Abstract
Background
Endometriosis is known to have an impact on fertility and it is common for women affected by endometriosis to require fertility treatments, including in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI), to improve the chance of pregnancy. It has been postulated that long‐term gonadotrophin‐releasing hormone (GnRH) agonist therapy prior to IVF or ICSI can improve pregnancy outcomes. This systematic review supersedes the previous Cochrane Review on this topic (Sallam 2006).
Objectives
To determine the effectiveness and safety of long‐term gonadotrophin‐releasing hormone (GnRH) agonist therapy (minimum 3 months) versus no pretreatment or other pretreatment modalities, such as long‐term continuous combined oral contraception (COC) or surgical therapy of endometrioma, before standard in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) in women with endometriosis.
Search methods
We searched the following electronic databases from their inception to 8 January 2019: Cochrane Gynaecology and Fertility Specialised Register of Controlled Trials, CENTRAL via the Cochrane CENTRAL Register of Studies ONLINE (CRSO), MEDLINE, Embase, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL). We searched trial registries to identify unpublished and ongoing trials. We also searched DARE (Database of Abstracts of Reviews of Effects), Web of Knowledge, OpenGrey, Latin American and Caribbean Health Science Information Database (LILACS), PubMed, Google and reference lists from relevant papers for any other relevant trials.
Selection criteria
Randomised controlled trials (RCTs) involving women with surgically diagnosed endometriosis that compared use of any type of GnRH agonist for at least three months before an IVF/ICSI protocol to no pretreatment or other pretreatment modalities, specifically use of long‐term continuous COC (minimum of 6 weeks) or surgical excision of endometrioma within six months prior to standard IVF/ICSI. The primary outcomes were live birth rate and complication rate per woman randomised.
Data collection and analysis
Two independent review authors assessed studies against the inclusion criteria, extracted data and assessed risk of bias. A third review author was consulted, if required. We contacted the study authors, as required. We analysed dichotomous outcomes using Mantel‐Haenszel risk ratios (RRs), 95% confidence intervals (CIs) and a fixed‐effect model. For small numbers of events, we used a Peto odds ratio (OR) with 95% CI instead. We analysed continuous outcomes using the mean difference (MD) between groups and presented with 95% CIs. We studied heterogeneity of the studies via the I2 statistic. We assessed the quality of evidence using GRADE criteria.
Main results
We included eight parallel‐design RCTs, involving a total of 640 participants. We did not assess any of the studies as being at low risk of bias across all domains, with the main limitation being lack of blinding. Using GRADE methodology, the quality of the evidence ranged from very low to low quality.
Long‐term GnRH agonist therapy versus no pretreatment
We are uncertain whether long‐term GnRH agonist therapy affects the live birth rate (RR 0.48, 95% CI 0.26 to 0.87; 1 RCT, n = 147; I2 not calculable; very low‐quality evidence) or the overall complication rate (Peto OR 1.23, 95% CI 0.37; to 4.14; 3 RCTs, n = 318; I2 = 73%; very low‐quality evidence) compared to standard IVF/ICSI. Further, we are uncertain whether this intervention affects the clinical pregnancy rate (RR 1.13, 95% CI 0.91 to 1.41; 6 RCTs, n = 552, I2 = 66%; very low‐quality evidence), multiple pregnancy rate (Peto OR 0.14, 95% CI 0.03 to 0.56; 2 RCTs, n = 208, I2 = 0%; very low‐quality evidence), miscarriage rate (Peto OR 0.45, 95% CI 0.10 to 2.00; 2 RCTs, n = 208; I2 = 0%; very low‐quality evidence), mean number of oocytes (MD 0.72, 95% CI 0.06 to 1.38; 4 RCTs, n = 385; I2 = 81%; very low‐quality evidence) or mean number of embryos (MD ‐0.76, 95% CI ‐1.33 to ‐0.19; 2 RCTs, n = 267; I2 = 0%; very low‐quality evidence).
Long‐term GnRH agonist therapy versus long‐term continuous COC
No studies reported on this comparison.
Long‐term GnRH agonist therapy versus surgical therapy of endometrioma
No studies reported on this comparison.
Authors' conclusions
This review raises important questions regarding the merit of long‐term GnRH agonist therapy compared to no pretreatment prior to standard IVF/ICSI in women with endometriosis. Contrary to previous findings, we are uncertain as to whether long‐term GnRH agonist therapy impacts on the live birth rate or indeed the complication rate compared to standard IVF/ICSI. Further, we are uncertain whether this intervention impacts on the clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, mean number of oocytes and mean number of embryos. In light of the paucity and very low quality of existing data, particularly for the primary outcomes examined, further high‐quality trials are required to definitively determine the impact of long‐term GnRH agonist therapy on IVF/ICSI outcomes, not only compared to no pretreatment, but also compared to other proposed alternatives to endometriosis management.
Plain language summary
Long‐term pituitary down‐regulation before in vitro fertilisation (IVF) for women with endometriosis
Review question
We reviewed the efficacy and safety of treating women with known endometriosis (a disease characterised by the presence of endometrial tissue outside the cavity of the womb) with gonadotrophin‐releasing hormone (GnRH) agonist medication for a period of three to six months prior to in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI). We aimed to compare this intervention to no treatment prior to IVF/ICSI, pretreatment with long‐term continuous combined oral contraception (COC) or surgical treatment to remove endometriomas (cysts forming in the ovaries as a consequence of endometriosis).
Background
Many women affected by endometriosis suffer with infertility and may, as a result, seek IVF/ICSI treatment. IVF/ICSI is known to be less successful in women with endometriosis and a variety of interventions prior to IVF/ICSI have been proposed to try and improve outcomes. These include long‐term GnRH agonist therapy, long‐term continuous COC therapy or surgery to remove endometriomas.
Study characteristics
We found eight randomised controlled trials comparing long‐term GnRH agonist therapy with no pretreatment including a total of 640 women with endometriosis prior to IVF/ICSI. The evidence is current to January 2019.
Key results
Compared to no pretreatment, we are uncertain whether long‐term GnRH agonist therapy prior to IVF/ICSI in women with endometriosis affects the live birth rate. The evidence suggests that if the chance of live birth rate is assumed to be 36% with no pretreatment, the chance following long‐term GnRH agonist therapy would be between 9% and 31%. We are also uncertain whether this intervention affects complication rate, clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, mean number of oocytes and mean number of embryos. No studies compared long‐term GnRH agonist therapy to long‐term continuous COC therapy or surgery to remove endometriomas.
Quality of the evidence
The evidence was of very low quality. The main limitations in the evidence were lack of blinding (the process where the women participating in the trial, as well as the research staff, are not aware of the intervention used), inconsistency (differences between different studies) and imprecision (random error and small size of each study).
Summary of findings
Summary of findings for the main comparison. Long‐term GnRH agonist therapy compared to no additional therapy in patients with endometriosis prior to standard in vitro fertilisation/intracytoplasmic sperm injection (IVF/ICSI).
| Long‐term GnRH agonist therapy compared to no additional therapy in patients with endometriosis prior to standard IVF/ICSI | ||||||
| Patient or population: patients with endometriosis prior to standard IVF/ICSI Setting: assisted reproduction clinic Intervention: long‐term GnRH agonist therapy Comparison: no additional therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no additional therapy | Risk with long‐term GnRH agonist therapy | |||||
| Live birth rate | Study population | RR 0.48 (0.26 to 0.87) | 147 (1 RCT) | ⊕⊝⊝⊝ Very low a,b | The evidence is very uncertain about the effect of long‐term GnRH agonist therapy on live birth rate. | |
| 355 per 1,000 | 171 per 1000 (92 to 309) | |||||
| Complication rate | Study population | Peto OR 1.23 (0.37 to 4.14) | 318 (3 RCTs) | ⊕⊝⊝⊝ Very low c,d,e | The evidence is very uncertain about the effect of long‐term GnRH agonist therapy on complication rate. | |
| 31 per 1,000 | 38 per 1000 (11 to 128) | |||||
| Clinical pregnancy rate | Study population | RR 1.13 (0.91 to 1.41) | 552 (6 RCTs) | ⊕⊝⊝⊝ Very low c,d,e | The evidence is very uncertain about the effect of long‐term GnRH agonist therapy on clinical pregnancy rate. | |
| 331 per 1,000 | 374 per 1000 (301 to 467) | |||||
| Multiple pregnancy rate | Study population | Peto OR 0.14 (0.04 to 0.56) | 208 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,f | The evidence is very uncertain about the effect of long‐term GnRH agonist therapy on multiple pregnancy rate. | |
| 74 per 1,000 | 10 per 1000 (3 to 41) | |||||
| Miscarriage rate | Study population | Peto OR 0.45 (0.10 to 2.00) | 208 (2 RCTs) | ⊕⊝⊝⊝ Very low b,f | The evidence is very uncertain about the effect of long‐term GnRH agonist therapy on miscarriage rate. | |
| 46 per 1,000 | 21 per 1000 (5 to 93) | |||||
| Mean number of oocytes | The mean number of oocytes was 8.43 | MD 0.72 higher (0.06 higher to 1.38 higher) | ‐ | 385 (4 RCTs) | ⊕⊝⊝⊝ Very low c,g | The evidence is very uncertain about the effect of long‐term GnRH agonist therapy on mean number of oocytes. |
| Mean number of embryos | The mean number of embryos was 4.74 | MD 0.76 lower (1.33 lower to 0.19 lower) | ‐ | 267 (2 RCTs) | ⊕⊝⊝⊝ Very low c,e,h | The evidence is very uncertain about the effect of long‐term GnRH agonist therapy on mean number of embryos. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GnRH: gonadotrophin‐releasing hormone; IVF/ICSI: in vitro fertilisation/intracytoplasmic sperm injection; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a data arising exclusively from an unpublished trial; downgraded one level.
b small number of events; downgraded two levels.
c incorporates at least one open‐label study; downgraded one level.
d high degree of heterogeneity; downgraded one level.
e wide confidence intervals; downgraded one level.
f data arising from an unpublished trial and a conference abstract; downgraded one level.
g very high degree of heterogeneity; downgraded two levels.
h significant contribution by an unpublished trial; downgraded one level.
Background
Description of the condition
Endometriosis is a challenging disease observed in 16% to 50% of infertile women (Hart 2003; Meuleman 2009; Prescott 2016; Zondervan 2018). A cause and effect relationship between endometriosis and infertility has not been established, but it has been postulated that a combination of distorted pelvic anatomy, altered peritoneal function, alteration of immunological milieu within the peritoneal cavity, diminished ovarian reserve and altered endometrial receptivity could be the cause of infertility (ASRM 2006; Giudice 2010). Women with endometriosis often require in vitro fertilisation (IVF) to improve the chance of pregnancy, and more than one‐third of women undergoing IVF have endometriosis (Ozkan 2008). The benefits of in vitro fertilisation‐embryo transfer (IVF‐ET) in endometriosis‐associated infertility are well established (Barnhart 2002; Olivennes 2003; Opoein 2012). A meta‐analysis of 22 non‐randomised studies on the effect of endometriosis on IVF outcomes concluded that endometriosis interferes with all aspects of the reproductive process and is associated with lower success rates than other indications for IVF (Barnhart 2002). The overall chance of achieving pregnancy with IVF in these 22 studies was 25%. Nevertheless, a fairly recent large retrospective cohort study of 2245 women reported comparable pregnancy rates and live birth rates of IVF/intracytoplasmic sperm injection (ICSI) treatment in women with endometriosis‐related infertility as in women with tubal factors, except for those with ovarian endometrioma (Opoein 2012).
The severity of endometriosis has been shown to have a direct impact on the outcomes of IVF/ICSI treatment (Azem 1999; Coccia 2011; Olivennes 2003). A recent meta‐analysis of 27 non‐randomised studies reported a slight reduction in fertilisation rates among women with ASRM (American Society of Reproductive Medicine) stage I/II endometriosis undergoing IVF/ICSI treatment (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.87 to 0.99, P = 0.03; (Harb 2013). In women with severe pelvic endometriosis and stage III/IV disease, implantation rates were significantly reduced (RR 0.79, 95% CI 0.67 to 0.93, P = 0.006), as were clinical pregnancy rates (RR 0.76, 95% CI 0.69 to 0.91, P = 0.0008). In stage I/II disease, a hostile pelvic environment due to alteration of immunological milieu within the peritoneal cavity may be responsible for impaired gamete and early embryo development (Ryan 1997; Surrey 1998). On the other hand, in stage III/IV endometriosis, lower oocyte yield and poor oocyte quality (Norenstedt 2001; Pal 1998), resulting in embryos of lesser quality have been reported to be responsible for lower pregnancy and implantation rates post‐IVF/ICSI (Arici 1996; Barnhart 2002).
Description of the intervention
The surgical management of asymptomatic ovarian endometrioma in women before IVF is debatable. A systematic review and meta‐analysis of 21 cohort studies (Raffi 2012), and recent non‐randomised studies (Tang 2013; Urman 2013), reported a negative impact of excision of endometriomas on ovarian reserve. Another systematic review of 20 non‐randomised studies (Tsoumpou 2009), concluded that excision of endometriomas has no significant effect on IVF pregnancy rates and ovarian response to stimulation compared with no treatment, nor does it improve fertility outcomes (Garcia‐Velasco 2004; Geber 2002; Suzuki 2005). On the other hand, non‐randomised studies have reported that resection of mild endometriosis and/or restoration of the pelvic anatomy (Harkki 2010; Marconi 2002), especially if done laparoscopically, may enhance the efficacy of assisted reproductive techniques, but that aggressive ovarian surgery should be avoided. Because of the negative effect of surgical management of endometriosis on the ovarian reserve, pituitary suppression therapy with gonadotrophin‐releasing hormone (GnRH) agonists before IVF has become a popular alternative chosen by many clinicians.
Long‐term therapy with GnRH agonists, synthetic peptide analogues of GnRH, has been shown to be effective in treating symptomatic endometriosis, including a reduction in the size of some endometriomas (Surrey 2010a). However, whether this improves fecundity is questionable. Several non‐randomised studies (Ma 2008; Marcus 1994; Nakamura 1992; Van der Hauven 2013), suggest that in women with endometriosis‐related infertility, long‐term treatment with GnRH agonists for at least three months (and up to six months) before IVF cycles are initiated may improve implantation and clinical pregnancy rates and reduce preclinical miscarriages, compared with a conventional IVF protocol. GnRH agonists (leuprorelin, goserelin, buserelin, gonadorelin, triptorelin, nafarelin), depending on their formulation, can be given intramuscularly, subcutaneously or intranasally. Upon completion of GnRH treatment, approximately two to four weeks from the last administration, an ovarian stimulation protocol with gonadotrophins is initiated according to standard IVF/ICSI procedures.
How the intervention might work
Continuous hypophyseal exposure to GnRH agonists leads to down‐regulation of GnRH receptors, which desensitises the pituitary gland. The hypogonadotrophic‐hypogonadal state results in prolonged amenorrhoea and a low estradiol (E2) level, thus depriving existing endometriotic lesions of their main growth stimulus. This may improve the symptoms of endometriosis, and it is plausible that it may also reverse the negative effects of endometriosis on IVF cycles, including poor folliculogenesis resulting in oocytes of reduced quality; a hostile peritoneal environment from macrophages, cytokines or vasoactive substances in the peritoneal fluid; mechanical interference with oocyte pickup and transportation; and anatomical dysfunction of the fallopian tubes and ovaries (Cahill 1996; Harb 2013; Harlow 1996). It has been suggested by several non‐randomised studies that poor oocyte quality results in decreased fertilisation rates (Bergendal 1998; Garrido 2000; Pal 1998). This in turn leads to embryos of lesser quality, along with a reduced implantation rate, especially in severe endometriosis (Arici 1996; Simon 1994). A higher rate of implantation and multiple pregnancy after ovarian stimulation has been reported in women in a hypogonadotrophic‐hypogonadal state (Edwards 1995); this might be due to improved endometrial responsiveness following amenorrhoea. However, other studies have reported contradictory findings (Haouzi 2010; Hickman 2002).
Why it is important to do this review
The previous version of this review suggested that long‐term GnRH agonist therapy before IVF/ICSI in women with endometriosis is associated with higher live birth and clinical pregnancy rates (Sallam 2006). However, the live birth data were extrapolated from a single study reporting on pregnancies that "reached viability" (Dicker 1992). Furthermore, the clinical pregnancy rate was calculated from meta‐analysis of three studies, of which two reported no significant difference (Rickes 2002; Surrey 2002), but with the data reaching statistical significance due to the size of the effect reported by Dicker 1992.
Based on the findings of Sallam 2006, IVF/ICSI with long‐term pituitary down‐regulation has been suggested as the first choice treatment for women with endometriosis‐associated infertility (ESHRE 2013). However, many clinicians still have doubts about its effectiveness. Concerns include the possibility that it may lower ovarian response to ovarian stimulation, especially among poor responders. The beneficial effects of prolonged GnRH treatment before IVF are seen most often for severe endometriosis, and the benefits and harms of the intervention in mild to moderate disease have not been fully evaluated. Other concerns focus on the unpleasant side effects of GnRH agonist therapy, such as vasomotor and psychological irritability, which may seriously affect women's quality of life. Complications associated with treatment such as infection, pelvic abscess and ovarian hyperstimulation syndrome (OHSS) have not been fully assessed. Alternatively, continuous use of combined oral contraceptives (COCs) (which have the fewest side effects) for six to eight weeks before IVF or ICSI in women with endometriosis has shown favourable fertility outcomes (de Ziegler 2010), although no comparison with long‐term GnRH treatment has been made.
Objectives
To determine the effectiveness and safety of long‐term gonadotrophin‐releasing hormone (GnRH) agonist therapy (minimum 3 months) versus no pretreatment or other pretreatment modalities, such as long‐term continuous combined oral contraception (COC) or surgical therapy of endometrioma, before standard vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) in women with endometriosis.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) that reported use of any type of GnRH agonist for at least three months before an IVF/ICSI protocol was started in women diagnosed with endometriosis. We included cross‐over trials only if we could extract phase one data. We excluded quasi‐randomised trials.
Types of participants
Infertile women diagnosed with endometriosis (regardless of the grade of the disease) and treated with IVF or ICSI. The diagnosis of endometriosis was exclusively based on laparoscopy or laparotomy. For severity of endometriosis, grades according to the American Society of Reproductive Medicine (ASRM) score at laparotomy or laparoscopy were used.
Types of interventions
Intervention
Any type of GnRH agonist preparation used to down‐regulate the hypothalamic‐pituitary complex for three months or longer before a standard IVF or ICSI protocol in women diagnosed with endometriosis.
Comparison
Standard IVF/ICSI protocol only
Continuous combined oral contraception (COC) therapy for a minimum of six weeks before a standard IVF/ICSI protocol
Surgical excision of endometrioma within six months before the start of a standard IVF/ICSI protocol
Studies with a cointervention (e.g. surgical treatment of endometriosis in both groups prior to the study intervention) were eligible for inclusion.
Types of outcome measures
We recorded the following outcomes if the information was available.
Primary outcomes
Live birth rate: delivery of a live foetus after 22 completed weeks of gestational age
Complication rate: adverse effects per woman (e.g. infection, ovarian hyperstimulation syndrome (OHSS), serious vasomotor instability, overall adverse effects)
Secondary outcomes
Clinical pregnancy rate per couple/woman: defined as pregnancy confirmed by visualisation of a foetal sac on ultrasound (Zegers‐Hochschild 2017)
Multiple pregnancy rate: number of twin, triplet or higher‐order pregnancies (specified if possible) per pregnancy and confirmed by ultrasound or delivery
Miscarriage rate: miscarriage defined as the spontaneous loss of an intrauterine pregnancy prior to 22 completed weeks of gestational age (Zegers‐Hochschild 2017)
Ectopic pregnancy rate: ectopic pregnancy defined as a pregnancy outside the uterine cavity, diagnosed by ultrasound, surgical visualisation or histopathology, per couple/woman (Zegers‐Hochschild 2017)
Foetal abnormalities: number of women giving birth to a child with a foetal abnormality
Mean number of oocytes retrieved per woman
Mean number of embryos obtained per woman
Search methods for identification of studies
We searched for all published and unpublished randomised controlled trials (RCTs) of women diagnosed with endometriosis and treated with GnRH agonists to down‐regulate the hypothalamic‐pituitary complex before IVF or ICSI, without language restriction and in consultation with the Cochrane Gynaecology and Fertility (CGF) Information Specialist.
Electronic searches
We searched the following electronic databases from their inception to 8 January 2019.
The CGF Specialised Register of Controlled Trials; searched 8 January 2019 (PROCITE platform) (Appendix 1).
CENTRAL via the Cochrane CENTRAL Register of Studies ONLINE (CRSO); searched 8 January 2019 (Web platform) (Appendix 2).
MEDLINE; searched from 1946 to 8 January 2019 (OVID platform) (Appendix 3).
Embase; searched from 1980 to 8 January 2019 (OVID platform) (Appendix 4).
PsycINFO; searched from 1806 to 8 January 2019 (OVID platform) (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL); searched from 1961 to 8 January 2019 (EBSCO platform) (Appendix 6).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2008). EMBASE, PsycINFO and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (www.sign.ac.uk/methodology/filters).
Other electronic sources of trials included the following.
-
Trial registers for ongoing and registered trials.
ClinicalTrials.gov; a service of the US National Institutes of Health (www.clinicaltrials.gov).
World Health Organization International Trials Registry Platform search portal (www.who.int/trialsearch).
DARE (Database of Abstracts of Reviews of Effects) in the Cochrane Library at onlinelibrary.wiley.com (for reference lists from relevant non‐Cochrane reviews).
Web of Knowledge; another source of trials and conference abstracts (wokinfo.com).
OpenGrey; for unpublished literature from Europe (www.opengrey.eu).
LILACS (Latin American and Caribbean Health Science Information Database; for trials from the Portuguese and Spanish speaking world; regional.bvsalud.org).
PubMed and Google (for recent trials not yet indexed in MEDLINE).
Searching other resources
We handsearched reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. We also handsearched relevant journals and conference abstracts that are not covered in the CGF register, in liaison with the Information Specialist.
Data collection and analysis
Selection of studies
Two review authors (EG and PM) carried out an initial screen of titles and abstracts retrieved by the search and identified potentially eligible studies. The full texts of these studies were retrieved. Two review authors (EG and PM or PB) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. Disagreements as to study eligibility were resolved by discussion or by discussion with a third review author (IG). The selection process was documented with a PRISMA flow chart (Moher 2009).
Data extraction and management
Two review authors (EG and PM or PB) independently extracted data from eligible studies. Any discrepancies were resolved by discussion or by discussion with a third review author (IG). If a study appeared to meet the eligibility criteria but had aspects of methodology that were unclear, or if the data were provided in a form that was unsuitable for meta‐analysis, the study authors were contacted, and additional information on trial methodology or actual original trial data, or both, was sought. When studies had multiple publications, the review authors collated all reports of the same study, so that each study rather than each report was the unit of interest in the review, and such studies were given a single study ID.
Assessment of risk of bias in included studies
Two review authors (EG and PM or PB) independently assessed the included studies for risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011) to assess allocation (random sequence generation and allocation concealment); blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data; selective reporting and other bias. Disagreements were resolved by discussion or by discussion with a third review author (IG). We fully described all judgements and presented the conclusions in the 'Risk of bias' table, which was incorporated into the interpretation of review findings by means of sensitivity analyses.
We took care to search for within‐trial selective reporting, such as trials failing to report obvious outcomes or reporting them in insufficient detail to allow inclusion. We sought published protocols and compared outcomes between the protocol and the final published study.
Measures of treatment effect
For dichotomous data (e.g. live birth rate) we used the numbers of events in the control and intervention groups of each study to calculate risk ratios (RRs). For continuous data we calculated mean differences (MDs) between treatment groups. We present 95% confidence intervals (CIs) for all outcomes. If appropriate, we used number needed to treat for an additional beneficial outcome (NNTB), number needed to treat for an additional harmful outcome (NNTH), or both, to describe significant findings.
Unit of analysis issues
The primary analysis was per woman randomised. A sensitivity analysis using per pregnancy data was also included for relevant outcomes (multiple pregnancy, miscarriage, ectopic pregnancy, foetal abnormalities). We planned to briefly summarise in an additional table data that did not allow valid analysis (e.g. 'per cycle' or 'per embryo' data), and these were not meta‐analysed.
If studies reported only 'per cycle' data, we contacted study authors to request 'per woman randomised' data.
We counted multiple live births (e.g. twins, triplets) as one live birth event.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and made attempts to obtain missing data from the original trialists. When data on live birth or clinical pregnancy could not be obtained, we assumed that the outcome did not occur. For other outcomes, we analysed only available data.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by measuring the I2 statistic. We considered an I2 measurement greater than 50% to indicate substantial heterogeneity (Higgins 2011). Where substantial heterogeneity was detected, we explored possible explanations in sensitivity analyses (see below) and considered a subgroup analysis. We took any statistical heterogeneity into account when interpreting the results, especially if variation in the direction of effect was noted.
Assessment of reporting biases
The review authors aimed to minimise the potential impact of publication bias and other reporting biases by ensuring a comprehensive search for eligible studies. If 10 or more studies were included in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies; Higgins 2011).
Data synthesis
Where studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons.
Long‐term GnRH agonist pretreatment followed by standard IVF/ICSI versus standard IVF/ICSI only.
Long‐term GnRH agonist pretreatment versus at least six weeks of COC pretreatment, both followed by standard IVF/ICSI.
Long‐term GnRH agonist treatment versus surgical excision of endometriomas, both followed by standard IVF/ICSI.
Analyses were stratified by months of GnRH agonist pretreatment: three months, six months, over six months.
We displayed an increase in the risk of a particular outcome which may be beneficial (e.g. live birth) or detrimental (e.g. adverse effects) graphically in the meta‐analyses to the right of the centre line, and a decrease in the odds of an outcome to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
Where data were available, we conducted subgroup analyses to determine separate evidence within the following subgroups.
Severity of endometriosis; stage I/II and stage III/IV disease.
Previous history of surgery to treat endometriosis.
Type of embryo transfer: fresh or cryopreserved.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcome measures to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
eligibility had been restricted to studies at low risk of bias (i.e. no high risk of selection bias);
the effect measure had been expressed as odds ratio (OR) rather than RR;
the unit of analysis had been per pregnancy rather than per woman, for relevant outcomes (multiple pregnancy, miscarriage, ectopic pregnancy, foetal abnormalities).
Where substantial heterogeneity was detected, we explored clinical or methodological differences between studies that might have accounted for the heterogeneity.
Overall quality of the body of evidence: 'Summary of findings' table
We generated a 'Summary of findings' table using GRADEpro software (GRADEpro GDT 2015). This table was prepared by two review authors (EG and PM) working independently, with disagreements resolved by consensus. It evaluates the overall quality of the body of evidence for the main review comparison (GnRH agonist pretreatment versus no pretreatment) and reports the main review outcomes (live birth rate, complication rate, clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, mean number of oocytes, and mean number of embryos), using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We have justified, documented and incorporated into the report of results for each outcome our judgements about evidence quality (high, moderate, low or very low).
Results
Description of studies
Results of the search
We ran the updated electronic search on 8 January 2019, and this yielded 817 articles. We identified 59 additional articles via other sources: 23 via ClinicalTrials.gov (www.clinicaltrials.gov) and 36 via the World Health Organization International Trials Registry Platform search portal (www.who.int/trialsearch). After removal of duplicates, 809 were left for screening. Of these, we excluded 770 as they were clearly not relevant. We reviewed 39 full‐text articles of which we excluded 27 (Characteristics of excluded studies). One study is awaiting classification as it was not clear how endometriomas were diagnosed (Characteristics of studies awaiting classification), and two studies were ongoing trials that had not yet reported their results (Characteristics of ongoing studies).
We identified eight studies (nine articles) that met the inclusion criteria for this review (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002; Surrey 2010; Totaro 2009). Of these, we included two in our qualitative analysis (Surrey 2010; Totaro 2009), and six in our quantitative analysis (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002). We have presented the trial flow diagram in Figure 1. The previous Sallam 2006 version of this review incorporated Dicker 1992, Rickes 2002 and Surrey 2002.
1.
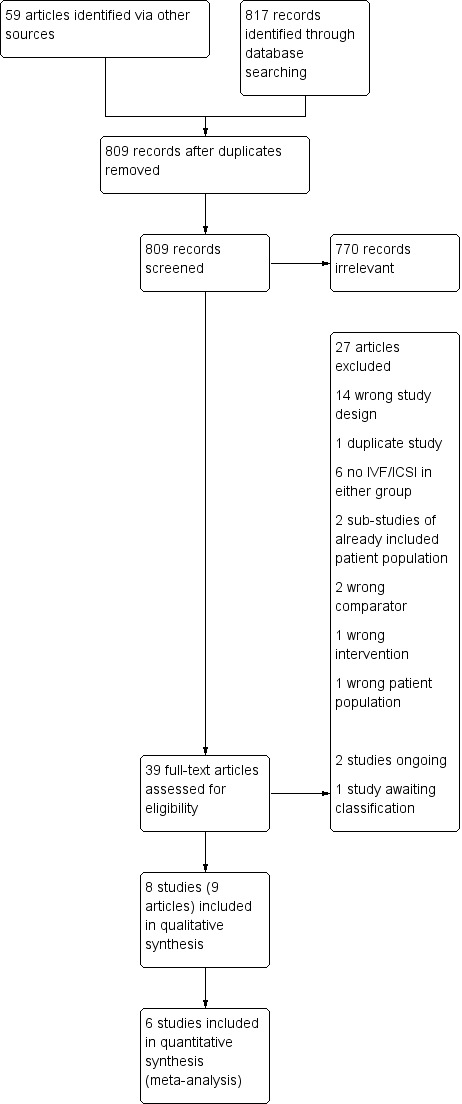
Study flow diagram.
Included studies
Study design and setting
We included eight parallel‐design randomised controlled trials (RCTs), five of which have been published as full articles (Decleer 2016; Dicker 1992; Rickes 2002; Surrey 2002; Surrey 2010), one was published as a conference abstract (Totaro 2009), and two are unpublished studies (NCT01269125; NCT01581359). Five studies were single‐centre (Decleer 2016; NCT01269125; NCT01581359; Rickes 2002; Surrey 2010), one study was multiple‐centre (Surrey 2002), and for two studies it was unclear (Dicker 1992; Totaro 2009). Two of the studies were carried out in the USA (Surrey 2002; Surrey 2010), and one each in Belgium (Decleer 2016), Germany (Rickes 2002), Greece (NCT01269125), Israel (Dicker 1992), Italy (Totaro 2009), and Spain (NCT01581359).
Participants
The eight included studies had a total of 640 participants: 322 women in the intervention group and 318 in the control group. All included patients had a diagnosis of endometriosis at laparoscopy or laparotomy. Decleer 2016 and NCT01269125 included patients with stages I or II endometriosis, Rickes 2002 included patients with stages II‐IV endometriosis and Totaro 2009 included patients with stages III or IV endometriosis. NCT01581359 and Surrey 2002 included patients with any severity of endometriosis. Dicker 1992 described endometriosis as severe and Surrey 2010 included patients with laparoscopically diagnosed endometriosis but "no current sonographic evidence of an ovarian endometrioma > 2 cm in mean diameter".
The mean age of participants ranged from 30.3 to 33.9 years, where reported (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Surrey 2002; Surrey 2010). Rickes 2002 reported that the age range in their study was 23 to 40 years old and Totaro 2009 did not comment on age. In five studies, patients underwent in vitro fertilisation (IVF) (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Surrey 2010), in two studies they had IVF or intracytoplasmic sperm injection (ICSI) (Rickes 2002; Surrey 2002), and in one study it was not clear which type of assisted reproductive technique was employed (Totaro 2009).
Interventions
Surgical treatment of endometriosis was performed prior to randomisation in five studies (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002), whereas in three studies it was not clear whether any treatment was performed (Surrey 2002; Surrey 2010; Totaro 2009).
Three studies used leuprolide (NCT01269125; Surrey 2002; Surrey 2010), two studies used goserelin (Decleer 2016; Rickes 2002), two studies used triptorelin (NCT01581359; Totaro 2009), and one used decapeptyl (Dicker 1992). Duration of treatment was for three months in five studies (Decleer 2016; NCT01269125; NCT01581359; Surrey 2002; Surrey 2010), six months in two studies (Dicker 1992; Totaro 2009), and five to six months in one study (Rickes 2002).
For all included studies, the control group consisted of standard IVF/ICSI (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002; Surrey 2010; Totaro 2009). None of the studies had control groups including the control group of continuous combined oral contraception (COC) therapy for a minimum of six weeks before standard IVF/ICSI or surgical excision of endometrioma within six months before the start of standard IVF/ICSI.
Outcomes
Primary outcomes
One study reported on the primary outcome of live birth (NCT01581359). Three studies reported on the primary outcome of complication rate (NCT01269125; NCT01581359; Surrey 2002); specifically ovarian hyperstimulation syndrome (OHSS) (NCT01269125; NCT01581359), local reaction at injection site (NCT01581359), and premature luteinisation (NCT01269125).
Secondary outcomes
Eight studies reported on clinical pregnancy rate (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002; Surrey 2010; Totaro 2009), and of these, six could be included for meta‐analysis (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002). The reasons for exclusion of Surrey 2010 and Totaro 2009 are detailed in Characteristics of included studies.
Two studies reported on multiple pregnancy rate (NCT01581359; Totaro 2009), two studies reported on miscarriage rate (NCT01581359; Totaro 2009), two studies reported on ectopic pregnancy rate (NCT01581359; Surrey 2002), and two studies reported on foetal abnormality rate (NCT01581359; Surrey 2002).
Six studies reported on the mean number of oocytes (Decleer 2016; Dicker 1992; NCT01581359; Surrey 2002; Surrey 2010; Totaro 2009), of which four could be included for meta‐analysis as per woman data (Decleer 2016; Dicker 1992; NCT01581359; Surrey 2002). The reasons for exclusion of Surrey 2010 and Totaro 2009 are detailed in Characteristics of included studies.
Two studies reported on the mean number of embryos obtained per woman (Decleer 2016; NCT01581359).
Author correspondence
We contacted the authors of Decleer 2016, NCT01269125, NCT01581359, Rickes 2002, Surrey 2010 and Totaro 2009 to obtain and clarify data. To date, we have received a response from NCT01581359 and Surrey 2010.
Excluded studies
We excluded 27 studies for the following reasons.
14 wrong study design
1 duplicate study
6 no IVF/ICSI in either group
2 sub‐studies of already included patient population
2 wrong comparator
1 wrong intervention
1 wrong patient population
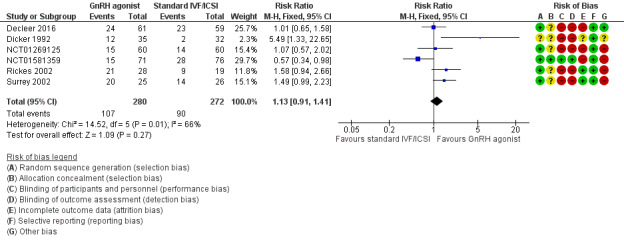
Risk of bias in included studies
We assessed risk of bias in all included studies as demonstrated in Figure 2 and Figure 3. Detailed information can be found in Characteristics of included studies.
2.
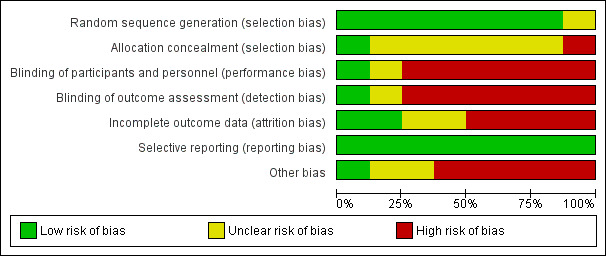
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
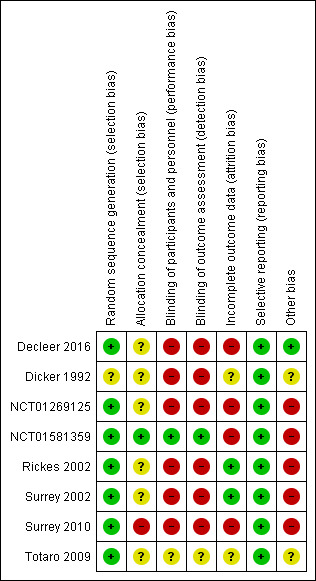
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Seven studies utilised adequate methods for random sequence generation, such as computer‐generated random number tables, and hence we deemed them to be at low risk of bias (Decleer 2016; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002; Surrey 2010; Totaro 2009). Dicker 1992 states that patients were "randomly and sequentially allocated" and attempts to contact the study authors at the time of the previous version of this review (Sallam 2006), were unsuccessful.
Allocation concealment
One study reported an adequate method of allocation concealment using "opaque‐sealed envelopes, sequentially numbered' and hence we deemed it to be at low risk of bias (NCT01581359). Four studies provided no details of allocation concealment and hence we deemed them to be at unclear risk of bias (Decleer 2016; Rickes 2002; Surrey 2002; Totaro 2009). We also judged Dicker 1992 to be at unclear risk of bias, based on patients being "randomly and sequentially allocated", as was NCT01269125, in which the random allocation was made by the first author. In correspondence with Surrey 2010, they confirmed that no allocation concealment was undertaken and hence we labelled the study as high risk of bias.
Blinding
Blinding of participants and personnel (performance bias)
One study confirmed blinding of participants and personnel and hence we judged it to be at low risk of bias (NCT01581359). Four studies reported being open label (Decleer 2016; NCT01269125; Rickes 2002; Surrey 2010), and the previous version of this review (Sallam 2006), mentions no placebo injections in another two studies (Dicker 1992; Surrey 2002); we judged all of these to be at high risk of bias. One study did not report on blinding and we labelled it as unclear risk of bias (Totaro 2009).
Blinding of outcome assessment (detection bias)
We judged NCT01581359 to be at low risk of bias, as blinding was maintained during the study until follow‐up was completed. We judged most other studies to be at high risk as they were open label (Decleer 2016; NCT01269125; Rickes 2002; Surrey 2010), or previously reported no blinding (Dicker 1992 and Surrey 2002 in Sallam 2006). We labelled Totaro 2009 as unclear risk of bias as no information was supplied.
Incomplete outcome data
An intention‐to‐treat analysis was performed in two studies (Rickes 2002; Surrey 2002), and hence we judged them to be at low risk of bias. For Surrey 2002, a lack of study dropouts was confirmed in author correspondence at the time of the previous version of this review (Sallam 2006). We marked four studies at high risk of bias in light of a lack of an intention‐to‐treat analysis (Decleer 2016; NCT01269125; NCT01581359; Surrey 2010). Where data were available, we used the number of participants randomised for this meta‐analysis (Decleer 2016; NCT01269125; NCT01581359). Where the data for meta‐analysis were not available, we included the study in the qualitative synthesis (Surrey 2010). We judged one study to be at unclear risk of bias as no information was supplied (Totaro 2009).
Selective reporting
We deemed all studies to be at low risk of bias as they all reported on a priori outcomes (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002; Surrey 2010; Totaro 2009). We did, however, note that in one study, there was a change in the secondary outcomes (NCT01269125), and in another study there was a change in the primary outcome (NCT01581359).
Other potential sources of bias
We deemed one study to be at low risk of bias on the basis of no difference in basal patient characteristics and no conflict of interest or relevant funding sources (Decleer 2016). We judged two studies to be at unclear risk of bias on the basis of a lack of information on conflict of interest or funding sources (Dicker 1992; Totaro 2009). We labelled five studies as high risk of bias; three due to the fact that they all received funding from pharmaceutical companies involved in making GnRH analogues (Rickes 2002; Surrey 2002; Surrey 2010); and two due to the fact that they are presently unpublished plus lack information on conflict of interest and funding sources (NCT01269125; NCT01581359). In addition, in one study there was a higher proportion of stage III or IV endometriosis in the intervention arm than the control arm (Surrey 2002).
Effects of interventions
See: Table 1
See: Table 1.
1. Comparison of gonadotrophin‐releasing hormone (GnRH) agonist to standard in vitro fertilisation/intracytoplasmic sperm injection (IVF/ICSI) protocol only
Primary outcomes
1.1 Live birth rate
We are uncertain whether long‐term GnRH agonist therapy affects the live birth rate compared to standard IVF/ICSI (risk ratio (RR) 0.48, 95% confidence interval (CI) 0.26 to 0.87; 1 RCT, n = 147; I2 not calculable; very low‐quality evidence; Analysis 1.1; Figure 4). This suggests that with a live birth rate of approximately 36% (355 per 1000) with standard IVF/ICSI, the equivalent live birth rate with long‐term GnRH agonist therapy prior to standard IVF/ICSI lies between 9% and 31% (92 to 309 per 1000).
1.1. Analysis.
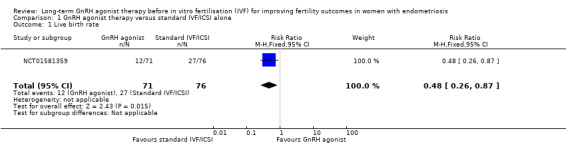
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 1 Live birth rate.
4.
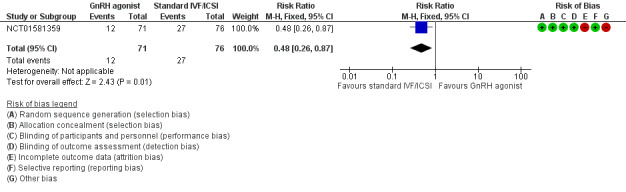
Forest plot of comparison: 1 GnRH agonist therapy versus standard IVF/ICSI alone, outcome: 1.1 Live birth rate.
We did not perform sensitivity analysis as only one unpublished trial reported on the primary outcome of live birth.
1.1.1 Subgroup analysis: severity of endometriosis
We are uncertain whether long‐term GnRH agonist therapy affects the live birth rate compared to standard IVF/ICSI in patients with either stage I/II endometriosis (Peto OR 0.16, 95% CI 0.03 to 0.83; 1 RCT, n = 33; I2 not calculable; very low‐quality evidence; Analysis 1.15) or stage III/IV endometriosis . (Peto OR 0.49, 95% CI 0.22 to 1.10; 1 RCT, n = 114; I2 not calculable; low‐quality evidence; Analysis 1.16).
1.15. Analysis.
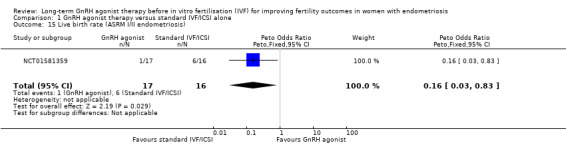
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 15 Live birth rate (ASRM I/II endometriosis).
1.16. Analysis.
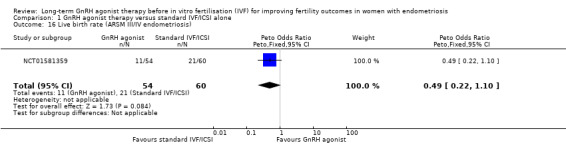
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 16 Live birth rate (ARSM III/IV endometriosis).
Subgroup analysis: previous history of surgery to treat endometriosis
None of the included studies reported on this comparison.
Subgroup analysis: type of embryo transfer
None of the included studies reported on this comparison.
1.2 Complication rate
We are uncertain whether long‐term GnRH agonist therapy affects the overall complication rate (Peto OR 1.23, 95% CI 0.37 to 4.14; 3 RCTs, n = 318; I2 = 73%; very low‐quality evidence; Analysis 1.10). This suggests that with a complication rate of approximately 3% (31 per 1000) with standard IVF/ICSI, the equivalent complication rate with long‐term GnRH agonist therapy prior to IVF/ICSI lies between 1% and 13% (11 to 128 per 1000).
1.10. Analysis.
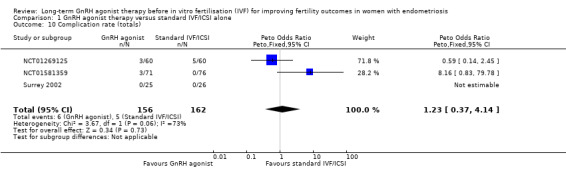
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 10 Complication rate (totals).
We did not perform sensitivity analysis as data for this outcome originated exclusively from unpublished trials. Looking at specific complications following long‐term GnRH agonist therapy, we are uncertain whether this intervention affects the incidence of ovarian hyperstimulation syndrome (OHSS) (Peto OR 0.75, 95% CI 0.17 to 3.40; 2 RCTs, n = 267; I2 = 39%; very low‐quality evidence), local reaction at the injection site (Peto OR 8.04, 95% CI 0.50 to 130.02; 1 RCT, n = 147; I2 not calculable; very low‐quality evidence) or premature luteinisation (Peto OR 1.00, 95% CI 0.06 to 16.18; 1 RCT, n = 120; I2 not calculable; very low‐quality evidence). See Analysis 1.2 and Figure 5.
1.2. Analysis.
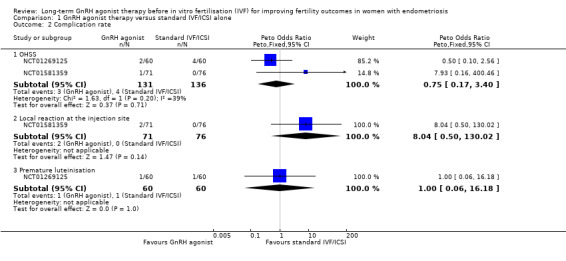
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 2 Complication rate.
5.
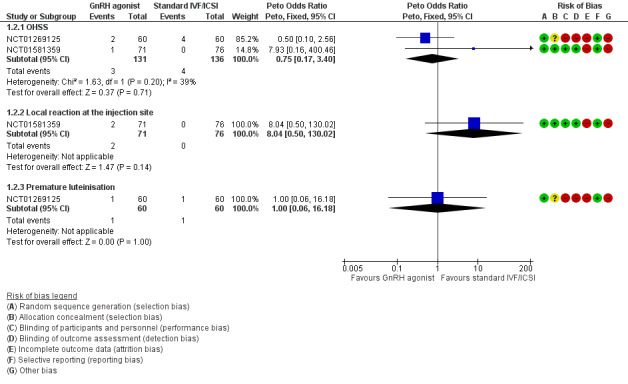
Forest plot of comparison: 1 GnRH agonist therapy versus standard IVF/ICSI alone, outcome: 1.2 Complication rate.
1.2.1 Subgroup analysis: severity of endometriosis
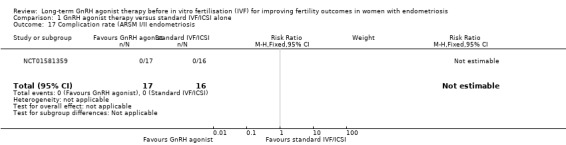
We are uncertain whether long‐term GnRH agonist treatment affects the complication rate compared to standard IVF/ICSI in patients with stage I/II endometriosis (no events in either group, see Analysis 1.17) or stage III/IV endometriosis (Peto OR 0.14, 95% CI 0.01 to 1.42; 1 RCT, n = 114; I2 not calculable; very low‐quality evidence; Analysis 1.18).
1.17. Analysis.
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 17 Complication rate (ARSM I/II endometriosis.
1.18. Analysis.
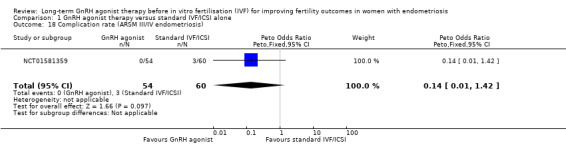
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 18 Complication rate (ARSM III/IV endometriosis).
1.2.2 Subgroup analysis: previous history of surgery to treat endometriosis
None of the included studies reported on this comparison.
1.2.3 Subgroup analysis: type of embryo transfer
None of the included studies reported on this comparison.
Secondary outcomes
1.3 Clinical pregnancy rate
We are uncertain of the effect of long‐term GnRH agonist therapy on clinical pregnancy rate (RR 1.13, 95% CI 0.91 to 1.41; six RCTs, n = 552; I2 = 66%; very low‐quality evidence; Analysis 1.3; Figure 6).
1.3. Analysis.
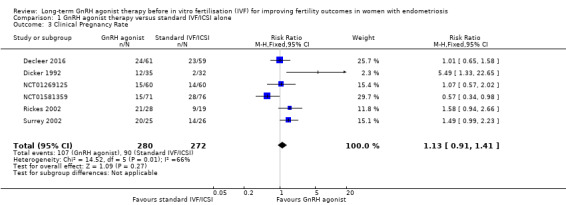
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 3 Clinical Pregnancy Rate.
6.
Forest plot of comparison: 1 GnRH agonist therapy vs Standard IVF/ICSI alone, outcome: 1.3 Clinical Pregnancy Rate.
Due to the high degree of heterogeneity, we repeated the analysis using a random‐effects model yielding similar results (RR 1.20, 95% CI 0.81 to 1.78; six RCTs, n = 552; I2 = 66%; very low‐quality evidence).
We could not include data from two studies in the meta‐analysis and we have summarised these in Table 2 and explained the reasons for this in Characteristics of included studies.
1. Data not suitable for meta‐analysis.
| Study ID | Clinical pregnancy rate | Mean number of oocytes |
| Surrey 2010 | GnRH agonist therapy: 9* out of 18 Standard IVF/ICSI: 9* out of 19 * these numbers are approximate, based on calculations done using % and assumption on number of patients per group |
GnRH agonist therapy (mean + SD): 10.3* + 7.19* Standard IVF/ICSI (mean + SD): 18* + 13.76* |
| Totaro 2009 | GnRH agonist therapy: 7 out of 29 Standard IVF/ICSI: 7 out of 32 |
GnRH agonist therapy: 4.3 + 2.5^ Standard IVF/ICSI: 5.4 + 4.7^ ^: unclear if SD |
GnRH: gonadotrophin‐releasing hormone; IVF/ICSI: in vitro fertilisation/intracytoplasmic sperm injection; SD: standard deviation
1.4. Multiple pregnancy rate
We are uncertain whether long‐term GnRH agonist therapy affects the multiple pregnancy rate per woman randomised (Peto OR 0.14, 95% CI 0.03 to 0.56; 2 RCTs, n = 208; I2 = 0%; very low‐quality evidence; Analysis 1.4). Similar results are obtained if the multiple pregnancy rate is expressed per clinical pregnancy (Peto OR 0.17, 95% CI 0.03 to 1.05; 1 RCT, n = 147; I2 not calculable; very low‐quality evidence; Analysis 1.11).
1.4. Analysis.
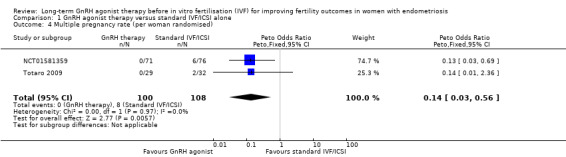
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 4 Multiple pregnancy rate (per woman randomised).
1.11. Analysis.
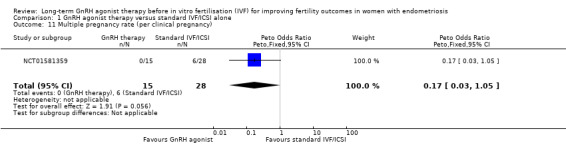
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 11 Multiple pregnancy rate (per clinical pregnancy).
1.5 Miscarriage rate
We are uncertain of the effect of long‐term GnRH agonist therapy on the miscarriage rate (Peto OR 0.45, 95% CI 0.10 to 2.00; 2 RCTs, n = 208; I2 = 0%; very low‐quality evidence; Analysis 1.5). Similar results are obtained if the miscarriage rate is expressed per clinical pregnancy (Peto OR 0.93, 95% CI 0.15 to 5.54; 1 RCTs, n = 57; I2 not calculable; very low‐quality evidence; Analysis 1.12).
1.5. Analysis.
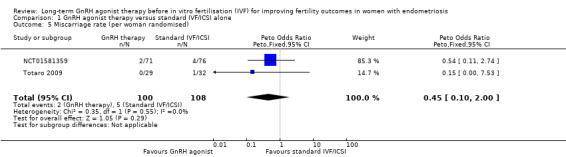
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 5 Miscarriage rate (per woman randomised).
1.12. Analysis.
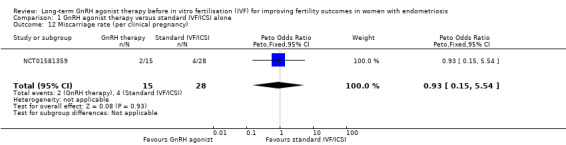
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 12 Miscarriage rate (per clinical pregnancy).
1.6 Ectopic pregnancy rate
We are uncertain of the effect of long‐term GnRH agonist therapy on the ectopic pregnancy rate (Peto OR 0.14, 95% CI 0.00 to 7.30; 2 RCTs, n = 198; I2 not calculable; very low‐quality evidence; Analysis 1.6).
1.6. Analysis.
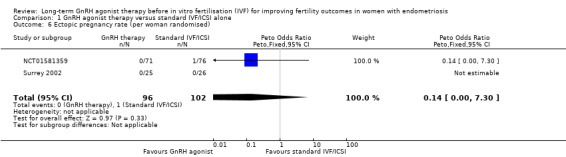
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 6 Ectopic pregnancy rate (per woman randomised).
Similar results are obtained if the ectopic pregnancy rate is expressed per clinical pregnancy (Peto OR 0.22, 95% CI 0.00 to 13.15; 2 RCTs, n = 198; I2 not calculable; very low‐quality evidence; Analysis 1.13).
1.13. Analysis.
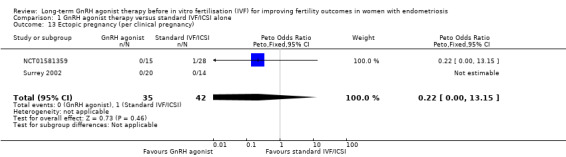
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 13 Ectopic pregnancy (per clinical pregnancy).
1.7 Foetal abnormality rate
We are uncertain of the effect of long‐term GnRH agonist therapy on the foetal abnormality rate (Peto OR 7.93, 95% CI 0.16 to 400.46; 2 RCTs, n = 198; I2 not calculable; very low‐quality evidence; Analysis 1.7).
1.7. Analysis.
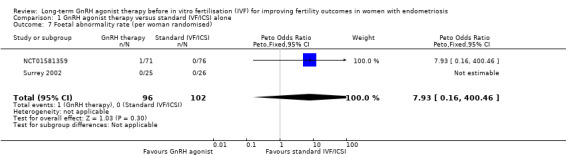
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 7 Foetal abnormality rate (per woman randomised).
Similar results are obtained if the foetal abnormality rate is expressed per clinical pregnancy (Peto OR 17.58, 95% CI 0.29 to 1073.88; 2 RCTs, n = 198; I2 not calculable; very low‐quality evidence; Analysis 1.14).
1.14. Analysis.
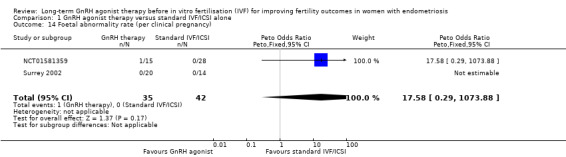
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 14 Foetal abnormality rate (per clinical pregnancy).
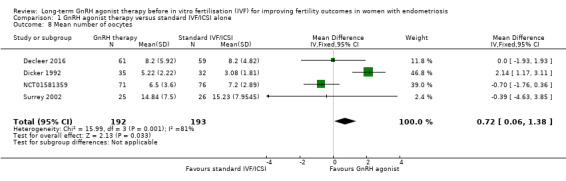
1.8 Mean number of oocytes
We are uncertain of the effect of long‐term GnRH agonist therapy on the mean number of oocytes (mean difference (MD) 0.72, 95% CI 0.06 to 1.38; 4 RCTs, n = 385; I2 = 81%; very low‐quality evidence; Analysis 1.8).
1.8. Analysis.
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 8 Mean number of oocytes.
Due to the high degree of heterogeneity, we repeated the analysis using a random‐effects model yielding similar results (MD 0.42, 95% CI ‐1.38 to 2.22; 4 RCTs, n = 385; I2 = 81%; very low‐quality evidence).
We could not include data from two studies in the meta‐analysis and we have summarised these in Table 2 and explained the reasons for this in Characteristics of included studies.
1.9 Mean number of embryos
We are uncertain of the effect of long‐term GnRH agonist therapy on the mean number of embryos (MD ‐0.76, 95% CI ‐1.33 to ‐0.19; 2 RCTs, n = 267; I2 = 0%; very low‐quality evidence; Analysis 1.9).
1.9. Analysis.
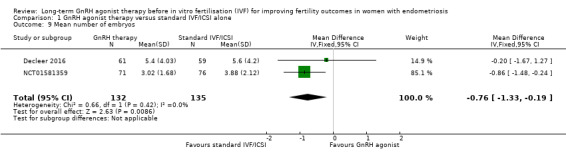
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 9 Mean number of embryos.
Comparison of GnRH agonist to continuous combined oral contraception (COC) therapy for a minimum of six weeks before a standard IVF/ICSI protocol
No studies reported on this comparison.
Comparison of GnRH agonist to surgical excision of endometrioma within six months before the start of a standard IVF/ICSI protocol
No studies reported on this comparison.
Discussion
Summary of main results
This Cochrane Review aimed to determine the effectiveness and safety of long‐term gonadotrophin‐releasing hormone (GnRH) agonist therapy versus no pretreatment or other pretreatment modalities, specifically long‐term continuous combined oral contraception (COC) or surgical therapy, before standard in vitro fertilisation/intracytoplasmic sperm injection (IVF/ICSI) in women with endometriosis. Our searches revealed no studies comparing long‐term GnRH agonist therapy to other pretreatment modalities, thus this review has focused entirely on long‐term GnRH agonist therapy versus no pretreatment prior to standard IVF/ICSI.
Our findings suggest that there is uncertainty about the effect of long‐term GnRH agonist therapy on the live birth rate compared to standard IVF/ICSI. Subgroup analysis by endometriosis severity further highlighted the uncertainty of the effect of long‐term GnRH agonist therapy on the live birth rate. Subgroup analysis by previous history of surgery to treat endometriosis was not possible due to a lack of data. Subgroup analysis by type of embryo transfer was not possible for the same reason.
The quality of the evidence for complication rate was very low and hence it is difficult to draw any conclusion on safety. The data available suggest that the overall complication rate as well as the complication rate by subtype are not associated with any safety concerns with long‐term GnRH agonist therapy.
Subgroup analysis by endometriosis severity, similarly had no impact on complication rate. We did not conduct an analysis by months of GnRH agonist pretreatment due to the paucity of data.
Very low‐quality evidence leaves us very uncertain on the impact of long‐term GnRH agonist therapy on clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, mean number of oocytes and mean number of embryos.
Our findings are summarised in Table 1.
Overall completeness and applicability of evidence
Based on our findings, it is evident that there is a paucity of data for the primary outcomes of live birth and complications. Of the included studies, only one reported on the outcome of live birth rate, which of course will be the outcome of most significance to women with endometriosis undertaking fertility treatment (NCT01581359). Furthermore, this was a relatively small study which at the time of writing of this systematic review was not published and hence had not been through the peer review process.
Only three studies looked at complication rate and hence it is difficult to definitively draw conclusions on safety (NCT01269125; NCT01581359; Surrey 2002). We noted a high degree of heterogeneity, and this could be a reflection of the small number of events identified on a background of small studies.
The majority of studies focused on clinical pregnancy rate (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002; Surrey 2010; Totaro 2009), although only some of these data could be used for meta‐analysis (Decleer 2016; Dicker 1992; NCT01269125; NCT01581359; Rickes 2002; Surrey 2002). Once again, there was no change in this outcome with long‐term GnRH agonist therapy. We noted a significant degree of heterogeneity, mainly due to the contribution of the older study by Dicker 1992 and the, as yet, not published study NCT01581359.
Similarly, we noted a significant degree of heterogeneity with the mean number of oocytes; once again, this was mainly due to the contribution of the older study by Dicker 1992. We speculate that this could be a reflection of older, outdated IVF protocols and techniques.
Overall, even though the findings of the review draw into question the use of long‐term GnRH agonists prior to IVF/ICSI in women with endometriosis, we feel that the overall quality of the evidence is too low to support a move away from the use of GnRH agonists. Further high‐quality research is required to draw definitive conclusions.
Quality of the evidence
In this study we identified and included data originating from eight randomised controlled trials (RCTs), two of which are not published and one which was published solely as an abstract. The total number of participants involved was 640. The risk of bias for individual studies is summarised in Figure 2 and Figure 3.
We rated the quality of the evidence based on the GRADE criteria. The quality of evidence was very low with issues arising due to lack of blinding, imprecision and inconsistency. See Table 1.
Potential biases in the review process
We aimed to reduce the risk of publication bias by conducting systematic searches of multiple databases as well as trial registries to identify unpublished and ongoing studies. We contacted trial authors for (more) information where necessary, although unfortunately data were not always available or we did not receive a response in all cases. It is possible that our searches did not identify all unpublished studies. We performed subgroup analysis by endometriosis severity but data were not available for subgroup analysis by status of previous surgery for endometriosis. As prespecified, where possible, we performed sensitivity analyses on the primary outcomes. We were unable to conduct a funnel plot due to the small number of included studies.
Agreements and disagreements with other studies or reviews
Our review findings are in sharp contrast to the previous version of this review which reported an association between long‐term GnRH agonist therapy and greater live birth and clinical pregnancy rates (Sallam 2006). Compared to the previous version of this review, the outcome of live birth includes one new RCT in the meta‐analysis and excludes a previously included RCT (Dicker 1992), as this paper does not truly report on live birth as per the Zegers‐Hochschild 2017 definition. Further, for the outcome of clinical pregnancy rate, this review includes three new RCTs in the meta‐analysis. The net effect of the addition of these RCTs, which in terms of bias are no worse in design, is to bring the results closer to the line of no effect. Indeed, in NCT01581359, which was the only adequately blinded study to date (i.e. patients in the control group received placebo injections rather than commencing standard IVF/ICSI immediately), both the live birth and clinical pregnancy rates favoured standard IVF/ICSI rather than pretreatment with long‐term GnRH agonists. Given that visible endometriosis was surgically treated in NCT01581359 prior to randomisation, the argument arises for a potential deleterious effect of long‐term GnRH therapy prior to IVF/ICSI. However, due to the very low quality of this evidence, it is difficult to reach any definitive conclusions at present. The trend for miscarriage rate did not differ from the previous version of this review, whereas in this review we found no trend towards a higher oocyte yield with GnRH agonist therapy.
Data from retrospective studies suggest that the live birth and clinical pregnancy rates are improved with cryopreserved embryo transfer after GnRH agonist therapy (Mohamed 2011; Xie 2018). Due to a lack of data we were unable to examine this outcome in this systematic review.
To our knowledge there are no other reviews that address long‐term GnRH agonist therapy compared no or to other pretreatments prior to standard IVF/ICSI. It is worth noting however that at least one other RCT, which did not meet our inclusion criteria and used continuous COC as the control group, also reported no difference in outcomes compared to long‐term GnRH agonist therapy (NCT02400801).
Authors' conclusions
Implications for practice.
This review raises important questions regarding the merit of long‐term gonadotrophin‐releasing hormone (GnRH) agonist therapy compared to no pretreatment prior to standard vitro fertilisation/intracytoplasmic sperm injection(IVF/ICSI) in women with endometriosis. Contrary to previous findings, we are uncertain as to whether long‐term GnRH agonist therapy impacts on the live birth rate or indeed the complication rate compared to standard IVF/ICSI. Further, we are uncertain whether this intervention impacts on the clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, mean number of oocytes and mean number of embryos. In light of the paucity and very low quality of existing data, particularly for the primary outcomes examined, further high‐quality trials are required to definitively determine the impact of long‐term GnRH agonist therapy on IVF/ICSI outcomes, not only compared to no pretreatment, but also compared to other proposed alternatives to endometriosis management.
Implications for research.
More high‐quality RCTs are needed to definitively address the questions of efficacy and safety of long‐term GnRH agonist therapy before IVF/ICSI in women with endometriosis. Specifically, we suggest that future studies need to focus on live birth rate and complication rate as the primary outcomes of choice. In addition, study design to address the comparison of long‐term GnRH agonist therapy versus no pretreatment will need to be improved by consistent blinding in terms of participants, personnel and outcome assessors. This will also ensure that the time frame between initiation of IVF/ICSI is the same in both groups, i.e. the control group does not potentially progress to IVF/ICSI sooner after the immediate postoperative period than the GnRH agonist group. We would also advocate for further studies specifically examining the effect of fresh versus cryopreserved embryo transfer following long‐term GnRH agonist therapy. This is on the basis of retrospective data suggesting outcomes, including clinical pregnancy and live birth, are improved with cryopreserved embryo transfer following GnRH agonist therapy in women with endometriosis (Mohamed 2011; Xie 2018).
Given the lack of RCTs comparing long‐term GnRH agonist therapy to other pretreatments prior to standard IVF/ICSI, these will need to be conducted. It would also be interesting for future studies to examine outcomes following continuous combined oral contraception (COC) therapy or surgical excision of endometrioma as compared to standard IVF/ICSI. In addition, in terms of basic science, it would be interesting to study the effect of long‐term GnRH agonist therapy on oocyte and/or embryo quality.
Notes
This review supersedes the Sallam 2006 Cochrane Review.
Acknowledgements
We wish to thank Cochrane Gynaecological and Fertility editorial group for their patience and kind support. In particular, we would like to thank Elena Kostova for her help with all our queries and Marian Showell for her help with conducting the searches.
We wish to thank Dr Sharifah Halimah Jaafar who was involved in the writing of the updated protocol of this review. We also wish to thank Dr Sofia Dias for her contributions to the Sallam 2006 Cochrane Review that will be replaced by this current work.
We wish to thank all the trial authors who took the time to engage with this systematic review by sharing data and answering multiple emails.
Finally, we are grateful to Roger Hart, Edgardo Somigliana, Katie Stocking and Devashana Gupta for their critical review of our work.
Appendices
Appendix 1. Gynaecology and Fertility Group Specialised Register search strategy
Searched 8 January 2019
PROCITE platform
Keywords CONTAINS "Gonadorelin" or "GnRHa‐gonadotropin" or "GnRHa" or "GnRh" or "GnRH analog" or "GnRH analogue" or "GnRH analogues" or "GnRH agonist" or "Gonadotrophin releasing hormones" or "Gonadotrophin releasing agonist" or "gonadotropin releasing hormone agonist" or "Goserelin" or "Gosereline " or "buserelin" or "busereline" or "Nafarelin" or "Zoladex" or "Lupron" or "triptoielin" or "triptorelin" or "triptoreline" or "triptorelyn" or "triptrolein" or Title CONTAINS "Gonadorelin" or "GnRHa‐gonadotropin" or "GnRHa" or "GnRh" or "GnRH analog" or "GnRH analogue" or "GnRH analogues" or "GnRH agonist" or "Gonadotrophin releasing hormones" or "Gonadotrophin releasing agonist" or "gonadotropin releasing hormone agonist" or "Goserelin" or "Gosereline " or "buserelin" or "busereline" or "Nafarelin" or "Zoladex" or "Lupron" or "triptoielin" or "triptorelin" or "triptoreline" or "triptorelyn" or "triptrolein"
AND
Keywords CONTAINS "endometriosis" or "adenomyosis" or Title CONTAINS "endometriosis" or "adenomyosis"
AND
Keywords CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "Embryo Transfer" or "ET" or "Blastocyst" or "implantation" or "poor implantation" or "poor prognostic patients" or "recurrent implantation failure" or "repeated implantation failure" or "Subfertility" or "pregnancy" or Title CONTAINS"IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or "intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "Embryo Transfer" or "ET" or "Blastocyst" or "implantation" or "poor implantation" or "poor prognostic patients" or "recurrent implantation failure" or "repeated implantation failure" or "Subfertility" or "pregnancy" (112 records)
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 8 January 2019
Web platform
#1 MESH DESCRIPTOR Endometriosis EXPLODE ALL TREES 689 #2 Endometrio*:TI,AB,KY 1804 #3 #1 OR #2 1804 #4 MESH DESCRIPTOR Gonadotropin‐Releasing Hormone EXPLODE ALL TREES 2406 #5 (Gonadotropin* or Gonadotrophin*):TI,AB,KY 5337 #6 (buserelin or goserelin):TI,AB,KY 1296 #7 (leuprolide or nafarelin):TI,AB,KY 982 #8 triptorelin:TI,AB,KY 692 #9 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh):TI,AB,KY 3220 #10 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin):TI,AB,KY 1388 #11 (dirigestran or factrel or gonadoliberin):TI,AB,KY 5 #12 leuprorelin:TI,AB,KY 429 #13 (down regulat*):TI,AB,KY 2508 #14 downregulat*:TI,AB,KY 1152 #15 (pituitary suppress*):TI,AB,KY 140 #16 (Ovar* adj2 suppress*):TI,AB,KY 233 #17 (suprecur or suprefact):TI,AB,KY 15 #18 (zoladex or lupron):TI,AB,KY 313 #19 GnRHa:TI,AB,KY 358 #20 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 11047 #21 #3 AND #20 595 #22 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 1952 #23 (vitro fertili?ation):TI,AB,KY 2479 #24 (ovar* stimulat*):TI,AB,KY 1581 #25 (ovar* adj3 hyperstimulat*):TI,AB,KY 1240 #26 (intracytoplasmic sperm injection*):TI,AB,KY 1468 #27 (ivf or icsi):TI,AB,KY 4768 #28 implantation:TI,AB,KY 14217 #29 (infertil* or subfertil*):TI,AB,KY 6046 #30 (assisted reproducti* treatment*):TI,AB,KY 92 #31 (assisted reproducti* techn*):TI,AB,KY 604 #32 (artificial* reproducti* techn*):TI,AB,KY 9 #33 pregnanc*:TI,AB,KY 35378 #34 embryo*:TI,AB,KY 5563 #35 blastocyst*:TI,AB,KY 938 #36 fertil*:TI,AB,KY 6429 #37 #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 54092 #38 #21 AND #37 248
Appendix 3. MEDLINE search strategy
Searched from 1946 to 8 January 2019
OVID platform
1 exp ENDOMETRIOSIS/ (20399) 2 Endometrio*.tw. (27613) 3 or/1‐2 (31305) 4 (buserelin or goserelin).tw. (2218) 5 (leuprolide or nafarelin).tw. (2019) 6 triptorelin.tw. (706) 7 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (30057) 8 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (5756) 9 (dirigestran or factrel or gonadoliberin).tw. (160) 10 leuprorelin.tw. (434) 11 down regulat*.tw. (127662) 12 downregulat*.tw. (117113) 13 pituitary suppress*.tw. (359) 14 (Ovar* adj2 suppress*).tw. (1681) 15 (suprecur or suprefact).tw. (30) 16 (zoladex or lupron).tw. (540) 17 exp Gonadotropin‐Releasing Hormone/ (31357) 18 (Gonadotropin* or Gonadotrophin*).tw. (61193) 19 GnRHa.tw. (1434) 20 or/4‐19 (324523) 21 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (39146) 22 vitro fertili?ation.tw. (21455) 23 ovar* stimulat*.tw. (5499) 24 (ovar* adj3 hyperstimulat*).tw. (4893) 25 intracytoplasmic sperm injection$.tw. (6614) 26 (ivf or icsi).tw. (25281) 27 implantation.tw. (151636) 28 (infertil* or subfertil*).tw. (58699) 29 assisted reproducti* treatment*.tw. (686) 30 assisted reproducti* techn*.tw. (9068) 31 artificial* reproducti* techn*.tw. (243) 32 pregnanc*.tw. (385346) 33 embryo*.tw. (331617) 34 blastocyst*.tw. (20675) 35 fertil*.tw. (155547) 36 or/21‐35 (965399) 37 randomized controlled trial.pt. (473863) 38 controlled clinical trial.pt. (92838) 39 randomized.ab. (430801) 40 randomised.ab. (85959) 41 placebo.tw. (199703) 42 clinical trials as topic.sh. (185645) 43 randomly.ab. (302952) 44 trial.ti. (192194) 45 (crossover or cross‐over or cross over).tw. (78777) 46 or/37‐45 (1249782) 47 exp animals/ not humans.sh. (4532405) 48 46 not 47 (1149881) 49 3 and 20 and 36 and 48 (205)
Appendix 4. Embase search strategy
Searched from 1980 to 8 January 2019
OVID platform
1 exp endometriosis/ (33417) 2 endometrio*.tw. (40059) 3 or/1‐2 (45891) 4 (buserelin or goserelin).tw. (2993) 5 (leuprolide or nafarelin).tw. (2992) 6 triptorelin.tw. (1133) 7 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (36055) 8 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (5400) 9 (dirigestran or factrel or gonadoliberin).tw. (274) 10 leuprorelin.tw. (667) 11 down regulat*.tw. (170918) 12 downregulat*.tw. (155595) 13 pituitary suppress*.tw. (491) 14 (Ovar* adj2 suppress*).tw. (2192) 15 (suprecur or suprefact).tw. (1457) 16 (zoladex or lupron).tw. (3687) 17 exp gonadorelin/ (31707) 18 (Gonadotropin* or Gonadotrophin*).tw. (63392) 19 GnRHa.tw. (2203) 20 or/4‐19 (412932) 21 exp in vitro fertilization/ (66279) 22 exp intracytoplasmic sperm injection/ (20610) 23 exp embryo transfer/ (30231) 24 vitro fertili?ation.tw. (29039) 25 ovar* stimulat*.tw. (9695) 26 (ovar* adj3 hyperstimulat*).tw. (7547) 27 intracytoplasmic sperm injection*.tw. (9451) 28 (ivf or icsi).tw. (46309) 29 implantation.tw. (214249) 30 (infertil* or subfertil*).tw. (82871) 31 assisted reproducti* treatment*.tw. (1273) 32 assisted reproducti* techn*.tw. (14303) 33 artificial* reproducti* techn*.tw. (413) 34 pregnanc*.tw. (458592) 35 embryo*.tw. (366628) 36 blastocyst*.tw. (27731) 37 fertil*.tw. (184393) 38 or/21‐37 (1133038) 39 Clinical Trial/ (943095) 40 Randomized Controlled Trial/ (525520) 41 exp randomization/ (80582) 42 Single Blind Procedure/ (33489) 43 Double Blind Procedure/ (153616) 44 Crossover Procedure/ (57605) 45 Placebo/ (314683) 46 Randomi?ed controlled trial$.tw. (193503) 47 Rct.tw. (30758) 48 random allocation.tw. (1845) 49 randomly.tw. (393172) 50 randomly allocated.tw. (31235) 51 allocated randomly.tw. (2383) 52 (allocated adj2 random).tw. (798) 53 Single blind$.tw. (21833) 54 Double blind$.tw. (186587) 55 ((treble or triple) adj blind$).tw. (868) 56 placebo$.tw. (276887) 57 prospective study/ (492047) 58 or/39‐57 (2181426) 59 case study/ (58345) 60 case report.tw. (359641) 61 abstract report/ or letter/ (1041120) 62 or/59‐61 (1449925) 63 58 not 62 (2131226) 64 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5594799) 65 63 not 64 (1983619) 66 3 and 20 and 38 and 65 (533)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 8 January 2019
OVID platform
1 Endometrio*.tw. (251) 2 exp Gonadotropic Hormones/ (4136) 3 (Gonadotropin* or Gonadotrophin*).tw. (1652) 4 (buserelin or goserelin).tw. (35) 5 (leuprolide or nafarelin).tw. (83) 6 triptorelin.tw. (28) 7 (GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh).tw. (1168) 8 (gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin).tw. (232) 9 (dirigestran or factrel or gonadoliberin).tw. (2) 10 leuprorelin.tw. (12) 11 down regulat*.tw. (4280) 12 downregulat*.tw. (4661) 13 pituitary suppress*.tw. (2) 14 (Ovar* adj2 suppress*).tw. (61) 15 (suprecur or suprefact).tw. (0) 16 (zoladex or lupron).tw. (23) 17 GnRHa.tw. (40) 18 or/2‐17 (13949) 19 1 and 18 (19) 20 random.tw. (54327) 21 control.tw. (418370) 22 double‐blind.tw. (21866) 23 clinical trials/ (11189) 24 placebo/ (5185) 25 exp Treatment/ (723622) 26 or/20‐25 (1130711) 27 19 and 26 (13)
Appendix 6. CINAHL search strategy
Searched from 1961 to 8 January 2019
EBSCO platform
S51 S38 AND S50 48 S50 S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 1,297,817 S49 TX allocat* random* 9,666 S48 (MH "Quantitative Studies") 21,628 S47 (MH "Placebos") 11,084 S46 TX placebo* 54,826 S45 TX random* allocat* 9,666 S44 (MH "Random Assignment") 52,593 S43 TX randomi* control* trial* 163,393 S42 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) 998,061 S41 TX clinic* n1 trial* 237,800 S40 PT Clinical trial 86,732 S39 (MH "Clinical Trials+") 254,342 S38 S21 AND S37 179 S37 S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S34 OR S35 OR S36 78,148 S36 TX fertil* 26,503 S35 TX blastocyst* 1,976 S34 TX embryo* 20,658 S33 TX pregnanc* 197,479 S32 TX artificial* reproducti* techn* 359 S31 TX assisted reproducti* techn* 3,168 S30 TX assisted reproducti* treatment* 1,673 S29 TX (infertil* or subfertil*) 15,562 S28 TX implantation 26,793 S27 TX intracytoplasmic sperm injection* 790 S26 TX vitro fertili?ation 6,385 S25 TX ovar* stimulat* 2,457 S24 TX ovar* N3 hyperstimulat* 770 S23 TX IVF or TX ICSI 4,478 S22 (MM "Fertilization in Vitro") 3,123 S21 S3 AND S20 390 S20 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 22,238 S19 TX GnRHa* 127 S18 TX (Gonadotropin* or Gonadotrophin*) 4,567 S17 TX (zoladex or lupron) 51 S16 TX (suprecur or suprefact) 2 S15 TX (Ovar* N2 suppress*) 288 S14 TX pituitary suppress* 458 S13 TX downregulat* 7,495 S12 TX down regulat* 8,387 S11 TX leuprorelin 47 S10 TX (dirigestran or factrel or gonadoliberin) 1 S9 TX(gonadorelin or luliberin or luteinizing hormone‐releasing hormone or cystorelin) 1,541 S8 TX(GnRH or lhrh or gn‐rh or lfrh or lh‐rh or lhfshrh) 1,091 S7 TX triptorelin 71 S6 TX (leuprolide or nafarelin) 433 S5 TX (buserelin or goserelin) 330 S4 (MM "Gonadorelin+") 1,010 S3 S1 OR S2 6,033 S2 TX Endometrio* 5,920 S1 (MM "Endometriosis") OR (MM "Adenomyosis") 3,129
Data and analyses
Comparison 1. GnRH agonist therapy versus standard IVF/ICSI alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.26, 0.87] |
| 2 Complication rate | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 OHSS | 2 | 267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.17, 3.40] |
| 2.2 Local reaction at the injection site | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.04 [0.50, 130.02] |
| 2.3 Premature luteinisation | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.06, 16.18] |
| 3 Clinical Pregnancy Rate | 6 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.91, 1.41] |
| 4 Multiple pregnancy rate (per woman randomised) | 2 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.03, 0.56] |
| 5 Miscarriage rate (per woman randomised) | 2 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.10, 2.00] |
| 6 Ectopic pregnancy rate (per woman randomised) | 2 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 7.30] |
| 7 Foetal abnormality rate (per woman randomised) | 2 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.93 [0.16, 400.46] |
| 8 Mean number of oocytes | 4 | 385 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [0.06, 1.38] |
| 9 Mean number of embryos | 2 | 267 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.33, ‐0.19] |
| 10 Complication rate (totals) | 3 | 318 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.37, 4.14] |
| 11 Multiple pregnancy rate (per clinical pregnancy) | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.17 [0.03, 1.05] |
| 12 Miscarriage rate (per clinical pregnancy) | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.15, 5.54] |
| 13 Ectopic pregnancy (per clinical pregnancy) | 2 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.00, 13.15] |
| 14 Foetal abnormality rate (per clinical pregnancy) | 2 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 17.58 [0.29, 1073.88] |
| 15 Live birth rate (ASRM I/II endometriosis) | 1 | 33 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.03, 0.83] |
| 16 Live birth rate (ARSM III/IV endometriosis) | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.22, 1.10] |
| 17 Complication rate (ARSM I/II endometriosis | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Complication rate (ARSM III/IV endometriosis) | 1 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 1.42] |
| 19 Clinical pregnancy rate (ARSM I/II endometriosis) | 3 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.74, 1.48] |
| 20 Clinical pregnancy rate (ARSM III/IV endometriosis) | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.53, 1.49] |
1.19. Analysis.
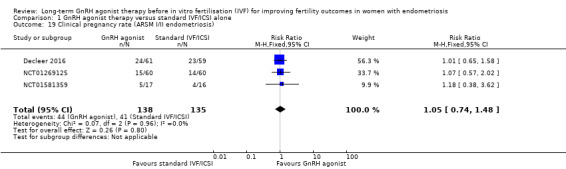
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 19 Clinical pregnancy rate (ARSM I/II endometriosis).
1.20. Analysis.
Comparison 1 GnRH agonist therapy versus standard IVF/ICSI alone, Outcome 20 Clinical pregnancy rate (ARSM III/IV endometriosis).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Decleer 2016.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Belgium Site: Fertility Center, Department of Obstetrics and Gynaecology, Jan Palfijn Hospital, Ghent Participants: 120 patients with stage I or II endometriosis presenting for advanced fertility treatment Mean age + SD: 30.3 + 3.63 for Group A and 31.7 + 4.28 for Group B Inclusion: American Fertility Society Stage I or II endometriosis diagnosed at laparoscopy, younger than 38 years with indication for IVF Exclusion: patients older than 38 years, severe male problems (e.g. indication for testicular sperm extraction), severe endometriosis, ovarian endometriotic cysts, deep fibrosing endometriosis of the rectovaginal septum, uterine pathology, such as congenital malformation of the uterine cavity or fibroids and patients with major endocrine problems |
|
| Interventions | All included patients underwent laparoscopy during which therapeutic measures were taken, such as adhesiolysis or coagulation of endometriotic lesions, whereby all visible lesions were destroyed by bipolar or laser coagulation. Following randomisation, patients in Group A were started on a 3‐month pituitary suppression with a long‐acting GnRH agonist (Zoladex 3.6 mg, AstraZeneca, Cheshire, UK), using one ampule in the abdominal subcutaneous fat tissue on a monthly basis. Ten days after the last dose of Zoladex was administered, the ovarian stimulation was initiated with Menopur, giving three ampules of 75 IU s.c. daily. The second group (Group B) was referred to IVF straight away, without hormonal treatment. To avoid possible bias from comparing long protocol stimulation with short protocol stimulation, the patients in Group B were given a long protocol schedule, using buserelin nasal spray (3×3puffs/day) (Suprefact, Sanofi‐Aventis, Frankfurt‐am‐Main, Germany) from day 20 of the pretreatment cycle. They were started on day 3 after initiation of menstruation, with progesterone level lower than 1.5 ng/ml, using three ampules of Menopur 75 IU (Ferring, Aalst, Belgium) s.c. on a daily basis. The so‐called pretreatment cycle (down‐regulation) was the first cycle after the endoscopic coagulation of endometriosis lesions. In both groups, an ultrasound evaluation of the size and number of the follicles, as well as the endometrial thickness was performed on day 7, starting from the first administration of Menopur. According to the result, the dose of FSH was adapted in order to achieve optimal follicular development. As soon as three follicles or more had reached an average diameter of 17 mm, oocyte maturation as well as ovulation triggering was performed by the i.m. administration of 5000 IU hCG (Pregnyl, MSD, Brussels, Belgium). Levels of oestrogen, progesterone, LH and FSH were measured at the start of the stimulation (baseline), on day 7, the day of triggering and the day after triggering, as in routine IVF monitoring (Unicell DXI 800 Beckman Coulter, USA). After exactly 36 h, a vaginal ultrasound guided oocyte retrieval took place. Embryo transfer was performed on day 3 after egg retrieval, using a soft tip catheter (Wallace, Smiths Medical International, Kent, UK). One or two embryos were transferred according to the Belgian legislation (one embryo at first attempt in women 36 years old or younger, one or two embryos depending on embryo quality in the second attempt, two embryos transferred from the third attempt onwards; for women over 36 years, two embryos per transfer). The luteal phase was supported by the vaginal application of 200 mg micronised progesterone (Utrogestan, Besins, Brussels, Belgium: vaginal tablets, three times a day), as well as s.c hCG (Pregnyl, MSD, Brussels, Belgium) 1500 IU on days 1 and 5 after transfer. hCG and progesterone were measured 13 days after transfer, and in case of positive results a first ultrasound examination was performed 10‐14 days later to confirm pregnancy, to exclude ectopic implantation, and to count the number of gestational sacs. Two weeks later assessment of heart activity was performed using a vaginal ultrasound probe, in which case ongoing pregnancy was diagnosed. |
|
| Outcomes |
|
|
| Notes | A power calculation was conducted as follows: "To significantly detect a difference of 2 Metaphase II (MII) oocytes between groups with a SD in each group equal to 4, 120 subjects in total are needed (80% power, 2‐sided t‐test, 5% significance level)". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was carried out through a computer program" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open label trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open label trial" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 explained loss: "lost to follow‐up (patient did not show up for follow‐up consultation) (n = 1)" Intention‐to‐treat analysis not performed. For the purposes of this meta‐analysis, the number of women randomised was used. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported |
| Other bias | Low risk | No significant difference in basal patient characteristics except for a trend in differences in age (patients in the control group tended to be slightly older, P = 0.060). No conflict of interest declared. No external funding was sought or obtained for this study. |
Dicker 1992.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Israel Site: Sherman Fertility Institute, Department of Obstetrics‐Gynecology, Golda Meir Medical Center, Hasharon Hospital, Petah‐Tikva, and Tel‐Aviv University Medical School, Tel‐Aviv Participants: 67 women with severe endometriosis referred for IVF Mean age + unclear if SD : 32 + 4 for Group A and 31 + 5 for Group B Inclusion: severe endometriosis as categorised by the American Fertility Society, failed to conceive; laparoscopic visualisation and biopsy of endometrial implants Exclusion: concomitant infertility factor other than mechanical |
|
| Interventions | 32 patients underwent ovarian stimulation for oocyte retrieval without any preparation (protocol A), whereas 35 women underwent a 6‐month period of hormonal suppression therapy with a GnRH agonist (protocol B), 3.2 mg of D‐Trp6 luteinising hormone‐releasing hormone microcapsules (Decapeptyl; Ferring, Kiel, Germany) before the IVF procedure. Induction of ovulation was done by the administration of pure follicle‐stimulating hormone (FSH) (Metrodin; Serono Laboratories, Randolph, MA) combined with human menopausal gonadotrophin (Pergonal; Serono Laboratories) starting on the 3rd day of the cycle with administration of 3 ampules per day of either preparation. Human chorionic gonadotrophin (hCG, Chorigon; Teva, Kfar‐Saba, Israel), 10,000 U, was administered in the presence of at least two follicles > 16 mm, serum 17β‐estradiol (E2) levels of 150 to 200 pg/mL for each follicle and progesterone (P) levels not > 1.5 ng/mL. Aspiration of oocytes was done 32 to 34 hours after hCG injection; they were classified as preovulatory, immature, or degenerated and inseminated 3 to 6 hours after collection. Pronuclei were identified 16 to 20 hours after insemination. Daily blood samples were taken for β‐hCG, E2, and P on the 11th day after ET. |
|
| Outcomes |
|
|
| Notes | The authors state: "Of the clinical pregnancies 1 of 2 in group A and 8 of 12 in group B reached viability". As this statement does not strictly meet the Zegers‐Hochschild 2017 definition of live birth, these data were not included as live births or extrapolated to calculate miscarriages. For the purposes of this review, 'severe' endometriosis was interpreted to fall within ASRM III/IV as per the previous AFS classification system. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly and sequentially allocated" |
| Allocation concealment (selection bias) | Unclear risk | "Randomly and sequentially allocated" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo injections in Group A |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding as reported in Sallam 2006 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated if participants were lost to follow‐up. Results presented per cycle so difficult to tell if women were lost to follow‐up or excluded. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported. |
| Other bias | Unclear risk | No significant difference in patient characteristics. No statement regarding competing interests. No declaration of funding source(s), if any. |
NCT01269125.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Greece Site: Department of Obstetrics and Gynecology, Ioannina University School of Medicine, Ioannina Participants: 120 infertile women referred for IVF Mean age + SD: 33.8 + 3.2 in Group A and 31.8 + 2.1 in Group B. Inclusion: 29‐38 years old, laparoscopically documented mild endometriosis (up stage II) Exclusion: Women with sonographic evidence of ovarian endometrioma > 2 cm in mean diameter, early follicular phase serum Follicular Stimulating Hormone (FSH) levels > 12 mIU/ml, male factor (defined as a concentration of motile sperm less than 10 x 106 /ml and sperm with normal morphology less than 4%) |
|
| Interventions | Prior to randomisation, the extent of endometriosis was documented at laparoscopy and all visible endometriotic lesions were cauterised with bipolar diathermy. Following randomisation, Group A consisted of 60 women who received a depot preparation of a GnRHa, 3.75 mg s.c, (leuprolide, Daronda depot, 3.75, Abbott, Hellas) every 28 days for three injections. Down‐regulation was initiated 30 to 45 days after the third GnRHa injection. Group B consisted of 60 women with endometriosis who received no pretreatment with GnRHa. They underwent controlled ovarian hyperstimulation (COH) after down‐regulation with a GnRHa (leuprolide, 20 IU/day, Daronda, 2.8, Abbott, Hellas) in a long protocol with a mid‐luteal start. Administration of recombinant follicle stimulating hormone (rFSH, Gonal‐F, Serono, Geneva, Switzeland) was started after at least 14 days of leuprolide therapy and when serum estradiol (E2) had been less than 100 pmol/L and when the thickness of the endometrium was less than 5 mm. A starting dose of 150 IU of follicle stimulating hormone (rFSH, Gonal‐F, Serono, Geneva, Switzerland) was adjusted individually from day 6 of the cycle according to estradiol (E2) values and ultrasonographic follicular measurements. An ovulatory dose of human chorionic gonadotropin (hCG) (Pregnyl, Organon, Oss, The Netherlands) 5000‐10,000 IU was administered I.M. when mean diameter of an average of two to four follicles was larger than 16 mm and the plasma estradiol concentration was higher than 1500 pmol/L. All women were given natural micronised progesterone as luteal‐phase support (Ultrogestan, Faran, Athens, Greece), 600 mg daily vaginally in three divided dosages, starting the day after embryo transfer. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization is performed by accessing a central internet‐based randomization program." |
| Allocation concealment (selection bias) | Unclear risk | "The random allocation sequence and the assignment of the participants to interventions were made by the first author of the study." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open label" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Women lost to follow‐up and excluded because of adverse reactions. Intention‐to‐treat analysis not performed. For the purposes of this meta‐analysis, the number of women randomised was used. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported. Change in secondary outcomes planned but all secondary outcomes reported nevertheless. |
| Other bias | High risk | This is an unpublished study. No difference in basal patient characteristics. No statement regarding competing interests. No declaration of funding source(s), if any. |
NCT01581359.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Spain Site: Human Reproduction Unit, La Fe University and Politechnic Hospital, Valencia Participants: 147 infertile patients with surgical diagnosis of endometriosis Mean age + SD: 33.85 + 3.17 in the intervention arm and 33.73 + 3.44 in the control arm Inclusion: Infertile women with endometriosis diagnosed by surgery, BMI < 28 kg/m2, age < 40 years old, signed informed consent to perform IVF and participation in this study Exclusion: Follicle stimulating hormone (FSH) 2nd‐5th cycle day > 12 IU/L, liver disease (sALAT > 80 IU/L), kidney disease (creatinine > 130 nmol/L), other relevant disease that contraindicates a pregnancy. |
|
| Interventions | Prior to randomisation, at time of laparoscopy, endometriosis was treated via cystectomy and adhesiolysis with bipolar. Intervention arm (66 patients): Triptorelin acetate 3.75 mg subcutaneous injection administered on days 1, 28 and 56 after menstrual cycle. Control arm (71 patients): Saline subcutaneous injection with same delivery device and same volume as intervention arm administered on days 1, 28 and 56 after menstrual cycle. Controlled ovarian stimulation was started 80 +/‐ 3 days after the first injection. In both groups, patients were treated with gonadotropins (hMG‐HP: Menopur, Ferring SAU, Madrid and/or recombinant FSH (rFSH): Puregon, MSD Barcelona or Gonal, Merck, Madrid) daily. GnRH antagonist (Ganirelix –Orgalutran, MSD, Madrid, or Cetrorelix –Cetrotide, Merck, Madrid) co‐treatment was initiated at daily dose of 0,25 mg on stimulation day 6 or when at least one follicle of mean diameter was 14 mm or more, and was continued until hCG administration. Transvaginal ultrasound guided oocyte retrieval, under venous sedation, was performed 36 hours after administration of 250 mcg of human chorionic gonadotropin (hCG) (Ovitrelle 250 mcg, Merck, Madrid) when there was at least one follicle was >17 mm and three or more follicles reached a mean diameter of 16 mm. On day 2 or 3 of the embryo culture, up to two embryos were selected and transferred. All the fresh embryo transfers were performed only in the progesterone levels < 1.5 ng/mL on the trigger day. For fresh transfers the luteal phase was supported by the vaginal application of 200 mg twice a day of micronised progesterone (Progeffik tablets 200 mg, Effik Laboratories, Madrid or Utrogestan tablets 200 mg, SEID, Barcelona) starting the day after the egg retrieval. For frozen embryo transfers, progesterone supplementation (200 mg three times a day) started when monitoring was adequate and one or two embryos were thawed and transferred 2, 3 or 5 days after the start of progesterone according to the day in which the embryos were frozen. Serum hCG was measured 15 days after the embryo transfer to confirm biochemical pregnancy, and in case of positive results the first ultrasound examination was performed two weeks later. |
|
| Outcomes |
|
|
| Notes | The study authors were contacted and provided further information for inclusion into this systematic review. "The sample size was calculated for a superiority trial of GnRHa over placebo for a two‐fold increase in the primary outcome measure (clinical pregnancy rate) from 20% to 40%. The calculation indicated that the minimal sample should be 180 patients (90 in each arm) to have an 80% chance of detecting the difference, at significance level of 0.05. Assuming a 10% withdrawal rate, we planned to enrol 200 patients." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was carried out by a statistician with no clinical involvement in the trial, through a computer program using a random block sequence. Subjects were allocated in blocks of four" |
| Allocation concealment (selection bias) | Low risk | "Patients were assigned to each group through opaque‐sealed envelopes, sequentially numbered" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The treating physicians, embryologists and patients were blinded for the randomization results during the study until the follow‐up was finished." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The treating physicians, embryologists and patients were blinded for the randomization results during the study until the follow‐up was finished." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 fewer patients (1 from intervention group and 3 from control group) were randomised but subsequently excluded because of "voluntary drop out or selection failure (BMI)" For the purposes of this meta‐analysis, the number of women randomised was used. |
| Selective reporting (reporting bias) | Low risk | Change in original primary outcome from live birth rate to clinical pregnancy rate. All a priori outcomes were reported. |
| Other bias | High risk | This is an unpublished study. No difference in basal patient characteristics. No statement regarding competing interests. No declaration of funding source(s), if any. |
Rickes 2002.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Germany Site: Clinic for Reproductive Medicine and Gynecologic Endocrinology, Faculty of Medicine, Otto‐von‐Guericke University, Magdeburg Participants: 110 patients with stage II‐IV endometriosis according to ASRM criteria Mean age + SD: no statistical difference in between groups in terms of mean age but no details provided Inclusion: Women in whom stage II to IV endometriosis had been diagnosed by videolaparoscopy Exclusion: Lack of desire to conceive, age older than 40 years, dependence on testicular sperm in ART. |
|
| Interventions | The authors state their aim at laparoscopy/laparotomy was to completely remove all endometriosis and carry out adhesiolysis prior to randomisation. Fifty‐five women randomised to therapy with goserelin (Zoladex‐Gyn; AstraZeneca, Wedel, Germany), 3.6 mg, received their first s.c. dose on day 3 after surgery. These women received GnRHa monthly over 5 or 6 cycles. Patients scheduled for IVF or ICSI had patent but poorly movable fallopian tubes, an unfavourable position of the fallopian tubes with respect to the ipsilateral ovary, or up to grade III oligoasthenoteratozoospermia in the partner. ICSI was performed if the progressive motile sperm count in the processed semen was less than 1 x 106 cells/mL. In patients who had surgery only, ovarian stimulation began after down‐regulation with 0.1 mg of GnRHa in the long protocol. The women received daily s.c. injections of GnRHa (Decapeptyl 0.1; Ferring, Kiel, Germany) from cycle day 18. Starting on day 3 of the new cycle, daily injections of recombinant FSH (Gonal‐F; Serono), 150 IU to300 IU, were given. Ovulation was induced with 10,000 IU of hCG (Pregnesin; Serono). Ultrasonography‐guided follicular puncture was performed 35 to 36 hours later. After processing of the eggs and sperm and cultivation of the pronuclei, embryos were transferred intrauterinely 72 hours later. At least two but no more than three embryos were transferred per cycle. The luteal phase was supported by vaginal application of progesterone (Utrogest; Kade‐Bessin), 300 mg/d, and hCG, 1500 to 2500 IU, on days 1, 3, and 6 after transfer. In patients who received GnRHa treatment, ovarian stimulation was begun exactly 2 weeks after the last depot injection of GnRHa by giving daily injections of recombinant FSH (Gonal‐F; Serono), 150 IU to 300 IU. The remainder of the course was identical to the procedure described above, including luteal phase support. A maximum of three IVF/ICSI cycles were included in the analysis. |
|
| Outcomes |
|
|
| Notes | Of the 110 women randomised, 55 were allocated to therapy with GnRHa and 55 were allocated to no GnRHa treatment. Of the 55 who had GnRHa treatment, 27 went on to have IUI and 28 went on to have IVF/ICSI. Of the 55 who did not have GnRHa treatment, 36 went on to have IUI and 19 went on to have IVF/ICSI. Only the patients undergoing IVF/ICSI were included in this systematic review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized by computer in blocks of six" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Neither patients nor physicians were blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Neither patients nor physicians were blinded to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | A priori outcome was reported |
| Other bias | High risk | No difference in basal patient characteristics. Sponsored by AstraZeneca. |
Surrey 2002.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: USA Site: 3‐centre study: 1. Colorado Center for Reproductive Medicine, Englewood, Colorado; 2. Texas Fertility Center, Austin, Texas; 3. Reproductive Medicine and Surgery Associates, Beverly Hills, California Participants: IVF‐ET candidates with surgically confirmed endometriosis within 60 months of cycle initiation Mean age + SE: 33.12 + 0.67 in Group 1 and 32.58 + 0.56 in Group 2 Inclusion: endometriosis documented at laparoscopy or laparotomy within 60 months of cycle initiation (stage I‐IV), regular menses (26‐33 days), candidates for autologous IVF‐ET undergoing fresh embryo transfer only Exclusion: early follicular FSH > 12 mIU/mL, evidence of ovarian endometrioma |
|
| Interventions | Group 1 consisted of 25 patients who received a depot preparation of the GnRH agonist leuprolide acetate (Lupron Depot; TAP Pharmaceuticals, Waukegan, IL), 3.75 mg i.m. every 28 days for three injections. The first injection was administered during the early follicular phase. Controlled ovarian hyperstimulation as described below was initiated within 45 days of the last leuprolide acetate injection. Group 2 consisted of 26 controls with endometriosis who did not receive the long‐acting GnRH agonist but instead underwent standard controlled ovarian hyperstimulation regimens. Controlled ovarian hyperstimulation consisted of standard GnRH agonist down‐regulation using leuprolide acetate (TAP Pharmaceuticals), 0.5 to 1.0 mg/d s.c. for 7 to 10 days, initiated 30 to 45 days after the third leuprolide acetate depot injection (group 1) or in the mid‐luteal phase (group 2). Once gonadotropin suppression was confirmed, the dose was reduced to 0.25 to 0.5 mg/d s.c., and exogenous gonadotropin stimulation was initiated. Other participants received a micro‐dose flare regimen. The specific regimen and gonadotropin dose to be used was determined before randomisation and based on the standard protocols of each institution. Human chorionic gonadotropin was administered when at least one follicle achieved a mean diameter of 19 mm and the participant’s serum estradiol level was at least 500 pg/mL. Techniques and indications for oocyte aspiration, oocyte and embryo culture, insemination, ICSI, assisted hatching, and embryo transfer were based on the protocols specific to each centre. |
|
| Outcomes |
|
|
| Notes | Only one cycle was included for each woman. Corresponse with the authors as part of the previous version of this review (Sallam 2006) confirmed no complications, no ectopic pregnancies and no foetal abnormalities. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a computer‐generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo injections |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding as reported in Sallam 2006 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Correspondence with authors at the time of the previous version of this review (Sallam 2006), confirmed no dropouts from the study |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported. |
| Other bias | High risk | A significantly greater proportion of intervention group patients than control group patients had stages III or IV endometriosis according to revised American Society for Reproductive Medicine scoring (P < 0.05). Supported by grant from TAP Pharmaceuticals, Waukegan, IL. |
Surrey 2010.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: USA Site: Colorado Center for Reproductive Medicine, Lone Tree, Colorado Participants: IVF candidates with regular menses, surgically confirmed endometriosis, and normal ovarian reserve. Inclusion: Participants with surgically documented endometriosis, evidence of normal ovarian reserve testing (day 3 serum FSH level < 11 mIU/mL, E2 level < 60 pg/mL, and bilateral antral follicle count > 4), normal clomiphene challenge test was required for all women > 38 years. All participants had a normal uterine cavity documented at pre‐cycle office hysteroscopy. Exclusion: Current sonographic evidence of an ovarian endometrioma > 2 cm in mean diameter, treatment with depot preparations of a GnRH agonist, danazol, or other hormonal suppressive therapy for endometriosis within 6 months of study entry. |
|
| Interventions | Participants were randomised into one of four groups based on αvβ3 vitronextin expression and treatment protocol. Patients randomised to Group 1 and Group A proceeded directly to controlled ovarian hyperstimulation and IVF. Patients randomised to Group 2 and Group B received an intramuscular preparation of the GnRH agonist leuprolide acetate (TAP Pharmaceuticals, Waukegan, IL) 3.75 mg every 28 days for three injections before initiation of controlled ovarian hyperstimulation. Standard GnRH agonist down‐regulation or microdose flare protocols were used for controlled ovarian hyperstimulation. The determination for which protocol was to be employed was based on ovarian reserve testing and response in previous cycles when appropriate. Indications for hCG administration, day 3 versus 5 embryo transfer and numbers of embryos to transfer were based on previously published protocols and ASRM/SART guidelines. |
|
| Outcomes |
|
|
| Notes | Intervention and control group further subdivided by integrin αvβ3 vitronectin expression. Study authors contacted and a reply containing further information was received but unfortunately not all raw data were available. In light of high risk of attrition bias and inability to deduce data per woman randomised, this study was not included for meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized by two separate computer generated randomized number tables" |
| Allocation concealment (selection bias) | High risk | Email correspondence with authors confirming no allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open label" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis not performed for 1 patient in Group B |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported |
| Other bias | High risk | No difference in basal patient characteristics. Supported by grant from TAP Pharmaceuticals, Waukegan, IL |
Totaro 2009.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Italy Site: Gynaecology and Obstetrics Unit, University of Bari, Bari, Italy; Department of Obstetrics and Gynaecology, Negrar‐VR Participants: 61 women with stage III‐IV endometriosis diagnosed by laparoscopy Mean age + SD: not stated Inclusion: age < 40 years, basal FSH < 12UI/mL, first IVF attempt Exclusion: not stated Group difference: unknown |
|
| Interventions | After surgery, group 1 (29 patients) received 3.75 mg triptorelin for 6 months and ovarian stimulation within 45 days of the last triptorelin injection. Group 2 (32 patients) underwent ovarian hyperstimulation with GnRH agonist 3.75 mg in a long protocol, within 2‐6 months of laparoscopy | |
| Outcomes |
|
|
| Notes | Response awaited from authors as to whether data presented are mean + SD and what the definition of pregnancy is. Data not included for meta‐analysis at present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported |
| Other bias | Unclear risk | No statement regarding basal patient characteristics. No statement regarding competing interests. No declaration of funding source(s), if any. |
2PN: 2 pronuclear stage embryo; AFS: American Fertility Society; ART: assisted reproductive technology; ASRM: American Society for Reproductive Medicine; BMI: body mass index; COH: controlled ovarian hyperstimulation; E2: oestradiol; ET: embryo transfer; FSH: follicle stimulating hormone; GnRH: gonadotrophin‐releasing hormone; GnRHa: gonadotrophin‐releasing hormone agonist; (β‐)hCG: human chorionic gonadotrophin; hMG‐HP: highly purified human menopausal gonadotropin; ICSI: intracytoplasmic sperm injection; IL‐1β: interleukin 1 beta; IL‐6: interleukin 6; IL‐8: interleukin 8; IL‐1‐ra: interleukin 1 receptor antagonist; IVF: in vitro fertilisation; IVF‐ET: in vitro fertilisation and embryo transfer; i.m.: intramuscular; IU: international units; LH: luteinising hormone; MII: metaphase II; OHSS: ovarian hyperstimulation syndrome; P: progesterone; rFSH: recombinant follicle stimulating hormone; sALAT: soluble alanine transaminase; SART: Society for Assisted Reproductive Technology; s.c.: subcutaneously; SD: standard deviation; SE: standard error of the mean; TNF‐α: tumour necrosis factor alpha; U: units.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Badawy 2003 | This is a review article. Reason for exclusion: wrong study design |
| Donnez 1990 | 225 patients treated for 6 months with: 60 mg danazol daily; 5 mg lynestrenol daily; 2.5 mg gestrinone 3 times per week; 300 μg buserelin intranasally 3 times daily; or, buserelin implant subcutaneously. Pregancy rate calculated for follow‐up of 18 months. The patients in this study did not undergo IVF/ICSI. Reason for exclusion: wrong study design |
| Ghosh 2005 | 42 women were split into 2 groups: 18 treated with GnRH agonist depot leuprolide acetate 3.75 mg once per month for 3 consecutive months before conventional long protocol IVF; 24 had conventional long protocol IVF treatment alone. This is not a randomised controlled trial. Reason for exclusion: wrong study design |
| Hefni 1997 | 70 women with laparoscopically documented symptomatic endometriosis were assigned treatment: 37 women for Zoladex 3.6 mg depot s.c. every 4 weeks; and laparoscopic diathermy of endometriosis in 33 women. This is not a randomised controlled trial. Reason for exclusion: wrong study design |
| Henzl 1989 | 143 patients randomly assigned to receive: intranasal naferelin (400 ug/day or 800 ug/day) with placebo capsules; or 800 mg danazol capsules with placebo nasal spray. Reason for exclusion: wrong comparator |
| Hosokawa 1994 | This is not a randomised controlled trial. Reason for exclusion: wrong study design |
| Karlstrom 2000 | A total of 172 couples with unexplained infertility (n = 88), endometriosis (n = 39), cervical (n = 24) or male (n = 21) factors were included, of whom 161 fulfilled the inclusion criteria and treatment. Eighty‐one women were treated with GnRH‐a/hMG and another 80 with hMG only. Artificial insemination was performed as direct intraperitoneal insemination (DIPI) with a volume of 0.3mL to 1.0 mL in 118 couples in the first part of the study and as intrauterine insemination (IUI) with a volume of 0.4 mL 0.7 mL in 33 couples in the latter part of the study. None of the patients underwent IVF/ICSI. Reason for exclusion: wrong intervention |
| Lemay 1987 | 13 patients randomised to 2 groups: 400 μg of buserelin nasal spray three times daily, or LH‐RH agonists by s.c. injection once daily. Reason for exclusion: wrong comparator |
| Loverro 2001 | 62 women diagnosed during a laparoscopy with AFS stages III or IV endometriosis, were randomised after surgical treatment in a prospective trial. Thirty‐three patients were treated with a GnRH agonist (decapeptyl 3.75‐Ipsen, one for 28 days) for 3 months after laparoscopy; the remaining 29 women received no therapy. Reason for exclusion: no IVF/ICSI in either group |
| Loverro 2008 | Sixty patients (mean age 28.6 years) with symptomatic stages III and IV endometriosis following laparoscopic surgery and without previous hormonal treatment were enrolled in a prospective, randomised, controlled trial to compare the effects of 3‐month treatment with triptorelin depot—3.75 i.m. (30 patients) versus expectant management using placebo injection (30 patients). Reason for exclusion: no IVF/ICSI in either group |
| Ma 2008 | 162 women surgically diagnosed as having moderate or severe endometriosis. Pituitary down‐regulation was achieved with injections of a GnRH agonist prior to the IVF procedures either for 7 to 10 days in the mid‐luteal phase (group 1 (standard protocol), 97 cycles in total), or for 2 months (group 2, 55 cycles), or 3 months (group 3, 75 cycles). Women could self‐allocate to groups hence this is not a randomised controlled trial. Reason for exclusion: wrong study design |
| Maged 2018 | 90 patients with an indication for ICSI and having unilateral single endometrioma of less than 5 cm in diameter were randomised into 2 groups. Group A (45 patients) received the standard long protocol; whereas group B (45 patients) received 3 consecutive Intramuscular (IM) injections of triptorelin 3.75 mg 28 days apart followed by the standard long protocol 28 days after the last injection. The diagnosis of endometrioma was made by ultrasound. Reason for exclusion: wrong patient population |
| Montanino 1996 | 36 women with ultrasonographic diagnosis of ovarian endometrioma were treated laparoscopically. After surgery the patients were assigned to one of three groups: GnRHa for 3 months, oral contraceptives, nothing. This is not a randomised controlled trial. Reason for exclusion: wrong study design |
| Monzo 2016 | As confirmed in correspondence with the authors, this is a sub‐analysis of NCT01581359 looking at CYP19A1 gene expression in granulosa cells. Reason for exclusion: sub‐study on same patient population |
| Nakagawa 2000 | This is not a randomised controlled trial. Reason for exclusion: wrong study design |
| NCT00654524 | GnRH‐a injection (goserelin 3.6 mg) every 4 weeks for 6 months compared to spontaneous pregnancy. Reason for exclusion: no IVF/ICSI in either group |
| NCT01682642 | This is a duplicate study of Decleer 2016 |
| NCT02400801 | Patients with prior laparoscopic treatment of endometriosis and an indication for ART were randomised into 2 groups; the control group underwent ART in a classical long agonist protocol using preparation with oral contraceptives, the ultra‐long group first underwent at least 3 months down‐regulation followed by a long agonist protocol for ART stimulation. The outcome measure was clinical pregnancy rate. Only 2 patients in the control received a minimum of 6 weeks of COC. Reason for exclusion: wrong study design |
| Parazzini 1994 | 75 women underwent laparotomy as first surgical treatment for debulking or radical surgery of endometriotic lesions. Patients were randomised to nasal nafarelin, 400 ug/day (36 subjects) or placebo nasal spray (39 subjects) for 3 months. Pelvic pain was assessed before first surgery and at the 12‐month follow‐up visit in women with pelvic pain. Pregancy during 1‐year follow‐up recorded. Reason for exclusion: no IVF/ICSI in either group |
| Rodriguez‐Tarrega 2016 | As confirmed in correspondence with the authors, these data originate from NCT01581359, but include patients diagnosed with endometriosis either surgically or via ultrasound. Reason for exclusion: sub‐study on same patient population |
| Sahebkashaf 2003 | 102 participants were randomised into two groups. Group 1 consisted of 50participants who received a depot preparation of the GnRH agonist decapeptyl 3.75 mg IM every 28 days for three injections. Controlled ovarian hyperstimulation consisted of standard GnRH agonist down‐regulation using LHRH agonist buserelin (suprefact) HMG combinations in the form of a long protocol and transvaginal ultrasound guided oocyte retrieval, ICSI and laparoscopic tubal transfer within 45 days
of the last decapeptyl injection. Group 2 consisted of 52 controls with endometriosis who did not receive the long‐acting GnRH agonist but instead underwent standard controlled ovarian hyperstimulation regimen. Participants did not undergo standard IVF/ICSI but instead had laparoscopic tubal transfer. Reason for exclusion: wrong study design |
| Shawki 2002 | 68 cases of stage I or II endometriosis were randomised for either: no further treatment (34 participants); or, postoperative zoladex 3.6 mg subcutaneous injection every 28 days for 6 months (34 participants). Cases were followed up for period of one year to assess pregnancy rate after completion of analogue treatment cycles. If no pregnancy occurs after 3 months of spontaneous cycles, clomiphene citrate stimulation is added for another 3 cycles. Reason for exclusion: no IVF/ICSI in either group |
| Song 2013 | No IVF/ICSI Reason for exclusion: wrong study design |
| Tamura 2012 | 23 women were randomly divided into 2 groups for IVF‐ET; ultra‐long protocol (UL group: n = 11) and long protocol (L group: n = 12). In the UL group, GnRHa (1.8 mg s.c. every 28 days) was administered for 3 months until hCG injection in the IVF‐ET cycle. In the L group, GnRHa (900 µg/day) was given from the mid‐luteal phase in the previous cycle to the time of hCG injection in the IVF‐ET cycle. Reported measures were number of matured follicles, the number of retrieved oocytes, fertilisation rates, implantation rates, clinical pregnancy rates, intrafollicular concentrations of TNF‐α, 8‐hydroxy‐2’‐deoxyguanosine, hexanoyl‐lysine, and melatonin and Cu, Zu‐superoxide dismutase. This is not a randomised controlled trial. Reason for exclusion: wrong study design |
| Tomassetti 2018 | This is a protocol for an ongoing study and diagnosis of endometriosis is by ultrasound. Reason for exclusion: wrong study design |
| Vercellini 1999 | 269 women underwent surgery and were then assigned to treatment with subcutaneous goserelin depot injections for six months or to expectant management. The women were evaluated regularly for two years. Main outcome measures: Postoperative pain recurrences (total symptoms scores > 5), time to recurrence, pregnancy rates and time to conception in the two study groups. Reason for exclusion: no IVF/ICSI in either group |
| Yang 2014 | 125 women were treated with laparoscopic bilateral ovarian endometrial cystectomies and then subsequently split into 2 groups: gonadotropin‐releasing hormone agonist (GnRH‐a) treatment for three months or no further treatment. Outcome measures: the changes of follicle stimulating hormone (FSH) and FSH/luteinising hormone (LH), estradiol (E2) in preoperative and postoperative three months or menstrual two to three days, menstrual two to three days after surgery, natural pregnancy, and cyst recurrence in 18th month during postoperative follow‐up. The participants did not undergo IVF/ICSI. Reason for exclusion: wrong study design |
AFS: American Fertility Society; ART: assisted reproductive technology; COC: combined oral contraception; DIPI: direct intraperitoneal insemination; E2: oestradiol FSH: follicle stimulating hormone; GnRH: gonadotrophin‐releasing hormone; GnRHa: gonadotrophin‐releasing hormone agonist; hCG: human chorionic gonadotrophin; hMG: human menopausal gonadotropin; IVF/ICSI: in vitro fertilisation/intracytoplasmic sperm injection; LH: luteinising hormone; LH‐RH: luteinising hormone‐releasing hormone; TNF‐α: tumour necrosis factor alpha.
Characteristics of studies awaiting assessment [ordered by study ID]
NCT02737800.
| Methods | Prospective open‐label randomised controlled trial |
| Participants | 18‐40 year‐old women with endometrioma |
| Interventions | Group 1 consists of 30 patients of endometrioma more than 5 cm subdivided into:
Group 2 consists of 30 patients of endometrioma less than 5 cm subdivided into :
|
| Outcomes | Primary outcome:
|
| Notes | Study authors contacted to clarify how endometrioma was diagnosed |
GnRH: gonadotrophin‐releasing hormone.
Characteristics of ongoing studies [ordered by study ID]
NCT02779387.
| Trial name or title | Reproductive outcome of EM treated by GnRH‐a associated with laparoscopy |
| Methods | Multicentre open‐label randomised controlled trial |
| Participants |
|
| Interventions | Participants in the experimental group are treated with GnRH‐a after surgery and outpatient guidance. Participants in the control group are treated with outpatient guidance only |
| Outcomes | Primary outcomes:
|
| Starting date | May 2016 |
| Contact information | Yu xiaoming Peking University People's Hospital China |
| Notes | Not entirely clear if patients underwent IVF/ICSI but the study intends to record "accept the assisted reproductive treatment rate" |
NCT03142035.
| Trial name or title | Dienogest versus GnRH‐a pre‐treatment in women with endometriosis undergoing IVF |
| Methods | Prospective non‐blinded randomised controlled trial |
| Participants |
|
| Interventions | This study has 3 groups, 2 of which are relevant. The first group will receive one injection of 3.75 mg of GnRH‐a every 28 days for three doses followed by a standard IVF/ICSI cycle 3 months later. The second group will not receive any medical interventions before the planned IVF/ICSI cycle. |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | 22 October 2017 |
| Contact information | Dina Chamsi, MD American University of Beirut Medical Center Beirut, Lebanon |
| Notes | Only patients with surgically diagnosed endometriosis will meet the inclusion criteria of this systematic review. |
BMI: body mass index; EM: endometriosis; FSH: follicle stimulating hormone; GnRH: gonadotrophin‐releasing hormone; GnRH‐a: gonadotrophin‐releasing hormone agonist; IVF: in vitro fertilisation; TSH: thyroid stimulating hormone; WHO: World Health Organisation.
Differences between protocol and review
In cases of low event rates, we calculated a Peto odds ratio (OR) rather than a risk ratio (RR). We used a random‐effects model for all outcomes (as opposed to just the primary outcomes) if we detected significant heterogeneity.
Contributions of authors
EXG took the lead on the review update postprotocol stage and was involved in preparing all sections of the review. PM and PB were involved in data extraction for the review. CB and IEG made substantial editorial amendments to the review.
JGV contributed to the background of the revised draft of the protocol by applying methodological and statistical expertise. HNS wrote the original review and commented on all drafts of the protocol, including search strategy methods. AA commented on the draft protocol. AMAS participated in the first review and commented on the draft protocol.
Sources of support
Internal sources
No sources of support supplied
External sources
None, Other.
Declarations of interest
EXG has no interests to declare.
PM has no interests to declare.
PB has no interests to declare.
AA has no interests to declare
AMAS has no interests to declare. HNS is a co‐editor of two textbooks on infertility and has received royalties from the publishers. JGV has received consultancy and lecture fees from MSD, Merck Serono, Ferring and Gedeon Richter. CMB has received research support by Bayer, Volition Rx, Roche Diagnostics and MDNA Life Sciences. He has received consultancy fees from ObsEva, AbbVie and Myovant. IEG has received research support from Finox and Bayer.
New
References
References to studies included in this review
Decleer 2016 {published data only}
- Decleer W, Osmanagaoglu K, Verschueren K, Comhaire F, Devroey P. RCT to evaluate the influence of adjuvant medical treatment of peritoneal endometriosis on the outcome of IVF. Human Reproduction 2016;31(9):2017‐23. [DOI] [PubMed] [Google Scholar]
Dicker 1992 {published data only}
- Dicker D, Goldman JA, Levy T, Feldberg D, Ashkenazi J. The impact of long‐term gonadotropin‐releasing hormone analogue treatment on preclinical abortions in patients with severe endometriosis undergoing in vitro fertilization‐embryo transfer. Fertility and Sterility 1992 Mar;57(3):597‐600. [DOI] [PubMed] [Google Scholar]
NCT01269125 {published data only}
- NCT01269125. GnRH‐a and pregnancy rate in in vitro fertilization (IVF) cycles [Ultralong administration of GnRH‐a before in vitro fertilization improves fertilization rate but not pregnancy rate in women with endometriosis. A prospective, randomized, controlled trial]. clinicaltrials.gov/show/NCT01269125 (first received 4 January 2011).
NCT01581359 {unpublished data only}
- NCT01581359. The effect of pre‐treatment with GnRH analogues prior in vitro fertilization in patients with endometriosis (ENDOFIV) [A comparative study, randomized, blinded, about the effect of pre‐treatment with GnRH analogues versus placebo in infertile patients with endometriosis undergoing in vitro fertilization treatment]. clinicaltrials.gov/ct2/show/record/NCT01581359 (first received 20 April 2012).
Rickes 2002 {published data only}
- Rickes D, Nickel I, Kropf S, Kleinstein J. Increased pregnancy rates after ultralong postoperative therapy with gonadotropin‐releasing hormone analogs in patients with endometriosis. Fertility and Sterility 2002 Oct;78(4):757‐62. [DOI] [PubMed] [Google Scholar]
Surrey 2002 {published data only}
- Surrey E, Silverberg K, Surrey M, Schoolcraft W. The effect of prolonged GnRH agonist (GnRHa) therapy on in vitro fertilization‐embryo transfer (IVF‐ET) cycle outcome in endometriosis (ENDO) patients: a multicenter randomized trial. Fertility and Sterility 2001;76(3 Suppl 1):S151. [Google Scholar]
- Surrey ES, Silverberg KM, Surrey MW, Schoolcraft WB. Effect of prolonged gonadotropin‐releasing hormone agonist therapy on the outcome of in vitro fertilization‐embryo transfer in patients with endometriosis. Fertility and Sterility 2002;78(4):699‐704. [DOI] [PubMed] [Google Scholar]
Surrey 2010 {published and unpublished data}
- Surrey ES, Lietz AK, Gustofson RL, Minjarez DA, Schoolcraft WB. Does endometrial integrin expression in endometriosis patients predict enhanced in vitro fertilization cycle outcomes after prolonged GnRH agonist therapy?. Fertility and Sterility 2010;93(2):646‐51. [DOI] [PubMed] [Google Scholar]
Totaro 2009 {published data only}
- Totaro I, Lorusso F, Scioscia M, Lorusso M, Mangiatordi G, Selvaggi L, et al. IVF outcome after ultralong postoperative therapy with gonadotrophin‐releasing hormone analogs in patients with advanced‐stage endometriosis. Twenty‐fifth meeting of European Society of Human Reproduction and Embryology; 2009 June 28‐July 1; Amsterdam. 2009:i190.
References to studies excluded from this review
Badawy 2003 {published data only}
- Badawy SZ, Etman AE, Aboulghar MA, Hassan HA. The outcome of in vitro fertilization in infertile women with endometriosis. Middle East Fertility Society Journal 2003;8(2):128‐38. [Google Scholar]
Donnez 1990 {published data only}
- Donnez J, Nisolle M, Clerckx F, Casanas F. Evaluation of preoperative use of danazol, gestrinone, lynestrenol, buserelin spray and buserelin implant, in the treatment of endometriosis associated infertility. Progress in Clinical and Biological Research 1990;323(pz5, 7605701):427‐42. [PubMed] [Google Scholar]
Ghosh 2005 {published data only}
- Ghosh S, Chattopadhyay R, Goswami SK, Chakravarty BN. Ultra‐long GnRH agonist and IVF‐ET in advanced endometriosis. The 21st Annual Meeting of the European Society of Human Reproduction and Embryology. 2005:i105.
Hefni 1997 {published data only}
- Hefni MA. Comparison controlled study of GNRH analogue (Goserelin) and laparoscopic diathermy for the treatment of endometriosis. Acta Obstetricia et Gynecologica Scandinavica 1997;76(Suppl):66. [Google Scholar]
Henzl 1989 {published data only}
- Henzl MR. Role of nafarelin in the management of endometriosis. The Journal of Reproductive Medicine 1989;34(12 Suppl):1021‐4. [PubMed] [Google Scholar]
Hosokawa 1994 {published data only}
- Hosokawa K, Ohno Y, Fujimoto Y, Okada H. Efficacy of GnRH analogue treatment for endometriosis associated with infertility. Japanese Journal of Fertility and Sterility 1994;39(4):36‐40. [Google Scholar]
Karlstrom 2000 {published data only}
- Karlstrom PO, Bergh T, Lundkvist O. Addition of gonadotrophin‐releasing hormone agonist and/or two inseminations with husband's sperm do not improve the pregnancy rate in superovulated cycles. Acta Obstetricia et Gynecologica Scandinavica 2000;79(1):37‐42. [PubMed] [Google Scholar]
Lemay 1987 {published data only}
- Lemay A, Maheux R, Quesnel G, Bureau M, Faure N, Merat P. LH‐RH agonist treatment of endometriosis. Contributions to Gynecology & Obstetrics 1987;16:247‐53. [PubMed] [Google Scholar]
Loverro 2001 {published data only}
- Loverro G, Santillo V, Pansini M, Lorusso F, Depalo R, Selvaggi L. Are GnRH agonists helpful in the therapy of endometriosis after surgical treatment?. Human Reproduction 2001;16(Suppl 1):96. [Google Scholar]
Loverro 2008 {published data only}
- Loverro G, Carriero C, Rossi AC, Putignano G, Nicolardi V, Selvaggi L. A randomized study comparing triptorelin or expectant management following conservative laparoscopic surgery for symptomatic stage III‐IV endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;136(2):194‐8. [DOI] [PubMed] [Google Scholar]
Ma 2008 {published data only}
- Ma C, Qiao J, Liu P, Chen G. Ovarian suppression treatment prior to in‐vitro fertilization and embryo transfer in Chinese women with stage III or IV endometriosis. International Journal of Gynaecology and Obstetrics 2008;100(2):167‐70. [DOI] [PubMed] [Google Scholar]
Maged 2018 {published data only}
- Maged AM, Rashwan H, Mahmood M, El‐Mazny A, Farouk M, Belal DS, et al. Effect of prolonged GnRH agonist downregulation on ICSI outcome in patients with endometriomas of less than 5 cm: a randomized controlled trial. Reproductive Sciences (Thousand Oaks, Calif.) 2018;25(10):1509‐14. [DOI] [PubMed] [Google Scholar]
Montanino 1996 {published data only}
- Montanino G, Porpora MG, Montanino OM, Gulemi L, Boninfante M, Cosmi EV. Laparoscopic treatment of ovarian endometrioma. One year follow‐up. Clinical and Experimental Obstetrics & Gynecology 1996;23(2):70‐2. [PubMed] [Google Scholar]
Monzo 2016 {published data only}
- Monzo A, Quiroga R, Rodriguez‐Tarrega E, Monterde M, Martinez‐Triguero L, Fernandez‐Colom P, et al. Effect of prolonged gonadotropin releasing hormone (GnRH) agonist before IVF versus placebo on CYP19 A1 gene expression in granulosa cells in infertile women with endometriosis. Human Reproduction 2016;31(Suppl 1):i268 Abstract no: P‐324. [Google Scholar]
Nakagawa 2000 {published data only}
- Nakagawa K, Yamano S, Nakasaka H, Komatsu J, Hinokio K, Aono T. Effectiveness of pre‐treatment with gonadotropin‐releasing hormone agonist to the patients with endometriosis in in vitro fertilization and embryo transfer. Japanese Journal of Fertility and Sterility 2000;45(1):1‐6. [Google Scholar]
NCT00654524 {published data only}
- NCT00654524. Randomized study of gonadotropin‐releasing‐hormone agonist (GnRH‐a) or expectant management for endometriosis [A randomized study comparing goserelin or expectant management following laparoscopic surgery for advanced endometriosis]. clinicaltrials.gov/ct2/show/NCT00654524 (first received 8 April 2008).
NCT01682642 {published data only}
- NCT01682642. The influence of adjuvant medical treatment of peritoneal endometriosis on the outcome of IVF. A prospective randomized analysis [IVF outcome in patients with peritoneal endometriosis. The impact of a hormonal treatment of the endometriosis prior to the IVF on the pregnancy rates]. clinicaltrials.gov/ct2/show/NCT01682642 (first received 11 September 2012).
NCT02400801 {published data only}
- NCT02400801. Gonadotropin‐releasing hormone (GnRH) downregulation versus oral anticonception prior to ART in postoperative endometriosis patients. clinicaltrials.gov/ct2/show/NCT02400801 (first received 27 March 2015).
Parazzini 1994 {published data only}
- Parazzini F, Fedele L, Busacca M, Falsetti L, Pellegrini S, Venturini PL, et al. Postsurgical medical treatment of advanced endometriosis: results of a randomized clinical trial. American Journal of Obstetrics and Gynecology 1994;171(5):1205‐7. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Tarrega 2016 {published data only}
- Rodriguez‐Tarrega E, Monzo A, Quiroga R, Romeu M, Polo P, Garcia‐Gimeno T, et al. Randomized controlled trial to evaluate the usefulness of GnRH agonist versus placebo on the outcome of IVF in infertile patients with endometriosis. Human Reproduction 2016;31(Suppl 1):i267. [Google Scholar]
Sahebkashaf 2003 {published data only}
- Sahebkashaf H, Aleyassin A, Saidi H, Ghalavand N, Ghavami AH. Effect of prolonged gonadotrophin‐releasing hormone agonist therapy on the outcome of rapid ICSI‐ZIFT in patients with endometriosis. Fertility and Sterility 2003;80(Suppl 3):S121, Abstract no: P‐2. [Google Scholar]
Shawki 2002 {published data only}
- Shawki O, Hamza H, Sattar M. Mild endometriosis, to treat or not treat: randomized controlled trial comparing diagnostic laparoscopy with no further treatment versus post‐operative Zoladex in cases with infertility associated with stage I, II endometriosis. Fertility and Sterility 2002;77(Suppl 1):S13. [Google Scholar]
Song 2013 {published data only}
- Song JH, Lu H, Zhang J, Li B. Clinical study on the effectiveness and safety of combined laparoscopy and gonadotropin‐releasing hormone agonist in the treatment of endometriosis. Zhonghua Fu Chan Ke Za Zhi 2013;48(8):584‐8. [PubMed] [Google Scholar]
Tamura 2012 {published data only}
- Tamura H, Tamura I, Maekawa R, Asada H, Sugino N. Effects of long term gonadtrophin‐releasing hormone agonist treatment on the pregnancy outcome of IVF‐ E in infertile patients with endometriosis. Human Reproduction 2012;27 Suppl 2:ii205‐ii223: Abstract number: P. [Google Scholar]
Tomassetti 2018 {published data only}
- Tomassetti C, Adamson D, Arici A, Canis M, Hompes P, Hummelshoj L, et al. EndoART: A proposed randomized controlled trial on endometriomas in assisted reproductive technologies, comparing the effect of no intervention, surgery, and prolonged GnRH downregulation on pregnancy rates. Journal of Endometriosis and Pelvic Pain Disorders 2018;10(3):158‐73. [Google Scholar]
Vercellini 1999 {published data only}
- Vercellini P, Crosignani PG, Fadini R, Radici E, Belloni C, Sismondi P. A gonadotrophin‐releasing hormone agonist compared with expectant management after conservative surgery for symptomatic endometriosis. British Journal of Obstetrics and Gynaecology 1999;106(7):672‐7. [DOI] [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Yang XH, Ji F, AiLi A, Tuer Xun H, He Y, Ding Y. Effects of laparoscopic ovarian endometriosis cystectomy combined with postoperative GnRH‐a therapy on ovarian reserve, pregnancy, and outcome recurrence. Clinical and Experimental Obstetrics and Gynecology 2014;41(3):272‐5. [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02737800 {published data only}
- NCT02737800. Effect of prolonged GnRh agonists on results of intracytoplasmic sperm injection (ICSI ) in endometrioma patients (GnRh) [Effect of prolonged GnRh agonist therapy on the outcome of in‐vitro fertilization & embryo transfer in endometrioma patients]. clinicaltrials.gov/ct2/show/NCT02737800 (first received 14 April 2016).
References to ongoing studies
NCT02779387 {published data only}
- NCT02779387. Reproductive outcome of EM treated by GnRH‐a associated with laparoscopy [Efficacy of laparoscopic surgery combined with GnRH‐a in the treatment of endometriosis associated infertility: a multicenter, prospective, randomized‐control‐trial]. clinicaltrials.gov/ct2/show/NCT02779387 (first received 20 May 2016).
NCT03142035 {published data only}
- NCT03142035. Dienogest versus GnRH‐a pre‐treatment in women with endometriosis undergoing IVF [Dienogest versus gonadotropin releasing hormone agonist pre‐treatment in women with endometriosis undergoing in vitro fertilization]. clinicaltrials.gov/ct2/show/NCT03142035 (first received 5 May 2017).
Additional references
Arici 1996
- Arici A, Oral E, Bukulmez O, Duleba A, Olive DL, Jones EE. The effect of endometriosis on implantation: results from the Yale University in vitro fertilization and embryo transfer program. Fertility and Sterility 1996;65(3):603‐7. [DOI] [PubMed] [Google Scholar]
ASRM 2006
- The Practice Committee of the American Society of Reproductive Medicine. Endometriosis and infertility. Fertility and Sterility 2006;86(4):S156‐60. [Google Scholar]
Azem 1999
- Azem F, Lessing JB, Geva A, Shahar A, Lerner‐Geva L. Patients with stages III and IV endometriosis have a poorer outcome of in vitro fertilization‐embryo transfer than patients with tubal infertility. Fertility and Sterility 1999;72(6):1107‐9. [DOI] [PubMed] [Google Scholar]
Barnhart 2002
- Banhart K, Dunsmoore‐Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertility and Sterility 2002;77(6):1148‐55. [DOI] [PubMed] [Google Scholar]
Bergendal 1998
- Bergendal A, Naffah S, Nagy C, Bergqvist A, Sjoblom P, Hillensjo T. Outcome of IVF in patients with endometriosis in comparison with tubal‐factor infertility. Journal of Assisted Reproduction and Genetics 1998;15(9):530‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 1996
- Cahill DJ, Wardle PG, Harlow CR, Hull MG. Ovarian dysfunction in endometriosis‐associated and unexplained infertility. Journal of Assisted Reproduction and Genetics 1997;14(10):554‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coccia 2011
- Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Impact of endometriosis on in vitro fertilization and embryo transfer cycles in young women: a stage‐dependent interference. Acta Obstetricia et Gynecologica Scandinavica 2011;90(11):1232‐8. [DOI] [PubMed] [Google Scholar]
de Ziegler 2010
- Ziegler D, Gayet V, Aubriot FX, Fauque P, Streuli I, Wolf JP, et al. Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcome. Fertility and Sterility 2010;94(7):2796‐9. [DOI] [PubMed] [Google Scholar]
Edwards 1995
- Edwards RG. Clinical approaches to improving uterine receptivity during human implantation. Human Reproduction 1995;10(S):60‐6. [DOI] [PubMed] [Google Scholar]
ESHRE 2013
- ESHRE Endometriosis Guideline Development Group. Management of women with endometriosis. Guideline of the European Society of Human Reproduction and Embryology 2013.
Garcia‐Velasco 2004
- Garcia‐Velasco JA, Mahutte MG, Corona J, Zuniga V, Giles J, Arici A, et al. Removal of endometriomas before in vitro fertilization does not improve fertility outcomes: a matched case‐control study. Fertility and Sterility 2004;81:1194‐7. [DOI] [PubMed] [Google Scholar]
Garrido 2000
- Garrido N, Nevarro J, Remohi J, Simon C, Pellicer A. Follicular hormonal environment and embryo quality in women with endometriosis. Human Reproduction Update 2000;6(1):67‐74. [DOI] [PubMed] [Google Scholar]
Geber 2002
- Geber S, Ferreira DP, Spyer Prates LF, Sales L, Sampaio M. Effects of previous ovarian surgery for endometriosis on the outcome of assisted reproduction treatment. Reproductive BioMedicine Online 2002;5:162‐5. [DOI] [PubMed] [Google Scholar]
Giudice 2010
- Guidice LC. Clinical practice. Endometriosis. New England Journal of Medicine 2010;362:2389‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 28 October 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).
Haouzi 2010
- Haouzi D, Assou S, Dechanel C, Anthony T, Dechaud S, Vos J. Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in human: protocol effects. Biology of Reproduction 2010;82:679‐83. [DOI] [PubMed] [Google Scholar]
Harb 2013
- Harb HM, Gallos ID, Chu J, Harb M, Coomarasamy A. The effect of endometriosis on in vitro fertilization outcome: a systematic review and meta‐analysis. BJOG 2013;120:1308‐20. [DOI] [PubMed] [Google Scholar]
Harkki 2010
- Härkki P, Tiitinen A, Ylikorkala O. Endometriosis and assisted reproduction techniques. Annals of the New York Academy of Sciences 2010;1205:207‐13. [DOI] [PubMed] [Google Scholar]
Harlow 1996
- Harlow CR, Cahill DJ, Maile LA, Talbot MW, Mears J, Wardle PG. Reduced perovulatory granulosa cell steroidogenesis in women with endometriosis. Journal of Clinical Endocrinology and Metabolism 1996;81:426‐9. [DOI] [PubMed] [Google Scholar]
Hart 2003
- Hart R. Unexplained infertility. Endometriosis and fibroids. BMJ 2003;27:721‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hickman 2002
- Hickman TN. Impact of endometriosis on implantation. Data from the Wilford Hall Medical Centre IVF‐ET Program. Journal of Reproductive Medicine 2002;47(10):801‐8. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration: Available from handbook.cochrane.org.
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Chichester (UK): John Wiley & Sons, 95‐150.
Marconi 2002
- Marconi G, Vilela M, Quintana R, Sueldo C. Laparoscopic ovarian cystectomy of endometriomas does not affect the ovarian response to gonadotropin stimulation. Fertility and Sterility 2002;78(4):876‐8. [DOI] [PubMed] [Google Scholar]
Marcus 1994
- Marcus SF, Edwards RG. High rates of pregnancy after long term down regulation of women with endometriosis. American Journal of Obstetrics and Gynaecology 1994;171:812‐7. [DOI] [PubMed] [Google Scholar]
Meuleman 2009
- Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normosperm partners. Fertility and Sterility 2009;92:64‐74. [DOI] [PubMed] [Google Scholar]
Mohamed 2011
- Mohamed AM, Chouliaras S, Jones CJ, Nardo LG. Live birth rate in fresh and frozen embryo transfer cycles in women with endometriosis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;156(2):177‐80. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement.. PLOS Medicine 2009;6(7):e1000097. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakamura 1992
- Nakamura K, Oosawa M, Kondou I, Ingaki S, Shibata H, Narita O. Menotrophin stimulation after prolonged gonadotrophin releasing hormone agonist pretreatment for in‐vitro fertilization in patients with endometriosis. Journal of Assisted Reproduction and Genetics 1992;9:113‐7. [DOI] [PubMed] [Google Scholar]
Norenstedt 2001
- Norenstedt SN, Linderoth‐Nagy C, Bergendal A, Sjoblom P, Bergqvist A. Reduced developmental potential in oocytes from women with endometriosis. Journal of Assisted Reproduction and Genetics 2001;18(12):644‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olivennes 2003
- Olivennes F. Result of IVF in women with endometriosis. Journal of Gynaecology Obstetrics and Biology of Reproduction 2003;32:547‐7. [PubMed] [Google Scholar]
Opoein 2012
- Opoein HO, Peter F, Anne KO, Thomas A, Sveere B, Gudvor E. In vitro fertilization is a successful treatment in endometriosis‐associated infertility. Fertility and Sterility 2012;97(4):913‐8. [DOI] [PubMed] [Google Scholar]
Ozkan 2008
- Ozkan S, Murk W, Arici A. Endometriosis and Infertility: epidemiology and evidence‐based treatments. Annals of New York Academy of Sciences 2008;1127:92‐100. [DOI] [PubMed] [Google Scholar]
Pal 1998
- Pal L, Shifren JL, Isaacson KB, Chang Y, Toth TL. Impact of varying stages of endometriosis on the outcome of in vitro fertilisation and embryo transfer. Journal of Assisted Reproduction and Genetics 1998;43:351‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prescott 2016
- Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Human Reproduction 2016;31(7):1475‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raffi 2012
- Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: a systematic review and meta‐analysis. Journal of Clinical Endocrinology and Metabolism 2012;97(9):3146‐54. [DOI] [PubMed] [Google Scholar]
Ryan 1997
- Ryan IP, Taylor RN. Endometriosis and infertility: new concept. Obstetrical and Gynaecological Survey 1997;52:365‐71. [DOI] [PubMed] [Google Scholar]
Simon 1994
- Simon C, Gutierrez A, Vidal A, Santos MJ, Tarin JJ, Remohi J, et al. Outcome of patients with endometriosis in assisted reproduction: results from in‐vitro fertilization and oocyte donation. Human Reproduction 1994;9(4):725‐9. [DOI] [PubMed] [Google Scholar]
Surrey 1998
- Surrey ES, Halme J. Effect of peritoneal fluid from endometriosis patient on endometrial stromal cell proliferation in vitro. Obstetrics and Gynaecology 1990;76:792‐7. [DOI] [PubMed] [Google Scholar]
Surrey 2010a
- Surrey ES. Gonadotrophin‐releasing hormone agonists and add‐back therapy: what do the data show?. Current Opinion on Obstetrics and Gynaecology 2010;22:283‐8. [DOI] [PubMed] [Google Scholar]
Suzuki 2005
- Suzuki T, Izumi S, Matsubayashi H, Awaji H, Yoshikata K, Makino T. Impact of ovarian endometrioma on oocytes and pregnancy outcome in in‐vitro fertilization. Fertility and Sterility 2005;83(4):908‐13. [DOI] [PubMed] [Google Scholar]
Tang 2013
- Tang Y, Chen SL, Chen X, He YX, Ye DS, Guo W, et al. Ovarian damage after laparoscopic endometrioma excision might be related to the size of cyst. Fertility and Sterility 2013;100(2):464‐9. [DOI] [PubMed] [Google Scholar]
Tsoumpou 2009
- Tsoumpou I, Kyrgiou M, Gelbaya TA, Nardo LG. The effect of surgical treatment for endometrioma on in vitro fertilization outcomes: a systematic review and meta‐analysis. Fertility and Sterility 2009;92:75‐87. [DOI] [PubMed] [Google Scholar]
Urman 2013
- Urman B, Alper E, Yakin K, Oktem O, Aksoy S, Alatas C, et al. Removal of unilateral endometriomas is associated with immediate and sustained reduction in ovarian reserve. Reproductive BioMedicine Online 2013;27(2):212‐6. [DOI] [PubMed] [Google Scholar]
Van der Hauven 2013
- Hauven LE, Mijatovic V, Leemhius E, Schats R, Heymans MW, Lambalk CB, et al. Efficacy & safety of IVF/ICSI in patients with severe endometriosis after long term pituitary down regulation. Reproductive BioMedicine Online 2013;28(1):39‐46. [http://www.rbmojournal.com/article/S1472‐6483(13)00523‐3/] [DOI] [PubMed] [Google Scholar]
Xie 2018
- Xie D, Chen F, Xie SZ, Chen ZL, Tuo P, Zhou R, et al. Artificial cycle with or without a depot gonadotropin‐releasing hormone agonist for frozen‐thawed embryo transfer: an assessment of infertility type that is most suitable. Current Medical Science 2018;38(4):626‐31. [DOI] [PubMed] [Google Scholar]
Zegers‐Hochschild 2017
- Zegers‐Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertility and Sterility 2017;108(3):393‐406. [DOI: 10.1016/j.fertnstert.2017.06.005] [DOI] [PubMed] [Google Scholar]
Zondervan 2018
- Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nature Reviews Disease Primers 2018;4(1):9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sallam 2006
- Sallam HN, Garcia‐Velasco JA, Dias S, Arici A. Long‐term pituitary down‐regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004635] [DOI] [PMC free article] [PubMed] [Google Scholar]


