Supplemental Digital Content is available in the text
Keywords: chemotherapy, diffuse large B-cell lymphoma, immunotherapy, mediation analysis, racial disparities
Abstract
Despite near universal health coverage under Medicare, racial disparities persist in the treatment of diffuse large B-cell lymphoma (DLBCL) among older patients in the United States. Studies evaluating DLBCL outcomes often treat socioeconomic status (SES) measures as confounders, potentially introducing biases when SES factors are mediators of disparities in cancer treatment.
To examine differences in DLBCL treatment, we performed causal mediation analyses of SES measures, including: metropolitan statistical area (MSA) of residence; census-tract poverty level; and private Medicare supplementation using the Surveillance, Epidemiology and End Results-Medicare linked database between 2001 and 2011. In this retrospective cohort study of DLBCL patients ages 66+ years, we conducted a series of multivariable logistic regression analyses estimating odds ratios (OR) and 95% confidence intervals (CI) relating chemo- and/or immuno-therapy treatment and each SES measure, comparing non-Hispanic (NH)-black, Hispanic/Latino, and Asian/Pacific Islander (API) to NH-white patients.
Compared to NH-white patients, racial/ethnic minority patients had lower odds of receiving chemo- and/or immuno-therapy treatment (NH-black: OR 0.84, 95% CI 0.65, 1.08; API: OR 0.80, 95% CI 0.64, 1.01; Hispanic/Latino: OR 0.78, 95% CI 0.64, 0.96) and higher odds of lacking private Medicare supplementation and residence within an urban MSA and poor census tracts. Adjustment for SES measures as confounders nullified observed racial differences. In causal mediation analyses, between 31% and 38% of race/ethnicity differences were mediated by having private Medicare supplementation.
Providing equitable access to Medicare supplementation may reduce disparities in receipt of chemo- and/or immuno-therapy treatment in older DLBCL patients.
1. Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma subtype among adults and the incidence rate of DLBCL is over 40 per 100,000 individuals in adults ages 70 years and older.[1–3] The introduction of rituximab to chemotherapy treatment regimens (e.g., R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has resulted in considerably improved survival in patients with DLBCL.[4–6] Despite the availability of highly effective regimens, racial differences persist in the treatment of DLBCL.[7–9] In the United States (U.S.), NH-black DLBCL patients typically present at younger ages, with more advanced stages of disease, and more frequently with B symptoms (e.g., fever night sweats and weight loss) compared to non-Hispanic (NH) white patients.[1,7] Hispanic/Latino patients present at later stages of follicular non-Hodgkin lymphoma when compared to NH-white and NH-black patients.[10] Although NH-black patients have a lower overall incidence of DLBCL, they are less likely to receive treatment and have worse survival compared to NH-white patients.[7]
Among the factors that contribute to the under treatment of DLBCL, insurance has a critical role given its direct relation to cost of treatment. Insurance status (uninsured and Medicaid vs privately insured) is associated with survival in DLBCL patients 18 to 64 years of age.[11] Less is known about whether and how insurance supplementation is associated with receipt of DLBCL treatment in the Medicare-aged U.S. population. Even though all adults over the age of 65 years have health coverage through Medicare, a greater proportion of NH-white Medicare beneficiaries have private supplementation plans and a lower proportion are dual Medicare-Medicaid eligible.[12] Patients without private Medicare supplementation incur higher out-of-pocket costs relative to their total income when they receive care,[13,14] representing a greater financial burden that is associated with poorer adherence to cancer treatment.[15,16]
Lower individual and community socioeconomic status (SES) are also associated with poorer health outcomes and increased risk of mortality among cancer patients.[17–19] Most epidemiologic studies account for SES by modeling it as a confounder or an effect modifier when examining the relationship between racial disparities and cancer treatment or mortality.[18,19] A confounder is defined as a characteristic associated with both the exposure and the outcome, though not in the causal pathway.[20] We hypothesize, as depicted in our a priori causal diagram (Supplemental Figure 1), that race and ethnicity may determine or predict SES measures and we therefore consider SES measures to be potential mediators in a causal pathway between race/ethnicity and treatment of DLBCL in older patients. With the development of mediation analysis, it is possible to better understand how incorporating a mediator as a confounder in statistical modeling can bias effect estimates; mis-specifying or possibly over adjusting the model could explain the conflicting findings and interpretations in observational studies of health disparities.[21]
No known study has conducted a mediation analysis evaluating the relationship between race and ethnicity and differences in the treatment of older patients with DLBCL using the Surveillance Epidemiology and End Results (SEER) Medicare-linked database.[22] Our objective was to conduct causal mediation analyses examining associations between race and ethnicity and receipt of chemo- and/or immuno-therapy (vs no chemo- and/or immuno-therapy) with potential mediation by patient- and community-level SES variables:
-
(i)
having Medicare private supplementation;
-
(ii)
metropolitan statistical area (MSA) of residence; and
-
(iii)
census-tract poverty level.
2. Methods
2.1. Data and participants
These analyses were conducted among patients ages 66 years and older diagnosed with DLBCL using the SEER-Medicare linked database between 2001 and 2011. Patients with DLBCL were identified using World Health Organization International Classification of Diseases for Oncology, third edition histology codes for ‘DLBCL, NOS [not otherwise specified]’ (codes: 9680, 9688, 9737, 9738, 9684, and 9735) available within the SEER dataset.[23] Data from this period coincides with the greater evidence and increasing use of rituximab in addition to standard multiple chemotherapy regimens to improve outcomes in older adults with DLBCL.[4] This database links population-based cancer registries in the U.S. with Medicare administrative claims data (medical claims from Parts A and B, and prescription claims from Part D).[22,24,25] Medicare is the primary payer for over 93% of the U.S. population ≥65 years of age,[26] making it possible to conduct epidemiologic studies to examine treatment and health outcomes in older adult cancer patients.[22,24,25]
Medicare beneficiaries ages 66 years and older with a diagnosis of histologically confirmed first primary DLBCL between 2001 and 2011 were eligible for inclusion in this study. Patients with first primary DLBCL include those with (1) DLBCL as their first and only cancer diagnosis or (2) DLBCL as the first of multiple cancer diagnoses. Patients were required to be continuously enrolled in Medicare Parts A and B for at least one year prior to and following diagnosis (unless died) with Medicare as their primary payer. If individuals died in <12 months following diagnosis, then they were required to have continuous coverage of Medicare Parts A and B prior to death. We did not condition enrollment on survival, which would have induced bias. Individuals with Medicare administered through a managed care organization were excluded because our data may not comprehensively capture administrative claims determining their treatment. Individuals with non-age-related eligibility for Medicare were also excluded from the analysis. Patients with missing data on race/ethnicity and our potential SES mediators of interest were also excluded from the sample. Our final analytic cohort included 9484 patients ages 66 years and older with DLBCL (Supplemental Figure 2).
The SEER-Medicare linked database is available to investigators only under a limited data use agreement. While we cannot directly share these data files, the programming code used for these analyses are available upon request. The institutional review board of the University of Illinois at Chicago approved this study and determined using de-identified data to be exempt from human subjects research requiring informed consent.
2.2. Covariates
Receipt of infused, systemic therapies (or oral equivalents) was determined using administrative claims with current procedure terminology and ICD-9-CM codes by providers.[27] Similar to other studies utilizing the SEER-Medicare linked database,[7–9] patients who received rituximab plus chemotherapy, rituximab monotherapy or other systemic chemotherapy in the year following diagnosis are here forward referred to as having received “any chemo- and/or immuno-therapy.” Patients with no documentation of these treatments in the year following diagnosis are classified here forward as receiving “no chemo- and/or immuno-therapy”.
Information on the exposure and other important covariates including race/ethnicity (NH-white, NH-black, Asian/Pacific Islander (API), Hispanic/Latino), age, sex, year of diagnosis, Ann Arbor stage, and presence of B symptoms at diagnosis were collected from SEER registry-reported data at cohort entry. The modified Charlson Comorbidity Score was determined using administrative medical claims data and was calculated from 12 months prior to diagnosis.[28] Finally, we also used these data to collect information on the 3 measures of SES mediators (i.e., private Medicare supplement status [yes vs no], MSA [urban vs nonurban], and ≥10% census-tract poverty level [yes vs no]), categorized as binary variables in these analyses. Beneficiaries were considered to have “Medicare without private supplement” if they lacked any supplement or had Medicaid (dual-eligible); all other beneficiaries had some form of private Medicare supplementation documented. Our definition of private Medicare supplementation did not include Medigap. These classifications were documented from the enrollment information at the time of DLBCL diagnosis. Urban MSAs were those with a population of at least 1,000,000; all others were considered non-urban MSAs.
2.3. Statistical analyses
Descriptive analyses described the difference in distribution of covariates overall and by receipt of any chemo- and/or immuno-therapy. Statistical significance was tested using t-test or Pearson's chi-squared for continuous and categorical variables, respectively. Mediation analyses follow the product method approach described by VanderWeele et al.[21,29] This method estimates the presence of mediation, direct effects, and indirect effects through a series of regression analyses. First, we conducted univariate (Eq. (1)) and multivariable (Eq. (2)) analyses regressing the outcome (i.e., any chemo- and/or immuno-therapy) on the exposure (i.e., race/ethnicity) and a priori measured confounders (Supplemental Figure 1). In our models below, a represents the level of the exposure, m represents fixing the mediator (M) at a constant level, and c are the measured confounders adjusted for in the model.
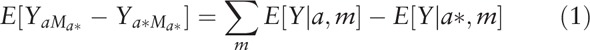 |
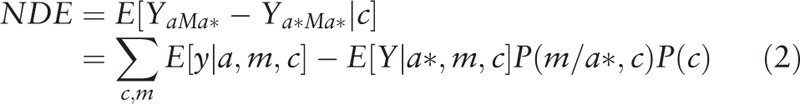 |
We then conducted univariate (Eq. (3)) and multivariate (Eq. (4)) analyses separately regressing mediators (i.e., the potential SES mediators of interest) on race/ethnicity and a priori measured confounders. Using these estimates, we calculated the natural direct effects (NDE) (Eq. (2)), natural indirect effects (NIE) (Eq. (4)), and total effects (TE) (Eq. (5)).[30] The direct effect describes the exposure-outcome relationship that does not include the mediator (θ); and the indirect effect describes the part of the exposure-outcome relationship that incorporates the mediator (1 − θ). While our approach accounted for the possibility of interaction among these SES measures and race/ethnicity, we did not find evidence of significant interaction with these mediators of interest and thus, interaction terms were not included in the final models.
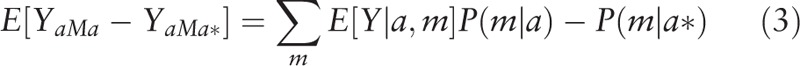 |
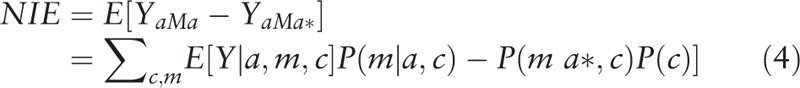 |
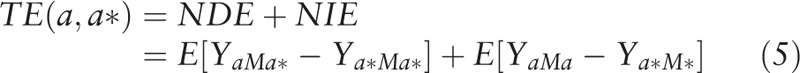 |
To estimate the extent to which the exposure-outcome relationship is affected by the mediator, we conducted a proportion-mediated calculation (Eq. (6)) using the PARAMED module in Stata. We used the calculated coefficients from the logistic regression and linear regression models to characterize the outcome and mediator variables respectively. All analyses were [30] conducted using Stata Release 14 (College Station, TX).[31]
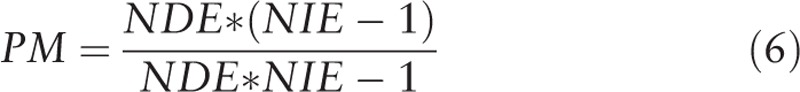 |
3. Results
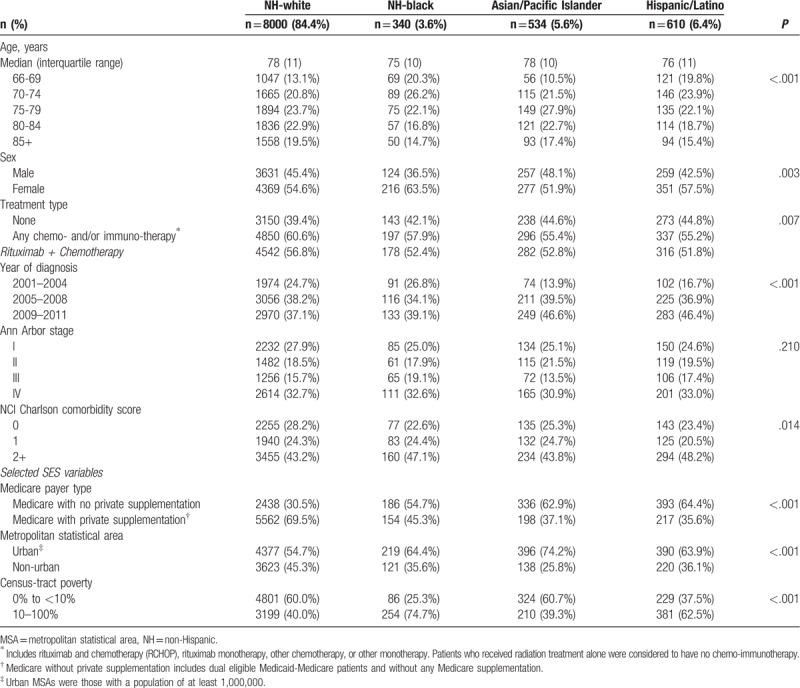
We report descriptive characteristics of Medicare beneficiaries diagnosed with DLBCL who received any chemo- and/or immuno-therapy and those who did not receive such therapy in Table 1. Those receiving any chemo- and/or immuno-therapy were younger (median 76 vs 80 years) and had slightly fewer female patients (54% vs 56%). Compared to patients that received any chemo- and/or immuno-therapy, a lower proportion of Medicare recipients that received no chemo- and/or immuno-therapy had private Medicare supplementation (61% vs 67%), and a greater proportion lived in an urban MSA (59% vs 56%) and in census-tracts with poverty levels of ≥10% (45% vs 41%). In Table 2, we describe characteristics of Medicare beneficiaries with DLBCL by race/ethnicity. A slightly greater proportion of NH-white Medicare beneficiaries (61%) received treatment with any chemo- and/or immuno-therapy compared to NH-black (58%), API (55%), and Hispanic/Latino beneficiaries (55%). Regardless of racial/ethnic group, the majority (>90%) of patients treated with any chemo- and/or immuno-therapy received R-CHOP regimens. A lower proportion of NH-black (45%), API (37%), and Hispanic/Latino patients (36%) had private Medicare supplementation compared to NH-white patients (70%); and more racial/ethnic minority patients lived in an urban MSA (64%, 74%, and 64% respectively) compared to their NH-white counterparts (55%). Furthermore, more NH-black (75%) and Hispanic/Latino patients (63%) lived in census-tracts with poverty levels of ≥10% compared to NH-white (40%) and API patients (39%).
Table 1.
Descriptive characteristics of Medicare recipients with diffuse large B-cell lymphoma in the Surveillance, Epidemiology and End Results Program registries by treatment status, 2001–2011.
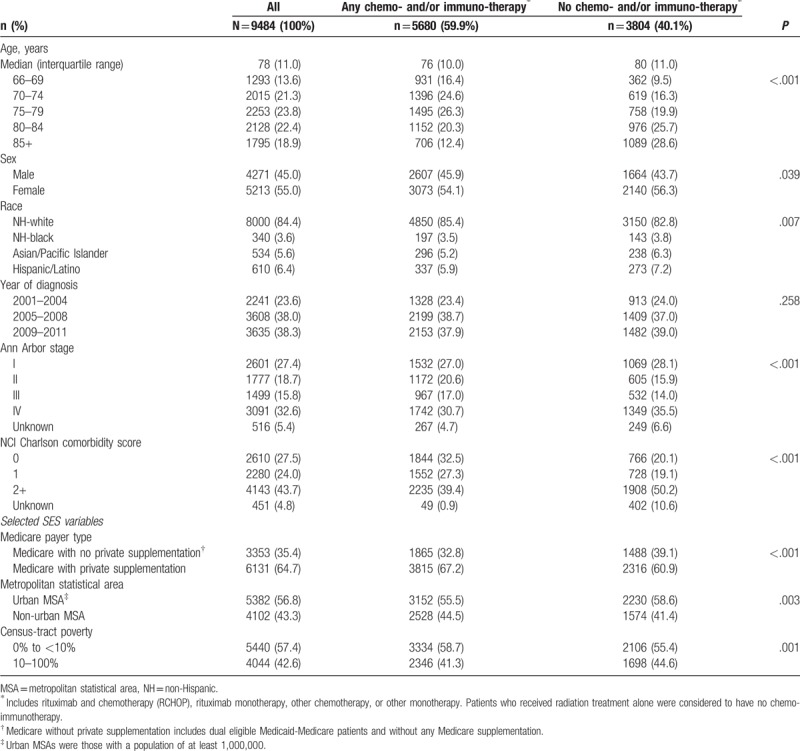
Table 2.
Descriptive characteristics of Medicare recipients with diffuse large B-cell lymphoma in the Surveillance, Epidemiology and End Results Program registries by race/ethnicity, 2001–2011.
In Table 3, we report results from logistic regression models for the association between race/ethnicity and odds of treatment with any chemo- and/or immuno-therapy. In multivariable models adjusting for measured confounders (C1), NH-black (OR 0.89, 95% CI 0.72, 1.11), API (OR 0.81, 95% CI 0.68, 0.96) and Hispanic/Latino patients (OR 0.80, 95% CI 0.68, 0.95) had lower odds of receiving treatment compared to NH-white patients. When adjusting for Medicare with private supplement as a confounder, the observed associations between race/ethnicity and treatment were attenuated toward the null or remained non-statistically significant (black: OR 0.89, 95% CI 0.69, 1.14; API: OR 0.86, 95% CI 0.68, 1.08; Hispanic/Latino: OR 0.84, 95% CI 0.69, 1.04). Adjustment for the other possible mediators as confounders in these models resulted in a smaller magnitude of attenuation in effect estimates.
Table 3.
Logistic regression models for the receipt of any chemo- and/or immuno-therapy∗ among black, Asian/PI and Hispanic compared to non-Hispanic white Medicare recipients with diffuse large B-cell lymphoma in the Surveillance, Epidemiology and End Results, 2001–2011.
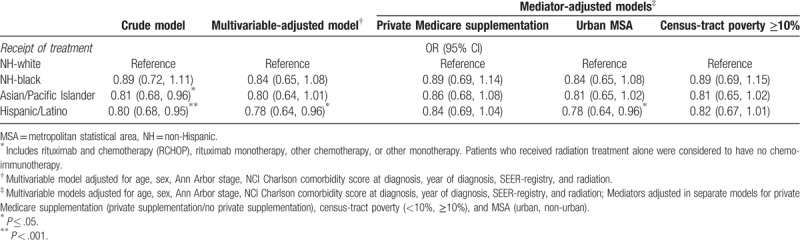
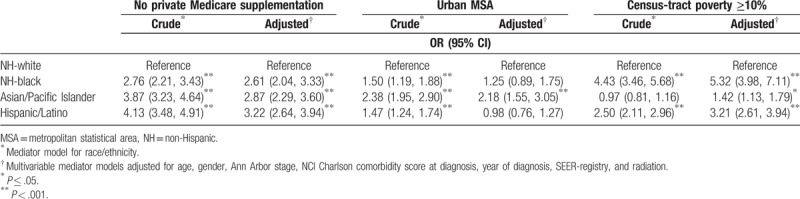
In Table 4, we report results from logistic regression models for the associations between race/ethnicity and the SES mediators of interest. NH-black and Hispanic/Latino patients had higher odds of having Medicare without private supplementation, residence in an urban MSA and in a census-tract with ≥10% poverty in both crude and multivariable adjusted logistic regressions among racial/ethnic minorities compared to NH-white patients. API patients also had higher odds of having Medicare without private supplementation and residence in an urban MSA but were similar to NH-white patients in respect to census-tract poverty levels.
Table 4.
Logistic regression models for lacking private Medicare supplementation, living in a big metro area, and living in a census-tract with <10% poverty among black, Asian/PI and Hispanic compared to non-Hispanic white Medicare recipients with diffuse large B-cell lymphoma in the Surveillance, Epidemiology and End Results, 2001–2011.
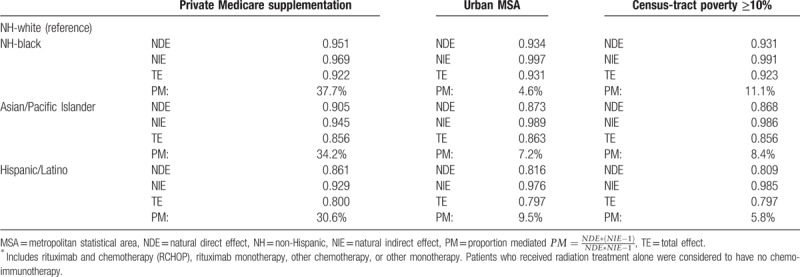
Based on the regression coefficients estimated in these multivariable analyses, Table 5 reports the calculated causal natural direct effect, natural indirect effect, total effect and the proportion mediated by the three SES mediators of interest in our casual pathway. The total effects from the mediation analyses were similar to the total effects found in the regression modeling that treats SES mediators as confounders (Table 3). For the observed differences in likelihood of treatment of patients ≥66 years of age with DLBCL with any chemo- and/or immuno-therapy, private Medicare supplementation accounted for the greatest proportion mediated for NH-black (38%), API (34%) and Hispanic/Latino patients (31%); as mediators, MSA and census-tract poverty levels of ≥10% accounted for only a modest amount of the observed differences in treatment by racial/ethnic group (between 5–10% and 6–11%, respectively) (Table 5).
Table 5.
Mediated association between race/ethnicity and receipt of any chemo- and/or immuno-therapy∗ by socioeconomic status (single mediation analyses).
4. Discussion
To our knowledge, this is the first study to investigate SES as a mediator for the association between race/ethnicity and the receipt of chemo- and/or immuno-therapy in patients 66 years of age and older with DLBCL. Past epidemiologic studies modeling SES as a confounder or effect modifier when evaluating receipt of cancer treatment and mortality have conflicting results.[9,11,19,32] While SES was demonstrated to attenuate any differences in cancer-specific and all-cause survival by race/ethnicity when adjusted as a confounder,[19] we contend that it is possibly more appropriate to consider SES measures as mediators to avoid introducing bias and minimize the possibility of spurious findings.[33] Mediation analyses that determine a proportion effect mediated provide an estimate of how much of the observed effect of race/ethnicity on treatment receipt would be reduced if we could fix the value of SES for the entire population,[29] an important interpretation for health policy decisions. These findings support the role of health policies, SES, and race/ethnicity on disparities in DLBCL survival.[18,34]
In previous studies, lack of insurance or having coverage with Medicaid only was associated with lower survival in younger lymphoma patients ages 18 to 64 years.[35] When comparing racial/ethnic minorities to NH-white DLBCL patients 66+ years of age in our analysis using SEER Medicare linked database, a large proportion (31–38%) of the effect of race/ethnicity on receiving any chemo- and/or immuno-therapy treatment was mediated by having private Medicare supplementation. Our findings are consistent with the available, although limited, literature describing differences in DLBCL treatment by racial/ethnic group and lower treatment rates among those with less comprehensive insurance coverage.[9,11,19] Given that the highest incidence of DLBCL occurs in the older adults covered by Medicare,[36] understanding substantial mediators to treatment access is critically important. This knowledge creates an opportunity for medical providers to optimize treatment outcomes and policymakers to provide equitable public health gains from highly effective breakthrough treatments such as rituximab-based therapy.
Despite the improvements in cancer care, racial disparities persist in treatment and survival in DLBCL and non-Hodgkin lymphoma in general. Access to medical treatments is characterized in the health services literature in multiple ways including affordability, availability, accessibility, accommodation, and acceptability.[37] This suggests that minority patients continue to experience barriers to effective treatments for DLBCL owed in a considerable extent to SES-related factors like private Medicare supplementation.[8,9,17] Treatment of non-Hodgkin lymphoma, and DLBCL specifically, with R-CHOP regimens is highly efficacious and improves survival regardless of race/ethnicity.[8,19] Beyond cancer treatment, racial disparities may also persist due to differences in cancer prevention, screening, and outreach.[17]
Among our mediators, private Medicare supplementation affected whether patients with DLBCL were treated with chemo- and/or immuno-therapy; however, population-level SES proxy measures (i.e., MSA and census-tract poverty level) accounted for only minimal mediated effects. In other studies, low neighborhood-level SES is associated with higher odds of not receiving cancer treatment when compared to patients from higher SES areas.[17,32,38] Therefore, further research using more detailed geographic data is warranted to determine the role of these factors in relation to racial/ethnic disparities in the treatment of DLBCL.
4.1. Policy implications
DLBCL five-year mortality has declined for the past two decades, in large part due to the introduction of rituximab-based treatment.[6] However, groups have benefited unequally from this advancement—racial minorities and individuals with no insurance or public insurance have higher rates of not receiving treatment and mortality.[9,11,19] According to Phelan and Link's theory of fundamental causes, disparities persist because individuals with higher SES have more resources and this leads to greater and more immediate access to new medical treatments and technologies.[39] This is a major cause for concern because as the field of oncology continues to progress toward more precision medicine and targeted therapy, the potential for worsening disparities among under resourced populations is daunting. Our findings suggest that improving access to private Medicare supplementation may reduce racial disparities in the receipt of treatment and may in turn have implications on cancer-specific mortality among Medicare recipients with DLBCL. This would be possible through policies that provide a subsidy for private supplementation for low-income Medicare beneficiaries. Medicare part D, for example, improved access to prescription medications among low-income adults by subsidizing the cost of the private supplement (i.e. Low Income Subsidy).[40] Expansion of Medicaid to a greater number of older adults is another option for policymakers. However, considering that dual-eligible Medicare beneficiaries have lower receipt of cancer treatments compared to beneficiaries with private insurance,[41] expanded Medicaid coverage would need to be comparable to that of private supplementation.
Rising costs of cancer treatment and out-of-pocket expenses represent a significant burden on cancer patients and barriers to care.[12,16] Out-of-pocket expenses for Medicare beneficiaries with cancer vary by supplemental insurance—beneficiaries with Medicaid have the lowest average expenditure and beneficiaries with no supplemental insurance have the highest expenditure,[13] although our study did not evaluate the role of out-of-pocket expenses on DLBCL outcomes. Together with our findings, these studies suggest that improving access to cancer treatments may lead to a reduction cancer disparities among racial/ethnic minorities.[42]
4.2. Limitations
There were limitations to our approach in this study. Analytically, applying the product method assumes that there are no unmeasured confounders in the exposure–outcome, mediator–outcome, and exposure–mediator relationships.[29] In this study using cancer registry and linked administrative claims data, residual confounding by unmeasured covariates is always possible given its observational nature and the use of mediator proxy measures. While SEER-Medicare captures many socio-demographic variables that are typically missing from other large administrative claims data, it was not possible to collect information on other important determinants for receipt of cancer treatments, such as: individual-level SES, ability to pay/cost-sharing, transportation, caregiver status, and important clinical considerations in treatment decision making (e.g., functional or performance status). Also, private Medicare supplementation plans are heterogeneous and vary in extent of additional coverage for beneficiaries. While we measured continuous Medicare enrollment and supplementation status at the time of DLBCL diagnosis, we were unable to distinguish whether supplementation (Part C) lapsed but patients remained enrolled on Parts A and B. An unmeasured confounder that is correlated with our mediators and may have contributed to the observed proportion mediated. However, we do not believe this could account entirely for the observed magnitude of the proportion mediated.
Given the dichotomous structure of our variables, we were unable to conduct a sensitivity analysis that estimated the proportion mediated using a different analytic method such as structural equation modeling (SEM), which requires the use of continuous variables.[43] Generalized SEM is a new method that accommodates dichotomous/categorical variables, but is unable to estimate indirect effects for comparison to our main approach.[44]
Our findings have limited generalizability due to sampling from SEER reporting regions (instead of the entire U.S.), exclusion of patients who did not have Medicare as the primary payer, and other inclusion/exclusion criteria. Other studies have reported lower rates (23%) of non-treatment of older DLBCL patients in the SEER-Medicare linked database,[45] while we identified that 40% of our cohort did not receive treatment. This difference in observed treatment (or absence thereof) may be explained by our exclusion of patients with a history of lymphoma diagnosis, non-age related Medicare eligibility, and requirements on continuous enrollment. Prior studies also classified patients as treated if they had only one chemo- and/or immuno-therapy diagnostic or procedural claim following DLBCL diagnosis.[45] We required two or more claims for a specific chemotherapeutic agent within 3 months of each other, not including a single date of service with the ICD-9-CM code indicating an encounter for antineoplastic chemotherapy (V58.11). Determining treatment from a sole claim for a single chemotherapeutic agent could represent intolerance (e.g., hypersensitivity) or the decision to not continue treatment (e.g., high cost or patient burden). Similarly, the use of a single ICD-9-CM code indicating the occurrence of an antineoplastic administration is not necessarily for a chemotherapeutic agent (e.g., Mesna administration post-cyclophosphamide). Indeed, the authors of the previous study of DLBCL treatment concluded that suboptimal duration of treatment was associated with poor outcomes, similar to that of having not received any treatment.[45]
Lastly, the age of onset of DLBCL among NH-black patients is lower compared to other racial/ethnic groups. Therefore, racial disparities in younger patients with respect to SES mediators such as having robust insurance were beyond the scope of this analysis.
5. Conclusion
Understanding health insurance-related disparities in lymphoma treatment and outcomes remains an important concern for oncologists and health policy makers. We found that between 31% and 38% of the observed differences in DLBCL treatment between racial/ethnic minority patients (i.e., NH-black, API, and Hispanic/Latino patients) and NH-white patients were mediated by having private Medicare supplementation. Census-tract-level SES factors (i.e., MSA and census-tract poverty level) yielded only modest mediation effects. Further research examining the role of other SES characteristics at the person and community level as possible mediators of differences in receipt of treatment by race/ethnicity is needed to confirm our findings. Still, understanding of mediation by socioeconomic measures such as Medicare supplementation provides important evidence for policymakers to consider actions to reduce cancer health disparities in the treatment of DLBCL.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Author contributions
Conceptualization: Jenny Guadamuz, Katharine Ozenberger, Brian Chiu, Gregory Sampang Calip.
Data curation: Jenny Guadamuz, Katharine Ozenberger, Sruthi Adimadhyam, Kellyn Moran, Gregory Sampang Calip.
Formal analysis: Jenny Guadamuz, Katharine Ozenberger, Kellyn Moran, Gregory Sampang Calip.
Funding acquisition: Brian Chiu, Gregory Sampang Calip.
Investigation: Jenny Guadamuz, Katharine Ozenberger, Naomi Ko, Ashley Cha, Kellyn Moran, Brian Chiu, Gregory Sampang Calip.
Methodology: Jenny Guadamuz, Katharine Ozenberger, Christopher Saffore, Brian Chiu, Gregory Sampang Calip.
Project administration: Gregory Sampang Calip.
Resources: Brian Chiu, Gregory Sampang Calip.
Software: Jenny Guadamuz, Katharine Ozenberger, Gregory Sampang Calip.
Supervision: Gregory Sampang Calip.
Validation: Naomi Ko, Karen Sweiss, Pritesh Patel, Gregory Sampang Calip.
Visualization: Ashley Cha, Kellyn Moran, Gregory Sampang Calip.
Writing – Original Draft: Jenny Guadamuz, Katharine Ozenberger, Gregory Sampang Calip.
Writing – Review & Editing: Jenny Guadamuz, Katharine Ozenberger, Dima Qato, Naomi Ko, Christopher Saffore, Sruthi Adimadhyam, Ashley Cha, Kellyn Moran, Karen Sweiss, Pritesh Patel, Brian Chiu, Gregory Sampang Calip.
Gregory Sampang Calip: 0000-0002-7744-3518.
Gregory Sampang Calip orcid: 0000-0002-7744-3518.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: API = Asian/Pacific Islander, CI = confidence intervals, DLBCL = diffuse large B-cell lymphoma, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, MSA = metropolitan statistical area, NDE = natural direct effects, NH = non-Hispanic, NIE = natural indirect effects, OR = odds ratios, PM = proportion mediated, R-CHOP = Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, SEER = Surveillance, Epidemiology and End Results, SEM = structural equation modeling, SES = socioeconomic status, TE = total effects, U.S. = United States.
How to cite this article: Guadamuz JS, Ozenberger K, Qato DM, Ko NY, Saffore CD, Adimadhyam S, Cha AS, Moran KM, Sweiss K, Patel PR, Chiu BH, Calip GS. Mediation analyses of socioeconomic factors determining racial differences in the treatment of diffuse large B-cell lymphoma in a cohort of older adults. Medicine. 2019;98:46(e17960).
Results from this study were presented, in part, in an oral presentation at the 58th Annual Meeting of the American Society of Hematology, San Diego, CA (December 5, 2016).
This study was supported by the National Institutes of Health, National Heart, Lung and Blood Institute (T32HL125294), National Center for Advancing Translational Sciences (KL2TR002002) and National Institute on Minority Health and Health Disparities (R21MD011439). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
PRP has consulted and received honoraria from Celgene. The other authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–59. doi:10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- [2].Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006;107:265–76. doi:10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smith A, Crouch S, Lax S, et al. Lymphoma incidence,;1; survival and prevalence 2004–2014: sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer 2015;112:1575–84. doi:10.1038/bjc.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–42. doi:10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- [5].Shah BK, Bista A, Shafii B. Survival in advanced diffuse large B-cell lymphoma in pre- and post-rituximab Eras in the United States. Anticancer Res 2014;34:5117–20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25202101 [access date May 27, 2018]. [PubMed] [Google Scholar]
- [6].Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin's lymphoma. Annu Rev Med 2008;59:237–50. [DOI] [PubMed] [Google Scholar]
- [7].Shenoy PJ, Malik N, Nooka A, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer 2011;117:2530–40. doi:10.1002/cncr.25765. [DOI] [PubMed] [Google Scholar]
- [8].Flowers CR, Shenoy PJ, Borate U, et al. Examining racial differences in diffuse large B-cell lymphoma presentation and survival. Leuk Lymphoma 2013;54:268–76. doi:10.3109/10428194.2012.708751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Griffiths R, Gleeson M, Knopf K, et al. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma (DLBCL). BMC Cancer 2010;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nabhan C, Byrtek M, Taylor MD, et al. Racial differences in presentation and management of follicular non-Hodgkin lymphoma in the United States: report from the National LymphoCare Study. Cancer 2012;118:4842–50. doi:10.1002/cncr.27513. [DOI] [PubMed] [Google Scholar]
- [11].Han X, Jemal A, Flowers CR, et al. Insurance status is related to diffuse large B-cell lymphoma survival. Cancer 2014;120:1220–7. doi:10.1002/cncr.28549. [DOI] [PubMed] [Google Scholar]
- [12].Brunt CS. Supplemental insurance and racial health disparities under Medicare Part B. Health Serv Res 2016;52:2197–218. doi:10.1111/1475-6773.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Narang AK, Nicholas LH. Out-of-pocket spending and financial burden among Medicare beneficiaries with cancer. JAMA Oncol 2017;3:757–65. doi:10.1001/jamaoncol.2016.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Commonwealth Fund. Medicare Beneficiaries’ High Out-of-Pocket Costs: Cost Burdens by Income and Health Status. doi:10.15868/socialsector.27426. [PubMed] [Google Scholar]
- [15].Kale HP, Carroll NV. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer 2016;122:283–9. doi:10.1002/cncr.29808. [DOI] [PubMed] [Google Scholar]
- [16].Zheng Z, Han X, Guy GP, Jr, et al. Do cancer survivors change their prescription drug use for financial reasons? Findings from a nationally representative sample in the United States. Cancer 2017;123:1453–63. doi:10.1002/cncr.30560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54:78–93. doi:10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- [18].Tao L, Foran JM, Clarke CA, et al. Socioeconomic disparities in mortality after diffuse large B-cell lymphoma in the modern treatment era. Blood 2014;123:3553–62. doi:10.1182/blood-2013-07-517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang M, Burau KD, Fang S, et al. Ethnic variations in diagnosis, treatment, socioeconomic status, and survival in a large population-based cohort of elderly patients with non-Hodgkin lymphoma. Cancer 2008;113:3231–41. doi:10.1002/cncr.23914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chubak J, Boudreau DM, Wirtz HS, et al. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst 2013;105:1456–62. doi:10.1093/jnci/djt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].VanderWeele TJ. Mediation and mechanism. Eur J Epidemiol 2009;24:217–24. doi:10.1007/s10654-009-9331-1. [DOI] [PubMed] [Google Scholar]
- [22].Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care 2002;408 Suppl: IV-55-61. doi:10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- [23].National Cancer Institute. Lymphoma Subtype Recode/WHO 2008. Available at: https://seer.cancer.gov/lymphomarecode/lymphoma-who2008.html [access date September 5, 2019]. [Google Scholar]
- [24].Mues KE, Liede A, Liu J, et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol 2017;9:267–77. doi:10.2147/CLEP.S105613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Engels EA, Pfeiffer RM, Ricker W, et al. Use of Surveillance, Epidemiology, and End Results-Medicare Data to conduct case-control studies of cancer among the US elderly. Am J Epidemiol 2011;174:860–70. doi:10.1093/aje/kwr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Smith JC, Medalia C. Health Insurance Coverage in the United States: 2014. US Census Bureau, Current Population Reports, P60-253. 2015. Available at: https://www.census.gov/content/dam/Census/library/publications/2015/demo/p60-253.pdf. [access date May 27, 2018]. [Google Scholar]
- [27].Bikov KA, Mullins CD, Seal B, et al. Algorithm for identifying chemotherapy/biological regimens for metastatic colon cancer in SEER-Medicare. Med Care 2015;53:e58–64. doi:10.1097/MLR.0b013e31828fad9f. [DOI] [PubMed] [Google Scholar]
- [28].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. doi:10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- [29].VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford: Oxford University Press; 2015. [Google Scholar]
- [30].Emsley R, Liu H. PARAMED: Stata Module to Perform Causal Mediation Analysis Using Parametric Regression Models. 2013. [Google Scholar]
- [31].StataCorp Stata Statistical Software: Release 14. 2015. [Google Scholar]
- [32].Byers TE, Wolf HJ, Bauer KR, et al. The impact of socioeconomic status on survival after cancer in the United States. Cancer 2008;113:582–91. doi:10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- [33].Kramer MS, Zhang X, Dahhou M, et al. Does fetal growth restriction cause later obesity? Pitfalls in analyzing causal mediators as confounders. Am J Epidemiol 2017;185:585–90. doi:10.1093/aje/kww109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Flowers CR, Nastoupil LJ. Socioeconomic disparities in lymphoma. Blood 2014;123:3530–1. doi:10.1182/blood-2014-04-568766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pulte D, Jansen L, Brenner H. Survival disparities by insurance type for patients aged 15–64 years with non-hodgkin lymphoma. Oncologist 2015;20:554–61. doi:10.1634/theoncologist.2014-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].De Lew N. Medicare – 35 years of service. Health Care Financ Rev 2000;22:75–103. [PMC free article] [PubMed] [Google Scholar]
- [37].Kullgren JT, McLaughlin CG, Mitra N, et al. Nonfinancial barriers and access to care for U.S. adults. Health Serv Res 2012;47(Pt 2):462–85. doi:10.1111/j.1475-6773.2011.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer 2011;117:180–9. doi:10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- [39].Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities theory, evidence, and policy implications. J Health Soc Behav 2010;511 Suppl:S28–40. [DOI] [PubMed] [Google Scholar]
- [40].Neuman P, Strollo MK, Guterman S, et al. Medicare prescription drug benefit progress report: findings from a 2006 national survey of seniors. Health Aff 2007;26:w630–43. doi:10.1377/hlthaff.26.5.w630. [DOI] [PubMed] [Google Scholar]
- [41].Warren JL, Butler EN, Stevens J, et al. Receipt of chemotherapy among Medicare patients with cancer by type of supplemental insurance. J Clin Oncol 33:312–8. doi:10.1200/JCO.2014.55.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol 2016;34:980–6. doi:10.1200/JCO.2015.64.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mackinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev 1993;17:144–58. doi:10.1177/0193841X9301700202. [Google Scholar]
- [44].Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008;40:879–91. doi:10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- [45].Hamlin PA, Satram-Hoang S, Reyes C, et al. Lymphoma treatment patterns and comparative effectiveness in elderly diffuse large B-cell lymphoma patients: a surveillance, epidemiology, and end results-medicare analysis. Oncologist 2014;19:1249–57. doi:10.1634/theoncologist.2014-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


