Abstract
Background:
This study will aim to systematically explore the efficacy of resveratrol for the treatment of patients with ulcerative colitis (UC).
Methods:
We will search the electronic databases of MEDLINE, EMBASE, Cochrane Library, Web of Science, Chinese Biomedical Literature Database, and China National Knowledge Infrastructure up to the September 1, 2019 for randomized controlled trials (RCTs) that report on UC who have undergone resveratrol compared with other interventions. All electronic databases will be searched without restrictions of language. Two authors will independently conduct study screen, data extraction, and risk of bias assessment. Any disagreements between 2 authors will be resolved with a third author by discussion or consultation if it is necessary. RevMan 5.3 software will be applied for statistical analysis.
Results:
Outcomes include clinical remission, improvement of clinical symptoms, maintenance of remission, relapse rate, endoscopic assessment, histological assessment, quality of life, and adverse events.
Conclusion:
This study will provide most recent evidence of resveratrol for the treatment of patients with UC.
PROSPERO registration number:
PROSPERO CRD42019150849.
Keywords: efficacy, resveratrol, safety, ulcerative colitis
1. Introducton
Ulcerative colitis (UC) is a chronic, idiopathic inflammatory disease.[1,2] It is characterized by relapsing and remitting mucosal inflammation.[3–5] It often manifests as diarrhea, rectal bleeding, urgent need to defecate, and abdominal pain.[6–9] It has reported that the incidence steadily increased around the world during the past 2 decades.[10,11] Although its precise etiology is still unknown, several treatments are reported to manage this condition, including nonsteroidal anti-inflammatory drug, antibiotics, anti inflammatory drug, steroid, analgesic, dietary supplement, surgery, supportive care, acupuncture, and moxibusion.[12–21] Of those, resveratrol has been reported to treat UC effectively.[22–24] However, no systematic review has been conducted to assess its efficacy and safety for the treatment of UC. Thus, this study will comprehensively and systematically investigate the efficacy and safety of resveratrol for the treatment of patients with UC.
2. Methods
2.1. Eligibility criteria for the selection of studies
2.1.1. Type of studies
We will only consider randomized controlled trials (RCTs) focusing on the efficacy and safety of resveratrol for the treatment of patients with UC. However, non-RCTs and quasi-RCTs will be excluded.
2.1.2. Type of participants
We will include patients who were diagnosed as UC without limitations of race, sex, and age.
2.1.3. Type of interventions
All patients in the experimental group must undergo resveratrol alone.
However, all patients in the control group can receive any interventions, but not any single or combined forms of resveratrol.
2.1.4. Type of outcomes
In this study, we will assess the outcomes of clinical remission, improvement of clinical symptoms, maintenance of remission, relapse rate, endoscopic assessment, histological assessment, quality of life, and adverse events.
2.2. Search strategy
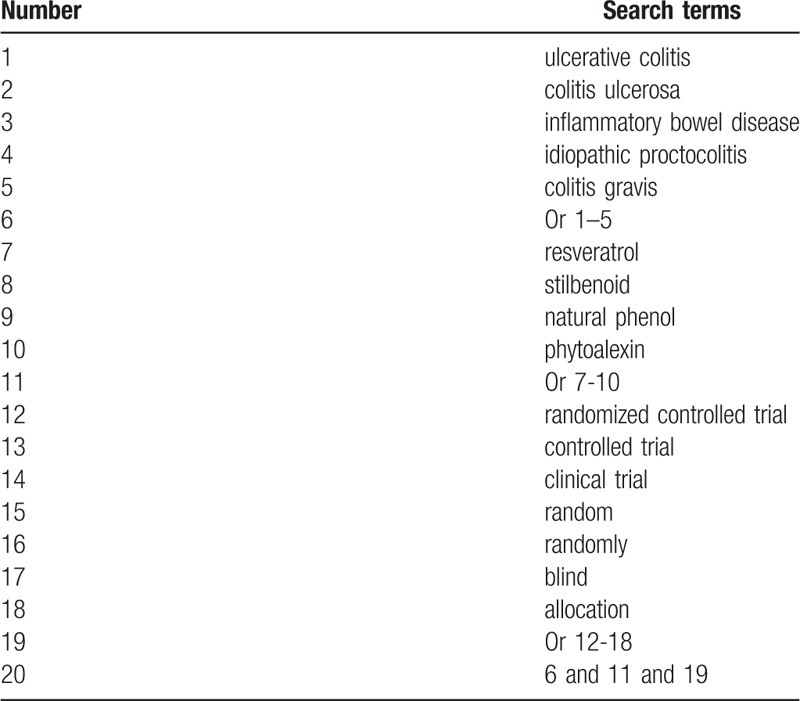
We will search MEDLINE, EMBASE, Cochrane Library, Web of Science, Chinese Biomedical Literature Database, and China National Knowledge Infrastructure up to the September 1, 2019 without language limitations. We will use the following search terms: ulcerative colitis, colitis ulcerosa, inflammatory bowel disease, resveratrol, randomized controlled trial, clinical study, controlled study, clinical trial, random, and blind. We will build a search strategy sample for MEDLINE in Table 1. We will also adapt similar search strategy to other electronic databases. In addition, we will also search the grey literatures, including dissertations, clinical registry, and reference lists of relevant reviews.
Table 1.
Search strategy for MEDLINE.
2.3. Data selection and extraction
2.3.1. Study selection
Two authors will scan the titles and abstracts of all searched studies to identify studies for inclusion initially, and all irrelevant studies will be excluded. At the second stage, we will assess the full texts of remaining studies for eligibility. Any different opinions of study selection between 2 authors will be solved by a third author through discussion. We will exert the process of study selection in the flowchart.
2.3.2. Data extraction
Two authors will independently collect data using piloted data extraction sheet. We will extract study characteristics (title, first author, time of publication, et al), patient characteristics (race, sex, age, inclusion and exclusion criteria, diagnostic criteria, sample size, et al), study setting, study methods (methods of randomization, blind and allocation details, et al), treatment details (intervention name, dosage, frequency, duration, et al), outcomes (all outcome measurements, safety, et al), and funding information. If there are any divergences between 2 authors, we will invite another author to solve them via discussion where required. We will contact primary study authors for any insufficient or missing data if it occurs during the period of data extraction.
2.4. Assessment of risk of bias
Two authors will assess the risk of bias for all eligible studies using Cochrane Risk of Bias Tool. This tool has 7 aspects, and we will further judge each aspect as low, unclear and high risk of bias. A third author will help to solve any disagreements between 2 authors by discussion or consultation if necessary.
2.5. Data synthesis and analysis
We will use RevMan 5.3 software for statistical analysis in this study. We will express continuous outcome data as mean difference or standardized mean difference with 95% confidence intervals (CIs), and dichotomous data as risk ratio with 95% CIs. We will evaluate heterogeneity among included studies using I2 test. I2 ≤ 50 means low heterogeneity, and a fixed-effects model will be applied. I2 > 50% exerts significant heterogeneity, and a random-effects model will be utilized. When the heterogeneity is low, we will perform meta-analysis with low heterogeneity if more than 2 studies using the same treatments, comparators, and outcomes. When the heterogeneity is substantial, we will carry out subgroup analysis and meta-regression to explore possible factors that may cause significant heterogeneity.
2.6. Subgroup analysis
We will conduct subgroup analysis based on the different study quality, treatments, and outcome measurements.
2.7. Sensitivity analysis
If sufficient data are available, we will plan for sensitivity analysis to check the robustness of pooled results by removing studies with high risk of bias.
2.8. Reporting bias
If sufficient studies are included, we will perform Funnel plot[25] and Egger regression test[26] to check the reporting bias among the eligible studies.
2.9. Ethics and dissemination
This study will not require ethic approval, because no individual patient data will be used. We are expected to publish this study at a peer-reviewed journal.
3. Discussion
Previous studies have found that resveratrol can effectively treat patients with UC. However, its results are still inconsistent and inconclusive. Thus, this study will systematically assess the efficacy and safety of resveratrol for the treatment of patients with UC. We hope this study may provide convincing evidence to the clinical practice and health related policy maker.
This study still has several potential limitations. First, different study quality may affect the heterogeneity among studies. Second, different doses of resveratrol may also cause clinical heterogeneity.
Author contributions
Conceptualization: Yan-hui Chen, Yi Xiang.
Data curation: Yan-hui Chen, Yi Xiang.
Formal analysis: Yan-hui Chen, Yi Xiang.
Investigation: Yan-hui Chen.
Methodology: Yi Xiang.
Project administration: Yan-hui Chen.
Resources: Yi Xiang.
Software: Yi Xiang.
Supervision: Yan-hui Chen.
Validation: Yan-hui Chen, Yi Xiang.
Visualization: Yan-hui Chen, Yi Xiang.
Writing – original draft: Yan-hui Chen, Yi Xiang.
Writing – review & editing: Yan-hui Chen, Yi Xiang.
Footnotes
Abbreviations: CIs = confidence intervals, RCTs = randomized controlled trials, UC = ulcerative colitis.
How to cite this article: Chen Yh, Xiang Y. Efficacy of resveratrol for the treatment in patients with ulcerative colitis. Medicine. 2019;98:46(e17938).
This study is supported by Science and Technology Research Project of Xianyang City (2016k02-101). The funder had no role in this study.
The authors have no conflicts of interests to disclose.
References
- [1].Johnson CM, Linzay CD, Dassopoulos T. Maneuvering clinical pathways for ulcerative colitis. Curr Gastroenterol Rep 2019;21:52. [DOI] [PubMed] [Google Scholar]
- [2].Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc 2019;94:1357–73. [DOI] [PubMed] [Google Scholar]
- [3].Kawachi H. Histopathological diagnosis of ulcerative colitis-associated neoplasia. Dig Endosc 2019;31Suppl 1:31–5. [DOI] [PubMed] [Google Scholar]
- [4].Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol 2018;30:1–0. [DOI] [PubMed] [Google Scholar]
- [5].Testa A, Castiglione F, Nardone OM, et al. Adherence in ulcerative colitis: an overview. Patient Prefer Adherence 2017;11:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pineton de Chambrun G, Tassy B, Kollen L, et al. The treatment of refractory ulcerative colitis. Best Pract Res Clin Gastroenterol 2018;32-33:49–57. [DOI] [PubMed] [Google Scholar]
- [7].Shrestha MP, Taleban S. Management of ulcerative colitis in the elderly. Drugs Aging 2019;36:13–27. [DOI] [PubMed] [Google Scholar]
- [8].Tripathi K, Feuerstein JD. New developments in ulcerative colitis: latest evidence on management, treatment, and maintenance. Drugs Context 2019;8:212572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology 2013;145:158–65. [DOI] [PubMed] [Google Scholar]
- [11].Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54. [DOI] [PubMed] [Google Scholar]
- [12].Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- [13].Blonski W, Buchner AM, Lichtenstein GR. Treatment of ulcerative colitis. Curr Opin Gastroenterol 2014;30:84–96. [DOI] [PubMed] [Google Scholar]
- [14].Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc 2014;89:1553–63. [DOI] [PubMed] [Google Scholar]
- [15].Brand S. Medical therapy of ulcerative colitis. MMW Fortschr Med 2015;157:45–8. [DOI] [PubMed] [Google Scholar]
- [16].Roose L, D’cunja J, Biedermann L. Ulcerative colitis. Praxis (Bern 1994) 2016;105:607–15. [DOI] [PubMed] [Google Scholar]
- [17].Palumbo VD, Romeo M, Marino Gammazza A, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: a clinical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2016;160:372–7. [DOI] [PubMed] [Google Scholar]
- [18].Uygun A, Ozturk K, Demirci H, et al. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine (Baltimore) 2017;96:e6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ji J, Huang Y, Wang XF, et al. Review of clinical studies of the treatment of ulcerative colitis using acupuncture and moxibustion. Gastroenterol Res Pract 2016;2016:9248589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee DH, Kim JI, Lee MS, et al. Moxibustion for ulcerative colitis: a systematic review and meta-analysis. BMC Gastroenterol 2010;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang C, Jiang M, Lu A. Considerations of traditional Chinese medicine as adjunct therapy in the management of ulcerative colitis. Clin Rev Allergy Immunol 2013;44:274–83. [DOI] [PubMed] [Google Scholar]
- [22].Samsami-Kor M, Daryani NE, Asl PR, et al. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res 2015;46:280–5. [DOI] [PubMed] [Google Scholar]
- [23].Samsamikor M, Daryani NE, Asl PR, et al. Resveratrol supplementation and oxidative/anti-oxidative status in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res 2016;47:304–9. [DOI] [PubMed] [Google Scholar]
- [24].Laurell A, Sjöberg K. Prebiotics and synbiotics in ulcerative colitis. Scand J Gastroenterol 2017;52:477–85. [DOI] [PubMed] [Google Scholar]
- [25].Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


