Abstract
Objective:
To determine the feasibility of pharmacologic beta-adrenergic blockade in women with newly diagnosed stage II-IV epithelial ovarian cancer (EOC) throughout primary treatment.
Methods:
Patients initiated propranolol prior to beginning chemotherapy or surgery. Feasibility was assessed as proportion able to complete 6 chemotherapy cycles while on adrenergic suppression. Descriptive statistics summarized surveys, and paired changes were analyzed using signed rank tests. Random-intercept Tobit models examined immune response.
Results:
Median age was 59.9; 88.5% were stage IIIC/IV; and 38.5% underwent primary debulking. Thirty-two patients were enrolled; 3 excluded because they never took propranolol; an additional 3 didn’t meet inclusion criteria, leaving 26 evaluable. Eighteen of 26 (69%), 90% credible interval (CI) of 53–81%, completed 6 chemotherapy cycles plus propranolol (an 82% posterior probability that the true proportion of success is ≥60%). Among the 23 patients with baseline and six month follow up data, overall QOL, anxiety, and depression improved (P<0.05) and leukocyte expression of pro-inflammatory genes declined (P=0.03) after completion of therapy. Decrease from baseline of serum IL-6 and IL-8 preceded response to chemotherapy (P<0.0014). Change from baseline IL-10 preceded complete response.
Conclusion:
Use of propranolol during primary treatment of EOC is feasible and treatment resulted in decrease in markers of adrenergic stress response. In combination with chemotherapy, propranolol potentially results in improved QOL over baseline.
Keywords: Propranolol, Ovarian Cancer, Adrenergic Blockade, Beta Blocker, IL10, IL 6, IL8
Precis:
Use of propranolol during primary treatment of ovarian cancer is feasible and potentially results in improved quality of life. Decreases in serum IL-6, IL-8, and IL10 correlated with response to cancer therapy.
INTRODUCTION
Continuous exposure to activated stress hormones, triggered via biobehavioral or other stressors, has been correlated with negative cancer survival outcomes [1]. Surgical and other forms of physiologic stress can activate the sympathetic nervous system (SNS) response and the hypothalamic-pituitary-adrenal (HPA) axis [2, 3] and stimulate production of pro-inflammatory cytokines [4–7]. Few pharmacologic interventions to reduce the perioperative stress response have been studied in oncology patients. In non-cancer settings, the perioperative use of beta-blockers has been explored to reduce cardiac risk induced by surgical stress, but some studies have shown increased risk of stroke and death [8]. While retrospective studies have shown that use of a beta-blocker correlates with better survival outcomes [9], many of the patients in these studies are on a beta-blocker for a specific reason (e.g., hypertension); therefore, a feasibility study, especially with known significant fluid shifts that occur in patients with ovarian cancer, was necessary to test the use of beta-blocker specifically for reducing the effects of the adrenergic stress response.
Preclinical models have shown that chronic stress can result in increased tumor growth and metastasis [10]. The effects of chronic stress were abrogated by the use of a propranolol, a nonselective beta-blocker in ovarian and other cancer models [10]. In a prospective melanoma clinical trial, propranolol use was inversely associated with recurrence.[11] In breast cancer patients, propranolol use was associated with reduced activity of pro-metastatic/pro-inflammatory transcription factors [12]. With due regard to potential for side effects from propranolol, we launched a prospective feasibility study in patients presenting for primary treatment of epithelial ovarian cancer (EOC), fallopian tube (FT) or primary peritoneal (PP) cancer. Our primary objective was to determine the feasibility of using propranolol through the first 6 cycles of standard intravenous chemotherapy administered in adjuvant or neoadjuvant setting. We also assessed biobehavioral states, circulating levels of cytokines, and leukocyte gene expression profiles pre- and post-primary chemotherapy.
METHODS
Patient selection
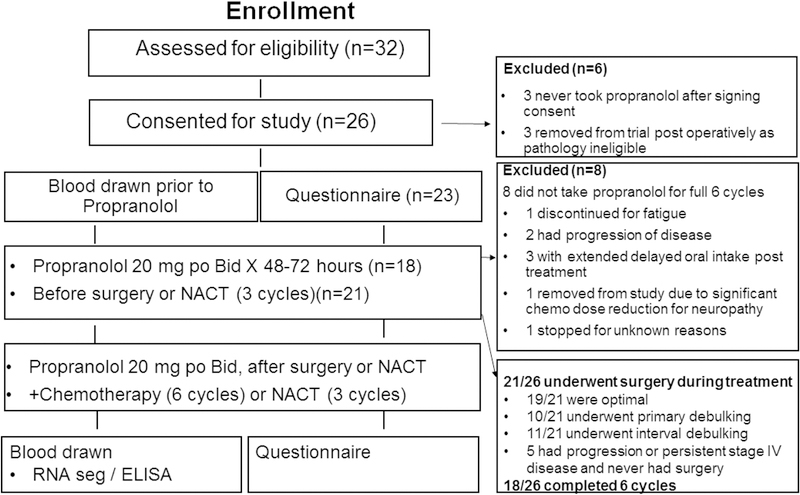
Following IRB approval, patients with EOC or FT or PP cancer were recruited between September 2012 and October 2015 from four Houston institutions. Patients were considered eligible if they had a preoperative diagnosis of suspected stage II-IV invasive disease based on imaging and serum CA-125 level. Patients were removed from the study if post-operative diagnosis was not high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer. A Zubrod performance status of 0–2 was required, as was a proposed treatment plan including primary or interval debulking surgery and neoadjuvant or adjuvant chemotherapy. Concurrent bevacizumab was an exclusion criterion because the study protocol included evaluation of VEGF levels. To ensure safety while initiating elective beta-blocker treatment, only normotensive individuals not already on a beta-blocker were eligible; patients on another antihypertensive agent were still eligible. Exclusion criteria included: non-epithelial, low-grade, or low malignant potential tumor, cirrhosis, Addison’s disease, hepatitis, HIV, connective tissue disease, rheumatoid arthritis, second- or third-degree heart block, or sick sinus syndrome; systemic glucocorticoid use in the month prior to enrollment or inability to answer questions or speak English or Spanish (Figure 1).
Figure 1:
Patient enrollment chart
Treatment protocols
Chemotherapy regimens comprised standard (weekly or q 21 day) carboplatin (AUC 4–6) and paclitaxel (150–175mg/m2 every 21 days or 60–80mg/m2 weekly) were allowed. Surgery or neoadjuvant chemotherapy was scheduled so that the patient could take propranolol for at least 48 hours ahead of time and have stable vital signs confirmed. Blood pressure and heart rate were checked prior to starting propranolol and 48–72 hours after starting the beta-blocker. Patients who had surgery resumed taking the beta-blocker if hemodynamically stable (pulse ≥60 beats per minute [bpm], systolic BP >110 mmHg, diastolic BP ≥60 mmHg) and tolerating oral food intake. Propranolol was started at 20 mg orally twice a day. If tolerated (hemodynamically stable), the dose was increased to the maximum 40 mg orally twice daily at the start of chemotherapy for postoperative patients or prior to cycle 2 of neoadjuvant chemotherapy. The dose was titrated to maintain a heart rate between 60 and 80 bpm without hypotension (systolic <110 mmHg or diastolic <50 mmHg). For patients aged >65 years, the initial dose was 10 mg twice daily and did not exceed 20 mg twice daily. If the patient had adverse events (Common Toxicity Criteria version 3.0) thought to be related to propranolol (bradycardia, hypotension, new onset fatigue), the dose could be decreased or discontinued. (Table S1) Patients could voluntarily withdraw from the study at any time. After completion of primary chemotherapy, patients were weaned off propranolol over 2 weeks by reducing the dose by 50% for 7 days then by an additional 25% for 7 days, after which propranolol was discontinued completely. Any hematologic delay >21 days mandated removal from study. Patients were monitored for progression-free survival for 12 months. To insure compliance, patients completed pill calendar diaries. Calendars, empty bottles, and any unused medication were collected at each chemotherapy clearance visit (Figure 1).
Quality of life and depression/anxiety measures
Quality of life (QOL) and mood were assessed by written instruments (see Supplementary Information for details) before chemotherapy or surgery (Time Point zero-TP0) and after completion of cycles 3 (TP1) and 6 (TP2) of chemotherapy. The demographic characteristics were collected once, TP 0.
Translational methods
Serum was collected from 23 eligible patients at TP0, TP1 and TP2 for responders, or at the time the patient went off treatment if before cycle 6.
RNA sequencing: The buffycoat samples were collected from ovarian cancer patients at TP0, TP1, and TP2. RNA was isolated from the buffy coat samples using Direct-zol RNA MiniPrep Plus (Zymo Research, Irvine, CA). Total RNA was tested for suitable mass (PicoGreen RNA) and integrity (Agilent TapeStation), converted to cDNA (Illumina TruSeq), depleted of ribosomal and globin RNA (Illumina RiboZero Globin), and sequenced on an Illumina HiSeq 4000 instrument in the UCLA Neuroscience Genomics Core Laboratory, following the manufacturers’ standard protocols. All serum samples were processed in duplicate and biomarker analysis was performed by those blinded to the outcome.
Statistical considerations
The primary endpoint was treatment feasibility represented by the proportion of patients who successfully completed 6 cycles of chemotherapy and concurrent propranolol treatment. Endpoints measuring biobehavioral stress were the QOL and mood state results of the FACT-O, HADS, and CES-D. Endpoints measuring immune response were serum levels of IL-6, IL-8, VEGF, and other cytokines. Demographic and clinical characteristics of patients were summarized with descriptive statistics.
To determine feasibility, we calculated the proportion of patients who were able to successfully complete 6 cycles of chemotherapy while on adrenergic suppression therapy, as well as the 90% credible interval for success and the posterior probability that success was ≥0.60. Furthermore, we monitored success using the method described by Thall et al. [13] and planned to stop the trial if there was less than a 5% chance that success was at least 60%. For calculating credible intervals and posterior probabilities, we assumed that success followed a uniform prior distribution. Failure was defined as any possible, probable, or definite propranolol-related reason for lack of completion of 6 chemotherapy cycles. Patients with progressive disease were not considered failures. They, like the patients with allergy to the initial treatment regimen, were replaced for full accrual. The operating characteristics of this stopping rule were based on 1,000 simulations and are presented in Table S2.
Descriptive statistics were used to summarize the scores on the various survey instruments at TP0, TP1, and TP2, and changes during this period were analyzed using a Wilcoxon signed rank test. Random-intercept Tobit models with response as the independent variable and cytokine as the dependent variable were used to examine immune response. In these models, cytokines were censored at the limits of detection. Prior to analysis, cytokines were transformed using the log base 2 function because of non-normality. Patients were included in the model each time they were assessed for tumor response, so intercept was treated as a random effect. Models were created to examine the effects of raw cytokine values upon response as well as to examine change from baseline and its association with response. In the latter set of models, change from TP 0 was calculated after the log transformation, and therefore change represented the log of the fold difference between TP0 and TP1 & TP2. In all models, the primary outcome of interest was CR; PR was only examined if CR was statistically significant. Given the large number of cytokines examined, p < 0.0014 was considered statistically significant. With 37 cytokines and 2 sets of models (raw cytokine values and change from baseline), the familywise Type I error for cytokine analyses was 1 - (1 – 0.0014)74 = 0.10. Statistical analyses were performed using SAS 9.4 for Windows (SAS Institute Inc., Cary, NC).
For analysis of QOL surveys, we reported counts and percentages for discrete variables. For continuous variables we reported mean, median and appropriate measures of dispersion. The Fisher exact test and the Wilcoxon rank sum test were used to test for differences between chemotherapy groups.
Analyses of buffy coat RNA samples were treated as conceptually distinct outcomes from the plasma cytokine measures, and used to test a single pre-specified biological hypothesis that propranolol exposure would be associated with reduced inflammatory signaling, based on previous pre-clinical findings [1, 12, 14, 15]. In primary analyses, we tested that hypothesis using change in the average expression of 19 pre-specified mRNAs encoding pro-inflammatory molecules (CXCL8, FOS, FOSB, FOSL1, FOSL2, IL1A, IL1B, IL6, JUN, JUNB, JUND, NFKB1, NFKB2, PTGS1, PTGS2, REL, RELA, RELB, TNF). Given the limited sample size, we did not conduct any statistical testing at the level of individual gene transcripts; we assessed only change in average expression of the pre-specified inflammatory gene set as a whole. We conducted two secondary bioinformatics analyses to validate the results of this primary analysis based on empirically observed change in the leukocyte transcriptome profile. We first examined the prevalence of transcription factor-binding sites in the promoters of all genes showing a point estimate of >1.5-fold change over time and tested the hypothesis that NF-κB response elements would be significantly more prevalent among the promoters of down-regulated genes relative to up-regulated genes [16]. Next, we tested whether the down-regulated subset of those genes would be more characteristic of the pro-inflammatory CD16− classical monocyte transcriptome relative to the more tissue-regenerative CD16+ non-classical monocyte transcriptome, using Transcript Origin Analysis [17] of previously derived monocyte subset transcriptome profiles [18] as reference data. These secondary analyses did not involve any statistical testing at the level of individual genes; each involved only a single statistical test of the differential prevalence of gene annotations (i.e., NF-κB-binding site or classical vs. non-classical monocyte expression) conducted at p < 0.05, utilizing standard errors derived from 200 cycles of bootstrap resampling of gene expression vectors (to account for correlation among genes [19]. We used point estimates of differential expression rather than p-value based statistical testing to identify differentially expressed genes because the former has been found to generate more replicable results when the genes serve as input into higher-order bioinformatics analyses which maintain their own control over statistical error rates[20]’.
RESULTS
Patients and treatments
Total enrollment was 32 patients, but 6 patients were excluded from analysis: 3 patients never took beta-blocker after signing consent and 3 were excluded due to incorrect post-operative pathology. Demographic data are presented in Table 1. QOL analysis (Table 2) was available for 23 patients; the other 3 patients did not complete QOL instruments beyond TP0. Surgery (either primary or interval) was performed on 21 of the 26 (80.7%) patients; 10 (38.5%) underwent primary debulking, and a total of 19 patients ultimately underwent optimal tumor reductive surgery. Five patients never underwent surgery during study period; 2 had disease progression, 3 had stage IV disease that did not respond.
Table 1.
Baseline demographic and clinical characteristics of study participants (N=26)
| Characteristic | N=26 | % |
|---|---|---|
| Age, years | ||
| 40–49 | 7 | 26.9% |
| 50–59 | 6 | 23.1% |
| 60–71 | 13 | 50.0% |
| Race | ||
| American Indian/Alaska Native | 1 | 3.8% |
| Asian | 1 | 3.8% |
| Black or African American | 5 | 19.2% |
| White | 19 | 73.1% |
| Ethnicity | ||
| Hispanic | 8 | 30.8% |
| Non-Hispanic | 18 | 69.2% |
| Disease stage | ||
| IIB | 2 | 7.7% |
| IIIB | 1 | 3.8% |
| IIIC | 11 | 42.3% |
| IV | 12 | 46.2% |
| Chemotherapy | ||
| Adjuvant | 10 | 38.5% |
| Neoadjuvant | 16 | 61.5% |
| BB dose (mg twice daily) | ||
| 10 mg | 2 | 7.7% |
| 20 mg | 15 | 57.7% |
| 40 mg | 9 | 34.6% |
| Surgery | ||
| No | 5 | 19.2% |
| Yes | 21 | 80.8% |
| Debulking 1 | ||
| Optimal | 19 | 90.5% |
| Suboptimal | 2 | 9.5% |
| Postoperative diagnosis 1 | ||
| Fallopian tube | 1 | 4.8% |
| Ovarian | 17 | 81.0% |
| Peritoneal | 3 | 14.3% |
| Completed 6th Cycle | ||
| No | 8 | 44.4% |
| Yes | 18 | 66.6% |
| PP Disease status 2 | ||
| Progressive disease | 1 | 5.6% |
| Stable disease | 1 | 5.6% |
| Partial response | 6 | 33.3% |
| Complete response | 10 | 55.5% |
21 surgery patients
18 patients who received beta-blockers per-protocol
At TP 3. PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response; BB, β-blocker
Table 2.
Change From baseline scores (TP0) on quality of life measures (N=23)
| Variable | Visit | N | Mean (95% CI) | P-value* |
|---|---|---|---|---|
| CESD | TP1 | 21 | −8.88 (−13.90, −3.86) | 0.009 |
| TP2 | 18 | −11.12 (−17.43, −4.81) | 0.010 | |
| FACT EWB | TP1 | 18 | 2.42 (−0.08, 4.93) | 0.097 |
| TP2 | 15 | 3.27 (0.84, 5.69) | 0.045 | |
| FACT FWB | TP1 | 18 | 1.62 (−0.47, 3.71) | 0.197 |
| TP2 | 15 | 1.87 (−0.85, 4.60) | 0.232 | |
| FACT PWB | TP1 | 19 | 2.61 (−1.35, 6.58) | 0.238 |
| TP2 | 16 | 3.38 (−0.51, 7.28) | 0.213 | |
| FACT SWB | TP1 | 16 | −0.78 (−3.34, 1.78) | 0.591 |
| TP2 | 14 | −1.59 (−4.18, 1.00) | 0.273 | |
| FACT-G Total | TP1 | 14 | 3.84 (−3.12, 10.81) | 0.239 |
| TP2 | 12 | 5.71 (−0.87, 12.28) | 0.220 | |
| FACT-O Total | TP1 | 14 | 9.22 (−0.42, 18.86) | 0.186 |
| TP2 | 12 | 12.46 (5.50, 19.41) | 0.034 | |
| HADS Anxiety | TP1 | 20 | −1.80 (−3.59, −0.01) | 0.117 |
| TP2 | 17 | −3.24 (−5.44, −1.03) | 0.036 | |
| HADS Depression | TP1 | 19 | −1.16 (−3.34, 1.02) | 0.273 |
| TP2 | 18 | −2.11 (−3.75, −0.48) | 0.045 | |
False discovery rate–adjusted signed rank test P-values.
Of the 26 evaluable patients, 18 (69%; 90% Credible interval 53%−81%) met the primary objective of completing 6 cycles of chemotherapy while on beta-blocker. The posterior probability that at least 60% of patients would complete all 6 cycles was 82%. Eight patients did not take beta-blocker for 6 cycles: 1 patient stopped after 2 weeks due to fatigue suspected related to beta-blocker; 2 had progression of disease after three cycles; 1 had delayed oral intake after interval surgery and 2 had delayed oral intake after primary treatment; 1 patient stopped because of chemotherapy dose reduction due to neuropathy, and 1 stopped post operatively for unknown reason. There were no other safety events to report.
Quality of life over treatment period
Of the 23 patients who completed QOL instruments at more than one time point, 18 completed the QOL instruments at TP2. QOL results are summarized in Table 2. The results, comparing QOL survey scores among patients who received neoadjuvant chemotherapy and those who received adjuvant chemotherapy (i.e., upfront surgery), are reported in Table 3. Among the 23 patients, overall quality of life, anxiety, and depression improved significantly (P = 0.03) on the combination of chemotherapy and propranolol. Two crossed the boundary of 15 in the direction of improvement in anxiety or depression.
Table 3.
Change from baseline (TP0) scores on quality of life scales by treatment group (N=23)
| Variable | Visit | Arm | N | Mean* (95% CI) | P-value† |
|---|---|---|---|---|---|
| CESD | TP1 | Neo | 13 | −6.16 (−11.89, −0.43) | 0.824 |
| Adj | 8 | −13.30 (−23.80, −2.79) | |||
| TP2 | Neo | 10 | −10.49 (−18.92, −2.06) | 0.953 | |
| Adj | 8 | −11.91 (−24.07, 0.25) | |||
| FACT EWB | TP1 | Neo | 12 | 1.55 (−1.88, 4.98) | 0.900 |
| Adj | 6 | 4.17 (−0.10, 8.44) | |||
| TP2 | Neo | 9 | 3.56 (0.25, 6.86) | 0.953 | |
| Adj | 6 | 2.83 (−2.28, 7.95) | |||
| FACT FWB | TP1 | Neo | 13 | 2.04 (−0.48, 4.56) | 0.949 |
| Adj | 5 | 0.53 (−5.12, 6.19) | |||
| TP2 | Neo | 10 | 2.51 (−1.30, 6.32) | 0.900 | |
| Adj | 5 | 0.60 (−4.64, 5.84) | |||
| FACT PWB | TP1 | Neo | 12 | 1.60 (−3.09, 6.29) | 0.953 |
| Adj | 7 | 4.36 (−4.79, 13.51) | |||
| TP2 | Neo | 10 | 3.71 (−0.38, 7.80) | 0.953 | |
| Adj | 6 | 2.83 (−7.71, 13.38) | |||
| FACT SWB | TP1 | Neo | 10 | −1.04 (−5.25, 3.16) | 1.000 |
| Adj | 6 | −0.33 (−3.04, 2.38) | |||
| TP2 | Neo | 8 | −2.40 (−7.09, 2.28) | 0.900 | |
| Adj | 6 | −0.50 (−3.05, 2.05) | |||
| FACT-G Total | TP1 | Neo | 9 | 3.85 (−5.26, 12.96) | 1.000 |
| Adj | 5 | 3.83 (−13.38, 21.05) | |||
| TP2 | Neo | 7 | 9.21 (1.60, 16.83) | 0.773 | |
| Adj | 5 | 0.80 (−13.95, 15.55) | |||
| FACT-O Total | TP1 | Neo | 9 | 10.11 (−1.23, 21.44) | 0.953 |
| Adj | 5 | 7.63 (−19.19, 34.45) | |||
| TP2 | Neo | 7 | 16.04 (7.66, 24.41) | 0.773 | |
| Adj | 5 | 7.44 (−7.90, 22.78) | |||
| HADS Anxiety | TP1 | Neo | 13 | −0.69 (−2.63, 1.24) | 0.773 |
| Adj | 7 | −3.86 (−7.80, 0.08) | |||
| TP2 | Neo | 9 | −1.78 (−3.73, 0.17) | 0.773 | |
| Adj | 8 | −4.88 (−9.35, −0.40) | |||
| HADS Depression | TP1 | Neo | 13 | −1.00 (−4.11, 2.11) | 1.000 |
| Adj | 6 | −1.50 (−4.80, 1.80) | |||
| TP2 | Neo | 10 | −2.80 (−5.41, −0.19) | 0.824 | |
| Adj | 8 | −1.25 (−3.56, 1.06) | |||
Mean change in score from baseline TP 0 to TP 1 and TP 2
False discovery rate–adjusted Wilcoxon rank sum test P-values.
Neo, neoadjuvant chemotherapy; Adj, adjuvant chemotherapy.
Longitudinal assessment of cytokine levels
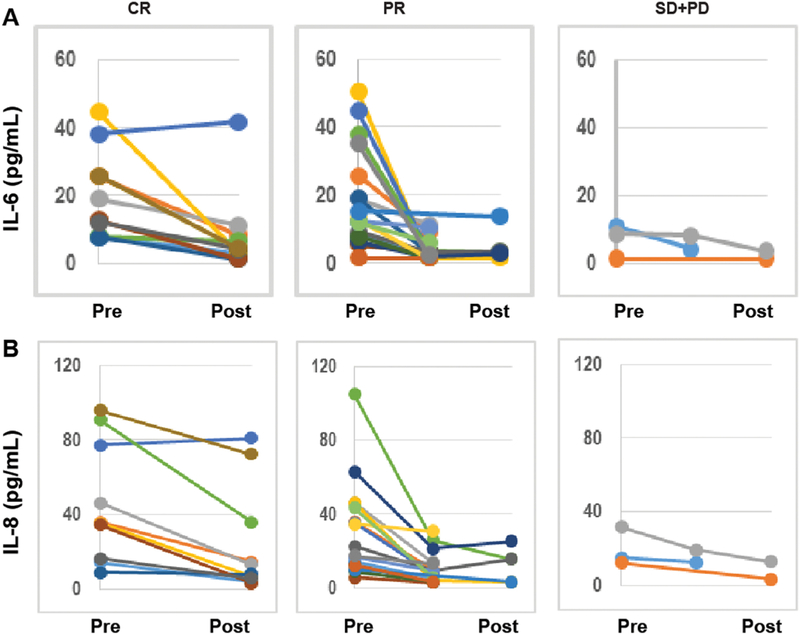
The cytokine analysis included all 26 patients. Complete cytokine data were available for only 14 patients; however, generalized linear mixed models, can handle data that are missing at random. Levels of 38 plasma markers were determined by a multiplex ELISA assay, IL-3, was excluded from the analysis because all had values below the limit of detection. We tested changes in levels of selected cytokines (IL-6, IL-8, IL-10 and VEGF) while on treatment; IL-6, IL-8 and IL-10 levels were markedly lower at TPs that coincided with complete or partial response to chemotherapy than at TPs that coincided with non-response (Figure 2; Suppl Table 3).
Figure 2:
Multiplex ELISA analyses of the dynamic changes in IL-6 (A), and IL-8 (B) in ovarian cancer patients who had complete response (CR), partial response (PR), or stable (SD)/progressive disease (PD) with chemotherapy during the study period.
Longitudinal assessment of leukocyte gene expression
Analyses of treatment effects on leukocyte gene expression showed a reduction over time in expression of a pre-specified set of 19 inflammation-related gene transcripts (e.g., IL1B, IL6, TNF, CXCL8; average change = −0.296 log2 expression units ± .127 standard error, P=0.031). To cross-validate these results for a priori-specified genes, we conducted targeted bioinformatics analyses of all 322 gene transcripts that empirically changed in average expression by >50% over time (122 up-regulated and 200 down-regulated). In analyses testing for differential prevalence of promotor binding motifs, results indicated decreased activity of the pro-inflammatory transcription factor, NF-κB (log2 motif prevalence ratio = −0.543 ± 0.263, P=0.040). Although not targeted a priori, based on relevance to inflammation, these analyses also indicated a complementary increase in interferon response factor (IRF) activity (+0.857 ± 0.369, P=0.021). As a third convergent analysis, transcript origin analyses applied to the same 322 genes indicated decreased activity of the immature, pro-inflammatory classical (CD16−) monocyte subset (mean cell type diagnosticity score for down-regulated genes: 1.37 ± .19, P< 0.001). Finally, we conducted a descriptive transcriptome representation analysis to assess changes in the abundance of specific leukocyte subsets and found reduced monocyte prevalence (−0.278 log2 mRNA abundance ± 0.063, p < .001) and increased prevalence of CD8+ T lymphocytes (+0.100 ± 0.046, p = 0.030).
DISCUSSION
Our study raises the hypotheses that the combination of chemotherapy with a beta-blocker is associated with improved QOL (specifically anxiety) as well as, a decrease in inflammatory markers. Preclinical evidence supports that sustained adrenergic activation can promote ovarian cancer growth and metastasis and many of the stimulatory effects of SNS on tumor biology can be blocked by propranolol [10, 21, 22].
Previously reported data on the benefits of beta-blockade in prolonging progression-free survival and overall survival in EOC are promising, but mixed [23]. Much of the research has been retrospective and in limited patient cohorts, typically women receiving a selective beta-blocker for hypertension. When no discrimination was made for selective vs. nonselective blockade, either no significant improvement in progression-free survival and overall survival was observed [24, 25] or the data were insufficient to determine significance [26]. In vitro studies have shown that ADRB2 is the receptor whose stimulation most contributes to ovarian cancer development and metastasis [10, 22, 26–29]. In a retrospective study, nonselective beta-blocker users had improved survival outcomes [30]. Since the initiation of this trial, reported data continue to support the benefit of nonselective beta-blockade in other types of cancers.[31, 32] De Giorgi et al. showed that in patients with stage IB to IIIA cutaneous melanoma [11], propranolol reduced risk of recurrence by 80% [11]. It is unclear if long-term use of a beta-blocker as treatment for hypertension can effectively lead to adrenergic blockade in cancer patients (i.e., someone diagnosed while already receiving a beta-blocker). Although data on breast cancer and melanoma are reassuring, prospective feasibility studies are needed in patients with different tumor types.
Over the past decade, the utility of perioperative beta blockade has been questioned, the previous practice of routine initiation of beta blockade before surgery has been replaced with a more nuanced approach [33]. Important questions as to the role and attenuation of the adrenergic pathways in perioperative medicine and cancer care remain. Tissue levels of the sympathetic catecholamine norepinephrine are known to be elevated in tumor samples from patients with a high biobehavioral risk profile (high depressive symptoms/low social support) [21, 34]. Studies have suggested meditation, yoga, and breathing techniques to reduce this risk [35, 36].
It is possible that perioperative adrenergic blockade may work better in combination with other drugs to counter stress responses during oncologic surgical interventions as well as with primary chemotherapy. Perioperative treatment with combinations of propranolol and COX2 inhibition have been evaluated as a potential method to reduce recurrence[12, 37]. Shaashua et al. have explored the safety of the combination of COX2 and beta-adrenergic signaling blockade in women with breast cancer in perioperative settings. In their study, treatment reduced activity of pro-metastatic/pro-inflammatory transcription factors, reduced IL-6 and CRP levels, and decreased tumor-infiltrating monocytes while increasing tumor-infiltrating B cells. [12]. Our data show similar changes in circulating immune cell gene expression profiles to those observed in that trial [38] including reduced expression of pro-inflammatory genes accompanied by increased expression of type I interferon-related genes, reduced activation of and prevalence of immature “classical” (CD16−) monocytes, and increased prevalence of CD8+ T cells. These changes in the circulating leukocyte transcriptome are consistent with a reduction in the so-called Conserved Transcriptional Response to Adversity (CTRA), which is a β-adrenergically mediated pattern typically observed in people confronting chronic stress [39]. Collectively, our prospective data collected on a small number of patients supports the concept that chronic stress reduces the antitumor immune response and blocks the stress response thus synergistically working with immune-targeted tumor therapy. Nevertheless, it is important to note that the present analyses examined only reductions in inflammatory biology as an a priori-specified hypothesis (tested using a primary analysis of pre-specified inflammatory genes, and re-confirmed using alternative analyses examining reductions in inflammatory biology at the level of NF-kB transcription factor activity and CD16− classical monocyte polarization). Differences in Type I interferon activity and CD8+ T cell biology are presented for descriptive purposes and their relevance to previous research findings; they should be considered incidental findings and were not primary targets of our pre-specified analysis plan. All of these biomarker observations need to be confirmed in future research.
The strengths of our study include prospective design in a primary treatment setting without use of potentially confounding anti-angiogenic therapies. However, potential limitations include non-randomized design with a control arm and inability to generalize to a larger population. Additionally, given the number of statistical tests, it is likely that some of the statistically significant results were observed by chance and not due to an underlying relationship between the data. While the study did produce robust translational correlates, more than three time point measurements will be required to more accurately model associations with response and survival and to differentiate the selective effects of the beta-blocker. As such, the present biomarker findings should be considered preliminary, given the limited sample size, limited follow-up, absence of a randomized control group, and the multiple biomarker outcomes examined. It will be important to confirm the findings observed here in future studies. Finally, changes in QoL might be attributable to the natural course of disease treatment. The next step is to determine if the beta blockade adds to the effect on QOL and inflammatory markers.
CONCLUSIONS
We demonstrated that the addition of propranolol to upfront ovarian cancer therapy is feasible and safe in this patient population. In addition, we demonstrated a significant reduction in a β-adrenergically mediated cytokine pattern typically observed in people confronting chronic stress.
Supplementary Material
Highlights.
Use of propranolol during primary chemotherapy and surgical treatment of ovarian cancer is feasible
Combination chemotherapy and propranolol may improve overall quality of life, anxiety, and depression
The combination of chemotherapy and propranolol were associated with a measured reduction in the Conserved Transcriptional Response to Adversity (CTRA)
Acknowledgments
Funding:
The partial work was supported by the Blanton-Davis Ovarian Cancer Research Program, and by the National Institutes of Health (P50 CA217685 and P30 CA016672) and the Frank McGraw Memorial Chair in Cancer Research, CPRIT RP210214, Judy Reis/Al Pisani, MD Ovarian Cancer Research Fund.
Conflict of interest disclosure:
AKS reports serving as consultant for Kiyatec and Merck, stock holder for Biopath, Research funding from M-Trap. WH reports research funding from Convergene LifeScience, Inc., Tracon Pharmaceuticals, Inc., and Bio-Path Holdings, Inc. PHT is a consultant and stock holder of Celsion, serves on advisory boards for Iovance, has research funding from Merck and Tesaro, and a consultant to Clovis, Astra Zeneca, Tesaro, Abbvie, and Immunogen. RLC has research funding from Abbvie, Janssen, Genmab, Merck, Clovis, AstraZeneca, Roche/Genentech, V-Foundation, Gateway Foundation; consultant to Ararvive, Abbvie, Roche/Genentech, Oncomed, Merck, AstraZeneca, Tesaro, Immunogen, Janssen, Gamamab, Genmab. RLC is supported by the Ann Rife Cox Chair in Gynecology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NCT ID:
NCI CTRP ID: NCI-2012-00056
References
- 1.Lutgendorf SK and Sood AK, Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med, 2011. 73(9): p. 724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JW, et al. , Surgical stress promotes tumor growth in ovarian carcinoma. Clin Cancer Res, 2009. 15(8): p. 2695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilmore DW, From Cuthbertson to fast-track surgery: 70 years of progress in reducing stress in surgical patients. Ann Surg, 2002. 236(5): p. 643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finnerty CC, et al. , The surgically induced stress response. JPEN J Parenter Enteral Nutr, 2013. 37(5 Suppl): p. 21S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackburn GL, Metabolic considerations in management of surgical patients. Surg Clin North Am, 2011. 91(3): p. 467–80. [DOI] [PubMed] [Google Scholar]
- 6.Mari G, et al. , ERAS Protocol Reduces IL-6 Secretion in Colorectal Laparoscopic Surgery: Results From a Randomized Clinical Trial. Surg Laparosc Endosc Percutan Tech, 2016. 26(6): p. 444–448. [DOI] [PubMed] [Google Scholar]
- 7.Kim YS, et al. , The immunomodulatory role of esmolol in patients undergoing laparoscopic gastrectomy due to gastric cancer. Anaesthesia, 2013. 68(9): p. 924–30. [DOI] [PubMed] [Google Scholar]
- 8.Bolton N, Perioperative beta-blockers for preventing surgery-related mortality and morbidity. J Perioper Pract, 2016. 26(3): p. 30–1. [PubMed] [Google Scholar]
- 9.Al-Niaimi A, et al. , The impact of perioperative beta blocker use on patient outcomes after primary cytoreductive surgery in high-grade epithelial ovarian carcinoma. Gynecol Oncol, 2016. 143(3): p. 521–525. [DOI] [PubMed] [Google Scholar]
- 10.Thaker PH, et al. , Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med, 2006. 12(8): p. 939–44. [DOI] [PubMed] [Google Scholar]
- 11.De Giorgi V, et al. , Propranolol for Off-label Treatment of Patients With Melanoma: Results From a Cohort Study. JAMA Oncol, 2018. 4(2): p. e172908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaashua L, et al. , Perioperative COX-2 and beta-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin Cancer Res, 2017. 23(16): p. 4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thall PF, Simon RM, and Estey EH, Bayesian Sequential Monitoring Designs for Single-Arm Clinical-Trials with Multiple Outcomes. Statistics in Medicine, 1995. 14(4): p. 357–379. [DOI] [PubMed] [Google Scholar]
- 14.Cole SW, et al. , Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer, 2015. 15(9): p. 563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole SW and Sood AK, Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res, 2012. 18(5): p. 1201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole SW, et al. , Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics, 2005. 21(6): p. 803–10. [DOI] [PubMed] [Google Scholar]
- 17.Cole SW, et al. , Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A, 2011. 108(7): p. 3080–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong KL, et al. , Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood, 2011. 118(5): p. e16–31. [DOI] [PubMed] [Google Scholar]
- 19.Efron B.a.T., R.J., An Introduction to the Bootstrap 1993, Chapman and Hall, New York. [Google Scholar]
- 20.Witten DMT, R. , A comparison of fold-change and the T-statistic for microarray data analysis . 2007.
- 21.Lutgendorf SK, et al. , Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun, 2009. 23(2): p. 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sood AK, et al. , Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res, 2006. 12(2): p. 369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunch KP and Annunziata CM, Are beta-blockers on the therapeutic horizon for ovarian cancer treatment? Cancer, 2015. 121(19): p. 3380–3. [DOI] [PubMed] [Google Scholar]
- 24.Johannesdottir SA, et al. , Use of ss-blockers and mortality following ovarian cancer diagnosis: a population-based cohort study. BMC Cancer, 2013. 13: p. 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eskander R, Bessonova L, Chiu C, Ward K, Culver H, Harrison T, Randall L, Beta blocker use and ovarian cancer survival: A retrospective cohort study. Gynecol Oncol, 2012. 127(1): p. S21. [Google Scholar]
- 26.Heitz F, et al. , Impact of beta blocker medication in patients with platinum sensitive recurrent ovarian cancer-a combined analysis of 2 prospective multicenter trials by the AGO Study Group, NCIC-CTG and EORTC-GCG. Gynecol Oncol, 2013. 129(3): p. 463–6. [DOI] [PubMed] [Google Scholar]
- 27.Tang J, et al. , beta-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol, 2013. 23(6 Pt B): p. 533–42. [DOI] [PubMed] [Google Scholar]
- 28.Diaz ES, Karlan BY, and Li AJ, Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol, 2012. 127(2): p. 375–8. [DOI] [PubMed] [Google Scholar]
- 29.Sood AK, et al. , Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest, 2010. 120(5): p. 1515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins JL, et al. , Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer, 2015. 121(19): p. 3444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kokolus KM, et al. , Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology, 2018. 7(3): p. e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonneau RH, Kiecolt-Glaser JK, and Glaser R, Stress-induced modulation of the immune response. Ann N Y Acad Sci, 1990. 594: p. 253–69. [DOI] [PubMed] [Google Scholar]
- 33.Oprea AD, Lombard FW, and Kertai MD, Perioperative beta-Adrenergic Blockade in Noncardiac and Cardiac Surgery: A Clinical Update. J Cardiothorac Vasc Anesth, 2019. 33(3): p. 817–832. [DOI] [PubMed] [Google Scholar]
- 34.Lutgendorf SK, et al. , Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer, 2002. 95(4): p. 808–15. [DOI] [PubMed] [Google Scholar]
- 35.Nelson G, et al. , Enhanced Recovery Program and Length of Stay After Laparotomy on a Gynecologic Oncology Service: A Randomized Controlled Trial. Obstet Gynecol, 2017. 129(6): p. 1139. [DOI] [PubMed] [Google Scholar]
- 36.Powell R, et al. , Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database Syst Rev, 2016(5): p. CD008646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorski L, et al. , Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through beta-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun, 2016. 58: p. 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haldar R, et al. , Perioperative inhibition of beta-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav Immun, 2018. 73: p. 294–309. [DOI] [PubMed] [Google Scholar]
- 39.Cole SW, Human social genomics. PLoS Genet, 2014. 10(8): p. e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.