Supplemental Digital Content is available in the text
Keywords: chest radiographic findings, computed tomography, scrub typhus, severe fever with thrombocytopenia syndrome
Abstract
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease caused by SFTS virus (SFTSV) which involves multiple organ systems, including lungs. However, there is limited data on lung involvement of SFTS. Therefore, the present study investigated the chest radiographic findings of SFTS, including computed tomography (CT), and compared these with those of scrub typhus, which is the most common tick-borne illness in South Korea and share risk factors and occur in similar settings.
Medical records of patients with confirmed SFTS and scrub typhus in a tertiary hospital in Seoul (South Korea), between January 2014 and June 2018, were reviewed. Initial chest radiography and CT were reviewed by 2 experienced radiologists.
A total of 39 patients with SFTS and 101 patients with scrub typhus were analyzed. All patients except 3 patients with scrub typhus in both groups received chest radiography. Cardiomegaly (90%) and patchy consolidation with ground glass opacity (GGO) pattern (31%) were more common in SFTS group than scrub typhus group (20%, P < .001 and 2%, P < .001, respectively). About half of each group received chest CT. Consolidation (29%) and pericardial effusion (24%) were more common in SFTS group than scrub typhus group (6%, P = .02 and 4%, P = .008, respectively). Interstitial thickening in chest radiography (58%) and chest CT (65%) was more frequent in scrub typhus group than SFTS group (18%, P < .001 and 19%, P < .001, respectively).
Cardiomegaly with/without pericardial effusion and patchy consolidation with GGO pattern were more frequent in SFTS group, whereas interstitial thickening was more frequent in scrub typhus group. These findings will assist the early differentiation of SFTS from scrub typhus.
1. Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease caused by novel bunyavirus, SFTS virus (SFTSV), which was first identified in China in 2011.[1] SFTS causes a characteristic clinical presentation which includes fever, thrombocytopenia, lymphadenopathy, bleeding tendency, vomiting, diarrhea, and multiple organ impairment.[1–7] Patients present with symptoms ∼4 to 15 days following exposure to SFTSV via a tick bite or exposure to body fluid of an infected individual. The mortality rate was reported to 6.3% to 30% in China,[1,4,8] 31% in Japan,[9] and 32.6% in Korea.[10] In Korea, the incidence of laboratory-confirmed SFTS has increased annually since 2013. A total of 272 cases of laboratory-confirmed SFTS were reported in 2017.[11] It shows seasonality from May to October and nationwide distribution.[10] SFTSV involves multiple organs, including the liver, muscles, central nervous system, and lungs.[1,12,13] It is reported that ∼9.6% to 28.7% of patients with SFTS experience respiratory symptoms including cough, sputum, and dyspnea, and radiographic abnormalities are present in 29% to 45% of patients with SFTS.[3,7,14–16] However, there is limited data regarding the detailed lung involvement of SFTS, including some case reports and a cohort study.[3,6,17]
Scrub typhus is caused by Orientia tsutsugamushi, transmitted via chigger bite.[18] In Korea, 5000 to 6000 cases were reported annually. The clinical features of scrub typhus are similar to those of SFTS, including fever, lymphadenopathy, and multiple organ impairment in severe cases.[19] Some clinical features including eschar and rash were more common in scrub typhus than SFTS.[14,16] Therefore, careful history taking and physical examination are important in differentiate between 2 diseases. However, these findings were also documented in some SFTS cases and absent in some scrub typhus cases.[14,16,19] Furthermore, risky behavior and seasonality overlap between the 2 diseases. These similarities make it difficult to distinguish SFTS from scrub typhus. However, cause of the differences in treatment, prognosis, and infection control in terms of human-to-human transmission between 2 diseases, early differential diagnosis between SFTS and scrub typhus is important in patients with suspected tick-borne illness in South Korea. Therefore, comparison of laboratory and radiologic findings, which can be obtained immediately, were needed to differentiate SFTS from scrub typhus. There were efforts to distinguish SFTS from scrub typhus by laboratory findings including leukopenia, thrombocytopenia, and low C-reactive protein (CRP) in previous studies.[14] However, there are few studies that compared the differences between radiologic findings of SFTS and scrub typhus.
In the present study, the characteristics of chest radiographic findings of SFTS, including chest computed tomography (CT) scan, were described and compared with those of scrub typhus, which is the most common tick-borne disease in South Korea.
2. Methods
2.1. Study population and clinical data
We performed a retrospective observational cross-sectional study including adult patients, aged ≥16 years, who were diagnosed with SFTS and scrub typhus at the Asan Medical Center in Seoul, South Korea, between January 2014 and June 2018. The patients’ medical charts were reviewed retrospectively and the data of the baseline characteristics, clinical presentations, laboratory findings, treatments, and outcomes were collected. The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2016–0748).
2.2. Diagnostic tests
SFTS was diagnosed when the SFTSV was identified by reverse transcription-polymerase chain reaction (RT-PCR) analysis of plasma samples, using a DiaStar 2X OneStep RT-PCR Pre-Mix kit (SolGent, Daejeon, South Korea), as described previously.[16,18,20] The diagnosis of scrub typhus was confirmed according to the following criteria, as described previously[16]: a single positive immunoglobulin G (IgG) result (titer ≥1:320) or an >4-fold increase in IgG titer between 2 consecutive samples. An immunofluorescence assay (IFA; SD Bioline Tsutsugamushi Assay; Standard Diagnostics) was used to measure antibody titer.[21]
2.3. Radiologic data acquisition
The anteroposterior chest radiographs and posteroanterior and lateral projection radiographs were obtained using digital radiography equipment (GE Definium 8000, GE Healthcare and GC85A, Samsung Healthcare) with a standardized technique (90 kV, 5 mAs, and 72-in. film-focus distance for a anteroposterior view, broad tube focus; 120 kVp, 2 mAs, and a 72-in. film-focus distance for a posteroanterior radiograph). All the images were reviewed on a PACS (Picture Archiving and Communication System) viewer. Chest CT examinations were performed using 128-slice CT scanners (Somatom Definition AS+, Siemens Healthcare, Forchheim, Germany). The parameters were set to 120 kV, 100 mA with dose modulation. The reconstruction intervals included 3/5-mm thickness and 3/5-mm intervals without gaps using the B50 algorithm and 1-mm reconstruction with a 5-mm gap using the B60 algorithm. Iodine contrast material was administered for 16 of 21 CT examinations in patients with SFTS and 39 of 49 CT examinations in patients with scrub typhus. For chest CT scan with enhancement, 100 mL of intravenous iopromide 300 (300 mg/mL iodine) (Ultravist; Bayer Pharma, Berlin, Germany) was administered at a rate of 2.5 mL/s using a power injector with a fixed delay (50-second). All axial and coronal CT images were reviewed in the PACS viewer using mediastinal (width, 450 HU; level, 50 HU), lung (width, 1500 HU; level, 2700 HU), and bone (width, 1000 HU; level, 200 HU) window settings.
2.4. Chest radiography and chest CT analysis
Two chest radiologists, who had 19 and 8 years of experience, respectively, reviewed all chest radiographic images and CT examinations in consensus. On the chest radiography at the time of admission, the lung parenchyma was classified as normal or abnormal. The abnormalities were characterized further as patchy consolidation (opacification obscuring the underlying vessels) and ground glass opacity (GGO; increased attenuation without obscuring the underlying vessels) pattern, interstitial pattern, lobar consolidation pattern, and diffuse GGO pattern. Cardiomegaly was defined as a cardiothoracic ratio >0.5 on chest radiography or the size of the cardiac silhouette on chest radiography at the time of admission was higher than that on a previous chest radiograph or the size of the cardiac silhouette was decreased on the chest radiograph taken following improvement of the disease. The abnormalities on chest CT were characterized as consolidation, GGO, centrilobular nodules, nodules, and interstitial thickening as a per-segment basis (6 lobes in each patient, i.e., right upper lobe, right middle lobe, right lower lobe, left upper lobe, lingula segment, and left lower lobe). The anatomic distribution was characterized as unilateral or bilateral, and the lobe involvement was assessed. The presence of lymph node enlargement, pleural effusion, and pericardial effusion was recorded. Any additional lung findings were recorded.
2.5. Statistical analysis
SPSS version 21.0 (SPSS, Inc., Chicago, IL) was used to analyze data. Categorical variables were analyzed using the chi-squared test or Fisher exact test and continuous variables were analyzed using Student t test. All tests were 2-tailed and differences were considered significant at P < .05.
3. Results
3.1. Study populations
A total of 39 patients with SFTS and 101 patients with scrub typhus were analyzed in the present study. All patients with SFTS were confirmed by RT-PCR analysis. All patients with scrub typhus were confirmed by IFA, which was used to evaluation antibodies. Among patients with scrub typhus, 58 patients (57%) exhibited a rise in IgG titer >4-folds in paired samples and 43 patients (43%) had IgG titer >1:320 at initial test; 3 patients (3%) with 1:320, 9 patients (9%) with 1:640, and 33 patients (33%) with ≥1:1280.
3.2. Baseline characteristics and clinical features
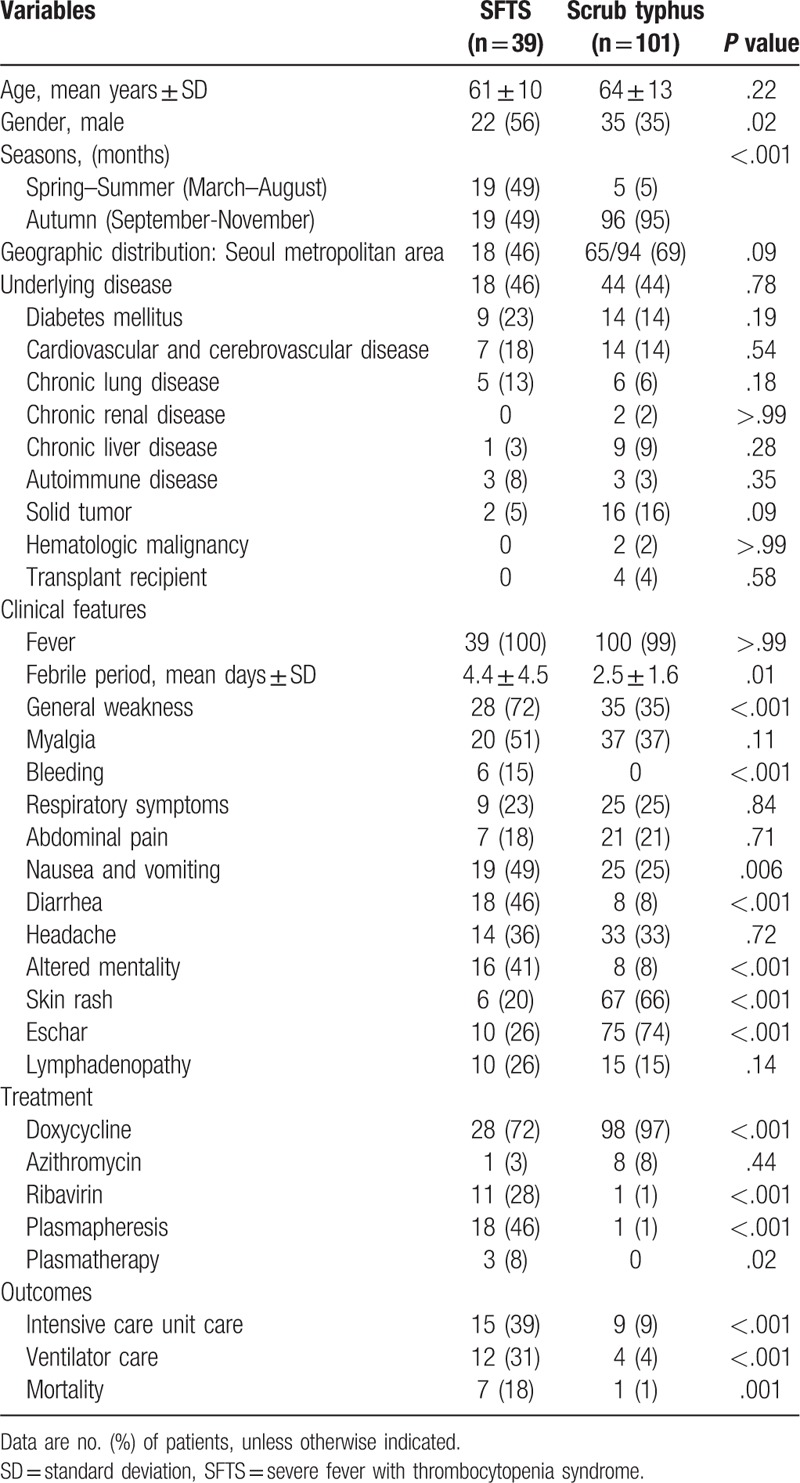
Table 1 shows the baseline characteristics between patients with SFTS and patients with scrub typhus. There were no significant differences in age, geographic distribution, or underlying diseases between the 2 groups. SFTS was more common in men (22 [56%]) and occurred more frequently in the spring–summer season (19 [49%]), between March and August, than scrub typhus (35 [35%], P = .02 and 5 [5%], P < .001, respectively). Patients with SFTS complained of general weakness (P < .001), gastrointestinal symptoms (P = .006), and central nervous system symptoms (P < .001) more frequently than scrub typhus patients did. Bleeding episodes, including epistaxis, bruising, petechiae, and gum bleeding, were observed in 6 (15%) of the patients with SFTS (P < .001). Respiratory symptoms, including a cough, sputum, and dyspnea, were similar between the 2 groups (9 [23%] in SFTS, vs 25 [25%] in scrub typhus, P = .84).
Table 1.
Demographic findings, clinical features, and outcomes of patients with severe fever with thrombocytopenia syndrome (SFTS) and patients with scrub typhus.
3.3. Laboratory findings, treatments, and outcomes
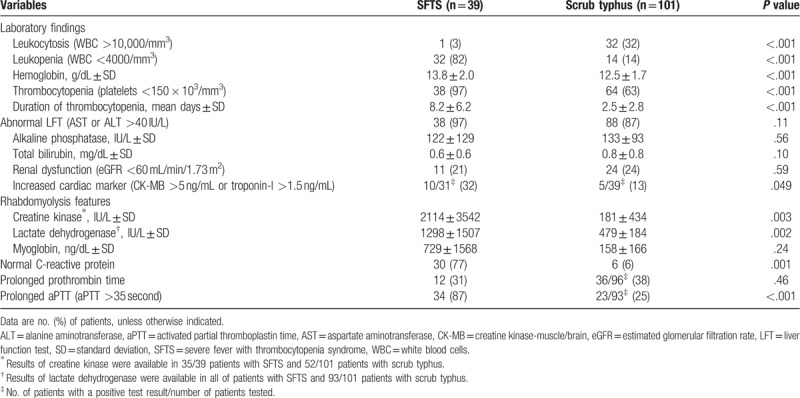
Tables 1 and 2 show comparison of laboratory findings, treatments, and outcomes between 2 groups. Leukopenia and thrombocytopenia were more common in the SFTS group than in the scrub typhus group (P < .001), and leukocytosis was more common in the scrub typhus group than in the SFTS group (P < .001). The proportion of patients with abnormal liver function tests (LFT) and renal function tests was similar between 2 groups. Increases in cardiac markers, including creatine kinase-muscle/brain and troponin-I, were more common in the SFTS group (10/31 [32%]) than in the scrub typhus group (5/39 [13%], P = .049). The levels of creatine kinase and lactate dehydrogenase were higher in the SFTS group than in the scrub typhus group (P = .003 and P = .002, respectively). A normal range of CRP and prolonged activated prothrombin time (aPTT) were observed more frequently in the SFTS group than in the scrub typhus group (P = .001 and P < .001, respectively).
Table 2.
Laboratory findings of patients with severe fever with thrombocytopenia syndrome (SFTS) and patients with scrub typhus.
The provision of care in an intensive care unit and use of a ventilator were more frequent in the SFTS group than in the scrub typhus group (P < .001). Mortality rate was higher in the SFTS group (18%) than in the scrub typhus group (1%, P = .001).
3.4. Radiologic findings
All of the patients with SFTS and 98 (97%) of the patients with scrub typhus underwent chest radiography. Time from symptoms onset to chest x-ray and time from admission to chest x-ray were comparable between 2 groups (P = .12 and P = .80, respectively) (Table 3). Anteroposterior portable bedside radiographs were obtained in 22 (56%) patients of SFTS group and in 55 (56%) patients of scrub typhus group and the remainders had posteroanterior projection radiographic images. The findings of chest radiography are summarized in Table 3 and shown in Figs. 1 and 2. In both groups, ∼60% of patients presented with abnormal initial chest radiographic findings. Cardiomegaly was the most common chest radiographic pattern of the SFTS group and was more common in the SFTS group (90%) than in the scrub typhus group (20%, P < .001).
Table 3.
Chest radiographic findings of patients with severe fever with thrombocytopenia syndrome (SFTS) and patients with scrub typhus.
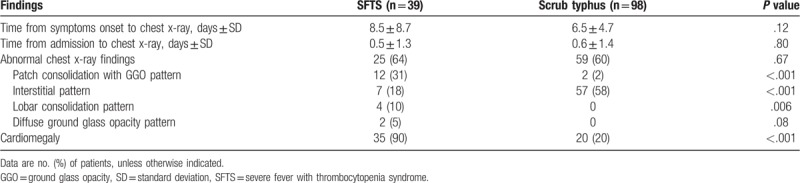
Figure 1.
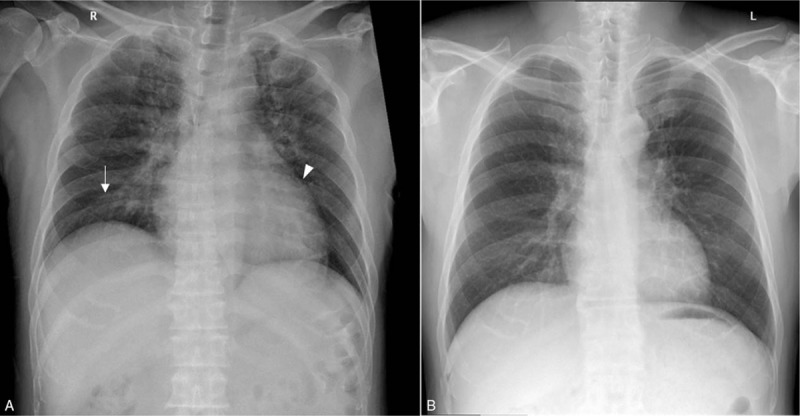
Chest radiography of a patient with severe fever with thrombocytopenia syndrome. A. Bilateral patchy consolidation with ground glass opacity in both upper lung zone ( ) and right lower lung zone are present. Cardiomegaly (▾) is also present. B. Following resolution of symptoms, the abnormal findings are no longer apparent.
) and right lower lung zone are present. Cardiomegaly (▾) is also present. B. Following resolution of symptoms, the abnormal findings are no longer apparent.
Figure 2.
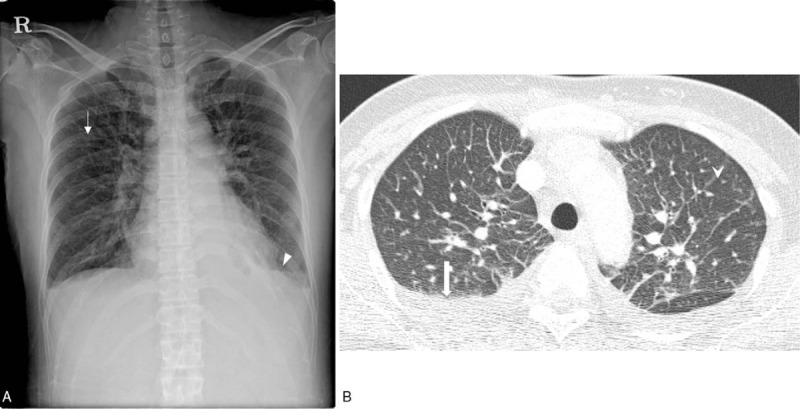
Chest radiography and CT of a patient with scrub typhus. A. Chest radiograph shows diffuse and mild interstitial thickening ( ) in the bilateral lungs. A small amount of bilateral pleural effusion (▾) is noted. B. Axial chest CT shows smooth interlobular septal line thickening (
) in the bilateral lungs. A small amount of bilateral pleural effusion (▾) is noted. B. Axial chest CT shows smooth interlobular septal line thickening ( ), axial interstitial thickening, and bilateral pleural effusion (
), axial interstitial thickening, and bilateral pleural effusion ( ). CT = computed tomography.
). CT = computed tomography.
Chest CT was performed in 21 (54%) patients of SFTS group and 49 (49%) patients of scrub typhus group. Time from symptoms onset to chest CT and time from admission to chest CT were comparable between 2 groups (P = .07 and P = .27, respectively) (Table 4). The CT findings are summarized in Table 4 and shown in Figs. 2 and 3. Lobar or segmental consolidation was the most common CT finding of the SFTS group (6/21 [29%]) and was more frequently observed in the SFTS group than in the scrub typhus group (3/49 [6%], P = .02). Most consolidation involved bilateral lungs (4/6 [67%]), and lower lobes (5/6 [83%]) (Supplement Table 1).
Table 4.
Chest computed tomography (CT) findings of patients with scrub typhus and patients with severe fever with thrombocytopenia syndrome (SFTS).
Figure 3.
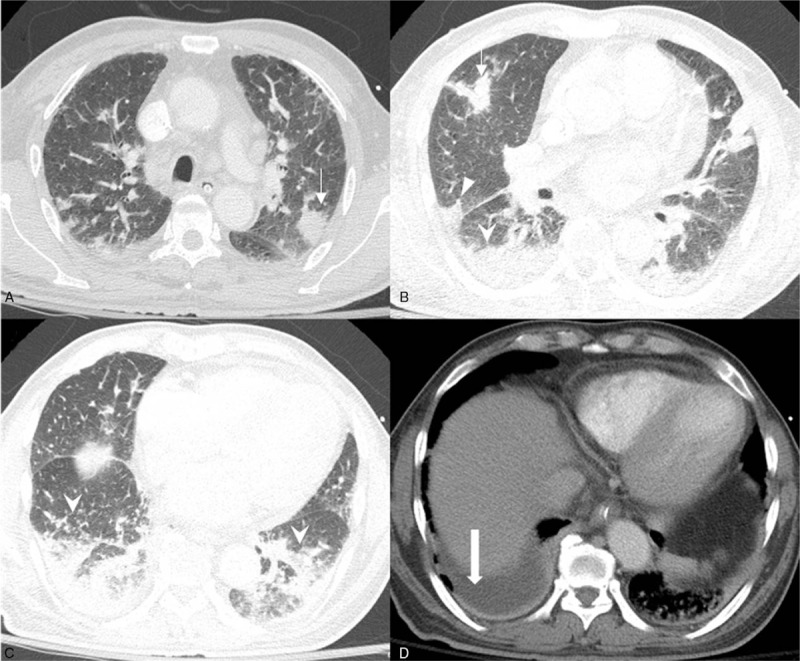
Chest CT of a patient with severe fever with thrombocytopenia syndrome. A. Multifocal patchy consolidations are present in both upper lobes ( ). B. Multifocal patchy consolidations are present in right upper, middle (▾), and both lower lobes. C. Multifocal patchy consolidations are present in both lower lobes (
). B. Multifocal patchy consolidations are present in right upper, middle (▾), and both lower lobes. C. Multifocal patchy consolidations are present in both lower lobes ( ). D. A small amount of right pleural effusion (
). D. A small amount of right pleural effusion ( ) is present, whereas no pericardial effusion is noted. CT = computed tomography.
) is present, whereas no pericardial effusion is noted. CT = computed tomography.
4. Discussion
In the present study, SFTS was found to develop more frequently in men and during the spring-summer seasons than scrub typhus. Clinical features, including general weakness, bleeding, nausea and vomiting, diarrhea, and altered mentality were more common in patients with SFTS than in patients with scrub typhus. The rate of symptoms was similar to the previous studies.[9,10,15] Skin rash and eschar were more common in patient with scrub typhus than in patients with SFTS. These findings were also compatible with the previous study.[16] In laboratory findings, leukopenia, thrombocytopenia, increased cardiac marker, and prolonged aPTT were more frequent in SFTS patients than in scrub typhus patients. Neutropenia and thrombocytopenia were marked findings of SFTS and observed in most of SFTS patients in previous study.[9,10,15] Normal range of CRP was more common in SFTS patients than in scrub typhus patients. Despite the differences in epidemiologic and clinical characteristics between the SFTS and scrub typhus groups, overlapping of seasonality and similarities in the risks of exposure[9] and clinical presentations sometimes make it difficult to distinguish SFTS from scrub typhus. So, rapid available conventional clinical tests may provide further clinical clues for the differentiation between 2 diseases. However, there are limited data on radiologic findings between 2 diseases.
Of note, the majority (90%) of patients with SFTS presented with cardiomegaly on chest radiographs, and this was more common in the SFTS group compared with the scrub typhus group. However, only 25% of patients with SFTS who underwent chest CT presented with pericardial effusion and one-third of patients with SFTS had elevated cardiac enzymes (Table 2). Therefore, these findings are not sufficient to explain the remaining patients with SFTS with cardiomegaly. Further investigations on this area are required.
Patchy consolidation with GGO pattern was the most common chest radiographic pattern of lung involvement in the SFTS group and patchy consolidation was the most common CT finding in SFTS group. These radiologic findings are nonspecific and can be manifestations of several diseases, including bacterial or viral infection. However, these findings may be distinguished from the typical radiologic findings of scrub typhus. Typical chest CT findings of scrub typhus have been reported as interlobular septal thickening and lymphadenopathy.[22,23] And the chest radiographic and CT findings of scrub typhus in the present study were consistent with those of previous reports. Therefore, the radiologic findings of SFTS may assist the physician to early differentiate SFTS from scrub typhus.
The proportion of patients with SFTS who had abnormal chest radiography, 64% in chest radiography and 62% in chest CT, was higher than in previous studies (29%–45%).[3,16] Patchy shadowing was observed in 21/44 (48%) SFTS patients with abnormal chest radiography in a previous study.[3] This finding is consistent with the present study which identified patchy consolidation with GGO in 12/25 (48%) SFTS patients with abnormal chest radiography. However, pleural effusion and pericardial effusion were more frequently observed in the present study (38% and 24%, respectively) than in the previous study (23% and 14%, respectively).[3] This may be associated with differences in modalities, disease severity, and the experience of the thoracic radiologists.
The present study has some limitations. First, the study was performed retrospectively. There were missing data in laboratory findings, including cardiac markers. In total, ∼50% of patients with SFTS and scrub typhus initially underwent chest CT scans because chest CT was performed to evaluate the other causes of fever in the patient with severe disease or abnormal chest x-ray, and/or no obvious fever focus. If the patients had typical clinical presentation of scrub typhus or SFTS, chest CT was not performed in patients with suspected SFTS and scrub typhus. Therefore, our estimates on abnormal chest CT findings might be overestimated. Although we did not access whether CT findings affected clinical decision in patients with suspected SFTS of scrub typhus, further studies are needed on the clinical impact of chest CT on the differential diagnosis of SFTS and scrub typhus, especially in patients who had atypical presentation of scrub typhus or SFTS. Second, the present study was conducted in a tertiary center in South Korea. So, some kind of selection bias toward more severe disease might occur. Third, although the majority of CT scans were performed in the emergency department, CT scans at heterogeneous clinical stages of patients with SFTS and scrub typhus were analyzed as hospital visits varied following symptom onset. Despite these limitations, to the best of our knowledge, the present study is the first to systemically evaluate the differences in chest radiography and chest CT findings between SFTS and scrub typhus, which are the most common tick-borne illness in South Korea.
5. Conclusions
Cardiomegaly with/without pericardial effusion, patchy consolidation with GGO pattern, and lobar consolidation were more frequent in patients with SFTS, whereas interstitial thickening was more frequent in patients with scrub typhus. These findings will assist in the early differentiation of SFTS from scrub typhus.
Author contributions
Conceptualization: Sung-Han Kim.
Data curation: Ji Hyun Yun, Hye Jeon Hwang, Mi Young Kim.
Formal analysis: Ji Hyun Yun.
Funding acquisition: Sung-Han Kim.
Investigation: Ji Hyun Yun, Hye Jeon Hwang, Mi-Young Kim.
Resources: Jun Hee Woo, Mi Young Kim.
Supervision: Jiwon Jung, Min Jae Kim, Yang Soo Kim, Jun Hee Woo, Sung-Han Kim.
Validation: Yong Pil Chong, Sang-Oh Lee, Sang-Ho Choi.
Writing – original draft: Ji Hyun Yun, Hye Jeon Hwang.
Writing – review & editing: Mi Young Kim, Sung-Han Kim.
Supplementary Material
Footnotes
Abbreviations: aPTT = activated prothrombin time, CRP = C-reactive protein, CT = computed tomography, GGO = ground glass opacity, IFA = immunofluorescence assay, IgG = immunoglobulin G, LFT = liver function test, RT-PCR = reverse transcription-polymerase chain reaction, SFTS = severe fever with thrombocytopenia syndrome, SFTSV = severe fever with thrombocytopenia syndrome virus.
How to cite this article: Yun JH, Hwang HJ, Jung J, Kim MJ, Chong YP, Lee SO, Choi SH, Kim YS, Woo JH, Kim MY, Kim SH. Comparison of chest radiographic findings between severe fever with thrombocytopenia syndrome and scrub typhus. Medicine. 2019;98:46(e17701).
JHY and HJH have equally contributed to this manuscript.
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korean [grant no. HI16C0272].
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 2011;364:1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cui F, Cao HX, Wang L, et al. Clinical and epidemiological study on severe fever with thrombocytopenia syndrome in Yiyuan County, Shandong Province, China. Am J Trop Med Hyg 2013;88:510–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Deng B, Zhou B, Zhang S, et al. Clinical features and factors associated with severity and fatality among patients with severe fever with thrombocytopenia syndrome Bunyavirus infection in Northeast China. PLoS One 2013;8:e80802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ding F, Zhang W, Wang L, et al. Epidemiologic features of severe fever with thrombocytopenia syndrome in China, 2011-2012. Clin Infect Dis 2013;56:1682–3. [DOI] [PubMed] [Google Scholar]
- [5].Kim KH, Yi J, Kim G, et al. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis 2013;19:1892–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hiraki T, Yoshimitsu M, Suzuki T, et al. Two autopsy cases of severe fever with thrombocytopenia syndrome (SFTS) in Japan: a pathognomonic histological feature and unique complication of SFTS. Pathol Int 2014;64:569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu S, Chai C, Wang C, et al. Systematic review of severe fever with thrombocytopenia syndrome: virology, epidemiology, and clinical characteristics. Rev Med Virol 2014;24:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li H, Lu QB, Xing B, et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011-17: a prospective observational study. Lancet Infect Dis 2018;18:1127–37. [DOI] [PubMed] [Google Scholar]
- [9].Kato H, Yamagishi T, Shimada T, et al. Epidemiological and clinical features of severe fever with thrombocytopenia syndrome in Japan, 2013-2014. PLoS One 2016;11:e0165207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Choi SJ, Park SW, Bae IG, et al. Severe fever with thrombocytopenia syndrome in South Korea, 2013-2015. PLoS Negl Trop Dis 2016;10:e0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Public Health Weekly Report Disease Surveillance Statistics. Available at: http://www.cdc.go.kr/CDC/cms/cmsFileSeDownload.jsp?fid=9712&cid=142361&fieldName=attach1&index=1&fk=5bbd612dc2bf83528a2ce2b11cbe3e96ee90b4e319ac45faa861824bf6887. Accessed January 10, 2019. [Google Scholar]
- [12].Uehara N, Yano T, Ishihara A, et al. Fatal severe fever with thrombocytopenia syndrome: an autopsy case report. Intern Med 2016;55:831–8. [DOI] [PubMed] [Google Scholar]
- [13].Li S, Li Y, Wang Q, et al. Multiple organ involvement in severe fever with thrombocytopenia syndrome: an immunohistochemical finding in a fatal case. Virol J 2018;15:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park SW, Lee CS, Kim JH, et al. Severe fever with thrombocytopenia syndrome: comparison with scrub typhus and clinical diagnostic prediction. BMC Infect Dis 2019;19:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guo CT, Lu QB, Ding SJ, et al. Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome (SFTS) in China: an integrated data analysis. Epidemiol Infect 2016;144:1345–54. [DOI] [PubMed] [Google Scholar]
- [16].Kim MC, Chong YP, Lee SO, et al. Differentiation of severe fever with thrombocytopenia syndrome from scrub typhus. Clin Infect Dis 2018;66:1621–4. [DOI] [PubMed] [Google Scholar]
- [17].Zhu Y, Wu H, Gao J, et al. Two confirmed cases of severe fever with thrombocytopenia syndrome with pneumonia: implication for a family cluster in East China. BMC Infect Dis 2017;17:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim JY, Koo B, Jin CE, et al. Rapid diagnosis of tick-borne illnesses by use of one-step isothermal nucleic acid amplification and bio-optical sensor detection. Clin Chem 2018;64:556–65. [DOI] [PubMed] [Google Scholar]
- [19].Xu G, Walker DH, Jupiter D, et al. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 2017;11:e0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kwon JS, Kim MC, Kim JY, et al. Kinetics of viral load and cytokines in severe fever with thrombocytopenia syndrome. J Clin Virol 2018;101:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Blacksell SD, Bryant NJ, Paris DH, et al. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis 2007;44:391–401. [DOI] [PubMed] [Google Scholar]
- [22].Choi YH, Kim SJ, Lee JY, et al. Scrub typhus: radiological and clinical findings. Clin Radiol 2000;55:140–4. [DOI] [PubMed] [Google Scholar]
- [23].Jo BS, Lee IJ, Im HJ, et al. High-resolution computed tomography findings of swine-origin influenza A (H1N1) virus (S-OIV) infection: comparison with scrub typhus. Acta Radiol 2012;53:657–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


