Abstract
Predicting Helicobacter pylori (Hp) status by endoscopic finding would be useful in recent clinical condition that the use of proton-pump inhibitors, anti-platelet, and anti-coagulant have become widespread. We aimed to elucidate the diagnostic accuracy of magnifying narrow-band imaging (M-NBI) endoscopy in distinguishing Hp status in patients with or without history of successful Hp eradication and compare this accuracy to the diagnostic accuracy of conventional white light (WL) endoscopy.
Two hundred seven endoscopic examinations before and after Hp eradication were performed in prospective 163 patients. Endoscopic images by using the M-NBI and conventional WL were stored electronically and randomly allocated to 2 readers for evaluation. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy were assessed by reference to Hp status assessed by conventional clinical test.
Sensitivity, specificity, PPV, NPV, and accuracy for predicting Hp status for the conventional WL was 72.2%, 75.5%, 72.2%, 75.5%, and 73.9% for the first reader; 86.6%, 57.3%, 64.1%, 82.9%, and 71.0% for the second reader. On the other hand, sensitivity, specificity, PPV, NPV, and accuracy for predicting Hp status for the M-NBI was 96.9%, 93.6%, 93.1%, 97.1%, and 95.2% for the first reader; 92.8%, 93.6%, 92.8%, 93.6%, and 93.2% for the second reader, respectively. The diagnostic accuracy of M-NBI was significantly higher than that of WL (P < .0001 for both readers). Inter-observer agreement of M-NBI (k = 0.83) was also better than that of WL (k = 0.53).
M-NBI was capable of distinguishing Hp status before and after eradication therapy.
Keywords: comparative study, conventional white light endoscopy, eradication, Helicobacter pylori, magnifying narrow-band imaging endoscopy
1. Introduction
Helicobacter pylori (Hp) infection is a major cause of gastric cancer.[1–3] It has been demonstrated that the Hp eradication significantly reduces the incidence of metachronous gastric cancer following endoscopic resection of early gastric cancer.[4] Eradication therapy for patients with Hp-positive chronic gastritis is now covered by the social insurance systems in Japan. New regimens for eradication therapy for Hp such as Nitazoxanide based regimens and vonoprazan based regimens have been proposed for the improvement of eradication rates.[5–7] Such conditions would increase the situation that we assess the Hp status undergoing endoscopic examination. Furthermore, considering the recent clinical condition that the use of proton-pump inhibitors (PPIs), anti-platelet, and anti-coagulant have become widespread, to establish the diagnostic criteria to distinguish Hp-positive or eradicated stomachs on the basis of endoscopic findings alone would be very useful.
Many clinical studies have reported the diagnostic performance of image enhanced endoscopy (IEE) systems such as narrow-band imaging (NBI) for diagnosing endoscopic lesions.[8–11] NBI enhances the visualization of the surface mucosal and vascular patterns using optical filters against a xenon lamp that allows narrow-band light to pass at wavelengths of 415 and 540 nm. Combining the NBI and magnifying endoscopy provides accurate real-time diagnostic performance in gastric neoplastic lesion compared with the conventional white light endoscopy.[9] The diagnostic utility of magnifying NBI (M-NBI) endoscopy for predicting Hp status as well as degree of Hp related gastritis was also been demonstrated in several studies.[12,13] Our group reported that M-NBI endoscopy is useful for predicting Hp status early after eradication therapy.[14]
Our study aimed to prospectively elucidate the diagnostic accuracy of M-NBI endoscopy in distinguishing Hp status in patients with or without history of successful Hp eradication and compare this accuracy to the diagnostic accuracy of conventional white light (WL) endoscopy.
2. Methods
2.1. Study population
Study participants were prospectively enrolled from 163 patients attending the endoscopy Center of Fujita Health University from January 2013 to March 2016. The 163 patients were either Hp positive or Hp negative after successful Hp eradication therapy. Since current study aimed to evaluate the diagnostic utility of M-NBI endoscopy in distinguishing Hp status in patients with or without history of successful Hp eradication, the Hp naïve was not included in this study. All participants underwent upper gastroscopy for various indications, including assessment of early gastric cancer (EGC) before endoscopic resection (ER), yearly follow up examination after ER of EGC, yearly screening for gastric cancer, secondary complete check-up after barium radiographic examination due to a suspicion of gastric cancer or peptic ulcer disease, and complaints of abdominal discomfort. At the study enrollment, 163 participants consisted of 91 Hp positive subjects, diagnosed by either histology, serum titer, or urea breath test (UBT). The remaining 72 subjects had a history of successful Hp eradication therapy for the various reasons, including gastric and duodenal ulcer, chronic gastritis, or after endoscopic ER of EGC. The success of Hp eradication in the 72 subjects with successful Hp eradication was confirmed at the study enrollment by the UBT. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was reviewed and approved by the Institutional Review Board of Fujita University School of Medicine (ID: HM16–225; complete date of approval from the Ethical committee, June 26, 2017). Written informed consent was obtained from all participants.
2.2. Endoscopic procedure
All participants underwent esophagogastroduodenoscopy (EGD) using a magnifying video endoscope (Olympus GIF-H260Z and a CV260SL/CV290SL endoscopic system [Olympus Medical Systems, Tokyo, Japan]). After the endoscope was inserted, the entire stomach was initially observed with conventional WL to exclude obvious lesions. At least 35 photographs were taken from the entire stomach, even if there was no obvious lesion. If obvious lesions, such as polyps, erosions, ulcers, or tumors were seen, additional scanning and photography of these lesions were performed. If necessary, a biopsy was also taken from the lesions.
For the evaluation of endoscopic feature of gastric mucosa by the M-NBI endoscopy, the non-pathological mucosa of the gastric body (fundic area) were carefully evaluated with complete magnification coupled with a NBI light source. We tried to scan the nonatrophic area by the Kimura–Takemoto classification[15] because judgment of Hp status by the endoscopic findings become difficult where severe gastric atrophy was present.[12–14] At least 5 representative photographs were taken from the gastric body using the M-NBI endoscopy. If obvious lesions, such as polyps, erosions, or cancers were seen, NBI scanning was performed in a distant enough site from these pathological lesions to avoid their influence.
During the study period, 1 patient who has a history of successful Hp eradication underwent follow up upper gastroscopy within 1 year. Forty Hp positive subjects underwent Hp eradication therapy and judgement of Hp eradication was performed by the UBT at least 3 months after the eradication. Follow up upper gastroscopy was also performed on the same day of the UBT. Thirty four out of the 40 subjects were considered as successful eradication by the UBT. On the other hand, 6 were considered as failure by the UBT. Secondary eradication therapy was performed for these patients and all were considered as successful eradication by the UBT at least after 3 months. Two out of the 6 participants underwent follow up upper gastroscopy on the same day of the UBT. In total, 207 endoscopic examinations were performed for the 163 participants during the study period. Ninety seven and 110 examinations were performed when patients were Hp positive, and negative after eradication, respectively (Fig. 1).
Figure 1.
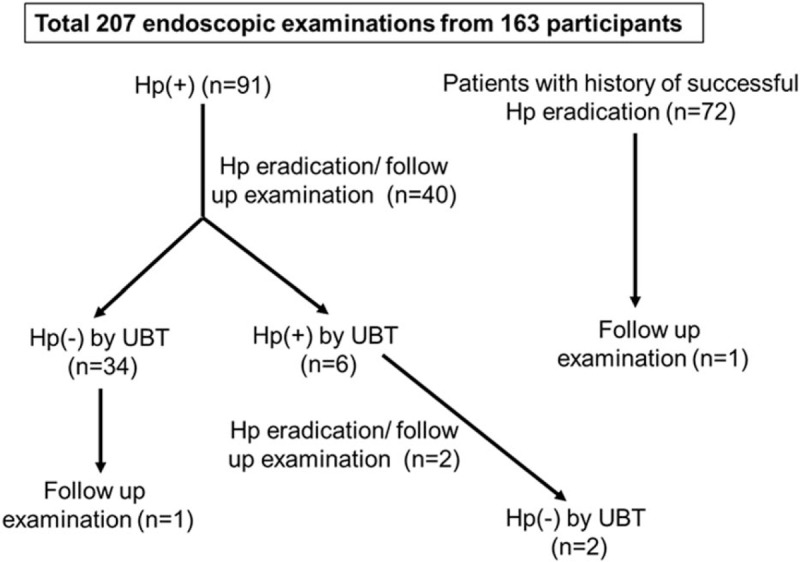
Patient distribution showing the clinical back ground. Hp = Helicobacter pylori, UBT = urea breath test.
2.3. Image evaluation
For each endoscopic examination, 4 representative photographs using the conventional white light were selected from the gastric fornix, greater, and lesser curvatures from the gastric body and the gastric antrum. Twoto 4 representative photographs were also selected from the gastric body using the M-NBI. The selection of images were based on the best image quality. All images were sorted by an endoscopist who were blinded to the patient's characteristics. The chart numbers of the patients on the endoscopic images were all processed and masked. Two other experienced endoscopists (NH and TT) participated in the image reading and both readers were blinded to the diagnosis of Hp status at the endoscopic examination. Endoscopic images by using the conventional WL and M-NBI from each examination were separated and arranged in random order for readers A and B. The judgement of Hp positive by the conventional WL endoscopy was based on the presence of either diffuse redness, rugal hypertrophy, or thick and whitish mucus, which is difficult to wash out using water,[16,17] while no evidence of all these findings were considered to be Hp negative after eradication. The judgement of Hp positive by the M-NBI endoscopy was based on the presence of enlarged or elongated pits with dense fine irregular vessels.[12] We have also reported that such M-NBI findings seen in Hp positive patients rapidly changes early after successful Hp eradication.[14] In such patients, enlarged or elongated pits with dense fine irregular vessels change to the small, round, or elliptical pits, accompanied with honeycomb-like subepithelial capillary networks or concentric white layer, which resemble to M-NBI findings seen in the Hp negative gastric body.[12–14] Therefore, we considered such M-NBI findings as Hp negative after eradication (Fig. 2). Diagnostic accuracies of the conventional WL and M-NBI were determined by reference to the Hp status at the endoscopic examination.
Figure 2.
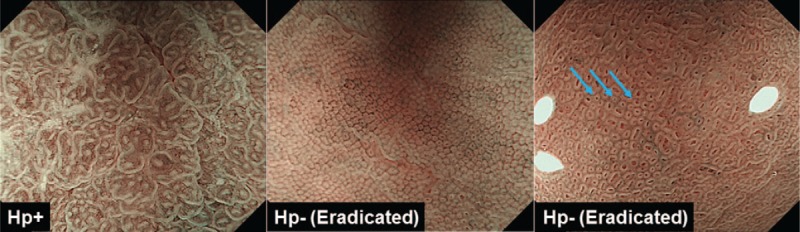
Magnifying NBI (M-NBI) features of Helicobacter pylori (Hp) infection positive (Hp+, left) and 2 Hp eradicated patients (middle and right). Hp+ gastric mucosa is characterized as enlarged or elongated pits with unclear subepithelial capillary networks or dense fine irregular vessels (left), while small, round, or elliptical pits, accompanied with honeycomb-like subepithelial capillary networks (center) or concentric white layer (right, light blue arrows) was considered to be after successful eradication.
2.4. Statistical analysis
For the conventional WL and M-NBI endoscopies, sensitivities, specificities, positive predictive values (PPV), negative predictive values (NPV), diagnostic accuracies for predicting Hp status were calculated by reference to the Hp status assessed by conventional clinical test. Diagnostic accuracy was defined as (true positive + true negative/total number of cases). Statistical differences of diagnostic accuracies were analyzed by the 2 times test of independence or Fisher exact test. P < .05 was considered significant. Inter-observer agreement for predicting Hp status was determined by calculation of k values. A k value below 0.4 was regarded as representing poor agreement, a k value of 0.41 to 0.60 fair agreement, a k value of 0.61 to 0.80 good agreement, and a k value >0.80 excellent agreement. Consistency was defined as (cases of same judgement among both readers/total number of cases).
3. Results
3.1. Study population
The clinic-pathological characteristics of 163 participants at the study enrollment are shown in the Table 1. During the study period, 40 Hp positive participants underwent Hp eradication therapy and 34 out of the 40 subjects were considered as successful eradication by the UBT after 3 months. Secondary eradication therapy was performed for 6 patients who were considered to be failure and all were considered as successful eradication by the UBT at least after 3 months. Since 2 out of the 6 participants underwent follow up upper gastroscopy on the same day of the UBT, these examinations were included for the analysis. In total, this study included 108 participants who underwent successful Hp eradication. The clinic-pathological characteristics of these 108 participants including age, sex, post-eradication period at endoscopy, reason for Hp eradication are shown in the Table 2.
Table 1.
Clinicopathological features of 163 participants at enrollment.

Table 2.
Clinicopathological features of 108 participants who underwent.

3.2. Diagnostic efficacy of WL and M-NBI for predicting Hp positive or eradicated patients
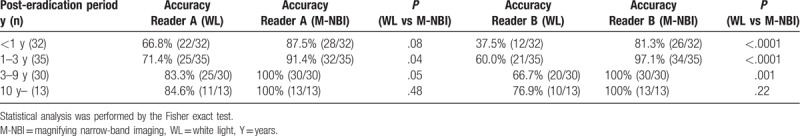
We used endoscopic photographs of a total of 207 endoscopic examinations from the 163 participants. Among the 207 endoscopic examinations, 97 examinations were performed when the Hp was positive, while the 110 examinations were performed when the Hp was eradicated (Fig. 2). Representative endoscopic photographs using the conventional WL as well as the M-NBI right source were separated and arranged in random order for the image reading by the 2 experienced endoscopists (NH and HY) who were blinded to the diagnosis of Hp status at the endoscopic examination. Sensitivity, specificity, PPV, NPV, and accuracy for predicting Hp status for the WL was 72.2%, 75.5%, 72.2%, 75.5%, and 73.9% for the reader A; 86.6%, 57.3%, 64.1%, 82.9%, and 71.0% for the reader B. On the other hand, sensitivity, specificity, PPV, NPV, and accuracy for predicting Hp status for the M-NBI was 96.9%, 93.6%, 93.1%, 97.1%, and 95.2% for the reader A; 92.8%, 93.6%, 92.8%, 93.6%, and 93.2% for the reader B, respectively (Table 3). The sensitivity, specificity, PPV, NPV, and accuracy were all superior by using the compared with the WL among the both readers. The accuracy of M-NBI was significantly greater than that of WL among the both readers (both P < .0001 by the by the Fisher exact test). Since the diagnostic efficacy for predicting eradicated patients by the endoscopic image would be influenced by the post-eradication period, we investigated the diagnostic accuracy of WL and M-NBI in the eradicated patients in relation to the post-eradication period. Although, the accuracy for predicting eradicated patients was inferior in cases with shorter post-eradication period for both WL and M-NBI, M-NBI demonstrated better accuracy compared with the WL in cases with shorter post-eradication period <3 years in the reader B (<1 year, WL vs M-NBI: 37.5% vs 81.3%, 1–3 year, WL vs M-NBI, WL vs M-NBI: 60.0% vs 97.1%, both P < .0001) (Table 4). We also noted that diagnostic accuracy of M-NBI was perfect with in cases with longer post-eradication (3–9 years, 10 years) in both readers.
Table 3.
Diagnostic efficacy of WL and M-NBI for predicting Hp positive or eradicated patients.
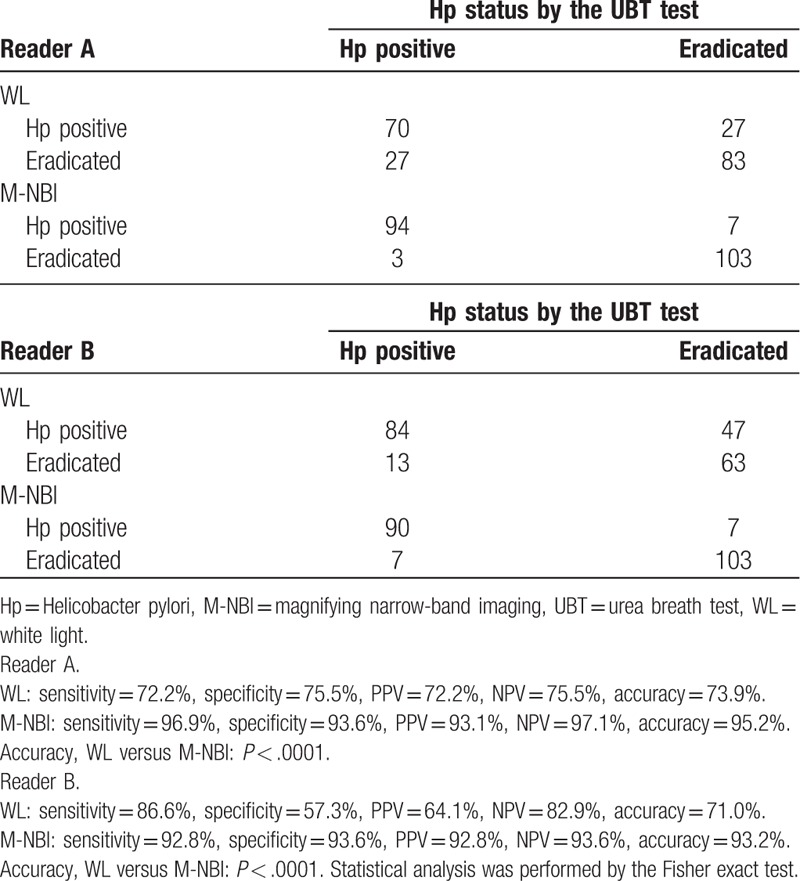
Table 4.
Diagnostic accuracy for predicting eradicated patients in relation to the post-eradication period.
We then analyzed inter-observer concordance using consistency and k coefficient value for the WL and M-NBI among 2 readers. The consistency and k coefficient value for WL was 75.8%, k = 0.53, respectively, while the consistency and k coefficient value for M-NBI was 91.3%, k = 0.83, respectively. The k coefficient value of M-NBI indicated excellent agreement and the consistency of this modality was also significantly greater than that of WL (P < .0001 by the Fisher exact test) (Table 5).
Table 5.
Inter-observer concordance among WL and M-NBI among 2 observers.

4. Discussion
We showed an excellent diagnostic ability of M-NBI to distinguish Hp status after or before eradication with >90% of sensitivity, specificity, PPV, NPV across 2 independent readers. The accuracy of M-NBI was also superior to that of WL for both readers.
On the other hand, the sensitivity, specificity, PPV, NPV of WL were inferior to that of M-NBI.
The result seems to be reasonable because diagnostic criteria of conventional WL for the Hp positive gastric mucosa is based on the presence of either diffuse redness, rugal hypertrophy, or thick and whitish mucus, most of which are non-specific and would become subjective among individual reader. Hence M-NBI was expected to have higher diagnostic accuracy in such patients. M-NBI allow as to visualize fine mucosal and vascular patterns, which is closely linked to the Hp status.[12–14] Since the M-NBI pattern of Hp infected gastric mucosa shows improvement after short term of eradication, which is in parallel with improvement of histological gastritis,[14] it is possible that M-NBI allows as to predict Hp status after or before eradication more accurately.
The diagnostic accuracy of WL and M-NBI in the eradicated patients showed that the accuracy for predicting eradicated patients was inferior in cases with shorter post-eradication period, which is probably due to the insufficient improvement of Hp induced gastritis within a short term. On the other hand, M-NBI demonstrated better accuracy compared with the WL in such shorter post-eradication in 1 reader, suggesting that M-NBI can more accurately predict Hp status after a short term of eradication compared with WL alone. In this study, we tried to targeted nonatrophic area of gastric body for the M-NBI scanning because judgment of Hp status become difficult where severe gastric atrophy was present.[12–14] We have previously shown that the patients who have severe atrophy of the entire stomach at eradication appeared to be unchanged by M-NBI after short term of eradication.[14] It should be noted that judgement of Hp status in severe gastric atrophy is difficult especially in shorter post-eradication period. We showed that the diagnostic accuracy for predicting eradicated patients was excellent after 3 years of eradication with 100% of accuracies in both readers. We have also demonstrated that interobserver concordance of M-NBI was excellent with a 0.83 of k value and 91.3% of consistency. These findings suggest that prediction of Hp status by M-NBI can be well applicable in the clinical condition.
The potential usefulness of N-NBI for predicting Hp status was previously reported in cases after ER of EGC,[14] which included subset of eradicated patients. The strength of our study is that we enrolled eradicated patients with various clinical conditions such as different reason for eradication and various post-eradication periods. We showed that the k coefficient value as well as the consistency of M-NBI among the different readers was favorable, suggesting that M-NBI would be applicable for the evaluation of Hp status at the endoscopic examination. The prevalence of patients who undergo eradication is now increasing in Japan. In addition, the use of PPIs, anti-platelet, and anti-coagulant have also become widespread. Our current result of usefulness of M-NBI in the diagnosis of Hp status would well fit with all these clinical conditions. Hp infection is well accepted as a major cause of gastric cancer.[1–3] The recent result also highlight the different clinicopathological characteristics of gastric cancer diagnosed after successful Hp eradication.[18–20] Since our result was from limited number of patients from single center, sample size, and the statistical power could not be assessed. Current result will lead us to further longitudinal investigation of M-NBI endoscopy to know whether our result can be applicable to gastric cancer risk stratification as well as endoscopic diagnosis of gastric cancer according to their Hp status.
Author contributions
Data curation: Tomoitsu Tahara, Noriyuki Horiguchi, Hyuga Yamada, Dai Yoshida, Tsuyoshi Terada.
Formal analysis: Tomoitsu Tahara, Dai Yoshida, Masaaki Okubo.
Investigation: Tomoitsu Tahara, Noriyuki Horiguchi, Hyuga Yamada, Dai Yoshida, Tsuyoshi Terada, Masaaki Okubo.
Supervision: Kohei Funasaka, Yoshihito Nakagawa, Naoki Ohmiya.
Validation: Tomoyuki Shibata.
Writing – original draft: Tomoitsu Tahara.
Writing – review & editing: Tomoitsu Tahara.
Footnotes
Abbreviations: EGC = early gastric cancer, EGD = esophagogastroduodenoscopy, ER = endoscopic resection, Hp = Helicobacter pylori, IEE = image enhanced endoscopy, M-NBI = magnifying narrow-band imaging, NPV = negative predictive value, PPIs = proton-pump inhibitors, PPV = positive predictive value, UBT = urea breath test, WL = white light.
How to cite this article: Tahara T, Horiguchi N, Yamada H, Yoshida D, Terada T, Okubo M, Funasaka K, Nakagawa Y, Shibata T, Ohmiya N. Comparative study of magnifying narrow-band imaging and conventional white light endoscopy in the diagnosis of Helicobacter pylori status after eradication therapy. Medicine. 2019;98:46(e17697).
The authors have no conflicts of interest to disclose.
References
- [1].Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991;325:1127–31. [DOI] [PubMed] [Google Scholar]
- [2].Huang JQ, Sridhar S, Chen Y, et al. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998;114:1169–79. [DOI] [PubMed] [Google Scholar]
- [3].Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- [4].Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomized controlled trial. Lancet 2008;372:392–7. [DOI] [PubMed] [Google Scholar]
- [5].Kang H, Kim BJ, Choi G, et al. Vonoprazan versus proton pump inhibitors for the management of gastric endoscopic submucosal dissection-induced artificial ulcer: a systematic review with meta-analysis. Medicine (Baltimore) 2019;98:e15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Abd-Elsalam S, Kobtan A, El-Kalla F, et al. A 2-week Nitazoxanide-based quadruple treatment as a rescue therapy for Helicobacter pylori eradication: a single center experience. Medicine (Baltimore) 2016;95:e3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shehata MA, Talaat R, Soliman S, et al. Randomized controlled study of a novel triple nitazoxanide (NTZ)-containing therapeutic regimen versus the traditional regimen for eradication of Helicobacter pylori infection. Helicobacter 2017;22:e12395. [DOI] [PubMed] [Google Scholar]
- [8].Nakayoshi T, Tajiri H, Matsuda K, et al. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy 2004;36:1080–4. [DOI] [PubMed] [Google Scholar]
- [9].Ezoe Y, Muto M, Uedo N, et al. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology 2011;141:2017–25. [DOI] [PubMed] [Google Scholar]
- [10].Goda K, Tajiri H, Ikegami M, et al. Usefulness of magnifying endoscopy with narrow band imaging for the detection of specialized intestinal metaplasia in columnar-lined esophagus and Barrett's adenocarcinoma. Gastrointest Endosc 2007;65:36–46. [DOI] [PubMed] [Google Scholar]
- [11].Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy 2004;36:1094–8. [DOI] [PubMed] [Google Scholar]
- [12].Tahara T, Shibata T, Nakamura M, et al. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc 2009;70:246–53. [DOI] [PubMed] [Google Scholar]
- [13].Yagi K, Saka A, Nozawa Y, et al. Prediction of Helicobacter pylori status by conventional endoscopy, narrow-band imaging magnifying endoscopy in stomach after endoscopic resection of gastric cancer. Helicobacter 2014;19:111–5. [DOI] [PubMed] [Google Scholar]
- [14].Okubo M, Tahara T, Shibata T, et al. Changes in gastric mucosal patterns seen by magnifying NBI during H. pylori eradication. J Gastroenterol 2011;46:175–82. [DOI] [PubMed] [Google Scholar]
- [15].Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969;3:87–97. [Google Scholar]
- [16].Nomura S, Terao S, Adachi K, et al. Endoscopic diagnosis of gastric mucosal activity and inflammation. Dig Endosc 2013;25:136–46. [DOI] [PubMed] [Google Scholar]
- [17].Kato M, Terao S, Adachi K, et al. Changes in endoscopic findings of gastritis after cure of H. pylori infection: multicenter prospective trial. Dig Endosc 2013;25:264–73. [DOI] [PubMed] [Google Scholar]
- [18].Kobayashi M, Hashimoto S, Nishikura K, et al. Magnifying narrow-band imaging of surface maturation in early differentiated-type gastric cancers after Helicobacter pylori eradication. J Gastroenterol 2013;48:1332–42. [DOI] [PubMed] [Google Scholar]
- [19].Saka A, Yagi K, Nimura S. Endoscopic and histological features of gastric cancers after successful Helicobacter pylori eradication therapy. Gastric Cancer 2016;19:524–30. [DOI] [PubMed] [Google Scholar]
- [20].Horiguchi N, Tahara T, Kawamura T, et al. A comparative study of white light endoscopy, chromoendoscopy and magnifying endoscopy with narrow band imaging in the diagnosis of early gastric cancer after Helicobacter pylori eradication. J Gastrointestin Liver Dis 2017;26:357–62. [DOI] [PubMed] [Google Scholar]


