Abstract
Background:
Non-arteritic anterior ischemic optic neuropathy (NAION) is the common cause of acute and subacute optic neuropathy in adults over the age of 50. Steroid administration in NAION seems to be in practice and is advised frequently by neurologists. The controversy regarding steroid usage in NAION is far from settled, with strong opinions on both sides. Despite a large amount of articles on this topic, but the results have not always been consistent. To address this gap, we decided to conduct a meta-analysis of all available published studies in order to better understand the effectiveness of steroids in treating NAION.
Objectives:
To identify the effectiveness of steroids in treating NAION.
Methods:
We performed a meta-analysis using databases, including PUBMED EMBASE, and the Cochrane library, to find relevant studies. The weighted mean difference (WMD) was determined for BCVA in steroid and nonsteroid groups.
Results:
Eight studies were included and summarized in this analysis. The studies included 720 eyes (392 NAION eyes and 328 eyes of normal controls). Heterogeneity among these studies was low (I2 = 0%). Because of the presence of heterogeneity, we conducted a fixed effects model to assess the effect of steroids on visual acuity in patients with NAION. The meta-analysis clearly demonstrated that in NAION, steroids did not significantly improve visual acuity (WMD = −0.02 [95% CI: −0.10 to 0.06], Z = 0.40, P = .69). After sensitivity analysis via the leave-one-out method, WMD was not significantly changed.
Conclusions:
Our meta-analysis found that steroids do not significantly improve visual acuity in NAION. In view of their long list of side effects, attempts at reversing ischemia should not involve the use of steroids.
Keywords: meta-analysis, nonarteritic anterior ischemic optic neuropathy, steroids
1. Introduction
Nonarteritic anterior ischemic optic neuropathy (NAION) is a common cause of acute and subacute optic neuropathy in adults over the age of 50 years old, and often results in severe visual loss.[1] It is thought to result from ischemic damage to the anterior optic nerve, which is predominantly supplied by the posterior ciliary arteries.[2] It is estimated that 2 to 10 persons per 100,000 persons have NAION (∼1500–6000 new cases per year in the United States).[3,4] The mechanisms involved in the development of optic disc ischemia in NAION are uncertain. Pathogenetically, the development of NAION is caused by hypoperfusion of the optic nerve head circulation, as well as transient occlusion of the capillaries of the posterior ciliary arteries, leading to ischemia of the optic nerve head.[5] There is no established treatment for NAION.[4] Numerous agents and procedures have been suggested for the treatment of NAION treatment, but most have not produced encouraging results.[4]
The earliest evidence of the beneficial effects of steroid treatment for NAION came from case series published in the late 1960s.[6] Experimental evidence obtained in a rodent model also showed that steroids exert neuroprotective effects on retinal ganglion cell survival in NAION.[5,7] A study published in 2007 provided strong support for the beneficial effects of steroids in NAION. A large, noncontrolled, retrospective study by Hayreh included 613 patients (696 eyes) treated with oral steroids over a period of 27 years.[8] Despite the heavy weighting and influence of the early Hayreh study, little other evidence, outside anecdotal evidence, has demonstrated that steroids provide any benefit in NAION. Outcomes of smaller trials have suggested that subtle benefits, at most, are achieved, but they also often demonstrate severe side effects. Indeed, steroids are selected by many physicians as a treatment option for NAION. A survey by Atkins et al showed that steroid administration is often applied in NAION and even frequently advised by neurologists.[2]
Recently, two randomized clinical trials (RCTs) were performed to investigate the effect of steroids on the course of NAION. Interestingly, the results showed that steroid use did not result in better final visual outcomes and potentially had harmful effects.[3,9] The controversy regarding steroid usage in NAION is far from settled, and there are strong opinions on both sides. Despite a large number of articles on this topic, to the best of our current knowledge, no published meta-analysis has focused on the effects of steroids in the treatment of NAION. To address this gap, we conducted a meta-analysis of all available published studies to improve understanding of the effectiveness of steroids in treating NAION.
2. Methods
2.1. Search strategy
We conducted a literature search using three databases (PUBMED, EMBASE, and Cochrane library) to identify records potentially relevant for this analysis, using the following search terms: (steroid OR corticosteroid) AND (ischemic optic neuropathy). A manual search was performed by checking the reference lists of the identified original reports and reviewing the relevant articles to identify studies not initially included in the computerized databases. The final search was carried out on April 4, 2019. The language was limited to English. Since all the data of this meta-analysis were collected from published literature and no patient consent were need, the ethical approval and written consent were not necessary in this study.
2.2. Inclusion criteria
The following inclusion criteria were applied in this meta-analysis: independent retrospective or prospective study that assessed the use of a corticosteroid to treat NAION that
-
(1)
assessed best-corrected visual acuity (BCVA) in patients with NAION, and
-
(2)
had sufficient data available to estimate WMD with 95% CI.
2.3. Exclusion criteria
If the study met the following selection criteria, it was excluded: abstracts from conferences, full text without raw data available for retrieval, duplicate publications, letters, and review articles. Studies published in a language different from English were excluded.
2.4. Data extraction
Two investigators independently extracted the data. Disagreement was resolved by discussion. For each study, we extracted information on the authors of each study, the year of report, the study design, the patient population, the number of eyes, the medication(s) given, their dosage and treatment duration, and BCVA. When studies reported data for several time intervals, we selected the one with the longest follow-up period for analysis. BCVA was measured in logMAR units. ETDRS letter scores were converted to logMAR units according to the formula logMAR = 1.7–0.02 × letter score.[10] Snellen visual acuities were converted to logMAR units according to the methods described by Holladay et al.[11] If ranges were provided instead of standard deviations (SD), the corresponding SD was calculated according to the method described by Hozo et al.[12]
2.5. Qualitative assessment
The quality of nonrandomized studies was evaluated using the Newcastle–Ottawa Scale (NOS) in this meta-analysis.[13] In this method, a study is judged on three categories: selection (four items, one star each), comparability (one item, up to two stars), and exposure/outcome (three items, one star each). A nine-point scale of the NOS (range, 0–9 points) has been developed for the evaluation of results.[14] In the NOS, poor, medium, and good quality are scored as 0–3, 4–6, and 7–9, respectively. Studies with NOS scores above 4 points were included in the final analysis. To analyze the quality of the RCTs, the Jadad scale was applied.[15]
2.6. Statistical analysis
All statistical analyses were performed in Review Manager 5.3 (Cochrane Collaboration, Copenhagen, Denmark) using extracted values for the mean, SD, and sample size. The weighted mean difference (WMD) was determined for BCVA in the steroid group and nonsteroid group, and the outcome was reported with a 95% CI. P < .05 was considered statistically significant in the test for an overall effect. Heterogeneity was explored using the Q-test to calculate the I2 statistic. In cases of low statistical heterogeneity (I2 < 30%), the fixed effects model was applied to the data. Given the low number (<10) of studies included in our meta-analysis, we chose not to test for publication bias to avoid the possibility of drawing a misleading conclusion.[16] Sensitivity analysis was performed via the leave-one-out method.[17]
3. Results
The study selection protocol used in this meta-analysis is shown in Figure 1. A total of 1444 articles were initially identified, and eight of these studies were included in this analysis; the studies are summarized in Tables 1 and 2.[3,5,8,9,18–21] All patients received treatment within 14 days (or median < 14 d) of onset. In the Hayreh published study, we extracted only the data on patients treated within 14 days for whom visual acuity was available at 6 months from the initial visit.[8,22] Two of the studies tested triamcinolone, two tested prednisolone, one tested prednisone, one tested methylprednisolone combined with prednisone, and two tested methylprednisolone combined with prednisolone.
Figure 1.
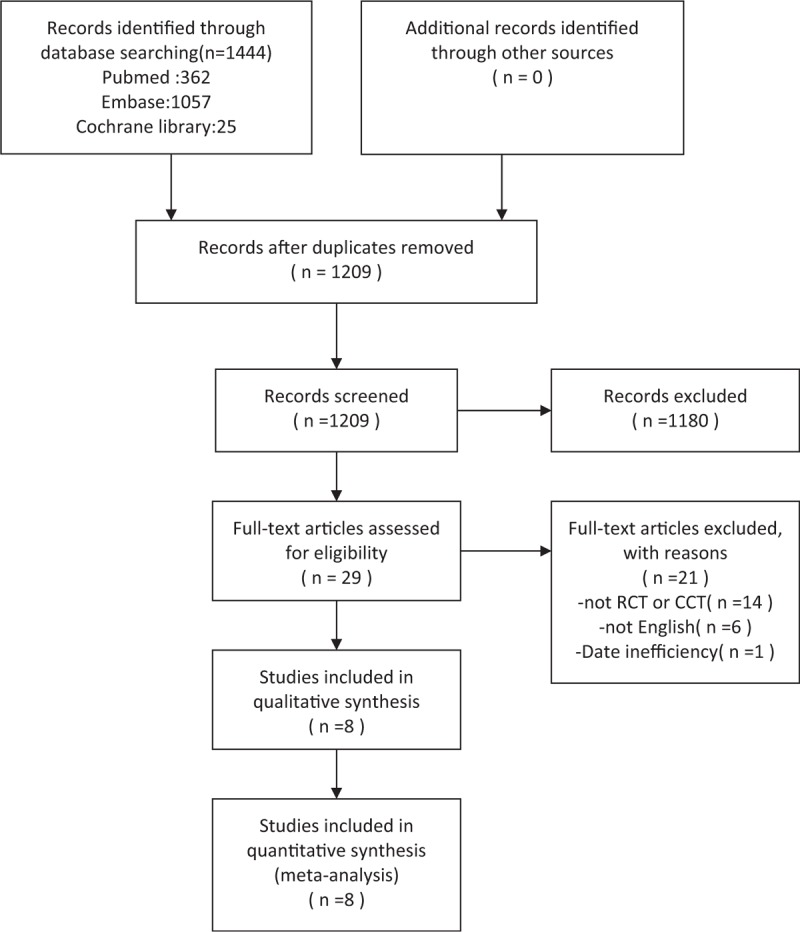
Flow diagram describing the identification and selection of relevant studies for the present meta-analysis.
Table 1.
Characteristics of the studies included in this meta-analysis.
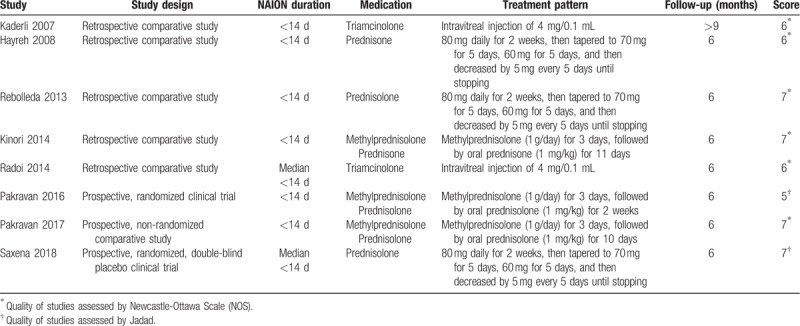
Table 2.
The patient characteristics of the included studies.
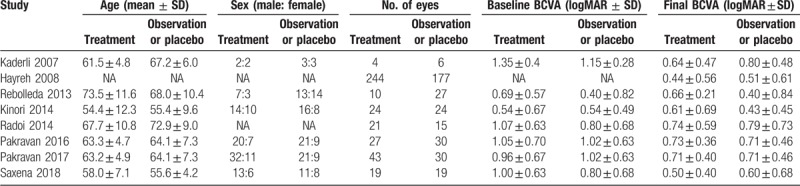
According to the NOS used for quality assessment, three nonrandomized studies had moderate quality scores of 5 or 6, while three nonrandomized studies had high quality scores of 7. When the quality of the RCTs was assessed according to the Jadad scale, the scores were 5 and 7, respectively. Overall, the risk of bias in the included studies was low, and all eight studies were deemed acceptable in the analysis (Table 1).
The trials in this meta-analysis examined 720 patient eyes with NAION (Figure 2). Heterogeneity among these studies was low (I2 = 0%). Because of the presence of heterogeneity, we conducted a fixed effects model to assess the effect of steroids on visual acuity in patients with NAION. This comparison clearly demonstrated that steroids in NAION did not significantly improve visual acuity (WMD = −0.02 [95% CI: −0.10 to 0.06], Z = 0.40, P = .69). After a sensitivity analysis performed via the leave-one-out method, we found that the WMD was not significantly changed.
Figure 2.
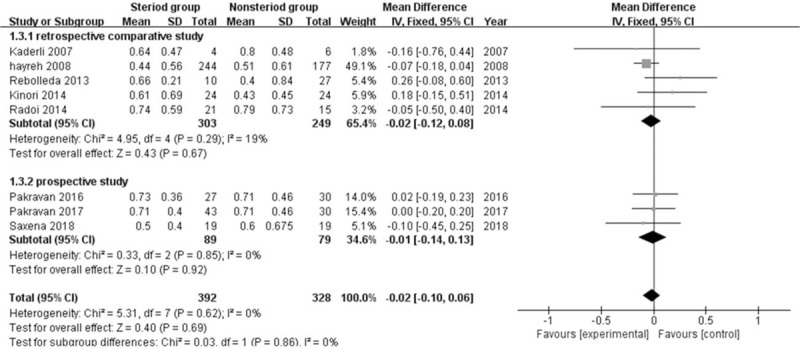
Forest plot demonstrating comparison of steroids versus nonsteroid in NAION on visual acuity.CI = confidence interval, IV = inverse variance, SD = standard deviation.
4. Discussion
In this meta-analysis, we summarized findings presented in the clinical literature on the effects of steroid administration on NAION patients. The results revealed that there was no significant change in BCVA after steroid administration.
The rationale for the use of steroids in NAION is based on a study published in the late 1960s in which several anecdotal case series demonstrated improved visual outcomes in patients with NAION treated with steroid therapy.[6] Theoretically, the effect of steroid therapy could be attributed to decreased compression of capillaries in the optic nerve head as a result of decreased edema and increased blood flow to the optic nerve head.[23] Based on these results, steroids are selected as a treatment option for NAION by many physicians, although conflicting results regarding its benefit have been reported over the past 2 to 3 decades.[8,24]
However, this meta-analysis demonstrates that steroids do not significantly improve visual acuity in NAION. If they act on optic disc edema, steroids should decrease the compression of capillaries in the optic nerve head.[23] Hence, the administration of steroids should result in effects similar to those achieved by optic nerve decompression surgery, which may be beneficial for optic disc edema. Regrettably, the Ischemic Optic Neuropathy Decompression Trial (IONDT), which was a randomized, single-blind, multicenter trial for NAION sponsored by the National Eye Institute, showed that surgical intervention provided no benefit.[25]
A number of questions have arisen regarding the pathogenesis of NAION with regards for whether it is solely ischemic in etiology, in which case, NAION should be steroid–responsive.[26] Furthermore, a study showed that the median time (25–75th percentile) to spontaneous resolution of optic disc edema from the onset of visual loss was 7.9 (5.8–11.4) weeks.[27] Although steroids might accelerate the resolution of optic disc edema, there is no evidence of a link between a shorter duration of optic disc edema and visual outcomes.[26,28] Moreover, a randomized double-blind clinical trial also showed that the use of steroids in acute NAION did not significantly improve visual acuity, although it did significantly improve the resolution of optic disc edema.[9]
Nonetheless, several limitations of this meta-analysis should be acknowledged. In the past, a large number of scattered case reports have been published on the use of steroids in NAION. This practice is not founded on any level I evidence and is potentially dangerous. Despite a broad literature search encompassing three major biomedical databases, one limitation of our study is the small number of patients included in the treatment group. The remaining eight studies were included and provided us with a total of 720 eyes for analysis. It is worth noting that our study included two RCTs, which provides some reliability for the results of this meta-analysis. Despite these variations, we observed low heterogeneity (I2 = 0%) in the BCVA analysis.
In addition, multiple drug administration methods were involved in this analysis. Due to the relatively small number of articles, the effectiveness of different corticosteroid administration methods in elevating BCVA in NAION could not be evaluated. However, we extracted only the data on BCVA obtained at 6 months from the initial visit. However, our results showed that steroids had not significantly improved visual acuity in NAION at 6 months. It is hard to say whether steroids accelerate the rate of recovery of vision without exerting a long-term visual benefit in NAION, such as that achieved by steroids in optic neuritis.[29]
5. Conclusion
This meta-analysis showed that steroids did not significantly improve visual acuity in NAION. In view of the long list of steroid side effects, steroids should not be used to reverse ischemia.
Author contributions
Conceptualization: Jun Chen.
Data curation: Yi Du.
Formal analysis: Jie Zhu.
Funding acquisition: Jun Chen.
Investigation: Jun Chen.
Methodology: Jun Chen.
Project administration: Jun Chen.
Resources: Chen Hu.
Software: Li Chen.
Supervision: Yi Du.
Validation: Jie Zhu.
Visualization: Jie Zhu.
Writing – original draft: Jun Chen.
Writing – review & editing: Yi Du.
Yi Du: 0000-0003-4879-5159.
Jun Chen: 0000-0001-5950-3276
Footnotes
Abbreviations: BCVA = best-corrected visual acuity, CI = confidence interval, IONDT = Ischemic Optic Neuropathy Decompression Trial, NAION = non-arteritic anterior ischemic optic neuropathy, NOS = Newcastle–Ottawa Scale, RCTs = randomized clinical trials, SD = standard deviations, WMD = weighted mean difference.
How to cite this article: Chen J, Zhu J, Chen L, Hu C, Du Y. Steroids in the treatment of nonarteritic anterior ischemic optic neuropathy: A PRISMA-compliant meta-analysis. Medicine. 2019;98:46(e17861).
This study was supported by the Startup Fund for Scientific Research, Fujian Medical University (No. 2018QH1170); and Youth Research Project of the Fujian Provincial Health Commission (NO.2019-1-94).
The authors have no conflicts of interest to disclose.
References
- [1].Atkins EJ. Nonarteritic anterior ischemic optic neuropathy. Curr Treat Options Neurol 2011;13:92–100. [DOI] [PubMed] [Google Scholar]
- [2].Atkins EJ, Bruce BB, Newman NJ, et al. Translation of clinical studies to clinical practice: survey on the treatment of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 2009;148:809. [DOI] [PubMed] [Google Scholar]
- [3].Pakravan M, Sanjari N, Esfandiari H, et al. The effect of high-dose steroids, and normobaric oxygen therapy, on recent onset non-arteritic anterior ischemic optic neuropathy: a randomized clinical trial. Graefe's Arch Clin Exp Ophthalmol 2016;254:2043–8. [DOI] [PubMed] [Google Scholar]
- [4].Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med 2015;372:2428–36. [DOI] [PubMed] [Google Scholar]
- [5].Radoi C, Garcia T, Brugniart C, et al. Intravitreal triamcinolone injections in non-arteritic anterior ischemic optic neuropathy. Graefe's Arch Clin Exp Ophthalmol 2014;252:339–45. [DOI] [PubMed] [Google Scholar]
- [6].Al-Zubidi N, Zhang J, Spitze A, et al. Systemic corticosteroids in nonarteritic ischemic optic neuropathy. Indian J Ophthalmol 2014;62:1022–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang TL, Wen YT, Chang CH, et al. Efficacy of intravitreal injections of triamcinolone acetonide in a rodent model of nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Visual Sci 2016;57:1878–84. [DOI] [PubMed] [Google Scholar]
- [8].Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefe's Arch Clin Exp Ophthalmol 2008;246:1029–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Saxena R, Singh D, Sharma M, et al. Steroids versus no steroids in nonarteritic anterior ischemic optic neuropathy: a randomized controlled trial. Ophthalmology 2018;125:1623–7. [DOI] [PubMed] [Google Scholar]
- [10].Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina (Philadelphia, Pa) 2011;31:1603–8. [DOI] [PubMed] [Google Scholar]
- [11].Holladay JT. Proper method for calculating average visual acuity. J Refract Surg 1997;13:388–91. [DOI] [PubMed] [Google Scholar]
- [12].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [14].Chen G, Tzekov R, Li W, et al. Subfoveal choroidal thickness in central serous chorioretinopathy: a meta-analysis. PLoS One 2017;12:e0169152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [16].Lau J, Ioannidis JP, Terrin N, et al. The case of the misleading funnel plot. BMJ 2006;333:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wei LK, Au A, Menon S, et al. Polymorphisms of MTHFR, eNOS, ACE, AGT, ApoE, PON1, PDE4D, and ischemic stroke: meta-analysis. J Stroke Cerebrovasc Dis 2017;26:2482–93. [DOI] [PubMed] [Google Scholar]
- [18].Kaderli B, Avci R, Yucel A, et al. Intravitreal triamcinolone improves recovery of visual acuity in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol 2007;27:164–8. [DOI] [PubMed] [Google Scholar]
- [19].Rebolleda G, Perez-Lopez M, Casas LP, et al. Visual and anatomical outcomes of non-arteritic anterior ischemic optic neuropathy with high-dose systemic corticosteroids. Graefe's Arch Clin Exp Ophthalmol 2013;251:255–60. [DOI] [PubMed] [Google Scholar]
- [20].Kinori M, Ben-Bassat I, Wasserzug Y, et al. Visual outcome of mega-dose intravenous corticosteroid treatment in non-arteritic anterior ischemic optic neuropathy – retrospective analysis. BMC Ophthalmol 2014;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pakravan M, Esfandiari H, Hassanpour K, et al. The effect of combined systemic erythropoietin and steroid on non-arteritic anterior ischemic optic neuropathy: a prospective study. Curr Eye Res 2017;42:1079–84. [DOI] [PubMed] [Google Scholar]
- [22].Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology 2008;115:298–305.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayreh SS. Management of ischemic optic neuropathies. Indian J Ophthalmol 2011;59:123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hayreh SS. Role of steroid therapy in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol 2010;30:388–9. [DOI] [PubMed] [Google Scholar]
- [25].Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. The Ischemic Optic Neuropathy Decompression Trial Research Group. JAMA 1995;273:625–32. [PubMed] [Google Scholar]
- [26].Lee AG, Biousse V. Should steroids be offered to patients with nonarteritic anterior ischemic optic neuropathy? J Neuroophthalmol 2010;30:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hayreh SS, Zimmerman MB. Optic disc edema in non-arteritic anterior ischemic optic neuropathy. Graefe's Arch Clin Exp Ophthalmol 2007;245:1107–21. [DOI] [PubMed] [Google Scholar]
- [28].Kelman SE. Intravitreal triamcinolone or bevacizumab for nonarteritic anterior ischemic optic neuropathy: do they merit further study? J Neuroophthalmol 2007;27:161–3. [DOI] [PubMed] [Google Scholar]
- [29].Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol (Chicago, Ill: 1960) 1993;111:773–5. [DOI] [PubMed] [Google Scholar]


