Supplemental Digital Content is available in the text
Keywords: brain abscess, complications, Glasgow outcome scale, reoperation
Abstract
This study aims to identify predictive factors related to clinical outcome, reoperation, and complications in patients with brain abscess.
Patients with a diagnosis of brain abscess at discharge at the Second Affiliated Hospital of Zhejiang University School of Medicine between 2008 and 2018 were reviewed. Logistic regression was used to identify predictive factors associated with Glasgow Outcome Scale (GOS) at discharge, GOS at 1 year after discharge, reoperation and complications.
Among 183 patients enrolled into the study, 142 patients had a good outcome at discharge (GOS ≥ 4) and 41 had a poor outcome (GOS ≤ 3). During the follow-up period, 20 additional patients had a good outcome. A total of 156 patients were treated by open craniotomy excision (n = 72) and aspiration (n = 84), 10 of whom underwent reoperation. Complications in surgical patients for brain abscess occurred in 54 patients. Poor outcome was related to Glasgow coma scale (P = .007) and ventricular proximity (P = .001). Surgical method was associated with reoperation (P = .04) and complications (P < .001). Seizure at admission was related to epilepsy (P < .001). Surgical method was related to postoperative intracranial hemorrhage (P = .02).
Glasgow coma scale (GCS) and ventricular proximity were associated with poor outcome. Further, patients who underwent aspiration were more likely to experience reoperation, while open craniotomy excision (OCE) was related to complications. Patients presenting seizure at admission were more likely to develop epilepsy. Patients who underwent OCE tended to experience postoperative intracranial hemorrhage.
1. Introduction
Brain abscess is a severe and life-threatening intracranial infectious disease occurring in 0.4% to 0.9% of 1000 people worldwide.[1,2] According to previous studies, the fatality rate has decreased from 40% to 10% over the past 5 decades because of the development of new neurosurgical techniques, modern neurosurgical imaging, better culturing methods, and better antimicrobial regimens. However there are still a considerable number of patients left with severe neurosurgical sequelae.[3]
Prognostic factors of brain abscess differ across previous studies. A study reported by Larsen et al showed that decreased Glasgow coma scale (GCS) at admission and presence of comorbidity were associated with poor outcome, while a study from China found that female gender was related to poor outcome.[2,4] The use of excision by craniotomy has declined, and currently the preferential method is aspiration.[2,5,6] While the choice of surgical method was still a debated subject. Zhang et al reported that excision and aspiration presented no significant difference in terms of clinical outcome and surgical complication,[4] and a study published in BMC Infectious Diseases found no significant difference in reoperation between the 2 procedures.[2] However, Sarmast et al found lower rate of reoperation (P = .024) in the excision group than that of the aspiration group, and a report from Turkey found that patients who underwent excision were more likely to develop seizures.[7]
This retrospective study is based on our experience with brain abscess diagnosed at 1 hospital over a 10-year period. The demographics data, predisposing factors, clinical characteristics, radiology findings, microbial findings, treatment, complications and clinical outcome of brain abscess were recorded, and we analyzed these variables to identify predictive factors of clinical outcome, reoperation, and complications.
2. Materials and methods
2.1. Patient series
We retrospectively reviewed patients diagnosed with brain abscess at the Second Affiliated Hospital of Zhejiang University School of Medicine between January 2008 and January 2018. Patients were identified through ICD-10 discharge codes. Demographic data, GCS at admission, predisposing factors, clinical manifestation, lesion location, ventricular proximity, size of lesion, microbial findings, white blood cell count, radiological findings, treatment strategies, complication, reoperation, length of hospital stay and GOS at discharge were collected from the electronic hospital data. Five patients were lost to follow-up, complete follow-up for the remaining patients was routinely conducted at 1 year after discharge by telephone interview. Inclusion criteria were characteristic computed tomography (CT)/magnetic resonance imaging (MRI) findings and evidence of brain abscess (postoperative pathological examination and/or appropriate microbiological specimens (pus, cerebro spinal fluid, or blood)) or characteristic CT/MRI findings, a clinical history, and treatment response compatible with brain abscess in patients with negative microbial findings. Patients were excluded from the study if,
-
1.
the diagnosis of brain abscess was not clear,
-
2.
the abscess was epidural or subdural,
-
3.
the patient required to transfer to another hospital for treatment,
-
4.
radiological information was not available,
-
5.
the patients had severe disease in other systems that affected their daily life or
-
6.
the patient was lost to follow-up.
All patients were initially treated with intravenous antibiotics once the diagnosis of brain abscess was suspected. Surgery was recommended in cases where abscesses >2.5 cm, there were signs of cerebral hernia, the abscess had ventricular proximity, or the abscess was located in an eloquent area with poor response to medical treatment. Aspiration was the preferential method of surgery, especially for abscesses located in deep or eloquent areas or for patients who could not tolerate OCE. In our study, burr hole aspiration was performed in 51 patients and 33 patients received stereotactically guided aspiration. Factors dictating the use of OCE included abscesses located in superficial non-eloquent areas, initial suspect of neoplasm, choice of patient, abscesses warranting a craniotomy, or individual surgeon preference. Aspiration would be repeated when little to no reduction in the size of the abscess was seen after the first aspiration despite combination with antibiotic therapy. Patients who had either inaccessible or multiple abscesses were recommended for conservative treatment first.
2.2. Medical treatment
Treatment in culture positive patients was guided by drug sensitivity tests. Ninety two patients received antibiotics before surgery. The use of antibiotics varied greatly. The empirical antimicrobial therapy of a combination of glycopeptide and carbapenem was used in 68 patients. One hundred forty five patients were treated with 2 or more different antibiotic regimens. Fifty two patients upgraded antibiotics during their hospitalization. Seizure prophylaxis or antiepileptic drugs were given in patients who underwent surgery during their hospitalization or who presented with seizure at admission.
2.3. Surgery approach
In our study, 84 patients were treated by aspiration, 72 patients underwent OCE and 27 patients were treated with antibiotics only. Seven patients with abscess size larger than 2.5 cm rejected the choice of surgery but they were recovered at discharge. Continuous monitoring of clinical status and brain imaging were performed in patients who were treated conservatively. All but 1 of the 27 non-surgical patients had recovered well (GOS ≥ 4) during the follow-up period.
2.4. Statistical analysis
Categorical variables were compared using the Chi-Squared test or Fisher exact test. Continuous variables within these groups were compared using an independent t test or nonparametric test. Logistic regression was used to detect predictive factors. Receiver operating characteristic (ROC) analyses were performed to evaluate the efficiency of predictive factors. All statistical analyses were conducted using SPSS v25.0, and P < .05 was considered statistically significant. Written approval for this study was obtained from the ethics committee of Zhejiang university school of medicine.
3. Results
During the 10-year period, 183 patients who met the standard were included in this study.
3.1. Demographics
The median age was 52 years (range, 3–81 years) and there were 131 (71.6%) males and 52 (28.4%) females (ratio 2.5:1) with an age distribution as shown in supplementary Fig. 1.
3.2. Symptoms and signs
Fever was the most common presenting symptom followed by headache, limb weakness, and vomiting (Table 1). Classic triad of brain abscess (headache, fever, and focal neurological deficits) was observed in 27 patients.
Table 1.
Clinical characteristics of patients at admission.
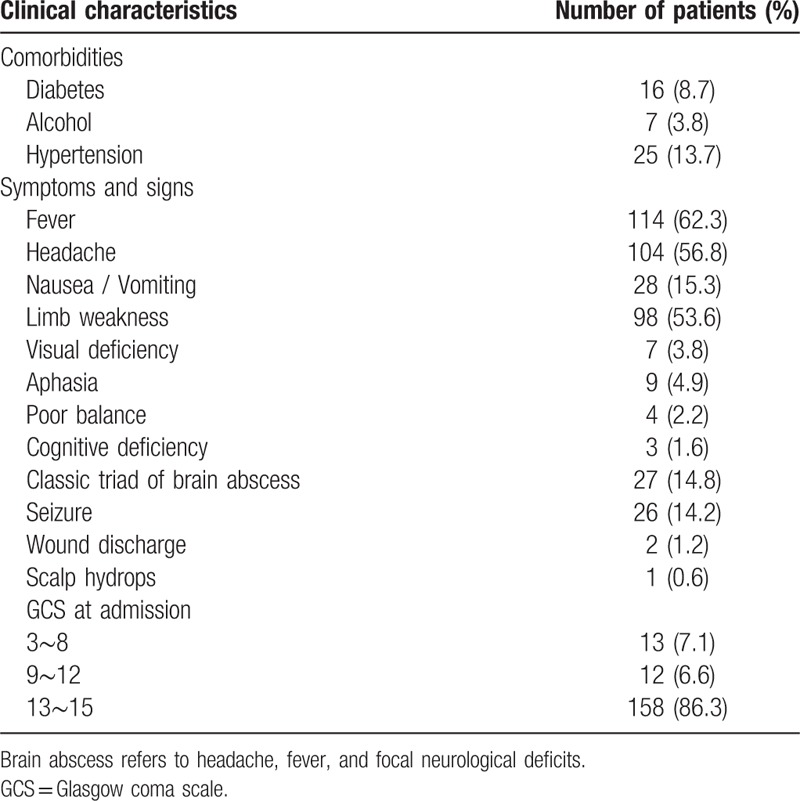
3.3. Comorbidities and predisposing factors
Comorbidities were present in 24.6% of the patients (Table 1). No patient was found to be affected with human immunodeficiency virus. Predisposing factors and numbers for each group are shown in Supplementary Fig. 2.
3.4. Radiological findings
Most of our patients (138 patients) underwent MRI with contrast enhancement, 12 patients had diffusion weighted imaging (DWI) alone. Thirteen patients had CT scans alone due to rapidly developing condition with high risk of mortality. Magnetic resonance spectroscopy (9 patients) and DWI (77 patients) were performed when it was difficult to distinguish a brain abscess from other cystic lesions. For most of our patients, brain abscesses were identified by isointense-to-slightly hyperintense rim in T1-weighted images, hypointense in T2-weighted images, and rim enhancement in MRI with contrast or restricted diffusion in DWI. The most frequent lesion location was the frontal lobe (45.9%). Additionally, among lesions located in frontal lobe, 31 lesions were located exactly in motor area. Multiple (from 2 to 6) abscesses were observed in 16 patients. In our study, ventricular proximity meant that the localized wall of brain abscess was the boundary between abscess and ventricle, and we could see localized enhancement of the ventricular wall adjacent to the abscess in enhanced MRI or CT images. A total of 63 patients’ lesions were categorized as ventricular proximity. The size of each brain abscess was represented by the maximum diameter, with an average measured 3.13 cm, Mid-line shift in CT/MRI at admission occurred in 135 patients, with a median size of 0.56 cm, ranging from 0.1 cm to 5.6 cm.
3.5. Microbial findings
By surgery, pus was available in 132 patients. Cerebrospinal fluid cultures were performed in all nonsurgical patients. Growth in culture from pus was positive in 34 patients. Cerebrospinal fluid cultures were positive in 2 of 33 patients. Four of 6 patients had positive blood cultures. Culture was not done in 20 surgical patients. Among nonsurgical patients, pathogenic microorganisms were found in only 2 patients. The most common species was Streptococcus. Multiple bacteria strains were observed in only 2 patients. Additionally, 11 patients had a negative pus culture but bacteria were seen by direct microscopy of a gram-stained pus smear. Among these patients, 10 had gram-positive bacteria, 1 had gram-negative bacteria. Further, there was 1 patient whose pus culture was negative, but the postoperative pathology of the wall of brain abscess indicated that the causative pathogen was likely to be Mycobacterium tuberculosis. Details of microbial findings was shown in supplementary Table 4.
3.6. Outcome, reoperation and complications
GOS ≤ 3 at discharge was observed in 41 patients (22.4%) at discharge and in 21 patients (11.5%). Mortality at discharge and 1 year after discharge were 4.9% (n = 9) and 6.0% (n = 11). The causes of death at discharge were cerebral hernia at admission in 3 patients, ventriculitis caused by abscess rupture into lateral ventricles after surgery in 2 patients, postoperative cerebral hernia after surgery in 2 patients, and postoperative intracranial hemorrhage in 2 patients. Another 2 patients who were in coma state at discharge died at 4 months after discharge and 6 months after discharge respectively.
Reoperation was conducted in 10 patients. Aspiration was repeated in 9 patients, because the size of the abscess after the first aspiration did not change significantly despite combination with antibiotic therapy. One patient received an open craniotomy excision as the first surgical procedure but because the wall of the abscess was not resected totally due to ventricular proximity location, the patient subsequently received stereotactically guided aspiration.
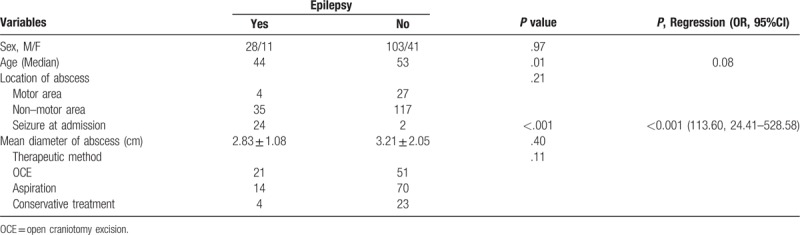
Epilepsy was the most common complication, which occurred both in surgical patients and patients who received conservative treatment only. Seizure at admission occurred in 26 patients and 24 patients progressed to epilepsy during their follow-up (P < .001), while size and location of brain abscess were not found to be related to epilepsy during follow-up (Table 2).
Table 2.
Comparison of clinical characteristics and measured variables between groups with and without epilepsy during follow-up.
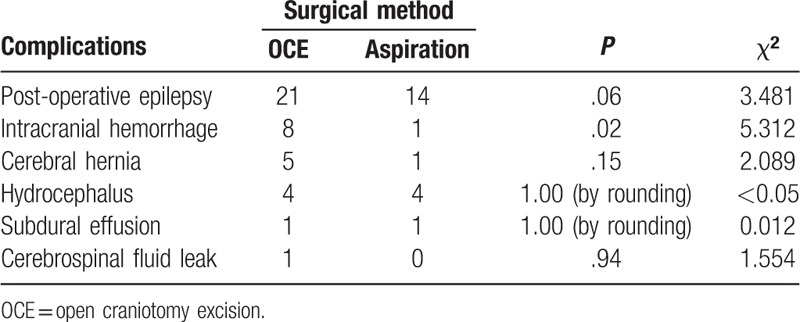
Complications in surgical patients was shown in Table 3. Patients who underwent OCE tend to have a higher incidence of epilepsy and cerebral hernia than those underwent aspiration, but no significant difference was found between the 2 surgical methods in terms of post-operative epilepsy and cerebral hernia. Intracranial hemorrhage was observed in 9 patients, of whom 8 patients (8/72) were treated by OCE, 1 patient (1/84) underwent aspiration (P = .02).
Table 3.
Complications in surgical patients.
3.7. Predictive factors
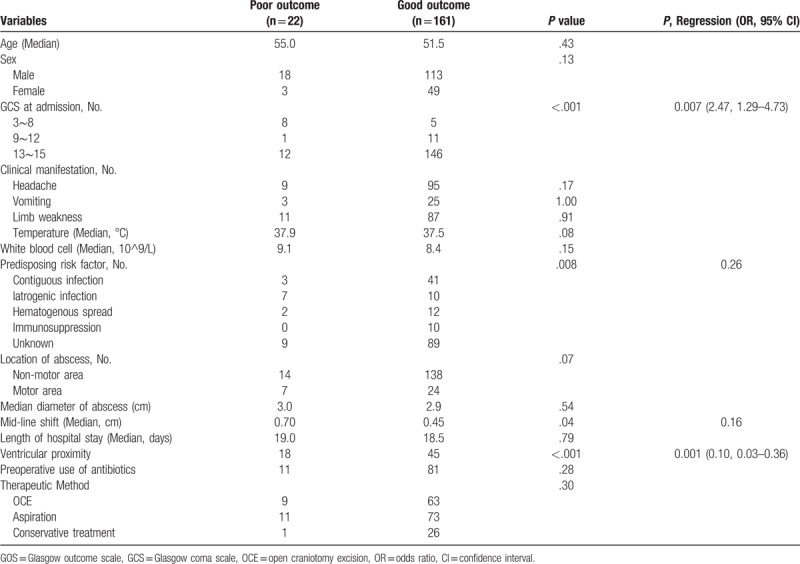
Univariate analysis and multivariate analysis were used to identify predictive factors that were associated with GOS at discharge (Supplementary, Table 1), GOS at 1 year after discharge (Table 4), reoperation (Supplementary, Table 2) and complications (Supplementary, Table 3). In univariate analysis, GCS at admission, headache, limb weakness, predisposing risk factors, location of abscess, ventricular proximity and middle shift were possible predictors associated with GOS at discharge. GCS at admission, ventricular proximity, predisposing risk factors, and mid-line shift were possible predictive factors related to GOS at 1 year after discharge. Surgical method was the only factor associated with reoperation, surgical method, and mid-line shift were possible factors related to complications in surgical patients. However, in multivariate analysis, GCS at admission (P = .007), headache (P = .03) ventricular proximity (P = .004), and motor area lesion location (P < .001) were associated with poor outcome (GOS ≤ 3) at discharge. GCS at admission (P = .007) and ventricular proximity (P = .001) were associated with poor outcome (GOS ≤ 3) at 1 year after discharge. Surgical method was related to reoperation (P = .045) and complications (P < .001).
Table 4.
Univariate and multivariate analysis of GOS at 1 year after discharge in intracranial abscesses.
ROC curves were performed to evaluate the efficiency of factors predicting clinical outcome, reoperation, and complications (Fig. 1). The area under curve (AUC) for predictive factors of GOS at discharge, GOS at 1 year after discharge, reoperation and complication were 0.870 (95% CI 0.809–0.931, P = .008), 0.828 (0.726–0.930, P < .001), 0.693 (95% CI: 0.553–0.834, P = .04) and 0.657 (95% CI: 0.566–0.747, P = .001).
Figure 1.
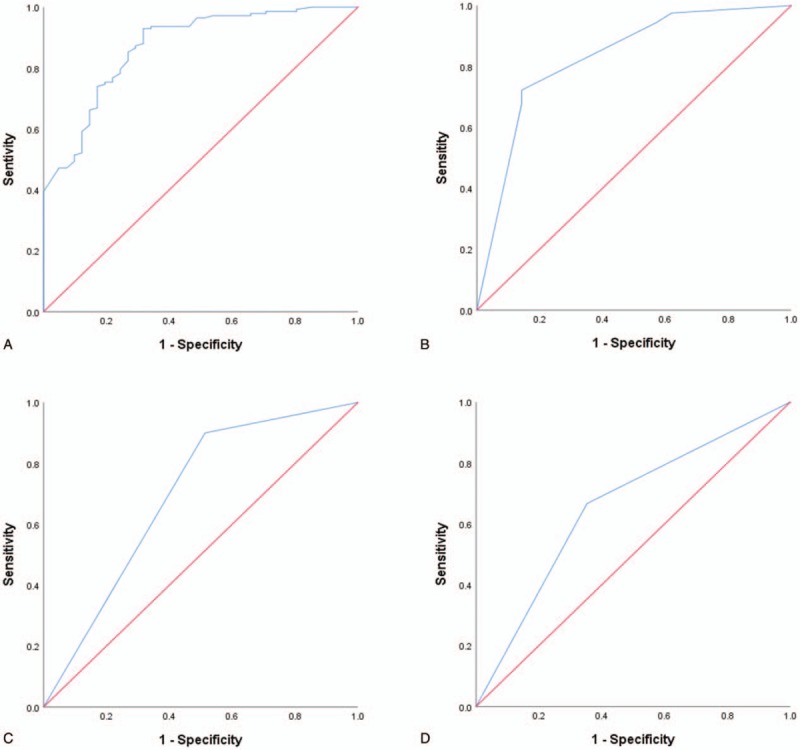
Receiver operating characteristic (ROC) curve for predictive factors of GOS at discharge (a), GOS at 1 year after discharge (b), reoperation (c) and complications (d).
4. Discussion
As patients with brain abscess are heterogeneous in terms of baseline characteristics, clinical outcome differs across previous studies. Mortality rate of brain abscess ranged from 9.8% to 25%, poor outcome (GOS ≤ 3) was reported from 21.6% to 38%.[2,4,7–10] The mortality rate and the rate of poor outcome at 1 year discharge in the present study was 6% and 12%, respectively, lower than previous observations, which may be attributed to the following reasons. First, there were lower proportion of patients (25/183) presenting a GCS ≤ 12 at admission in our study. Besides, patients with severe disease in other systems that affected their daily life were excluded from our study. On the other hand, it is also an inspiration to us that the prognosis of brain abscess can be better when diagnosis is made “in time”, and basic rules of management are applied.[11]
It was noticed that a literature published in 2014 found that GCS at admission had no effect on clinical outcome, a result not compatible with our clinical experience and most previous observations. In our study, we again found that decreased GCS score at admission was an independent factor related to poor outcome. Intraventricular rupture of brain abscess was a strong predictor of poor outcome in previous studies.[2,5] Further, signs of meningeal irritation and localized enhancement of the ventricular wall adjacent to the abscess (ventricular proximity as it was defined in our study) was a strong predictor of subsequent intraventricular rupture of brain abscess.[12] These factors could explain why ventricular proximity was associated with poor outcome our study, indicating that attention should be paid to abscesses adjacent to the ventricular wall. To prevent intraventricular rupture of brain abscess, surgery should be considered as a first line of treatment, especially when abscess has shown poor response to antibiotics. It has been well documented that brain abscess is a destructive lesion, the capsule of a brain abscess formed at the later stage after the necrotic center reaches its maximum size.[13] Lesions located in the motor area and edema surrounding the abscess often result in limb weakness. A subgroup analysis found a direct correlation between lesion located in motor area and limb weakness at discharge, which is important in determining GOS score at discharge. This could explain the finding that brain abscess located in motor area was associated with GOS at discharge. In our study, GOS ≤ 3 at discharge due to abscess located in motor area was observed in 20 patients (20/31) in all, 13 patients showed improvements in limb strength at 1 year after discharge and recovered (GOS ≥ 4). Therefore, abscesses located in motor area were not found to be related with GOS at 1 year after discharge, a result that may be related to edema subsiding and rehabilitation after surgery or conservative treatment. Thus, for abscesses located in motor area, the primary goal of treatment was to reduce the size of abscess and edema surrounding the abscess as quickly as possible. In patients with large abscesses and severe edema surrounding the abscess, aspiration may be the preferred choice if patients can tolerate surgery. Conservative treatment could be considered when patients showed good response to antibiotics and edema surrounding abscess was not obvious or patient could not tolerate surgery. An imaging severity index (ISI) score for brain abscess was first proposed by Demir et al and they found that imaging severity index score correlated strongly with patients’ outcome for all parameters studied.[14] ISI was scored according to the following radiological features: number, location, and largest diameter of the abscesses, presence of surrounding edema, and presence of mid-line shift. In our study, only mid-line shift was not found to be related with GOS, implying that we should consider more characteristics of brain abscess in imaging findings to judge the prognosis of brain abscess. The AUC value for predictive factors of GOS at discharge was 0.870 and GOS at 1 year after discharge was 0.828, indicating that these predictive factors had significant value in judging the prognosis of patients with brain abscess.
The choice of surgical method for brain abscess was still a debated subject. The mean mortality for aspiration post-1990 was 6.6% for publications with more than 5 patients. With surgical excision by craniotomy, the mean mortality in the same period was 12.7%.[6] Excision was found to be better than aspiration in terms of length of hospital stay and reoperation.[15] In our study, surgical method presented no significance in terms of clinical outcome and length of hospital stay, while aspiration was again found to be associated with reoperation. Additionally, we found that post-operative intracranial hemorrhage was more likely to occur in patients who underwent OCE (P = .021), this may explain the result that OCE was a risk factor of complication for brain abscess. Post-operative epilepsy was observed both in patients who underwent aspiration (14/72) and excision (22/84), but no significant difference was found between the 2 surgical procedures, in contrast to the result of a previous study.[7] Furthermore, excision of the abscess was also found to be superior to aspiration in terms of efficiency of surgical intervention, overall cost of treatment, length of hospital stay, and duration of antibiotic use,[15,16] indicating that excision still retained a significant role in surgical treatment of brain abscess, especially in superficially located non-eloquent area.[17]
According to ROC curve analyses, the AUC for predictive factors of complication and reoperation was 0.657 and 0.693, respectively, implying that surgical method had some predictive value in reoperation. However, surgical method may have limited value for assessing complications. A large sample prospective study is needed to confirm this result.
Patients in the conservative treatment group tended to have a more favorable outcome (26/27) compared with those in the surgical intervention group (136/156), though a subgroup analysis revealed no significant difference, indicating that conservative treatment could be considered if continuous monitoring of clinical signs and brain imaging showed improvement of patients’ condition. Additionally, in the conservative treatment group, 7 patients with abscesses larger than 2.5 cm which was considered to be threshold value, were recovered at discharge, indicating that conservative treatment could be used in a wider range of patients, but a further prospective study is still needed to demonstrate it.
Complications of brain abscess in our study included those directly related to abscess (epilepsy was included) and those related to therapeutic method. This may explain why the overall complications rate (34.6%) of brain abscess in surgical patients in our study was higher than a previous report that only included surgical complications (epilepsy was not included).[7]
Brain abscess was found to be a main risk factor for the development of post-infectious epilepsy in patients with central nervous infections.[18] The incidence of epilepsy following brain abscess differed across previous studies, ranging from 18.5% to 35.3%,[19–22] all of these data were based on retrospective studies with various follow-up period. In our study, the incidence of epilepsy was 21.3% (39/183), in spite of a relatively long follow-up times. Of the 26 patients who presented seizures at admission, 24 patients progressed to epilepsy during follow-up period, thus seizure at admission was found to be a strong predictors of epilepsy following brain abscess (P < .001), a result in line with previous observations.[21,22] However, sex, age, location, and volume of brain abscess were also discussed as possible predictive factors of epilepsy in previous reports.[19–21] Furthermore, Koszewski proposed that the capsule of brain abscess may not take part in the process of epileptic focus formation but probably to some reactions going on extracapsularly, which could explain why surgical method was not related to epilepsy, as was found in our study.[20] Koszewski also proposed a hypothesis that there is probably something like a minimal “critical mass” of brain tissue which must be affected by the inflammatory process in the same predisposed structural region of the brain at the same time, to begin the changes for forming an epileptic focus in future. However, among those with abscess size > 4 cm, epilepsy occurred only in 7 (7/34) patients while 33 (33/149) patients with epilepsy had an abscess size <4 cm in our study, therefore the size of brain abscess was not found to be related to epilepsy. Thus, we hypothesize that epilepsy could be affected by other factors rather than the size of abscess. Further research should be conducted to explain these controversial results and reveal the etiology and mechanism of epilepsy following brain abscess.
This study included a relatively large number of patients. Further, complete availability of detailed clinical characteristics, radiological information, microbial findings, management of patients, and clinical outcome was a strength in our study, making it possible to accurately identify predictive factors related to clinical outcome, epilepsy, reoperation, and complications. At the same time, there are some limitations. As a retrospective study in a single center, our findings may not be easily generalized to a wider population. Patients whose radiological information was unavailable were excluded, which resulted in selection bias in our study. Categories and duration of antibiotics used in our study differed greatly due to different period and neurosurgeons, experience, therefore it is difficult to analysis the relationship between antibiotics and clinical outcome. Although we have documented some radiological findings (location, size and middle line shift) of brain abscess, the extent of edema around brain abscess was not documented in our study, making it defective to explore the relationship between radiological findings and clinical outcome. A large-scale prospective multicenter study is needed to demonstrate wider reliability of these findings.
5. Conclusions
This retrospective study of 183 patients treated between 2008 and 2018 has shown that brain abscess still poses a threat to public health despite advances in surgical method, antibiotic regimens, and cranial imaging. GCS at admission and ventricular proximity were associated with poor outcome at 1 year after discharge. Patients who underwent aspiration tended to experience reoperation, while OCE was related to complications. Patients presenting seizure at admission were more likely to develop epilepsy. Patients who underwent OCE tended to experience postoperative intracranial hemorrhage.
Author contributions
Conceptualization: Shenglian Wu, Yiting Wei, Xiaobo Yu. Yucong Peng, Pingyou He, Hangzhe Xu, Cong Qian, Gao Chen.
Data curation: Shenglian Wu, Yiting Wei, Pingyou He.
Formal analysis: Shenglian Wu, Xiaobo Yu, Yucong Peng,. Pingyou He, Hangzhe Xu, Cong Qian.
Funding acquisition: Xiaobo Yu, Cong Qian, Gao Chen.
Investigation: Shenglian Wu.
Methodology: Yiting Wei, Xiaobo Yu, Gao Chen.
Project administration: Gao Chen.
Software: Shenglian Wu, Xiaobo Yu, Hangzhe Xu.
Supervision: Yucong Peng.
Validation: Yiting Wei.
Visualization: Yiting Wei, Yucong Peng.
Writing – original draft: Shenglian Wu, Yiting Wei.
Writing – review & editing: Xiaobo Yu, Cong Qian, Gao Chen.
Supplementary Material
Footnotes
Abbreviations: AUC = area under curve, CI = confidence interval, CT = computed tomography, DWI = diffusion weighted imaging, GCS = Glasgow coma scale, GOS = Glasgow outcome scale, MRI = magnetic resonance imaging, OCE = open craniotomy excision, OR = odds ratio, ROC = receiver operating characteristic.
How to cite this article: Wu S, Wei Y, Yu X, Peng Y, He P, Xu H, Qian C, Chen G. Retrospective analysis of brain abscess in 183 patients. Medicine. 2019;98:46(e17670).
This work was supported by grants from the National Natural Science Foundation of China [Grant No. 81401011, 81571106, 81701152].
For this type of study formal consent is not required.
The authors declare that they have no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Nicolosi A, Hauser WA, Musicco M, et al. Incidence and prognosis of brain abscess in a defined population: Olmsted County, Minnesota, 1935–1981. Neuroepidemiology 1991;10:122–31. [DOI] [PubMed] [Google Scholar]
- [2].Helweg-Larsen J, et al. Pyogenic brain abscess, a 15 year survey. BMC Infect Dis 2012;12:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brouwer MC, Coutinho JM, van de Beek D. Clinical characteristics and outcome of brain abscess: systematic review and meta-analysis. Neurology 2014;82:806–13. [DOI] [PubMed] [Google Scholar]
- [4].Zhang C, Hu L, Wu X, et al. A retrospective study on the aetiology, management, and outcome of brain abscess in an 11-year, single-centre study from China. BMC Infect Dis 2014;14:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Takeshita M, Kagawa M, Izawa M, et al. Current treatment strategies and factors influencing outcome in patients with bacterial brain abscess. Acta neurochirurgica 1998;140:1263–70. [DOI] [PubMed] [Google Scholar]
- [6].Ratnaike TE, Das S, Gregson BA, et al. A review of brain abscess surgical treatment--78 years: aspiration versus excision. World Neurosurg 2011;76:431–6. [DOI] [PubMed] [Google Scholar]
- [7].Aras Y, Sabanci PA, Izgi N, et al. Surgery for pyogenic brain abscess over 30 years: evaluation of the roles of aspiration and craniotomy. Turkish Neurosurg 2016;26:39–47. [DOI] [PubMed] [Google Scholar]
- [8].Tseng JH, Tseng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol 2006;65:557–62. discussion 562. [DOI] [PubMed] [Google Scholar]
- [9].Xiao F, Tseng MY, Teng LJ, et al. Brain abscess: clinical experience and analysis of prognostic factors. Surg Neurol 2005;63:442–9. discussion 449-450. [DOI] [PubMed] [Google Scholar]
- [10].Amornpojnimman T, Korathanakhun P. Predictors of clinical outcomes among patients with brain abscess in Thailand. J Clin Neurosci 2018;53:135–9. [DOI] [PubMed] [Google Scholar]
- [11].Sonneville R, Ruimy R, Benzonana N, et al. An update on bacterial brain abscess in immunocompetent patients. Clin Microbiol Infect 2017;23:614–20. [DOI] [PubMed] [Google Scholar]
- [12].Takeshita M, Kawamata T, Izawa M, et al. Prodromal signs and clinical factors influencing outcome in patients with intraventricular rupture of purulent brain abscess. Neurosurgery 2001;48:310–6. [PubMed] [Google Scholar]
- [13].Brouwer MC, Tunkel AR, McKhann GM, 2nd, et al. Brain abscess. N Engl Med 2014;371:447–56. [DOI] [PubMed] [Google Scholar]
- [14].Demir MK, Hakan T, Kilicoglu G, et al. Bacterial brain abscesses: prognostic value of an imaging severity index. Clinical radiology 2007;62:564–72. [DOI] [PubMed] [Google Scholar]
- [15].Sarmast AH, Showkat HI, Kirmani AR, et al. Aspiration versus excision: a single center experience of forty-seven patients with brain abscess over 10 years. Neurologia medico-chirurgica 2012;52:724–30. [DOI] [PubMed] [Google Scholar]
- [16].Mut M, Hazer B, Narin F, et al. Aspiration or capsule excision? Analysis of treatment results for brain abscesses at single institute. Turkish Neurosurg 2009;19:36–41. [PubMed] [Google Scholar]
- [17].Zhai Y, Wei X, Chen R, et al. Surgical outcome of encapsulated brain abscess in superficial non-eloquent area: a systematic review. Br J Neurosurg 2016;30:29–34. [DOI] [PubMed] [Google Scholar]
- [18].Sellner J, Trinka E. Clinical characteristics, risk factors and pre-surgical evaluation of post-infectious epilepsy. Eur J Neurol 2013;20:429–39. [DOI] [PubMed] [Google Scholar]
- [19].Chuang MJ, Chang WN, Chang HW, et al. Predictors and long-term outcome of seizures after bacterial brain abscess. J Neurol Neurosurg Psychiatry 2010;81:913–7. [DOI] [PubMed] [Google Scholar]
- [20].Koszewski W. Epilepsy following brain abscess. The evaluation of possible risk factors with emphasis on new concept of epileptic focus formation. Acta Neurochir 1991;113:110–7. [DOI] [PubMed] [Google Scholar]
- [21].Lee HS, Kim JH, Kim YH, et al. Predictors of unprovoked seizures in surgically treated pyogenic brain abscess: does perioperative adjunctive use of steroids has any protective effect? Clin Neurol Neurosurg 2018;173:46–51. [DOI] [PubMed] [Google Scholar]
- [22].Kilpatrick C. Epilepsy and brain abscess. J Clin Neurosci 1997;4:26–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


