Supplemental Digital Content is available in the text
Keywords: diabetes, diabetic nephropathy, insulin resistance, prognosis, TyG index
Abstract
Insulin resistance is usually a key factor in the development of type 2 diabetes. The triglyceride glucose (TyG) index is a marker of insulin resistance which is also implicated in the risk of nephropathy among people with type 2 diabetes. This study aimed to examine associations and potential thresholds between TyG index and the risk of newly diagnosed biopsy-proven diabetic nephropathy in people with type 2 diabetes. A nested case–control study incorporating 950 incident biopsy-proven diabetic nephropathy cases and age, gender matched 4750 patients with treated type 2 diabetes as controls selected by risk-set sampling method was implemented. The dose–response association between TyG index with subsequent risk of newly diagnosed biopsy-proven diabetic nephropathy after adjustment for age, gender, blood pressure, and other major cardiovascular risk factors were examined by conditional logistic regression model. A non-linear relationship was identified between TyG index and the risk of newly diagnosed biopsy-proven diabetic nephropathy with a potential threshold of TyG at 9.05–9.09. Similar relationships with the same threshold were also found in the analyses by fasting glucose and triglyceride levels. TyG index might be a prognostic factor in predicting newly development of biopsy-proven diabetic nephropathy among patients with treated type 2 diabetes. In people with type 2 diabetes, TyG index above 9.05–9.09 could be a prognostic threshold to identify individuals at high risk of diabetic nephropathy. Further replication studies are warranted.
1. Introduction
Diabetic nephropathy (DN) is the leading cause of chronic kidney disease in patients initialising renal replacement therapy, and is associated with increased cardiovascular mortality.[1,2] In the UK Prospective Diabetes Study (UKPDS), the randomized controlled trial of glycemic management of newly diagnosed type 2 diabetes, the annual incidence of clinically diagnosed DN was 2.0% with a 10-year prevalence of 25%.[3] DN is more common in Asian populations.[4] It has been estimated that in the 1990s, DN doubled as an indication for initializing renal replacement therapy.[5]
Insulin resistance (IR) has been reported to be associated with an increased risk of developing progressive DN among patients with diabetes, but only in cross-sectional studies.[6,7] Some prospective studies have also reported that IR may precede and predict microalbuminuria in patients with diabetes.[8] Furthermore, the frequency of factors associated with IR, like hypertension, central obesity, and dyslipidaemia also increase as DN progresses.[6] However, prospective studies demonstrating that IR contributes to the development and progression of DN in type 2 diabetes are yet to be reported, probably due to the cost of insulin measurement and required study duration.
Several recent studies have shown that the triglyceride glucose (TyG) index is associated with IR,[9,10] assessed by both hyperinsulinemic euglycemic clamp testing and homeostasis model assessment of insulin resistance (HOMA-IR). The clamp is considered as the gold standard method for measuring IR.[12] But the clamp method is complex and not used in clinical practice, as it involves a primed-continuous infusion of insulin administered to raise the plasma insulin concentration to a predetermined physiological or pharmacological level. The plasma glucose concentration is then measured at 5-min intervals with a variable infusion of exogenous glucose administered to maintain the plasma glucose concentration constant at the fasting level. Since the plasma glucose concentration remains unchanged, the amount of exogenous glucose infused must equal the amount of glucose utilized in response to the hyperinsulinemia and, thus, provides a direct measure of whole-body sensitivity to insulin.[11]
The other, more widely used HOMA-IR is calculated as (fasting plasma insulin level [in μU/mL] × fasting plasma glucose level [in mmol/L])/22.5. A value of 1.00 is considered normal and higher values indicate progressively severe states of IR. This method is much more variable than the clamp due to the wide range of “normal” values for fasting plasma insulin. Also, it cannot be used in patients with diabetes, where the normal homeostatic relationship between plasma glucose and insulin levels no longer exists. It has theoretical limitations based on the fact that it attempts to measure insulin sensitivity in the fasting state when the majority of glucose uptake is independent of insulin. Thus, the TyG index has shown direct correlation with IR and been proposed as a reliable and simple surrogate marker of IR in clinical practice.[13,14]
Consistent with these data, there is growing evidence to suggest that the TyG index is associated with cardiovascular disease.[15–17] However, to the best of our knowledge, few studies have examined the relationship between the TyG index and the risk of development of DN among patients with type 2 diabetes. Therefore, in the present study, we have investigated the relationship between the TyG index and risk of newly diagnosed biopsy-proven DN among patients with type 2 diabetes.
2. Materials and methods
2.1. Data setting
We conducted this nested case–control study in Zhengzhou, Henan, China, which has 109 million residents. Both cases and controls were enrolled in the First Affiliated Hospital, Zhengzhou University, which is the largest hospital in China and provides both primary and secondary care to Henan residents. Health insurance coverage has been 90% since 2008, allowing most patients with DN to be diagnosed during a hospital admission. As the provincial renal center, most renal biopsies among patients with diabetes were processed in the hospital.
2.2. Case definition
A total of 950 patients with type 2 diabetes who were newly diagnosed with DN at the First Affiliated Hospital, Zhengzhou University between February 1, 2012 and February 28, 2018 were included in this study. The diagnosis of diabetes for cases and controls was based on the American Diabetes Association criteria.[18] The diagnosis of DN was made based on histological characteristics, such as glomerular hypertrophy, thickened capillary basement membranes, diffuse mesangial expansion (sclerosis), nodular mesangial sclerosis, exudative lesions such as capsular drop or fibrin cap, mesangiolysis, mescapillary microaneurysm, or hyalinosis of afferent and efferent arterioles, using appropriate standard for renal biopsy including light microscopy, electron microscopy, and immunofluorescence examination.[9] Patients with other glomerular diseases concomitant with DN were excluded from this study. Renal biopsy was performed for precise diagnosis of renal lesions with the consent of each patient.
2.3. Matched controls
We used the inpatient administration system to select 5 controls for each case, matched for age and gender. Controls were patients with type 2 diabetes who attended outpatient departments or were admitted to the hospital between January 1, 2016 and December 31, 2017. Patients with estimated glomerular filtration rate (eGFR) <90 mL/min/1.73 m2 and urine total protein >30 mg/24 h were excluded.[9,19] Controls were selected using a risk set sampling method,[10] by which the odds ratios estimated the incidence rates ratios. Controls were assigned an index date identical to that of corresponding cases.
2.4. Exposure definition
TyG index was calculated as the ln[fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2].[20] In the dose–response analysis between TyG and risk of DN, TyG was treated as continuous variable. To understand the outcome distribution further, a histogram is shown of incidence rates ratios of outcome by TyG quartile group (Supplemental Figure S1): Group 1 (TyG index <8.60), Group 2 (TyG index in 8.60–9.09); Group 3 (TyG index in 9.09–9.60), and Group 4 (TyG index ≥ 9.60).
2.5. Ethics approval
Ethics approval was granted by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Written informed consent was obtained from all participants before inclusion.
2.6. Co-variables and missing information
There was missing information on body mass index (56.2%), systolic blood pressure (37.4%), diastolic blood pressure (38.2%), fasting glucose (6.1%), HbA1c (6.7%), hematocrit (13.6%), mean corpuscular hemoglobin (13.6%), mean platelet volume (13.6%), monocyte (13.6%), red blood cell distribution width (13.6%), magnesium (22.1%), sodium (22.1%), chlorine (22.1%), activated partial thromboplastin time (32.8%), D-Dimer (32.8%), thrombin time (32.8%), fibrinogen (32.8%), and fibrinogen degradation products (32.8%). Multiple imputations were applied to replace missing values by using a chained equation method based on all candidate predictors and primary outcome. Fifty-six imputed datasets were generated for missing predictors that were then combined across all datasets by using Rubin's rule to generate final model estimates.[21]
2.7. Statistical analysis
In descriptive analyses, differences in participant characteristics by TyG index categories were assessed by logistic regression model for categorical variables, and generalized linear model for continuous variables.
Newly diagnosed biopsy-proven DN was defined as a binary outcome measure. A conditional logistic regression model was used to estimate the crude and adjusted incidence rates ratios of newly diagnosed biopsy-proven DN by TyG index categories. The dose–response relationships between TyG index and risks of newly diagnosed biopsy-proven DN were estimated using a linear model, a natural cubic spline model with three equally spaced knots determined from the levels of TyG index measures, and a quadratic spline model. The natural cubic spline model was chosen as the best fit model for the relationship curve by its minimum Akaike information criterion (AIC) compared with the linear model or quadratic spline model. The linear test was used in the natural cubic spline model to test the linearity of the relationship. The break-point test[22] was carried out to target the potential thresholds (P5 to P95 of TyG index measures) by incorporating the piecewise term into the cubic spline model. The threshold with a significant break in the regression coefficients and achieving the minimum AIC was chosen as the final threshold.[23] The 95% CI of the threshold was obtained from 1000 bootstrap samples. The associations between the TyG index and the risk of newly diagnosed biopsy-proven DN below and above the threshold were analysed such that the TyG index was treated as a continuous variable to observe the risk of DN with each 1 unit increase of the TyG index.
In the sensitivity analyses, the dose–response association between the TyG index and risk of DN was re-analyzed by the level of confounders (glucose and triglyceride) to examine whether the dose–response associations were consistent with those found in the main analyses. At each level of confounders, we also quantified the association between TyG index and risk of DN below and above threshold.
All analyses were performed using STATA (STATA/MP 15.0 StataCorp, College Station, TX). All P values were calculated using two-tailed tests and a P value < .05 was taken to indicate statistical significance. All methods were performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
3. Results
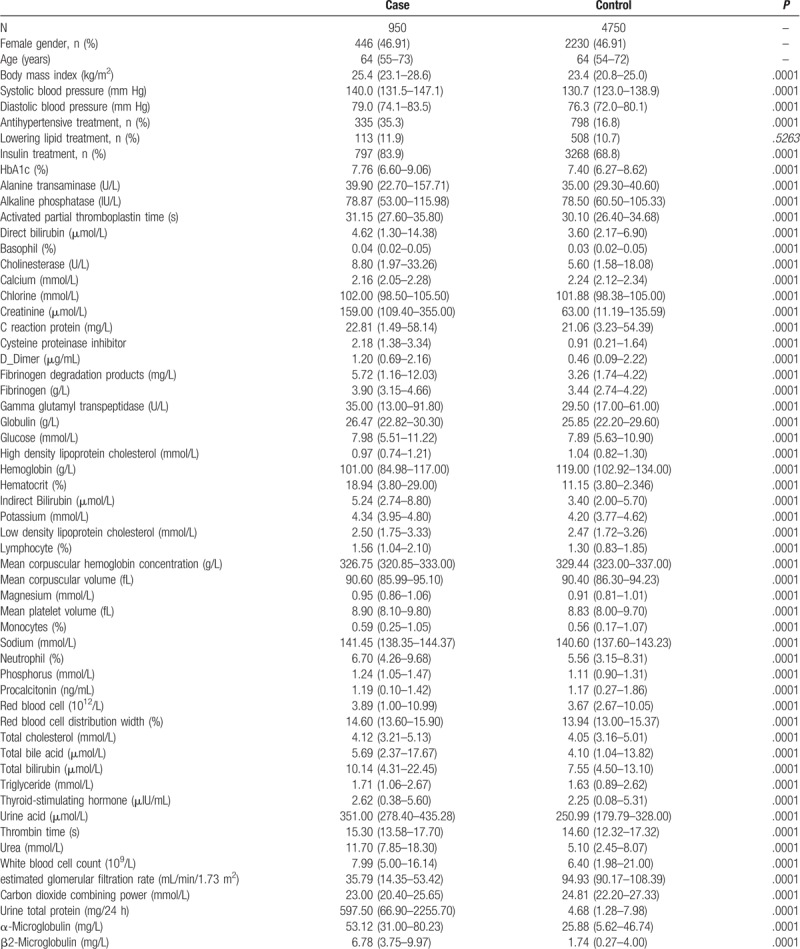
Potential prognostic factors measured in cases and controls are shown in Table 1. Cases with newly diagnosed biopsy-proven DN had lower levels of hemoglobin, mean corpuscular hemoglobin, lymphocyte, monocyte, total bile acid, high density lipoprotein cholesterol, calcium, carbon dioxide combining power and eGFR, and higher levels of the remaining factors.
Table 1.
Clinical measurements among diabetic nephropathy cases and matched controls with type 2 diabetes.
A non-linear (“J-shape”) relationship was found between TyG index and risk of development of newly diagnosed biopsy-proven DN (P-values for linearity test < .0001). The dose–response relationship was derived from the natural cubic spline model with adjustment of covariables in Figure 1. In the sensitivity analysis modeling the associations with fasting glucose and serum triglyceride (fasting glucose <8.0 mmol/L and fasting glucose ≥8.0 mmol/L; triglyceride <1.5 mmol/L and triglyceride ≥1.5 mmol/L), similar dose–response relationships were identified by fasting glucose level (Figure 1) and by serum triglyceride level (Figure 2). The individual-level incidence rates ratio distribution in four TyG categories based on the quartile of TyG (Group-1: TyG ≤ 8.60; Group-2: TyG 8.60 < TyG ≤ 9.09; Group-3: 9.09 < TyG≤9.60; and Group-4: TyG > 9.60) were presented in Supplemental Figure S1, which indicated most patients’ incidence rates ratio covering the estimated range of risk of DN shown in Figure 1.
Figure 1.
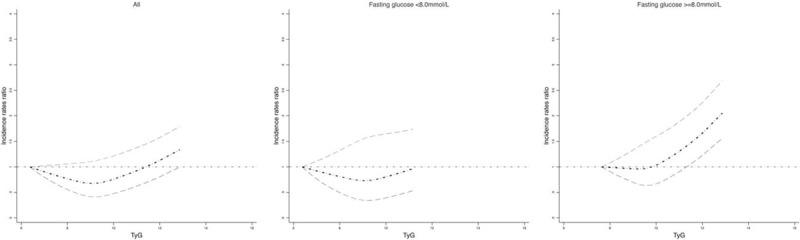
The dose–response association between TyG and risk of biopsy-proven diabetic nephropathy overall and by fasting glucose level. Body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive treatment, lowering lipid treatment, insulin treatment, HbA1c, alanine transaminase, alkaline phosphatase, activated partial thromboplastin time, direct bilirubin, Basophil, cholinesterase, calcium, chlorine, creatinine, C reaction protein, cysteine proteinase inhibitor, D_Dimer, fibrinogen Degradation Products, gamma glutamyl transpeptidase, globulin, glucose, high density lipoprotein cholesterol, hemoglobin, hematocrit, indirect Bilirubin, potassium, low density lipoprotein cholesterol, lymphocyte, mean corpuscular hemoglobin concentration, mean corpuscular volume, magnesium, mean platelet volume, monocytes, sodium, neutrophil, phosphorus, procalcitonin, red blood cell, red blood cell distribution width, total cholesterol, total bile acid, total bilirubin, triglyceride, thyroid-stimulating hormone, urine acid, thrombin time, white blood cell count, estimated glomerular filtration rate, carbon dioxide combining power, urine total protein, α-microglobulin, β2-microglobulin were adjusted.
Figure 2.
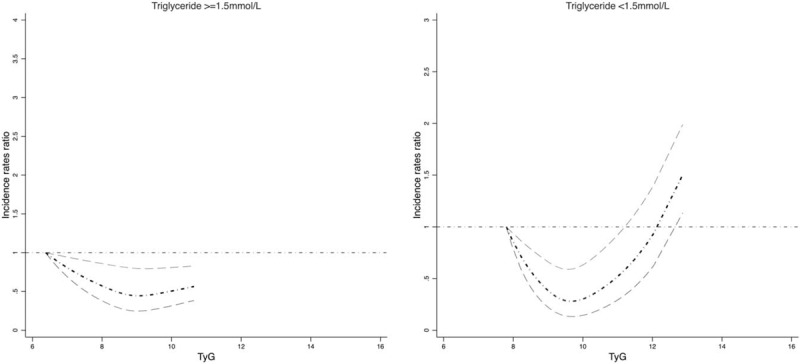
The dose–response association between TyG and risk of biopsy-proven diabetic nephropathy by triglyceride level. Body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive treatment, lowering lipid treatment, insulin treatment, HbA1c, alanine transaminase, alkaline phosphatase, activated partial thromboplastin time, direct bilirubin, Basophil, cholinesterase, calcium, chlorine, creatinine, C reaction protein, cysteine proteinase inhibitor, D_Dimer, fibrinogen Degradation Products, gamma glutamyl transpeptidase, globulin, glucose, high density lipoprotein cholesterol, hemoglobin, hematocrit, indirect Bilirubin, potassium, low density lipoprotein cholesterol, lymphocyte, mean corpuscular hemoglobin concentration, mean corpuscular volume, magnesium, mean platelet volume, monocytes, sodium, neutrophil, phosphorus, procalcitonin, red blood cell, red blood cell distribution width, total cholesterol, total bile acid, total bilirubin, triglyceride, thyroid-stimulating hormone, urine acid, thrombin time, white blood cell count, estimated glomerular filtration rate, carbon dioxide combining power, urine total protein, α-microglobulin, β2-microglobulin were adjusted.
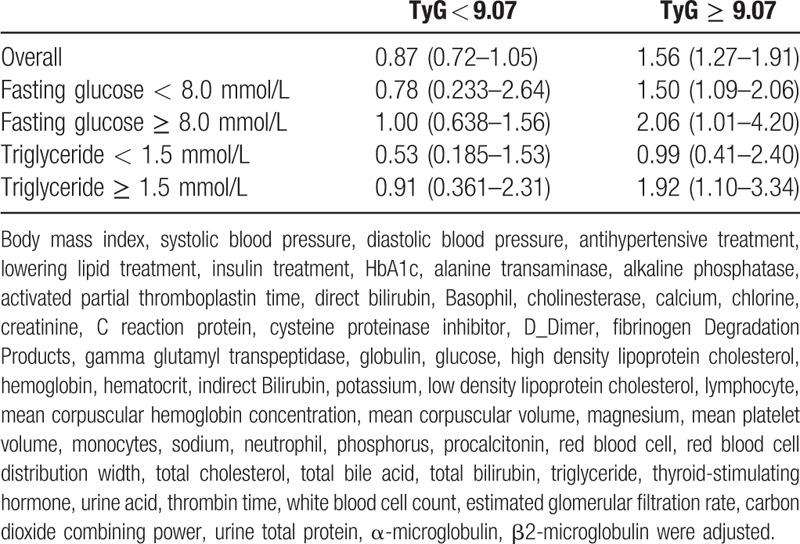
A TyG index below 9.07 (95% confidence interval: 9.05–9.09) was estimated to be associated with the lowest risk of newly diagnosed biopsy-proven DN, as tested by linear threshold models. The thresholds were the same with fasting glucose levels and serum triglyceride levels. Table 2 shows that the risks of newly diagnosed biopsy-proven DN increase significantly with each 1 unit increase of TyG index above the TyG threshold (9.07) overall and by glucose and triglyceride levels (except triglyceride <1.5 mmol/L): adjusted incidence rates ratio (IRR) per TyG index unit for risk of newly diagnosed biopsy-proven DN 1.56 (95% CI: 1.27–1.91, P < .0001) overall; adjusted IRR for risk of newly diagnosed biopsy-proven DN 1.50 (1.09–2.06, P < .0001) in those with glucose <8.0 mmol/L and 2.06 (1.01–4.20, P < .0001)) in those with glucose ≥ 8.0 mmol/L; adjusted IRR for risk of newly diagnosed biopsy-proven DN 0.99 (0.41–2.40, P = .3562) in those with triglyceride <1.5 mmol/L and 1.92 (1.10–3.34, P < .0001) in those with triglyceride ≥ 1.5 mmol/L. The risks of newly diagnosed biopsy-proven DN did not increase significantly with 1 unit increase of TyG index above the TyG threshold overall and by glucose and triglyceride levels (Table 2).
Table 2.
Adjusted incidence rates ratio for risk of newly diagnosed biopsy-proven diabetes nephropathy by 1 unit increase in TyG overall and in groups classified by TyG threshold (9.07).
4. Discussion
Our study was undertaken to relate TyG index, as a measure of insulin resistance, to the risks of newly diagnosed biopsy-proven DN in patients with type 2 diabetes. We focused our investigation on the dose–response relationships assessing the evidence for a nonlinear association, and for the existence of a threshold. In all our analyses, we found evidence that the associations are nonlinear. Threshold analysis provided evidence of a TyG index threshold: 9.07 (9.05–9.09). The significantly higher risks of newly diagnosed biopsy-proven DN were found above 9.07 of TyG index in people with type 2 diabetes.
Our investigation is the first study to explore the association between TyG index, as a surrogate for IR and the risk of newly diagnosed biopsy-proven DN in people with type 2 diabetes. In previous reports, a high TyG index had been found to be associated with the risk of type 2 diabetes in the general population,[24] with subclinical atherosclerosis and arterial stiffness in postmenopausal women[25] and with the risk of other diabetes related complications, such as coronary artery stenosis[26] and Cardiac Autonomic Neuropathy.[27] Consistent with these previous findings, we have found that a high TyG index (in particular TyG index ≥ 9.07) is associated with a high risk of DN, which is the leading cause of end-stage renal disease in people with type 2 diabetes.
Non-linear dose–response relationships between TyG index and non-DN outcomes have previously been shown. For example, among patients with type 2 diabetes, a previous study was found that compared with patients with TyG index at 8.2, people with TyG index at 8.9 had a higher risk of coronary artery stenosis and this risk significantly increased among those with a TyG index of 9.7.[26] In another study, in the general population, compared with people whose TyG index was below 8.21, the risk of incident type 2 diabetes significantly increased with a TyG index of 8.21 to 5.56, plateaued and then increased at a TyG index > 8.97.[24] However, in those studies, the TyG index was modeled as a categorical variable grouped by percentiles, a method no longer recommended as it is found to be less informative.[28] In our study, TyG was modeled as a continuous variable, applying a flexible cubic spline regression model to derive a more accurate dose–response relationship, “J-shape” and a convincible threshold, TyG at 9.05 to 9.09 for future replications and clinical practice.
The mechanism behind the association between TyG index and risk of newly diagnosed biopsy-proven DN could be the progression of IR in people with type 2 diabetes.[29] Previous findings suggest that the deteriorated IR in people with type 2 diabetes is associated with significantly changed levels of hormone, like parathyroid hormone,[30] cortisol,[31] peptide hormone[32,33] that could lead to microalbuminuria and declined eGFR in people with type 2 diabetes.
Our findings have some potential implications for the clinical practice. First, this dose–response relationship and especially the threshold found in our study could be used as a prognostic index in clinical practice to identify potentially high-risk individuals that could warrant more intensive treatment to postpone the progress of DN. In practical terms, the need to reduce and then cease metformin, an insulin sensitizer, could accelerate progression to end-stage renal disease.[34] The careful use of a thiazolidinedione, while monitoring for and avoiding fluid overload, could be a strategy to reduce progression while maintaining euglycemia.[35] Finally, while the clinical utilisation of HOMA is restricted by its cost and lack of utility in insulin-treated diabetes, the TyG Index, a measurement of fasting glucose and triglycerides, could be used as a marker of IR.[20,36] This index has the advantage of being clinically applicable as both triglyceride and glucose concentrations are inexpensive and routinely measured in those with diabetes.[20]
Our study has some limitations. First, the control population were patients admitted to hospital, and therefore unlikely to be representative of the Chinese type 2 diabetes population. However, those with DN were also hospital patients, partly because there are no distinctive differences between primary and secondary care settings in China. The potential selection bias in our study would therefore be lower than in settings without this continuum. Future validation studies are warranted in other type 2 diabetes populations, including those from ambulatory care (primary and secondary). Secondly, the proportion of people with missing data was significant, particularly for some covariables. Although our analyses were carried out using imputed datasets, further validation studies with datasets with less missing data are also warranted.
In summary, we have identified a non-linear relationship between the TyG index, a proxy for IR, and risk of newly diagnosed biopsy-proven DN in people with type 2 diabetes. The TyG index threshold of 9.05 to 9.09 may be useful for identifying high-risk individuals for further intensive intervention. These findings may suggest a greater role for insulin sensitizers in the prevention of DN. Further replication studies are warranted.
Acknowledgments
We thank the First Affiliated hospital of Zhengzhou University approved this study. We thank Henan Peritoneal Dialysis Registry (HPDR) to provide the data for this study.
Author contributions
Conceptualization: Dahai Yu, Bin Zhao, Zhanzheng Zhao, David Simmons.
Data curation: Jin Shang, Zheng Wang, Zhanzheng Zhao.
Formal analysis: Dahai Yu, Yamei Cai, Bin Zhao, David Simmons.
Funding acquisition: Dahai Yu, Zhanzheng Zhao.
Investigation: Jin Shang, Zheng Wang, Bin Zhao, Zhanzheng Zhao, David Simmons.
Methodology: Dahai Yu, Yamei Cai, Bin Zhao, Zhanzheng Zhao, David Simmons.
Project administration: Jin Shang, Zheng Wang, Bin Zhao, Zhanzheng Zhao, David Simmons.
Supervision: Zhanzheng Zhao.
Validation: Zheng Wang.
Visualization: Dahai Yu, Yamei Cai.
Writing – original draft: Jin Shang, Dahai Yu, Yamei Cai, David Simmons.
Writing – review & editing: Jin Shang, Dahai Yu, Yamei Cai, Zheng Wang, Bin Zhao, Zhanzheng Zhao, David Simmons.
Supplementary Material
Footnotes
Abbreviations: DN = diabetic nephropathy, eGFR = estimated glomerular filtration rate, HOMA-IR = homeostasis model assessment of insulin resistance, IR = insulin resistance, TyG = triglyceride glucose index, UKPDS = UK Prospective Diabetes Study.
How to cite this article: Shang J, Yu D, Cai Y, Wang Z, Zhao B, Zhao Z, Simmons D. The triglyceride glucose index can predict newly diagnosed biopsy-proven diabetic nephropathy in type 2 diabetes. Medicine. 2019;98:46(e17995).
JS and DY are the first authors.
The data used to support the findings of this study are available from the corresponding author upon request.
This work was supported by the National Natural Science Foundation of China (Grant No. 81873611), Science and Technology Innovation Team of Henan (Grant No. 17IRTSTHN020); Foundation for Leading Personnel of Central Plains of China (Grant No. 194200510006) the Excellent Youth Foundation of Henan Scientific Committee (Grant No. 154100510017). Dr Dahai Yu was funded by Honorary Public Health Academic Contract from Public Health England.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011;80:572–86. [DOI] [PubMed] [Google Scholar]
- [2].Yu D, Cai Y, Chen Y, et al. Development and validation of risk prediction models for cardiovascular mortality in Chinese people initialising peritoneal dialysis: a cohort study. Sci Rep 2018;8:1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225–32. [DOI] [PubMed] [Google Scholar]
- [4].Roderick P, Byrne C, Casula A, et al. Survival of patients from South Asian and Black populations starting renal replacement therapy in England and Wales. Nephrol Dial Transplant 2009;24:3774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nitsch D, Burden R, Steenkamp R, et al. Patients with diabetic nephropathy on renal replacement therapy in England and Wales. QJM 2007;100:551–60. [DOI] [PubMed] [Google Scholar]
- [6].Thorn LM, Forsblom C, Fagerudd J, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 2005;28:2019–24. [DOI] [PubMed] [Google Scholar]
- [7].Liang S, Cai GY, Chen XM. Clinical and pathological factors associated with progression of diabetic nephropathy. Nephrology (Carlton) 2017;22Suppl 4:14–9. [DOI] [PubMed] [Google Scholar]
- [8].Lane JT. Microalbuminuria as a marker of cardiovascular and renal risk in type 2 diabetes mellitus: a temporal perspective. Am J Physiol Renal Physiol 2004;286:F442–50. [DOI] [PubMed] [Google Scholar]
- [9].Yu D, Cai Y, Graffy J, et al. Development and external validation of risk scores for cardiovascular hospitalization and rehospitalization in patients with diabetes. J Clin Endocrinol Metab 2018;103:1122–9. [DOI] [PubMed] [Google Scholar]
- [10].Yu D, Cai Y, Graffy J, et al. Derivation and external validation of risk algorithms for cerebrovascular (re)hospitalisation in patients with type 2 diabetes: two cohorts study. Diabetes Res Clin Pract 2018;144:74–81. [DOI] [PubMed] [Google Scholar]
- [11].Tam CS, Xie W, Johnson WD, et al. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 2012;35:1605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95. [DOI] [PubMed] [Google Scholar]
- [13].Bloomgarden ZT. Diabetic nephropathy. Diabetes Care 2005;28:745–51. [DOI] [PubMed] [Google Scholar]
- [14].Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005;28:164–76. [DOI] [PubMed] [Google Scholar]
- [15].American Diabetes, Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013;36Suppl 1:S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shimizu M, Furuichi K, Toyama T, et al. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care 2013;36:3655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Molitch ME, DeFronzo RA, Franz MJ, et al. Nephropathy in diabetes. Diabetes Care 2004;27Suppl 1:S79–83. [DOI] [PubMed] [Google Scholar]
- [18].Yu D, Simmons D. Association between pulse pressure and risk of hospital admissions for cardiovascular events among people with Type 2 diabetes: a population-based case-control study. Diabet Med 2015;32:1201–6. [DOI] [PubMed] [Google Scholar]
- [19].Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–9. [DOI] [PubMed] [Google Scholar]
- [20].Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010;95:3347–51. [DOI] [PubMed] [Google Scholar]
- [21].Zhang X, Yu D, Cai Y, et al. Derivation and validation of risk scores to predict cerebrovascular mortality among incident peritoneal dialysis patients. Kidney Blood Press Res 2018;43:1141–8. [DOI] [PubMed] [Google Scholar]
- [22].Zhang X, Yu D, Cai Y, et al. Dose-response between cardiovascular risk factors and cardiovascular mortality among incident peritoneal dialysis patients. Kidney Blood Press Res 2018;43:628–38. [DOI] [PubMed] [Google Scholar]
- [23].Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–73. [DOI] [PubMed] [Google Scholar]
- [24].Lee DY, Lee ES, Kim JH, et al. Predictive value of triglyceride glucose index for the risk of incident diabetes: a 4-year retrospective longitudinal study. PLoS One 2016;11:e0163465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lambrinoudaki I, Kazani MV, Armeni E, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ 2018;27:716–24. [DOI] [PubMed] [Google Scholar]
- [26].Lee EY, Yang HK, Lee J, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis 2016;15:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Akbar M, Bhandari U, Habib A, et al. Potential association of triglyceride glucose index with cardiac autonomic neuropathy in type 2 diabetes mellitus patients. J Korean Med Sci 2017;32:1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006;25:127–41. [DOI] [PubMed] [Google Scholar]
- [29].Svensson M, Eriksson JW. Insulin resistance in diabetic nephropathy – cause or consequence? Diabetes Metab Res Rev 2006;22:401–10. [DOI] [PubMed] [Google Scholar]
- [30].DeFronzo RA, Andres R, Edgar P, et al. Carbohydrate metabolism in uremia: a review. Medicine (Baltimore) 1973;52:469–81. [DOI] [PubMed] [Google Scholar]
- [31].Schmitz O, Alberti KG, Christensen NJ, et al. Aspects of glucose homeostasis in uremia as assessed by the hyperinsulinemic euglycemic clamp technique. Metabolism 1985;34:465–73. [DOI] [PubMed] [Google Scholar]
- [32].Jansson PA, Eliasson B, Lindmark S, et al. Endocrine abnormalities in healthy first-degree relatives of type 2 diabetes patients – potential role of steroid hormones and leptin in the development of insulin resistance. Eur J Clin Invest 2002;32:172–8. [DOI] [PubMed] [Google Scholar]
- [33].Girard J. Is leptin the link between obesity and insulin resistance? Diabetes Metab 1997;23Suppl 3:16–24. [PubMed] [Google Scholar]
- [34].Pham H, Utzschneider KM, de Boer IH. Measurement of insulin resistance in chronic kidney disease. Curr Opin Nephrol Hypertens 2011;20:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 2016;311:F1087–108. [DOI] [PubMed] [Google Scholar]
- [36].Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008;6:299–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


