Supplemental Digital Content is available in the text.
Keywords: acute kidney injury, cell cycle biomarkers, outcome prediction, renal replacement therapy, sepsis, soluble urokinase-type plasminogen activator receptor
Objectives:
Sepsis-induced acute kidney injury is the dominant acute kidney injury etiology in critically ill patients and is often associated with a need for renal replacement therapy. The indication and timing of renal replacement therapy are controversially discussed. We hypothesized that the product of the G1-cell cycle arrest biomarkers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 ([TIMP-2] × [IGFBP7]), and the soluble urokinase-type plasminogen activator receptor are of diagnostic value for the prediction of septic acute kidney injury courses requiring renal replacement therapy.
Design:
In this prospective study, critically ill patients were enrolled immediately after the fulfillment of Sepsis-3 criteria. Urinary [TIMP-2] × [IGFBP7] levels over time and serum soluble urokinase-type plasminogen activator receptor levels once at inclusion were measured. The primary endpoint was the development of septic acute kidney injury with the need for renal replacement therapy. Area under the receiver operating characteristic curves, de Long’s tests, and logistic regression models were calculated.
Setting:
Two ICUs at Heidelberg University Hospital between May 2017 and July 2018.
Patients:
One-hundred critically ill patients with positive Sepsis-3 criteria.
Interventions:
None.
Measurement and Main Results:
Nineteen patients required renal replacement therapy. Diagnostic performance of urinary [TIMP-2] × [IGFBP7] improved over time with the highest area under the receiver operating characteristic curve of 0.89 (95% CI, 0.80–0.98) 24 hours after study inclusion. Soluble urokinase-type plasminogen activator receptor levels at inclusion showed an area under the receiver operating characteristic curve of 0.83 (0.75–0.92). The best discrimination ability for the primary outcome measure was achieved for [TIMP-2] × [IGFBP7] at 24 hours after inclusion by applying a cutoff value of greater than or equal to 0.6 (ng/mL)2/1,000 (sensitivity 90.9, specificity 67.1). Soluble urokinase-type plasminogen activator receptor performed best by using a cutoff value of greater than or equal to 8.53 ng/mL (sensitivity 84.2, specificity 82.7). A combination of newly tested biomarkers with cystatin C resulted in a significantly improved diagnostic accuracy. Cystatin C in combination with [TIMP-2] × [IGFBP7] 24 hours outperformed all standard renal parameters (area under the receiver operating characteristic curve 0.93 [0.86–1.00]).
Conclusions:
[TIMP-2] × [IGFBP7] and soluble urokinase-type plasminogen activator receptor are promising biomarker candidates for the risk stratification of septic acute kidney injury patients with the need for renal replacement therapy.
Sepsis-induced acute kidney injury (AKI) is the most common type of AKI in the ICU (1). Up to 59% of critically ill patients suffer from AKI and sepsis is found to be the causative factor in almost 50% of patients (2, 3). So far, there is no pharmacological therapy to prevent or treat septic AKI, except for the treatment of the underlying sepsis (4–6). Thus, AKI management is limited to supporting measures such as the avoidance of secondary hemodynamic or toxic insults (5). In 15–20% of patients with sepsis-induced AKI, renal replacement therapy (RRT) is required to preserve metabolic and body fluid homeostasis (2). The optimal timing of RRT, however, is an ongoing controversy (7). Randomized controlled trials comparing “early” versus “late” initiation strategies found conflicting results (8–10).
There is growing evidence that improved outcomes and avoidance of unnecessary RRT are possible under restrictive RRT strategies by allowing sufficient time for autonomous renal recovery, which is seen in up to 49% of AKI patients (7, 9, 10). On the other hand, in patients with progressive AKI, the same strategies lead to the highest reported mortality rates; therefore, these patients may benefit from an earlier initiation of RRT (9, 10). Hence, the early risk stratification of patients with regard to the necessity of RRT by the use of biomarkers of renal stress, damage, and prognosis is urgently needed.
The soluble urokinase-type plasminogen activator receptor (suPAR) is discussed as a molecule of interest in several kidney diseases. This includes focal segmental glomerulosclerosis, diabetic nephropathy, as well as diagnostic qualities for the prediction of chronic kidney disease (CKD) and AKI (11–14). Its potential role as a (surrogate) parameter of ongoing inflammatory drivers in the kidneys, suggests suPAR as a biomarker for outcome prediction in septic AKI (11).
In a recent systematic review, G1-cell cycle arrest and tubular stress biomarkers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 ([TIMP-2] × [IGFBP7]) were reported as the best biomarker for the prediction of RRT (15). However, the underlying studies were not designed to predict the necessity of RRT, and the lack of predefined criteria for RRT initiation is likely to affect biomarker performances. In addition, no comparison of biomarker accuracy with routinely available parameters of renal impairment was performed, preventing a final assessment of their additive value for implementation in clinical routine.
We hypothesized that the product of the two cell cycle arrest biomarkers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 ([TIMP-2] × [IGFBP7]), and the suPAR are of diagnostic value for the prediction of septic AKI courses with the need for RRT.
MATERIAL AND METHODS
Study Protocol and Population
This prospective, observational study was conducted at two ICUs at Heidelberg University Hospital. The study protocol was approved by the local Ethics Committee of the Medical Faculty of Heidelberg and registered at DRKS.de (German Clinical Trials Register: DRKS00012446). The patients were selected constitutively from May 2017 to July 2018. The criterion for inclusion was the fulfillment of sepsis-3 criteria at ICU admission (≥ 18 yr old) (16). Written informed consent was obtained from all participants or their legal representatives. Exclusion criteria are provided in the online supplemental methods (Supplemental Digital Content 1, http://links.lww.com/CCM/E952). All patients were treated according to the Surviving Sepsis Campaign sepsis guidelines (17). Derived from the Kidney Disease Improving Global Outcomes, patients were categorized in three distinct AKI groups, whereby patients with need for RRT were treated as an additional and independent group (18): 1) No or mild AKI (AKI 0/1); 2) moderate or severe AKI without the need for RRT (AKI 2/3); and 3) need for RRT (RRT). Furthermore, we defined five divergent AKI courses, comparing AKI stage at day 1 and day 7: 1) stable AKI 0/1 (stable AKI 0/1), 2) AKI progression form AKI 0/I to AKI 2/3 (AKI 0/1 to 2/3), 3) recovery from AKI 2/3 to AKI 0/1 (AKI 2/3 to 0/1), 4) stable AKI 2/3 (stable AKI 2/3), and 5) AKI progression with need for RRT (RRT).
Urinary [TIMP-2] × [IGFBP7] concentrations were measured at 0, 12, 24, and 48 hours ([TIMP-2] × [IGFBP7] 12, 24, and 48 hr) (Table S1, Supplemental Digital Content 2, http://links.lww.com/CCM/E953). SuPAR levels were measured once at the time of study inclusion (stabile kinetics over 7 d [19]). Figure 1 shows a flow chart of study design and data analysis.
Figure 1.
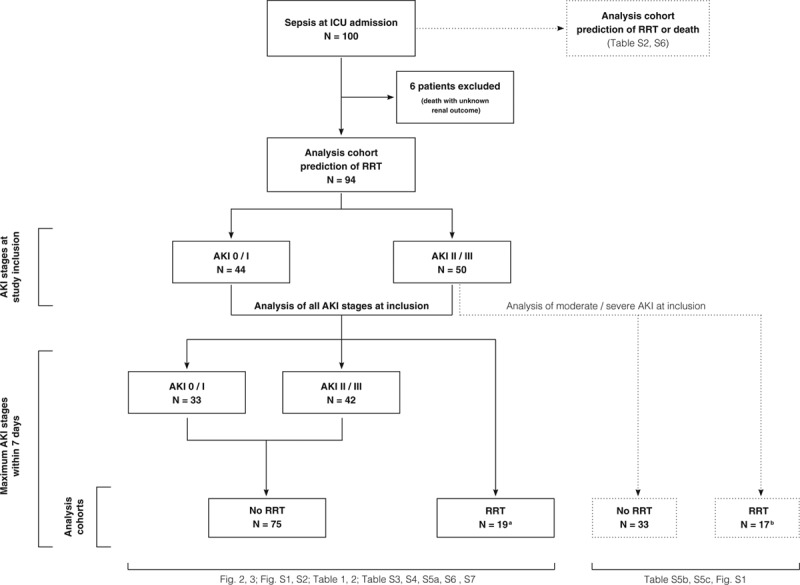
Flow chart of study design and data analysis. aDespite the fulfillment of renal replacement therapy (RRT) criteria, initiation of RRT has been declined retrospectively in eight patients; and bsix patients (unfavorable outcome in the context of a malignant disease). AKI = acute kidney injury (AKI 0/1 no or moderate AKI, AKI 2/3 moderate or severe AKI without RRT, RRT AKI with need for RRT), n = number of patients, For Table S2, see Supplemental Digital Content 2 (http://links.lww.com/CCM/E953).
Clinical Endpoint and Definitions
The primary endpoint was the development of AKI with the need for RRT within 7 days after study inclusion. We used a “delayed strategy” for RRT initiation, as recently described by Gaudry et al (9) to give enough time for autonomous renal recovery: Urea greater than 240 mg/dL, serum potassium greater than 6 mmol/L or greater than 5.8 despite treatment, pH less than 7.15 in the context of pure metabolic acidosis or mixed acidosis (Paco2 of 50 mm Hg or more without the possibility of increasing alveolar ventilation), acute pulmonary edema due to fluid overload requiring greater than 5 L oxygen to maintain a peripheral capillary oxygen saturation greater than 95% or a Fio2 greater than 50%. The definition of baseline serum creatinine (SCr) is provided in the online supplemental methods (Supplemental Digital Content 1, http://links.lww.com/CCM/E952). The secondary endpoint was a combinatory endpoint consisting of death or RRT within 7 days (Tables S2, S6a, and S6b, (Supplemental Digital Content 2, http://links.lww.com/CCM/E953).
Data Collection and Laboratory Methods
Data collection and laboratory methods are displayed in the online supplemental methods (Supplemental Digital Content 1, http://links.lww.com/CCM/E952). [TIMP-2] × [IGFBP7] was measured with a point-of-care device for the simultaneous quantification of TIMP-2 and IGFBP7 (NephroCheck Test; Astute Medical, San Diego, CA), utilizing a sandwich immunoassay. All values for [TIMP-2] × [IGFBP7] are reported in units of (ng/mL)2/1,000.
Statistical Analyses
Statistical analyses were performed using SPSS Statistics 25 (IBM, Armonk, NY) and Graph Pad Prism 8 (GraphPad Software, La Jolla, CA). For all analyses, two-sided p values of less than 0.05 were considered statistically significant. Receiver operating characteristics (ROCs) curves were generated to analyze individual biomarker performances. The optimal cutoff level was defined by the highest Youden index (sensitivity + specificity–1). Logistic regression models were generated to assess an additive predictive value of biomarker combinations. DeLong’s test was used for the comparison of individual area under the ROC curves (AUCs).
RESULTS
Patient Characteristics and Clinical Outcomes
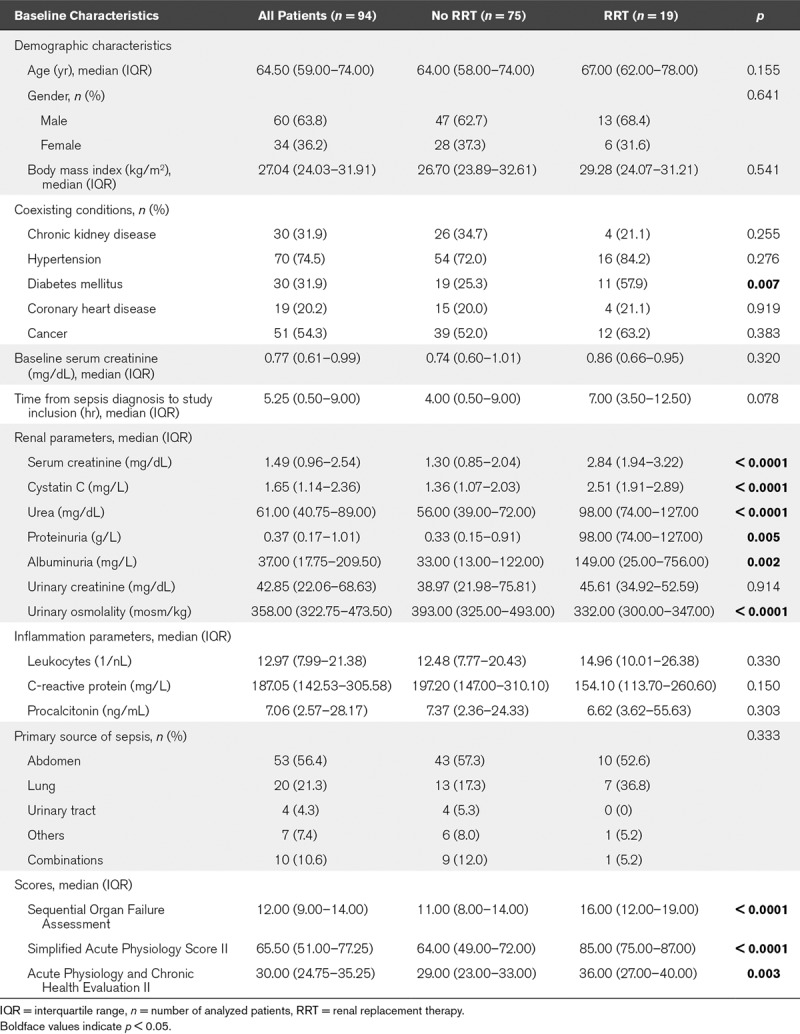
A total of 100 patients were included into the study (Table S2, Supplemental Digital Content 2, http://links.lww.com/CCM/E953). Six patients died (6%) within 7 days without fulfilling RRT criteria (AKI 0/1: n = 2; AKI 2/3: n = 4) and were therefore excluded from the analyses for RRT prediction (unknown renal outcome), but were considered in the analysis for the combinatory endpoint “RRT or death” (Fig. 1). In total, 86 of the remaining 94 patients (91%) developed AKI. No or mild AKI (AKI 0/1) occurred in 33 patients (35%), 42 patients (45%) suffered from moderate or severe AKI without the need for RRT (AKI 2/3) and 19 patients (20%) met the primary endpoint of AKI with the need for RRT. Baseline characteristics of the 94 patients are shown in Table 1. The baseline SCr levels before sepsis manifestation were comparable in RRT and non-RRT patients while RRT patients had a significantly higher SCr level at the time of sepsis diagnosis and study inclusion. Patients who developed need for RRT were also more likely to have higher disease severity scores. The median time from sepsis diagnosis to study inclusion was not significantly different between groups. The outcome parameters are summarized in Table S3 (Supplemental Digital Content 2, http://links.lww.com/CCM/E953). The frequency of septic shock and the maximum severity of illness were higher in the RRT cohort. Types of ventilation, vasopressor use, and cumulative administered furosemide dose did not differ between groups, but RRT patients had a significantly higher fluid input of 9.34 (4.90–12.93) versus 5.75 L (4.25–8.12 L) within the first 24 hours after study inclusion. Patients suffering from AKI with need for RRT had an excess mortality of 57.9% versus 14.7%.
TABLE 1.
Baseline Characteristics
Biomarker Kinetics
Patients with the need for RRT showed significantly higher [TIMP-2] × [IGFBP7] levels at all times points compared with patients without the need for RRT (Fig. S1, Supplemental Digital Content 2, http://links.lww.com/CCM/E953). When dividing non-RRT patients in maximum AKI stages, a stepwise increase in [TIMP-2] × [IGFBP7] concentrations was observed with higher AKI stages (Fig. 2A). At baseline, patients with AKI 2/3 without the need for RRT showed higher [TIMP-2] × [IGFBP7] concentrations than patients with AKI stage 0/1; however, [TIMP-2] × [IGFBP7] levels decreased in those patients, whereas [TIMP-2] × [IGFBP7] levels remained elevated throughout the first 48 hours in patients with the future need for RRT.
Figure 2.
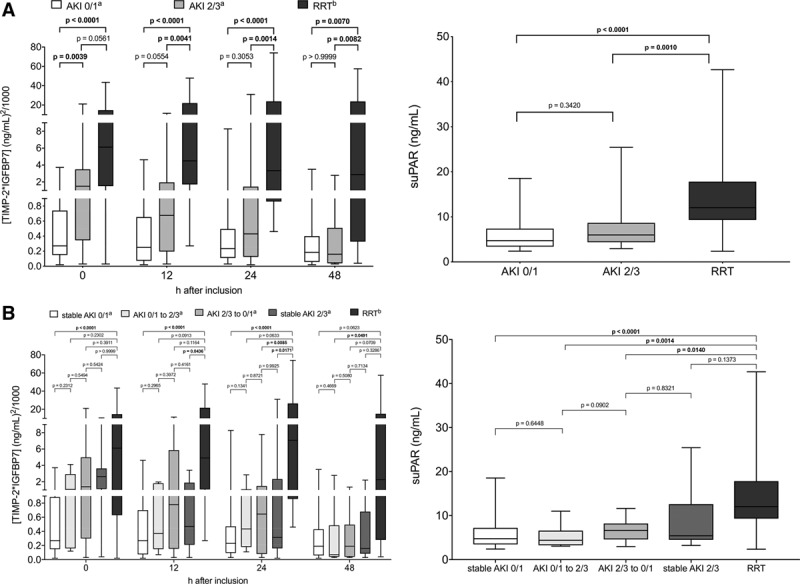
Biomarker kinetics of [TIMP-2] × [IGFBP7] over time and suPAR at baseline. A, Biomarker kinetics in relation to maximum acute kidney injury (AKI) stage within 7 d: AKI 0/1 = no or mild AKI, AKI 2/3 = moderate or severe AKI without the need for RRT, RRT = AKI with the need for RRT. B, Biomarker kinetics in relation to AKI course, comparing AKI stage at day 1 and day 7: stable AKI 0/1 = stable AKI 0/1 within 7 d, AKI 0/1 to 2/3 = AKI progression form AKI 0/1 to AKI 2/3, AKI 2/3 to 0/1 = recovery from AKI 2/3 to AKI 0/1, stable AKI 2/3 = stable AKI 2/3 within 7 d, RRT = AKI progression with need for RRT. aFor [TIMP-2] × [IGFBP7] measurement, three urinary samples were missing for 12 hr, two for 24 hr, and six for 48 hr in non-RRT patients, and one sample for 48 hr in the RRT group (anuria, ongoing surgery, administration of toluidine blue/interference with the urinary immunoassay). bPatient with the fulfillment of RRT criteria earlier than 12 hr, 24 hr, and 48 hr were excluded from the respective analysis of [TIMP-2] × [IGFBP7] values at the corresponding time points (six patients < 12 hr, eight patients < 24 hr, 11 patients ≤ 48 hr). RRT = renal replacement therapy, suPAR = soluble urokinase-type plasminogen activator receptor, [TIMP-2] × [IGFBP7] = product of tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7. Boldface values indicate p < 0.05.
Significantly higher serum suPAR concentrations were observed for patients requiring RRT (Fig. S1, Supplemental Digital Content 2, http://links.lww.com/CCM/E953). In patients with maximum AKI 2/3 without the need for RRT, suPAR levels remained in a lower range, matching patients with no or mild AKI (Fig. 2A).
When observing the AKI course instead of maximum AKI (Fig. 2B), patients with stable AKI 0/1 over the observation period showed consistently low median [TIMP-2] × [IGFBP7] levels below 0.3 (ng/mL)2/1,000 with no difference between AKI 0 and 1 (Fig. S2, Supplemental Digital Content 2, http://links.lww.com/CCM/E953). Patients with progressive AKI (AKI 0/1 to AKI 2/3), improving AKI (AKI 2/3 to AKI 0/1) or stable AKI 2/3 showed increased median [TIMP-2] × [IGFBP7] levels at the time of inclusion, without reaching statistical significance. This trend decreased over 12–48 hours, and patients reached [TIMP-2] × [IGFBP7] levels comparable to levels in patients with stable AKI 0/1. In contrast, the biomarker elevation was prolonged in patients who developed need for RRT. The biggest differences in [TIMP-2] × [IGFBP7] levels were seen 24 hours after inclusion. The highest suPAR levels at baseline were seen in patients who developed need for RRT (Fig. 2B), whereas median suPAR levels of other AKI courses remained in a lower range.
Spearman correlation analyses showed no relevant association of the two biomarkers with age, BMI, inflammation, or impairment of liver function and only a moderate correlation of suPAR levels with surrogate parameters of renal function (Table S4, Supplemental Digital Content 2, http://links.lww.com/CCM/E953).
Biomarker Performance for Outcome Prediction
Table 2 illustrates the performance of both biomarkers for RRT prediction in relation to a set of routinely available parameters of kidney impairment, inflammation, and disease severity with AUCs above 0.50 (for AUC < 0.50; see Tables S5a and S5b, Supplemental Digital Content 2, http://links.lww.com/CCM/E953). [TIMP-2] × [IGFBP7] at 24 hours after study inclusion and cystatin C (CysC), suPAR and SCr at the time of study inclusion showed the highest AUCs of 0.89 (95% CI, 0.80–0.98), 0.84 (95% CI, 0.75–0.92), 0.83 (95% CI, 0.72–0.95), and 0.80 (95% CI, 0.70–0.90), respectively. The best diagnostic accuracy for the tested biomarkers was achieved by using a cutoff of greater than or equal to 0.60 (ng/mL)2/1,000 for [TIMP-2] × [IGFBP7] 24 hours (sensitivity 90.9%, specificity 67.1%) and greater than or equal to 8.53 ng/mL for suPAR (sensitivity 84.2%, specificity 82.7). Using deLong’s test, [TIMP-2] × [IGFBP7] 24 hours showed a significantly improved diagnostic accuracy compared with [TIMP-2] × [IGFBP7] at study inclusion, [TIMP-2] × [IGFBP7] 12 hours and standard urinary parameters. Despite higher nominal AUC of [TIMP-2] × [IGFBP7] 24 hours, no statistical improvement was seen compared with surrogate parameters of glomerular filtration rate (GFR). The same applies to suPAR, where no diagnostic benefit could be statistically demonstrated with respect to standard parameters. The best discrimination for the combinatorial endpoint “RRT or death” was found for CysC, [TIMP-2] × [IGFBP7] 24 hours and suPAR with AUCs of 0.79 (95% CI, 0.69–0.88), 0.78 (95% CI, 0.63–0.92), and 0.76 (95% CI, 0.65–0.88), respectively (Table S6a, Supplemental Digital Content 2, http://links.lww.com/CCM/E953).
TABLE 2.
Receiver Operating Characteristic Analyses in Comparison to Standard Parameters (deLong’s test) and Best Performing Cutoff Values for the Prediction of Renal Replacement Therapy

Of note, when only patients with moderate to severe AKI at study inclusion were analyzed (Fig. 1), nominal diagnostic performances for the primary and secondary outcome measure were obtained for both [TIMP-2] × [IGFBP7] 24 hours and suPAR, whereas the nominal AUCs of CysC and SCr diminished in the same subset of patients (Tables S5b and S6b, Supplemental Digital Content 2, http://links.lww.com/CCM/E953).
The normalization of urinary biomarkers to urinary creatinine (biomarker/creatinine ratio) did not lead to significantly improved performances (Table S7, Supplemental Digital Content 2, http://links.lww.com/CCM/E953).
Additive Predictive Value of Biomarker Combinations
Figure 3 shows the ROC curves and corresponding AUCs for the newly tested biomarkers in combination with the best standard parameter CysC. Combining CysC and [TIMP-2] × [IGFBP7] 24 hours as well as CysC and suPAR significantly improved the individual ROC characteristics with AUCs of 0.93 (95% CI, 0.86–1.00) and 0.89 (95% CI, 0.82–0.96). CysC in combination with [TIMP-2] × [IGFBP7] 24 hours outperformed all standard renal parameters for the prediction of RRT (Fig. 3). The combination of CysC and suPAR resulted in a significantly improved diagnostic performance compared with standard urinary parameters and showed a trend toward superior performance as compared with SCr (p = 0.0603), CysC (p = 0.1053) and Sequential Organ Failure Assessment score (p = 0.1212).
Figure 3.
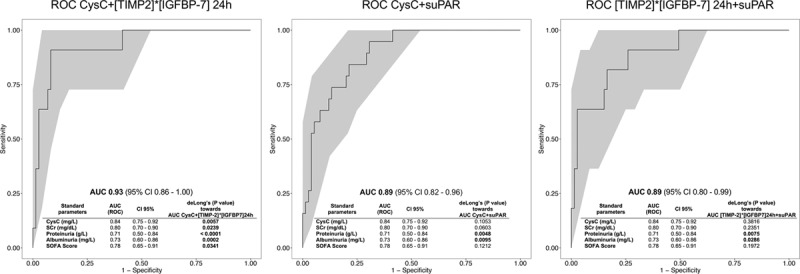
Bivariate logistic regression models and ROC analyses of biomarker combinations in comparison to standard parameters (deLong’s test) using [TIMP-2] × [IGFBP7] 24 hr, suPAR, and CysC. AUC = area under the ROC curve, CysC = Cystatin C, ROC = receiver operating characteristics, SCr = serum creatinine, SOFA = Sequential Organ Failure Assessment, suPAR = soluble urokinase-type plasminogen activator receptor, [TIMP-2] × [IGFBP7] = product of tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7. Boldface values indicate p < 0.05.
Combining [TIMP-2] × [IGFBP7] 24 hours and suPAR, however, did not provide any additive diagnostic benefit with a comparable AUC to that of [TIMP-2] × [IGFBP7] 24 hours alone (AUC, 0.89; 95% CI, 0.80–0.99).
DISCUSSION
To the best of our knowledge, this is the first prospective study in septic AKI patients assessing the diagnostic accuracy of [TIMP-2] × [IGFBP7] and suPAR in comparison to standard renal parameters for the prediction of RRT requirement using predefined RRT criteria. The main finding of our study was an improved diagnostic performance of [TIMP-2] × [IGFBP7] values over time with the best discrimination ability 24 hours after study inclusion and a diagnostic superiority toward standard urinary parameters. Furthermore, both [TIMP-2] × [IGFBP7] 24 hours and suPAR provided additive predictive value in combination with the best performing standard renal parameter CysC. The combination of [TIMP-2] × [IGFBP7] 24 hours with CysC outperformed all other tested parameters for the prediction of RRT. RRT patients showed the highest [TIMP-2] × [IGFBP7] and suPAR levels, whereas patients with maximum or stable AKI 0/1 showed consistently low median [TIMP-2] × [IGFBP7] levels below 0.3 (ng/mL)2/1,000 over 48 hours and the lowest suPAR levels at baseline. Remarkably, regardless of whether maximum AKI stage or AKI course was studied, the initial increase in [TIMP-2] × [IGFBP7] levels in patients with AKI 2/3 decreased within 12–48 hours, reaching levels of those with maximum AKI 0/1. In contrast, [TIMP-2] × [IGFBP7] elevation persisted longer in patients who developed RRT criteria, suggesting prolonged renal stress despite initiation of therapeutic measures. Unlike AKI after cardiac surgery, usually, no specific time point of injury can be identified in septic AKI. Therefore, and due to the extremely short time frame of biomarker elevation (12–24 hr) in response to renal stress (20, 21), sequential measurement of [TIMP-2] × [IGFBP7] is essential to predict septic AKI progression.
Although the relevance of this finding is limited by the small sample size, the nominal diagnostic accuracy of [TIMP-2] × [IGFBP7] and suPAR persisted in a subgroup analysis of patients with already AKI 2/3 at the time of study inclusion, whereas the performance of surrogate parameters of GFR have tended to decrease.
The current assessment of renal function in critically ill patients is based on the measurement of surrogate parameters of glomerular filtration such as SCr, urea, and urine output (7). Unfortunately, none of these markers reflect the true damage to the kidneys and are therefore inefficient parameters for renal outcome prediction. Newly discovered biomarkers of renal damage and stress including neutrophil gelatinase-associated lipocalin (NGAL) and [TIMP-2] × [IGFBP7] were suggested as more accurate diagnostic markers and have been intensively investigated for the early prediction of AKI (22, 23). In particular, the combination of [TIMP-2] and [IGFBP7] ([TIMP-2] × [IGFBP7]) led to a significant improvement in diagnostic accuracy and the development of a point-of-care device (23). Their value for the prediction of need for RRT, however, is still unclear. Recent evidence comes from a systematic review by Klein et al (15) who investigated the value of several urinary and blood biomarkers for the prediction of RRT. The largest evidence existed for urinary and blood NGAL and CysC with pooled AUCs of only 0.72 (95% CI, 0.64–0.80), 0.76 (95% CI, 0.71–0.80), and 0.77 (95% CI, 0.73–0.81), respectively. Of note, the same review identified [TIMP-2] × [IGFBP7] as the best urinary and overall predictor for RRT with a pooled AUC of 0.86 (95% CI, 0.79–0.93) (15). However, the data are limited to only four small studies originally designed for general AKI prediction (24–27). Three out of the four studies reported AUCs comparable to the results from our study (AUCs 0.83–0.92) (24, 25, 27) with only the study by Koyner et al (26) showing an inferior performance of [TIMP-2] × [IGFBP7] (AUC 0.61). The study of Koyner et al (26) differs, however, in that [TIMP-2] × [IGFBP7] was predominantly measured in patients with mild AKI and once at the time of study inclusion. These results are consistent with our study, which found only fair discrimination abilities for [TIMP-2] × [IGFBP7] at baseline, while we could demonstrate an improved diagnostic accuracy over time. This observation is supported by other authors who showed the best results for [TIMP-2] × [IGFBP7] levels after 12–24 hours, but for AKI prediction in general (21, 24, 28, 29). One explanation for this time-dependent performance of [TIMP-2] × [IGFBP7] might be the short time frame of biomarker elevation after episodes of renal stress (20, 21) together with a varying extent of renal injury in individual septic patients at the time of study inclusion. The initiation of therapeutic measures may therefore lead to a more dichotomous biomarker pattern in the following 12–24 hours depending on the individual therapeutic success with respect to the kidneys.
It is noteworthy that two other studies examined the performance of [TIMP-2] × [IGFBP7] in septic AKI patients focusing on general AKI prediction (28, 29). Honore et al (28) investigated the performance of [TIMP-2] × [IGFBP7] for the prediction of moderate to severe AKI (RRT not reported) within 12 hours in 232 septic patients, of whom 40 patients developed AKI (AUC, 0.84; 95% CI, 0.77–0.90; sensitivity, 95.0%; specificity, 37.5%). Cuartero et al (29) prospectively analyzed the diagnostic value of [TIMP-2] × [IGFBP7] for the prediction of AKI in both septic and nonseptic critically ill patients, showing an AUC of 0.80 (95% CI, 0.71–0.89; sensitivity, 73.5%; specificity, 71.4%). The conclusions for the RRT prediction, however, are limited by the small number of RRT patients (n = 5) and the absence of predefined RRT criteria.
Taken together, our results suggest that urinary [TIMP-2] × [IGFBP7] is a potential tool for monitoring the success of renal-directed sepsis therapy with at least comparable sensitivity and specificity characteristics compared with its use as an early predictor of moderate to severe AKI (21, 28–31). However, in patients who develop the need for RRT prior to 12–24 hours, a different biomarker with more constant kinetics is required to provide reliable performance at the time of study inclusion.
It is known that suPAR is stably elevated in the first week in critically ill patients (19) and considered a predictor of mortality (32, 33). In addition, suPAR is increasingly recognized as a predictive biomarker for the future decline of GFR in CKD and discussed as a parameter of ongoing inflammatory processes in the kidneys (11, 13). Our data suggest that suPAR may also be a valuable biomarker for predicting AKI progression with need for RRT, irrespective of acute impairments of renal function and extent of inflammation. In contrast to [TIMP-2] × [IGFBP7], baseline suPAR values already predicted the future need for RRT with promising diagnostic accuracy.
There are some limitations, which need to be addressed. First, the small sample size represents a main limitation of our study. Although we add novel insights to the literature, we could not prove the statistical superiority of [TIMP-2] × [IGFBP7] or suPAR values overall standard parameters. Therefore, the diagnostic thresholds and trends presented need to be further validated in larger cohorts. Second, despite a comparable time between sepsis diagnosis and study inclusion, most patients with future need for RRT suffered already from AKI 2/3 at the time of study inclusion. This could lead to an overestimation of the diagnostic accuracy of surrogate parameters of GFR such as CysC and SCr as compared with the newly tested biomarkers [TIMP-2] × [IGFBP7] and suPAR. Nevertheless, renal impairment remains a common finding at the time of sepsis diagnosis (28, 34), especially when Sepsis-3 criteria are applied. Third, [TIMP-2] × [IGFBP7] after 24 hours as compared with baseline values may be confounded by additional renal stress.
CONCLUSIONS
In conclusion, [TIMP-2] × [IGFBP7] levels after 24 hours and suPAR levels at baseline are promising biomarker candidates for the prediction of septic AKI courses requiring RRT. In future studies, a biomarker-assisted stratification of patients to the rather early or later start of RRT compared with a one-size-fits-all strategy needs to be tested to clarify whether biomarker-assisted risk stratification may result in improved outcomes in AKI patients.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Juliane Gehlen in preparing the tables and figures.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
References
- 1.Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol 2018; 307:2265–230 [DOI] [PubMed] [Google Scholar]
- 2.Peters E, Antonelli M, Wittebole X, et al. A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: Results from The Intensive Care Over Nations audit. Crit Care 2018; 22:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005; 294:813–818 [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, et al. ; Early Goal-Directed Therapy Collaborative Group: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 5.Bellomo R, Kellum JA, Ronco C, et al. Acute kidney injury in sepsis. Intensive Care Med 2017; 43:816–828 [DOI] [PubMed] [Google Scholar]
- 6.Romagnoli S, Ricci Z, Ronco C. CRRT for sepsis-induced acute kidney injury. Curr Opin Crit Care 2018; 24:483–492 [DOI] [PubMed] [Google Scholar]
- 7.Nusshag C, Weigand MA, Zeier M, et al. Issues of acute kidney injury staging and management in sepsis and critical illness: A narrative review. Int J Mol Sci 2017; 18:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 2016; 315:2190–2199 [DOI] [PubMed] [Google Scholar]
- 9.Gaudry S, Hajage D, Schortgen F, et al. ; AKIKI Study Group: Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375:122–133 [DOI] [PubMed] [Google Scholar]
- 10.Barbar SD, Clere-Jehl R, Bourredjem A, et al. ; IDEAL-ICU Trial Investigators and the CRICS TRIGGERSEP Network: Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018; 379:1431–1442 [DOI] [PubMed] [Google Scholar]
- 11.Saleem MA. What is the role of soluble urokinase-type plasminogen activator in renal disease? Nephron 2018; 139:334–341 [DOI] [PubMed] [Google Scholar]
- 12.Dande RR, Peev V, Altintas MM, et al. Soluble urokinase receptor and the kidney response in diabetes mellitus. J Diabetes Res 2017; 2017:3232848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayek SS, Sever S, Ko YA, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med 2015; 373:1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossanen JC, Pracht J, Jansen TU, et al. Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci 2017; 18:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein SJ, Brandtner AK, Lehner GF, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: A systematic review and meta-analysis. Intensive Care Med 2018; 44:323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–377 [DOI] [PubMed] [Google Scholar]
- 18.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group: Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care 2013; 17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch A, Voigt S, Kruschinski C, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care 2011; 15:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostermann M, McCullough PA, Forni LG, et al. ; all SAPPHIRE Investigators: Kinetics of urinary cell cycle arrest markers for acute kidney injury following exposure to potential renal insults. Crit Care Med 2018; 46:375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One 2014; 9:e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alge JL, Arthur JM. Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 2015; 10:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dusse F, Edayadiyil-Dudásova M, Thielmann M, et al. Early prediction of acute kidney injury after transapical and transaortic aortic valve implantation with urinary G1 cell cycle arrest biomarkers. BMC Anesthesiology 2016; 16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gocze I, Koch M, Renner P, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One 2015; 10:e0120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyner JL, Davison DL, Brasha-Mitchell E, et al. Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc VNephrol 2015; 26:2023–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pianta TJ, Peake PW, Pickering JW, et al. Evaluation of biomarkers of cell cycle arrest and inflammation in prediction of dialysis or recovery after kidney transplantation. Transpl Int 2015; 28:1392–1404 [DOI] [PubMed] [Google Scholar]
- 28.Honore PM, Nguyen HB, Gong M, et al. ; Sapphire and Topaz Investigators: Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med 2016; 44:1851–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuartero M, Ballús J, Sabater J, et al. Cell-cycle arrest biomarkers in urine to predict acute kidney injury in septic and non-septic critically ill patients. Ann Intensive Care 2017; 7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014; 189:932–939 [DOI] [PubMed] [Google Scholar]
- 31.Hoste EA, McCullough PA, Kashani K, et al. ; Sapphire Investigators: Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant 2014; 29:2054–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Backes Y, van der Sluijs KF, Mackie DP, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: A systematic review. Intensive Care Med 2012; 38:1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall A, Crichton S, Varrier M, et al. suPAR as a marker of infection in acute kidney injury - a prospective observational study. BMC Nephrol 2018; 19:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medeiros P, Nga HS, Menezes P, et al. Acute kidney injury in septic patients admitted to emergency clinical room: Risk factors and outcome. Clin Exp Nephrol 2015; 19:859–866 [DOI] [PubMed] [Google Scholar]


