Abstract
Background:
Trauma-induced coagulopathy can present as abnormalities in a conventional or viscoelastic coagulation assay or both. We hypothesized that patients with discordant coagulopathies reflect different clinical phenotypes.
Methods:
Blood samples were collected prospectively from critically injured patients upon arrival at two urban Level I trauma centers. International normalized ratio (INR), partial thromboplastin time (PTT), thromboelastography (TEG), and coagulation factors were assayed.
Results:
278 patients (median ISS 17, mortality 26%) were coagulopathic: 20% with isolated abnormal INR and/or PTT (CONVENTIONAL), 49% with isolated abnormal TEG (VISCOELASTIC), and 31% with abnormal INR/PTT and TEG (BOTH). Compared with VISCOELASTIC, CONVENTIONAL and BOTH had higher ISS, lower GCS, larger base deficit, and decreased factor activities (all p<0.017). They received more blood products and had more ICU/ventilation days (all p<0.017). Mortality was higher in CONVENTIONAL (40%) and BOTH (49%) than VISCOELASTIC (6%, p<0.017).
Conclusions:
Although TEG-guided resuscitation improves survival after injury, INR and PTT identify coagulopathic patients with highest mortality regardless of TEG and likely represent distinct mechanisms independent of biochemical clot strength.
Keywords: Trauma-induced coagulopathy, Thromboelastography, Resuscitation, Transfusion, Precision medicine
SUMMARY
Only 31% of patients with trauma-induced coagulopathy present with abnormalities in both conventional and viscoelastic coagulation assays. Although thromboelastography (TEG) has been shown to improve survival when used to guide resuscitation after injury, international normalized ratio and partial thromboplastin time identify coagulopathic patients with highest mortality regardless of TEG and reflect coagulation factor deficiencies.
INTRODUCTION
Trauma-induced coagulopathy (TIC) occurs in one-third of severely injured patients and is associated with increased bleeding, morbidity, and mortality.1–3 Unlike congenital hematologic disorders, which often result from isolated abnormalities in the clotting system, TIC reflects a multifactorial biologic response initiated by tissue injury and shock and mediated by numerous mechanisms including coagulation factor depletion, protein C activation and depletion, fibrinolysis, and platelet and endothelial dysfunction.4–10
When it was first recognized that trauma patients can present with coagulopathy prior to resuscitation, TIC was defined as prolongation of either international normalized ratio (INR) or partial thromboplastin time (PTT).1,2 Over the last decade, the use of viscoelastic hemostatic assays like thromboelastography (TEG) has increased in trauma because of their ability to identify specific clotting deficits underlying TIC and guide targeted treatment of these deficits.11–14 However, patients can arrive with an abnormality in either a conventional or viscoelastic test but not both, suggesting a discordance of unknown biochemical and clinical significance. These discordances might exist because of the multifactorial etiology of TIC and represent an important avenue of scientific investigation to facilitate individualized approaches to its diagnosis and treatment.15
Although the significance of discordant abnormalities between conventional coagulation tests like INR and PTT has previously been examined,16 discrepancies between conventional and viscoelastic tests remain poorly understood. The purpose of this prospective study was to identify injury and coagulation factor characteristics associated with differences between conventional and viscoelastic tests and to determine the outcomes associated with such discrepancies. We hypothesized that patients with discordant coagulopathies (isolated abnormal INR/PTT and isolated abnormal TEG) reflect distinct clinical phenotypes that provide prognostic and mechanistic insight into TIC.
METHODS
Arrival blood samples were collected prospectively from patients meeting criteria for highest-level trauma activation at two urban Level I trauma centers: Denver Health Medical Center in Denver, Colorado, and Zuckerberg San Francisco General Hospital in San Francisco, California. Patients were excluded if they were <18-years-old, pregnant, incarcerated, transferred from another hospital, or taking outpatient anticoagulant or antiplatelet medications. Samples were obtained under protocols approved by the institutional review board for each center.
Our procedures for sample collection and analysis at each center have previously been described.14,17 In addition to conventional coagulation tests including INR and PTT, rapid TEG was performed on each sample following recalcification using a TEG® 5000 Thrombelastograph® Hemostasis Analyzer (Haemonetics Corporation; Braintree, Massachusetts). Parameters measured by TEG included activated clotting time (ACT, the time from the start of the test to initial clot formation), alpha angle (α; the slope of the tracing, which represents the rate of clot formation), and maximum amplitude (MA; the greatest amplitude of the tracing, which illustrates clot strength). Samples were also assayed for coagulation factors II, V, VII, VIII, IX, and X; protein C; D-dimer; and antithrombin III using a STA Compact Max® (Diagnostica Stago; Parsippany, New Jersey).
TIC was classified into three phenotypes using the results of INR, PTT, and TEG (Table 1). Patients with isolated abnormal INR and/or PTT (INR ≥1.4 and/or PTT ≥35 s) but normal TEG were designated CONVENTIONAL. Those with isolated abnormal TEG (ACT >128 s, angle <65°, and/or MA <55 mm) but normal INR/PTT were designated VISCOELASTIC.14 Those with abnormalities in both a conventional and viscoelastic test were designated BOTH. Patients were considered noncoagulopathic if their INR was <1.4, PTT <35 s, ACT ≤128 s, angle ≥65°, and MA ≥55 mm.
TABLE 1.
Coagulopathic Phenotypes.
| CONVENTIONAL | VISCOELASTIC | BOTH |
|---|---|---|
|
|
|
ACT, activated clotting time; INR, international normalized ratio; MA, maximum amplitude; PTT, partial thromboplastin time; TEG, thromboelastography.
Demographic, injury, resuscitation, and outcome data were collected for all patients. Summary statistics are reported as mean with standard deviation for normally distributed data, median with interquartile range for skewed data, and percentage for binary data. Univariate differences between groups were evaluated using Student’s t-test or one-way analysis of variance (ANOVA) for normally distributed data, Wilcoxon rank-sum or Kruskal-Wallis tests for skewed data, and Fisher’s exact test for binary data. Multinomial logistic regression was performed following a stepwise selection procedure to identify independent predictors of discordant coagulopathies while controlling for significant covariates. Kaplan-Meier curves were generated to compare survival times by coagulopathic phenotype. A p-value <0.05 was considered significant, except when adjusted for multiple comparisons with Bonferroni correction. Statistical analyses were performed with Stata/SE 15 (StataCorp; College Station, Texas).
RESULTS
Study Population
From December 2010 to June 2017, 839 patients were enrolled. These patients were mostly male (81%) and young [median age 33 y (IQR 25–47)], representing a typical trauma population. They had a median Injury Severity Score (ISS) of 10 (IQR 2–26) and median base deficit of 5.0 (IQR 1.0–9.1). 51% sustained blunt trauma, and the entire cohort received minimal intravenous fluid prior to sample collection [median 100 mL (IQR 0–350)].
Coagulopathic versus Noncoagulopathic Patients
278 patients (33%) arrived with coagulopathy by at least one conventional or viscoelastic parameter (Table 2). Consistent with previous studies on TIC, these patients were more severely injured than noncoagulopathic patients (ISS 17 vs 10, p<0.001) and presented with a larger base deficit (7.0 vs 4.0, p<0.001). Coagulopathic patients required more blood transfusions within the first 24 hours and experienced more intensive care unit (ICU) and ventilator days. Mortality was higher for coagulopathic patients (26%) than for noncoagulopathic patients (4%, p<0.001).
TABLE 2.
Characteristics of Coagulopathic versus Noncoagulopathic Patients.
| Noncoagulopathic (n=561) |
Coagulopathic (n=278) |
p | |
|---|---|---|---|
| Age (y) | 34 (26, 47) | 31 (24, 46) | 0.050 |
| Male | 81% | 82% | 0.641 |
| BMI (kg/m2) | 26.6 (23.3, 30.5) | 25.4 (23.0, 29.0) | 0.029 |
| Blunt mechanism | 47% | 59% | 0.002 |
| ISS | 10 (2, 22) | 17 (5, 34) | <0.001 |
| Head AIS score | 0 (0, 2) | 0 (0, 4) | <0.001 |
| GCS score | 15 (12, 15) | 11 (3, 15) | <0.001 |
| Platelet count (× 109/L) | 273 (233, 322) | 221 (171, 289) | <0.001 |
| Fibrinogen (mg/dL) | 237 (186, 295) | 174 (137, 232) | <0.001 |
| D-dimer (μg/mL) | 0.8 (0.3, 4.0) | 3.7 (0.7, 8.9) | <0.001 |
| pH | 7.34 (7.27, 7.39) | 7.28 (7.16, 7.37) | <0.001 |
| Base deficit | 4.0 (0.4, 7.9) | 7.0 (3.0, 12.8) | <0.001 |
| Lactate (mg/dL) | 3.2 (2.3, 5.0) | 4.7 (2.8, 8.6) | <0.001 |
| Factor II (% activity) | 77 (63, 91) | 68 (51, 81) | <0.001 |
| Factor V (% activity) | 54 (36, 71) | 43 (24, 69) | 0.003 |
| Factor VII (% activity) | 83 (62, 105) | 72 (51, 89) | <0.001 |
| Factor VIII (% activity) | 159 (95, 253) | 177 (93, 277) | 0.505 |
| Factor IX (% activity) | 123 (104, 153) | 107 (77, 136) | <0.001 |
| Factor X (% activity) | 82 (65, 98) | 72 (54, 85) | <0.001 |
| ATIII (% activity) | 89 (71, 107) | 73 (50, 98) | <0.001 |
| Protein C (% activity) | 93 (±28) | 76 (±31) | 0.149 |
| Crystalloid, first 24 h (mL) | 3500 (1502, 5779) | 4130 (2301, 7082) | <0.001 |
| RBC, first 24 h (u) | 0 (0, 1) | 2 (0, 7) | <0.001 |
| Plasma, first 24 h (u) | 0 (0, 0) | 2 (0, 6) | <0.001 |
| Platelets, first 24 h (u) | 0 (0, 0) | 0 (0, 1) | <0.001 |
| Hospital LOS (d) | 5 (2, 13) | 5 (2, 14) | 0.528 |
| ICU LOS (d) | 2 (0, 5) | 2 (0, 7) | 0.001 |
| Ventilation days | 0 (0, 2) | 2 (0, 4) | <0.001 |
| Mortality | 4% | 26% | <0.001 |
AIS, Abbreviated Injury Scale; ATIII, antithrombin III; BMI, body mass index; GCS, Glasgow Coma Scale; ICU, intensive care unit; ISS, Injury Severity Score; LOS, length of stay; RBC, red blood cells.
Coagulopathic Phenotypes
Among coagulopathic patients, 55 (20%) had isolated abnormal INR/PTT (CONVENTIONAL), 137 (49%) isolated abnormal TEG (VISCOELASTIC), and 86 (31%) abnormal INR/PTT and TEG (BOTH). The characteristics of these coagulopathic phenotypes are depicted in Table 3. Compared with VISCOELASTIC, patients with abnormal INR/PTT (CONVENTIONAL and BOTH) were more severely injured, especially with intracranial injuries as demonstrated by their higher head Abbreviated Injury Scale (AIS) and lower Glasgow Coma Scale (GCS) scores (all p<0.017 for multiple comparisons). Likewise, they were more acidotic with greater base deficit (all p<0.017).
TABLE 3.
Characteristics of Patients by Coagulopathic Phenotype.
| CONVENTIONAL (n=55) |
VISCOELASTIC (n=137) |
BOTH (n=86) |
p | |
|---|---|---|---|---|
| Age (y) | 33 (24, 49) | 32 (25, 45) | 30 (23, 44) | 0.511 |
| Male | 75% | 85% | 81% | 0.209 |
| BMI (kg/m2) | 26.3 (22.6, 30.1) | 25.1 (22.9, 29.0) | 25.4 (23.9, 28.4) | 0.722 |
| Blunt mechanism | 69% | 53% | 60% | 0.123 |
| ISS | 27 (9, 38) | 9 (1, 20) | 34 (26, 43) | <0.001 |
| Head AIS score | 3 (0, 5) | 0 (0, 3) | 3 (0, 5) | <0.001 |
| GCS score | 6 (3, 15) | 14 (10, 15) | 3 (3, 13) | <0.001 |
| Prehospital crystalloid (mL) | 150 (0, 400) | 100 (0, 300) | 200 (0, 500) | 0.079 |
| Platelets (× 109/L) | 227 (179, 305) | 231 (193, 299) | 179 (123, 261) | <0.001 |
| Fibrinogen (mg/dL) | 161 (141, 191) | 214 (169, 274) | 134 (106, 173) | <0.001 |
| D-dimer (μg/mL) | 5.1 (1.3, 9.4) | 0.8 (0.3, 4.7) | 8.2 (3.6, 15.4) | <0.001 |
| pH | 7.22 (7.08, 7.32) | 7.34 (7.26, 7.38) | 7.18 (7.05, 7.33) | <0.001 |
| Base deficit | 7.6 (2.8, 13.0) | 4.8 (1.7, 9.0) | 10.8 (4.5, 17.2) | <0.001 |
| Lactate (mg/dL) | 4.4 (2.7, 8.5) | 4.1 (2.6, 5.5) | 7.9 (3.9, 12.3) | <0.001 |
| Factor II (% activity) | 57 (40, 72) | 73 (63, 84) | 59 (46, 70) | <0.001 |
| Factor V (% activity) | 30 (21, 47) | 61 (41, 76) | 23 (17, 40) | <0.001 |
| Factor VII (% activity) | 58 (45, 79) | 79 (62, 96) | 55 (43, 87) | 0.009 |
| Factor VIII (% activity) | 111 (71, 226) | 186 (141, 322) | 177 (74, 289) | 0.054 |
| Factor IX (% activity) | 75 (66, 129) | 132 (103, 153) | 86 (55, 113) | <0.001 |
| Factor X (% activity) | 68 (42, 79) | 78 (67, 92) | 58 (52, 77) | 0.002 |
| ATIII (% activity) | 56 (30, 87) | 82 (68, 101) | 57 (37, 80) | 0.002 |
| Protein C (% activity) | 64 (±28) | 87 (±30) | 61 (±30) | 0.962 |
| Crystalloid, first 24 h (mL) | 5238 (3000, 7926) | 3043 (2000, 5200) | 5873 (2700, 9814) | <0.001 |
| RBC, first 24 h (u) | 2 (0, 6) | 0 (0, 2) | 7 (3, 22) | <0.001 |
| Plasma, first 24 h (u) | 2 (0, 8) | 0 (0, 2) | 5 (3, 14) | <0.001 |
| Platelets, first 24 h (u) | 0 (0, 1) | 0 (0, 0) | 1 (0, 2) | <0.001 |
| Hospital LOS (d) | 6 (2, 20) | 4 (2, 10) | 5 (1, 19) | 0.153 |
| ICU LOS (d) | 3 (2, 9) | 2 (0, 5) | 3 (1, 13) | 0.007 |
| Ventilation days | 2 (1, 5) | 1 (0, 3) | 2 (1, 10) | <0.001 |
| Mortality | 40% | 6% | 49% | <0.001 |
AIS, Abbreviated Injury Scale; ATIII, antithrombin III; BMI, body mass index; GCS, Glasgow ComaScale; ICU, intensive care unit; ISS, Injury Severity Score; LOS, length of stay; RBC, red blood cells.
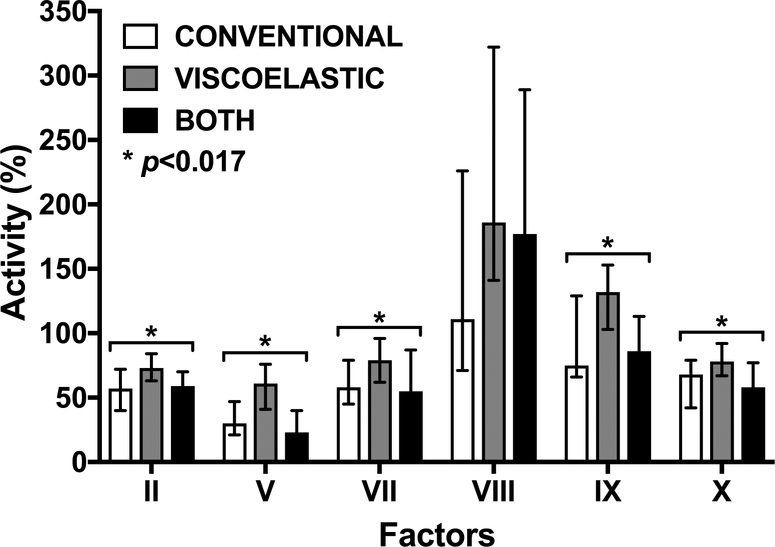
Factor activities differed significantly among the coagulopathic phenotypes (Table 3 and Figure 1). Compared with VISCOELASTIC, patients with abnormal INR/PTT (CONVENTIONAL and BOTH) had decreased activities of factors in the tissue factor (VII), contact (IX), and common (X, V, and II) coagulation pathways (all p<0.017). Factor VIII activity was not different among groups. In addition, CONVENTIONAL and BOTH were associated with lower fibrinogen, higher D-dimer, and lower antithrombin III activity than VISCOELASTIC (Table 3, all p<0.017).
FIGURE 1.
Coagulation factor activities by coagulopathic phenotype.
Resuscitation requirements and outcomes differed among the three coagulopathic phenotypes (Table 3). CONVENTIONAL and BOTH received more blood transfusions during the first 24 hours and had more ICU and ventilator days (all p<0.017). Overall mortality was higher for CONVENTIONAL (40%) and BOTH (49%) than VISCOELASTIC (6%, p<0.001). This latter finding was consistent with Kaplan-Meier survival analysis (Figure 2).
FIGURE 2.
Kaplan-Meier survival analysis by coagulopathic phenotype.
Discordant Coagulopathies
After adjusting for significant covariates, ISS and factor II (a common pathway factor) were the only independent predictors of discordance (Table 4).
TABLE 4.
Predictors of Discordant Coagulopathies.
| Phenotype | OR (95% CI) | p | |
|---|---|---|---|
| ISS | CONVENTIONAL | 1.073 (1.019, 1.129) | 0.007 |
| VISCOELASTIC | Reference | — | |
| BOTH | 1.104 (1.042, 1.169) | <0.001 | |
| Factor II | CONVENTIONAL | 0.953 (0.916, 0.990) | 0.014 |
| VISCOELASTIC | Reference | — | |
| BOTH | 0.950 (0.908, 0.993) | 0.024 |
Model performance was assessed using deviance (p=0.975) and Pearson’s chi-squared test (p=0.081), for both of which higher p indicates a better fit. CI, confidence interval; ISS, Injury Severity Score; OR, odds ratio.
DISCUSSION
In the current prospective observational study, we identified the characteristics of patients with discrepancies between conventional and viscoelastic tests. Coagulopathic patients with abnormal INR/PTT, regardless of the results of their TEG, were more severely injured with more profound shock, required more blood transfusions, and experienced higher mortality than those with normal INR/PTT but abnormal TEG. Most interestingly, these patients (CONVENTIONAL and BOTH) had lower activity levels for all coagulation factors except VIII. Multinomial logistic regression analysis, which controlled for age, sex, injury severity, and shock, identified factor II as an independent predictor of discordance between conventional and viscoelastic tests. Because factor II contributes to the common coagulation pathway, these findings suggest that patients with elevated INR and/or PTT but normal TEG should continue to be resuscitated aggressively with blood products to replete these factor deficiencies.18 Conventional coagulation tests remain an important indicator of coagulopathy after trauma and should continue to be obtained with initial trauma labs.
When first recognized as a direct sequela of tissue injury and shock, TIC was described as a prolongation of either PTT or prothrombin time, which had been designed to monitor anticoagulant therapy.1,2 Upon arrival at a trauma center, abnormalities of these tests portended increased bleeding, morbidity, and mortality.1–3 Although this finding challenged the notion at the time that post-injury coagulopathy resulted iatrogenically from resuscitation, it did not identify specific clotting deficits that could be targeted to treat TIC. Consequently, patients were resuscitated empirically with fixed ratios of blood products.19,20
As we progress into an era of precision medicine, much work has been done to individualize treatment of TIC.11–16,21–23 Critical to these efforts has been the increased use of viscoelastic hemostatic assays, which assess whole-blood clot formation and degradation and can identify specific coagulation deficits for targeted treatment with blood products or hemostatic adjuncts.11–14 Our group previously conducted a prospective, randomized trial which demonstrated that viscoelastic assays improve survival compared with conventional tests when used to guide resuscitation.13 Nevertheless, discrepancies between these two types of tests exist and can create a management dilemma for clinicians.
Although this study supports the need for combined use of conventional and viscoelastic assays, viscoelastic assays should still play the primary role in guiding resuscitation, as they have been shown to be superior to conventional tests for this purpose.13 Furthermore, while conventional tests identify coagulopathy, viscoelastic assays supply mechanistic details that facilitate targeted treatment of a specific derangement. In addition, they are superior in their ability to identify hypercoagulability and quantify clot degradation by percent lysis.24 Most importantly, TEG can be performed rapidly, with ACT available in five minutes. While this feature allows early identification and treatment of TIC, certain coagulopathic patients can be missed by TEG, as suggested in this study. This finding highlights the need for a test that promptly identifies patients with greatest need for transfusion, as INR and PTT do, and can guide targeted treatment of these patients, as TEG does.
We recognize several limitations of this study beyond those inherent in its prospective observational design. While conducting this study at two institutions provided adequate patient numbers, transfusion practices were not identical at each center and inevitably varied among providers. Specifically, resuscitation is guided by TEG in Denver but not San Francisco. Likewise, the thresholds used to define coagulopathy by INR, PTT, and TEG are not absolute and were developed for different purposes. Elevated INR and PTT are associated with increased mortality, while TEG thresholds have previously been chosen based on the need for massive transfusion.14 It is also important to note that the TEG parameter percent lysis at 30 minutes (LY30) was not included in this analysis because there is no corollary conventional test that quantifies clot degradation.
This study represents an important attempt to describe discrepancies between conventional and viscoelastic assays in identifying TIC, and while it suggests that such discrepancies reflect differences in coagulation factor activities, future studies are needed to clarify the utility of this finding. Specifically, subsequent studies should address whether correction of lower factor activity levels with transfusions improves survival. Although the phenotypic characters of patients with abnormal INR versus PTT have already been addressed in prior work from our group, the individual contributions of each TEG parameter to coagulopathy and patient outcomes should be addressed.
CONCLUSIONS
Only 31% of patients with TIC present with an abnormality in both conventional and viscoelastic hemostatic assays. Although TEG-guided resuscitation has been shown to improve survival after injury, INR and PTT identify coagulopathic patients with highest mortality regardless of TEG and likely represent distinct mechanisms independent of biochemical clot strength. Specifically, elevated INR and PTT reflect decreased coagulation factor activities, suggesting that factor repletion might be the appropriate therapy for patients who present with these abnormalities but normal TEG.
HIGHLIGHTS.
31% of patients with TIC have both abnormal conventional and viscoelastic assays.
Conventional assays identify more severely injured patients than viscoelastic.
Abnormal conventional assays are associated with higher mortality.
Abnormal conventional assays might reflect coagulation factor deficiencies.
Funding
This study was supported by the National Institutes of Health (grants UM1HL120877 to MJC; K01ES026834 to RAC; and T32GM08315 to GRS, GRN, and HBM) and the United States Department of Defense (contract W911QY-15-C-0044 to MJC and RAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation History This study was presented at the 70th annual meeting of the Southwestern Surgical Congress; April 8–11, 2018; in Napa, California.
REFERENCES
- 1.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–30. [DOI] [PubMed] [Google Scholar]
- 2.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. [DOI] [PubMed] [Google Scholar]
- 3.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–63. [DOI] [PubMed] [Google Scholar]
- 4.Shaz BH, Winkler AM, James AB, et al. Pathophysiology of early trauma-induced coagulopathy: emerging evidence for hemodilution and coagulation factor depletion. J Trauma. 2011;70:1401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunitake RC, Howard BM, Kornblith LZ, et al. Individual clotting factor contributions to mortality following trauma. J Trauma Acute Care Surg. 2017;82:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MJ, Call M, Nelson M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashuk JL, Moore EE, Sawyer M, et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252:434–44. [DOI] [PubMed] [Google Scholar]
- 8.Kutcher ME, Redick BJ, McCreery RC, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74:1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen MJ, Kutcher M, Redick B, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75:S40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashuk JL, Moore EE, Sawyer M, et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251:604–14. [DOI] [PubMed] [Google Scholar]
- 12.Cotton BA, Faz G, Hatch QM, et al. Rapid thrombelastography delivers real-time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71:407–7. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Einersen PM, Moore EE, Chapman MP, et al. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MJ. Translational approaches to coagulopathy after trauma: towards targeted treatment. PLoS Med. 2017;14(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie SA, Kornblith LZ, Howard BM, et al. Characterization of distinct coagulopathic phenotypes in injury: pathway-specific drivers and implications for individualized treatment. J Trauma Acute Care Surg. 2017;82:1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornblith LZ, Howard B, Kunitake R, et al. Obesity and clotting: body mass index independently contributes to hypercoagulability after injury. J Trauma Acute Care Surg. 2015;78:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–84. [DOI] [PubMed] [Google Scholar]
- 19.Cotton BA, Au BK, Nunez TC, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–9. [DOI] [PubMed] [Google Scholar]
- 20.Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Díaz I, Hubbard A, Decker A, et al. Variable importance and prediction methods for longitudinal problems with missing variables. PLoS One. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore SE, Decker A, Hubbard A, et al. Statistical machines for trauma hospital outcomes research: application to the PRospective, Observational, Multi-Center Major Trauma Transfusion (PROMMTT) study. PLoS One. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menezes AA, Vilardi RF, Arkin AP, et al. Targeted clinical control of trauma patient coagulation through a thrombin dynamics model. Sci Transl Med. 2017;9. [DOI] [PubMed] [Google Scholar]
- 24.Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]