and anterior ROI. Because the same posterior ROI coordinates were used for the functional and structural analysis, the posterior ROI was placed at the posterior cingulum PCC (MNI coordinate 0, −53, 26) based on a previous rs‐fMRI connectivity study [Van Dijk et al., 2010]. However, to ensure that the ROI reached into the WM, which is essential for tractography, we created a spherical ROI with 12 mm radius to track the cingulum bundle (vs. 6 mm see below). The anterior ROIs for the tractography and resting state analysis differed. The anterior ROI to track the cingulum was placed in accordance with Wakana et al. [2007]. This ROI is situated just above the middle of the genu of the corpus callosum in the coronal plane, a location within WM, which is not suitable for rs‐fMRI analysis. To find the middle of the corpus callosum's genu we first selected the mid‐sagittal plane from the genu mask (obtained from the JHU ICBM‐DTI‐81 White‐Matter Labels Atlas). Using fslstats the center of gravity was determined indicating the middle of the genu. A box was created with the following dimensions 31×5×30 mm. To track the cingulum bundle in the right hemisphere, the left lower corner of the ROI cuboid was placed at 30, 22, 12 (MNI space); for the left cingulum bundle, the left lower corner of the ROI was placed at 0, 22, 12 (MNI space). Both ROIs were used as seed and waypoint masks. Posterior and frontal ROIs are depicted in Figure 1.
Figure 1.
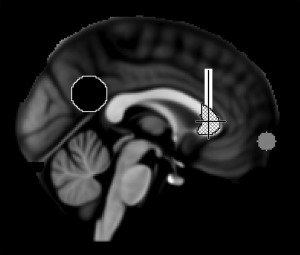
Mid‐sagittal image of the MNI152 template brain. Black indicates the posterior cingulate cortex and white the anterior region of interest, plaid indicates the genu of corpus callosum and gray the medial prefrontal cortex region of interest. Crosshairs represent the center of gravity of the genu of the corpus callosum.
For further analyses we transformed the ROIs from MNI into native diffusion space with the following steps:
First, the averaged T1w image was rigidly registered to the DTI images using FSL's Linear Image Registration Tool (FLIRT) with mutual information used as a cost function. The co‐registered averaged T1w image was then normalized into MNI space using linear (FLIRT) and nonlinear registration FNIRT (FSL's Non‐linear Image Registration Tool). The obtained warp‐fields were inverted using the invwarp command and subsequently applied to the ROI to obtain the PCC and anterior ROI in native diffusion space.
FSL's diffusion toolbox was used to determine the probabilistic tractography. First, we used the BEDPOSTX (Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques) command to calculate the distribution of fiber orientations at each brain voxel. Second, the probtrackx2 command was used to initiate probabilistic tractography from each voxel within the seed ROI. We applied the following parameters: streamlines=25,000; step length=0.5 mm; curvature threshold = 0.2. To ensure that only the cinglum bundle was tracked, tractography for each hemisphere was conducted twice, once using the PCC ROI as a seed mask and the anterior ROI as a waypoint mask (PCC‐tract), and vice versa (i.e., anterior ROI=seed mask; PCC=waypoint mask; anterior‐tract). All tracts were further thresholded based on the individual maximum connectivity value within a tract. The maximum connectivity value was obtained with fslstats and voxels which had values of more than 5% of the maximum connectivity value were kept in the analysis [Bennett et al., 2011]. Furthermore, tracts were binarized and combined by masking the PCC with the anterior‐tract (final‐tract; see Fig. 2). Finally, we masked diffusion maps with the binarized final‐tract to compute the mean value of FA/MD/AD and RD within the final‐tract mask. We did not trace the entire cingulum bundle but just the posterior part. This procedure was applied to obtain more robust tract length and to ensure that only the cingulum bundle was traced. All tracts were visually inspected by SH. Subjects were excluded if tractography failed or a tract was touching the edge of the ROI (leading to 25 exclusions). In addition, participants were excluded if the transformation for the ROI into diffusion space failed (e.g., ROI depicted a hole). ROI transformation failed for 17 participants. The excluded participants did not differ in age, education, MMSE and gender (t‐test, for all P > 0.07).
Figure 2.
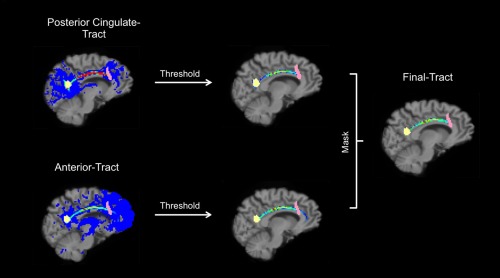
Steps to create the final cingulum tract. Heat colors in the cingulum tract represent higher probability. Threshold level is set on 5%. Yellow depicts the spherical ROI used for the posterior cingulate cortex, the pink color indicates the anterior ROI. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Analysis of Resting‐State Data
Rs‐fMRI data preprocessing
The first 10 volumes of the time‐series were discarded. Images were corrected for slice‐timing using sinc interpolation to the median reference slice and realigned for head motion correction using the Statistical Parametric Mapping software (SPM8, Welcome Department of Imaging Neuroscience, Institute of Neurology, London, United Kingdom). Whole brain rs‐fMRI images were normalized to MNI space using a multi‐step approach. First, the averaged T1w image was co‐registered to the mean rs‐fMRI echo planar image (EPI) using SPM8. The co‐registered averaged T1w image was smoothed with a Gaussian kernel of 0.9 mm and normalized to MNI152 space using non‐linear transformation with symmetric normalization (SyN) using Advanced Normalization Tools (ANTs; http://picsl.upenn.edu/software/ants/). Further, the resulting warp parameters were applied to the 4D EPI images, which were subsequently smoothed using FSL with a Gaussian kernel of 4 mm. Four participants were excluded because of excessive head motion (>3 mm, mean for included participants: 0.35 mm).
Rs‐fMRI connectivity
The Data Processing Assistant for Resting‐State fMRI (DPARSF) software package [Chao‐Gan and Yu‐Feng, 2010] and the Resting‐State fMRI Data Analysis Toolkit (REST; http://www.restfmri.net) [Song et al., 2011] were used for calculation of the FC maps. After normalization and pre‐processing of the functional images the linear drift was removed using DPARSF's detrend. A temporal bandpass filter (0.01–0.08 Hz) was applied to reduce low‐frequency drifts and physiological high‐frequency noise. To generate FC maps, we created a 6 mm spherical seed in the medial PCC cortex (MNI coordinate 0, −53, 26) based on Van Dijk and colleagues [2010]. Head motion realignment parameters (three rotations and three translations) and signals from the WM and cerebrospinal fluid (CSF) were regressed out during the calculation of FC maps. For whole brain analysis, WM and CSF signals were extracted based on the mask provided by the REST toolkit. Pearson's correlation coefficient between the averaged time course at the seed ROI and each brain voxel's time course were computed. The correlation maps were converted to z‐scores using Fisher's r‐to‐z transformation. The individual z‐maps were then entered into a random effects analysis. In order to determine the peak correlation between the PCC ROI and the mPFC region, a one‐sample t‐test was performed on the z‐maps that were created from the PCC ROI in a voxel‐wise manner. Because of the relatively large number of subjects in our analysis, we chose a rather conservative statistical threshold, (P < 0.01 × 10−8, FWE corrected) for the initial voxel‐wise connectivity maps. We then created a second 6 mm spherical ROI in the mPFC (MNI coordinate 0, 62, −6; Fig. 1) and subsequently reran the analysis. This time we concentrated our analysis on the correlation strengths between the PCC and mPFC seed ROIs. Again, the resulting Pearson's correlation coefficient between two regions was converted to z‐values using Fisher's r‐to‐z transformation.
Behavioral Assessment
All participants completed an extensive cognitive and sensory‐motor test battery.
Cognitive performance
The following cognitive outcome measures were assessed: (a) processing speed, (b) memory and (c) executive functions.
Processing speed (PS): PS was measured with the Digit Symbol‐Coding Test of the Wechsler Adult Intelligence Scale [WAIS; Aster et al., 2006]. In this test, numbers from 1 to 9 are presented, each with a corresponding symbol. The aim is to fill in the blanks under additional rows of numbers by drawing the corresponding symbol below the number as fast as possible. The number of correct symbols drawn in 120 seconds was recorded. The second PS measure was time to task completion of the Trail Making Test (TMT) part A [Reitan and Wolfson, 1985]. The third PS measure was assessed using the Regensburger word fluency test [Regensburger Wortflüssigkeits‐Test; RWT; Aschenbrenner et al., 2000]. Participants were asked to name as many animals as possible within two minutes.
Memory: Immediate and delayed episodic memory was measured using the German version of the Rey Auditory Verbal Learning Task [RAVLT; Rey, 1964] [Verbaler Lern‐ und Merkfähigkeitstest (VLMT); Helmstaedter et al., 2001]. In the immediate verbal recall phase, participants had to recall a list of 20 words immediately after an examiner presented the list verbally. This procedure was repeated five times. The score for immediate verbal recall was the sum of words recalled per session. Delayed verbal recall score was the number of words that participants remembered after an unexpected retrieval succeeding a 20 minute break. Figural memory capacity was assessed using the DCS (Diagnosticum für Cerebralschädigung a diagnostic method for cerebral impairment). Participants were shown consecutively and without time constraints 9 figures printed on cards. Each figure consisted of five lines. Participants were instructed to look at the figures carefully and to remember them as accurately as possible. After the last card was shown, participants were encouraged to reconstruct all previously presented figures using 5 12cm long, wooden sticks. The test consisted of 6 runs and was either finished after 6 trials or when all 9 figures were reconstructed. All remaining runs were then scored as 9. The maximum score for the DCS was 54. There was no constraint concerning the order in which participants reproduced the figures.
Executive functions (EF): Two sub‐components of executive functioning, inhibition and task‐switching, were assessed. Inhibition was measured using a computerized version of the Stroop task (Vienna Test System, Schuhfried). In the Stroop task, participants are asked to name the color in which the words are displayed as fast as possible. The inhibition score is the ratio between the median reaction time for congruent and incongruent stimuli. Task‐switching was measured using the TMT. The task‐switching score is the difference between time required to complete TMT part B minus time required for TMT part A.
Motor behavior
We obtained the following motor outcome measures: (a) motor speed and (b) grip force.
Motor speed: Motor speed was measured using the grooved pegboard (Lafayette Instrument Company, Lafayette, IN 47903 USA; model 32025) and a tapping task. The grooved pegboard test consists of a board with 25 holes, which have randomly aligned slots and pegs with a key along the side, which fit into the holes when rotated accordingly. Participants were asked to fill all of the holes with pegs as fast as possible. Pegs were placed from left to right with the right hand and from right to left with the left hand. Time was measured from the moment participants picked up the first peg until all the holes of the board were filled. The amount of motor taps executed in 32 seconds was measured using the tapping test from the motor performance series (MLS, Vienna Test System, Schuhfried). Participants were asked to tap as many times as possible on a 4 × 4 cm metal plate with a pen‐like device. Both hands were tested.
Grip force was measured using a hydraulic hand dynamometer (Model SH5001, Saehan Corporation, Korea). Participants were asked to sit upright, with the shoulder in a neutral position, elbow in 90° flexion, the forearm in a neutral position and with a 0°–30° wrist extension. Data was collected alternately for the right and left hand with a break of 30 seconds between measurements. Participants were asked to keep the force stable for 4 seconds for each trial. Three measurements per hand were collected. If the third measurement was the highest, data collection continued until performance dropped under a previous measurement. Grip force score represents the mean of the three highest measurements for each participant in kilograms.
Covariates
Analyses were adjusted for several potential confounders which have been demonstrated to be associated with age, behavioral and brain outcome measures: blood pressure [Beauchet et al., 2013; Marcus et al., 2011], years of education [Alley et al., 2007; Noble et al., 2013] and gender [Hsu et al., 2008; Inano et al., 2012; Maylor et al., 2007].
Blood pressure was obtained using an automated blood pressure cuff (Model M6, HEM‐7211‐E; Omron Corporation, Kyoto, Japan). Measurements were conducted three times at the same day (roughly 2.5–3 hours apart) and the average for the diastolic measurement was used for analysis.
Education depict years spend until achievement of highest degree of education based on self‐report.
Statistical Analyses
All statistical analyses were performed using IBM SPSS Statistics for Mac OS X, Version 19. To ensure that extreme cases did not drive associations, outliers of the dependent and independent variables (i.e., values three standard deviations above or below the mean) were removed.
Raw data for all behavioral tests were z‐transformed and if necessary reversed (multiplied by −1) so that higher numbers always represent better performance. Test parameters were reduced to four behavioral domains based on literature (PS, memory, EF, and motor speed) by equally integrating the above‐mentioned tests (see Table 1 for the correlation matrix of the test parameters). Brain parameter scores were additionally standardized using z‐scores for better comparisons.
Table 1.
Correlations between behavioral tasks
| Digital symbol test | TMT‐A | Verbal fluency | Immediate recall | Delayed recall | Visual memory | Inhibition | Task‐switching | Pegboard | Tapping | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Digital symbol test | r | ||||||||||
| P | |||||||||||
| TMT‐A | r | 0.446 | |||||||||
| P | <0.001 | ||||||||||
| Verbal fluency | r | 0.478 | 0.343 | ||||||||
| P | <0.001 | <0.001 | |||||||||
| Immediate recall | r | 0.409 | 0.244 | 0.395 | |||||||
| P | <0.001 | 0.002 | <0.001 | ||||||||
| Delayed recall | r | 0.378 | 0.254 | 0.373 | 0.801 | ||||||
| P | <0.001 | 0.001 | <0.001 | <0.001 | |||||||
| Visual memory | r | 0.337 | 0.241 | 0.392 | 0.501 | 0.456 | |||||
| P | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | ||||||
| Inhibition | r | 0.259 | 0.159 | 0.172 | 0.169 | 0.122 | 0.172 | ||||
| P | 0.001 | 0.046 | 0.031 | 0.033 | 0.126 | 0.031 | |||||
| Task‐switching | r | 0.359 | 0.174 | 0.279 | 0.223 | 0.211 | 0.307 | 0.227 | |||
| P | <0.001 | 0.029 | <0.001 | 0.005 | 0.008 | <0.001 | 0.005 | ||||
| Pegboard | r | 0.369 | 0.408 | 0.325 | 0.236 | 0.256 | 0.249 | 0.144 | 0.207 | ||
| P | <0.001 | <0.001 | <0.001 | 0.002 | 0.001 | 0.001 | 0.072 | 0.009 | |||
| Tapping | r | 0.342 | 0.219 | 0.233 | 0.160 | 0.129 | 0.162 | 0.120 | 0.182 | 0.260 | |
| P | <0.001 | 0.005 | 0.003 | 0.042 | 0.102 | 0.041 | 0.135 | 0.022 | 0.001 | ||
| Grip force | r | 0.178 | 0.129 | 0.137 | −0.100 | −0.050 | 0.162 | 0.147 | 0.182 | 0.085 | 0.276 |
| P | 0.023 | 0.103 | 0.083 | 0.205 | 0.526 | 0.040 | 0.066 | 0.022 | 0.281 | <0.001 |
Abbreviations: TMT‐A, trail making test part A.
r: Pearson's correlation coefficient.
To assess a potential association between SC and FC, correlations between FC and diffusion measures were calculated.
To determine the associations between SC or FC with behavioral outcome measures we created general linear models (GLM) that were adjusted for age, gender, education, and diastolic blood pressure. To explore the possibility if overall brain atrophy (one covariate controlling for both, gray matter (GM) and WM volume atrophy ((WM+GM Volume)/ICV Volume)) could confound our associations between behavioral measures and brain parameters we run all analyses with atrophy included as a covariate. No significant differences in results were found and thus, only models without atrophy are shown. As FA and MD are derived from the 3 eigenvectors of the diffusion ellipsoid which also depict axial (first eigenvector) and radial (mean of second and third eigenvectors) diffusivity, significant associations in AD or RD should be reflected in significant associations in either MD or FA. Thus, for the association between behavioral measures and SC only GLMs for FA and MD were conducted in a first step. If significant relations could be found, additional analyses for AD and RD were conducted. Second, to test if the FC explains significant additional variance in our behavioral data on top of the variance explained by SC, we used hierarchical regression analysis. Separate regressions were conducted for each SC measure. All models included age, gender, education and diastolic blood pressure as covariates of no interest. The level of significance for all tests was set to P=0.05, uncorrected for multiple comparisons unless otherwise indicated.
Eta‐squared (η 2) was calculated as effect size by dividing the Type III Sum of Squares of the factor of interest by the Total Sum of Squares (“Corrected Total” in SPSS). Because we were interested in the dominant (right) hand performance and WM integrity of the cingulum bundle was significantly correlated between the left and right hemispheres (FA: r(162)=0.581 P < 0.001; MD: r(162) = 0.798 P < 0.001; AD: r(160) = 0.575 P < 0.001; RD: r(163) = 0.740 P < 0.001), we decided to only include the left cingulum tract in our analyses. In addition, supplementary analyses, which included the mean of the left and the right diffusion measures for the cingulum tract, revealed no differences in associations between behavioral measures and SC.
RESULTS
Sample characteristics and overview of all behavioral tasks and brain parameters can be found in Table 2. All behavioral measures and several SC measures (i.e., MD, AD and RD) showed associations with age.
Table 2.
Demographic characteristics of the study participants
| Variable | N | M (SD) | rage | P |
|---|---|---|---|---|
| Age (years) | 165 | 70.15 (4.50) | ||
| Men [N (%)] | 165 | 81 (49%) | ||
| MMSE | 165 | 28.85 | ||
| Education years | 165 | 14.53 (3.46) | ||
| Diastolic blood pressure (mmHg) | 165 | 81.70 (9.85) | ||
| Cognition | ||||
| Processing speed | 161 | −0.28 | <0.001 | |
| Memory | 162 | −0.31 | <0.001 | |
| Executive functions | 154 | −0.26 | 0.001 | |
| Motor performance: | ||||
| Motor speed | 161 | −0.40 | <0.001 | |
| Grip force (kg) | 163 | −0.21 | 0.008 | |
| Cingulum measures | ||||
| Volume (ml) | 164 | 1992.48 (437.77) | −0.04 | 0.635 |
| FA | 164 | 4.7 × 10−1 (3.6 × 10−2) | −0.06 | 0.484 |
| MD (mm2/s) | 165 | 7.6 × 10−4 (3.0 × 10−5) | 0.32 | <0.001 |
| AD (mm2/s) | 164 | 1.2 × 10−3 (4.5 × 10−5) | 0.27 | 0.001 |
| RD (mm2/s) | 165 | 5.5 × 10−4 (3.9 × 10−5) | 0.22 | 0.005 |
| rs‐fMRI* | 165 | 7.7 × 10−1 (2.7 × 10−1) | −0.08 | 0.306 |
Abbreviations: AD, axial diffusivity; FA, fractional anisotropy; kg, kilogram; M, mean; MD, mean diffusivity; ml, milliliter; mmHg, millimeter of mercury; MMSE, mini‐mental state examination; RD, radial diffusivity; r age, Pearson's correlation with age; rs‐fMRI, resting‐state functional connectivity; s, seconds; SD, standard deviation.
Mean of rs‐fMRI represents the Pearson's correlation coefficient (z‐values).
There was no significant relationship between SC and FC measures (Table 3).
Table 3.
Correlations between neuroimaging parameters
| rs‐fMRI | FA | MD | AD | ||
|---|---|---|---|---|---|
| rs‐fMRI | r | ||||
| P | |||||
| FA | r | −0.004 | |||
| P | 0.964 | ||||
| MD | r | −0.019 | −0.490 | ||
| P | 0.810 | <0.001 | |||
| AD | r | 0.026 | 0.470 | 0.486 | |
| P | 0.745 | <0.001 | <0.001 | ||
| RD | r | −0.029 | −0.864 | 0.851 | −0.028 |
| P | 0.709 | <0.001 | <0.001 | 0.719 |
Abbreviations: AD, axial diffusivity; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity; rs‐fMRI, resting‐state functional connectivity.
r: Pearson's correlation coefficient.
None of the cognitive composite scores were significantly associated with SC measures whereas FC was significantly associated with PS performance (Table 4). For motor behavior, we observed a significant association between SC measures (i.e., FA and MD) and grip force. Furthermore, FC strength was positively associated with motor speed performance (Table 4). Additional linear regression analyses for investigating the relationship between AD and RD and grip force revealed significant associations between grip force and RD (β = −0.130, P = 0.006, η 2 = 0.015) whereas no relation was found for AD (β = −0.021, P = 0.652, η 2 < 0.001). After controlling for multiple comparisons using the procedure suggested by Holm [1979] the association between FC and motor speed remained significant.
Table 4.
Associations between brain connectivity and behavior measures
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| N | β | Low | High | η2 | P | ||
| Processing speed | FA | 160 | 0.081 | −0.033 | 0.195 | 0.010 | 0.161 |
| MD | 161 | −0.026 | −0.143 | 0.092 | <0.001 | 0.667 | |
| rsfMRI | 161 | 0.154 | 0.044 | 0.264 | 0.039 | 0.006 | |
| Memory | FA | 161 | −0.032 | −0.156 | 0.091 | 0.001 | 0.606 |
| MD | 162 | 0.052 | −0.078 | 0.181 | 0.003 | 0.432 | |
| rsfMRI | 162 | 0.011 | −0.114 | 0.137 | <0.001 | 0.857 | |
| Executive functions | FA | 153 | 0.074 | −0.046 | 0.195 | 0.009 | 0.225 |
| MD | 154 | −0.049 | −0.173 | 0.075 | 0.004 | 0.435 | |
| rsfMRI | 154 | −0.030 | −0.149 | 0.088 | 0.002 | 0.613 | |
| Motor speed | FA | 160 | 0.084 | −0.031 | 0.199 | 0.012 | 0.151 |
| MD | 161 | 0.018 | −0.105 | 0.140 | <0.001 | 0.777 | |
| rsfMRI | 161 | 0.168 | 0.058 | 0.278 | 0.044 | 0.003 a | |
| Grip force | FA | 162 | 0.106 | 0.016 | 0.196 | 0.011 | 0.022 |
| MD | 163 | −0.116 | −0.212 | −0.020 | 0.012 | 0.018 | |
| rsfMRI | 163 | −0.051 | −0.143 | 0.040 | 0.003 | 0.270 | |
Abbreviations: Cl, confidence interval; FA, fractional anisotropy; MD, mean diffusivity; rsfMRI, resting state functional connectivity.
β Values represent the increase in standard deviation (s.d.) in behavior performance with 1 s.d. increase in connectivity measures.
Adjusted for age, gender, education, and diastolic blood pressure.
Bonferroni‐corrected for multiple comparisons using the procedure by Holm [1979].
For the hierarchical regression analyses the SC measures (FA or MD) were entered first, followed in the second step by the FC measure. The combination of SC and FC did not explain unique variance in behavioral performance. Thus, significant increase in model fit when adding FC in a second step was only observed for PS and motor speed composite scores, which were significantly associated with FC in the first place (as stated above). In addition, non‐significant associations between PS, memory, EF and motor speed composite scores and SC remained after including FC in a second step, hence, the lack of associations between SC and these behavioral measures cannot be explained by a masking effect of differences in FC.
DISCUSSION
Our study showed that WM integrity within the cingulum bundle is associated with age, which corroborates previous findings. In general, WM integrity throughout the entire brain is reported to decline with age [Bennett and Madden, 2013]. Previous studies that investigated more specifically the relationship between WM integrity of the cingulum bundle and age also observed an age‐related decline. Principally, FA was persistently negatively associated with age whereas the relationship between diffusion metrics was not consistent across studies. Using the tractography approach, previous work showed that older participants differ significantly from young participants in the cingulum's FA and RD but not MD or AD [Davis et al., 2009; Zahr et al., 2009]. Interestingly, in a tract‐based spatial statistics study by Salami et al. [2011] the MD within the cingulum was not associated with age whereas a decrease in MD with age on the whole brain level was apparent. However, Borghesani and colleagues [2013] recently observed an age‐related decline in WM integrity for all diffusion metrics within the cingulum bundle. Whereas we found associations for MD, AD and RD, no relationship was apparent between FA and age. Since RD is commonly associated with myelin integrity, while AD is assumed to reflect axonal integrity [Song et al., 2003] we suggest that differences in myelin integrity and axial density arise with increasing age, however, the overall directionality of diffusion independent of its rate within this specific well‐defined tract does not vary among older participants. Discrepancies between our findings and former studies could be attributed to large differences in sample characteristics between studies, which make it difficult to relate our results to previous findings. First, Salami and colleagues [2011] investigated WM integrity over a large age range, including participants as young as 25 which clearly leads to larger variability within the data. Even though the age range was smaller in the study conducted by Borghesani et al. [2013] (age range = 54–89), it spanned 35 years while our study is restricted to an age range of 21 years (age range = 64–85).
In contrast to SC, we observed no relationship between FC and age. In a recent review, Dennis and Thompson [2014] concluded that DMN FC decreased with age. It is important to mention that most of the studies included in the review based their assumption on group comparisons between young and older participants [Andrews‐Hanna et al., 2007; Damoiseaux et al., 2008; Wu et al., 2011]. However, in addition to a group comparison Andrews‐Hanna and colleagues [2007] also investigated the relationship between age and FC within a group of older participants aged 60 to 90. They found a significant decrease in connectivity with increasing age. To gain a more clear‐cut picture about the difference of FC strength over the life span Onoda and colleagues [2012] as well as Mevel and colleagues [2013] included a sample between 36 to 86 and 19 to 80 years of age respectively in their cross‐sectional analyses. Results from both studies delineated a linear negative association between age and FC strength within the DMN. More precisely, Mevel et al. [2013] also reported a negative linear association of the PCC–mPFC FC strength and increasing age. To the best of our knowledge, Persson et al. [2014] were the first to systematically investigate longitudinal FC changes within the DMN in a relatively large sample of healthy aging participants. In contrast to the aforementioned studies, the authors reported no relationship between age and FC change between the PCC and mPFC ROI within a test‐retest period of six years. Thus, including our findings, the picture about the relationship between FC and age is not as clear‐cut as previously stated.
The cingulum bundle was previously shown to connect the PCC and the mPFC of the DMN [e.g., Greicius et al., 2009]. Using a new fully automatic approach we were able to track the cingulum bundle for more than 100 participants using ROIs based on functional data from literature, thus, supporting the notion of a monosynaptic connection between the PCC and the mPFC. However, our main goal was to determine if there is an association between the SC and FC strength and if structural and FC data will serve as a better predictor for behavioral measures than each connectivity measure separately. In contrast to previous findings suggesting a relationship between SC/FC strength between the two key regions of the DMN (PCC‐mPFC) in young adults [Khalsa et al., 2013; van den Heuvel et al., 2008] our data did not support such a notion. Analyzing data from 45 young participants, van den Heuvel and colleagues [2008] found a positive relationship between the mean FA value within the cingulum bundle and the FC strength between the PCC and mPFC ROI. Interestingly, a relationship between SC and FC strength within the DMN can also be seen when age‐related declines in both WM integrity and FC strength are apparent [Andrews‐Hanna et al., 2007]. The reported association between SC and FC in aging participants also supports the disconnection hypothesis according to which deterioration in SC between linked brain regions could lead to a decrease in FC within the two connected areas [Bennett and Madden, 2013]. However, we did not observe an association between any measure of WM integrity and FC in the cingulum bundle. This could be due to insufficient variation in FC strength with age in our sample of healthy older adults. Further reasons for the absence of a SC and FC relationship could be an existent compensation mechanism in healthy aging participants, which would also explain the differing results compared to younger adults. As we found significant associations between FC and behavioral outcome measures we could argue that the maintenance of FC strength is desirable. Thus, the preservation of FC strength could be attributable to indirect WM tracts that could compensate for any loss of FC through the deteriorated WM of the cingulum bundle. As Andrews‐Hanna and colleagues [2007] extracted SC measures from a relatively large and coarsely defined ROI, they may have included tracts involved in such a compensation mechanism. As we did not find an association between FC and age we could also argue that a relationship between SC and FC in aging can only be established after a detectable age‐related decline in FC strength. In other words such a relationship is masked by a running compensation strategy and can only be detected after FC strength can no more be maintained. It seems that previous longitudinal studies support this idea. While the age‐related decrease in SC seems established [Barrick et al., 2010], first longitudinal FC data in healthy older adults depict stability over a 6 year time period [Persson et al., 2014].
One of the main goals of this study was to investigate if the combined information of SC and FC between brain areas connected through the cingulum tract could be a better predictor for cognitive and motor behavior outcome measures. We therefore investigated different cognitive domains and motor behaviors, which are consistently reported to be associated with age [Grady, 2012; Seidler et al., 2010]. First, we examined the relationship between behavioral measures and SC. Structural connectivity was in previous studies reported to be associated with PS or EF [Fjell and Walhovd, 2010; Raz and Rodrigue, 2006]. In contrast to our hypothesis we did not observe such a relation. However, grip force strength was significantly related to measures of WM integrity. An additional analysis revealed that besides FA and MD, grip force is significantly associated with RD whereas no association with AD was found. Investigating the relationship between several behavioral measures and long WM tract integrity Davis and colleagues [2009] found stronger associations with RD as compared with AD. Based on animal studies, RD is often interpreted as marker for myelin integrity [Song et al., 2002]. Thus, we could argue that demyelination of the cingulum bundle could have driven the association between grip force and SC rather than axonal integrity. However, the neuroanatomical substrates of AD and RD are poorly understood so far [Bennett and Rypma, 2013]. In general, grip strength is associated not only with physical but also mental health (e.g., depression) [Rantanen et al., 2000a, 2000b; Syddall et al., 2003]. It is reported to be clearly associated with age [Frederiksen et al., 2006] but underlying mechanisms of grip strength ability still need to be clarified. There is clear evidence that not only muscle mass/quality but also neural control affects motor performance in older adults [Seidler et al., 2010]. In a functional MRI analysis Noble et al. [2011] found that with increased generated strength brain regions including motor cortical areas, the cingulate gyrus and medial frontal areas showed higher activation in older compared to younger participants. It could therefore be argued that grip strength decline is associated with neuroanatomical and functional age‐related alterations. It would be interesting to focus on tracts more specifically related to motor control (e.g., superior longitudinal fasciculus and corticospinal tract) to see if degeneration of these tracts is an even better predictor of grip strength. Discrepancies between our findings and results from former studies could be ascribed to the fact that we investigated a very homogenous sample of older highly educated participants, who did not show any signs of cognitive impairment or dementia, whereas other studies often include less cognitive healthy samples in which the association between cingulum bundle integrity and function might be more apparent because of the larger variability in these measures. In addition, the age range of our participants was relatively small, which could have led to less variance in the variables of interest compared with other studies.
Second, we analyzed the relationship between FC strength and behavioral measurements. We observed that performance on both cognitive and motor behavioral tasks were related to FC strength. Interestingly, both composite scores associated with FC are measures for speed with which a task can be completed (in terms of cognition and motor behavior). Although after controlling for multiple comparisons only motor speed still reached significance. Nevertheless, our results show that maintaining higher synchronous intrinsic FC protects for age‐related decrease in PS tasks or tasks with a high PS or dexterity component. This is of high interest since PS ability is not only reported to be clearly associated with increasing age but also PS is one of the strongest predictors of cognitive performance in older adults. Slowed PS is assumed to be the underlying mechanism of age‐related decline in many higher‐order cognitive functions, such as working memory and EF [Salthouse, 1996, 2003]. Associations between PS and FC strength have been reported previously. Andrews‐Hanna et al. [2007] found significant associations between PCC‐mPFC connectivity strength and PS ability in older adults. A study, investigating a sample with a comparable age range as in our study, also showed an association between cognition and connectivity strength [Damoiseaux et al., 2008]. More specifically, it showed that PS and other cognitive measures such as attention, concentration, and executive functioning were related to connectivity strength in the anterior DMN, which comprised the superior frontal gyrus, posterior cingulate and bilateral middle temporal gyrus and superior parietal regions.
Besides cognitive alterations, decreases in sensorimotor control and functioning are prevalent with increasing age leading, among other things, to slowing of movements [Aoki and Fukuoka, 2010; Seidler et al., 2010]. Along with the association between performance and FC strength in the DMN, recent results from our laboratory showed that tapping speed (incorporated in the motor speed composite score) is associated with FC strength within the sensorimotor network [Seidler et al., 2015]. Our data therefore supports the notion of our previous findings as it shows that participants with stronger FC between the PCC and mPFC outperformed participants with lower FC strength in motor speed.
We explored if models that incorporated both SC as well as FC were better predictors of cognitive and motor behavior than models who only incorporated SC. Contrary to our hypothesis, our results demonstrated that this was not the case. Although hierarchical regression analysis indicated an improvement of the model, no significant additive effect was found and the same results were apparent when analyzing the influence of SC and FC separately.
This study has some limitations. First, all findings are based on cross‐sectional data and observed associations cannot be interpreted as causal. In addition, the associations found in this study were fairly small. However, we found brain‐behavioral relations in a very healthy, highly functioning sample with a narrow age range even after controlling for several covariates such as age. Therefore, we believe that these observations contribute to our understanding of how brain structure and function and cognitive and motor performance are related in normal aging.
This study stands out by applying an automatic procedure to analyze SC and FC measures of the cingulum tract in a large sample. Combining these multimodal findings with an extensive set of cognitive and motor behavioral outcome measures helps to gain specific insight into the neuroanatomical and physiological underpinnings of healthy aging. Further, by applying strict eligibility criteria the LHAB project aims to focus on the course of healthy aging.
CONCLUSION
We found no relationship between SC and FC of the cingulum bundle in a large well‐educated sample of healthy older participants. In addition, we found no association between FC strength and age. However, FC strength was a predictor for individual differences in cognitive performance, especially in tasks of visuomotor speed and dexterity. Based on the relevance of PS for age‐related cognitive decline in general [Salthouse, 1996], it seems that maintained FC strength with age despite WM integrity loss may be of great importance for healthy cognitive aging. Our data underline the value for combining multimodal imaging and cognitive/motor performance measures to gain a better understanding of integrated brain functioning in healthy aging.
ACKNOWLEDGMENTS
SH, SM and LJ are supported by the Velux Stiftung (project No. 369) and University Research Priority Program (URPP) “Dynamics of Healthy Aging” of the University of Zurich awarded to LJ and Mike Martin. The current analysis incorporates data from the Longitudinal Healthy Aging Brain (LHAB) database project, a core project at the International Normal Aging and Plasticity Imaging Center/INAPIC and the URPP “Dynamics of Healthy Aging”. The following members of the core INAPIC team were involved in the design, set‐up, maintenance and support of the LHAB database: A. Eschen, L. Jäncke, M. Martin, S. Mérillat, C. Röcke, and J. Zöllig. RDS and LJ are faculty members of the LIFE Course: Evolutionary and Ontogenetic Dynamics. The authors disclose no conflicts of interest.
REFERENCES
- Alley D, Suthers K, Crimmins E (2007): Education and cognitive decline in older Americans: results from the AHEAD sample. Res Aging 29:73–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007): Disruption of large‐scale brain systems in advanced aging. Neuron 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner S, Tucha O, Lange KW. ( 2000): RWT. Regensburger Wortflüssigkeits‐Test. Göttingen: Hogrefe. [Google Scholar]
- Aoki T, Fukuoka Y (2010): Finger tapping ability in healthy elderly and young adults. Med Sci Sports Exercise 42:449–455. [DOI] [PubMed] [Google Scholar]
- Aster Von M, Neubauer A, Horn R (2006): Hamburg‐Wechsler‐Intelligenz‐Test für Erwachsene III. Frankfurt: Harcourt. [Google Scholar]
- Barrick TR, Charlton RA, Clark CA, Markus HS (2010): White matter structural decline in normal ageing: a prospective longitudinal study using tract‐based spatial statistics. NeuroImage 51:565–577. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J (2010): Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging 31:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Celle S, Roche F, Bartha R, Montero‐Odasso M, Allali G, Annweiler C (2013): Blood pressure levels and brain volume reduction: a systematic review and meta‐analysis. J Hypertens 31:1502–1516. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ (2014): Disconnected aging: Cerebral white matter integrity and age‐related differences in cognition. Neuroscience 276:187–205. [DOI] [PMC free article] [PubMed]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Howard DV (2011): White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging 32:2317.e1–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Rypma B (2013): Advances in functional neuroanatomy: a review of combined DTI and fMRI studies in healthy younger and older adults. Neurosci Biobehav Rev 37:1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesani PR, Madhyastha TM, Aylward EH, Reiter MA, Swarny BR, Schaie KW, Willis SL (2013): The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia 51:1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB Toolbox for “Pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SARB (2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex 18:1856–1864. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R (2009): Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage 46:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM (2014): Functional brain connectivity using fMRI in aging and Alzheimer's disease. Neuropsychol Rev 24:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF (2013): Resting‐state functional connectivity in normal brain aging. Neurosci Biobehav Rev 37:384–400. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB (2010): Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci 21:187–221. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K (2006): Age trajectories of grip strength: cross‐sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol 16:554–562. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1965): Disconnexion syndromes in animals and man. Brain 88:237–294, 585–564. [DOI] [PubMed] [Google Scholar]
- Grady C (2012): The cognitive neuroscience of ageing. Nat Rev Neurosci 13:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Lendt M, Lux S (2001): Verbaler Lern‐ und Merkfähigkeitstest [Verbal Learning and Memory Test]. Beltz.
- Holm S (1979): A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics.
- Hsu J‐L, Leemans A, Bai C‐H, Lee C‐H, Tsai Y‐F, Chiu H‐C, Chen W‐H (2008): Gender differences and age‐related white matter changes of the human brain: A diffusion tensor imaging study. NeuroImage 39:566–577. [DOI] [PubMed] [Google Scholar]
- Inano S, Takao H, Hayashi N, Yoshioka N, Mori H, Kunimatsu A, Abe O, Ohtomo K (2012): Effects of age and gender on neuroanatomical volumes. J Magn Reson Imaging 2013:1072–1076. [DOI] [PubMed] [Google Scholar]
- Khalsa S, Mayhew SD, Chechlacz M, Bagary M, Bagshaw AP (2013): The structural and functional connectivity of the posterior cingulate cortex: Comparison between deterministic and probabilistic tractography for the investigation of structure–function relationships. NeuroImage 102:118–127. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N‐K, Song AW (2012): Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta 1822:386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus J, Gardener H, Rundek T, Elkind MSV, Sacco RL, Decarli C, Wright CB (2011): Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume: the northern Manhattan study. Stroke 42:2639–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylor EA, Reimers S, Choi J, Collaer ML, Peters M, Silverman I (2007): Gender and sexual orientation differences in cognition across adulthood: age is kinder to women than to men regardless of sexual orientation. Arch Sex Behav 36:235–249. [DOI] [PubMed] [Google Scholar]
- Metzler‐Baddeley C, Jones DK, Steventon J, Westacott L, Aggleton JP, O'Sullivan MJ (2012): Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. J Neurosci 32:17612–17619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevel K, Landeau B, Fouquet M, La Joie R, Villain N, Mézenge F, Perrotin A, Eustache F, Desgranges B, Chételat G (2013): Age effect on the default mode network, inner thoughts, and cognitive abilities. Neurobiol Aging 34:1292–1301. [DOI] [PubMed] [Google Scholar]
- Noble JW, Eng JJ, Kokotilo KJ, Boyd LA (2011): Aging effects on the control of grip force magnitude: an fMRI study. Exp Gerontol 46:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, Brickman AM (2013): Higher education is an age‐independent predictor of white matter integrity and cognitive control in late adolescence. Dev Sci 16:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS (2001): Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 57:632–638. [DOI] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S (2012): Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci 24:2186–2198. [DOI] [PubMed] [Google Scholar]
- Persson J, Pudas S, Nilsson L‐G, Nyberg L (2014): Longitudinal assessment of default‐mode brain function in aging. Neurobiol Aging 35:2107–2117. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM (2000a): Muscle strength and body mass index as long‐term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55:M168–M173. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Penninx BW, Masaki K, Lintunen T, Foley D, Guralnik JM (2000b): Depressed mood and body mass index as predictors of muscle strength decline in old men. J Am Geriatr Soc 48:613–617. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM (2006): Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev 30:730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D (1985): The Halstead‐Reitan neuropsychological test battery. Tucson, AZ: Neuropsychological Press. [Google Scholar]
- Rey A (1964): L'examen clinique en psychologie. Paris: Presses Universitaires de France. [Google Scholar]
- Salami A, Eriksson J, Nilsson L‐G, Nyberg L (2011): Age‐related white matter microstructural differences partly mediate age‐related decline in processing speed but not cognition. BBA Mol Basis Disease:1–8. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996): The processing‐speed theory of adult age differences in cognition. Psychol Rev 103:403–428. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE (2003): Executive functioning as a potential mediator of age‐related cognitive decline in normal adults. J Exp Psychol Gen 132:566–594. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010): Motor control and aging: links to age‐related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R, Erdeniz B, Koppelmans V, Hirsiger S, Mérillat S, Jäncke L (2015): Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. NeuroImage 108:47–59. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- Song S‐K, Sun S‐W, Ju W‐K, Lin S‐J, Cross AH, Neufeld AH (2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 20:1714–1722. [DOI] [PubMed] [Google Scholar]
- Song S‐K, Sun S‐W, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 17:1429–1436. [DOI] [PubMed] [Google Scholar]
- Song X‐W, Dong Z‐Y, Long X‐Y, Li S‐F, Zuo X‐N, Zhu C‐Z, He Y, Yan C‐G, Zang Y‐F (2011): REST: a toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A (2003): Is grip strength a useful single marker of frailty? Age Ageing 32:650–656. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Bokde ALW, Meindl T, Amaro E, Soldner J, Reiser MF, Herpertz SC, Möller H‐J, Hampel H (2010): White matter microstructure underlying default mode network connectivity in the human brain. NeuroImage 49:2021–2032. [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC (2010): N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H (2008): Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci 28:10844–10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010): Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J‐T, Wu H‐Z, Yan C‐G, Chen W‐X, Zhang H‐Y, He Y, Yang H‐S (2011): Aging‐related changes in the default mode network and its anti‐correlated networks: a resting‐state fMRI study. Neurosci Lett 504:62–67. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV (2009): Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: A quantitative fiber tracking study. NeuroImage 44:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllig J, Mérillat S, Eschen A, Röcke C, Martin M, Jäncke L (2011): Plasticity and imaging research in healthy aging: core ideas and profile of the International Normal Aging and Plasticity Imaging Center (INAPIC). Gerontology 57:190–192. [DOI] [PubMed] [Google Scholar]


