Abstract
The purpose of this study was to examine how adherence to the physical activity (PA), screen-time (ST), and sleep duration guidelines differ between youth with autism spectrum disorder (ASD) and youth with typical development (TD). A secondary objective was to assess how PA, ST, and sleep duration varied among youth with ASD by age and ASD severity. Utilizing the 2016 National Survey of Children’s Health data, parental reports of time spent by youth in PA, ST, and sleep were used to determine adherence to the 24-hr movement guidelines for 1008 youth with ASD and 34 489 youth with TD. Multivariate logistic regression analyses determined that children with ASD were less likely to meet the guidelines for PA, ST, and sleep duration, and adolescents with ASD were less likely to meet the guidelines for PA and ST than participants with TD. Furthermore, logistic regression analyses determined adolescents with severe ASD to be less likely to meet the sleep guideline compared to adolescents with mild ASD. Overall, youth with ASD were significantly less likely to adhere to all three guidelines. The findings highlight the breadth of health behaviors that require intervention to counteract the poorer health status among youth with ASD.
Keywords: physical activity, screen-time, sleep, autistic, exercise, sedentary behavior, health, obesity
Lay Summary:
New health recommendations suggest children and adolescents should have at least 1 hr of physical activity, no more than 2 hr of screen-time (e.g., television), and 9–11 hr of sleep (or 8–10 hr for children aged 14 or older) every day. This article looked at how children and adolescents with autism meet these new guidelines. The two main results were that: (a) children with autism were less likely to meet all three guidelines compared to children without autism, and (b) adolescents with autism were less likely to meet the guidelines for physical activity and screen-time.
Introduction
Compared to youth with typical development (TD), youth with autism spectrum disorder (ASD) have significantly higher rates of overweight (19.4% vs. 14.9%, OR = 1.48) and obesity (23.05% vs. 15.91%, OR= 1.49) [Healy, Aigner, & Haegele, 2018], and have disproportionately higher rates of obesity-related conditions, such as Type II diabetes (1.1% vs. 04%, respectively; OR = 2.68), hypertension (1% vs. 0.5%, respectively; OR = 2.04), and hyperlipidemia (3.3% vs. 1.7%, respectively; OR = 2.01) [Shedlock et al., 2016]. Modifiable risk factors proposed to contribute to obesity among this population include lower levels of physical activity (PA) [Ayvazoglu, Kozub, Butera, & Murray, 2015;Dreyer Gillette et al., 2015;McCoy, Jakicic, & Gibbs, 2016;Pan et al., 2016;Tyler, MacDonald, & Menear, 2014], high levels of screen-time (ST) [Macmullin, Lunsky, & Weiss, 2016; Must, Phillips, Curtin, & Bandini, 2015;Orsmond & Kuo, 2011], and poorer sleep health [Liu, Hubbard, Fabes, & Adam, 2006;Reynolds & Malow, 2011].
PA has been identified as a key factor in the promotion of health among children [Centers for Disease Control and Prevention [CDC], 2014;Poitras et al., 2016], including children with ASD [Healy, Nacario, Braithwaite, & Hopper, 2018;Sam, Chow, & Tong, 2015;Sowa & Meulenbroek, 2012]. For example, a meta-analysis by Healy et al. [2018] indicated that moderate to large positive effects are available for children with ASD who are exposed to PA interventions targeting the development of manipulative skills, locomotor skills, skill-related fitness, social functioning, and muscular strength and endurance. Worrisome, however, is evidence that youth with ASD tend to be less active and less likely to meet the PA guidelines compared to children with TD [Srinivasan, Pescatello, & Bhat, 2014]. For example, Pan [2008], utilizing objective data from 70 male children with ASD (n = 35) and TD (n = 35), indicated that only 37% of participants with ASD met the daily guideline of 60 min or more of moderate-to-vigorous physical activity (MVPA), compared to 60% of participants with TD. Other studies give credence to this difference, particularly among older children [Healy, Haegele, Grenier, & Garcia, 2017;Stanish et al., 2017]. Conversely, some data deviates from this trend and demonstrates that among younger children with and without ASD, PA levels are comparable [Bandini et al., 2013; Sandt & Frey, 2005]. Bandini and colleagues, for example, reported that objectively measured weekday PA levels of 53 children with ASD were comparable to that of 58 children with TD (ages 3–11 years) after adjustment for age and sex (50 vs. 57.1 min/day, respectively). In the same study, however, during weekends, PA levels of the children with ASD were significantly lower.
Sedentary behavior, often represented by ST, has also shown to be an independent risk factor for a plethora of negative outcomes, including unfavorable body composition, decreased fitness, lower self-esteem and pro-social behavior, and decreased academic achievement [Tremblay et al., 2011; van Ekris et al., 2016]. For children with ASD, who have a well-documented preferenzce for screen-based activities over more active pursuits [Eversole et al., 2016; Russell, Healy, & Braithwaite, 2019; Stanish et al., 2015], high levels of sedentariness is prevalent and problematic. Substantial evidence demonstrates that youth with ASD, particularly younger children, are more sedentary than their TD peers [Chonchaiya, Nuntnarumit, & Pruksananonda, 2011; Macmullin et al., 2016; Mazurek & Sohl, 2016;Must et al., 2014; Stiller & Mößle, 2018]. Must et al. [2014], for example, reported that children with ASD spent an hour more in sedentary behaviors, particularly screen-based activities, on weekdays compared to children with TD (5.2 vs. 4.2 hr). In contrast to this, however, are data showing that levels of sedentariness are comparable between youth with and without ASD, particular among adolescents (Dreyer Gillette et al., 2015; McCoy et al., 2016; Montes, 2016]. Regardless of differences compared to children with TD, high levels of ST among youth with ASD is a cause for concern as it has been suggested that the negative consequences of excessive ST are compounded for children with ASD. For example, Mcmullin and colleagues’ [2016] survey study of parents of children with (n = 139) and without (n = 172) ASD found that parents of children with ASD were significantly more likely to report that use of electronics (e.g., video games) negatively impacted their child’s life compared to parents of children without ASD. Furthermore they highlight that children with ASD with high levels of ST have less opportunities for developing social and academic skills [Macmullin et al., 2016]. Conversely, the beneficial uses of screen-based technologies for children with ASD should not be ignored, even if they contribute to additional minutes of sedentariness. For example, technology can be effectively used for the delivery of interventions targeting the core social-communication deficits of the disorder [Wainer & Ingersoll, 2011], and for facilitating teaching and learning via strategies such as video modeling and computer-based instruction [Burton, Anderson, Prater, & Dyches, 2013; McCoy, Holloway, Healy, Rispoli, & Neely, 2016].
The significance of sleep for the promotion of health among youth is supported by multiple meta-analyses, demonstrating positive relationships between longer sleep duration and lower adiposity, better emotional regulation, better academic achievement, and better quality of life/well-being [Chaput et al., 2016;Wu, Gong, Zou, Li, & Zhang, 2017]. Additionally, among children with ASD, poorer sleep health is associated with increases in daytime challenging behaviors, including physical aggression, irritability, inattention, and hyperactivity [Cortesi, Giannotti, Ivanenko, & Johnson, 2010;Johnson et al., 2018;Mazurek & Sohl, 2016], and increased family stress [Johnson et al., 2018]. There is a general consensus in the literature that children with ASD have poorer sleep health (including shorter sleep duration) compared to their TD peers [Krakowiak, Goodlin-Jones, Hertz-Picciotto, Croen, & Hansen, 2008;Richdale & Schreck, 2009;van der Heijden, Stoffelsen, Popma, & Swaab, 2018]. For example, utilizing parent reports of sleep behaviors among 529 children (303 ASD and 163 TD), children with ASD tended to sleep about half an hour less than their TD counterparts [Krakowiak et al., 2008]. The literature on adolescents with ASD is less conclusive. Although some evidence suggests sleep duration is comparable between adolescents with ASD and TD [Baker, Richdale, Short, & Gradisar, 2013;Dreyer Gillette et al., 2015], other studies report contradictory findings, demonstrating that sleep problems tend to persist among children with ASD into adolescence, whereas remission with age is typical among children with TD [Hodge, Carollo, Lewin, Hoffman, & Sweeney, 2014;Sivertsen, Posserud, Gillberg, Lundervold, & Hysing, 2012].
Traditionally, among youth with TD and ASD, the health behaviors of PA, SB, and sleep have been largely viewed as independent contributors to health, and patterns of these behaviors have been studied in isolation. Now, researchers are increasingly recognizing the multi-etiological nature of health outcomes, such as obesity, and have therefore proposed 24-hr movement guidelines that provide recommended amounts of time, across the 24-hr period, that should be spent in PA, ST, and sleep. The guidelines state that youth should engage in at least 1 hr of PA, no more than 2 hr of ST, and 9–11 hr of sleep (or 8–10 for children aged 14 or older) [Tremblay et al., 2016]. The utility of the 24-hr movement guidelines has been demonstrated in youth with TD where those who met the 24-hr movement guidelines were 72% less likely to be obese than those who do not meet them [Roman-Viñas et al., 2016]. Moreover, meeting less than three guidelines is associated with a higher BMI and lower fitness compared to meeting all three guidelines [Carson, Chaput, Janssen, & Tremblay, 2017]. Adherence to the 24-hr movement guidelines in youth (children and adolescents) with ASD as compared to youth with TD has yet to be determined.
The purpose of this descriptive study was to utilize the 2016 National Survey of Children’s health (NSCH) data to examine adherence to the individual guidelines for PA, ST, and sleep duration, and concurrent adherence to portions of the guidelines (meeting all, some, or none), in youth with ASD and youth with TD. A secondary purpose was to assess how adherence to the guidelines differed among youth with ASD by age and ASD severity (mild, moderate, and severe). An updated understanding of PA, ST, and sleep behaviors among children with ASD may inform the development of policy, allocation of resources, and the prioritization of intervention targets [Skinner, Perrin, & Skelton, 2016].
Methods
Data Source
To examine the proposed research questions, the NSCH’s 2016 data were used. The NSCH is a population-based cross-sectional survey of the health and well-being of US children, sponsored by the Health Resources and Services Administration’s Maternal and Child Health Bureau, and undertaken by the US Census Bureau [Maternal and Child Health Bureau in collaboration with the US Census Bureau, 2018]. The 2016 NSCH data collection cycle was conducted between June 2016 and February 2017, and integrates two surveys, the NSCH and the National Survey of Children with Special Health Care Needs. Initially, a sample of 364 150 households was selected from the Census Master Address File; the sample was stratified by state and child-presence indicator to allow for the Census Bureau to oversample households that were more likely to have children. During data collection, a screener was used to identify households with children. Of households with children, one was randomly selected to be the subject of the topical survey. The survey respondent was a parent or guardian with knowledge about the child’s health and health care needs. The weighted response rate for the 2016 NSCH was 40.7%. Of the 138 009 screener questionnaires completed, 67 047 households were identified as being eligible for participation. Of the eligible households 50 212 completed the topical questionnaire. The study sample is 1008 youth (ages 6–17) with ASD and 34 489 youth with TD. Information on the sampling, administration, and questionnaires can be accessed on http://childhealthdata.org/learn/NSCH.
Study Measures
Dependent Variables
PA was determined based on the parents’ report of how many days, during the past week, did the child exercise, play a sport, or participate in PA for at least 60 min. Parents selected from one of eight response options: “0 days,” “1 day,” … to “every day.” A dichotomous variable was created, categorizing the children as meeting the guidelines of 1 hr per day of PA (“every day”) or not (6 days or less) [Tremblay et al., 2016;US Department of Health and Human Services, 2008].
Sleep Duration was measured by the parents’ response to the question “during the past week, how many hours of sleep did this child get on an average weeknight?” Response options were “Less than 6 hr,” “7–8 hr,” “9–10 hr,” or “11 or more hours.” A dichotomous variable for adherence to sleep duration guidelines was generated. Children (ages 6–12 years) reporting 9–12 hr per 24 hr, and adolescents (ages 13–18 years) reporting 8–10 hr per 24 hr were defined as adherent [Paruthi et al., 2015;Tremblay et al., 2016].
Screen time was determined based on the parents’ response to the question “on an average weekday about how much time does this child usually spend in front of a TV watching TV programs, videos, or playing video games?” Response options were “none,” “less than 1 hr,” “1 hr,” “2 hr,” “3 hr,” or “4 or more hours.” A dichotomous variable for adherence to the guideline of less than or equal to 2 hr of ST per day was created: 0 = ≤2 hr (i.e., meeting the guideline), and 1 = >2 hr [Tremblay et al., 2016].
Independent Variable
ASD status was determined by a response of “yes” to two questions posed to the parents;(a) “Has a doctor or other health care provider ever told you that this child has Autism or Autism Spectrum Disorder?” Parents were prompted to include diagnoses of Asperger’s Disorder or Pervasive Developmental Disorder, and (b) “does this child currently have the condition?” (Yes/No). As used in previous research [Must et al., 2017], a positive response was required to both questions. Respondents also reported if their child’s diagnosis was “mild,” “moderate,” or “severe.”
Demographic Variables
Demographic variables included age (years), sex (male/female), race/ethnicity (Hispanic, white, black, multiracial, and other), and household income level (percentage of the federal poverty level; FPL). Age was also included in the original NSCH dataset as a dichotomized variable with children (6–11 years) and adolescents (12–17 years) (together defined as “youth”).
Data Analysis
All analyses were conducted using the Statistical Package for the Social Science (SPSS) Complex Samples module. The 2016 NSCH sampling plan (.csaplan) for SPSS was used in all analysis to apply sampling weights. Subpopulations were specified in SPSS Complex Samples for children ages 6–12 and adolescents ages 13–17. All analyses were performed separately for children and adolescents. Descriptive statistics for all study variables were generated. Continuous variables were described using mean and standard deviation, categorical variables using counts and percentages. Separate multivariable logistic regressions were generated for the outcomes of meeting PA, ST, and sleep duration guidelines. ASD status (ASD vs. TD) was entered into each model as the predictor of interest and all models were adjusted for race/ethnicity, income, age, and sex. To assess how adherence to PA, ST, and sleep duration guidelines differed among youth with ASD by age and ASD severity, separate logistic regressions were generated for each outcome; age and ASD severity were entered as the predictors of interest, and sex, race, income, and age included as covariates. Only children and adolescents with ASD were included in this analysis. Lastly, to examine if the number of guidelines met (meeting all, some, or none) differed by ASD status, binary variables were created representing meeting all guidelines versus none, and meeting no guidelines versus meeting some guidelines. A logistic regression was generated for the binary outcomes of numbers of guidelines met. ASD status (ASD vs. TD) was entered as the predictor of interest and models were adjusted for race/ethnicity, income, age, and sex. Statistical significance was set at the 0.05 level.
Results
Sample Characteristics
The child sample (ages 6–12 years) included 507 youth with ASD (weighted n = 752 152) and 17 326 youth with TD (weighted n = 27 867 416). The adolescent sample (ages 13–17 years) included 501 youth with ASD (weighted n = 541 725) and 17 163 youth with TD (weighted n = 20 054 377). The percentage of youth with an ASD diagnosis was 2.6% among both children and adolescents. Among children with ASD, the percentage with mild, moderate, and severe ASD were 44.25%, 47.38%, and 8.36%, respectively. Among adolescents with ASD, the percentage with mild, moderate, and severe ASD were 46.34%, 44.83%, and 8.83%, respectively.
Compared to children with TD, children with ASD were significantly more likely to be male (84.02% vs. 49.97%, P < 0.01), and at or above the FPL (31.97% vs. 21.78%, P = 0.04). Race/ethnicity did not significantly differ between groups; approximately 50% of children were white/non-Hispanic. Compared to adolescents with TD, adolescents with ASD were significantly more likely to be male (80.64% vs. 50.35%, P < 0.01), and white/non-Hispanic (66.67% vs. 55.53%, P < 0.01). Fewer adolescents with ASD were Hispanic, compared to adolescents with TD (10.68% vs. 24.41%, P < 0.01). See Table 1 for a full listing of the descriptive statistics of the samples.
Table 1.
Descriptive Statistics for Model Variables by Children (Ages 6–12) and Adolescents (Ages 13–17)
| N (%) or Mean (SE) | Children (ages 6–12) | Adolescents (ages 13–17) | ||||
|---|---|---|---|---|---|---|
| ASD diagnosis | P value | ASD diagnosis | P value | |||
| No | Yes | No | Yes | |||
| (n = 27 867 416) | (n = 752 152) | (n = 20 054 377) | (n = 541 725) | |||
| Sex (male) | 49.97 | 84.02 | <0.01** | 50.35 | 80.64 | <0.01** |
| Age (M, SD) | 9.0 (0.03) | 8.94 (0.20) | 0.77 | 15.0 (0.02) | 14.78 (0.11) | 0.07 |
| Poverty level of household | 0.04* | 0.35 | ||||
| 0–99% FPL | 21.78 | 31.97 | 21.45 | 25.39 | ||
| 100% FPL or greater | 78.22 | 69.03 | 78.54 | 74.61 | ||
| Race/ethnicity | 0.81 | <0.01** | ||||
| Hispanic | 25.83 | 26.58 | 24.41 | 10.68 | ||
| White/non-Hispanic | 50.65 | 50.19 | 52.53 | 66.67 | ||
| Black/non-Hispanic | 12.62 | 13.32 | 13.82 | 16.29 | ||
| Other/multiracial non-Hispanic | 10.90 | 9.91 | 9.22 | 6.36 | ||
| Physical activity | 0.09 | 0.01* | ||||
| Does not meet guidelines | 71.38 | 77.82 | 81.45 | 89.39 | ||
| Meets guidelines | 28.62 | 22.18 | 18.55 | 10.63 | ||
| Screen time | 0.02* | <0.01** | ||||
| 2 hr or less | 79.77 | 70.07 | 72.05 | 59.90 | ||
| >2 hr | 20.23 | 29.93 | 27.98 | 40.10 | ||
| Sleep | <0.01** | 0.61 | ||||
| Does not meet | 35.72 | 51.59 | 30.07 | 32.19 | ||
| Guidelines | ||||||
| Meets guidelines | 64.27 | 48.41 | 69.93 | 67.81 | ||
| Portions of guidelines meet | 0.03* | <0.01** | ||||
| Meet all | 12.89 | 6.54 | 10.69 | 4.38 | ||
| Meet 2 of 3 | 42.79 | 36.38 | 46.70 | 42.28 | ||
| Meet 1 of 3 | 25.17 | 45.98 | 35.23 | 40.84 | ||
| Meet none | 9.14 | 12.11 | 7.37 | 12.51 | ||
P < 0.05
P < 0.01.
Physical Activity
Across both child and adolescent samples, those with ASD were less likely to meet PA guidelines (see Table 2). Specifically, in a multivariable model of PA, children with ASD had a 39% reduced odds of meeting activity guidelines than TD children [22.18% vs. 28.62%, respectively; adjusted odds ratio (aOR) = 0.61, 95% CI: 0.40, 0.92]. Age and severity of ASD were not related to meeting the PA guideline among children. Among adolescents, those with an ASD diagnosis were 59% less likely to meet PA guidelines than TD adolescents [10.63% vs. 18.55%; aOR = 0.41, 95% CI: 0.24, 0.68] (see Table 3). Older adolescents with ASD were less likely to meet the PA guidelines than younger adolescents with ASD [OR = 0.68, 95% CI: 0.48,0.96].
Table 2.
Odds Ratios for the Adjusted Models for Sleep, Physical Activity, and Screen Time among Children Ages 6–12
| Odds ratio or beta, adjusted model for meeting sleep guidelines |
Odds ratio or beta, adjusted model for meeting physical activity guidelines |
Odds ratio or beta, adjusted model for meeting screen time guidelines |
|
|---|---|---|---|
| Sex | |||
| Male | 1.03 (0.88, 1.20) | 1.36 (1.17, 1.59)** | 0.83 (0.69, 0.99)* |
| Female | 1.0 | 1.0 | 1.0 |
| Age | 0.86 (0.83, 0.89)** | 0.88 (0.85, 0.92)** | 0.94 (0.89, 0.98)* |
| Autism | |||
| Yes | 0.52 (0.36, 0.75)** | 0.61 (0.40, 0.92)* | 0.64 (0.42, 0.98)* |
| No | 1.0 | 1.0 | 1.0 |
| Poverty level of household | |||
| 0–99% FPL | 1.0 | 1.0 | 1.0 |
| 100% FPL or greater | 1.64 (1.33, 2.03)** | 0.66 (0.54, 0.82)** | 1.28 (1.01, 1.62)* |
| Race/ethnicity | |||
| Hispanic | 0.70 (0.53, 0.93)* | 0.90 (0.65, 1.25) | 0.64 (0.46, 0.87)** |
| White/non-Hispanic | 1.30 (1.06, 1.60)* | 1.36 (1.07, 1.71)* | 1.19 (0.93, 1.52) |
| Black/non-Hispanic | 0.43 (0.33, 0.53)** | 1.33 (0.98, 1.83) | 0.48 (0.35, 0.66)** |
| Other/multiracial non-Hispanic | 1.0 | 1.0 | 1.0 |
FLP, Federal poverty level.
P < 0.05
P < 0.01.
Table 3.
Odds Ratios for the Adjusted Models for Sleep, Physical Activity, and Screen Time among Adolescents Ages 13–17
| Odds ratio or beta, adjusted model for sleep for meeting sleep guidelines |
Odds ratio or beta, adjusted model for physical activity for meeting physical activity guidelines |
Odds ratio or beta, adjusted model for meeting screen time guidelines |
|
|---|---|---|---|
| Sex | |||
| Male | 1.06 (0.91, 1.23) | 2.17 (1.78, 2.65)** | 0.60 (0.51, 0.70)** |
| Female | 1.0 | 1.0 | 1.0 |
| Age | 0.71 (0.67, 0.75)** | 0.98 (0.91, 1.06) | 1.02 (0.97, 1.08) |
| Autism | |||
| Yes | 0.83 (0.57, 1.19) | 0.41 (0.24, 0.68)** | 0.67 (0.47, 0.95)* |
| No | 1.0 | 1.0 | 1.0 |
| Poverty level of household | |||
| 0–99% FPL | 1.0 | 1.0 | 1.0 |
| 100% FPL or greater | 1.26 (1.0, 1.59)* | 0.64 (0.48, 0.84)** | 1.28 (1.03, 1.59)* |
| Race/ethnicity | |||
| Hispanic | 1.22 (0.90, 1.65) | 1.03 (0.71, 1.51) | 0.85 (0.63, 1.14) |
| White/non-Hispanic | 1.32 (1.06, 1.64)* | 1.02 (0.79, 1.34) | 1.01 (0.82, 1.26) |
| Black/non-Hispanic | 0.68 (0.50, 0.93)* | 1.14 (0.77, 1.69) | 0.47 (0.35, 0.63)** |
| Other/multiracial non-Hispanic | 1.0 | 1.0 | 1.0 |
FLP, Federal poverty level.
P < 0.05
P < 0.01.
Screen-Time
Across both child and adolescent samples, those with ASD were less likely to meet ST guidelines. More specifically, children with ASD were less likely to meet the ST guidelines compared to children with TD, in the adjusted model including covariates [70.07% vs. 79.77%, respectively, aOR = 0.64, 95% CI: 0.42, 0.98]. Similarly, for adolescents, those with ASD were less likely to meet the ST guidelines, in the adjusted model [59.90% vs. 72.05%, respectively, aOR = 0.67, 95% CI: 0.47, 0.95] (see Table 3). For children and adolescents with ASD, age and severity of ASD were not related to meeting ST guidelines (see Table 2).
Sleep Duration
Children with ASD were less likely to meet the sleep duration guideline than children with TD, in the adjusted model [48.41% vs. 64.27%, respectively, aOR = 0.52, 95% CI: 0.36, 0.75]. For children with ASD, age and severity of ASD were not related to meeting sleep duration guidelines (see Table 2). Having an ASD diagnosis was not associated with meeting the sleep duration guidelines in the adjusted model;32.19% of adolescents with ASD and 30.07% of adolescents with TD met the sleep duration guideline (see Table 3). For adolescents with ASD, age was not related to the likelihood of meeting sleep duration guidelines. Severity of ASD status was related to meeting the sleep duration guideline; adolescents with severe ASD were less likely to meet the recommended levels of sleep duration compared to adolescents with mild ASD [OR = 0.23, 95% CI: 0.09, 0.61].
Level of Adherence to 24-hr Movement Guidelines
Children with ASD most commonly adhered to one 24-hr movement guideline (45.98%); conversely, children with TD most commonly adhered to two guidelines (42.79%). Children with ASD were significantly less likely to adhere to all three guidelines compared to children with TD (6.54% vs. 12.89%, respectively, aOR = 0.40, 95% CI: 0.17, 0.91). Adolescents with ASD and adolescents with TD most commonly adhered to two 24-hr movement guidelines, 42.28% and 46.70%, respectively. Adolescents with ASD were significantly less likely to adhere to all three guidelines compared to adolescents with TD (4.38% vs. 10.69%, respectively, aOR = 0.30, 95% CI: 0.17, 0.52) (see Table 1).
Discussion
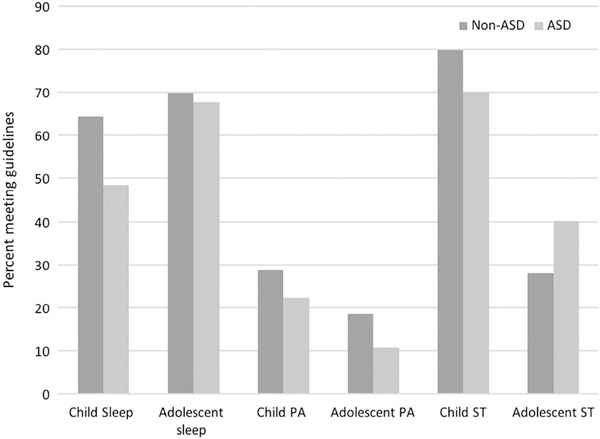
This study examined how adherence to the individual 24-hr movement guidelines for PA, ST, and sleep duration, and concurrent adherence to portions of the guidelines (meeting all, some, or none), compared between youth with ASD and youth with TD. A secondary purpose was to assess how PA, ST, and sleep duration varied among youth with ASD by age and ASD severity. The main findings from this study are that children with ASD were less likely to meet PA, ST, and sleep guidelines, and adolescents with ASD were less likely to meet PA and ST guidelines (see Fig. 1). Furthermore, older adolescents with ASD were less likely to meet the PA guidelines than younger adolescents with ASD, and adolescents with severe ASD were less likely to meet the recommended levels of sleep duration compared to adolescents with mild ASD. Finally, both children and adolescents with ASD were significantly less likely to adhere to all three 24-hr movement guidelines compared to children and adolescents with TD.
Figure 1.
Proportion of children and adolescents meeting health behaviors guidelines by ASD status.
The finding that children with ASD were 39% less likely to meet the PA guideline as compared to their TD counterparts both converges and contrasts with the current literature. Previous studies comparing PA levels among children with and without ASD are mixed. Some studies report that levels of PA among children with ASD are lower than in children with TD [Pan et al., 2016; Tyler et al., 2014]. For example, Tyler et al. [2014] reported that 17 children with ASD (mean age = 12.6 years) spent 165.9 min in MVPA compared to 218.3 min spent in MVPA by 12 peers with TD. Conversely, other studies have reported that children with ASD have comparable levels of PA to children with TD [Bandini et al., 2013; Sandt & Frey, 2005]. For example, Bandini et al. [2013] reported the mean minutes of MVPA per day among 53 children ages 3–11 years with ASD to be 50 min as compared to 57.1 min in 58 children with TD (P = NS). In terms of the adolescent sample, the current study’s finding that adolescents with ASD were 59% less likely to meet the PA guidelines as compared to their TD counterparts converges with the general consensus in the literature (Dreyer Gillette et al., 2015;McCoy et al., 2016;Pan et al., 2016;Stanish et al., 2017;Tyler et al., 2014]. For example, McCoy et al. [2016] reported adolescents (ages 10–17) with ASD from the 2012 NSCH dataset to be 60% less likely to engage in regular PA compared to adolescents with TD. It should be noted that there remains considerably heterogeneity in the literature regarding measurement of PA (e.g., subjective and objective measures) and attributes of the participants (e.g., severity of ASD); this heterogeneity may contribute the variability seen in the reported PA patterns in this population.
Regarding the effect of age, levels of adherence to the PA guideline were seen to be lower for adolescents compared to children;a finding that reflects trends among TD youth [National Physical Activity Plan Alliance, 2018]. This difference was particularly apparent for the sample with ASD; the percentage of adolescents with ASD adhering to the PA guideline was less than half that of children with ASD (10.63% vs. 22.18%, respectively). Furthermore, age was associated with meeting the PA guidelines among the adolescents with ASD; older adolescents were less likely to meet the PA guidelines. This is consistent with the previous studies that report a decline in PA levels with age among youth with ASD; for example, Macdonald, Esposito, and Ulrich’s [2011] analysis of the PA levels of 72 youth with ASD demonstrated older children to be significantly more physical inactive compared to younger children. Similarly, Pan and Frey [2005] reported elementary school children with ASD to be significantly more active than middle and high school adolescents with ASD, as measured using accelerometers.
Another key finding from this study is that children with ASD were 36% less likely to meet the ST guideline than children with TD. These data are consistent with the literature showing elevated levels of ST among this population [Chonchaiya et al., 2011;Must et al., 2014; Stiller & Mößle, 2018]. For example, Must et al. [2014] compared time spent in sedentary behaviors between children with ASD and children with TD (ages 3–11); children with ASD were shown to spend an hour more in sedentary behaviors on weekdays compared to children with TD (5.2 vs. 4.2 hr) and most of this difference was due to ST activities. Data from the current study also showed that adolescents with ASD were 33% less likely to meet the ST guidelines compared to adolescents with TD. Previous research comparing ST behavior between adolescents with ASD and adolescents with TD has been mixed (Dreyer Gillette et al., 2015;Mazurek, Shattuck, Wagner, & Cooper, 2012;Mazurek & Wenstrup, 2013; McCoy et al., 2016;Stiller & Mößle, 2018]. For example, research utilizing data from the 2012 NSCH showed insignificant differences in screen time between youth, ages 10–17, with ASD and TD [Dreyer Gillette et al., 2015; McCoy et al., 2016]. Conversely, there is also evidence for higher rates of ST behavior among adolescents with ASD. For example, Mazurek and Wenstrup [2013] compared screen-based activities between children with ASD and siblings with TD (ages 8–18) and found that children with ASD spent more time (approximately 50 min) playing video games per day. It should also be noted the screen-based devices for youth with ASD may be utilized as facilitators of PA. For example, video modeling and exergaming may be used to promote PA for youth with ASD [Caro, Tentori, Martinez-Garcia, & Alvelais, 2017; Edwards, Jeffre, Jeffre, Rinehart, & Barnett, 2016]. Future research should seek to differentiate how ST can facilitate and inhibit PA among this population.
Data from the current study showed that children with ASD were nearly half as likely to meet the sleep duration guideline compared to children with TD. This is in-line with the general consensus in the literature that sleep problems (including shorter sleep duration) are pervasive among children with ASD [Krakowiak et al., 2008;Richdale & Schreck, 2009;van der Heijden et al., 2018]. For example, van der Heijden et al. [2018] reported sleep duration to be lower among children ages 6–12 years with ASD compared to controls with TD during weekdays (588 vs. 628 min) and weekends (604 vs. 636 min). In the current adolescent samples, adherence to the sleep duration guideline was comparable between the ASD and TD groups: this finding is supported by previous work [Baker et al., 2013; Dreyer Gillette et al., 2015]. Baker et al. [2013], for example, reported that the total sleep time of 14 adolescents with ASD did not significantly differ from 25 adolescents with TD, as measured using actigraphy (470 vs. 492 min per night). Other research, however, has demonstrated contradictory findings in that sleep problems tended to persist among children with ASD, whereas remission with age is typical among children with TD [Hodge et al., 2014; Sivertsen et al., 2012]. These data also showed that severity of ASD status was related to meeting sleep duration guidelines; adolescents with severe ASD were 76.7% less likely to meet the recommended levels of sleep duration compared to adolescents with mild ASD. This result reflects the findings of Sivertsen et al. [2012] who documented a significant association between sleep problems and severity of ASD symptoms.
Adherence to 24-hr Movement Guidelines
The current study extends use of the new 24-hr movement guidelines to youth with ASD. Of particular interest in the current study are the data showing that children with ASD were 60% less likely to meet all three guidelines compared to children with TD, and adolescents with ASD were 70% less likely to meet all three guidelines compared to adolescents with TD, when adjusting for race/ethnicity, income, age, and sex. The low level of adherence to the 24-hr movement guidelines, identified in the current study, has several implications for future research and practice. First, the current findings prompt examination of the associations between combinations of time spent in PA, ST, and sleep with health indicators among youth with ASD. The relationship between the movement behaviors and health outcomes is well established for children with TD. For example, meeting less than three guidelines, compared to meeting all three guidelines, is associated with higher triglycerides and lower HDL-cholesterol [Chaput, Carson, Gray, & Tremblay, 2014], higher levels of obesity [Roman-Viñas et al., 2016], and lower levels of aerobic fitness [Carson et al., 2017]. The cumulative effect of multiple risk factors relating to PA, ST, and sleep has yet to be examined among youth with ASD. Longitudinal and intervention studies are required to better understand the effects of replacing time in one behavior with another [Saunders et al., 2016]. Furthermore, in addition to a focus on health outcomes, the movement behaviors’ associations with other outcomes relevant for individuals with ASD should also be examined, such as behavioral, social, and psychological outcomes.
Second, the current study’s use of the 24-hr movement framework has implications for practice. To date, interventions impacting on the health of youth with ASD tend to have a singular focus. A singular focus on PA, for example, is evident in obesity prevention interventions with children with ASD, and has produced limited success [Lourenco, Esteves, Corredeira, & Seabra, 2015; Pan, 2011;Pitetti, Rendoff, Grover, & Beets, 2007]. Similarly, PA is commonly examined in isolation as a means to affect outcomes such as academic engagement, self-stimulatory behaviors, and challenging behaviors [Cannella-Malone, Tullis, & Kazee, 2011;Celiberti, Bobo, Kelly, Harris, & Handleman, 1997;Nicholson, Kehle, Bray, & Van Heest, 2011;0riel, George, Peckus, & Semon, 2011]. Our finding that multiple negative health behaviors co-occur at a higher frequency among youth with ASD suggests multimodal health behavior change approaches should be considered in future research (i.e., interventions that tackle multiple health behaviors simultaneously). A precursor to this may be the objective longitudinal measurement of each 24-hr movement behavior and outcomes of interest (e.g., body mass index, quality of life), therefore allowing for the independent and the cumulative effects of these behaviors to be better understood. The potential temporal relationships that exist between the behaviors also require examination (e.g., how does PA influence sleep, and vice versa). This understanding will assist intervention developers in identifying the behaviors that should be prioritized for optimal intervention efficacy.
Limitations
Several limitations must be considered when interpreting the results of this study. First, the response biases and error that may be associated with parental report variables should be noted. Due to the large-scale nature of the NSCH data collection, there was a reliance on subjective measures for all variables used. Furthermore, despite frequent usage of the health behaviors measures utilized in the current study for youth with ASD [e.g., Corvey, Menear, Preskitt, Goldfarb, & Menachemi, 2016; Dreyer Gillette et al., 2015; McCoy et al., 2016], they are not validated measures. Indeed, there is little research on the validity of parental report for health behaviors of children with ASD. In one of the few studies pertaining to this issue, Veatch et al. [2016] examined the relationship between measures of sleep captured by parent report and objective measures, demonstrating the subjective measures were effective in accurately capturing sleep duration. Furthermore, parent education improved the accuracy of parental report method. Future researchers should continue to strive to utilize objective measures, and work to educate parents when they are in the role of reporting on health behaviors. This rings true for the parent reporting of ASD severity as well. More objective measures, such as utilizing the ADOS, are required for a more accurate portrayal of this heterogeneous population. The effect of social desirability on the accuracy of parental reporting, for children with ASD, should also be examined. It is logical to suggest that parenting a child with ASD can affect the social desirability experience. A second limitation involves the inability for the NSCH 2016 data to be used for examining trends with previous iterations of the NSCH. Although efforts were made in the discussion section to compare and contrast with results from previous research on the NSCH (e.g., 2011/2012 and 2007), statistical trend analyses are not possible. Questions, for example, on sleep have changed from previous years. The redesigned NSCH will allow for trend analysis using future waves of the NSCH.
Conclusions
The current study provides an update on how adherence to the 24-hr movement guidelines compared between youth with ASD and youth with TD. Analysis of the 2016 NSCH data showed children with ASD to be less likely to meet the guidelines for PA, ST, and sleep duration and adolescents with ASD to be less likely to meet the PA and ST guidelines. Moreover, children and adolescents with ASD were significantly less likely to adhere to all three 24-hr movement guidelines compared to children and adolescents with TD. The findings highlight the breadth of health behaviors that require intervention to counteract the poorer health status among this population of youth.
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
References
- Ayvazoglu NR, Kozub FM, Butera G, & Murray MJ (2015). Determinants and challenges in physical activity participation in families with children with high functioning autism spectrum disorders from a family systems perspective. Research in Developmental Disabilities, 47, 93–105. 10.1016/j.ridd.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Baker E, Richdale A, Short M, & Gradisar M (2013). An investigation of sleep patterns in adolescents with high-functioning autism spectrum disorder compared with typically developing adolescents. Developmental Neurorehabilitation, 16(3), 155–165. 10.3109/17518423.2013.765518. [DOI] [PubMed] [Google Scholar]
- Bandini LG, Gleason J, Curtin C, Lividini K, Anderson SE, Cermak SA, … Must A (2013). Comparison of physical activity between children with autism spectrum disorders and typically developing children. Autism, 17(1), 44–54. 10.1177/1362361312437416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CE, Anderson DH, Prater MA, & Dyches TT (2013). Video self-modeling on an iPad to teach functional math skills to adolescents with autism and intellectual disability. Focus on Autism and Other Developmental Disabilities, 28(2), 67–77. 10.1177/1088357613478829. [DOI] [Google Scholar]
- Cannella-Malone HI, Tullis CA, & Kazee AR (2011). Using antecedent exercise to decrease challenging behavior in boys with developmental disabilities and an emotional disorder. Journal of Positive Behavior Interventions, 13(4), 230–239. 10.1177/1098300711406122. [DOI] [Google Scholar]
- Caro K, Tentori M, Martinez-Garcia AI, & Alvelais M (2017). Using the FroggyBobby exergame to support eye-body coordination development of children with severe autism. International Journal of Human-Computer Studies, 105, 12–27. 10.1016/JJJHCS.2017.03.005. [DOI] [Google Scholar]
- Carson V, Chaput J-P, Janssen I, & Tremblay MS (2017). Health associations with meeting new 24-hour movement guidelines for Canadian children and youth. Preventive Medicine, 95, 7–13. https://doi.org/10.1016Zj.ypmed.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Celiberti DA, Bobo HE, Kelly KS, Harris SL, & Handleman JS (1997). The differential and temporal effects of antecedent exercise on the self-stimulatory behavior of a child with autism. Research in Developmental Disabilities, 18(2), 139–150. 10.1016/S0891-4222(96)00032-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2014). 2014 State Indicator Report on Physical Activity, Atlanta, GA: Retrieved from http://www.cdc.gov/physicalactivity/resources/reports.html. [Google Scholar]
- Chaput J-P, Carson V, Gray C, & Tremblay M (2014). Importance of all movement behaviors in a 24 hour period for overall health. International Journal of Environmental Research and Public Health, 11(12), 12575–12581. 10.3390/ijerph111212575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput J-P, Gray CE, Poitras VJ, Carson V, Gruber R, Olds T, … Tremblay MS (2016). Systematic review of the relationships between sleep duration and health indicators in school-aged children and youth. Applied Physiology, Nutrition, and Metabolism, 41(6 (Suppl. 3)), S266–S282. 10.1139/apnm-2015-0627. [DOI] [PubMed] [Google Scholar]
- Chonchaiya W, Nuntnarumit P, & Pruksananonda C (2011). Comparison of television viewing between children with autism spectrum disorder and controls. Acta Paediatrica, 100(7), 1033–1037. 10.1111/j.1651-2227.2011.02166.x. [DOI] [PubMed] [Google Scholar]
- Cortesi F, Giannotti F, Ivanenko A, & Johnson K (2010). Sleep in children with autistic spectrum disorder. Sleep Medicine, 11(7), 659–664. 10.1016/j.sleep.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Corvey K, Menear KS, Preskitt J, Goldfarb S, & Menachemi N (2016). Obesity, physical activity and sedentary behaviors in children with an autism spectrum disorder. Maternal and Child Health Journal, 20(2), 466–476. 10.1007/s10995-015-1844-5. [DOI] [PubMed] [Google Scholar]
- Dreyer Gillette M. L., Borner KB, Nadler CB, Poppert KM, Odar Stough C., Swinburne Romine R., & Davis AM (2015). Prevalence and health correlates of overweight and obesity in children with autism spectrum disorder. Journal of Developmental and Behavioral Pediatrics, 36(7), 489–496. 10.1097/DBP.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jeffre S, Jeffre S, Rinehart NJ, & Barnett LM (2016). Does playing a sports active video game improve object-control skills of children with autism spectrum disorder? Journal of Sport and Health Science, 6, 17–24. 10.1016/j.jshs.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eversole M, Collins DM, Karmarkar A, Colton L, Quinn JP, Karsbaek R, … Hilton CL (2016). Leisure activity enjoyment of children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 46(1), 10–20. 10.1007/s10803-015-2529-z. [DOI] [PubMed] [Google Scholar]
- Healy S, Aigner CJ, & Haegele JA (2018). Prevalence of overweight and obesity among US youth with autism spectrum disorder. Autism. 10.1177/1362361318791817. [DOI] [PubMed] [Google Scholar]
- Healy S, Haegele JA, Grenier M, & Garcia JM (2017). Physical activity, screen-time behavior, and obesity among 13-year olds in Ireland with and without autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(1), 49–57. 10.1007/s10803-016-2920-4. [DOI] [PubMed] [Google Scholar]
- Healy S, Nacario A, Braithwaite RE, & Hopper C (2018). The effect of physical activity interventions on youth with autism spectrum disorder: A meta-analysis. Autism Research, 11(6), 818–833. 10.1002/aur.1955. [DOI] [PubMed] [Google Scholar]
- Hodge D, Carollo TM, Lewin M, Hoffman CD, & Sweeney DP (2014). Sleep patterns in children with and without autism spectrum disorders: Developmental comparisons. Research in Developmental Disabilities, 35(7), 1631–1638. 10.1016/j.ridd.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Smith T, DeMand A, Lecavalier L, Evans V, Gurka M, … Scahill L (2018). Exploring sleep quality of young children with autism spectrum disorder and disruptive behaviors. Sleep Medicine, 44, 61–66. 10.1016/J.SLEEP.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, & Hansen RL (2008). Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. Journal of Sleep Research, 17(2), 197–206. 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hubbard JA, Fabes RA, & Adam JB (2006). Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry and Human Development, 37(2), 179–191. 10.1007/s10578-006-0028-3. [DOI] [PubMed] [Google Scholar]
- Lourenco C, Esteves D, Corredeira R, & Seabra A (2015). Children with autism spectrum disorder and trampoline training. Wulfenia Journal, 22(5), 1–11. [Google Scholar]
- Macdonald M, Esposito P, & Ulrich D (2011). The physical activity patterns of children with autism. BMC Research Notes, 4, 422 10.1186/1756-0500-4-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmullin JA, Lunsky Y, & Weiss JA (2016). Plugged in: Electronics use in youth and young adults with autism spectrum disorder. Autism, 20(1), 45–54. 10.1177/1362361314566047. [DOI] [PubMed] [Google Scholar]
- Maternal and Child Health Bureau in collaboration with the US Census Bureau. (2018). Child and Adolescent Health Measurement Initiative, Data Resource Center for Child and Adolescent Health. 2016 National Survey of Children’s Health (NSCH) [(SPSS) Indicator Data Set]. [Google Scholar]
- Mazurek MO, Shattuck PT, Wagner M, & Cooper BP (2012). Prevalence and correlates of screen-based media use among youths with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(8), 1757–1767. 10.1007/s10803-011-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, & Sohl K (2016). Sleep and behavioral problems in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(6), 1906–1915. 10.1007/s10803-016-2723-7. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, & Wenstrup C (2013). Television, video game and social media use among children with ASD and typically developing siblings. Journal of Autism and Developmental Disorders, 43(6), 1258–1271. 10.1007/s10803-012-1659-9. [DOI] [PubMed] [Google Scholar]
- McCoy A, Holloway J, Healy O, Rispoli M, & Neely L (2016). A systematic review and evaluation of video modeling, role-play and computer-based instruction as social skills interventions for children and adolescents with high-functioning autism. Review Journal of Autism and Developmental Disorders, 3(1), 48–67. 10.1007/s40489-015-0065-6. [DOI] [Google Scholar]
- McCoy SM, Jakicic JM, & Gibbs BB (2016). Comparison of obesity, physical activity, and sedentary behaviors between adolescents with autism spectrum disorders and without. Journal of Autism and Developmental Disorders, 46(7), 2317–2326. 10.1007/s10803-016-2762-0. [DOI] [PubMed] [Google Scholar]
- Montes G (2016). Children with autism spectrum disorder and screen time: Results from a large, nationally representative US study. Academic Pediatrics, 16(2), 122–128. 10.1016/j.acap.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Must A, Eliasziw M, Phillips SM, Curtin C, Kral TVE, Segal M,… Bandini LG (2017). The effect of age on the prevalence of obesity among US youth with autism spectrum disorder. Childhood Obesity, 13(1), 25–35. 10.1089/chi.2016.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Phillips S, Curtin C, & Bandini LG (2015). Barriers to physical activity in children with autism spectrum disorders: Relationship to physical activity and screen time. Journal of Physical Activity and Health, 12(4), 529–534. 10.1123/jpah.2013-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Phillips SM, Curtin C, Anderson SE, Maslin M, Lividini K, & Bandini LG (2014). Comparison of sedentary behaviors between children with autism spectrum disorders and typically developing children. Autism, 18(4), 376–384. 10.1177/1362361313479039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Physical Activity Plan Alliance. (2018). The 2018 United States Report Card on Physical Activity for Children and Youth, Washington, DC: Retrieved from https://health.gov/news/blog-bayw/2016/11/2016-united-states-report-card-on-physical-activity-for-children-and-youth-released/. [Google Scholar]
- Nicholson H, Kehle TJ, Bray MA, & Van Heest J (2011). The effects of antecedent physical activity on the academic engagement of children with autism spectrum disorder. Psychology in the Schools, 48(2), 198–213. 10.1002/pits.20537. [DOI] [Google Scholar]
- Oriel KN, George CL, Peckus R, & Semon A (2011). The effects of aerobic exercise on academic engagement in young children with autism spectrum disorder. Pediatric Physical Therapy, 23(2), 187–193. 10.1097/PEP.0b013e318218f149. [DOI] [PubMed] [Google Scholar]
- Orsmond GI, & Kuo H-Y (2011). The daily lives of adolescents with an autism spectrum disorder: discretionary time use and activity partners. Autism, 15(5), 579–599. 10.1177/1362361310386503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CY, & Frey GC (2005). Identifying physical activity determinants in youth with autistic spectrum disorders. Journal of Physical Activity and Health, 2(4), 412–422. [Google Scholar]
- Pan C (2011). The efficacy of an aquatic program on physical fitness and aquatic skills in children with and without autism spectrum disorders. Research in Autism Spectrum Disorders, 5(1), 657–665. 10.1016/j.rasd.2010.08.001. [DOI] [Google Scholar]
- Pan C, Tsai CL, Chu CH, Sung MC, Ma WY, & Huang CY (2016). Objectively measured physical activity and health-related physical fitness in secondary school-aged male students with autism spectrum disorders. Physical Therapy, 96(4), 511–520. 10.2522/ptj.20140353. [DOI] [PubMed] [Google Scholar]
- Pan C-Y (2008). Objectively measured physical activity between children with autism spectrum disorders and children without disabilities during inclusive recess settings in Taiwan. Journal of Autism and Developmental Disorders, 38(7), 1292–1301. 10.1007/s10803-007-0518-6. [DOI] [PubMed] [Google Scholar]
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, … Malhotra A (2015). Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. American Journal of Respiratory and Critical Care Medicine, 12(12), 1450–1458. 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitetti KH, Rendoff AD, Grover T, & Beets MW (2007). The efficacy of a 9-month treadmill walking program on the exercise capacity and weight reduction for adolescents with severe autism. Journal of Autism and Developmental Disorders, 37(6), 997–1006. 10.1007/s10803-006-0238-3. [DOI] [PubMed] [Google Scholar]
- Poitras VJ, Gray CE, Borghese MM, Carson V, Chaput J-P, Janssen I, … Tremblay MS (2016). Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Applied Physiology, Nutrition, and Metabolism, 41(6 (Suppl. 3)), S197–S239. 10.1139/apnm-2015-0663. [DOI] [PubMed] [Google Scholar]
- Reynolds AM, & Malow BA (2011). Sleep and autism spectrum disorders. Pediatric Clinics of North America, 58(3), 685–698. 10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Richdale AL, & Schreck KA (2009). Sleep problems in autism spectrum disorders: Prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews, 13(6), 403–411. https://doi.org/10.1016Zj.smrv.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Roman-Viñas B, Chaput J-P, Katzmarzyk PT, Fogelholm M, Lambert EV, Maher C, … ISCOLE Research Group. (2016). Proportion of children meeting recommendations for 24-hour movement guidelines and associations with adiposity in a 12-country study. The International Journal of Behavioral Nutrition and Physical Activity, 13(1), 123 10.1186/s12966-016-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S, Healy S, & Braithwaite RE (2019). Hobby preferences and physical activity participation among children with and without autism spectrum disorder. European Journal of Adapted Physical Activity, 11(2), 1–9. 10.5507/euj.2018.008. [DOI] [Google Scholar]
- Sam K-L, Chow B-C, & Tong K-K (2015). Effectiveness of exercise-based interventions for children with autism: A systematic review and meta-analysis. International Journal of Learning and Teaching, 1(2), 98–103. 10.18178/ijlt.1.2.98-103. [DOI] [Google Scholar]
- Sandt DDR, & Frey GC (2005). Comparison of physical activity levels between children with and without autistic spectrum disorders. Adapted Physical Activity Quarterly, 22(2), 146–159. 10.1123/apaq.22.2.146. [DOI] [Google Scholar]
- Saunders TJ, Gray CE, Poitras VJ, Chaput J-P, Janssen I, Katzmarzyk PT, … Carson V (2016). Combinations of physical activity, sedentary behaviour and sleep: relationships with health indicators in school-aged children and youth. Applied Physiology, Nutrition, and Metabolism, 41(6 (Suppl. 3)), S283–S293. 10.1139/apnm-2015-0626. [DOI] [PubMed] [Google Scholar]
- Shedlock K, Susi A, Gorman GH, Hisle-Gorman E, Erdie-Lalena CR, & Nylund CM (2016). Autism spectrum disorders and metabolic complications of obesity. Journal of Pediatrics, 178, 183–187.e1. 10.1016/j.jpeds.2016.07.055. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Posserud M-B, Gillberg C, Lundervold AJ, & Hysing M (2012). Sleep problems in children with autism spectrum problems: a longitudinal population-based study. Autism, 16(2), 139–150. 10.1177/1362361311404255. [DOI] [PubMed] [Google Scholar]
- Skinner AC, Perrin EM, & Skelton JA (2016). Prevalence of obesity and severe obesity in US children, 1999–2014. Obesity, 24(5), 1116–1123. 10.1002/oby.21497. [DOI] [PubMed] [Google Scholar]
- Sowa M, & Meulenbroek R (2012). Effects of physical exercise on Autism Spectrum Disorders: A meta-analysis. Research in Autism Spectrum Disorders, 6(1), 46–57. 10.1016/j.rasd.2011.09.001. [DOI] [Google Scholar]
- Srinivasan SM, Pescatello LS, & Bhat AN (2014). Current perspectives on physical activity and exercise recommendations for children and adolescents with autism spectrum disorders. Physical Therapy, 94(6), 875–889. 10.2522/ptj.20130157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanish H, Curtin C, Must A, Phillips S, Maslin M, & Bandini L (2015). Enjoyment, barriers, and beliefs about physical activity in adolescents with and without autism spectrum disorder. Adapted Physical Activity Quarterly, 32(4), 302–317. 10.1123/APAQ.2015-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanish HI, Curtin C, Must A, Phillips S, Maslin M, & Bandini LG (2017). Physical activity levels, frequency, and type among adolescents with and without autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(3), 785–794. 10.1007/s10803-016-3001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller A, & Mofile T (2018). Media use among children and adolescents with autism spectrum disorder: A systematic review. Review Journal of Autism and Developmental Disorders., 5, 227–246. 10.1007/s40489-018-0135-7. [DOI] [Google Scholar]
- Tremblay MS, Carson V, Chaput J-P, Connor Gorber S., Dinh T, Duggan M, … Zehr L (2016). Canadian 24-hour movement guidelines for children and youth: An integration of physical activity, sedentary behaviour, and sleep. Applied Physiology, Nutrition, and Metabolism, 41(6 (Suppl. 3)), S311–S327. 10.1139/apnm-2016-0151. [DOI] [PubMed] [Google Scholar]
- Tremblay MS, LeBlanc AG, Kho ME, Saunders TJ, Larouche R, Colley RC, . Gorber, S. (2011). Systematic review of sedentary behaviour and health indicators in school-aged children and youth. International Journal of Behavioral Nutrition and Physical Activity, 8(1), 98 10.1186/1479-5868-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K, MacDonald M, & Menear K (2014). Physical activity and physical fitness of school-aged children and youth with autism spectrum disorders. Autism Research and Treatment, 2014, 1–6. 10.1155/2014/312163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2008). 2008 Physical Activity Guidelines for Americans. Washington, DC: US Department of Health and Human Services. [Google Scholar]
- van der Heijden KB, Stoffelsen RJ, Popma A, & Swaab H (2018). Sleep, chronotype, and sleep hygiene in children with attention-deficit/hyperactivity disorder, autism spectrum disorder, and controls. European Child and Adolescent Psychiatry, 27(1), 99–111. 10.1007/s00787-017-1025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ekris E, Altenburg TM, Singh AS, Proper KI, Heymans MW, & Chinapaw MJM (2016). An evidence-update on the prospective relationship between childhood sedentary behaviour and biomedical health indicators: A systematic review and meta-analysis. Obesity Reviews, 17(9), 833–849. 10.1111/obr.12426. [DOI] [PubMed] [Google Scholar]
- Veatch OJ, Reynolds A, Katz T, Weiss SK, Loh A, Wang L, & Malow BA (2016). Sleep in children with autism spectrum disorders: How are measures of parent report and actigraphy related and affected by sleep education? Behavioral Sleep Medicine, 14(6), 665–676. 10.1080/15402002.2015.1065408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer AL, & Ingersoll BR (2011). The use of innovative computer technology for teaching social communication to individuals with autism spectrum disorders. Research in Autism Spectrum Disorders, 5(1), 96–107. 10.1016/j.rasd.2010.08.002. [DOI] [Google Scholar]
- Wu Y, Gong Q, Zou Z, Li H, & Zhang X (2017). Short sleep duration and obesity among children: A systematic review and meta-analysis of prospective studies. Obesity Research & Clinical Practice, 11(2), 140–150. 10.1016/J.ORCP.2016.05.005. [DOI] [PubMed] [Google Scholar]