Supplemental Digital Content is available in the text
Keywords: GLP-1, liraglutide, type 2 diabetes
Abstract
Background:
Liraglutide is a novel, long-acting glucagon-like peptide-1 (GLP-1) analogue used to treat type 2 diabetes mellitus. However, the cardiovascular safety and benefits of liraglutide treatment on type 2 diabetes patients remain in debate. In this study, we aimed to examine the overall cardiovascular outcomes of liraglutide in patients with type 2 diabetes.
Methods:
In this systematic review and meta-analysis, we searched the PubMed, Embase, and Web of Knowledge databases up to September 1st, 2017 for randomized trials in which type 2 diabetes patients were assigned to liraglutide and placebo or other comparators groups.
Results:
Eight studies fulfilled the eligibility criteria for inclusion and 14,608 patients were analyzed in this systematic review and meta-analysis. We found patients in the liraglutide group had a lower risk of major cardiovascular events (MACE) (RR = 0.89, 95% CI: 0.82–0.96, P = .002), acute myocardial infarction (AMI) (RR = 0.85, 95% CI: 0.74–0.99, P = .036), all-cause death (RR = 0.84, 95% CI: 0.74–0.96, P = .009), and cardiovascular death (RR = 0.77, 95% CI: 0.65–0.91, P = .002) than all comparator groups. However, liraglutide treatment did not decrease incidence of stroke (RR = 0.86, 95% CI: 0.70–1.04, P = .124). But among the MACE subgroups analysis, a significant reduction of MACE with liraglutide was only observed in placebo-controlled trials (RR = 0.89, 95% CI: 0.83–0.96, P = .004) but not in studies concerning other comparators (RR = 0.58, 95% CI: 0.29–1.16, P = .122).
Conclusions:
In conclusion, our results suggest that liraglutide treatment decreases the risk of MACE, AMI, all-cause death and cardiovascular death among patients with type 2 diabetes.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a chronic and progressive disease which characterized by beta cell function decline and insulin resistance, which is often associated with both microvascular and macrovascular complications.[1] As previously reported, patients with diabetes have a three-fold increase in cardiovascular mortality and a two-fold increase in all-cause mortality compared to those without diabetes.[2,3] However, among the currently available glucose lowering agents’ therapies, parts of therapies were suspected of adverse cardiovascular effects, although it has not been confirmed yet.[4,5] Thus, the US Food and Drug Administration (FDA) announced an updated Guidance for industry which requires any new diabetes drug must be proved that it shows no substantial increase in cardiovascular risk. For regulatory requirements from the FDA, a huge collection of data from randomized trials must be conducted to evaluate the effects of a diabetes drug on the cardiovascular risk of diabetes patients.
Glucagon-like peptide-1 (GLP-1) is an essential incretin hormone which shows trophic effects on the beta cells. GLP-1 could promote insulin biosynthesis and insulin gene expression which make GLP-1 as a potent blood-glucose-lowering agent, thus GLP-1 may modify the natural history of T2DM.[6] Liraglutide is a novel, long-acting GLP-1 analogue drug which is a recombinant once-daily human GLP-1 analog with 97% amino acid sequence identity to endogenous human GLP-1. The efficacy of liraglutide on lowering glucose levels was confirmed, and many previous clinical trials have demonstrated that liraglutide is associated with slight reductions in weight and blood pressure.[7–9] Recently, a large number of studies were conducted to evaluate the cardiovascular safety and efficacy of liraglutide for patients with T2DM. Thus, we conducted a system review and meta-analysis of the cardiovascular safety and efficacy of liraglutide as stipulated by the FDA recommendations for the evaluation of new treatments for diabetes.
2. Methods
This meta-analysis and systematic review based on a predefined protocol following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. All analyses in this study were on the basis of previously reported studies, thus, ethical approval and patient consents were not required.
2.1. Search strategy and selection criteria
We searched Web of Knowledge, PubMed, and Embase databases through September 1st, 2017. Key words were identified as follows: “type 2 diabetes” AND “liraglutide” AND “cardiovascular”. The literature search, data extraction, and quality assessments were conducted independently by 2 authors (T.-F.W., C.-M.D.). We also searched references cited in all included articles to avoid missing other relevant articles. If the effective data were not included in the original articles, we contacted the authors to get them. The studies were screened and evaluated by 2 authors (T.-F.W., C.-M.D.) independently for eligibility. The inclusion criteria are as follows:
-
1.
all randomized clinical trials to assess the efficacy and safety of liraglutide in the treatment of T2DM were considered eligible for analysis;
-
2.
adult T2DM patients with a duration of at least 12 weeks (with a glycated hemoglobin level of 7.0% or more);
-
3.
comparing liraglutide with another antidiabetic therapy or placebo, the cases with liraglutide treatment and controls with another antidiabetic therapy or placebo;
-
4.
reporting the available data on cardiovascular events. Studies with overlapping data or insufficient data to calculate or extract effect estimates were excluded.
Besides, the trials enrolling nondiabetic, or type 1 diabetic were also excluded.
2.2. Data extraction and outcomes
Two investigators (T.-F.W., C.-M.D.) managed data extraction independently to ensure the reliability of the results. Disagreement was resolved by consensus, and if necessary, consultation with a third reviewer (Y.W.). The information of studies meeting the inclusion criteria were extracted using a standardized tool. Relevant information included the first author's name, publication year, study country, intervention, the numbers of cases and controls, baseline patient characteristics (age, sex, race, diabetes duration, HbA1c level, body weight index), cardiovascular events, and the Jadad Score.
The primary outcome of this analysis was the effect of liraglutide, compared with either another antidiabetic therapy or placebo, on the incidence of major cardiovascular events (MACE), including cardiovascular death, nonfatal acute myocardial infarction (AMI) and stroke, and acute coronary syndromes and/or heart failure. Secondary outcomes included AMI, stroke, all-cause and cardiovascular mortality.
2.3. Assessment of study quality and statistical analysis
Two independent investigators (T.F.W., C.M.D.) evaluated the quality of the studies according to the Jadad Scale,[10] which is used for assessing the quality of case-control studies and contains 3 parts: randomization (0–2 points), double-blind (0–2 points), and withdrawals (0–1 point). The Jadad Scale is a validated 5-point scale to assess the following 5 criteria:
-
1.
whether the study was randomized (0–1 point);
-
2.
whether randomization was described appropriately (0–1 point);
-
3.
whether the study was double-blind (0–1 point);
-
4.
whether the double-blinding was described appropriately (0–1 point); and
-
5.
whether the dropouts and withdrawals were described (0–1 point). If the study meets a criterion, it would get 1 point.
The quality score ranges from 0 to 5 points. If a study got less than 3 points, it is defined as a low-quality report study. While a high quality study score is at least 3 points.
For each study, risk ratios (RRs) and their corresponding 95% confidence intervals (CIs) were derived from patient numbers with each outcome categorized by liraglutide treatment. All statistical analyses were conducted using STATA, Version 12.0 software (StataCorp, College Station, TX, USA). The statistical significance of pooled RRs and 95% CIs was performed by a Z test. The Chi-Squared-based Q-tests and I-squared (I2) statistic were used to evaluate the heterogeneity across studies.[11] Besides, as previously reported, the effects model we used must refer to our heterogeneity test. The selection criterions were as follows: if the P value of the Q test was more than .1 and I2 values was less than 50%, which may suggest no obvious heterogeneity across studies. Then Mantel-Haenszel fixed-effects model was applied;[12] otherwise, DerSimonian-Laird random-effects model was used.[13] In addition, to explore the source of between-study heterogeneity, the Galbraith plots were used. Further, we also conducted sensitivity analysis by removing each included study and then assess the stability of our results. Publication bias was quantified by the Egger regression[14] and Begg[15] methods, and showed by funnel plots. Statistical significance was defined as a P-value < .05.
3. Results
3.1. Characteristics of eligible studies
The search strategy identified 1909 records by different search strategies in PubMed, Web of Science, and Embase. After removing 463 duplications and 1420 unrelated records, 26 records were needed to further screen through full-text reading. Among the residual records, 17 records were excluded (6 records with insufficient data, 7 reviews, and 4 comments, Supplemental Table 1). Besides, there was 1 record[16] which was excluded for having overlapping data with other included records.[17] Finally, 8 studies fulfilled the eligibility criteria for inclusion in the primary analysis.[17–24] The study selection diagram is shown in Figure 1.
Figure 1.
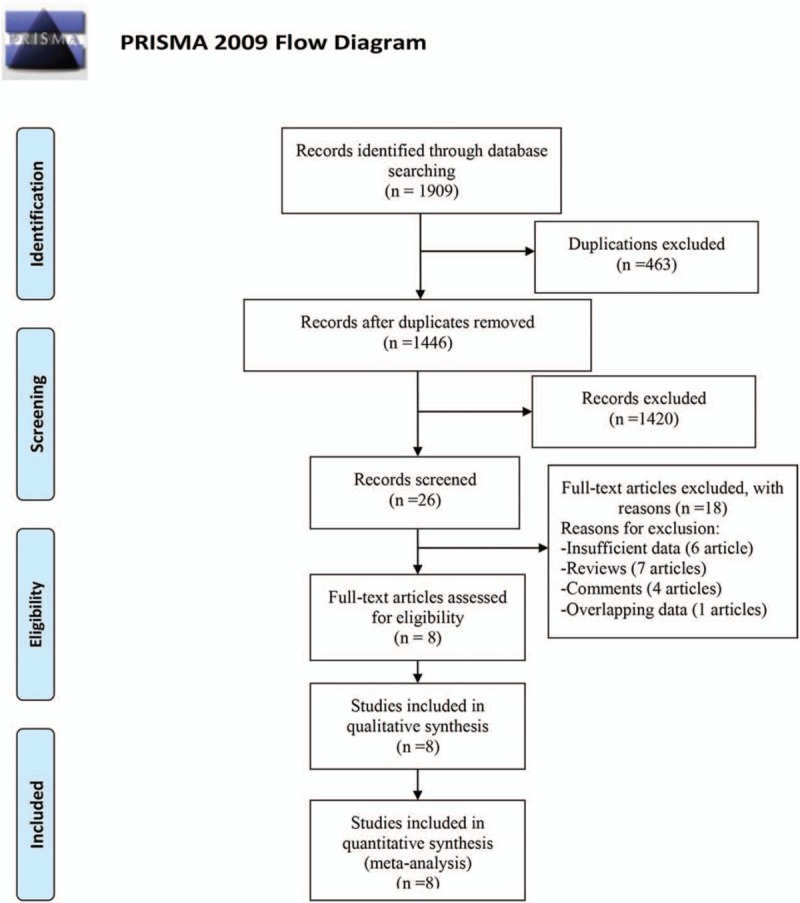
Flow diagram of study identification.
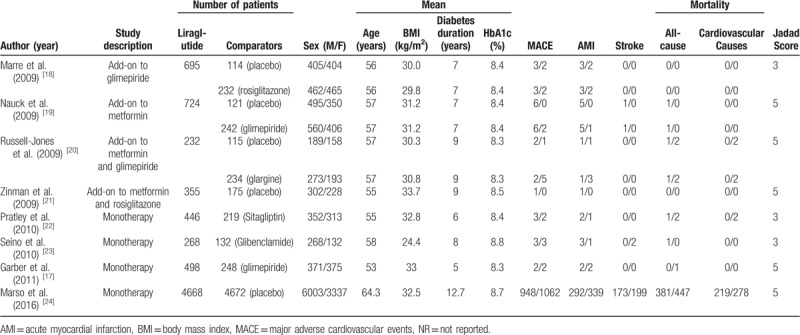
The characteristics of the included studies are listed in Table 1. All of the included studies were published during 2009 to 2016. Information on major cardiovascular events (MACE) and mortality were reported in all the trials. And only 8 studies were involved in the analysis of acute myocardial infarction (AMI) and stroke events.
Table 1.
Characteristics of the studies included in the meta-analysis.
3.2. Cardiovascular events and morbidity
Among all included studies, 14,608 patients in total were included. Of the 8 studies with available information on MACE and reporting at least 1 event were therefore included in the main analysis. The results of our meta-analysis suggested that the incidence of MACE differ significantly between the liraglutide group and the all-comparator groups (RR = 0.89, 95% CI: 0.82–0.96, P = .002) (Fig. 2A, Table 2). Moreover, we found few numerical differences for the other adjudicated endpoints with liraglutide versus all comparator, including AMI (RR = 0.85, 95% CI: 0.74–0.99, P = .036) (Fig. 2B, Table 2), all-cause death (RR = 0.84, 95% CI: 0.74–0.96, P = .009) (Fig. 2C, Table 2) and cardiovascular death (RR = 0.77, 95% CI: 0.65–0.91, P = .002) (Fig. 2D, Table 2). In contrast, among T2DM patients, liraglutide treatment did not decrease incidence of stroke (RR = 0.86, 95% CI: 0.70–1.04, P = .124) (Fig. 3A, Table 2). As subgroups analysis, a significant reduction of MACE with liraglutide was observed in placebo-controlled trials (RR = 0.89, 95% CI: 0.83–0.96, P = .004) (Fig. 3B, Table 2) but not in studies concerning other comparators (RR = 0.58, 95% CI: 0.29–1.16, P = .122) (Fig. 3C, Table 2).
Figure 2.
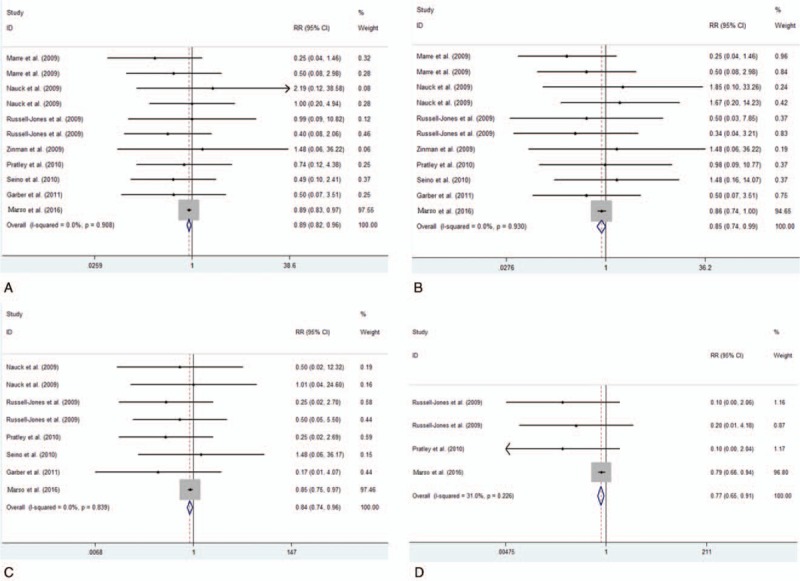
Frequency and incidence ratios for MACE (A), AMI (B), all-cause death (C) and cardiovascular death (D) in liraglutide vs. total comparator.
Table 2.
Summary of meta-analysis results.
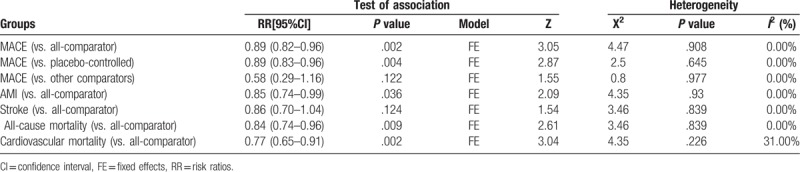
Figure 3.
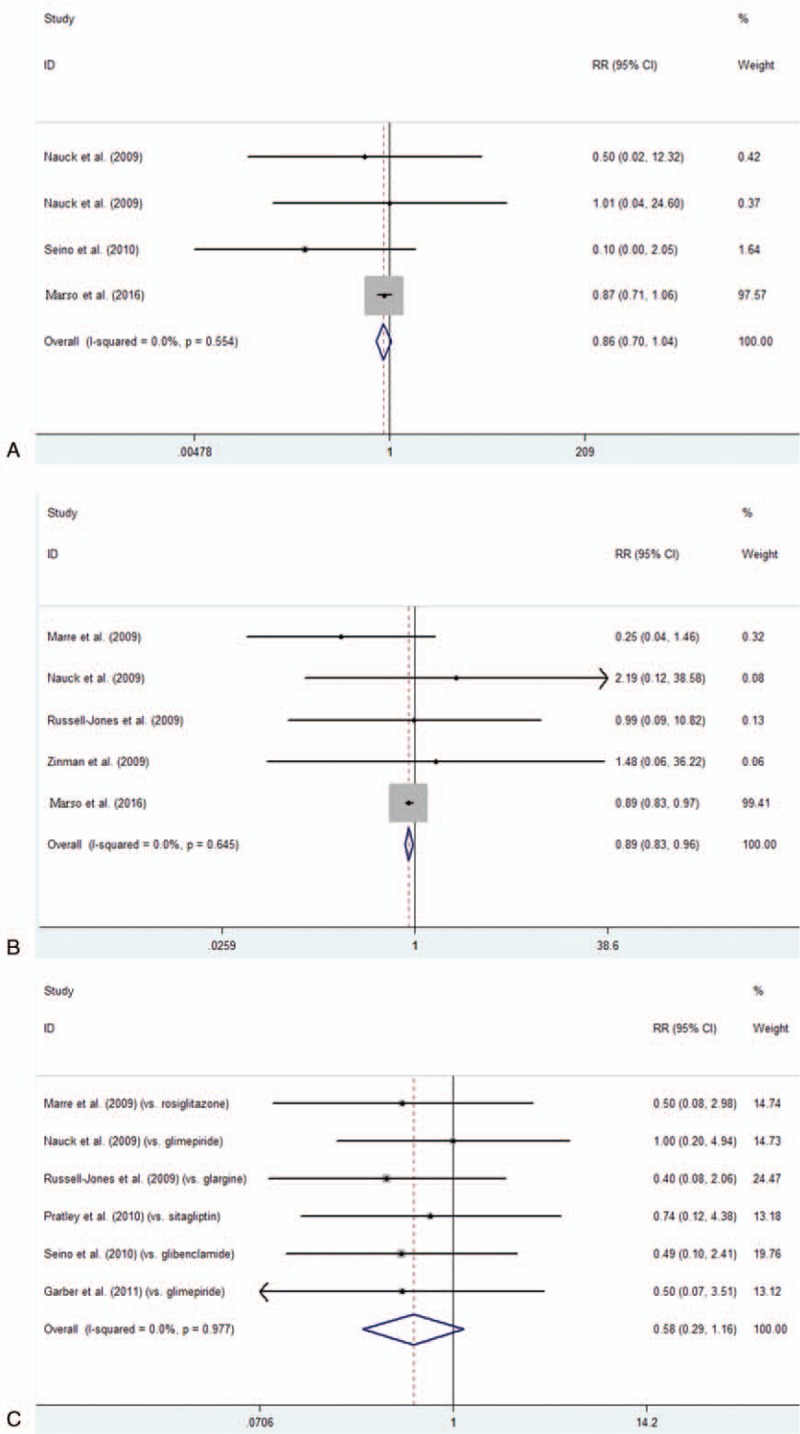
Frequency and incidence ratios for stroke in liraglutide vs. total comparator (A), and subgroup analysis the frequency and incidence ratios for MACE in liraglutide vs. placebo (B) and in liraglutide vs. other comparators (C).
3.3. Heterogeneity analysis
To investigate the heterogeneity across studies, the I-squared (I2) statistic and Chi-Squared-based Q tests were performed. Importantly, all the I2 values in this study was less than 50% and P > .10 for the Q test, which suggested that there is no obvious heterogeneity across studies, as shown in Table 2.
3.4. Sensitivity analysis and publication bias
Each study was removed sequentially to verify the effect of each individual study on our results. No obvious changes were found after excluding any study. Therefore, our results were reliable (data not shown). Both Egger and Begg methods were applied to explore the publication bias in our meta-analysis. There was no significant publication bias among the included studies (Begg's test: P = .392). The funnel plots are shown in Supplemental Figure 1.
4. Discussion
In the present meta-analysis including data from 14,608 patients, we found that patients in the liraglutide group had a lower risk of MACE, AMI, all-cause death and cardiovascular death than all-comparator groups. In contrast, for T2DM patients, liraglutide treatment did not decrease incidence of stroke. However, among the MACE subgroup analysis, a significant reduction of MACE with liraglutide treatment was only observed in placebo-controlled trials but not in studies versus other comparators. For the safety of drugs for type 2 diabetes, the FDA requires a formal demonstration of the absence of any risk or MACE, with an upper confidence limit of 1.30. This meta-analysis found the incidence ratio for MACE was <1.0 compared with total comparator and the upper 95% CI (CI: confidence interval) was also <1.0. Results from this study may provide reliable information on cardiovascular safety and efficacy of liraglutide for T2DM patients.
Liraglutide as a type of GLP-1 analogue drugs, was recommended once-daily injection for T2DM patients. Interestingly, liraglutide has been used in clinical practice before the FDA guidance was made. The major finding in our study was reporting CV outcomes in T2DM for liraglutide only (not as a class effect). However, previous meta-analysis mainly focused on evaluating the cardiovascular safety of all the GLP-1 receptor agonists (GLP-1 RA) class drugs. A meta-analysis found the difference in the incidence of MACE between GLP-1 RA and comparators did not reach statistical significance and the incidence radio was 0.78 (95% CI 0.54–1.13).[25] This study only confirmed the cardiovascular safety of all the GLP-1 RA class drugs, but did not find a beneficial effect on the incidence of MACE and mortality compared with all comparators. This disparity may be resulted from which in some of the trials included, cardiovascular events were reported only as adverse events, without being prospectively adjudicated. Another study also only found no detrimental effect of GLP-1 RA on cardiovascular events, but did not find any significant benefits in regard to rates of cardiovascular events or death.[26] Compared to those studies, our meta-analysis mainly focuses on the liraglutide but not all GLP-1 RA drugs. Importantly, we revealed liraglutide treatment can reduce the cardiovascular events and death, but previous meta-analysis just found the cardiovascular safety of liraglutide.[27] Therefore, the meta-analysis of clinical trials at different endpoints not only provides reliable safety information, but also provides information on efficacy.
In fact, our study found that the treatment of liraglutide was associated with a reduction of MACE risk. However, this effect disappeared when the placebo-comparator trials were excluded. This may attribute to the sample size of all included trials which compared the MACE risk between liraglutide and other comparative drugs were too small. In addition, as far as the existing knowledge is concerned, MACE risk was correlated with HbA1c level for T2DM patients.[28] And previous studies found that GLP-1 RA shows no significant difference in regard to reducing HbA1c levels compared with insulin glargine and lixisenatide.[29,30] Overall, those evidences may explain why liraglutide treatment shows no significant difference compared with other comparative drugs in reducing risk of MACE for T2DM patients. In order to confirm this finding, more large-scale, long-term and well-conducted randomized controlled trials are needed.
Furthermore, it is worth noting that the endpoints in this study provides reliable information on efficacy of liraglutide treatment compared with placebo. Speculatively, some mechanisms may account for the favorable effects of liraglutide on cardiovascular risk. It is well known that metabolic risk factors were closely linked with the morbidity and mortality in T2DM patients, and controlling the metabolic risk factors effectively could decrease the morbidity and mortality.[31] As previously reported, GLP-1 RA could reduce some of the metabolic risk factors effectively, including HbA1c levels, bodyweight and blood pressure.[32] And a randomized, placebo-controlled, double-blind, crossover trial has found liraglutide treatment could decrease the cardiovascular risk biomarkers significantly, including TNF-α (tumor necrosis factor α) and MR-proADM (mid-regional pro-adrenomedullin), while the decrease in MR-proANP levels effectively show a clinically related benefit for heart failure.[33] In contrast, recent study found albiglutide, a type of GLP-1 RA, shows no decrease effects on the MACE risk for T2DM patients.[34] This conflict may attribute to the greater statistical power in our study than previous. Moreover, other factors may also have been involved. For example, the different effects of glucagon-like peptide-1 receptor agonists on heart rate, as an increase in heart rate is considered to be associated with higher cardiovascular risk.[35,36] Thus, the details of factors which contributed to those differences among albiglutide and liraglutide should be explored in the future studies.
To our knowledge, we must admit there were several limitations in our study. Firstly, the diagnosis standard for incident cardiovascular disease were not normalized among the studies included. This would cause misdiagnosis and underdiagnosis. Secondly, some of included studies were intended to evaluate the effects of liraglutide on glycaemic control and the safety of liraglutide treatment for T2DM patients, but not designed for the assessment of cardiovascular outcomes. Thus, more large-scale, well-conducted RCTs are required.
In conclusion, available data from clinical trials confirm that liraglutide treatment could decrease the risk of MACE, AMI, all-cause death and cardiovascular death among patients with type 2 diabetes. However, the mechanisms of action of liraglutide on the cardiovascular outcomes in patients with T2DM are needed to confirm in the future studies.
Acknowledgments
We thank Dr. Liu Miao for helping us to proofread this manuscript.
Author contributions
Q.W.Y. conceived the study. T.F.W. and C.M.D. collected the data and drafted the manuscript. Y.W. revised the manuscript and language.
Supplementary Material
Footnotes
Abbreviations: AMI = acute myocardial infarction, CIs = confidence intervals, FDA = Food and Drug Administration, GLP-1 = Glucagon-like peptide-1, MACE = major cardiovascular events, MR-proADM = mid-regional pro-adrenomedullin, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR = risk ratio, T2DM = type 2 diabetes mellitus, TNF-α = tumor necrosis factor α.
How to cite this article: Duan CM, Wan TF, Wang Y, Yang QW. Cardiovascular outcomes of liraglutide in patients with type 2 diabetes. Medicine. 2019;98:46(e17860).
C-MD and T-FW contributed equally to this work.
This work was supported by grants from the National Natural Science Fund for Distinguished Young Scholars (81525008).
The authors have no conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Meigs JB. Epidemiology of cardiovascular complications in type 2 diabetes mellitus. Acta Diabetol 2003;40Suppl 2:S358–361. [DOI] [PubMed] [Google Scholar]
- [2].Preis SR, Hwang SJ, Coady S, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation 2009;119:1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Taylor KS, Heneghan CJ, Farmer AJ, et al. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large U.K. primary care database. Diabetes Care 2013;36:2366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71. [DOI] [PubMed] [Google Scholar]
- [5].Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007;370:1129–36. [DOI] [PubMed] [Google Scholar]
- [6].Lovshin JA. Glucagon-like peptide-1 receptor agonists: A class update for treating type 2 diabetes. Can J Diabetes 2017;10.1016/j.jcjd.2017.02.003. [DOI] [PubMed] [Google Scholar]
- [7].Du Q, Wang YJ, Yang S, et al. Liraglutide for the treatment of type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials. Adv Ther 2014;31:1182–95. [DOI] [PubMed] [Google Scholar]
- [8].Robinson LE, Holt TA, Rees K, et al. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 2013;3:e001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iepsen EW, Torekov SS, Holst JJ. Liraglutide for Type 2 diabetes and obesity: a 2015 update. Expert Rev Cardiovasc Ther 2015;13:753–67. [DOI] [PubMed] [Google Scholar]
- [10].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [11].Higgins J, Thompson S, Deeks J, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [13].Borenstein M, Higgins J. Meta-analysis and subgroups. Prev Sci 2013;14:134–43. [DOI] [PubMed] [Google Scholar]
- [14].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [16].Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473–81. [DOI] [PubMed] [Google Scholar]
- [17].Garber A, Henry RR, Ratner R, et al. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab 2011;13:348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 2009;26:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009;52:2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes care 2009;32:1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 2010;375:1447–56. [DOI] [PubMed] [Google Scholar]
- [23].Seino Y, Rasmussen MF, Nishida T, et al. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin 2010;26:1013–22. [DOI] [PubMed] [Google Scholar]
- [24].Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Monami M, Dicembrini I, Nardini C, et al. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 2014;16:38–47. [DOI] [PubMed] [Google Scholar]
- [26].Monami M, Cremasco F, Lamanna C, et al. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials. Exp Diabetes Res 2011;2011:215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marso SP, Lindsey JB, Stolker JM, et al. Cardiovascular safety of liraglutide assessed in a patient-level pooled analysis of phase 2: 3 liraglutide clinical development studies. Diabetes Vasc Dis Res 2011;8:237–40. [DOI] [PubMed] [Google Scholar]
- [28].Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–31. [DOI] [PubMed] [Google Scholar]
- [29].Li WX, Gou JF, Tian JH, et al. Glucagon-like peptide-1 receptor agonists versus insulin glargine for type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Curr Ther Res Clin Exp 2010;71:211–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017;19:524–36. [DOI] [PubMed] [Google Scholar]
- [31].Yakoob MY, Micha R, Khatibzadeh S, et al. Impact of dietary and metabolic risk factors on cardiovascular and diabetes mortality in south asia: analysis from the 2010 Global Burden of Disease Study. Am J Public Health 2016;106:2113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sivertsen J, Rosenmeier J, Holst JJ, et al. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol 2012;9:209–22. [DOI] [PubMed] [Google Scholar]
- [33].von Scholten BJ, Persson F, Rosenlund S, et al. Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: A sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes Obes Metab 2017;10.1111/dom.12884. [DOI] [PubMed] [Google Scholar]
- [34].Fisher M, Petrie MC, Ambery PD, et al. Cardiovascular safety of albiglutide in the Harmony programme: a meta-analysis. Lancet Diabetes Endocrinol 2015;3:697–703. [DOI] [PubMed] [Google Scholar]
- [35].Cooney MT, Vartiainen E, Laatikainen T, et al. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J 2010;159:612–9. e613. [DOI] [PubMed] [Google Scholar]
- [36].Perret-Guillaume C, Joly L, Benetos A. Heart rate as a risk factor for cardiovascular disease. Prog Cardiovasc Dis 2009;52:6–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


