Abstract
Background:
Alcohol use causes significant disruption of intestinal microbial communities, yet exactly how these dysbiotic communities interact with the host is unclear. We sought to understand the role of microbial products associated with alcohol-dysbiosis in mice on intestinal permeability and immune activation in an in-vitro model system.
Methods:
Microbiota samples from binge-on-chronic alcohol-fed and pair-fed male and female mice were cultured in Gifu Anaerobic Broth for 24 hours under anaerobic conditions. Live/whole organisms were removed, microbial products were collected, and added to human peripheral blood mononuclear cells (PBMC) or polarized C2BBe1 intestinal epithelial monolayers. Following stimulation, transepithelial electrical resistance (TEER) was measured using a volt/ohm meter and immune activation of PBMC was assessed via flow cytometry.
Results:
Microbial products from male and female alcohol-fed mice significantly decreased TEER (mean percentage change from baseline alcohol-fed 0.86 Ω/cm2 v. pair-fed 1.10 Ω/cm2) compared to microbial products from control mice. Following ex-vivo stimulation immune activation of PBMC was assessed via flow cytometry. We found that microbial products from alcohol-fed mice significantly increased the percentage of CD38+ CD4+ (mean alcohol-fed 17.32%+0.683% SD v. mean pair-fed 14.2%+1.21% SD, P<0.05) and CD8+ (mean alcohol-fed 20.28%+0.88% SD v. mean pair-fed 12.58%+3.59% SD, P<0.05) T-cells.
Conclusions:
Collectively, these data suggest that microbial products contribute to immune activation and intestinal permeability associated with alcohol dysbiosis. Further, utilization of these ex-vivo microbial product assays will allow us to rapidly assess the impact of microbial products on intestinal permeability and immune activation and to identify probiotic therapies to ameliorate these defects.
Keywords: Alcohol, dysbiosis, microbial products, immune activation, intestinal permeability
Introduction:
Chronic alcohol ingestion leads to small and large intestinal bacterial overgrowth and dysbiosis in animals and humans.(Bode, Bode, Heidelbach, Durr, & Martini, 1984; Casafont Morencos et al., 1996; Engen, Green, Voigt, Forsyth, & Keshavarzian, 2015; Hartmann et al., 2013; Yan et al., 2011) The increase in both aerobic and anaerobic bacteria is most pronounced in the proximal small intestine in alcohol-fed mice,(Yan et al., 2011) but also extends to the large intestine as early as 1 week after alcohol feeding.(Hartmann et al., 2013) Metabolomics studies in rodents and humans have demonstrated that chronic alcohol administration results in marked alterations to intestinal metabolites, which suggest that functional changes to the intestinal microbiota occur in conjunction with the phylogenetic changes associated with chronic alcohol consumption. Chronic ethanol administration to rats over an 8 week period results in a marked reduction in amino acid metabolism, as well as perturbations of steroid, lipid, carnitine(Xie et al., 2013a), and bile acid metabolism.(Xie et al., 2013b) Additionally, 1-nonanol, hexane, and styrene metabolites were only found in alcohol using subjects but not in controls. In contrast, volatile organic compounds (e.g., 2-methyl-1-butanol) could only be detectable in healthy humans but not in alcohol-dependent patients.(Leclercq et al., 2014) Further, intestinal levels of short-chain fatty acids (SCFAs) are lower after ethanol administration except for levels of acetic acid, which is generated by ethanol metabolism.(Xie et al., 2013a) Ethanol also directly decreases the biosynthesis of saturated long-chain fatty acids (LCFAs) by gut microbes. Consequently, intestinal LCFAs levels are reduced after alcohol feeding.(Chen, Torralba, et al., 2015; Ronis, Korourian, Zipperman, Hakkak, & Badger, 2004)
The intestinal tract occupies a central location at the intersection between alcohol consumption, the microbiota, and immune system homeostasis. Alcohol use increases intestinal permeability and translocation of bacterial components with associated local and systemic inflammation.(Leclercq et al., 2014) The alcohol-associated increases in intestinal permeability and inflammation are, in part, mediated by the intestinal microbial communities and epithelial TNF-α signaling, as treatment with non-absorbable antibiotics or knockout of the TNF-α receptor mitigate alcohol induced intestinal permeability and inflammation.(Adachi, Moore, Bradford, Gao, & Thurman, 1995; Chen, Starkel, Turner, Ho, & Schnabl, 2015) Ethanol also has a wide range of immunologic effects (Cook, 1998), including increased T-cell activation.(Cook et al., 1995; Cook et al., 1991; Cook et al., 1994; Song et al., 2002) However, chronic alcohol feeding decreases the total number of circulating lymphocytes (Geissler, Gesien, & Wands, 1997), which suggests that alcohol affects both the number and function of immune cells. Similarly, our recent work demonstrated that mice recolonized with microbiota from alcohol-fed mice had increased intestinal permeability and increased numbers of intestinal immune cells when compared to mice recolonized with control pair-fed microbiota.(Samuelson et al., 2017) These data suggest that the alcohol-associated changes to the intestinal microbiota contribute to impaired intestinal permeability, inflammation, and altered immune regulation. However, the direct contribution of specific alcohol-associated microbial products (bacterial components and/or metabolites) to intestinal barrier function and CD4+ and CD8+ T-cell immune activation, independent of the direct effects of alcohol consumption, have not been examined. We hypothesized that intestinal microbial products from alcohol-fed animals would increase intestinal permeability and increase T-cell immune activation. Specifically, we sought to establish the link between alcohol-associated microbial products and intestinal permeability and immune activation.
Materials and Methods:
Mice:
Female and male 8 to 10 week old C57BL/6 mice were obtained from Charles Rivers Breeding Laboratories (Wilmington, MA) and maintained in a temperature controlled room for two days prior to experimental manipulation. Animals remained in the animal care facility at Louisiana State University Health Sciences Center (LSUHSC) throughout the experiment. The LSUHSC Institutional Animal Care and Use Committee approved all experiments.
Binge-on-chronic alcohol model:
We have adapted and modified the chronic-binge alcohol model (Bertola, Mathews, Ki, Wang, & Gao, 2013) to generate alcohol-dysbiotic microbiota and pair-fed microbiota for microbial products, as described in our recent publication (Samuelson et al., 2017). Briefly, mice were acclimated to liquid diet for 5 days using Lieber-DeCarli ‘82 Shake and Pour control liquid diet. Groups of mice (n = 1–2 per cage) were ten randomized into ethanol fed (Lieber-DeCarli ‘82 Shake and Pour 5% vol/vol ethanol liquid diet) or pair-fed groups (control-liquid diet). Pair-fed mice were on control-liquid diet adjusted daily according to the consumption of ethanol-fed mice. Alcohol-fed mice were administered 4 g kg−1 (24.03% vol/vol) ethanol by gavage (binge) following 5 days of chronic-ethanol consumption. Alcohol-fed mice were maintained for an additional 5 days on the 5% ethanol diet and on day 10, mice received a second and final ethanol binge (4 g kg−1). Pair-fed control mice were gavaged with 9 g kg−1 (45% wt/vol) maltose dextrin on days 5 and 10. Mice achieved blood alcohol concentrations of ~200 mg/dL during the chronic alcohol consumption and ~400 mg/dL following binge alcohol administration (data not shown). Weight gain and daily food intake was similar in alcohol- and pair-fed mice (data not shown).
Ex-vivo Microbial Product Generation:
Cecal and colonic microbiota from alcohol-fed and pair-fed male and female mice were collected, homogenized in sterile PBS (1:2, weight/volume), and then cultured in Gifu Anaerobic Broth (diluted 1:20) for 24 hours under anaerobic conditions. The anaerobic environment was generated using the AnaeroPack System (Mitsubishi Gas Chemical Co., Japan) and GasPas EZ Anaerobe Gas Generating Pouch System with Indicator (BD, Sparks, MD). Microbial products (i.e., metabolites, bacterial components, and remaining culture media constituents) were collected by the removal of live and/or whole organisms by a two-step process of centrifugation (10,000 × g for 10 min) followed by filtration through a 0.22 μm membrane filter. Microbial products were then used immediately or stored at −80oC for later use. Our experimental schema for testing the microbial products is shown in Figure 1. Microbial products were used at 20% (vol/vol) in all in vitro experiments.
Figure 1: Schematic outline of the experimental protocol used in this study.
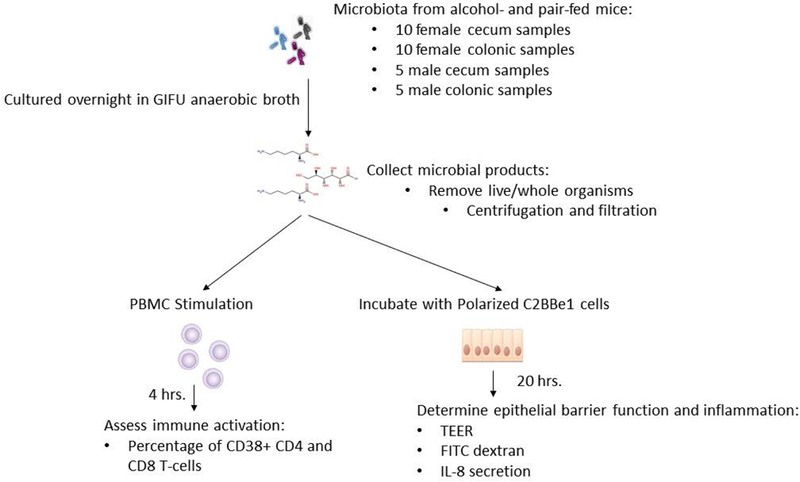
Cecal and colonic microbiota from alcohol-fed and pair-fed male and female mice were collected, homogenized in sterile PBS and cultured in Gifu Anaerobic for 24 hours under anaerobic conditions. Microbial products were collected by the removal of live and/or whole organisms. Microbial products were then used to assess intestinal permeability and inflammation, as well as peripheral immune activation.
Collection of in vivo microbial products:
Colonic microbial products from alcohol-fed and pair-fed male and female mice were collected, homogenized in sterile PBS (1:2, weight/volume) and the microbial products were isolated by the removal of live and/or whole organisms by centrifugation (10,000 × g for 10 min) and filtration through a 0.22 μm membrane filter.
DNA sequencing of the 16S rRNA gene:
16S rRNA gene sequencing was performed by The Louisiana State University School of Medicine Microbial Genomics Resource Group (http://metagenomics.lsuhsc.edu/mgrg), as described previously (Samuelson, Burnham, et al., 2018).
Sequence curation and analysis:
Analysis and curation of 16S sequencing reads were performed using R and the following R packages: DADA2 v1.1.5, Phyloseq v1.16.2, DESeq2 v1.20.0, and vegan v2.3–5 (Anders & Huber, 2010; Callahan et al., 2016; McMurdie & Holmes, 2012, 2014; Oksanen, 2007), as described elsewhere (Samuelson, Siggins, et al., 2018).
Data Repository:
16S rRNA gene sequencing data (accession number PRJNA540293) is deposited in the National Center for Biotechnology Information Sequence Read Archive.
Peripheral Blood Mononuclear Cell (PBMC) Immune activation:
Human leukapheresis products were purchased from the local blood bank, PBMCs were then isolated using ficoll gradient centrifugation and cryopreserved. One million PBMCs were added to each well of a 96 well plate and cultured for 24 hours in RPMI1640 containing 10% FBS and penicillin/streptomycin. Following overnight recovery, the media was replaced with 150 μL of fresh RPMI and PBMCs were stimulated with 50 μL cecal and colonic microbial products from alcohol-fed and pair-fed mice (male and female). PBMCs were stimulated for 4 hours and immune activation was assessed via polychromatic flow cytometry.
Staining and Flow Cytometry:
Stimulated human PBMCs were stained with the eFlour780 Fixable live/dead cell stain (Invitrogen Eugene, OR) followed by immunological staining with various combinations of fluorochrome-conjugated Abs specific for human CD45, CD3e, CD4, CD8a, and CD38 (BioLegend) suspended in BD Brilliant Stain Buffer at pre-determined concentrations for 30 min at 4°C. Cells were then washed with staining buffer and fixed with PBS + 1% formalin. For all experiments, cells were acquired using an LSR II flow cytometer (BD Biosciences, San Jose, CA), and analyses were performed using FlowJo software Version 10.5.3 (Tree Star, Ashland, OR).
Transepithelial Electrical Resistance (TEER):
Cell lines were obtained from the American Type Culture Collection and used at a passage number ≤13 for all experiments. C2BBe1 human colorectal adenocarcinoma cells (CRL-2012) were maintained in DMEM (Gibco) containing 10 μg/mL human transferrin (Sigma) and 10% (vol/vol) heat-inactivated FBS (Gibco). Polarized monolayers were established by seeding 2 × 105 C2BBe1 cells on BIOCOAT fibrillar collagen 24-well inserts with a 1-μm membrane pore size (Becton Dickinson). Cells were incubated in medium for a total of 3 d, replaced with fresh medium each day, until the transepithelial electrical resistance (TEER) was ≥250 Ω/cm2, as measured using a Millicell ERS-2 Voltohmmeter (Millipore). Polarized monolayers were stimulated apically with 100 μL of cecal or colonic microbial products from alcohol-fed and pair-fed mice (male and female) for 20 hours, with measurements taken every 1 hour for the first 6 hours. Results are expressed as the percent change from baseline.
Dextran Translocation:
FITC-dextran (3,000 kDa) and Texas Red-dextran (40,000 kDa) were added to the apical chamber of polarized C2BBe1 cells. Thirty-minutes following the addition of dextran molecules the levels of FITC-dextran and Texas Red-dextran in the basal lateral chamber of polarized C2BBe1 monolayers treated with microbial products was measured via fluorescent plate reader.
IL-8 and i-FABP ELISA:
The levels of i-FABP (R&D Systems Minneapolis, MN, Cat#DFBP20) and IL-8 (R&D Systems, Cat#D8000C) in the culture supernatant of C2BBe1 cells treated with microbial products were assessed via ELISA according to manufactures’ instructions.
MTT Assay:
Cellular viability was determined using the MTT reagent (ThermoFisher, Cat#M6494) according to manufactures’ instructions.
Statistical Analysis:
Statistical analyses were performed using GraphPad Prism 5 (La Jolla, CA. USA), as well as the R package vegan v2.3–5 (Oksanen, 2007). Results are shown as the mean ± S.E.M and significance was measured at a p < 0.05 and an FDR q-value < 0.1. Specific statistical analyses are indicated in the figure legends. Briefly, for in-vitro assays statistical significance were assessed using a two-way ANOVA with Sidak’s multiple comparisons test or by using a three-way ANOVA. The statistical significance of the different microbiome measurements was assessed as follows. Alpha diversity (observed sample richness) was assessed using generalized linear model. Beta-diversity analysis was performed using a distance-based redundancy analysis (dbRDA) on sample-wise Bray-Curtis dissimilarity distances. Significance was then inferred via permutational multivariate analysis of variance with corrections for multiple comparison via False Discovery Rate (FDR) (Benjamini, 1995). Finally, differentially abundant OTUs between groups was determined via DESeq2.
Results:
Binge-on-chronic alcohol feeding alters the intestinal microbial communities:
We assessed the composition of the intestinal microbial communities in binge-on-chronic alcohol consuming male and female mice. Binge-on-chronic alcohol feeding resulted in changes to the microbial alpha (α)-diversity characterized by a reduction (q = 0.048) in the number of observed species in alcohol-fed male mice compared to pair-fed male mice in the cecal microbiota (Figure 2A). However, α-diversity between alcohol-fed and pair-fed mice was not significantly different in male colonic microbiota samples or in either cecal or colonic female microbiota (figure 2A). Beta (β)-diversity of the microbial communities from alcohol-fed and pair-fed male and female mice was also assessed. We found a significant difference in microbiota β-diversity between; 1) alcohol-fed mice compared to pair-fed mice (p = 0.0001), 2) male mice and female mice (p = 0.0001), and 3) a significant interaction between sex and alcohol consumption (p = 0.0009), as determined by the distance-based redundancy analysis (dbRDA) and permutational multivariate analysis of variance analysis via vegan (Figure 2B). However, no significant difference (p = 0.0710) was observed between microbiota sampling site (Figure 2B). We then examined changes in the relative abundance of specific operational taxonomic units (OTUs) using a negative binomial mixture model via DESeq2. Significant changes in the relative abundance of specific OTUs were seen between alcohol-fed and pair-fed cecal (Figure 3A) and colonic samples (Figure 3B) from male mice, as well as, alcohol-fed and pair-fed cecal (Figure 3C) and colonic samples (Figure 3D) from female mice. In addition, changes in the relative abundance of specific OTUs were seen between alcohol-fed male and female mice in both cecal (Figure 4A) and colonic (Figure 4B) samples. Significant changes were also seen between male and female pair-fed cecal (Figure 4C) and colonic (Figure 4D) samples. Weight gain and daily food intake in both male and female mice did not differ between the alcohol- and pair-fed male or female mice or between sexes (Supplemental Figure 1). We then sought to examine the effects of the alcohol-associated microbial products on intestinal permeability and inflammation, as well as peripheral immune activation, using an ex-vivo microbial products stimulation assay.
Figure 2: Binge-on-chronic alcohol feeding alters the intestinal microbial community structure.

Alpha- and beta-diversity of cecal and colonic microbial communities from alcohol-fed and pair-fed mice were assessed using R. (A) Significant differences in α-diversity of the male cecal microbial communities were seen between alcohol-fed mice compared to pair-fed mice. No significance diffrences in α-diversity were seen between alcohol-fed and pair-fed male colonic, or female cecal and colonic microbial communitie in mice (B) Significant differences in β-diversity were seen between; alcohol-fed and pair-fed mice, as well as between male and female mice. No significant difference was observed between the microbial communities in the cecum and colon. Alpha-diversity was determined by multiple general linear regression and beta-diversity was determined by permutational multivariate analysis of variance within R. N=5–10.
Figure 3: Binge-on-chronic alcohol feeding alters the realative abundance of specific bacterial taxa.

Differentially abundant OTUs was assessed using R and DESeq2. (A) Alcohol-feeding alters the relative abundance of specific OTUs in the male cecal microbiota compared to pair-fed mice. (B) Alcohol-feeding alters the relative abundance of specific OTUs in the male colonic microbiota compared to pair-fed mice. (C) Alcohol-feeding alters the relative abundance of specific OTUs in the female cecal microbiota compared to pair-fed mice. (D) Alcohol-feeding alters the relative abundance of specific OTUs in the female colonic microbiota compared to pair-fed mice. Differentially abundant taxa was determined via negative binomial mixture model using DESeq2 and only statistically significant OTUs were plotted. OTUs with a positive log2 fold change indicates that the OTU (genus) is more abundant in the pair-fed animals, while OTUs with a negative log2 fold change indicate that the OTU (genus) is more abundant in the alcohol-fed animals. N=5–10. Each dot represents a unique differentially abundant OTU.
Figure 4: Sex alters the relative abundance of specific bacterial taxa in alchol-fed and pair-fed mice.
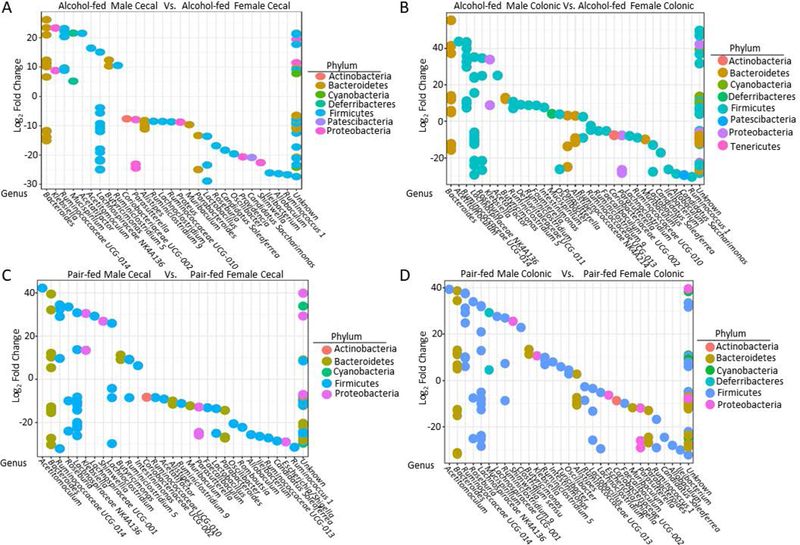
Differentially abundant OTUs was assessed using R and DESeq2 (A) Sex alters the relative abundance of specific OTUs in the alcohol-fed cecal microbiota. (B) Sex alters the relative abundance of specific OTUs in the alcohol-fed colonic microbiota. (C) Sex alters the relative abundance of specific OTUs in the pair-fed cecal microbiota. (D) Sex alters the relative abundance of specific OTUs in the pair-fed colonic microbiota. Differentially abundant taxa was determined via negative binomial mixture model using DESeq2. (A and B) OTUs with a positive log2 fold change indicates that the OTU (genus) is more abundant in the microbial communities from female alcohol-fed mice, while OTUs with a negative log2 fold change indicate that the OTU (genus) is more abundant in microbial communities from male alcohol-fed mice. (C and D) OTUs with a positive log2 fold change indicates that the OTU (genus) is more abundant in the microbial communities from female pair-fed mice, while OTUs with a negative log2 fold change indicate that the OTU (genus) is more abundant in microbial communities from male pair-fed mice. N=5–10. Each dot represents a unique differentially abundant OTU.
Alcohol-associated microbial products increase permeability of polarized intestinal epithelial cells:
We examined the effects of alcohol-associated intestinal microbial products on intestinal permeability. Polarized C2BBe1 were treated apically with microbial products from cecal or colonic alcohol-associated microbial products for 20 hours. TEER measurements were taken every hour for the first 6 hours. Both alcohol-associated cecal and colonic microbial products significantly decreased TEER (fold change from baseline) 20 hrs. post treatment (Figures 5A and 5B). In addition, both alcohol-associated cecal and colonic microbial products decreased TEER significantly over the 20-hr. time course (Supplemental Figure 2A and 2B, respectively). Specifically, changes in TEER were significantly associated with; 1) time, 2) sex of the mouse, and 3) alcohol consumption, via repeated measures 3-way ANOVA. In addition, there was a significant interaction of time and sex, as well as time and alcohol consumption, but not between sex and alcohol consumption.
Figure 5: Transepithelial electrical resistance (TEER) of polarized intestinal epithlieal cells decreases following treatment with alcohol-associated microbial products.
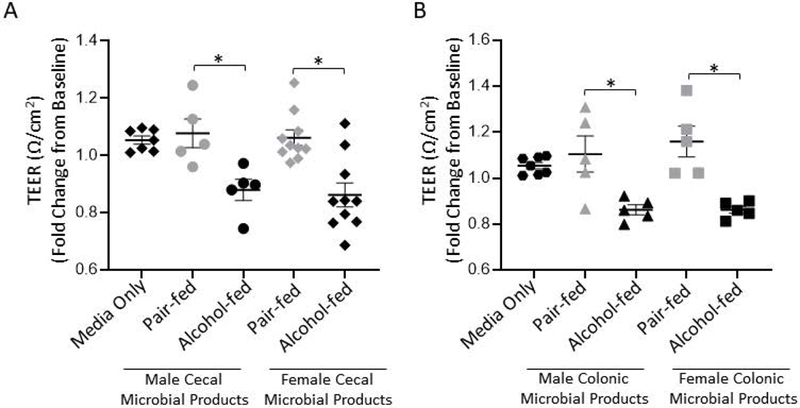
The effect of microbial products on polarized epitheilal TEER was deterimed via a volt/ohm meter. (A) Cecal microbial products from alcohol or pair-fed microbiota harvested from male and female mice were added to the apical chamber of polarized C2BBe1 cells for a total of 20 hrs. and TEER was assessed 20 hrs. post stimulation. (B) Colonic microbial products from alcohol or pair-fed microbiota collected from male and female mice were added to the apical chamber of polarized C2BBe1 cells for a total of 20 hrs. and TEER was assessed 20 hrs. post stimulation. N=5–10, repeated 3x. *indicates P<0.05, by 2-way ANOVA with Sidak’s multiple comparisons test. The same media only controls are shown in panel (A and B) for visualization, however all samples were ran at the same time.
Polarized C2BBe1 were treated with microbial products from cecal or colonic alcohol-associated microbial products and intestinal permeability assessed by dextran translocation from apical to the basolateral wells. Two sizes of dextran molecules were used to assess permeability through the polarized intestinal epithelial cells. Alcohol-associated cecal or colonic microbial products significantly increased translocation of 3,000-kDa dextran to the basolateral surface (Figure 6A and Figure 6B, respectively). Similarly, alcohol-associated cecal or colonic microbial products significantly increased translocation of 40,000-kDa dextran to the basolateral surface (Figure 6C and Figure 6D, respectively).
Figure 6: Treatment with alcohol-associated microbial products increased translocation of dextran to the basal lateral surface of polarized intestinal epithlieal cells.

Microbial products from alcohol or pair-fed intestinal samples harvested from male and female mice were added to the apical chamber of polarized C2BBe1 cells for a total of 20 hrs. and dextran translocation was assessed. Male and female alcohol-associated (A) cecal and (B) colonic microbial products increase translocation of a 3,000 kDa FITC-dextran to the basal lateral surface. Male and female alcohol-associated (C) cecal and (D) colonic microbial products increase translocation of a 40,000 kDa Texas Red-dextran to the basal lateral surface via a fluorescence spectroscopy. N=5–10, repeated 3x. *indicates P < 0.05, by 2-way ANOVA with Sidak’s multiple comparisons test. The same media only controls are shown in panel (A and B) and in panel (C and D) for visualization, however all samples were ran on the same plate.
We confirmed cellular viability of the C2BBe1 monolayers following treatment with cecal and colonic microbial products. First, we assessed trypan blue staining of epithelial cells following treatment. No trypan blue staining was seen following treatment with microbial products (data not shown). In addition, we assessed cell viability using MTT with different doses of microbial products (10% - 30% microbial products). We found no significant differences in cellular viability following treatment with microbial products (Supplemental Figure 3). We further assessed the levels of soluble i-FABP following treatment with microbial products. I-FABP is an intracellular protein expressed in the epithelial cells of the mucosal layer of the small and large intestine tissue, which is released from enterocytes when intestinal mucosal damage occurs.(Voth et al., 2017) We found no difference in the levels of soluble i-FABP following treatment with either cecal or colonic microbial products (Supplemental Figure 4).
Alcohol-associated microbial products increase basolateral secretion of IL-8 from polarized intestinal epithelial cells:
We examined the effects of alcohol-associated intestinal microbial products on intestinal inflammation. Polarized C2BBe1 were treated apically with microbial products from cecal or colonic alcohol-associated microbiota for 20 hours and the levels of IL-8 were assessed in the apical and basolateral chambers. Treatment with male cecal and colonic pair-fed microbial products increased the levels of IL-8 in the apical chamber (Figure 7A and 7B, respectively). In contrast, no differences were observed in apical secretion of IL-8 in cells treated with female cecal or colonic microbial products (Figure 7A and 7B, respectively), as determined by 2-way ANOVA with Sidak’s multiple comparisons test. No changes in basolateral secretion of IL-8 were seen following treatment with either male or female cecal microbial products (Figure 7C). However, male colonic alcohol-associated microbial products, as well as female colonic alcohol-associated microbial products increased the levels of IL-8 in the basolateral chamber (Figure 7D), via 2-way ANOVA with Sidak’s multiple comparisons test.
Figure 7: Treatment with alcohol-associated microbial products increased secretion of IL-8 from polarized intestinal epithlieal cells.
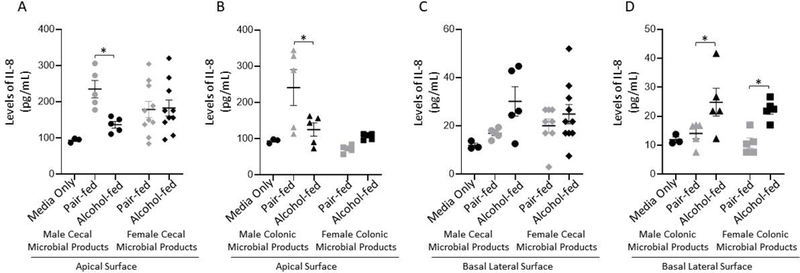
Microbial products from alcohol or pair-fed intestinal samples harvested from male and female mice were added to the apical chamber of polarized C2BBe1 cells for a total of 20 hrs. and IL-8 levels were assessed (A) IL-8 levels in the apical chamber culture supernatant following treatment with cecal microbial products from alcohol or pair-fed mice. (B) IL-8 levels in the apical chamber culture supernatant following treatment with colonic microbial products. (C) IL-8 levels in the basal lateral chamber culture supernatant following treatment with cecal microbial products from alcohol or pair-fed mice. (D) IL-8 levels in the basal lateral chambers culture supernatant following treatment with colonic microbial products. N=5–10, repeated 3x. *indicates P < 0.05, 2-way ANOVA with Sidak’s multiple comparisons test. The same media only controls are shown in panel (A and B) and in panel (C and D) for visualization, however all samples were ran on the same plate.
Ex-vivo generated alcohol-associated microbial products increase T-cell immune activation:
We next examined the effects of alcohol-associated intestinal microbial products on peripheral immune activation. Healthy human PMBCs were treated with microbial products from cecal or colonic alcohol-associated microbiota for 4 hours and CD4+ and CD8+ T-cell immune activation was determined. We defined immune activation for this assay as the percentage of CD4+ or CD8+ T-cells that express the immune activation marker CD38. We found that male and female alcohol-associated cecal microbial products increased the number of CD38+ CD4+ T-cells (Figure 8A) and CD38+ CD8+ T-cells (Figure 8B) compared to PBMCs treated with pair-fed microbial products. Further, male and female alcohol-associated colonic microbial products also increased the number of CD38+ CD4+ T-cells (Figure 8C) and CD38+ CD8+ T-cells (Figure 8D) compared to PBMCs treated with pair-fed microbial products. In addition, we found that alcohol-associated cecal and colonic microbial products from male mice caused more immune activation compared to alcohol-associated cecal and colonic microbial products from female mice (Figure 8A–D), via 2-way ANOVA with Sidak’s multiple comparisons test.
Figure 8: Alcohol-associated microbial products increase PBMC immune activation.
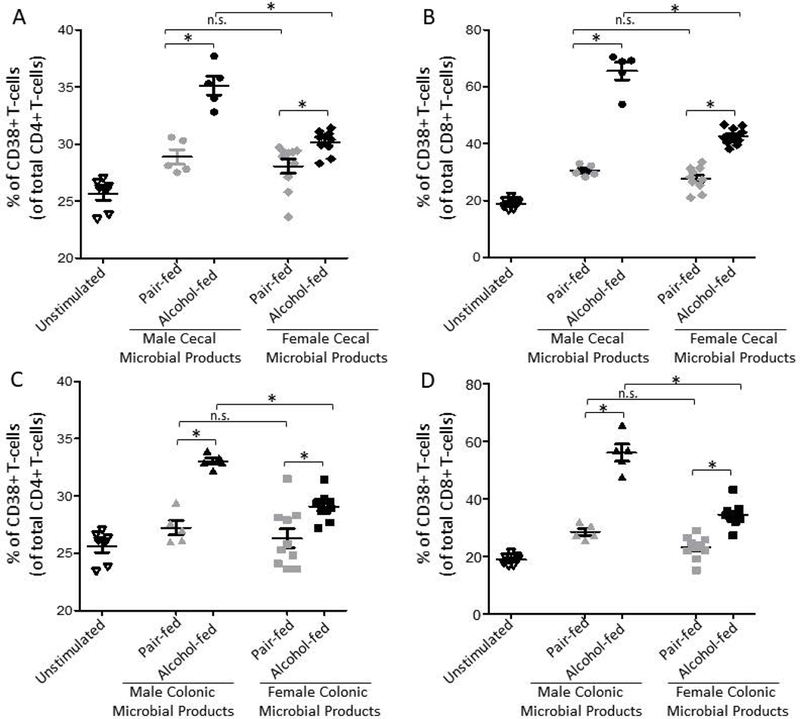
Cecal microbial products from alcohol or pair-fed intestinal samples harvested from male and female mice were added to normal PBMCs for 4 hrs. and immune activation the percentage of (A) CD38+ CD4+ T-cells and (B) CD38+ CD8+ T-cells via a flow cytometry. Colonic microbial products from alcohol or pair-fed intestinal samples collected from male and female mice were added to normal PBMCs for 4 hrs. and immune activation the percentage of (C) CD38+ CD4+ T-cells and (D) CD38+ CD8+ T-cells via a flow cytometry. N=5–10, repeated 3x. *indicates P < 0.05, by 2-way ANOVA with Sidak’s multiple comparisons test. The same unstimulated controls are shown in panel (A and C) and in panel (B and D) for visualization, however all samples were ran at the same time.
In vivo alcohol-associated microbial products increase T-cell immune activation:
As the intestinal microbiota samples were grown in anaerobic culture media the bacterial products that are present in the supernatant may only represent a fraction of the total functional capacity of the in vivo intestinal microbiota. To investigate the differences between in vivo and ex-vivo conditions we performed an additional assay to assess the effects alcohol-associated intestinal microbial products on peripheral immune activation, without the intermediate step of culture. Specifically, in vivo collected microbial products (colonic supernatants collected from colonic content homogenized in sterile PBS, followed by filtration and centrifugation) were added to human PBMCs for 4 hours and CD4+ and CD8+ T-cell immune activation was assessed. We found that male and female alcohol-associated colonic microbial products significantly increased the number of CD38+ CD4+ T-cells (Figure 9A) and CD38+ CD8+ T-cells (Figure 9B) compared to PBMCs treated with pair-fed microbial products, by 2-way ANOVA with Sidak’s multiple comparisons test. However, no sex effect was observed using the in vivo collected microbial products.
Figure 9: In vivo alcohol-associated microbial products increase PBMC immune activation.

In vivo collected colonic microbial products from alcohol or pair-fed intestinal samples collected from male and female mice were added to normal PBMCs for 4 hrs. and immune activation the percentage of (A) CD38+ CD4+ T-cells and (B) CD38+ CD8+ T-cells via a flow cytometry. N=5, repeated 2x. *indicates P < 0.05, by 2-way ANOVA with Sidak’s multiple comparisons test.
Discussion:
The results from this study demonstrate that alcohol-associated microbial products contribute to impaired intestinal barrier function and inflammation, as well as peripheral immune activation. Specifically, microbial products from alcohol-fed mice significantly decreased TEER of polarized C2BBE1 monolayers compared to microbial products from control mice. In addition, microbial products from alcohol-fed mice significantly increased the percentage of CD38+ CD4+ and CD8+ T-cells compared to microbial products from pair-fed mice.
Considerable work has detailed the deleterious effects of alcohol-consumption on the intestinal microbiota, and the intestinal track. For example, in experimental animal models, chronic alcohol consumption leads to marked intestinal dysbiosis and increases intestinal permeability.(Bode et al., 1984; Bull-Otterson et al., 2013; Casafont Morencos et al., 1996; Engen et al., 2015) Specifically, alcohol-consumption increases intestinal permeability and inflammation through alterations to the intestinal microbial communities and epithelial TNF-α signaling.(Chen, Starkel, et al., 2015) In addition, we and others have investigated the role of alcohol-associated intestinal dysbiosis on intestinal permeability, independent of the direct effects of alcohol. We found that mice recolonized with an alcohol-dysbiotic microbiota exhibited increased levels of intestinal fatty-acid binding protein, a biomarker of intestinal barrier damage, compared to mice recolonized with pair-fed microbiota.(Samuelson et al., 2017) Similarly, conventionalization of germ-free mice with intestinal contents from alcohol-fed mice induces inflammation in the small intestine.(Canesso et al., 2014) In line with these studies, we found that polarized C2BBe2 cells treated with alcohol-associated microbial products had increased intestinal permeability, as measured by a decrease in TEER and increased levels of dextran in the basolateral compartment. While the microbial composition of male and female mice were different the effects of the microbial products on TEER were the same, suggesting that while the microbial community structure changed; the biological function of these communities on TEER may be an alcohol-dependent effect, independent of sex. In addition, we found that polarized C2BBe2 cells treated with alcohol-associated microbial products had higher levels of IL-8 secretion into the basolateral chamber of the transwells. In contrast, cells treated with control microbial products had higher levels of apical IL-8 secretion. The mechanism for alcohol-associated directional secretion of IL-8 is unclear but may represent the host’s response to a potential pathogenic insult through the recruitment of neutrophils to the sub mucosa. Consistent with our studies, acute-on-chronic alcohol feeding has been shown to significantly increase the expression of the proinflammatory cytokines TNF-α, Mcp1, Hmgb1, Il-17, and Il-23 in the intestine, and antibiotic treatment attenuated the expression of the alcohol-induced proinflammatory cytokines in the intestinal track.(Lowe et al., 2018) Our data along with the published literature suggest that microbiota-derived signals contribute to intestinal inflammation following alcohol exposure.
Humans and animals chronically exposed to excess ethanol have a range of immunologic alterations (Cook, 1998), including persistent increases in T-cell activation.(Cook et al., 1995; Cook et al., 1991; Cook et al., 1994; Song et al., 2002) The activated T cells have features of effector cells and produce a rapid burst of interferon-gamma (IFN-γ) upon stimulation of the T-cell receptor.(Song et al., 2001; Song et al., 2002) We have previously shown that mice recolonized with an alcohol-dysbiotic microbiota exhibit increased levels of intestinal CD4+ and CD8+ T-cells, as well as an increased number of effector CD8+ T-cells compared to mice recolonized with pair-fed microbiota.(Samuelson et al., 2017) We here further investigate the role of intestinal dysbiosis on peripheral immune activation by examining the role of alcohol-associated microbial products on immune activation. We found that alcohol-associated cecal or colonic microbial products from male and female mice increased the number of CD38+ CD4+ T-cells and CD38+ CD8+ T-cells compared to PBMCs treated with pair-fed microbial products. Our results are similar to those recently published examining mucosal associated invariant T-cells (MAIT) cells in liver disease due to alcohol consumption.(Riva et al., 2018) This study demonstrated that intestinal bacterial antigens and metabolites from patients with alcoholic liver disease differentially alter the expression of the MAIT cell activation markers CD69 and HLA-DR. Specifically, normal PBMCs treated with fecal bacterial antigens from patients with alcoholic liver disease exhibited increase HLA-DR induction and decreased CD69 expression.(Riva et al., 2018) These data along with our current study suggest that alcohol-associated microbial products contribute to the immune cell dysfunction observed following chronic alcohol consumption. Additionally, we found that alcohol-associated cecal and colonic microbial products from male mice caused increased immune activation compared to alcohol-associated cecal and colonic microbial products from female mice. To our knowledge, this is the first time an alcohol-associated microbiota mediated effect has been reported to be sex-specific. These data also suggest that the biological role of sex should be examined when investigating the role of the intestinal microbiota on alcohol-associated health outcomes.
There are several limitations to our current study. First, we microbiota samples were grown in anaerobic culture media, which will select for culturable organisms. Thus, the bacterial products that are present in the supernatant may only represent a fraction of the total capacity of the intestinal microbiota. However, we performed an additional assay were in vivo microbial products (supernatants collected from colonic content homogenized in sterile PBS, followed by filtration and centrifugation) were added to human PBMCs to assess the effects of microbial products from alcohol-fed mice that are directly harvested from the colonic content without the intermediate step of culture. We found very similar results to those of the cultured supernatants; however, the sex affect was not statistically different in these experiments. Second, the addition of mouse microbial products to human immune and epithelial cells represent a potential limitation. As human cells are most likely reacting to novel components, which may not account for mucosal immune tolerance that typically develops to the intestinal microbiota or for the potential of antigen-specific activation. However, given that we still observed a differential response between alcohol-associated microbial products and control microbial products we believe that these model systems represent a practical approach to screen microbial products associated with alcohol consumption for biological effects. Finally, while we did observe sex differences between male and female microbial products on peripheral immune activation, we have only used cells (PBMCs and C2BBE1) from male donors. It is unknown if sex of the donor cell matters when stimulated with microbial products. Similarly, as male and female mice were not littermates there is a possibility that unknown environmental factors contribute to the observed differences in the microbial communities independent of sex. However, sex dependent microbial differences have been reported in both littermate and non-littermate animals.(Liang, Bushman, & FitzGerald, 2015; Org et al., 2016) We sought to minimize these effects by obtaining all of the mice from the same vendor at the same time, as well as adapting them to our animal care facility for 1 week prior to experimental manipulation. Diets were also the same between male and female mice.
In summary, these data indicate that microbial products contribute to immune activation and intestinal permeability and inflammation associated with alcohol dysbiosis. These data further suggest that functional changes in the intestinal microbiota may be more biologically relevant than the phylogenetic shifts in community structure observed with alcohol use.
Supplementary Material
Acknowledgments
Funding: This work was supported by The National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center grant #U54-GM104940, and by The National Institute on Alcohol Abuse and Alcoholism grants #P60-AA009803, and #K99-AA026336. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
Works Cited:
- Adachi Y, Moore LE, Bradford BU, Gao W, & Thurman RG (1995). Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology, 108(1), 218–224. [DOI] [PubMed] [Google Scholar]
- Anders S, & Huber W (2010). Differential expression analysis for sequence count data. Genome Biol, 11(10), R106. doi: 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Yosef. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing on JSTOR. Journal of the Royal Statistical Society. Series B, 57(1), 289–300. [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, & Gao B (2013). Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc, 8(3), 627–637. doi: 10.1038/nprot.2013.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode JC, Bode C, Heidelbach R, Durr HK, & Martini GA (1984). Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology, 31(1), 30–34. [PubMed] [Google Scholar]
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, Kong M, Barker D, McClain C, Barve S. Barve S (2013). Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One, 8(1), e53028. doi: 10.1371/journal.pone.0053028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, & Holmes SP (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods, 13(7), 581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canesso MCC, Lacerda NL, Ferreira CM, Gonçalves JL, Almeida D, Gamba C, Cassali G, Pedroso SH, Moreira C, Martins FS, Nicoli JR, Teixeira MM, Godard ALB Vieira AT. (2014). Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiology, 14(1), 240. doi: 10.1186/s12866-014-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casafont Morencos F, de las Heras Castano G, Martin Ramos L, Lopez Arias MJ, Ledesma F, & Pons Romero F (1996). Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci, 41(3), 552–556. [DOI] [PubMed] [Google Scholar]
- Chen P, Starkel P, Turner JR, Ho SB, & Schnabl B (2015). Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology, 61(3), 883–894. doi: 10.1002/hep.27489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Torralba M, Tan J, Embree M, Zengler K, Starkel P, van Pijkeren JP, DePew J, Loomba R, Ho SB, Bajaj JS, Mutlu EA, Keshavarzian A, Tsukamoto H, Nelson KE, Fouts DE, Schnabl B. (2015). Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology, 148(1), 203–214.e216. doi: 10.1053/j.gastro.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT (1998). Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res, 22(9), 1927–1942. [PubMed] [Google Scholar]
- Cook RT, Ballas ZK, Waldschmidt TJ, Vandersteen D, LaBrecque DR, & Cook BL (1995). Modulation of T-cell adhesion markers, and the CD45R and CD57 antigens in human alcoholics. Alcohol Clin Exp Res, 19(3), 555–563. [DOI] [PubMed] [Google Scholar]
- Cook RT, Garvey MJ, Booth BM, Goeken JA, Stewart B, & Noel M (1991). Activated CD-8 cells and HLA DR expression in alcoholics without overt liver disease. J Clin Immunol, 11(5), 246–253. [DOI] [PubMed] [Google Scholar]
- Cook RT, Waldschmidt TJ, Ballas ZK, Cook BL, Booth BM, Stewart BC, & Garvey MJ (1994). Fine T-cell subsets in alcoholics as determined by the expression of L-selectin, leukocyte common antigen, and beta-integrin. Alcohol Clin Exp Res, 18(1), 71–80. [DOI] [PubMed] [Google Scholar]
- Engen PA, Green SJ, Voigt RM, Forsyth CB, & Keshavarzian A (2015). The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol Res, 37(2), 223–236. [PMC free article] [PubMed] [Google Scholar]
- Geissler M, Gesien A, & Wands JR (1997). Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. J Immunol, 159(10), 5107–5113. [PubMed] [Google Scholar]
- Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, Stärkel P, Belzer C, Hellerbrand C, Tsukamoto H, Ho SB, Schnabl B. (2013). Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology, 58(1), 108–119. doi: 10.1002/hep.26321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, Delzenne NM. (2014). Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A, 111(42), E4485–4493. doi: 10.1073/pnas.1415174111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Bushman FD, & FitzGerald GA (2015). Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci U S A, 112(33), 10479–10484. doi: 10.1073/pnas.1501305112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe PP, Gyongyosi B, Satishchandran A, Iracheta-Vellve A, Cho Y, Ambade A, & Szabo G (2018). Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J Neuroinflammation, 15(1), 298. doi: 10.1186/s12974-018-1328-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie PJ, & Holmes S (2012). Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data. Pac Symp Biocomput, 235–246. [PMC free article] [PubMed] [Google Scholar]
- McMurdie PJ, & Holmes S (2014). Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol, 10(4), e1003531. doi: 10.1371/journal.pcbi.1003531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Kindt Roeland, Legendre Pierre, Bob O’Hara, Henry M Stevens H. (2007). “The Vegan Package.” Community Ecology Package 10: 631–37. [Google Scholar]
- Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, & Lusis AJ (2016). Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes, 7(4), 313–322. doi: 10.1080/19490976.2016.1203502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva A, Patel V, Kurioka A, Jeffery HC, Wright G, Tarff S, Shawcross D, Ryan JM, Evans A, Azarian S, Bajaj JS, Fagan A, Patel V, Mehta K, Lopez C, Simonova M, Katzarov K, Hadzhiolova T, Pavlova S, Wendon JA, Oo YH, Klenerman P, Williams R, Chokshi S. (2018). Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut, 67(5), 918–930. doi: 10.1136/gutjnl-2017-314458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronis MJ, Korourian S, Zipperman M, Hakkak R, & Badger TM (2004). Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr, 134(4), 904–912. [DOI] [PubMed] [Google Scholar]
- Samuelson DR, Burnham EL, Maffei VJ, Vandivier RW, Blanchard EE, Shellito JE, Luo M, Taylor CM, Welsh DA. (2018). The respiratory tract microbial biogeography in alcohol use disorder. Am J Physiol Lung Cell Mol Physiol, 314(1), L107–l117. doi: 10.1152/ajplung.00277.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson DR, Shellito JE, Maffei VJ, Tague ED, Campagna SR, Blanchard EE, Luo M, Taylor CM, Ronis MJJ, Molina PE, Welsh DA. (2017). Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae. PLoS Pathog, 13(6), e1006426. doi: 10.1371/journal.ppat.1006426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson DR, Siggins RW, Ruan S, Amedee AM, Sun J, Zhu QK, Marasco WA, Taylor CM, Luo M, Welsh DA, Shellito JE. (2018). Alcohol consumption increases susceptibility to pneumococcal pneumonia in a humanized murine HIV model mediated by intestinal dysbiosis. Alcohol. doi: 10.1016/j.alcohol.2018.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Coleman RA, Alber C, Ballas ZK, Waldschmidt TJ, Mortari F, LaBrecque DR, Cook RT. (2001). TH1 cytokine response of CD57+ T-cell subsets in healthy controls and patients with alcoholic liver disease. Alcohol, 24(3), 155–167. [DOI] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, & Cook RT (2002). Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol, 72(6), 1109–1116. [PubMed] [Google Scholar]
- Voth M, Duchene M, Auner B, Lustenberger T, Relja B, & Marzi I (2017). I-FABP is a Novel Marker for the Detection of Intestinal Injury in Severely Injured Trauma Patients. World J Surg, 41(12), 3120–3127. doi: 10.1007/s00268-017-4124-2 [DOI] [PubMed] [Google Scholar]
- Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, Chen H, Zhao X, Zhang S, Zhou Z, Zeisel SH, Jia W (2013b). Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. Faseb j, 27(9), 3583–3593. doi: 10.1096/fj.13-231860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Zhong W, Zheng X, Li Q, Qiu Y, Li H, Chen H, Zhou Z, Jia W (2013a). Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J Proteome Res, 12(7), 3297–3306. doi: 10.1021/pr400362z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B (2011). Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology, 53(1), 96–105. doi: 10.1002/hep.24018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


