Abstract
Nonalcoholic fatty liver disease (NAFLD) is recognized as a hepatic manifestation of metabolic syndrome because of the association with visceral obesity. However, the association between NAFLD and subcutaneous fat accumulation remains unclear.
The study population included 3197 participants in regular health checkups, who were both hepatitis B virus surface antigen and hepatitis C virus antibody-negative, and consumed <20 g of alcohol per day. They were divided according to 4 quantiles of subcutaneous fat area (SFA) and visceral fat area (VFA) on computed tomography. Fatty liver was diagnosed using ultrasonography (FL-US).
The prevalence of FL-US increased across the SFA categories, even after adjusting for the VFA, in both men (P < .001) and women (P < .001). This significant association between FL-US and the SFA was already detected from the second SFA quantile. It is noteworthy that the mean body mass index (BMI) of the subjects in the second quantile was 23.7 kg/m2 in men and 22.6 kg/m2 in women. Independent positive associations were observed between alanine aminotransferase elevation, and both the SFA and VFA in men, while gamma glutamyl transpeptidase elevation was independently associated with the VFA, but not the SFA, in both men and women. Similarly, the components of metabolic syndrome were independently associated with the VFA, but were less strongly associated (or not associated at all) with the SFA.
This cross-sectional study suggests that NAFLD is independently associated with both visceral and subcutaneous adiposity ab initio, which is a characteristic that distinguishes NAFLD from other components of metabolic syndrome.
Keywords: metabolic syndrome, nonalcoholic fatty liver disease, subcutaneous obesity, visceral obesity
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of noncancerous liver disease ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), and has been recognized as a hepatic manifestation of metabolic syndrome (ie, visceral obesity and insulin resistance).[1] An increased prevalence of NAFLD is a problem around the world, including in Japan.[1,2] Adiposity due to a positive energy balance starts with the accumulation of fat in the subcutaneous adipose tissue, with relatively little influence on insulin sensitivity, until the limit is expanded through adipose tissue dysfunction.[3,4] The lipids then overflow, and the accumulation of visceral and ectopic fat sets in, resulting in insulin resistance and related cardiometabolic problems. Several factors differentiate the subcutaneous and visceral adipose tissues, including adipokine and cytokine production, adipogenic potential, and the ability to store and mobilize lipids.[5,6] Visceral adipose tissue is anatomically linked to the liver via the portal vein.[7] It has been widely accepted that liver fat is a type of ectopic fat strongly associated with visceral obesity.
However, the prevalence of NAFLD in the nonobese population has not been low in the Japanese population.[8] In addition, there is not yet a unified view concerning the association between NAFLD and subcutaneous obesity. We therefore aimed to clarify the association between the subcutaneous fat area (SFA) (as determined by computed tomography [CT]) and fatty liver (FL) (as determined by ultrasonography [US]) in the Japanese population.
2. Methods
We conducted a cross-sectional study from April, 2007 to March, 2017. Participants in regular health checkups who had their SFA and visceral fat area (VFA) evaluated were enrolled. We collected the data from the records of these subjects. All subjects were both hepatitis B virus surface antigen and hepatitis C virus antibody-negative, and consumed less than 20 g of ethanol per day. We excluded cases with data loss. Ultimately, 1723 men and 1474 women were analyzed.
The VFA and SFA were determined by CT at an umbilical slice, and measured using the Fat Scan software program (East Japan Institute of Technology Co., Ltd, Ibaraki, Japan). A diagnosis of FL was made using US. The subjects were divided into 4 groups according to the SFA (cm2) quantiles (men: 17.8 ≤ S-Q1 ≤ 106.6, S-Q2 ≤ 138.3, S-Q3 ≤ 174.2, S-Q4 ≤ 452.5; women: 22.7 ≤ S-Q1 ≤ 142.1, S-Q2 ≤ 183.6, S-Q3 ≤ 231.8, S-Q4 ≤ 514.1) or VFA (cm2) quantiles (men: 10.0 ≤ V-Q1 ≤ 73.8, V-Q2 ≤ 103.2, V-Q3 ≤ 139.6, V-Q4 ≤ 447.8; women: 2.7 ≤ V-Q1 ≤ 44.1, V-Q2 ≤ 67.3, V-Q3 ≤ 94.2, V-Q4 ≤ 346.0).
The subjects were examined for concomitant metabolic abnormalities. We defined clinical terms such as dyslipidemia (DL) according to the Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017,[9] and hypertension (HT) according to the metabolic syndrome definition.[10] Specifically, HT was defined as the use of medication for hypertension, a systolic blood pressure of ≥130 mm Hg, and/or a diastolic blood pressure of ≥85 mm Hg. DL was defined as the use of medication for DL, a triglyceride level ≥150 mg/dL, a high-density lipoprotein (HDL)-cholesterol level of <40 mg/dL, and/or a low-density lipoprotein (LDL)-cholesterol level ≥140 mg/dL (total cholesterol minus HDL–cholesterol ≥170 mg/dL in the subjects in whom the LDL-cholesterol level had not been determined). An impaired glucose metabolism (IGM) was defined as the use of antidiabetic agents, a fasting blood glucose level ≥110 mg/dL, and/or a hemoglobin A1c concentration of >6.2%.[11] Alanine aminotransferase elevation (ALT-E) and gamma glutamyl transpeptidase elevation (GGTP-E) were defined as levels of >30 and >50 IU/L, respectively. Obesity was defined as a body mass index (BMI) ≥25 kg/m2, according to the criteria of the Japan Society for the Study of Obesity.[12]
This study was cross-sectional in nature and was approved by the ethics committee of the Kagoshima Prefectural Federation of Agricultural Cooperatives for Health and Welfare. The participants’ information was anonymized and de-identified before the analyses; this observational research did not require informed consent.
Continuous variables were analyzed using a t test or an analysis of variance. Categorical variables were examined using the chi-square test and Mantel-Haenszel test. The maximum likelihood of odds ratios (ORs) for the risk of fatty liver and 95% confidence interval (95% CI) were calculated using logistic regression models. We calculated the P for trend across the mean values of 4 groups according to the SFA and VFA quantiles. All P values were 2-sided. P values <.05 were considered to indicate statistical significance. Statistical analyses were performed using the R software program (version 2.13.0) and the Statistical Package for Social Sciences software program, version 18 (SPSS, Inc., Chicago, IL).
3. Results
3.1. The baseline features of the subjects
The characteristic features of the subjects are summarized in Table 1. The mean age was 55.2 ± 11.2 years in men, and 58.6 ± 9.4 years in women. The mean BMI was 24.5 ± 3.0 kg/m2 in men and 23.6 ± 3.3 kg/m2 in women. The SFA in men was significantly lower than that in women (146.1 ± 59.2 vs 190.0 ± 71.6 cm2; P < .001), whereas the VFA in men was significantly larger than that in women (115.5 ± 55.0 vs 74.4 ± 41.8 cm2; P < .001). The prevalence of fatty liver diagnosed using ultrasonography (FL-US), ALT-E, and GGTP-E in men was significantly higher than that in women (FL-US, 44.8% vs 27.9%, P < .001; ALT-E, 31.7% vs 11.3%, P < .001; GGTP-E, 24.4% vs 5.6%, P < .001).
Table 1.
The characteristics of the study population.
3.2. Comparisons among 4 groups divided according to the SFA or VFA
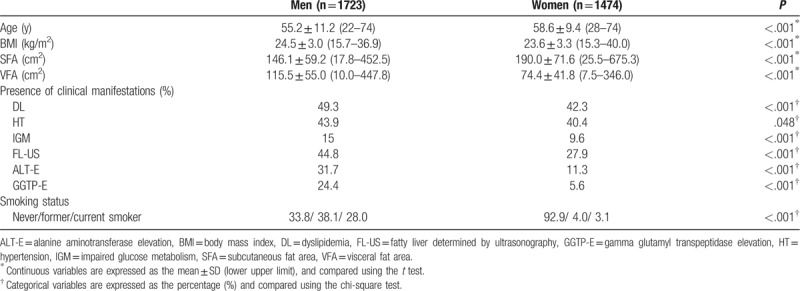
The comparisons among 4 groups divided according to the SFA or VFA are summarized in Tables 2 and 3 (men), and 4 and 5 (women). Age significantly decreased across the SFA categories in men (P < .001) (Table 2). There were no significant differences among the 4 SFA groups in women (P = .238) (Table 4). Age significantly increased across the VFA categories in both men and women (both P < .001) (Tables 3 and 5). These results suggested that the gravity of visceral adipose tissue in the body composition increases with age in both men and women. The BMI values significantly increased across the SFA and VFA categories in both men and women (all P < .001) (Tables 2–5).
Table 2.
Comparison of the 4 subcutaneous fat area groups (men).
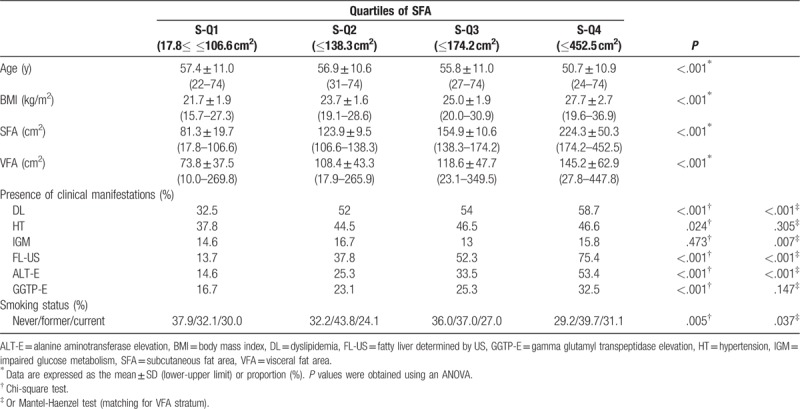
Table 3.
Comparison of the 4 visceral fat area groups (men).
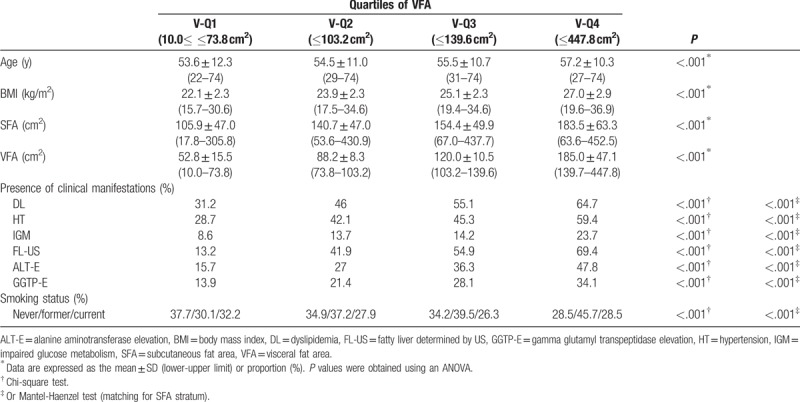
Table 4.
Comparison of the 4 subcutaneous fat area groups (women).
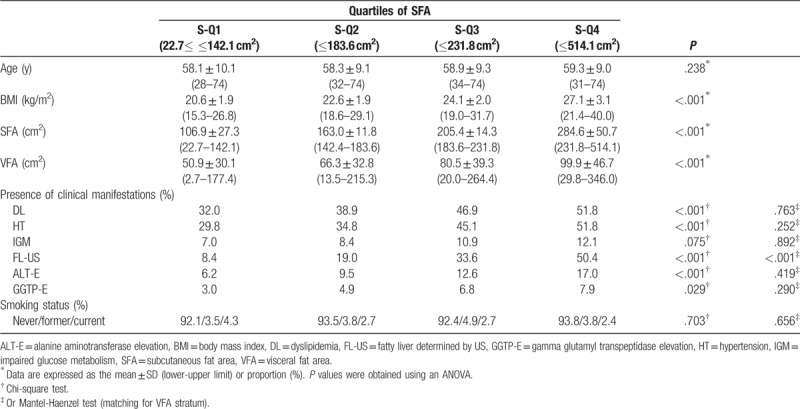
Table 5.
Comparison of the 4 visceral fat area groups (women).
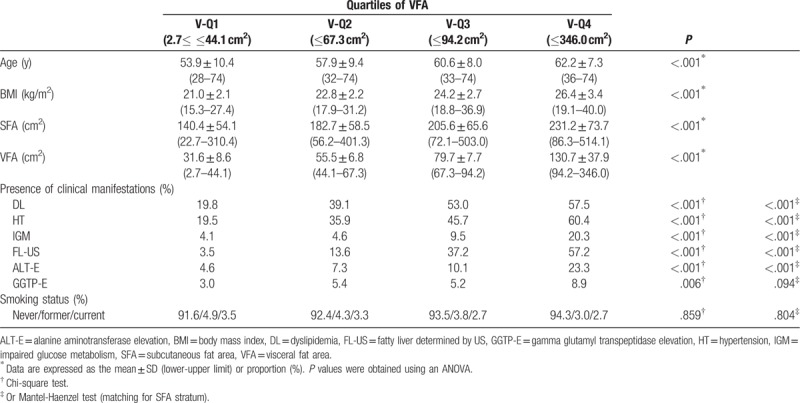
Dyslipidemia, HT, and IGM are components of metabolic syndrome. The prevalence of these diseases increased significantly across the VFA categories even after adjusting for the SFA in both men and women (all P < .001) (Tables 3 and 5).
The prevalence of DL was significantly increased across the SFA categories in both men and women (both P < .001) (Tables 2 and 4). The difference remained significant even after adjusting for the VFA in men (P < .001), but not in women (P = .763) (Tables 2 and 4).
Although the prevalence of HT in both men and women significantly increased with the SFA in both men (P = .024) and women (P < .001), the difference did not remain significant after adjusting for the VFA (men, P = .305; P = .075) (Tables 2 and 4).
There was no significant difference in the prevalence of IGM among the 4 SFA groups in men (P = .473) or women (P = .075) (Tables 2 and 4).
The prevalence of FL-US increased significantly across the SFA categories, even after adjusting for the VFA, in both men and women (all P < .001) (Tables 2 and 4). The prevalence of FL-US also increased significantly across the VFA categories, even after adjusting for the SFA, in both men and women (all P < .001) (Tables 3 and 5).
The prevalence of ALT-E significantly increased across the SFA categories in both men and women (both P < .001, chi-square test) (Tables 2 and 4). A significant difference was seen even after adjusting for the VFA in men (P < .001) (Table 2), but not in women (P = .419) (Table 4). The prevalence of ALT-E increased significantly across the VFA categories even after adjusting for the SFA, in both men and women (all P < .001) (Tables 3 and 5).
The prevalence of GGTP-E significantly increased across the SFA categories in both men (P < .001) and women (P = .029); however, the difference did not remain significant after adjusting for the VFA in men (P = .147) (Table 3) or women (P = .290) (Table 5), even after adjusting for the SFA (both P < .001). The prevalence of GGTP-E increased significantly across the VFA categories even after adjusting for the SFA in men (P < .001), but not in women (P = .094) (Tables 3 and 5).
3.3. Risk estimation for metabolic components and NAFLD
The risk factors, as estimated by a logistic regression analysis, are summarized in Tables 6 (men) and 7 (women). There was a significant independent association between DL and the SFA in men (S-Q1, 1; S-Q2, 1.60 [1.19–2.15]; S-Q3, 1.57 [1.16–2.12]; S-Q4, 1.44 [1.04–2.00]), but not in women (S-Q1, 1; S-Q2, 1.06 [0.77–1.46]; S-Q3, 1.22 [0.88–1.69]; S-Q4, 1.23 [0.87–1.73]). In contrast, there was no independent association between HT and the SFA in men (S-Q1, 1; S-Q2, 1.06 [0.78–1.44]; S-Q3, 1.15 [0.84–1.57]; S-Q4, 1.36 [0.96–1.91]), and in women (S-Q1, 1; S-Q2, 1.04 [0.75–1.45]; S-Q3, 1.38 [0.99–1.93]), the significant association found in S-Q4 only (1.49 [1.05–2.11]).
Table 6.
Associations between the SFA and VFA with metabolic diseases (men).
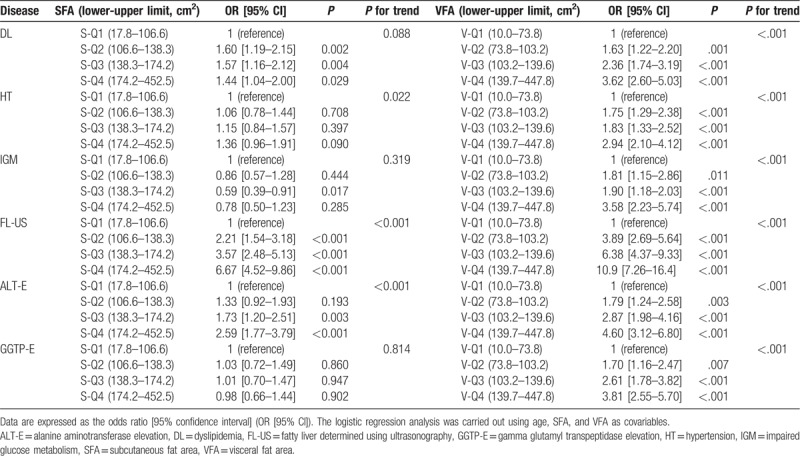
Table 7.
Associations between the SFA and VFA with metabolic diseases (women).
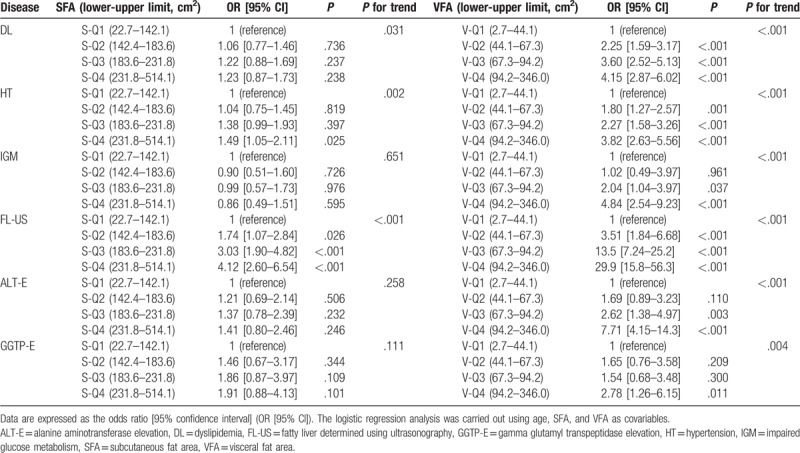
Of note, a significant reverse association was observed between IGM and the SFA in men in S-Q3 (0.59 [0.39–0.91]). However, there was no significant association between IGM and the SFA in women (S-Q1, 1; S-Q2, 0.90 [0.51–1.60]; S-Q3, 0.99 [0.57–1.73]; S-Q4, 0.86 [0.49–1.51]).
Dyslipidemia, HT, and IGM were strongly associated with the VFA in both men and women.
Fatty liver diagnosed using ultrasonography was independently associated with the SFA in both men (S-Q1, 1; S-Q2, 2.21 [1.54–3.18]; S-Q3, 3.57 [2.48–5.13]; S-Q4, 6.67 [4.52–9.86]) and women (S-Q1, 1; S-Q2, 1.74 [1.07–2.84]; S-Q3, 3.03 [1.90–4.82]; S-Q4, 4.12 [2.60–6.54]).
Alanine aminotransferase elevation was independently associated with the SFA in men (S-Q1, 1; S-Q2, 1.30 [0.92–1.93]; S-Q3, 1.73 [1.20–2.51]; S-Q4, 2.59 [1.79–3.79]), but not in women (S-Q1, 1; S-Q2, 1.21 [069–2.14]; S-Q3, 1.37 [0.78–2.39]; S-Q4, 1.41 [0.80–2.46]). ALT-E was strongly associated with the VFA in both men and women.
Gamma glutamyl transpeptidase elevation was not independently associated with the SFA in either men (S-Q1, 1; S-Q2, 1.03 [0.72–1.49]; S-Q3, 1.01 [0.70–1.47]; S-Q4, 0.98 [0.66–1.44]) or women (S-Q1, 1; S-Q2, 1.46 [0.67–3.17]; S-Q3, 1.86 [0.87–3.97]; S-Q4, 1.91 [0.88–4.13]). GGTP-E was strongly associated with the VFA in men, but not in women (an association with the VFA in women was found in V-Q4 only).
We conducted a trend test to analyze the association between SFA and VFA with metabolic diseases or NAFLD. In men, there was a significant trend across the mean values of 4 quantiles of SFA for HT (P = .002), FL-US (P < .001), and ALT-E (P < .001), but not for DL, IGM, or GGTP-E. In women, there was a significant trend across the mean values of 4 quantiles of SFA for DL (P = .031), HT (P = .002), and FL-US (P < .001), but not for IGM, ALT-E, or GGTP-E. While, there was a significant trend across the mean values of 4 quantiles of VFA for DL, HT, IGM, FL-US, ALT-E, and GGTP-E in men and women.
Furthermore, as shown in Table 8, there was a significant trend across the mean values of 4 quantiles of SFA for FL-US in men and women, even after adjusting for the age, VFA, presence of HT, DL, and IGM, and smoking status. However, no significant association between GGTP-E and SFA was detected, suggesting the influence of these metabolic components on this association.
Table 8.
Associations of SFA and VFA with NAFLD in men and women.
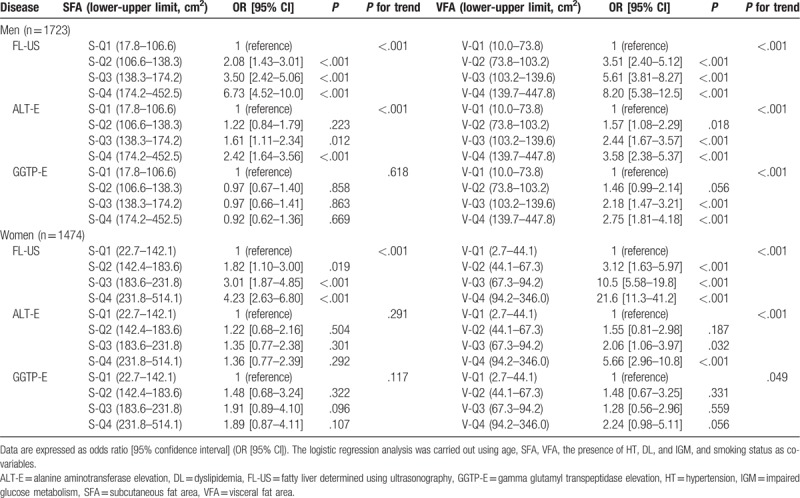
The same results were obtained in the subgroup of subjects who were not taking medications for HT, DL, and/or diabetes mellitus (data not shown).
4. Discussion
The present study was carried out to clarify the association between NAFLD and subcutaneous adiposity, providing valuable evidence concerning the role of NAFLD in the pathophysiology of obesity. The present study revealed, using a logistic regression analysis, that FL-US has an independent positive association with not only the VFA but also the SFA, whereas the components of metabolic syndrome (HT, DL, and IGM) and GGTP-E are strongly associated with the VFA, but less closely associated (or not associated at all) with the SFA. The association between FL-US and the SFA was not weak, even when compared with the association between FL-US and the VFA, especially in men. The same results were obtained using Mantel-Haenszel: the prevalence of FL-US significantly increased across the SFA categories, even after adjusting for the VFA. In addition, there was a significant trend across the mean values of 4 quantiles of SFA for FL-US even after adjusting for the age, VFA, presence of HT, DL, and IGM, and smoking status. These results suggested that NAFLD is correlated with subcutaneous obesity—independently of visceral obesity, which is a characteristic that distinguishes NAFLD from the other components of metabolic syndrome. In addition, this significant association between FL-US and the SFA was already detected from the second SFA quantile. It is noteworthy that the mean BMI values of the subjects in the second quantile were 23.7 kg/m2 in men and 22.6 kg/m2 in women, as these values are close to the normal BMI range according to the Japanese criteria (obesity >25 kg/m2 in both men and women). These results suggested that NAFLD is correlated with subcutaneous adiposity ab initio, even without obesity.
Liver fat is derived directly from meals, adipose tissue lipolysis, or de novo lipogenesis (DNL). Donnelly et al[13] found that the plasma nonesterified fatty acid (NEFA) pool accounts for approximately 60% of the triacylglycerol content in the livers of NAFLD patients. Although visceral adipose tissue is anatomically linked to the liver via the portal vein,[7] a large part of NEFA in the portal vein originates from the subcutaneous adipose tissue even in subjects with visceral obesity.[6] In addition, increased DNL is a distinct characteristic of NAFLD.[14] Recent accumulating evidence has indicated a relationship between body weight gain and increased DNL in the liver. Fabbrini et al[15] reported that body weight gain due to an excess energy intake induces an increase in DNL and a decrease in fat oxidation in the liver. A study using an animal model reported that high-fat diet-induced insulin resistance is initiated by the accumulation of fat in both the liver and the adipose tissue,[16] and that the induction of an increase in the hepatic DNL by weight gain is correlated with metabolic alterations in adipose tissue, including subcutaneous adipose tissue.[17] These present and previous findings therefore suggest that metabolic alteration in the NAFLD liver plays a pathogenic role in the development of subcutaneous obesity from an early stage.
However, some investigators have reported that subcutaneous fat can have a reverse association with NAFLD.[18] Emerging evidence suggests that the ability to retain fat in the subcutaneous adipose tissue is beneficial in human obesity because of the association with reduced visceral and ectopic fats and improved insulin sensitivity.[19] For example, thiazolidinediones act by stimulating adipogenesis, particularly in the subcutaneous compartments, and recruit new small adipocytes, resulting in the improvement of NAFLD.[20] These previous findings suggest that, for subjects with ectopic and visceral fat accumulation, the increase in subcutaneous fat may indicate the recovery of the expanding capacity, which results in the alteration of the body fat distribution. NAFLD can therefore be negatively associated with subcutaneous fat under such conditions. In contrast, as our present study revealed, NAFLD is positively correlated with the development of subcutaneous obesity from an early stage. We therefore consider that the results of epidemiological studies may differ according to the characteristics of the study subjects. Furthermore, Azuma et al[21] reported that the liver fat of Japanese individuals increases with reduced levels of subcutaneous and visceral fat accumulation compared with non-Hispanic whites, even in nonobese individuals. Some ethnic factors may therefore influence these results. In the present study, we found evidence concerning a possible role of NAFLD in the pathophysiology of obesity, probably because our analysis was carried out in a Japanese population.
Independent positive associations were observed between ALT-E, and both the SFA and VFA in men, while GGTP-E was independently associated with the VFA, but not the SFA in men—similarly to the components of metabolic syndrome (DL, HT, and IGM). ALT is the most popular surrogate marker of liver injury.[22] GGTP is also a well-established serum marker of liver injury, bile duct condition, and alcohol consumption.[23] Recently, the predictive utility of GGTP has been applied beyond cases of liver disease to cases of cardiovascular disease, diabetes mellitus, and metabolic syndrome. The major function of GGTP is the cleavage of glutathione, which is the main thiol antioxidant in humans.[24] It is now accepted that GGTP elevation contributes to its pro-oxidant activity, particularly in the presence of iron (hyperferritinemia).[25,26] We therefore hypothesize that ALT elevation reflects liver injury in NAFLD in association with both subcutaneous and visceral adiposity. In contrast, GGTP elevation may reflect oxidative stress due to visceral adiposity.
In the present study, a positive association between ALT-E and SFA was not detected in women, suggesting a potential sex-based difference. However, Bertoli et al[27] reported a positive association between ALT alteration and subcutaneous fat, as evaluated by US, in both men and women. It may therefore be insufficient to discuss this issue without considering the presence of menopause and the appropriate cut-off value for ALT in women. In addition, the prevalence of ALT-E in women was considerably lower than that in men. Thus, it is possible that this analysis was underpowered and that we failed to reach statistical significance. This is 1 of the limitations of the present study, and further studies should be performed to discuss this problem.
The present study is associated with several additional limitations. First, NAFLD was determined using US. Although US is the most common method for assessing fatty steatosis, a liver biopsy is still the golden standard for the diagnosis of NAFLD. In addition, both the SFA and VFA were measured only at 1 umbilical slice using CT, rather than via standard methods, such as dual-energy x-ray absorptiometry or a bioelectrical impedance analysis. Finally, a multivariate analysis was carried out using stratums of age, SFA, VFA, DL, HT, and IGM as co-variables. It is possible that other factors influenced the results.
5. Conclusions
In conclusion, it was epidemiologically revealed that NAFLD has an independent association with subcutaneous adiposity ab initio, which highlights a possible role of NAFLD in the development of obesity. Given our present study findings, we propose that the improvement of NAFLD is important for preventing the development of obesity and/or obesity-related diseases. Further studies should be performed to discuss this issue.
Author contributions
Conceptualization: Takeshi Kure, Seiichi Mawatari, Yasushi Imamura, Kohei Oda, Kotaro Kumagai, Akio Ido.
Data curation: Takeshi Kure, Seiichi Mawatari, Yasushi Imamura, Yasunari Hiramine, Hironori Miyahara.
Formal analysis: Takeshi Kure, Seiichi Mawatari, Yasushi Imamura, Yasunari Hiramine, Hironori Miyahara.
Investigation: Takeshi Kure, Seiichi Mawatari, Yasushi Imamura, Kotaro Kumagai, Yasunari Hiramine, Hironori Miyahara, Masahisa Horiuchi.
Methodology: Takeshi Kure, Seiichi Mawatari, Yasushi Imamura, Masahisa Horiuchi.
Project administration: Takeshi Kure, Seiichi Mawatari, Yasushi Imamura, Kohei Oda, Akio Ido.
Supervision: Shuji Kanmura, Akihiro Moriuchi, Hirofumi Uto, Masahisa Horiuchi, Akio Ido.
Writing – original draft: Takeshi Kure, Seiichi Mawatari, Yasushi Imamura.
Writing – review & editing: Takeshi Kure, Seiichi Mawatari, Yasushi Imamura, Akio Ido.
Seiichi Mawatari orcid: 0000-0002-3847-1065.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ALT-E = alanine aminotransferase elevation, BMI = body mass index, CT = computed tomography, DL = dyslipidemia, DNL = de novo lipogenesis, FL = fatty liver, FL-US = fatty liver diagnosed using ultrasonography, GGTP-E = gamma glutamyl transpeptidase elevation, HDL = high-density lipoprotein, HT = hypertension, IGM = impaired glucose metabolism, LDL = low-density lipoprotein, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, NEFA = nonesterified fatty acid, SFA = subcutaneous fat area, US = ultrasonography, VFA = visceral fat area.
How to cite this article: Kure T, Mawatari S, Imamura Y, Oda K, Kumagai K, Hiramine Y, Miyahara H, Kanmura S, Moriuchi A, Uto H, Horiuchi M, Ido A. Nonalcoholic fatty liver disease is associated with both subcutaneous and visceral adiposity -A cross-sectional study-. Medicine. 2019;98:46(e17879).
The authors have no conflicts of interest to disclose.
References
- [1].Eguchi Y, Eguchi T, Mizuta T, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol 2006;41:462–9. [DOI] [PubMed] [Google Scholar]
- [2].Imamura Y, Uto H, Hiramine Y, et al. Increasing prevalence of diabetes mellitus in association with fatty liver in a Japanese population. J Gastroenterol 2014;49:1406–13. [DOI] [PubMed] [Google Scholar]
- [3].Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7. [DOI] [PubMed] [Google Scholar]
- [4].Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404. [DOI] [PubMed] [Google Scholar]
- [5].Fontana L, Eagon JC, Trujillo ME, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007;56:1010–3. [DOI] [PubMed] [Google Scholar]
- [6].Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest 2004;113:1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bjorntorp P. Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990;10:493–6. [PubMed] [Google Scholar]
- [8].Nishioji K, Sumida Y, Kamaguchi M, et al. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011-2012. J Gastroenterol 2015;50:95–108. [DOI] [PubMed] [Google Scholar]
- [9].Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb 2018;25:846–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alberti KG, Zimmet P, Shaw J. The metabolic syndrome: a new worldwide definition. Lancet 2005;366:1059–62. [DOI] [PubMed] [Google Scholar]
- [11].Japanese Clinical Practice Guideline for Diabetes 2016–2017. Nankodo; 2016 Tokyo; Available at: http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2016-2017.pdf [Accession date is September 30, 2019]. [Google Scholar]
- [12].Chin R, Miyazaki S. [Criteria of obesity and obesity disease in Japan]. Nihon Rinsho 2009;67:297–300. [PubMed] [Google Scholar]
- [13].Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lambert JE, Ramos-Roman MA, Browning JD, et al. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014;146:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fabbrini E, Tiemann Luecking C, Love-Gregory L, et al. Physiological mechanisms of weight gain-induced steatosis in people with obesity. Gastroenterology 2016;150:79–81. e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Turner N, Kowalski GM, Leslie SJ, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 2013;56:1638–48. [DOI] [PubMed] [Google Scholar]
- [17].Eissing L, Scherer T, Todter K, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat Commun 2013;4:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim D, Chung GE, Kwak MS, et al. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2016;14:132–8. e134. [DOI] [PubMed] [Google Scholar]
- [19].Porter SA, Massaro JM, Hoffmann U, et al. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 2009;32:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bajaj M, Suraamornkul S, Pratipanawatr T, et al. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes 2003;52:1364–70. [DOI] [PubMed] [Google Scholar]
- [21].Azuma K, Kadowaki T, Cetinel C, et al. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism 2009;58:1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342:1266–71. [DOI] [PubMed] [Google Scholar]
- [23].Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001;38:263–355. [DOI] [PubMed] [Google Scholar]
- [24].Koenig G, Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers 2015;2015:818570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Banderas DZ, Escobedo J, Gonzalez E, et al. Gamma-glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol 2012;24:805–10. [DOI] [PubMed] [Google Scholar]
- [26].Turgut O, Tandogan I. Gamma-glutamyltransferase to determine cardiovascular risk: shifting the paradigm forward. J Atheroscler Thromb 2011;18:177–81. [DOI] [PubMed] [Google Scholar]
- [27].Bertoli S, Leone A, Vignati L, et al. Metabolic correlates of subcutaneous and visceral abdominal fat measured by ultrasonography: a comparison with waist circumference. Nutr J 2016;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]


