Supplemental Digital Content is available in the text
Keywords: muscle pain, network meta-analysis, temporomandibular joint disorders
Abstract
Background:
Numerous treatment modalities have been attempted for masticatory muscle pain in patients with temporomandibular disorders (TMD). To compare the treatment efficacy of more than 2 competing treatments, a network meta-analysis (NMA) was conducted.
Methods:
This study was reported with reference to the extended Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting of systematic reviews incorporating network meta-analyses. Medline via Pubmed, Embase via OVID, and Cochrane Library Central were searched (up to February 11, 2019). Axis I protocol of Diagnostic Criteria or Research Diagnostic Criteria for Temporomandibular Disorders (DC/TMD, RDC/TMD) were chosen as diagnostic standards. The PICOS (Problem/patient, Intervention, Comparison, Outcome, Study design) method was used to screen trials under eligibility criteria. And the NMA was performed with mvmeta commands in Stata (StataCorp, Tex).
Results:
Of 766 studies searched, 12 randomized clinical trials (RCTs) were finally included. Nineteen different therapies were found and further categorized into 9 treatment modalities. The general heterogeneity was not found among included trials. But predictive intervals (PrIs) were conspicuously wider than confidential intervals (CIs) of all pairwise comparisons, indicating that heterogeneity may exist between studies. Complementary therapy showed the greatest probability (42.7%) to be the best intervention. It also had the highest mean rank (2.3) in the rankogram and the biggest value of surface under the cumulative ranking (SUCRA, 84.1%).
Conclusions:
Based on the limited evidence of available trials, complementary therapy seemed to be slightly more effective than remaining treatment modalities for pain reduction in TMD patients with masticatory muscle pain. High-quality randomized controlled trials are expected to validate the findings.
1. Introduction
Temporomandibular disorders (TMD) have been reported to be a significant public health problem affecting approximately 5% to 12% of the population.[1] It is the second most common musculoskeletal condition (after chronic low back pain) leading to disability and pain.[1] American Academy of Orofascial Pain (AAOP) has attributed the pain to muscular or articular origin.[2] And myogenic pain is more frequently seen in clinical practice.[3] It is persistent in most cases,[4,5] which becomes the primary cause for TMD patients seeking medical assistance.[6,7] Patients’ quality of life would decrease when suffering from long-lasting pain.[8]
Masticatory muscle pain or myalgia has been classified into 3 clinical types according to Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): local myalgia, myofascial pain, and myofascial pain with referral.[1] Oral functions could be impaired, especially in chewing.[9] The etiology of masticatory muscle pain has not been fully unveiled.[10,11] Recent studies have shown its complex association with physical, behavioral, social, and psychological factors.[12] Treatment modalities targeting different factors have been attempted.[10,13,14] And the mainstream treatment is non-invasive and reversible.[6,7,14,15]Yet the agreement has not been reached on which conservative treatment is more effective. The option of proper therapy has triggered controversies.[16]
In order to compare the treatment efficacy among various treatments, network meta-analysis (NMA) has been introduced.[17,18] For a long time, NMA was criticized for its complexity and was inaccessible to nonstatisticians.[19] However, the development of software has made it accessible to clinical researchers.[20] With the application of NMA, it is realizable to compare more than 2 competing treatments for masticatory muscle pain. Given that the current evidence is insufficient, the purpose of this study is utilizing NMA to analyze current treatment modalities. And results of this study could be integrated with clinical practitioners’ experience to provide evidence-based medical care.
2. Methods
This study was reported with reference to the extended Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions.[21] NMA and graphical presentation of outcomes were performed with mvmeta commands in Stata (StataCorp, Tex).[22,23]
2.1. Eligibility criteria
We used the PICOS (Problem/patient, Intervention, Comparison, Outcome, Study design) method to screen studies.[24] Selection criteria were as following:
-
1.
Participants: adult patients with diagnosis of masticatory muscle pain by Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) axis I or Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) axis I (group Ia or Ib both eligible). Pain duration was no less than 6 months.
-
2.
Interventions and comparisons: various interventions including substantial pain management of masticatory muscle pain.
-
3.
Outcome measures: quantitative report of pain intensity.
-
4.
Study design: randomized clinical trials (RCTs).
2.2. Information sources and searches
The following electronic databases were searched: Medline via Pubmed, Embase via OVID, and Cochrane Library Central without language restrictions. The last access was on February 11, 2019. A manual review of reference lists was done to find related trials. Two independent reviewers (JF, ML) completed study selection. And a third reviewer (DB) was involved when distributes existed. Search algorithm was designed for Medline and modified for the other 2 databases (see Fig. 1, Supplemental Content, which showed the records of research strategy).
2.3. Data extraction and summery measures
Pain reduction was the primary outcome. Both numeric rating score (NRS) and visual analogue scale (VAS) were adoptable. Range of the scale was adjusted to 0 to 10 (0 = no pain to 10 = most intensive pain). Data were extracted and saved in a customized form for the analysis in Stata. When several time-point follow-ups were reported, only the longest follow-up duration was considered.[25] Differences in means of the pain reduction were adopted as summery measures. Standard deviation of median difference was calculated if necessary.[19] Medians and quartiles were transformed into means and standard deviation by Luo method.[26]
A standardized form was used to display the characteristics of studies, including items of gender, age, intervention, sample size, outcome measurement, follow-up period, and drop-out. All therapies in individual studies were further categorized into different treatment modalities.[3,14,27] Two independent reviewers (JF, ML) finished data extraction and fulfilled the standardized form. A group discussion (JM, YT, and XH) was held to resolve disagreements in this process.
2.4. Quality assessment
Quality of included studies were evaluated by 2 independent reviewers (JF, ML) with risk of bias under the instructions of Cochrane Collaboration.[28] Another independent reviewer (DB) was consulted when necessary.
2.5. Geometry of the network
In a NMA, the indirect comparison of a-c could be resulted from direct comparison of a-b and b-c (Fig. 1a). For a triangular closed loop like a-b-d, the comparison of a-d could result from a-d and a-b with b-d. Treatment effects of a-d and a-b/b-d were both calculated to estimate the treatment effects of a vs d. And the foundation of such estimates was that the treatment effects were transitive.[19] Transitivity was examined prior to further analysis.
Figure 1.
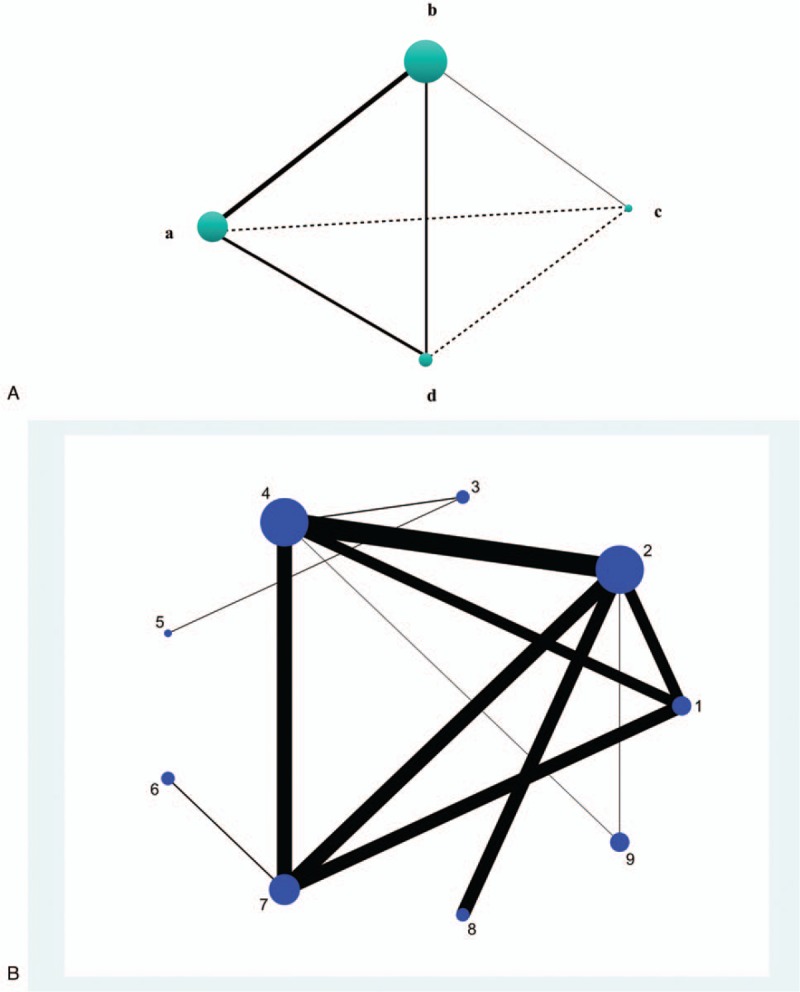
(a) Model of NMA structure. Solid line linked direct comparisons. (b) The network plot. Nudes were weighted according to the number of intervention being compared directly. And thickness of lines indicated the amount of direct pairwise comparisons (1 = splint therapy, 2 = physiotherapy, 3 = pharmacotherapy, 4 = placebo, 5 = acupuncture or needling, 6 =psychological intervention, 7 = complementary therapy, 8 = bi-physiotherapy, 9 = trigger-point injection.).
In avoidance of misunderstandings, there were several concepts to be explained:
Direct comparison: pairwise comparisons from two-arms or multi-arms trials.
Indirect comparison: pairwise comparisons established through the same comparator (intervention) and could not be detected in an individual trial.
Closed loop: usually a triangular structure, displaying direct pairwise comparisons among 3 interventions.
Direct estimate: the treatment effects calculated from direct comparisons.
Indirect estimate: the treatment effects calculated though the same comparator (intervention).
2.6. Assessment of inconsistency
Inconsistency plot was made to examine the transitivity in closed loops.[22,29] If inconsistency factor (IF) was larger than 2, then direct estimate could be at least twice larger than indirect estimate or vice versa.[22] In this case, the inconsistency in a closed loop would be deemed high.
2.7. Planned methods of analysis
Contributions of treatment effects from direct and indirect comparisons were presented by contribution plot. Confidential interval (CI) and mean summary effects were displayed in forest plot. Moreover, rankograms and surface under the cumulative ranking (SCURA) were produced to show the probabilities of efficacy ranking among all treatment modalities. [22,29]
2.8. Risk of bias across studies
The multivariate heterogeneity measures were used to detect heterogeneity in a general level.[29] Predictive interval (PrI) together with CI of pairwise comparisons were reported to interpret heterogeneity in a local level.[22,23] If PrI was wider enough than CI, then heterogeneity may exist between studies. Considering that study size would influence the credibility of comparative results, funnel plot was drawn to show if any small-study effects existed between direct comparions.[22,29] Small-study effect was deemed insignificant if the dots were on the zero line or symmetrical to the zero line in the funnel plot.
3. Results
3.1. Study selection and characteristics
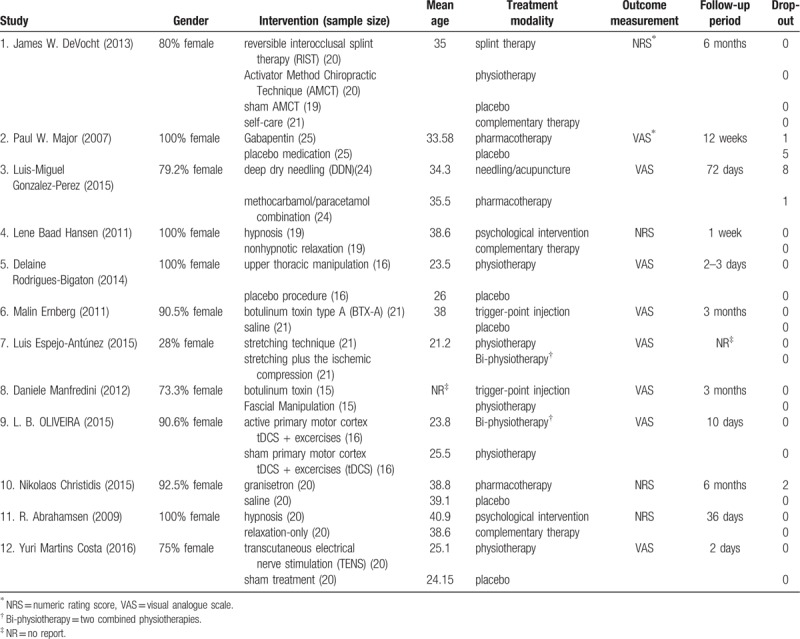
Of 766 studies identified through the search algorithm, 12 RCTs were finally included for quantitative synthesis[30–31] (Fig. 2). Except for 1 study,[32] the majority of participants were females. Participants’ age ranged from 21.2 to 40.9 years old. The follow-up period varied from 2 days to 6 months. Three studies used NRS for outcome reporting, and 9 used VAS. A total of 19 therapies were found and categorized into 9 treatment modalities (Table 1).
Figure 2.
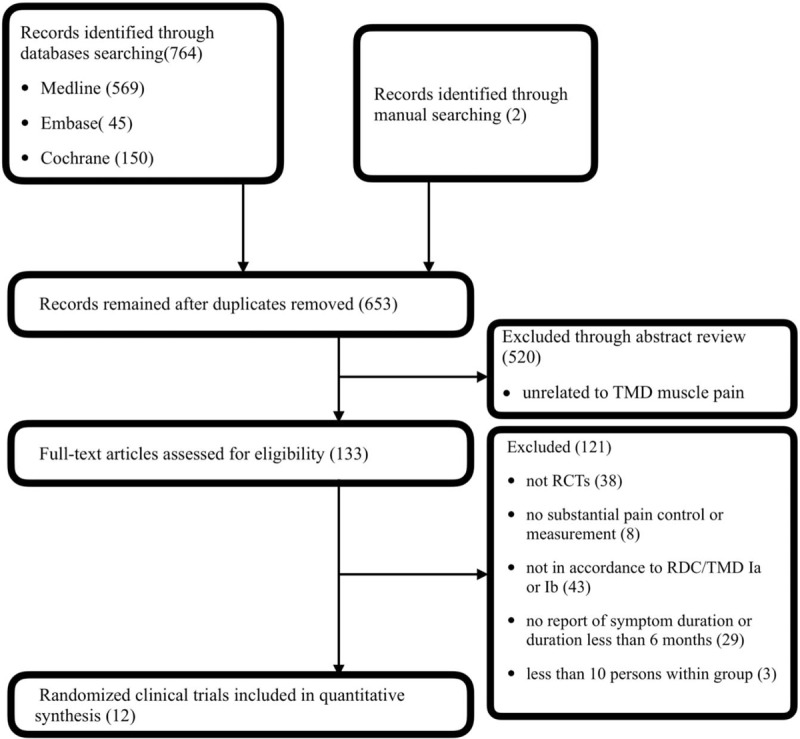
Flowchart of searching.
Table 1.
Characteristics of included studies.
3.2. Risk of bias within studies
Quality of included studies and summary of bias were shown in Figure 3. The main bias came from allocation concealment and binding of outcome measurement. According to the authors’ review, no obvious selective reporting bias existed. Overall quality of included studies was moderate.
Figure 3.
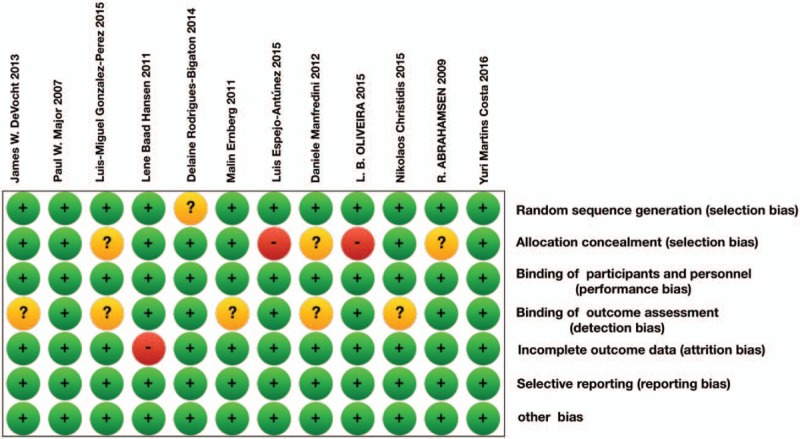
Risk of bias evaluation. Green represented positive risk of bias. Red indicated negative risk of bias. And yellow showed unknown risk of bias.
3.3. Network geometry
In the network plot, 17 direct pairwise comparisons were found. The physiotherapy, pharmacotherapy, and placebo were the 3 most common comparators (Fig. 1b). Besides, 24 indirect pairwise comparisons were established.
3.4. Inconsistency in NMA
Three closed loops were found, including the treatment modalities of splint therapy, physiotherapy, placebo, complementary therapy, and trigger-point injection. The IF values in these loops were acceptable, which meant that direct and indirect estimates were relatively consistent (see Fig. 2, Supplemental Content, which was the inconsistency plot).
3.5. NMA outcomes
The direct comparison of “pharmacotherapy vs placebo” had the largest proportion of treatment effect to the whole NMA structure (see Fig. 3, Supplemental Content, which was the contribution plot). Direct comparison of “placebo vs complementary therapy” and “physiotherapy vs complementary therapy” was the second and third most weighted contributor. Taking “physiotherapy vs bi-physiotherapy” as an example, the mixed estimates derived 100% from the direct comparison of “physiotherapy vs bi-physiotherapy”. Because there were no other pathways leading to it. Another example was “placebo vs acupuncture/needling”. This indirect comparison was established only through “pharmacotherapy vs placebo” and “pharmacotherapy vs acupuncture/needling”. Therefore, the indirect estimate of “placebo vs acupuncture/needling” was calculated from “pharmacotherapy vs placebo” and “placebo vs acupuncture/needling”. They happened to weight 50% respectively. More details could be discovered in the matrix of contribution plot.
Compared to complementary therapy, acupuncture/needling (95% CI: -5.76 to -0.13; 95% PrI: -23.27 to 17.39) and psychological intervention (95% CI: −2.95 to −0.16; 95% PrI: −14.28 to 11.17) seemed to have higher treatment effect in the forest plot (Fig. 4). Meanwhile, acupuncture/needling showed higher treatment effect than pharmacotherapy (95% CI: 0.52 to 4.18; 95% PrI: −12.53 to 17.23) (Fig. 4). Noteworthily, PrIs were much wider than CIs in these pairwise comparisons. A clear hierarchy of all treatment modalities in the network structure was seen in the rankogram (Fig. 5a Complementary therapy showed the greatest probability (42.7%) to be the best intervention. It also had the highest mean rank (2.3) in the rankograms and the biggest value of surface under the cumulative ranking (SUCRA, 84.1%) (Fig. 5b). However, the SUCRA scores and mean ranks of splint therapy, physiotherapy, pharmacotherapy, and trigger-point injection differed very slightly to placebo. And physiotherapy even showed a higher probability of being the best intervention. For these therapies, uncertainties may exist in the hierarchy.[33]
Figure 4.
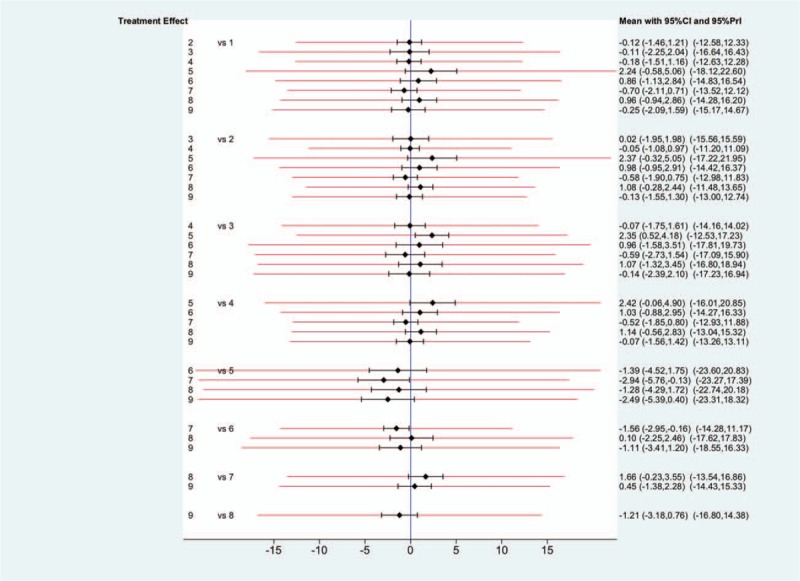
Confidential intervals (CIs) and predictive intervals (PrIs). The black solid lines represented CIs and the red lines represented PrIs. The blue line was the line of no effect (odds ratio equal to 1) (1 = splint therapy, 2 = physiotherapy, 3 = pharmacotherapy, 4 = placebo, 5 = acupuncture or needling, 6 = psychological intervention, 7 = complementary therapy, 8 = bi-physiotherapy, 9 = trigger-point injection.).
Figure 5.
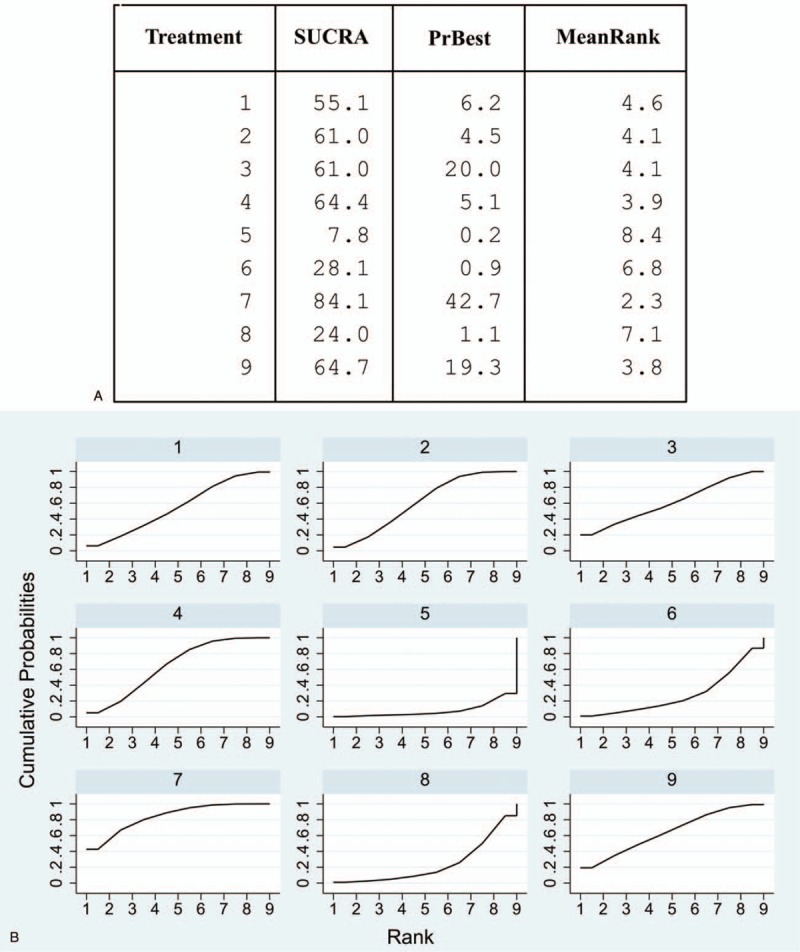
(a) The rankogram showed mean ranks of all treatment modalities, probabilities of being the best intervention and the SUCRA values. (b) Surface under the cumulative ranking (SCURA). Cumulative probabilities were shown graphically (1 = splint therapy, 2 = physiotherapy, 3 = pharmacotherapy, 4 = placebo, 5 = acupuncture or needling, 6 = psychological intervention, 7 = complementary therapy, 8 = bi-physiotherapy, 9 = trigger-point injection.).
3.6. Risk of bias across studies
The general heterogeneity assumption was rejected based on multivariate heterogeneity measures (see Fig. 4, Supplemental Content, which presented the results of multivariate heterogeneity test). However, all pairwise comparisons had much wider PrIs than CIs (Fig. 4). This might attribute to potential heterogeneity between studies, although general heterogeneity among studies were not found. Except 1 comparison between physiotherapy and placebo, nearly all dots were on the zero lone or symmetrical to the zero line in the funnel plot (Fig. 6). In this case, we presumed that the small-study effect had minor influence to the NMA outcomes.
Figure 6.
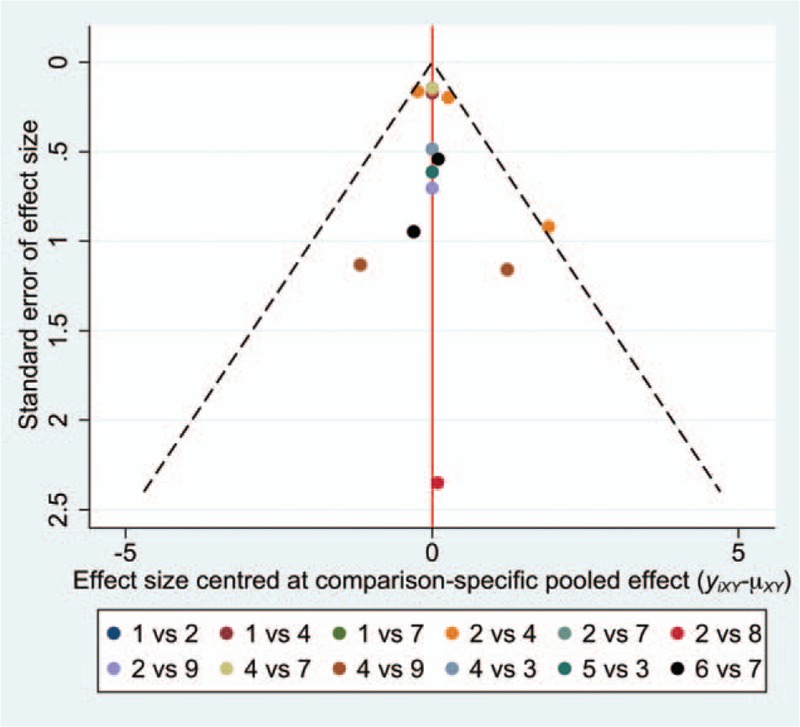
The funnel plot (1 = splint therapy, 2 = physiotherapy, 3 = pharmacotherapy, 4 = placebo, 5 = acupuncture or needling, 6 = psychological intervention, 7 = complementary therapy, 8 = bi-physiotherapy, 9 = trigger-point injection.).
4. Discussion
Complementary therapy and trigger-point injection seemed to be slightly more effective than other treatment modalities. Empirically efficient treatments such as splint therapy and physiotherapy showed no distinct advantage over placebo. But the outcomes of the NMA should be comprehended prudently. There were conflicting results between the NMA and individual trials. Taking hypnosis therapy as an example, it belonged to psychological intervention which showed no distinct advantage over placebo in the NMA. However, it was reported more effective than relaxation by 2 of the included trials.[34,35] But relaxation was in the categorization of complementary therapy with the greatest probability to be the best intervention, which was in contrast to previous trials. The seemingly inconsistent results might derive from the heterogeneity through the NMA which could not be calculated so far.[36] PrI was adopted as a supporting indicator of heterogeneity in pairwise comparisons. It could help verify the credibility of comparative results.[22] In this study, PrIs were wider than CIs in each pairwise comparison, which meant the possibility of reversed outcomes with more available data. This was a further proof that current evidence may not be sufficient enough to justify whether complementary therapy and trigger-point injection had higher treatment efficacy of pain reduction than other treatment modalities.
The diagnostic standards of eligible trials were relatively rigorous and precise. In literature review, there were synonymous but confusing concepts such as myofacial pain dysfunction syndrome (MPDS), pain dysfunction syndrome, facial arthromylagia, etc.[37,38] It might be attributed to different diagnostic systems. And the incidence of TMD was noticed to range from 4% up to 40%, which may also result from diverse diagnostic criteria.[39,40,41] In 1992, the RDC/TMD was intended to be the first step toward improved TMD classification.[1] The Axis I diagnostic algorithms were used for physical assessment, and Axis II were designed for psychological and disability evaluation. It proved to be a reliable protocol in multi-site practice.[42,43] Then the Diagnostic Criteria for TMD (DC/TMD) were newly recommended in 2014, including both a valid screener for detecting any pain-related TMD as well as valid diagnostic criteria for differential diagnosis (sensitivity ≥ 0.86, specificity ≥ 0.98).[1]
Findings of the NMA could be interpreted in the context of other studies. The occlusal appliances have been found in quite widely use and effective in most patients.[7,44] And 3 million splints were expected to be made per year, at a cost of approximately $990 million in the United States.[45] However, Huang et al summarized that there was insufficient evidence for or against the use of stabilization splint therapy over other active interventions for the treatment of temporomandibular myofascial pain.[46] It complied with the results in the NMA. And participants in this study were mostly females, which coincided with previous conditions.[47,48] Accumulating data have shown hormonal fluctuation in TMD patients.[11,49] This may explain why women were more vulnerable. But targeting the self-management treatment to menstrual cycle-related symptoms has not increased the treatment's efficacy so far.[49]
Moreover, Henrikson et al found that the muscle relaxant cyclobenzaprine had a positive effect for TMD muscle pain in their NMA.[50] Noteworthily, these TMD patients were associated with but no limited to myalgia. PrI or any other analytical means were not utilized to examine the credibility of comparative results either. Besides, the average age of participants in this study was relatively younger compared to the research of Anders et al. They found that 50-year-old women had statistically significantly higher prevalence of masticatory muscle pain.[51] If age was a potential influential factor, then age distribution of participants should be considered properly. Edward et al alleged that physiotherapy was probably the most common treatment.[2] And it has been encouraged for its cost-efficiency in a long time.[2] But complementary therapy including self-care and relaxation seemed to have better cost performance. To provide reliable reference for clinicians and public health policy makers, cost performance should be quantified and evaluated through statistical analyses.
There are limitations in the generalizability of NMA. The grouping of treatment modalities mainly depended on the substantial character of interventions,[3,14,27] which may differ with the provider's training, expertise, and clinical experience.[27] On account of multidimensional nature of masticatory muscle pain, standardized interventions should be employed in future trials.[52] For symptom duration, 6 months was adopted as the minimum.[40] But 3 months or shorter duration were also seen in other studies.[13,39] In terms of follow-up, 1 included trial did not report any detail.[32] Though duration of follow-ups was not settled, longer observing time was preferred.[53] As for outcome presentation, pain intensity was the only outcome measurement. It was the subjective evaluation of patients by themselves.[54] Comparatively, pressure pain threshold (PPT) and opening range of mouth (RMO) would be more objective.[54] For extensive results reporting, self judgment could be combined with objective parameters.
In conclusion, complementary therapy seemed to be slightly more effective than placebo for pain reduction in TMD patients with masticatory muscle pain. Evidence should be further reinforced by improving trial designing in optimizing allocation, binding outcome measurement, refining age distribution, initiating standardized interventions, and detailing follow-ups. To provide comprehensive reference, cost performance should be quantified. And future research may integrate subjective and objective parameters into results reporting.
Author contributions
Conceptualization: Ding Bai.
Formal analysis: Jie Feng, Mengqi Luo.
Investigation: Jianbin Ma, Ye Tian, Xianglong Han.
Methodology: Jie Feng, Ding Bai.
Supervision: Xianglong Han, Ding Bai.
Validation: Jianbin Ma, Ye Tian.
Writing – original draft: Jie Feng, Mengqi Luo.
Writing – review & editing: Ding Bai.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AAOP = American Academy of Orofascial Pain, CI = confidential interval, DC/TMD = Diagnostic Criteria Criteria for Temporomandibular Disorders, IF = inconsistency factor, MPDS = myofacial pain dysfunction syndrome, NMA = network meta-analysis, NRS = numeric rating score, PICOS = Problem/patient, Intervention, Comparison, Outcome, Study design, PPT = pressure pain threshold, PrI = predictive interval, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized clinical trial, RDC/TMD = Research Diagnostic Criteria for Temporomandibular Disorders, RMO = opening range of mouth, SUCRA = surface under the cumulative ranking, TMD = temporomandibular disorders, VAS = visual analogue scale.
How to cite this article: Feng J, Luo M, Ma J, Tian Y, Han X, Bai D. The treatment modalities of masticatory muscle pain a network meta-analysis. Medicine. 2019;98:46(e17934).
This study did not involve any information of patients or individuals. And there was no substantial intervention to any patients or individuals in this study. The processed data in this study were extracted from published literatures via available databases. Therefore, it was not necessary to acquire the ethical approval from local ethics committee.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network∗ and Orofacial Pain Special Interest Groupdagger. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Edward GG, Eleni S, Britt R. The use of an oral exercise device in the treatment of muscular TMD. Cranio XXX. [DOI] [PubMed] [Google Scholar]
- [3].Kalamir A, Bonello R, Graham P, et al. Intraoral myofascial therapy for chronic myogenous temporomandibular disorder: a randomized controlled trial. J Manipulative Physiol Ther 2012;35:26–37. [DOI] [PubMed] [Google Scholar]
- [4].Wiendels NJ, van Haestregt A, Knuistingh Neven A, et al. Chronic frequent headache in the general population: comorbidity and quality of life. Cephalalgia 2006;26:1443–50. [DOI] [PubMed] [Google Scholar]
- [5].Rizzatti-Barbosa CM, Martinelli DA, Ambrosano GM, et al. Therapeutic response of benzodiazepine, orphenadrine citrate and occlusal splint association in TMD pain. Cranio 2003;21:116–20. [DOI] [PubMed] [Google Scholar]
- [6].Magri LV, Carvalho VA, Rodrigues FC, et al. Effectiveness of low-level laser therapy on pain intensity, pressure pain threshold, and SF-MPQ indexes of women with myofascial pain. Lasers Med Sci 2017;32:419–28. [DOI] [PubMed] [Google Scholar]
- [7].Paco M, Peleteiro B, Duarte J, et al. The effectiveness of physiotherapy in the management of temporomandibular disorders: a systematic review and meta-analysis. J Oral Facial Pain Headache 2016;30:210–20. [DOI] [PubMed] [Google Scholar]
- [8].Venancio Rde A, Alencar FG, Zamperini C. Different substances and dry-needling injections in patients with myofascial pain and headaches. Cranio 2008;26:96–103. [DOI] [PubMed] [Google Scholar]
- [9].de Moraes Maia ML, Ribeiro MA, Maia LG, et al. Evaluation of low-level laser therapy effectiveness on the pain and masticatory performance of patients with myofascial pain. Lasers Med Sci 2014;29:29–35. [DOI] [PubMed] [Google Scholar]
- [10].Daif ET. Correlation of splint therapy outcome with the electromyography of masticatory muscles in temporomandibular disorder with myofascial pain. Acta Odontol Scand 2012;70:72–7. [DOI] [PubMed] [Google Scholar]
- [11].Roldan-Barraza C, Janko S, Villanueva J, et al. A systematic review and meta-analysis of usual treatment versus psychosocial interventions in the treatment of myofascial temporomandibular disorder pain. J Oral Facial Pain HeadacheV 28 2014;205–22. [DOI] [PubMed] [Google Scholar]
- [12].Benoliel R, Svensson P, Heir GM, et al. Persistent orofacial muscle pain. Oral Dis 2011;17Suppl 1:23–41. [DOI] [PubMed] [Google Scholar]
- [13].Sattayut S, Bradley P. A study of the influence of low intensity laser therapy on painful temporomandibular disorder patients. Laser Ther 2012;21:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kalamir A, Graham PL, Vitiello AL, et al. Intra-oral myofascial therapy versus education and self-care in the treatment of chronic, myogenous temporomandibular disorder: a randomised, clinical trial. Chiropr Man Therap 2013;21:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Michelotti A, Iodice G, Vollaro S, et al. Evaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw muscles. J Am Dent Assoc 2012;143:47–53. [DOI] [PubMed] [Google Scholar]
- [16].Francisco Guedes Pereira de Alencar Júnior, Patricia Gabriela Sabino Viana, Camila Andrade Zamperini, Anne Buss, Becker,. Patient education and self-care for the management of jaw pain upon awakening: a randomized controlled clinical trial comparing the effectiveness of adding pharmacologic treatment with cyclobenzaprine or tizanidine. J Oral Facial Pain Headache 2014;28:119–27. [DOI] [PubMed] [Google Scholar]
- [17].Schwendicke F, Tu YK, Hsu LY, et al. Antibacterial effects of cavity lining: a systematic review and network meta-analysis. J Dent 2015;43:1298–307. [DOI] [PubMed] [Google Scholar]
- [18].Schwendicke F, Paris S, Tu YK. Effects of using different criteria for caries removal: a systematic review and network meta-analysis. J Dent 2015;43:1–5. [DOI] [PubMed] [Google Scholar]
- [19].Pandis N, Fleming PS, Spineli LM, et al. Initial orthodontic alignment effectiveness with self-ligating and conventional appliances: a network meta-analysis in practice. Am J Orthod Dentofacial Orthop 2014;1454 Suppl:S152–63. [DOI] [PubMed] [Google Scholar]
- [20].Jones B, Roger J, Lane PW, et al. Statistical approaches for conducting network meta-analysis in drug development. Pharm Stat 2011;10:523–31. [DOI] [PubMed] [Google Scholar]
- [21].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [22].Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chaimani A, Mavridis D, Salanti G. A hands-on practical tutorial on performing meta-analysis with Stata. Evid Based Ment Health 2014;17:111–6. [DOI] [PubMed] [Google Scholar]
- [24].Major MP, Major PW, Flores-Mir C. Benchmarking of reported search and selection methods of systematic reviews by dental speciality. Evid Based Dent 2007;8:66–70. [DOI] [PubMed] [Google Scholar]
- [25].Barbato L, Kalemaj Z, Buti J, et al. Effect of surgical intervention for removal of mandibular third molar on periodontal healing of adjacent mandibular second molar: a systematic review and bayesian network meta-analysis. J Periodontol 2016;87:291–302. [DOI] [PubMed] [Google Scholar]
- [26].Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–805. [DOI] [PubMed] [Google Scholar]
- [27].Bogart RK, McDaniel RJ, Dunn WJ, et al. Efficacy of group cognitive behavior therapy for the treatment of masticatory myofascial pain. Mil Med 2007;172:169–74. [DOI] [PubMed] [Google Scholar]
- [28].Julian H, James T, Tianjing L, et al. Cochrane Handbook For Systematic Review Of Intervention 5.1.0. 2011. XXX. [Google Scholar]
- [29].Shim S, Yoon BH, Shin IS, et al. Network meta-analysis: application and practice using Stata. Epidemiol Health 2017;39:e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].DeVocht JW, Goertz CM, Hondras MA, et al. A pilot study of a chiropractic intervention for management of chronic myofascial temporomandibular disorder. J Am Dent Assoc 2013;144:1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abrahamsen R, Zachariae R, Svensson P. Effect of hypnosis on oral function and psychological factors in temporomandibular disorders patients. J Oral Rehabil 2009;36:556–70. [DOI] [PubMed] [Google Scholar]
- [32].Espejo-Antunez L, Castro-Valenzuela E, Ribeiro F, et al. Immediate effects of hamstring stretching alone or combined with ischemic compression of the masseter muscle on hamstrings extensibility, active mouth opening and pain in athletes with temporomandibular dysfunction. J Bodyw Mov Ther 2016;20:579–87. [DOI] [PubMed] [Google Scholar]
- [33].Trinquart L, Attiche N, Bafeta A, et al. Uncertainty in treatment rankings: reanalysis of network meta-analyses of randomized trials. Ann Intern Med 2016;164:666–73. [DOI] [PubMed] [Google Scholar]
- [34].Abrahamsen R, Baad-Hansen L, Zachariae R, et al. Effect of hypnosis on pain and blink reflexes in patients with painful temporomandibular disorders. Clin J Pain 2011;27:344–51. [DOI] [PubMed] [Google Scholar]
- [35].Packer AC, Pires PF, Dibai-Filho AV, et al. Effects of upper thoracic manipulation on pressure pain sensitivity in women with temporomandibular disorder: a randomized, double-blind, clinical trial. Am J Phy Med Rehabil 2014;93:160–8. [DOI] [PubMed] [Google Scholar]
- [36].Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 2012;31:3805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Talaat AM, el-Dibany MM, el-Garf A. Physical therapy in the management of myofacial pain dysfunction syndrome. Ann Otol, Rhinol Laryngol 1986;95(3 Pt 1):225–8. [DOI] [PubMed] [Google Scholar]
- [38].Al-Ani Z, Gray RJ, Davies SJ, et al. Stabilization splint therapy for the treatment of temporomandibular myofascial pain: a systematic review. J Dent Educ 2005;69:1242–50. [PubMed] [Google Scholar]
- [39].Diracoglu D, Vural M, Karan A, et al. Effectiveness of dry needling for the treatment of temporomandibular myofascial pain: a double-blind, randomized, placebo controlled study. J Back Muscul Rehabil 2012;25:285–90. [DOI] [PubMed] [Google Scholar]
- [40].Salmos-Brito JA, de Menezes RF, Teixeira CE, et al. Evaluation of low-level laser therapy in patients with acute and chronic temporomandibular disorders. Lasers Med Sci 2013;28:57–64. [DOI] [PubMed] [Google Scholar]
- [41].Kalamir A, Pollard H, Vitiello A, et al. Intra-oral myofascial therapy for chronic myogenous temporomandibular disorders: a randomized, controlled pilot study. J Man Manip Ther 2010;18:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang C, Wu JY, Deng DL, et al. Efficacy of splint therapy for the management of temporomandibular disorders: a meta-analysis. Oncotarget 2016;7:84043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schmitter M, Ohlmann B, John MT, et al. Research diagnostic criteria for temporomandibular disorders: a calibration and reliability study. Cranio 2005;23:212–8. [DOI] [PubMed] [Google Scholar]
- [44].Limchaichana N, Nilsson H, Petersson A, et al. Resilient appliance-therapy treatment outcome in patients with TMD pain correlated to MRI-determined changes in condyle position. Cranio 2009;27:185–93. [DOI] [PubMed] [Google Scholar]
- [45].Pierce CJ, Weyant RJ, Block HM, et al. Dental splint prescription patterns: a survey. J Am Dent Assoc 1995;126:248–54. [DOI] [PubMed] [Google Scholar]
- [46].Thurman MM, Huang GJ. Insufficient evidence to support the use of stabilization splint therapy over other active interventions in the treatment of temporomandibular myofascial pain. J Am Dent Assoc 2009;140:1524–5. [DOI] [PubMed] [Google Scholar]
- [47].La Touche R, Fernández-de-las-Peñas C, Fernández-Carnero J, et al. The effects of manual therapy and exercise directed at the cervical spine on pain and pressure pain sensitivity in patients with myofascial temporomandibular disorders. J Oral Rehabil V 36 2009;644–52. [DOI] [PubMed] [Google Scholar]
- [48].Ebrahim S, Montoya L, Busse JW, et al. The effectiveness of splint therapy in patients with temporomandibular disorders: a systematic review and meta-analysis. J Am Dent Assoc 2012;143:847–57. [DOI] [PubMed] [Google Scholar]
- [49].Turner JA, Mancl L, Huggins KH, et al. Targeting temporomandibular disorder pain treatment to hormonal fluctuations: a randomized clinical trial. Pain 2011;152:2074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haggman-Henrikson B, Alstergren P, Davidson T, et al. Pharmacological treatment of oro-facial pain - health technology assessment including a systematic review with network meta-analysis. J Oral Rehabil 2017;44:800–26. [DOI] [PubMed] [Google Scholar]
- [51].Yekkalam N, Wanman A. Prevalence of signs and symptoms indicative of temporomandibular disorders and headaches in 35-, 50-, 65- and 75-year-olds living in Vasterbotten, Sweden. Acta Odontol Scand 2014;72:458–65. [DOI] [PubMed] [Google Scholar]
- [52].Fricton JR, Ouyang W, Nixdorf DR, et al. Critical appraisal of methods used in randomized controlled trials of treatments for temporomandibular disorders. J Orofac Pain 2010;24:139–51. [PMC free article] [PubMed] [Google Scholar]
- [53].Doepel M, Nilner M, Ekberg E, Y, et al. Long-term effectiveness of a prefabricated oral appliance for myofascial pain. J Oral Rehabil 2012;39:252–60. [DOI] [PubMed] [Google Scholar]
- [54].Tuncer AB, Ergun N, Tuncer AH, et al. Effectiveness of manual therapy and home physical therapy in patients with temporomandibular disorders: a randomized controlled trial. J Bodyw Mov Ther 2013;17:302–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


