Abstract
Intracellular survival of pathogenic bacteria leads to high chances of bacterial persistence and relapse in the bacteria-infected host. However, many antibiotics fail to clear the intracellular bacteria due to their low internalization by cells. In order to increase delivery of antibiotics in cells and eliminate intracellular bacteria, we developed antibiotic-derived lipid nanoparticles. First, we synthesized antibiotic-derived lipid conjugates using two widely used antibiotics including penicillin G (PenG) and levofloxacin (Levo). Then, we formulated them into antibiotic-derived lipid nanoparticles and evaluated their antibacterial effects. We found that penicillin G derived phospholipid nanoparticles (PenG-PL NPs) were able to enhance cellular uptake of penicillin G as compared with free penicillin G and eliminate up to 99.9998% of ~108.5 intracellular methicillin sensitive Staphylococcus aureus (S. aureus) in infected A549 cells, a lung epithelial cell line. The PenG-PL NPs showed the potential for inhibiting intracellular S. aureus and are promising to be further studied for in vivo antibacterial applications.
Keywords: intracellular bacteria, penicillin G derived phospholipid, antibiotic-derived lipid nanoparticles
Graphical Abstract
INTRODUCTION:
The formidable prevalence of pathogenic bacteria remains a severe threat to the public health.1 Meanwhile, an increasing concern has been raised upon intracellular survival of bacteria due to the limitation of treatment options for approaching and eliminating these intracellular pathogens.2 Two major types of intracellular bacteria have been reported including facultative intracellular bacteria that can reproduce both inside and outside the host cell, e.g. Mycobacterium tuberculosis and S. aureus, and obligate intracellular bacteria that can only reproduce inside the host cell, e.g. Chiamydophiia pneumoniae.3
S. aureus is a gram-positive, facultative intracellular bacterium that can cause serious infection.4 Previous studies demonstrated that intracellular S. aureus was related to several diseases, including S. aureus bacteremia,5 bone and joint tissue infection,6 chronic rhinosinusitis7 and pneumonia.8 These studies also pointed out that the host cells, for example, lung epithelial cells may provide a protective compartment for local or metastatic infection of S. aureus.9 Apart from the intracellular survival behavior of S. aureus, poor penetration of antibiotics across the mammalian cell membrane also leads to the difficulty in the treatment and relapse of infectious disease.2 β-lactam antibiotics are the major treatment option for the methicillin sensitive S. aureus infection.10 However, the cellular pharmacodynamics studies revealed that the β-lactam antibiotics cannot effectively penetrate the mammalian cell membrane due to their physicochemical characteristics.2 A few strategies were studied to increase the accumulation of antibiotics in cells, such as increasing the dose and prolonging the infusion administration, 11 using efflux transporter inhibitors,2 and developing antibiotic nanoparticle drug delivery systems.12–17 In this study, we aimed to develop antibiotic- derived lipid nanoparticles to improve the internalization of antibiotics in infected cells and enable the clearance of intracellular bacteria.
Drug conjugated liposomal antimicrobials were used as a potential strategy to inhibit intracellular pathogens because of their enhanced drug internalization by host cells.18–22 Herein, we synthesized a series of lipid conjugates using FDA approved antibiotics and formulated them to antibacterial nanoparticles for treating intracellular bacteria. Using the methicillin sensitive S. aureus infected lung epithelial cells as an infection model, we identified that optimized penicillin G derived phospholipid nanoparticles (PenG-PL NPs) were able to eliminate up to 99.9998% of ~108.5 intracellular S. aureus, which were significantly more efficacious than free penicillin G under the same drug concentration. Furthermore, this enhanced antibacterial effect was consistent with the high internalization of penicillin G after treatment of PenG-PL NPs in infected cells. Compared to other antibiotic drug delivery systems, such as drug encapsulated liposomal nanoparticles, antibiotic-derived lipid nanoparticles may provide a more efficient drug loading and reduce burst release.18 The results showed that PenG-PL NPs may be promising to treat intracellular bacteria in the future.
RESULTS:
Synthesis of antibiotic-derived lipid conjugates
To synthesize the antibiotic-derived lipids (Figure 1, Figure S1), two FDA approved antibiotics, penicillin G (PenG) and levofloxacin (Levo) were conjugated with two types of lipids [alkane-based lipid chain (L) and phospholipid chain (PL)] through a multiple step synthetic route. Briefly, penicillin G derived lipid, PenG-L was prepared by installing two stearoyl chains to the extended hydroxyl groups on penicillin G (Figure S1A). Similarly, levofloxacin derived lipid, Levo-L was made by adding two stearoyl chains to the extended hydroxyl groups on levofloxacin (Figure S1C). Penicillin G derived phospholipid, PenG-PL and levofloxacin derived phospholipid, Levo-PL were synthesized by adding modified dicetyl phosphate to penicillin G and levofloxacin, respectively (Figure S1B and 1D). All products were confirmed by 1H NMR and high-resolution mass spectrum.
Figure 1.
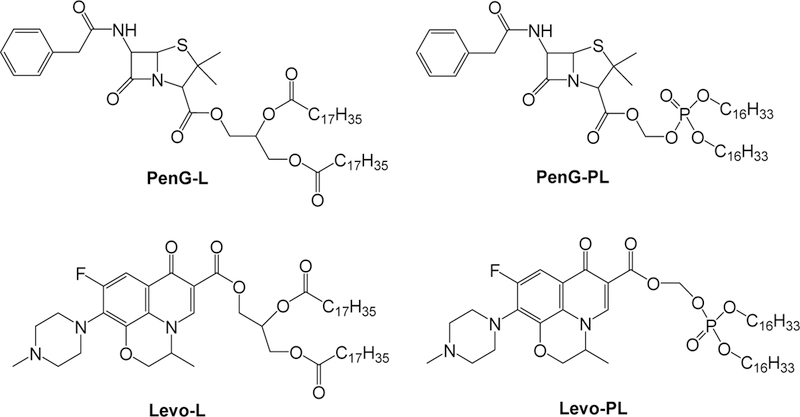
Chemical structures of the four antibiotic-lipid conjugates.
Formulation and characterization of antibiotic-derived lipid nanoparticles
The resulting antibiotic-lipid conjugates were formulated with 2-diphytanoyl-sn-glycero- 3-phosphocholine (DPhPC), cholesterol, and 1,2-dimyristoyl-rac-glycero-3- methylpolyoxyethylene (DMG-PEG2000) to prepare the antibacterial nanoparticles through a thin film hydration method.23 In order to find an appropriate formulation, we first used PenG-L to prepare the nanoparticles. Sixteen different PenG-L based formulations with diverse molar ratio of four components were made according to the formulation table (Table S1).23 Among these sixteen formulations, most formulations displayed two or more particle peaks (Figure S2A), indicating the particle heterogeneity. Formulation 6 (Figure 2A) and formulation 14 showed a relatively homogeneous peak. Previous studies reported that cholesterol may hinder the disassembly of nanoparticles.24 Due to the higher antibiotic percentage and simpler formulation composition without cholesterol in formulation 6 over 14, the molar ratio of formulation 6 was selected and further used for characterization of other antibiotic-derived lipid nanoparticles.
Figure 2.
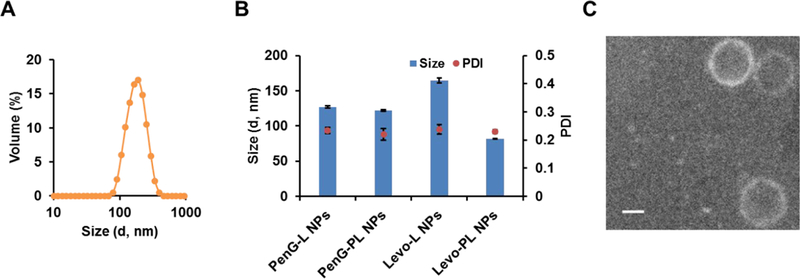
Characterization of antibiotic-derived lipid nanoparticles. (A) Size distribution of the selected formulation 6, PenG-L NPs. (B) Size distribution of antibiotic-derived lipid nanoparticles. Mean ± SD (n=3). (C) A TEM image of PenG-PL NPs. Scale bar = 100 nm.
The other three newly synthesized antibiotic-lipid conjugates with similar structure as PenG-L were included in the formulation (Table S2) following the selected molar ratio of nanoparticles components (phospholipid/antibiotic-lipid conjugates/PEG=45/15/2). The size and distribution were measured, and these nanoparticles displayed similar properties as PenG-L NPs (Figure 2B). To study the morphology of the PenG-PL NPs, a TEM imaging was conducted. As shown in Figure 2C and Figure S2B, PenG-PL NPs were spherical with a lipid membrane outside.
Bacterial growth inhibition by the antibiotic-derived lipid nanoparticles
After characterizing the antibiotic-derived lipid nanoparticles, we evaluated their antibacterial effects by incubating them with methicillin sensitive S. aureus (MSSA) in 96 wells plates. As shown in Figure 3, penicillin G (free PenG) and levofloxacin (free Levo) completely inhibited bacterial growth under the tested concentrations. The inhibition rates of PenG-L nanoparticles (PenG-L NPs), Levo-L nanoparticles (Levo-L NPs), and Levo-PL nanoparticles (Levo-PL NPs) were all less than 80% at the highest drug concentration. Importantly, the inhibition rate of PenG-PL nanoparticles (PenG-PL NPs) at the lowest tested concentration (0.17 μg/ml, equivalent penicillin G concentration) was still high (97.39 ± 3.65) %, indicating that PenG-PL NPs had the strongest antibacterial potential among these antibiotic-derived lipid nanoparticles. Further statistical test revealed that the antibacterial effect of PenG-PL NPs was significantly higher than other formulations. In addition, the MIC was 0.34–0.68 μg/ml for PenG-PL NPs.
Figure 3.
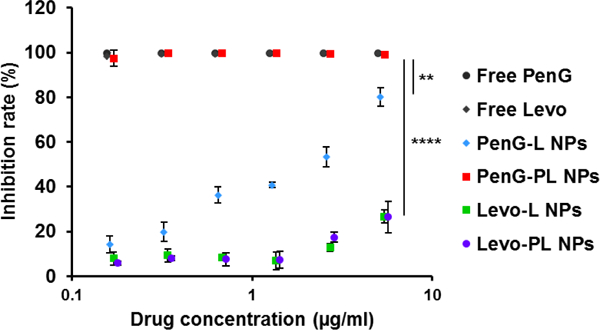
Bacterial growth inhibition by the antibiotic-derived lipid nanoparticles. Comparisons were made between the four nanoparticles at their highest concentration tested. **, PenG-PL vs PenG-L; ****, PenG-PL vs Levo-L and PenG-PL vs Levo-PL. Mean ± SD (n=3). (One-way ANOVA with Bonferroni post-hoc test).
To study the mechanism of antimicrobial activity of PenG-PL NPs, drug release rate was first measured. PenG-PL NPs displayed relatively faster drug release rate in the first 3 hours when compared to other three nanoparticles (Figure S3). The inhibition test also showed that PenG-PL had the strongest antimicrobial activity among the four conjugates when directly incubating with MSSA (Figure S4). These data may explain the different potency of antimicrobial activities observed in Figure 3.
Inhibition of intracellular bacteria by PenG-PL NPs
Next, we evaluated PenG-PL NPs for its inhibitory effect on intracellular bacteria in the MSSA infected A549 cells, a lung epithelial cancer cell line. The inhibitory effect was determined by counting and analyzing the colony forming units (CFU).25 After eliminating the extracellular bacteria by gentamicin, the number of intracellular bacteria in A549 was determined to be 108.49 ± 0.15 (n=3) after 20 hr of incubation. To compare the inhibitory effect of free penicillin G and PenG-PL NPs to the intracellular bacteria, different concentrations of free drug and nanoparticles were added to the MSSA infected A549 cells. The CFU number of each treatment group was quantified (Figure S5A) and the percentage losses of log10CFU in free PenG treated groups were less than 10% (Figure 4A). However, in PenG-PL NPs treated groups, the percentage losses of log10CFU were all significantly higher than that of free drug treated group, with the highest percentage loss at (67.4 ± 0.6) % in 20 μg/ml (equivalent PenG concentration) group, which was equal to the elimination of 99.9998% of ~108.5 bacteria. Meanwhile, no significant differences of cytotoxicity were found in free PenG and PenG-PL NPs treated cells (Figure S6). The results suggested that the enhanced anti-intracellular bacteria effect of PenG-PL NPs was not relevant to the particle cytotoxicity. Moreover, these cells were well tolerated with the PenG-PL NPs.
Figure 4.
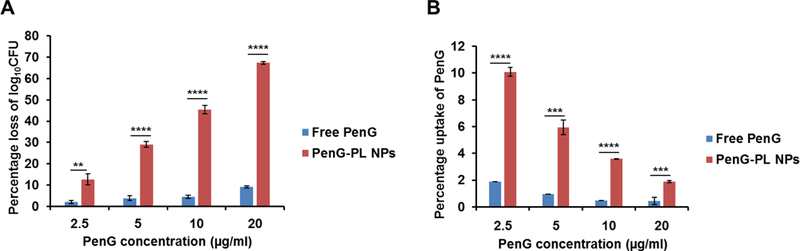
Growth inhibition of intracellular MSSA by PenG-PL NPs. (A) Inhibitory effect of free penicillin G (free PenG) and PenG-PL NPs on intracellular bacteria. Mean ± SD (n=3). (**, p<0.01, ****, p<0.0001, t-test, double-tailed). (B) Cellular internalization of free penicillin G (free PenG) and PenG-PL NPs in A549 cells. Mean ± SD (n=3). (***, p<0.001, ****, p<0.0001, t-test, double-tailed).
Next, we quantified cellular uptake of PenG and PenG-PL NPs (Figure 4B). After incubating cells with free PenG for 1 hr, the PenG uptake was less than 2% of total PenG treatment in all groups. On the other hand, the percentage of intracellular PenG uptake in the PenG-PL NPs treatment groups were significantly higher than that in the free PenG treatment group, with the highest percentage at (10.1 ± 0.3) % in 2.5 μg/ml equivalent PenG treatment group. Although the percentage of uptake decreased from the more concentrated nanoparticles, the total amount of internalized PenG were gradually increased (Figure S5B).
To study the intracellular trafficking profile of the nanoparticles, we imaged the MSSA infected A549 cells after treating with PenG-PL NPs. The MSSA were stained with Hoechst33342, the nanoparticles were labeled with FITC, and the lysosomes were labeled with lysotracker. As shown in Figure 5, bacteria were found in the cytoplasm. No co-localization between bacteria and PenG-PL NPs was noticed. In addition, apparent co-localization was found between FITC-labeled PenG-PL NPs and lysosomes. These results indicated that PenG-PL NPs underwent an endocytic pathway.
Figure 5.
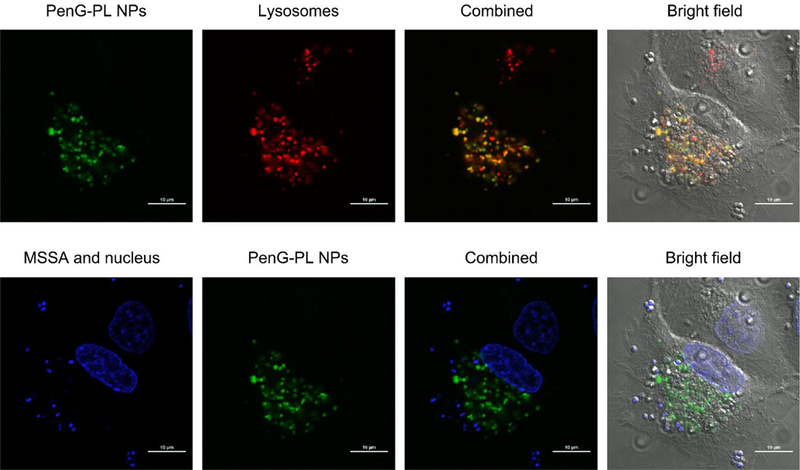
Intracellular trafficking of PenG-PL NPs in MSSA infected A549 cells. The PenG-PL NPs were labeled with FITC. Lysosomes were labeled with lysotracker. MSSA were stained with Hoechst33342. Co-localization of PenG-PL NPs and lysosomes was observed. No co-localization of MSSA and PenG-PL NPs was found. Scale bar = 10 μm.
DISCUSSION:
Previous studies reported that free penicillin G can hardly enter cells to eliminate intracellular bacteria, which leads to serious complications and makes several infectious diseases hard to cure.2 In order to address this issue and construct antibacterial nanoparticles, antibiotic-derived lipid conjugates were firstly synthesized, and their structures were confirmed via NMR and mass spectra. In our experiments, the antibiotics were conjugated with two forms of lipid chains forming carbonate and phosphate linkers. These antibiotic-lipid conjugates were formulated with phospholipids and PEG-lipids to prepare antibiotic-derived lipid nanoparticles. Results showed that nanoparticles with PenG-PL NPs had significantly stronger antibacterial effect than PenG-L NPs. Then, we quantified the drug release rate from the nanoparticles. The data indicated that PenG-PL NPs released the drug much faster than PenG-L NPs in the early time point. Next, we performed an inhibition assay using free antibiotic-derived lipid conjugates. Interestingly, PenG-PL displayed antibacterial activity, while the other conjugates were not. Previous studies reported that phospholipids (PL) helped anchoring the antibiotic to the bacteria membrane through its lipid part26, which may lead to stronger antimicrobial activities of PenG-PL conjugates in comparison to PenG-L conjugates. In order to test whether PenG-PL NPs can be efficiently internalized into cells and kill the intracellular bacteria, the MSSA infected lung epithelial cells were treated with either free penicillin G or PenG-PL NPs after eliminating the extracellular bacteria. The results showed that the PenG-PL NPs underwent an endocytic pathway when uptaken by infected cells. More importantly, the PenG-PL NPs were more potent than free penicillin G to inhibit intracellular bacteria. This strong antibacterial effect was consistent to the observation of increased cellular internalization of PenG-PL NPs. Meanwhile, these nanoparticles induced little cytotoxicity to the treated cells. The findings suggested that PenG-PL NPs were able to efficiently eliminate the intracellular bacteria MSSA by increasing cellular internalization.
With this proof-of-concept study, these antibiotic-derived lipid nanoparticles can be applied to a wide variety of infected cells, such as macrophages or neutrophils, and in vivo studies in bacterial infection models.27–31 Moreover, this conjugation and formulation approach is applicable to other types of antibiotics. Since there is an unmet and urgent need for treating drug-resistant bacteria, we anticipate developing diverse antibiotics-conjugated nanoparticles for killing intracellular drug-resistant bacteria with new antimicrobial mechanism32 or a combinational strategy.33
CONCLUSION:
In this study, we designed and synthesized antibiotic-derived lipid conjugates, and then developed a series of antibiotic-derived lipid nanoparticles. Among them, the PenG-PL NPs incorporating phosphate linker of antibiotic-lipid conjugates formed relatively homogeneous particle distribution and displayed strong antibacterial effects in cells. PenG-PL NPs enhanced the cellular internalization of penicillin G, and efficiently inhibit the intracellular bacteria in the MSSA infected lung epithelial cells. Our study demonstrated the potential of using antibiotic-derived lipid nanoparticles to treat intracellular bacteria.
EXPERIMENTAL SECTION:
Materials:
Penicillin G sodium salt was purchased from Alfa Aesar (Tewksbury, MA). Levofloxacin was purchased from Ark Pharm (Arlington Heights, IL). 1, 2-diphytanoyl- sn-glycero-3-phosphocholine (DPhPC) was purchased from Avanti Polar Lipids. 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amresco (Solon, OH). Other chemicals and solvents were purchased from Sigma- Aldrich (St. Louis, MO).
Bacteria and cell line:
Methicillin sensitive Staphylococcus aureus subsp. Aureus Rosenbach strain (MSSA, ATCC 6538) was obtained from the ATCC and cultured in tryptic soy broth/agar (BD) at 37 °C.
Lung epithelial cancer cell line (A549, ATCC CCL-185) was cultured in Ham's F-12K (Kaighn's) (Thermo Fisher, Waltham, MA) supplemented with 10% inactivated fetal bovine serum (FBS) (Thermo Fisher) at 37 °C in humidified atmosphere containing 5% CO2.
Synthesis and characterization of antibacterial drug-lipid conjugates:
In order to prepare the antibiotic-derived lipid nanoparticles, four antibiotic-lipid conjugates were afforded. These conjugates are amphiphilic materials, which can be formed with phospholipid, cholesterol and PEG to construct stable lipid nanoparticles.
Synthesis of PenG-L:
Penicillin G sodium salt (100 mg, 0.3 mmol) and 3-bromo-1,2- propanediol (156 g, 1 mmol) were dissolved in 2 mL DMF. The mixture was stirred at 35 °C for 24 hr. CH2Cl2 and water were then added to the reaction mixture. The organic phase was collected and dried with sodium sulfate. The crude product was purified by column chromatography with CH2Cl2/CH3OH (98:2, v/v) to give the product (1) (30 mg, yield 26.3 %). Triethylamine (22 mg, 0.22 mmol) and 4-(N, N-dimethylamino) pyridine (1 mg) were added to the solution of (1) (40 mg, 0.1 mmol) in CH2Cl2 (2 mL). The reaction mixture was cooled to 0 °C, and then stearoyl chloride (75.5 mg, 0.25 mmol) was added dropwise. The reaction was warmed to room temperature (RT) and quenched after 4 hr by pouring over saturated aqueous NaHCO3 (5 mL). The aqueous layer was separated, and the organic layer was washed successively with saturated aqueous NaHCO3, water, brine, and then dried with sodium sulfate. The crude product was purified by column chromatography to give the PenG-L in a white solid (100 mg, yield 33.4 %).1H NMR (400 MHz, CDCl3) d 7.38 (m, 5H, Ph), 6.06 (d, 1H, CHS), 5.69 (m,1H, CHNH), 5.52 (m, 1H, CHN), 5.30 (s, 1H, CHN), 4.14–4.41 (m, 5H, 2CH2O,CHO), 3.66 (s, 2H, CH2Ph), 2.32 (t, 4H, 2CH2C(O)), 1.62 (m, 4H, 2CH2), 1.45 (d, 6H, 2CH3), 1.28 (m, 56H, 28CH2), 0.90 (t, 6H, 2CH3); MS (m/z): [M+Na]+ calcd.for C55H92N2O8SNa, 963.6472; found, 963.6469.
Synthesis of PenG-PL:
Dicetyl phosphate (440 mg, 0.8 mmol), sodium bicarbonate (84 mg, 1 mmol) and tetra-n-butylammonium hydrogen sulfate (272 mg, 0.8 mmol) were dissolved in 8 ml water. Dichloromethane (8 mL) was then added to the mixture. The reaction was vigorously stirred at 0 °C for 10 min, followed by the addition of chloromethyl chlorosulfate (160 mg, 0.96 mmol) in DCM (4 mL) with continuous vigorous stirring. NaHCO3 (200 mg, 2.4 mmol) was added to the solution and the reaction was stirred overnight at room temperature. The organic layer was separated, washed with brine, and dried with sodium sulfate. The residue was purified by flash silica gel column chromatography using ethyl hexane/acetate (85:15, v/v) to give the product (2) in a white solid (236 mg, yield: 49.6 %). Penicillin G (100 g, 0.27 mmol) and (2) (80 mg, 0.13 mmol) were dissolved in 8 mL dry DMF and stirred at 35–40 °C for 4 hr. The mixture was dissolved in CH2Cl2 and washed with water, and then the organic layer was dried with sodium sulfate. The crude product solution was concentrated in vacuum. The residue was purified by column chromatography with EA/PE (30:70, v/v) to give the phosphate (PE) product in a white solid (35 mg, yield 29.2 %).1H NMR (400 MHz, CDCl3) d 7.34 (m, 5H, Ph), 6.18 (d, 1H, CHS), 5.61–5.75 (m, 3H, OCH2O, CHNH), 5.51 (m, 1H, CHN), 4.40 (b, 1H, NH), 4.05 (m, 4H, 2CH2O), 3.64 (s, 2H, CH2Ph), 1.49 (m,4H, 2CH2), 1.45 (d, 6H, 2CH3), 1.27 (m, 52H, 26CH2), 0.90 (m, 6H, 2CH3); MS (m/z): [M+H]+ calcd.for C49H86N2O8PS, 893.5842; found, [M+H]+=893.5839.
Synthesis of Levo-L:
Levofloxacin (300 mg, 0.83 mmol) was dissolved in 15 ml CH2Cl2. Then 2,2-DiMethyl-1,3-dioxolane-4-methanol (132 mg, 1 mmol), trimethylamine (303 mg, 3 mmol) and HBTU (380 mg, 1 mmol) were added successively. The reaction mixture was stirred at room temperature for 72hr. The mixture was then concentrated in vacuum, and the residue was purified by flash silica gel column chromatography to give the product (3) (210mg, yield: 44.21%). 120 mg product (3) was dissolved in 4 ml DMC. The solution was cooled to 0 °C, and 200 ul trifluoroacetic acid was added (solution turned yellow). The mixture was stirred at 0 °C for 1 hr and then stirred at room temperature for 3 hr. The NaHCO3 and 5 drops of water were added, and the solution turned no color. The solution was dried by sodium sulfate to give the product (4) (35mg, yield: 32.11%). Product (4) (20 mg, 0.046 mmol) was dissolved in 2 mL CH2Cl2. Triethylamine (33 mg) and 4-(N, N-dimethylamino) pyridine (1 mg) were then added to the solution. The reaction mixture was cooled to 0 °C, and then stearoyl chloride (38 mg) was added dropwise. The reaction was warmed to room temperature and quenched after 4 h by pouring over saturated aqueous NaHCO3 (5 mL). The aqueous layer was separated, and the organic layer was washed successively with saturated aqueous NaHCO3, water, and brine, and then dried with sodium sulfate. The crude product was concentrated in vacuum, and was purified by flash column chromatography with CH2Cl2/methanol (100:0–90:10, v/v) to give the product (14 mg, yield: 31.4 %).1H NMR (400 MHz, CDCl3) d 8.31 (d, 1H, Ph), 7.70 (dd, 1H, Ar), 5.43 (m,1H, CHO), 4.30-4.51 (m, 7H, 3CH2O, CHN), 3.41 (m, 4H, 2CH2N), 2.68(m, 4H, 2CH2N), 2.44 (s, 3H, CH3N), 2.35(m, 2CH2C(O), 4H), 1.57-1.63 (m, 7H, 2CH2, CH3), 1.27 (m, 56H, 28CH2), 0.90 (t, 6H, 2CH3); MS (m/z): [M+H]+ calcd.for C57H95FN3O8, 968.7103; found, 968.7151.
Synthesis of Levo-PL:
Following the same procedures as PenG-PL, the last reaction was performed at 90 °C for 2 hr. The product is 14.4 mg, yield 15.7%.1H NMR (400 MHz, CDCl3) d 8.46 (s, 1H, Ph), 7.76 (d, 1H, Ar), 5.87 (m,1H, CHN), 4.40 (m, 3H, OCH2, CHN), 4.08 (m, 4H, 2CH2O), 3.41 (m, 4H, 2CH2N), 2.58 (m, 4H, 2CH2N), 2.40 (s, 3H, NCH3), 1.58 (m, 4H, 2CH2), 1.28 (m, 52H, 26CH2), 0.90 (m, 6H, 2CH3); MS (m/z): [M+H]+ calcd.for C51H88FN3O8P, 920.6293; found, 920.6273.
Preparation and characterization of antibiotic-derived lipid nanoparticles:
The antibiotic-derived lipid nanoparticles (NPs) were prepared by thin film dispersion method.23 To prepare PenG-L NPs formulations, DPhPC, cholesterol, antibiotic-lipid conjugates, and 1,2-dimyristoyl-rac-glycero-3-methylpolyoxyethylene (DMG-PEG2000) were dissolved in chloroform at 16 different ratios (Table S1).23 The solvent was then removed at 50 degrees by a rotary vacuum evaporator (Hedolph G5, Schwabach, Germany). The remaining lipid film was hydrated with H2O using water bath sonication for 1 min, and further homogenized by ultrasonic sonication at 50 W, 20 KHz (QSonica, Newtown, CT) for 5 min. The theoretical concentration of antibacterial drug-lipid conjugates was 160 μg/ml. Particle size distribution of the NPs were determined using a NanoZS Zetasizer (Malvern, Worcestershire, U.K.). FITC-labeled PenG-PL NPs were prepared using the same method as to prepare the PenG-PL NPs with addition of fluorescein (fluorescein to total lipid, 1:200, w/w) when dissolving the formulation components in chloroform.
For TEM imaging, samples were prepared by applying a small aliquot (3 μL) of PenG- PL NPs on carbon coated copper grids. After blotting away excess liquid, the grids were air dried. Electron micrographs were collected under STEM mode on a Tecnai F20 S/TEM (Thermo Fisher Scientific, Hillsboro) with high angle angular dark field (HAADF) detector. Microscope was operated at an acceleration voltage of 200kV.
The encapsulation efficiency (EE%) of drug-lipid conjugates was calculated using the formula:EE%=(Wencap/Wtotal)×100%, where the Wencap is the measured amount of conjugates in the antibiotic-derived lipid nanoparticles precipitate after centrifuging the freshly made nanoparticles solution at 10,000 rpm for 20 min, and the Wtotal is the measured amount of conjugates in an equal amount of nanoparticle solution. The NPs were disrupted by CHCl3, and the concentration of drug-lipid conjugates was quantified by UV absorbance at 215 nm with standard curve (Figure S7) (Molecular Devices, Sunnyvale, CA). The encapsulation efficiency of PenG-PL in PenG-PL NPs was 48.2 ± 3.9 % (n=3).
We then quantified drug release rate by measuring the remaining amount of antibioticlipid conjugates after incubating the nanoparticles in water solution at 37 degree. The rationale is that Penicillin G derived phospholipid, PenG-PL, will be hydrolyzed by esterase after cell internalization. Thus, drug release was calculated by subtracting the remaining PenG-PL conjugates from the total conjugates in the solution. After 0, 3, 6, and 24 hr, chloroform was added to extract the remaining antibiotic lipid conjugates. The organic phase and the water phase were then separated by centrifuge at 10000 g for 10 min. Next, the chloroform phase was transferred and mixed with methanol (chloroform: methanol=1:1, v/v) for MS quantification. Drug release (%) = (1-(wtconjugates at different time points/wtconjugates at 0 hr)) ×100%.
Antibacterial effect of antibiotic-derived lipid nanoparticles:
Antibacterial effect was analyzed by the turbidity (0D600 nm) after incubating the NPs with MSSA.34 The MSSA was first prepared at ~105 bacterial cells/well in the 96 wells plate. After 2 fold serial dilution, each newly formulated antibacterial NPs was added to the bacteria suspension, and the final concentration of each drug was determined by the formula: drug concentration (μg/ml) = M.W.free drug/M.W.drug-lipid conjugates × EE% × 160 μg/ml. The NPs treated bacteria plates were then incubated at 37 degrees with continuous shaking at 150 rpm for 24 hrs. After incubation, the inhibition effect of antibacterial NPs was evaluated by a plate reader at 0D600 nm (SpectraMax M5, Molecular Devices, LLC., Sunnyvale, CA). When studying the bacterial growth inhibition by the antibiotic-derived lipid conjugates, drug concentration (μg/ml) = M.W.free drug/M.W.drug-lipid conjugates × Conc.drug-lipid conjugates. The inhibition rates of MSSA were calculated using the following formula: inhibition rate %= (1-A600 nm for treated bacteria cells/ A600 nm for non-treated control bacteria cells) × 100%. To measure the minimum inhibitory concentration (MIC) of drug loaded NPs and free drug, the pour plate method was used. Briefly, the colony forming units of drug treated bacteria were determined by serial diluting and plating on tryptic soy agar (BD) followed by incubation overnight at 37 °C. The MIC was determined by the lowest concentration of drugs that completely inhibited the growth of bacteria on the agar plate.
Inhibition of intracellular bacteria by antibiotic-derived lipid nanoparticles:
To quantify anti-intracellular bacteria effects, MSSA infected A549 cells were treated with antibiotic- derived lipid nanoparticles, and then the intracellular bacteria were quantified by pour plate method. A549 cells were seeded in 24 wells plate at 105 cells/0.5 ml/well and allowed it to grow for 24 hr at 37 degrees. Then the cells were washed with F12K 0.5% FBS media. MSSA suspension containing approximate 106 bacteria cells were added to A549 cells at MOI=10 (multiplicity of infection) and incubated with the cells for 2 hr. Then the cells were washed with F12K containing 0.5% FBS and treated with gentamicin 50 μg/ml in F12K for 2hr to eliminate the extracellular bacteria. A549 cells were then washed with F12K 0.5% FBS three times and incubated with penicillin G or antibacterial NPs at 37 degrees for 1 hr. After incubation, the cells were washed with F12K 0.5% FBS again, and incubated with full media at 37 degrees for 18 hr. The intracellular bacteria were then fully released by adding Triton X-100 0.1% (PBS, v/v) at 37 degrees for 10 min, and then counted by serial dilution and pour plate technique. Percentage loss of log10(colony forming units, CFU) = log10(CFU of untreated control/CFU of treated group)/log10(CFU of untreated control) × 100%.
Comparison of cytotoxicity between antibiotic-derived lipid nanoparticles and free drug:
To test if cells treated after PenG-PL NPs or penicillin G can still be intact, the cytotoxicity of these two formulations was evaluated. A549 cells were seeded at 5000 cells/well in 96 wells plate. After 24 hr of incubation, the free PenG or PenG-PL NPs were added. Following 18 hr of further incubation, the cytotoxicity of both treatment groups was analyzed by MTT assay. Briefly, 20 μl of MTT was added to 200 μl of treated cells. After 4 hr of incubation at 37 degrees, the media was removed and 150 μl DMSO was added. The absorbance was then recorded at 570 nm. The cell viability of A549 cells was calculated using the following formula: cell viability %= (A570 nm for treated cells-A570 nm blank well)/ (A570 nm for non-treated cells-A570 nm blank well) × 100%.
Intracellular uptake of PenG-PL NPs in A549 cells:
To further study the internalization of PenG-PL NPs in A549 cells, the intracellular PenG was quantified using mass spectrum (MS, LTQ Orbitrap XL, Thermo Scientific). The A549 cells were seeded at 105 cells/0.5 ml/well in 24 wells plate for 24 hr. The cells were then washed with the PBS, and free PenG or PenG-PL NPs in F12K 0.5% FBS were added. After incubation for 1hr, the cells were washed with PBS, and then disrupted with 0.5 ml ice cold water for 30 min. For PenG-PL NPs treated group, the cells solution was transferred to a 1.5 ml tube, and 0.5 ml CHCl3 was added to disrupt the NPs. The mixture was when centrifuged to separate water/CHCl3 layer, and 100 μl of the CHCl3 layer was diluted with 900 μl methanol for MS measurement. For free PenG treated group, after the cells disruption, 0.5 ml methanol was added. The mixture was then filtered with 0.22 μm membrane and diluted with methanol for further MS measurement.
Intracellular trafficking profile of the nanoparticles in MSSA infected A549 cells:
Intracellular trafficking profile of the nanoparticles in infected A549 cells was determined by the confocal imaging. A549 cells were seeded on cover slips in 6 wells plate at a density of 3×105 cells/2 ml/well and were cultured for 24 hr at 37 degrees. Then, the cells were washed with F12K 0.5% FBS media. MSSA suspension containing approximate 106 bacteria were added to A549 cells at MOI=10 (multiplicity of infection) and incubated with the cells for 2 hr. Next, the cells were washed with F12K containing 0.5% FBS and treated with FITC-labeled PenG-PL NPs for 2hr. A549 cells were then washed with F12K 0.5% FBS three times and incubated with lysotracker and Hoechst33342 at 37 degrees for 15 min. After incubation, the cells were fixed with 4% paraformaldehyde and washed with PBS for confocal imaging (Nikon A1R, Melville, NY).
Statistics:
Data were presented as the mean ± standard deviation. GraphPad Prism 6 (La Jolla, CA) was used to analyze the data. Double-tailed student t-test and one-way ANOVA were used in different experiments to determine the significance among groups. A value of p < 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgements
C.Z. acknowledges the support from the Professor Sylvan G. Frank Graduate Fellowship. Electron microscopy was performed at the Center for Electron Microscopy and Analysis (CEMAS) at The Ohio State University. Confocal images were generated using the instruments and services at the Campus Microscopy and Imaging Facility, The Ohio State University. This facility is supported in part by grant P30 CA016058, National Cancer Institute, Bethesda, MD.
Funding Sources
This work was supported by CAMS Innovation Fund for Medical Sciences (2017-I2M-1-012) for Dr.Xiaofang Chen, and by the Maximizing Investigators’ Research Award 1R35GM119679 from the National Institute of General Medical Sciences as well as the start-up fund from the College of Pharmacy at The Ohio State University for Dr.Yizhou Dong.
Footnotes
Author Contributions
C.Z., X.C., and Y.D. conceived and designed the experiments; C.Z., W.Z., C.B., X.H., D., D.M., and X.C. performed the experiments; C.Z., X.C., and Y.D. analyzed the data; C.Z., X.C., X.H., and Y.D. wrote the paper with edits and comments from all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Wilson JW; Schurr MJ; LeBlanc CL; Ramamurthy R; Buchanan KL; Nickerson CA, Mechanisms of Bacterial Pathogenicity. Postgrad. Med. J 2002, 78, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Bambeke F; Barcia-Macay M; Lemaire S; Tulkens PM, Cellular Pharmacodynamics and Pharmacokinetics of Antibiotics: Current Views and Perspectives. Curr. Opin. Drug Discov. Devel. 2006, 9, 218–230. [PubMed] [Google Scholar]
- 3.McClure EE; Chavez ASO; Shaw DK; Carlyon JA; Ganta RR; Noh SM; Wood DO; Bavoil PM; Brayton KA; Martinez JJ; McBride JW; Valdivia RH; Munderloh UG; Pedra JHF, Engineering of Obligate Intracellular Bacteria: Progress, Challenges and Paradigms. Nat. Rev. Microbiol. 2017, 15, 544–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawes T; Lopez-Lozano JM; Nebot CA; Macartney G; Subbarao-Sharma R; Dare CR; Wares KD; Gould IM, Effects of National Antibiotic Stewardship and Infection Control Strategies on Hospital-Associated and Community-Associated Meticillin-Resistant Staphylococcus aureus Infections across a Region of Scotland: A Non-Linear Time-Series Study. Lancet Infect. Dis. 2015, 15, 1438–1449. [DOI] [PubMed] [Google Scholar]
- 5.Horn J; Stelzner K; Rudel T; Fraunholz M, Inside Job: Staphylococcus aureus Host-Pathogen Interactions. Int. J. Med. Microbiol. 2018, 308, 607–624. [DOI] [PubMed] [Google Scholar]
- 6.Yang D; Wijenayaka AR; Solomon LB; Pederson SM; Findlay DM; Kidd SP; Atkins GJ, Novel Insights into Staphylococcus aureus Deep Bone Infections: the Involvement of Osteocytes. mBio 2018, 9, e00415–e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan NC; Foreman A; Jardeleza C; Douglas R; Vreugde S; Wormald PJ, Intracellular Staphylococcus aureus: the Trojan Horse of Recalcitrant Chronic Rhinosinusitis? Int. Forum Allergy Rhino/. 2013, 3, 261–266. [DOI] [PubMed] [Google Scholar]
- 8.Thwaites GE; Gant V, Are Bloodstream Leukocytes Trojan Horses for the Metastasis of Staphylococcus aureus? Nat. Rev. Microbiol. 2011, 9, 215–222. [DOI] [PubMed] [Google Scholar]
- 9.Garzoni C; Kelley WL, Staphylococcus aureus: New Evidence for Intracellular Persistence. Trends Microbiol. 2009, 17, 59–65. [DOI] [PubMed] [Google Scholar]
- 10.Lother SA; Press N, Once-Daily Treatments for Methicillin-Susceptible Staphylococcus aureus Bacteremia: Are They Good Enough? Curr. infect. Dis. Rep. 2017, 19, 43. [DOI] [PubMed] [Google Scholar]
- 11.Levison ME; Levison JH, Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. infect. Dis. Clin. North Am. 2009, 23, 791–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L; Hu C; Shao L, The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. int. J. Nanomedicine 2017, 12, 1227–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong MH; Bao Y; Yang XZ; Zhu YH; Wang J, Delivery of Antibiotics with Polymeric Particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [DOI] [PubMed] [Google Scholar]
- 14.Liu L; Xu K; Wang H; Tan PK; Fan W; Venkatraman SS; Li L; Yang YY, Self-Assembled Cationic Peptide Nanoparticles as an Efficient Antimicrobial Agent.Nat. Nanotechnol. 2009, 4, 457–463. [DOI] [PubMed] [Google Scholar]
- 15.Lam SJ; O'Brien-Simpson NM; Pantarat N; Sulistio A; Wong EHH; Chen Y-Y; Lenzo JC; Holden JA; Blencowe A; Reynolds EC; Qiao GG, Combating Multidrug-Resistant Gram-Negative Bacteria with Structurally Nanoengineered Antimicrobial Peptide Polymers. Nat. Microbiol. 2016, 1, 16162. [DOI] [PubMed] [Google Scholar]
- 16.Onyeji CO; Nightingale CH; Marangos MN, Enhanced Killing of Methicillin- Resistant Staphylococcus aureus in Human Macrophages by Liposome-Entrapped Vancomycin and Teicoplanin. infection 1994, 22, 338–342. [DOI] [PubMed] [Google Scholar]
- 17.Gao W; Chen Y; Zhang Y; Zhang Q; Zhang L, Nanoparticle-Based Local Antimicrobial Drug Delivery. Adv. Drug Deiiv. Rev. 2018, 127, 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalhapure RS; Suleman N; Mocktar C; Seedat N; Govender T, Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. J Pharm. Sci. 2015, 104, 872–905. [DOI] [PubMed] [Google Scholar]
- 19.Xiong LH; Cui R; Zhang ZL; Yu X; Xie Z; Shi YB; Pang DW, Uniform Fluorescent Nanobioprobes for Pathogen Detection. ACS nan? 2014, 8, 5116–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semiramoth N; Di Meo C; Zouhiri F; Said-Hassane F; Valetti S; Gorges R; Nicolas V; Poupaert JH; Chollet-Martin S; Desmaele D; Gref R; Couvreur P, Self-Assembled Squalenoylated Penicillin Bioconjugates: An Original Approach for the Treatment of Intracellular Infections. ACS nan? 2012, 6, 3820–3831. [DOI] [PubMed] [Google Scholar]
- 21.Xie S; Manuguri S; Proietti G; Romson J; Fu Y; Inge AK; Wu B; Zhang Y; Hall D; Ramstrom O; Yan M, Design and Synthesis of Theranostic Antibiotic Nanodrugs that Display Enhanced Antibacterial Activity and Luminescence. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 8464–8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abed N; Said-Hassane F; Zouhiri F; Mougin J; Nicolas V; Desmaele D; Gref R; Couvreur P, An Efficient System for Intracellular Delivery of Beta-Lactam Antibiotics to Overcome Bacterial Resistance. Sci. Rep. 2015, 5,13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang CX; Zhao WY; Liu L; Ju RJ; Mu LM; Zhao Y; Zeng F; Xie HJ; Yan Y; Lu WL, A Nanostructure of Functional Targeting Epirubicin Liposomes Dually Modified with Aminophenyl Glucose and Cyclic Pentapeptide Used for Brain Glioblastoma Treatment . Oncotarget 2015, 6, 32681–32700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng X; Lee RJ, The Role of Helper Lipids in Lipid Nanoparticles (LNPs) Designed for Oligonucleotide Delivery. Adv. Drug. De/iv. Rev. 2016, 99, 129–137. [DOI] [PubMed] [Google Scholar]
- 25.Dumont M; Villet R; Guirand M; Montembault A; Delair T; Lack S; Barikosky M; Crepet A; Alcouffe P; Laurent F; David L, Processing and Antibacterial Properties of Chitosan-Coated Alginate Fibers. Carbohydr. Potym. 2018, 190, 31–42. [DOI] [PubMed] [Google Scholar]
- 26.Anikin A; Buchynskyy A; Kempin U; Stembera K; Welzel P; & Lantzsch G, Membrane Anchoring and Intervesicle Transfer of a Derivative of the Antibiotic Moenomycin A. Angew Chem. int. Ed. Engl. 1999, 38, 3703–3707. [DOI] [PubMed] [Google Scholar]
- 27.Pei Y; Mohamed MF; Seleem MN; Yeo Y, Particle Engineering for Intracellular Delivery of Vancomycin to Methicillin-Resistant Staphylococcus aureus (MRSA)-Infected Macrophages. J Control. Release. 2017, 267, 133–143. [DOI] [PubMed] [Google Scholar]
- 28.Li J; Cha R; Mou K; Zhao X; Long K; Luo H; Zhou F; Jiang X, Nanocellulose-Based Antibacterial Materials. Adv. Hea/thc. Mater. 2018, e1800334. [DOI] [PubMed] [Google Scholar]
- 29.Lin LC; Chattopadhyay S; Lin JC; Hu CJ, Advances and Opportunities in Nanoparticle- and Nanomaterial-Based Vaccines against Bacterial Infections. Adv. Healthc. Mater. 2018, 7,e1701395. [DOI] [PubMed] [Google Scholar]
- 30.Hao N; Chen X; Jeon S; Yan M, Carbohydrate-Conjugated Hollow Oblate Mesoporous Silica Nanoparticles as Nanoantibiotics to Target Mycobacteria. Adv. Healthc. Mater. 2015, 4, 2797–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mi G; Shi D; Wang M; Webster TJ, Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 2018, 7,e1800103. [DOI] [PubMed] [Google Scholar]
- 32.Kim W; Zhu W; Hendricks GL; Van Tyne D; Steele AD; Keohane CE; Fricke N; Conery AL; Shen S; Pan W; Lee K; Rajamuthiah R; Fuchs BB; Vlahovska PM; Wuest WM; Gilmore MS; Gao H; Ausubel FM; Mylonakis E, A New Class of Synthetic Retinoid Antibiotics Effective against Bacterial Persisters. Nature 2018, 556, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brochado AR; Telzerow A; Bobonis J; Banzhaf M; Mateus A; Selkrig J; Huth E; Bassler S; Zamarreno Beas J; Zietek M; Ng N; Foerster S; Ezraty B; Py B; Barras F; Savitski MM; Bork P; Gottig S; Typas A, Species-Specific Activity of Antibacterial Drug Combinations. Nature 2018, 559, 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang G; Shen X; Gong Y; Dong Z; Zhao X; Shen W; Wang J; Hu F; Peng Y, Antibacterial Properties of Acinetobacter Baumannii Phage Abp1 Endolysin (PlyABI). BMC Infect. Dis. 2014, 14, 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


