Staphylococcus aureus extracellular DNA (eDNA) plays a crucial role in the structural stability of biofilms during bacterial colonization; on the contrary, host immune responses can be induced by bacterial eDNA. Previously, we observed production of S. aureus thermonuclease during the early stages of biofilm formation in a mammalian cell culture medium.
KEYWORDS: biofilm, eDNA, thermonuclease, NETs, ROS, early biofilm, NETosis, Staphylococcus aureus
ABSTRACT
Staphylococcus aureus extracellular DNA (eDNA) plays a crucial role in the structural stability of biofilms during bacterial colonization; on the contrary, host immune responses can be induced by bacterial eDNA. Previously, we observed production of S. aureus thermonuclease during the early stages of biofilm formation in a mammalian cell culture medium. Using a fluorescence resonance energy transfer (FRET)-based assay, we detected thermonuclease activity of S. aureus biofilms grown in Iscove’s modified Dulbecco’s medium (IMDM) earlier than that of widely studied biofilms grown in tryptic soy broth (TSB). The thermonuclease found was Nuc1, confirmed by mass spectrometry and competitive Luminex assay. These results indicate that biofilm development in IMDM may not rely on eDNA for structural stability. A bacterial viability assay in combination with wheat germ agglutinin (WGA) staining confirmed the accumulation of dead cells and eDNA in biofilms grown in TSB. However, in biofilms grown in IMDM, minimal amounts of eDNA were found; instead, polysaccharide intercellular adhesin (PIA) was detected. To investigate if this early production of thermonuclease plays a role in immune modulation by biofilm, we studied the effect of thermonuclease on human neutrophil extracellular trap (NET) formation using a nuc knockout and complemented strain. We confirmed that thermonuclease produced by early-stage biofilms grown in IMDM degraded biofilm-induced NETs. Additionally, neither the presence of biofilms nor thermonuclease stimulated an increase in reactive oxygen species (ROS) production by neutrophils. Our findings indicated that S. aureus, during the early stages of biofilm formation, actively evades the host immune responses by producing thermonuclease.
INTRODUCTION
Staphylococcus aureus is a well-known human pathogen that can cause mild subcutaneous infections but also life-threatening invasive infections (1–3). When infections caused by S. aureus become chronic, biofilm formation is often observed (4–7). The noncellular component of the biofilm, known as bacterial extracellular matrix (ECM), is a complex matrix that consists of polysaccharides, glycolipids, proteins, and extracellular DNA (eDNA) (8–14). eDNA formation in S. aureus biofilms is facilitated by an autolysis process that mimics apoptosis of eukaryotic cells (12) and is thought to play a crucial role in the structural stability of biofilms (14).
In a previous study from our group (15), we observed that during the early stages of biofilm formation, S. aureus produces immune modulators like staphylococcal complement inhibitor (SCIN), chemotaxis inhibitory protein of staphylococci (CHIPS), and formyl peptide receptor-like 1 inhibitor (FLIPr). These immunomodulators facilitate the defense of the developing biofilms against the host early immune responses. In addition to these immune modulators, we observed an early production of thermonuclease when S. aureus was grown in Iscove’s modified Dulbecco’s medium (IMDM) (15). S. aureus can produce the following two types of thermonucleases: Nuc1 and Nuc2 (16, 17). The major type of thermonuclease produced by S. aureus is Nuc1 (18). Thermonuclease Nuc1 production is particularly conserved across all strains of S. aureus, and it has been used as one of the specific markers to identify S. aureus (19, 20). Thermonuclease is an exoenzyme that catalyzes the hydrolysis of both DNA and RNA (21–23).
While eDNA stabilizes the biofilm structure, it also induces host immune responses. For example, unmethylated cytosine-phosphate-guanine (CpG) motifs of bacterial eDNA can be recognized by Toll-like receptor 9 (TLR9) (24, 25). In biofilm, thermonuclease is an enzyme that can dissolve and degrade eDNA that interconnects bacterial cells within the biofilm (26). Furthermore, in a study with planktonic S. aureus, thermonuclease was associated with the evasion of neutrophil extracellular traps (NETs), formed by a mechanism of cell death of neutrophils, that trap and kill bacteria (27, 28). Additionally, Berends et al. (27) demonstrated in a murine respiratory tract infection model that S. aureus thermonuclease also plays a role in immune avoidance against NETs in vivo. Taken together, thermonuclease production seems to be an important strategy for S. aureus to modulate the innate host response, allowing a biofilm to accumulate; conversely, thermonuclease is thought to destabilize biofilms as well.
To investigate this controversy, we studied, in the context of a developing biofilm, the consequences of thermonuclease production, particularly Nuc1, on (i) biofilm accumulation and (ii) defense against the innate host immune response. We first determined biofilm accumulation, composition, and thermonuclease production in IMDM, a mammalian cell culture medium, compared to those in the classical bacterial growth medium tryptic soy broth (TSB). Furthermore, we studied the NETosis-inducing abilities of early biofilms and the effect of thermonuclease Nuc1 on NET degradation, reactive oxygen species (ROS) production, and the activation of human TLR9.
RESULTS
Thermonuclease expression by early biofilms.
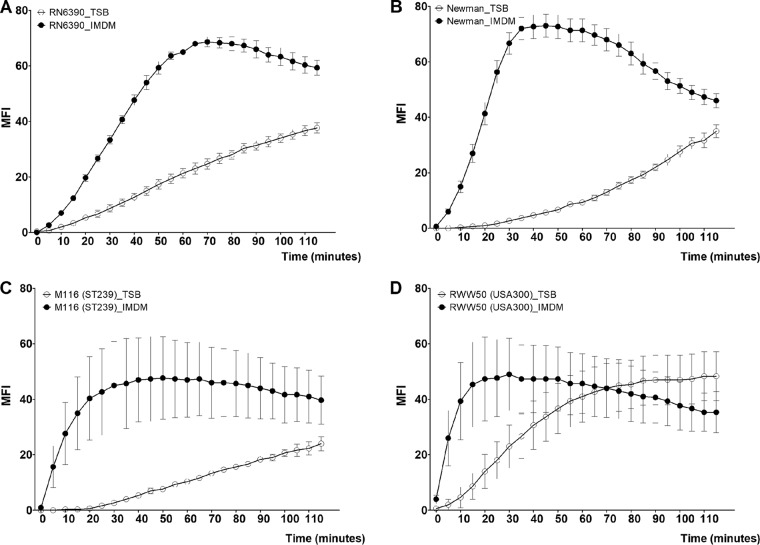
To determine the production of thermonuclease during the early stages of biofilm formation in both TSB and IMDM, we quantified thermonuclease activity for 2 h, starting with 3-h-old biofilms, using a fluorescence resonance energy transfer (FRET)-based assay. We saw that the thermonuclease activity of strains RN6390, Newman, and M116 was higher when grown in IMDM than when grown in TSB (P ≤ 0.001) (Fig. 1A to C). Strain RWW50 initially showed higher production of thermonuclease in IMDM than in TSB; however, this difference is lost over time (P = 0.05) (Fig. 1D).
FIG 1.
Thermonuclease production of S. aureus biofilms in TSB and IMDM. The mean fluorescence intensity (MFI) from FRET-based measurement of thermonuclease production (y axis) during the 4th and 5th hour (x axis) of biofilm formation of S. aureus RN6390 (A), Newman (B), M116 (C), and RWW50 (D) in TSB and IMDM. Figures have been generated from three independent experiments. Error bars represent SEM.
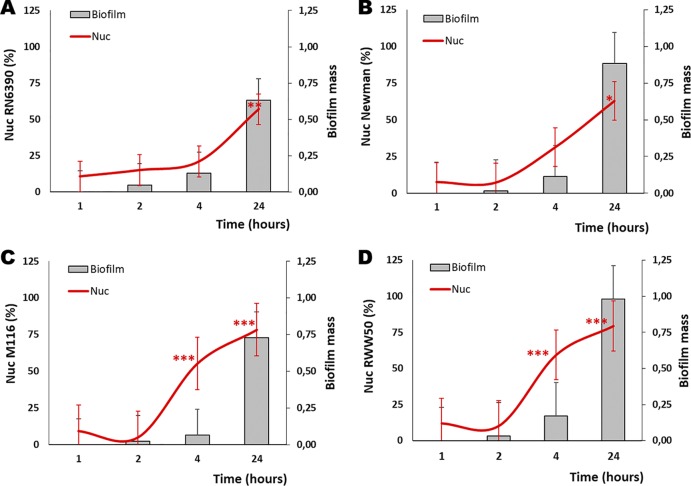
To demonstrate the timing of thermonuclease production, specifically of Nuc1, during biofilm formation in IMDM, biofilm supernatants from the 1st, 2nd, 4th, and 24th hours of biofilm formation were studied using a competitive Luminex assay (15, 29). Nuc1 was found in the supernatant of biofilms from all isolates (Fig. 2). Nuc1 expression was significantly higher in M116 (P ≤ 0.001) and RWW50 (P ≤ 0.001) (Fig. 2C and D) during the 4th hour of biofilm formation than during the 1st hour of biofilm formation. Furthermore, a positive correlation between thermonuclease production and biofilm mass was found for RN6390 (r = 0.999; P = 0.0002), Newman (r = 0.947; P = 0.053), M116 (r = 0.813; P = 0.187), and RWW50 (r = 0.847; P = 0.152) (Fig. 2). The presence of thermonuclease Nuc1 in the supernatant of the 4th hour of biofilm formation was additionally confirmed with mass spectrometry (Table 1).
FIG 2.
Thermonuclease Nuc1 expression during biofilm formation. With a competitive Luminex assay, we monitored the presence of thermonuclease Nuc1 (red line, left y axis) in the supernatants of the 1st, 2nd, 4th, and 24th hour during biofilm formation of S. aureus RN6390 (A), Newman (B), M116 (C), and RWW50 (D). Presented are biofilm mass (bar, right y axis) and thermonuclease Nuc1 (red line, left y axis). Significant statistical differences are indicated by asterisks (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). These figures have been generated from three independent experiments. Error bars presented are mean and SEM.
TABLE 1.
Mass spectrometry analysis of thermonuclease Nuc1 at the 4th and 24th hours of biofilm formation
| Strain | Hours of biofilm formation | No. of unique peptides detecteda
|
Sequence coverage (%)a
|
Protein identification probability (%)a
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | ||
| Newman | 4 | 5 | 25.60 | 100 | ||||||
| 24 | 11 | 3 | 1 | 40.8 | 16.7 | 6.58 | 100 | 100 | 92.9 | |
| RWW50 | 4 | 5 | 5 | 11 | 21.1 | 24.6 | 46.9 | 100 | 100 | 100 |
| 24 | 16 | 10 | 47.8 | 36.8 | 100 | 100 | ||||
Three separate experiments (experiment I, II, and III). Thermonuclease accession number is Q5HHM4.
Contribution of eDNA to biofilm mass in the early stages of biofilm formation.
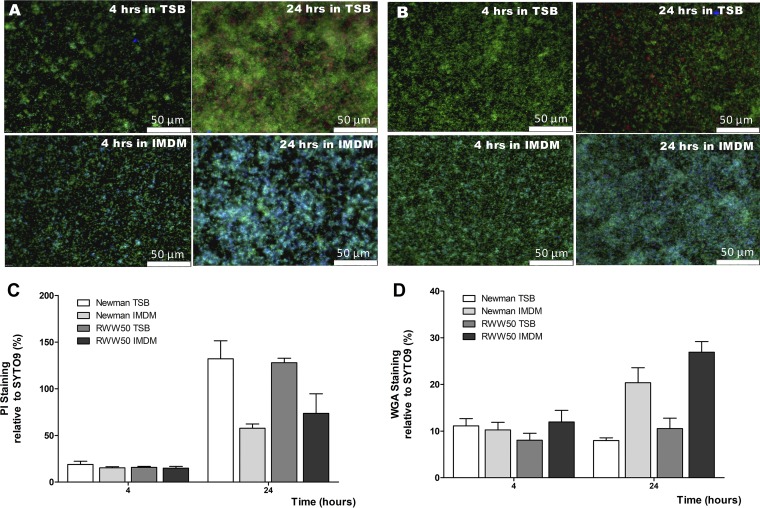
If eDNA is an important factor in the accumulation of bacterial cells during biofilm formation, how is it possible that the early stages of biofilm formation in IMDM do not seem to be hampered by the presence of thermonuclease? To understand this, we investigated the biofilm content of 4- and 24-h biofilms grown in TSB and IMDM. After 4 h of biofilm formation, dead cells and eDNA were minimally present in each growth medium (Fig. 3A to C). However, after 24 h of biofilm formation, eDNA was observed, in particular, in the biofilm formed in TSB medium (Fig. 3A to C). Interestingly, in biofilms grown in IMDM, accumulation of N-acetylglucosamine, presumably the consequence of an increase of the polysaccharide intercellular adhesin (PIA), was visible after 4 h of biofilm formation and much more abundant after 24 h than in biofilms grown in TSB (Fig. 3A, B, and D).
FIG 3.
Presence of extracellular DNA and PIA in biofilms of S. aureus. Four- and twenty-four-hour biofilms of S. aureus Newman (A) and RWW50 (USA300) (B) grown in TSB and IMDM were stained with SYTO 9 (DNA/life cells, green), PI (extracellular DNA/dead cells, red), and lectin WGA (N-acetylglucosamine/PIA, blue). Figures are a representative result of three separate experiments. For relative quantification, the same staining procedure was followed, only the different stains were applied in separate wells. Results are reported as the mean ratio of the PI (C) and WGA (D) signal to the SYTO9 signal (in %) of three separate experiments. Error bars represent SEM.
Biofilm activates ROS-independent NET formation.
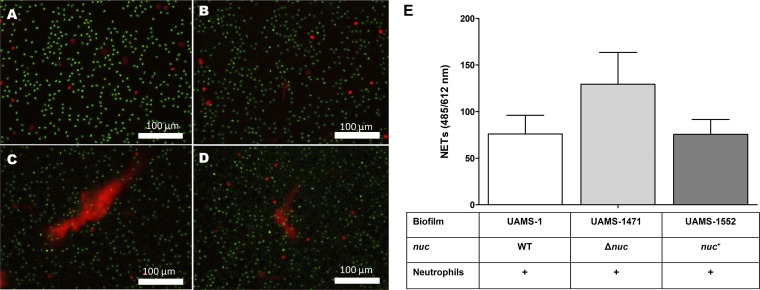
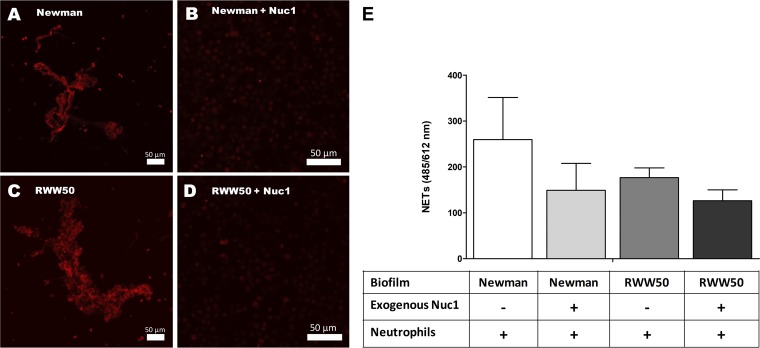
It was described previously in a planktonic setting that thermonuclease can degrade NETs to ensure the survival of S. aureus (27, 28). Prior to preforming a NETosis assay in a biofilm setup, we determined the thermonuclease production of a wild-type (WT) (UAMS-1), a thermonuclease (nuc) mutant (UAMS-1471), and a nuc complementation strain (UAMS-1552) during the 3rd and the 4th hour of biofilm formation using the FRET assay (see Fig. S1 in the supplemental material). In order to investigate whether thermonuclease production by young biofilms had an effect on NETosis, we added freshly isolated neutrophils to 3-h-old washed biofilms. After a coincubation period of 90 min, we saw massive NET formation with the nuc mutant (Fig. 4C and E), while minimal amounts were seen with the wild-type (Fig. 4B and E) or the nuc complementation strain (Fig. 4D and E). A 5-min coincubation of neutrophils with freshly washed, 3-h-old biofilms of Newman and RWW50 gave visible NET formation (Fig. 5A, C, and E). However, if biofilms were pretreated with 5 μg/ml of exogenous thermonuclease Nuc1 (eNuc1), almost no NETs were observed (Fig. 5B, D, and E).
FIG 4.
Three-hour-old biofilms produce enough thermonuclease to degrade NETs. Massive NET (red diffuse) formation can be observed when 3-h-old biofilms of the nuc mutant (Δnuc) are coincubated with human neutrophils (green, alive; red [localized], dead) (C, E). Thermonuclease produced by the biofilms degrades NETs induced by 3-h-old biofilms of the wild-type (WT) (B, E) and the nuc complemented (nuc+) (D, E) strains. No spontaneous NET formation was observed in the control neutrophils only (A). Figures have been generated from three independent experiments. Scale bar = 100 μm. Error bars represent SEM.
FIG 5.
Inductions of NETosis by early biofilm. Exogenous thermonuclease Nuc1 degrades the NETs (red diffuse) formed after coincubation of neutrophils with 3-h-old washed biofilms of Newman (A, B, E) and USA300 (RWW50) (C, D, E). Figures have been generated from three independent experiments. Scale bar = 50 μm. Error bars represent SEM.
During the coincubation of neutrophils with the 3-h-old biofilms of these strains, we found that reactive oxygen species (ROS) levels from these neutrophils were not significantly different than spontaneous ROS production by neutrophils by themselves (see Fig. S2 in the supplemental material). These findings indicate that S. aureus biofilm-induced NET formation is ROS independent. Meanwhile, the addition of eNuc1 alone had no effect on ROS activation (Fig. S2).
Upon encountering bacteria, neutrophils can be activated via TLR9 (30). TLR9 recognizes specifically the unmethylated cytosine-phosphate-guanine (CpG) dinucleotide part of bacterial or viral DNA (30, 31). After coincubation of S. aureus biofilms with HEK-Blue hTLR9 cells (32, 33), which are human embryonic kidney 293 cells that express human TLR9, no activation of human TLR9 was observed (see Fig. S3 in the supplemental material). However, when these HEK-Blue hTLR9 cells were pretreated with a TLR9 agonist, TLR9 activation was significantly higher than that of the unstimulated control cell line (P < 0.01) (Fig. S3).
Biofilm-associated S. aureus survives in the presence of human neutrophils during the early stages of biofilm formation.
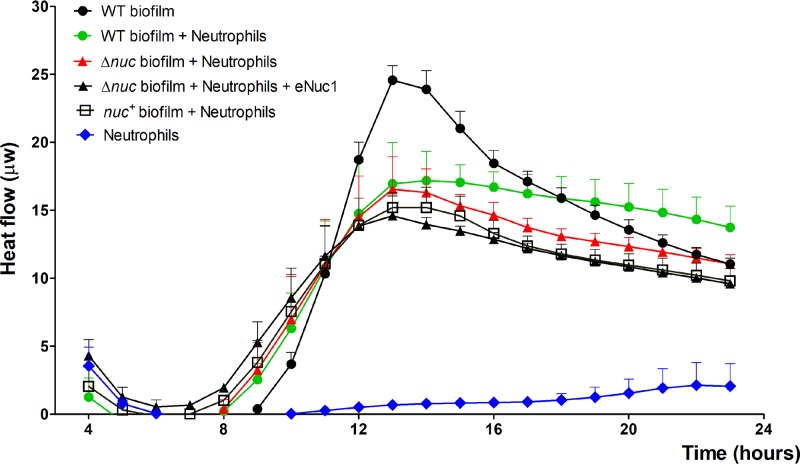
Considering that S. aureus biofilms can already inhibit ROS and NET production during the early stages of biofilm formation, it comes into question whether these S. aureus biofilms can survive exposure to neutrophils. Therefore, we monitored the metabolic activity of biofilm-associated S. aureus cells coincubated with human neutrophils using isothermal microcalorimetry. Interestingly, we saw that the metabolic rates of the biofilm-associated S. aureus cells were not significantly affected by the presence of neutrophils (Fig. 6). This confirms previous results that show that biofilm-associated S. aureus cells are able to withstand human neutrophils (28, 34). Although the metabolic rates of the biofilms formed by the nuc mutant (Δnuc) were lower than those of biofilms formed by the wild-type (WT) strain during coincubation with neutrophils, the differences were not statistically significant. Overall, no significant differences in metabolic rates were found between the WT, Δnuc, and complemented nuc (nuc+) strains during coincubation with neutrophils. Finally, addition of exogenous thermonuclease Nuc1 to biofilms of the Δnuc mutant, before coincubation with neutrophils, did not result in significant differences in survival compared to that of untreated biofilms of the same strain (Fig. 6).
FIG 6.
Effect of neutrophils and thermonuclease on the survival of biofilm-associated bacterial cells. Neutrophils were added to 3-h-old biofilms of wild-type (WT), nuc mutant (Δnuc) (with or without an addition of exogenous thermonuclease Nuc1 [eNuc1]), and complementary nuc (nuc+) UAMS S. aureus strains. The effect of neutrophils on bacterial survival was monitored using isothermal microcalorimetry. The metabolic rates of the bacteria and neutrophils are depicted as heat flow versus time. Figures have been generated from three independently performed experiments. Error bars represent SEM.
DISCUSSION
Previous studies in conventional growth media showed that thermonuclease transcription and production could be detected after approximately 10 h of incubation of biofilm in brain heart infusion (BHI) broth (35) and after 6 h in TSB (36). In addition, our previous study in IMDM (15), a mammalian cell culture medium, showed that thermonuclease was produced as early as the 4th hour of biofilm formation. Using three different methods—FRET-based assay, Luminex competitive assay, and mass-spectrometry—we confirmed that thermonuclease was indeed already produced during the early stages of biofilm formation.
It is widely accepted that an important role of thermonuclease is to release the bacteria from the eDNA-containing biofilm matrix, which will lead to the destabilization and remodeling of biofilms (36, 37). However, most of the experiments leading to this assumption were performed in classical growth media, such as TSB or BHI broth. When we grew biofilms in IMDM, we observed a steady accumulation of biofilm, even though the amount of thermonuclease, specifically the Nuc1, increased as well. These observations are in line with previous studies, which showed that early biofilms were insensitive to DNase I (36, 38). Our observations further indicate that biofilm formation is independent of eDNA in IMDM. Analysis of biofilm composition formed in IMDM and TSB revealed that, indeed, after 4 h of growth, very minimal amounts of eDNA were seen in any of the biofilms. However, biofilms formed in IMDM did contain N-acetylglucosamine-containing components, presumably PIA, which was visualized by wheat germ agglutinin (WGA) lectin staining (39). After 24 h of growth, dead cells and eDNA were observed in biofilms grown in TSB but not biofilms grown in IMDM. In addition, after 24 h, accumulation of N-acetylglucosamine was abundant in the IMDM-cultured biofilms but was not present in the TSB-cultured biofilms. This might be due to the activity of the SaeRS locus (40). The SaeRS locus, which regulates nuc transcription in vitro (40), may be activated differently in these two growth media. In mammalian cell culture medium that contains plasma, the Sae locus is required for biofilm formation, while in TSB, the Sae locus is dispensable (41). SaeRS can be activated by limited or no free iron in medium, such as in IMDM (41), and may stimulate both the production of PIA and thermonuclease (42–44). This could also explain why biofilms grown in IMDM produce more thermonuclease. Because thermonuclease breaks down DNA, it has been described to be produced by biofilms to regulate biofilm mass (26, 35). This would indeed be the case for biofilms grown in TSB, as eDNA is abundantly present. However, when grown in IMDM, the early and robust production of thermonuclease might break down the eDNA in these biofilms. The lack of eDNA in the tested S. aureus biofilms may be the reason why we were unable to demonstrate the activation of human TLR9 by S. aureus biofilms since it is known that TLR9 is expressed at the surface of human polymorphonuclear leukocytes (PMNs) (45) and can be activated by unmethylated CpG motifs of bacterial DNA (25).
Neutrophils are the first immune cells to respond to injury, and one of their defense responses is the formation of NETs (46). NETs have been described as an immune response against pathogens (46) and can be induced by bacteria (47, 48), fungi (49), and bacterial products such as lipopolysaccharides (LPS) (50). S. aureus is a very strong inducer of NETosis (47, 48, 51, 52; T. Hoppenbrouwers, A. R. Sultan, T. E. Abraham, N. Lemmens-den Toom et al., submitted for publication), the process of NET formation in which neutrophils excrete their DNA, histones, and antimicrobial proteins into the ECM (27). NETs trap the bacteria and limit their spreading (46). When we incubated these early-stage biofilms with neutrophils for 90 min, only very limited amounts of NETs were present in biofilms of the wild-type and the nuc complementation strains. In contrast, NETs were visible after 90 min of incubation with neutrophils in biofilms of the nuc mutant strain. Planktonic S. aureus has been described to break down NETs with thermonuclease (27, 28). We confirmed that early-stage biofilms are strong inducers of NETosis, but we also showed that these early biofilms are able to produce thermonuclease to degrade NETs as well as planktonic S. aureus. Therefore, this study provides the first step in understanding the interaction between the early stages of S. aureus biofilms and NETosis. Future studies need to be conducted to further elucidate how the balance between the parallel induction and degradation of NETs by biofilms is regulated. Also of interest is how this balance affects the role of NETosis in the pathophysiology of biofilm, particularly during the early stages of biofilm formation.
Finally, we did not observe ROS production of neutrophils in our biofilm experiments. Neutrophils typically have three different antimicrobial mechanisms as follows: (i) phagocytosis, where pathogens are engulfed into a phagosome and destroyed by NADPH-dependent mechanisms (ROS production) or antimicrobial proteins; (ii) degranulation, where pathogens are killed by ROS and antimicrobial proteins that are released into the extracellular matrix by PMN; and (iii) NETosis (53). NETosis can be induced by both ROS-dependent and ROS-independent pathways (54). In our biofilm experiments, we saw ROS-independent NETosis, indicated by NET formation without visible ROS activation. This is in line with previous literature on planktonic S. aureus (48) and Leishmania (55).
In this biofilm study, we were able to confirm that the protective role of thermonuclease towards NETs during the early stages of biofilm formation is similar to what has been described previously for planktonic cells (27, 28). The mechanism by which biofilm can avoid recognition and activation of hTLR9 and ROS still needs to be addressed properly. Since there are indications that thermonuclease does not act alone in the modulation of early host innate responses (52, 56), other immune modulators present during the early stage of biofilm formation should be explored. Investigation into the role of other SaeRS-related immune modulators such as Eap (map) (57) and sasH (AdsA) should be prioritized, since the role of these immune modulators in host immune evasion related to thermonuclease has already been described elegantly in planktonic S. aureus studies (52, 56, 58–60) but not for biofilms grown in mammalian cell culture medium.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. aureus strains used in this study are listed in Table 2. The wild-type and nuc mutant strains were plated on Trypticase soy agar (TSA) with 5% sheep blood for overnight incubation at 37°C (Becton Dickinson, Breda, The Netherlands). The nuc complementation strain was plated on chloramphenicol-containing TSA-blood agar.
TABLE 2.
Strains of S. aureus that were used in this study
| Strain | Genetic background | Description | Reference |
|---|---|---|---|
| Newman | ST8 | Laboratory strain | 71 |
| RN6390 | ST8 | Laboratory strain | 72 |
| M116 | ST8, ST239 | Clinical isolate from a patient with an osteomyelitis infection in Indonesia | 15 |
| RWW50 | ST8, USA300 | Clinical isolate from the collection of MMIZ Erasmus MC, The Netherlands | 15 |
| UAMS-1 | ST30 | Wild-type (WT) clinical isolate from a patient with an osteomyelitis infection in Arkansas, USA | 73 |
| UAMS-1471 | ST30 | Δnuc, thermonuclease mutant strain of UAMS-1 | 74 |
| UAMS-1552 | ST30 | nuc+, complementation of the nuc mutant strain with a plasmid-borne version of the nuc gene | 74 |
Bacterial growth and biofilm assessment.
Biofilm formation was assessed in vitro using a dynamic microtiter plate biofilm formation assay as previously described (61–64) with slight modifications (15). S. aureus bacteria cultured overnight on TSA were suspended in 4 ml of 0.9% NaCl to reach an optical density at 660 nm (OD660) of 2.0 (±0.2). One microliter of this suspension was added to 199 μl Iscove’s modified Dulbecco’s medium (IMDM) (Gibco, Bleiswijk, The Netherlands), without the presence of human serum, in separate wells of a sterile flat-bottom 96-well polystyrene tissue culture plate (Cellstar; Greiner Bio-One, Alphen aan den Rijn, The Netherlands) (64). Plates were incubated for 1, 2, 4, and 24 h under 150 rpm orbital shaking at 37°C. At these time points, the formed biofilms were washed once with 200 μl sterile water, air dried for 30 min at room temperature, and stained for 2 min with 50 μl of 1% crystal violet (Sigma-Aldrich, Zwijndrecht, The Netherlands). Excess crystal violet was removed by washing the plates five times with 200 μl of sterile water. The biofilms were dissolved in 200 μl extraction solution consisting of 50% distilled water (dH2O), 40% ethanol (Sigma-Aldrich), and 10% acetic acid (Sigma-Aldrich). Lastly, to determine the biofilm mass, the absorbance of crystal violet was measured at OD490 in a microplate reader (Epoch 2 microplate reader; BioTek Instruments, Inc., Winooski, VT, USA).
Determination of nuc presence.
The presence of nuc was determined by a PCR protocol as described previously (65).
Collecting biofilm supernatants.
Biofilm supernatants were collected according to protocol described previously (15). Briefly, S. aureus RN6390 (8325-4), Newman, and clinical strains M82 (ST20), M116 (ST239), and RWW50 (USA300) were cultured as described above. We cultured the biofilm for 1, 3, or 23 h. At these time points, we discarded the supernatant to remove all nonattached or planktonic bacteria, washed the biofilm with 200 μl IMDM once, added 200 μl of fresh IMDM, and proceeded for 1 h of incubation at the conditions described above. Subsequently, we collected the supernatants and labeled them as the 2nd, 4th, and 24th hour biofilm supernatants. All individual supernatants from all time points and all different strains were filtered through a 0.2-μm sterile filter (Whatman; GE Healthcare Life Sciences, Little Chalfont, UK) and stored at −20°C until further usage. The supernatant collected after 1 h of biofilm formation served as the negative reference control.
Quantification of thermonuclease Nuc1 production with competitive Luminex assay.
Thermonuclease Nuc1 production during biofilm formation was quantified using a multiplex competitive Luminex assay. With the competitive Luminex assay, we can assign semiquantitatively the amount of an antigen present by determining the ratio of bound and unbound antibodies to the antigen after preexposing serum antibodies to a liquid containing an unknown number of free antigens. The thermonuclease Nuc1 production was measured in the biofilm supernatant of the 1st, 2nd, 4th, and 24th hours of biofilm formation, and the assay was performed according to previously described protocols (15, 29, 61). Briefly, 30 μl of the diluted biofilm-derived supernatants was incubated separately with 30 μl of 1/200 diluted human pooled serum (HPS) (29, 61). The mixture was incubated at 22°C in a U shape 96-well microplate (Greiner Bio-One) placed within Thermostat plus (Eppendorf, Nijmegen, The Netherlands) for 35 min and continuously shaken at 800 rpm. Next, 50 μl of this mixture was directly assayed for the semiquantification of the noncaptured IgGs specific for S. aureus biofilm-derived thermonuclease Nuc1 using Luminex bead-based flow cytometry (Luminex Corporation, Austin, TX, USA). The thermonuclease Nuc1 (N3755; Sigma-Aldrich, Zwijndrecht, The Netherlands) used in this assay was coupled to MagPlex beads (Luminex Corporation) according to the manufacturer’s instructions. The antigen concentration used for coupling was 2 μg per antigen per 106 MagPlex beads as optimized by Hansenová Maňásková et al. (29). The beads were diluted to a final concentration of 1,500 beads/μl. Negative control beads (beads to which no antigen was added during coupling) were included in all assays. The Luminex assay was performed as described previously (66) with one modification; that is, we measured 50 beads per region (29). All samples were analyzed on the Luminex Bio-Plex 200 system using Bio-Plex Manager software version 6.1 (Bio-Rad Laboratories, Inc., USA). All data are based on three separate experiments. The median fluorescence intensity (MFI) values of these triplicates were averaged if the coefficient of variation (CV) value was lower than 25%, and the standard error of the mean (SEM) was calculated. For each sample, we calculated the proportion (%) of uncaptured IgG specific for the thermonuclease Nuc1 using the following formula: Y = (A/B) × 100, in which A is the unbound IgG specific for thermonuclease Nuc1 present in the HPS-biofilm supernatant mix, and B is the level of thermonuclease Nuc1-specific IgG present in untreated HPS. Y can be considered a semiquantitative measurement of the antigen-specific antibody absorption from HPS by biofilm supernatant, thus indirectly reflecting the immune modulators present in the biofilm supernatant (67, 68) and subtracting this percentage from 100%. Statistical differences between the time points were calculated by comparing the percent proportions of the 2nd, 4th, and 24th hour time points and using the 1st hour as the reference control.
Identification of the thermonuclease by mass spectrometry.
Protocol was previously described (15). Briefly, 100 μl of supernatants derived from the 4th hour during biofilm formation of the RWW50 (USA300) and Newman strains was precipitated at 1:10 (vol/vol) with ice-cold acetone and centrifuged for 10 min at 18,407 × g at 4°C. Subsequently, pellets were washed twice with 50 μl of cold acetone. Then, pellets were air dried for 30 min and dissolved in 10 μl of 50 mM NH4HCO3. Solution digestion was performed according to an adjusted protocol as described by Dekker et al. (69). Ten microliters of 0.2% RapiGest (Waters Corporation, Milford, MA, USA), which was previously diluted in 50 mM NH4HCO3, was added to the 10-μl sample. This mixture was then reduced with 5 mM dithiothreitol (DTT) (Thermo Fisher Scientific, Waltham, MA, USA) at 60°C and 450 rpm for 30 min. After cooling down to room temperature, this mixture was alkylated in the dark with 15 mM iodoacetamide (Thermo Fisher Scientific) at ambient temperature for 30 min and digested overnight with 1:100 (wt/wt) trypsin (Promega Corporation, Fitchburg, WI, USA) at 37°C and 450 rpm. Five percent trifluoroacetic acid was added to obtain a final concentration of 0.5% trifluoroacetic acid (pH < 2). After 45 min of incubation at 37°C and 450 rpm, the samples were centrifuged at 13,000 × g for 10 min, and the supernatant was used for liquid chromatography-mass spectrometry (LC-MS). Mass spectrometry and data analyses were performed as described elsewhere (69). A database search was performed against the uniprot_sprot_v151112 database (selected for Bacteria, 332,280 entries).
Quantification of thermonuclease activity with FRET-based assay.
We quantified the thermonuclease activity using a previously described fluorescence resonance energy transfer (FRET)-based assay (37). The FRET substrate was purchased from Eurogentec (Maastricht, The Netherlands) (35). The FRET substrate was diluted to 2 μM in Tris buffer (20 mM Tris, pH 8.0, and 10 mM CaCl2) (35, 37). To measure the activity of the biofilm-associated nuclease of each strain, biofilms were grown in a flat-bottom 96-well black polystyrene tissue culture plate (Costar; Corning Incorporated, NY, USA) at 37°C with continuous 150-rpm agitation. After incubation for 3 h, biofilms were washed once with IMDM or TSB. Twenty-five microliters of new IMDM or TSB (with or without tested compounds) and 25 μl diluted FRET substrate were added to the biofilms. The biofilms were then incubated in a Fluostar Optima microplate fluorescence reader (BMG Lab Technologies) at 30°C with 150-rpm periodic rotational shaking for 2 h. The accumulation of fluorescence during these 2 h, used as a measurement for thermonuclease activity, was determined automatically every 5 min (excitation, 552 nm; emission, 580 nm; gain, 800). Results are reported as fluorescence units. The mean fluorescence intensity (MFI) of three separate experiments was calculated.
LIVE/DEAD-WGA staining for biofilm.
Biofilms were stained with a LIVE/DEAD BacLight bacterial viability kit (Thermo Fisher Scientific, Bleiswijk, The Netherlands) and wheat germ agglutinin (WGA)-Alexa Fluor 350 conjugate (Invitrogen BV, Breda, The Netherlands) according to the manufacturer’s protocol with slight modification. This staining method used propidium iodide (PI) and SYTO 9 to stain the DNA. The affinity of SYTO 9 to DNA is stronger than that of PI, and contrary to PI, SYTO 9 can penetrate live or intact cells. Therefore, in this double staining, SYTO 9 will bind the DNA of live cells while PI will bind to the dead ones or to extracellular DNA (eDNA). As an addition, wheat germ agglutinin (WGA) lectin staining is used to detect the presence of N-acetylglucosamine, which is, for instance, the primary building block of PIA/PNAG (poly-N-acetylglucosamine) (70). In this experiment, 4-h-old biofilms were washed once with 200 μl of IMDM. The biofilms were then incubated with 50 μl of IMDM, 50 μl of 15 μM propidium iodide (PI), 50 μl of 2.5 μM SYTO 9, and 0.5 μl of 1 mg/ml WGA-Alexa Fluor 350 conjugate on each well. The plate was incubated at 22°C on an orbital shaker (300 rpm) in the dark for 35 min. The biofilms were imaged using an Olympus IX51 fluorescence microscope (Olympus Nederland B.V., Zoeterwoude, The Netherlands) with ×20 and ×40 magnification. For relative quantification, the same staining procedure was followed, only the stains were applied individually to separate wells in phosphate-buffered saline (PBS) containing S. aureus biofilms. After staining, the PBS in the wells was replaced with new PBS, and the plate was measured in a Fluostar Optima microplate fluorescence reader (BMG Lab Technologies) at 37°C at an excitation/emission for SYTO of 485 nm/520 nm (gain, 1,280), for PI of 544 nm/612 nm (gain, 2,560), and for WGA-Alexa Fluor 350 conjugate of 340 nm/360 nm (gain, 1,280). Results for both PI (eDNA) and WGA (PIA) are reported as the mean ratio (of three separate experiments) of the SYTO 9 signal (%).
NETosis assay.
Human neutrophils were isolated as described previously (47, 51) and stained with Hoechst 34580 (1:10,000; Life Technologies, Landsmeer, The Netherlands) and PI (1:400; Sigma-Aldrich, Zwijndrecht, The Netherlands). One hundred microliters of 107/ml neutrophils was added to a 3-h-old biofilm formed by Newman, RWW50, UAMS-1, UAMS-1471, and UAMS-1552 in the presence or absence of 5 μg/ml exogenous thermonuclease Nuc1 (N3755; Sigma-Aldrich) in a flat-bottom 96-well polystyrene tissue culture plate (Cellstar; Greiner Bio-One, Alphen aan den Rijn, The Netherlands). Biofilm and neutrophil interactions were imaged with a confocal microscope (Leica SP5 AOBS) after 20 min of incubation at 37°C. Hoechst and PI were excited by 405-nm and 561-nm lasers. NETs were visible as PI-positive elongated structures and were quantified. Furthermore, using the same experimental setup, the presence or absence of NETs was quantified by measuring the amount of fluorescence in a Fluostar Optima microplate fluorescence reader (BMG Lab Technologies) at an excitation and emission of 485 nm and 612 nm, respectively (gain, 1280). One exception to this assay was that the neutrophils were only stained with PI (1:400) before addition to the biofilms. The mean fluorescence intensities (MFIs) of three separate experiments were calculated.
All data were corrected for the background signal (neutrophils without bacteria).
Cellular ROS activation assay.
NETosis can be induced by both ROS-dependent and ROS-independent pathways (54). Therefore, to determine which pathway of NET formation is induced during the early stages of S. aureus biofilm formation, we quantified ROS activation of neutrophils during coincubation with S. aureus biofilm. Three-hour-old biofilms grown in IMDM were washed and refreshed with 100 μl of new IMDM (in the presence or absence of 5 μg/ml exogenous thermonuclease Nuc1 [N3755; Sigma-Aldrich]). One hundred microliters of 107/ml neutrophils was then added to the biofilms. The biofilms were then prepared for the cellular ROS activation assay (GeneCopoeia, Nivelles, Belgium) according to the manufacturer’s protocol. The kinetics of the first 145 min of ROS activation was measured in a Fluostar Optima microplate fluorescence reader (excitation, 552 nm; emission, 580 nm; gain, 800). Results were reported as fluorescence units. The MFIs of three independent experiments were calculated.
Human TLR9 activation assay.
To study the effect of bacterial DNA accumulation in biofilm toward host immune responses, we measured the activity of human Toll-like receptor 9 (TLR9), which is known to be able to bind to bacterial DNA (30, 31). For this experiment, 3-h-old biofilms prepared in IMDM were washed once with 200 μl endotoxin-free water. Two hundred microliters of HEK-Blue detection medium (InvivoGen, Toulouse, France) containing 5 × 104 human embryonic kidney 293 cells expressing human TLR9 (HEK-Blue hTLR9) (InvivoGen, Toulouse, France) was added to the biofilms (in the presence or absence of 5 μg/ml exogenous thermonuclease Nuc1 [N3755; Sigma-Aldrich]). As a positive control, HEK-Blue hTLR9 cells were pretreated with class B CpG oligonucleotides (ODN 2006; InvivoGen). After overnight incubation at 37°C in a 5% CO2 incubator, the OD620 was measured in a microplate reader (Epoch 2 microplate reader; BioTek Instruments, Inc., Winooski, VT, USA).
Biofilm survival assay with microcalorimeter.
Using isothermal microcalorimetry, we observed the effects of neutrophils on the metabolic status of biofilm-associated S. aureus in real time. This metabolic activity directly reflects the fitness of the biofilm-associated bacterial cells and the consequence of coincubation with neutrophils. Wild-type, nuc mutant, and nuc complemented S. aureus UAMS strains (Table 2) cultured overnight on TSA were suspended in 5 ml of 0.9% NaCl to reach an OD600 of 0.5 (±0.05). Ten microliters of this suspension was added to 9,990 μl 0.9% NaCl in a sterile 15-ml tube (Greiner Bio-One, Alphen aan de Rijn, The Netherlands). Then, 10 μl of this bacterial solution was added to 190 μl IMDM (Gibco, Bleiswijk, The Netherlands) in a sterile flat-bottom calWell insert (CalScreener; Symcel Sverige AB, Kista, Sweden). After 3 h of incubation under 150-rpm orbital shaking at 37°C, the biofilms within each individual calWell insert were washed and refreshed with 100 μl of new IMDM and 100 μl of medium containing 107 human neutrophils per milliliter. These individual calWell inserts that contain biofilms and neutrophils were inserted into a titanium tube set (SymCel Sverige AB, Kista, Sweden) and incubated at 37°C overnight in the isothermal microcalorimeter (CalScreener; SymCel Sverige AB, Kista, Sweden) to measure the metabolic activity of the bacteria within the biofilms.
Statistical analysis.
Statistical analysis was performed using the Prism 5.0 package (Graph Pad Software, San Diego, CA, USA) and Microsoft Excel 2010. We used the unpaired t test or one-way analysis of variance (ANOVA) for data analysis, where a P value of ≤0.05 was considered statistically significant. All experiments were independently repeated three times and depicted with standard error of the mean (SEM).
Supplementary Material
ACKNOWLEDGMENTS
We kindly thank Mark S. Smeltzer for sharing the strains UAMS-1, UAMS-1471, and UAMS-1552. We also kindly thank Rezin Majied for the isolation of human neutrophils and Kirby Lattwein for correcting the manuscript.
This study was partly funded by a DIKTI scholarship 2014, grant number 3340/E4.4/K/2014, of the Republic of Indonesia.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00605-19.
REFERENCES
- 1.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuchat A, Hilger T, Zell E, Farley MM, Reingold A, Harrison L, Lefkowitz L, Danila R, Stefonek K, Barrett N, Morse D, Pinner R, Active Bacterial Core Surveillance Team of the Emerging Infections Program Network. 2001. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis 7:92–99. doi: 10.3201/eid0701.010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi SD, Malachowa N, DeLeo FR. 2015. Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol 185:1518–1527. doi: 10.1016/j.ajpath.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 5.Lew DP, Waldvogel FA. 2004. Osteomyelitis. Lancet 364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 6.Ashong CN, Raheem SA, Hunter AS, Mindru C, Barshes NR. 2017. Methicillin-resistant Staphylococcus aureus in foot osteomyelitis. Surg Infect (Larchmt) 18:143–148. doi: 10.1089/sur.2016.165. [DOI] [PubMed] [Google Scholar]
- 7.Ferrando A, Part J, Baeza J. 2017. Treatment of cavitary bone defects in chronic osteomyelitis: biogactive glass S53P4 vs. calcium sulphate antibiotic beads. J Bone Jt Infect 2:194–201. doi: 10.7150/jbji.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol 178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arciola CR, Campoccia D, Ravaioli S, Montanaro L. 2015. Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Front Cell Infect Microbiol 5:7. doi: 10.3389/fcimb.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadovskaya I, Vinogradov E, Flahaut S, Kogan G, Jabbouri S. 2005. Extracellular carbohydrate-containing polymers of a model biofilm-producing strain, Staphylococcus epidermidis RP62A. Infect Immun 73:3007–3017. doi: 10.1128/IAI.73.5.3007-3017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penadés JR, Lasa I. 2009. Protein A-mediated multicellular behavior in Staphylococcus aureus. J Bacteriol 191:832–843. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montanaro L, Poggi A, Visai L, Ravaioli S, Campoccia D, Speziale P, Arciola CR. 2011. Extracellular DNA in biofilms. Int J Artif Organs 34:824–831. doi: 10.5301/ijao.5000051. [DOI] [PubMed] [Google Scholar]
- 13.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 15.Sultan AR, Swierstra JW, Lemmens-den Toom NA, Snijders SV, Hansenová Maňásková S, Verbon A, van Wamel WJB. 2018. Production of staphylococcal complement inhibitor (SCIN) and other immune modulators during the early stages of Staphylococcus aureus biofilm formation in a mammalian cell culture medium. Infect Immun 86:e00352-18. doi: 10.1128/IAI.00352-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang J, Zhou R, Shi X, Kang M, Wang H, Chen H. 2008. Two thermostable nucleases coexisted in Staphylococcus aureus: evidence from mutagenesis and in vitro expression. FEMS Microbiol Lett 284:176–183. doi: 10.1111/j.1574-6968.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Meng J, Shi C, Hervin K, Fratamico PM, Shi X. 2013. Characterization and comparative analysis of a second thermonuclease from Staphylococcus aureus. Microbiol Res 168:174–182. doi: 10.1016/j.micres.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Xie Y, Tang J, Shi X. 2012. Comparative expression analysis of two thermostable nuclease genes in Staphylococcus aureus. Foodborne Pathog Dis 9:265–271. doi: 10.1089/fpd.2011.1033. [DOI] [PubMed] [Google Scholar]
- 19.Lagace-Wiens PR, Alfa MJ, Manickam K, Karlowsky JA. 2007. Thermostable DNase is superior to tube coagulase for direct detection of Staphylococcus aureus in positive blood cultures. J Clin Microbiol 45:3478–3479. doi: 10.1128/JCM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alarcon B, Vicedo B, Aznar R. 2006. PCR-based procedures for detection and quantification of Staphylococcus aureus and their application in food. J Appl Microbiol 100:352–364. doi: 10.1111/j.1365-2672.2005.02768.x. [DOI] [PubMed] [Google Scholar]
- 21.Anfinsen CB. 1968. Characterization of staphylococcal nuclease and the status of studies on its chemical synthesis. Pure Appl Chem 17:461–517. doi: 10.1351/pac196817030461. [DOI] [PubMed] [Google Scholar]
- 22.Sandel MK, McKillip JL. 2004. Virulence and recovery of Staphylococcus aureus relevant to the food industry using improvements on traditional approaches. Food Control 15:5–10. doi: 10.1016/S0956-7135(02)00150-0. [DOI] [Google Scholar]
- 23.Foster TJ. 2005. Immune evasion by staphylococci. Nat Rev Microbiol 3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 24.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A 98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. 2000. A toll-like receptor recognizes bacterial DNA. Nature 408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 26.Hall-Stoodley L, Nistico L, Sambanthamoorthy K, Dice B, Nguyen D, Mershon WJ, Johnson C, Hu FZ, Stoodley P, Ehrlich GD, Post JC. 2008. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol 8:173. doi: 10.1186/1471-2180-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berends ETM, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. 2010. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun 2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thammavongsa V, Missiakas DM, Schneewind O. 2013. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansenová Maňásková S, Bikker FJ, Veerman ECI, van Belkum A, van Wamel WJB. 2013. Rapid detection and semi-quantification of IgG-accessible Staphylococcus aureus surface-associated antigens using a multiplex competitive Luminex assay. J Immunol Methods 397:18–27. doi: 10.1016/j.jim.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi F, Means TK, Luster AD. 2003. Toll-like receptors stimulate human neutrophil function. Blood 102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 31.Krieg AM. 2004. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep 6:88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- 32.Haile LA, Puig M, Kelley-Baker L, Verthelyi D. 2015. Detection of innate immune response modulating impurities in therapeutic proteins. PLoS One 10:e0125078. doi: 10.1371/journal.pone.0125078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano T, Yamamura E. June 2014. Patent WO2014088087 A1; PCT/JP2013/082774.
- 34.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol 164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 35.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. 2011. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One 6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moormeier DE, Bose JL, Horswill AR, Bayles KW. 2014. Temporal and stochastic control of Staphylococcus aureus biofilm development. mBio 5:e01341-14. doi: 10.1128/mBio.01341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beenken KE, Spencer H, Griffin LM, Smeltzer MS. 2012. Impact of extracellular nuclease production on the biofilm phenotype of Staphylococcus aureus under in vitro and in vivo conditions. Infect Immun 80:1634–1638. doi: 10.1128/IAI.06134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grande R, Nistico L, Sambanthamoorthy K, Longwell M, Iannitelli A, Cellini L, Di Stefano A, Hall Stoodley L, Stoodley P. 2014. Temporal expression of agrB, cidA, and alsS in the early development of Staphylococcus aureus UAMS-1 biofilm formation and the structural role of extracellular DNA and carbohydrates. Pathog Dis 70:414–422. doi: 10.1111/2049-632X.12158. [DOI] [PubMed] [Google Scholar]
- 39.Yoshii Y, Okuda KI, Yamada S, Nagakura M, Sugimoto S, Nagano T, Okabe T, Kojima H, Iwamoto T, Kuwano K, Mizunoe Y. 2017. Norgestimate inhibits staphylococcal biofilm formation and resensitizes methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics. NPJ Biofilms Microbiomes 3:18. doi: 10.1038/s41522-017-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson ME, Nygaard TK, Ackermann L, Watkins RL, Zurek OW, Pallister KB, Griffith S, Kiedrowski MR, Flack CE, Kavanaugh JS, Kreiswirth BN, Horswill AR, Voyich JM. 2013. Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infect Immun 81:1316–1324. doi: 10.1128/IAI.01242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zapotoczna M, McCarthy H, Rudkin JK, O'Gara JP, O'Neill E. 2015. An essential role for coagulase in Staphylococcus aureus biofilm development reveals new therapeutic possibilities for device-related infections. J Infect Dis 212:1883–1893. doi: 10.1093/infdis/jiv319. [DOI] [PubMed] [Google Scholar]
- 42.Cue D, Junecko JM, Lei MG, Blevins JS, Smeltzer MS, Lee CY. 2015. SaeRS-dependent inhibition of biofilm formation in Staphylococcus aureus Newman. PLoS One 10:e0123027. doi: 10.1371/journal.pone.0123027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lou Q, Zhu T, Hu J, Ben H, Yang J, Yu F, Liu J, Wu Y, Fischer A, Francois P, Schrenzel J, Qu D. 2011. Role of the SaeRS two-component regulatory system in Staphylococcus epidermidis autolysis and biofilm formation. BMC Microbiol 11:146. doi: 10.1186/1471-2180-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Yeo WS, Bae T. 2016. The SaeRS two-component system of Staphylococcus aureus. Genes (Basel) 7:81. doi: 10.3390/genes7100081. [DOI] [Google Scholar]
- 45.Lindau D, Mussard J, Wagner BJ, Ribon M, Ronnefarth VM, Quettier M, Jelcic I, Boissier MC, Rammensee HG, Decker P. 2013. Primary blood neutrophils express a functional cell surface toll-like receptor 9. Eur J Immunol 43:2101–2113. doi: 10.1002/eji.201142143. [DOI] [PubMed] [Google Scholar]
- 46.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 47.Hoppenbrouwers T, Autar ASA, Sultan AR, Abraham TE, van Cappellen WA, Houtsmuller AB, van Wamel WJB, van Beusekom HMM, van Neck JW, de Maat M. 2017. In vitro induction of NETosis: comprehensive live imaging comparison and systematic review. PLoS One 12:e0176472. doi: 10.1371/journal.pone.0176472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. 2010. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 49.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. 2014. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Avondt K, van der Linden M, Naccache PH, Egan DA, Meyaard L. 2016. Signal inhibitory receptor on leukocytes-1 limits the formation of neutrophil extracellular traps, but preserves intracellular bacterial killing. J Immunol 196:3686–3694. doi: 10.4049/jimmunol.1501650. [DOI] [PubMed] [Google Scholar]
- 51.Hoppenbrouwers T, Sultan AR, Abraham TE, Lemmens-den Toom NA, Hansenová Maňásková S, van Cappellen WA, Houtsmuller AB, van Wamel WJB, de Maat MPM, van Neck JW. 2018. Staphylococcal protein A is a key factor in neutrophil extracellular traps formation. Front Immunol 9:165. doi: 10.3389/fimmu.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. 2015. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol 13:529–543. doi: 10.1038/nrmicro3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 54.Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, Noppen S, Delforge M, Willems J, Vandenabeele P. 2011. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rochael NC, Guimaraes-Costa AB, Nascimento MT, DeSouza-Vieira TS, Oliveira MP, Garcia e Souza LF, Oliveira MF, Saraiva EM. 2015. Classical ROS-dependent and early/rapid ROS-independent release of neutrophil extracellular traps triggered by Leishmania parasites. Sci Rep 5:18302. doi: 10.1038/srep18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thammavongsa V, Schneewind O, Missiakas DM. 2011. Enzymatic properties of Staphylococcus aureus adenosine synthase (AdsA). BMC Biochem 12:56. doi: 10.1186/1471-2091-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harraghy N, Kormanec J, Wolz C, Homerova D, Goerke C, Ohlsen K, Qazi S, Hill P, Herrmann M. 2005. sae is essential for expression of the staphylococcal adhesins Eap and Emp. Microbiology 151:1789–1800. doi: 10.1099/mic.0.27902-0. [DOI] [PubMed] [Google Scholar]
- 58.Stapels DA, Ramyar KX, Bischoff M, von Kockritz-Blickwede M, Milder FJ, Ruyken M, Eisenbeis J, McWhorter WJ, Herrmann M, van Kessel KP, Geisbrecht BV, Rooijakkers SH. 2014. Staphylococcus aureus secretes a unique class of neutrophil serine protease inhibitors. Proc Natl Acad Sci U S A 111:13187–13192. doi: 10.1073/pnas.1407616111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng AG, DeDent AC, Schneewind O, Missiakas D. 2011. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol 19:225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. 2009. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med 206:2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.den Reijer PM, Haisma EM, Lemmens-den Toom NA, Willemse J, Koning RI, Demmers JA, Dekkers DH, Rijkers E, El Ghalbzouri A, Nibbering PH, van Wamel W. 2016. Detection of alpha-toxin and other virulence factors in biofilms of Staphylococcus aureus on polystyrene and a human epidermal model. PLoS One 11:e0145722. doi: 10.1371/journal.pone.0145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luong TT, Lei MG, Lee CY. 2009. Staphylococcus aureus Rbf activates biofilm formation in vitro and promotes virulence in a murine foreign body infection model. Infect Immun 77:335–340. doi: 10.1128/IAI.00872-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan X, Qin N, Wu C, Sheng J, Yang R, Zheng B, Ma Z, Liu L, Peng X, Jia A. 2015. Transcriptome analysis of the biofilm formed by methicillin-susceptible Staphylococcus aureus. Sci Rep 5:11997. doi: 10.1038/srep11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22:996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.den Reijer PM, Lemmens-den Toom N, Kant S, Snijders SV, Boelens H, Tavakol M, Verkaik NJ, van Belkum A, Verbrugh HA, van Wamel WJ. 2013. Characterization of the humoral immune response during Staphylococcus aureus bacteremia and global gene expression by Staphylococcus aureus in human blood. PLoS One 8:e53391. doi: 10.1371/journal.pone.0053391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verkaik N, Brouwer E, Hooijkaas H, van Belkum A, van Wamel W. 2008. Comparison of carboxylated and Penta-His microspheres for semi-quantitative measurement of antibody responses to His-tagged proteins. J Immunol Methods 335:121–125. doi: 10.1016/j.jim.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 67.den Reijer PM, Sandker M, Snijders SV, Tavakol M, Hendrickx AP, van Wamel WJ. 2017. Combining in vitro protein detection and in vivo antibody detection identifies potential vaccine targets against Staphylococcus aureus during osteomyelitis. Med Microbiol Immunol 206:11–22. doi: 10.1007/s00430-016-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hansenová Maňásková S, van Belkum A, Endtz HP, Bikker FJ, Veerman ECI, van Wamel WJB. 2016. Comparison of non-magnetic and magnetic beads in bead-based assays. J Immunol Methods 436:29–33. doi: 10.1016/j.jim.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Dekker LJ, Zeneyedpour L, Brouwer E, van Duijn MM, Sillevis Smitt PA, Luider TM. 2011. An antibody-based biomarker discovery method by mass spectrometry sequencing of complementarity determining regions. Anal Bioanal Chem 399:1081–1091. doi: 10.1007/s00216-010-4361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshii Y, Okuda KI, Yamada S, Nagakura M, Sugimoto S, Nagano T, Okabe T, Kojima H, Iwamoto T, Kuwano K, Mizunoe Y. 2017. Norgestimate inhibits staphylococcal biofilm formation and resensitizes methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics. NPJ Biofilms Microbiomes 3:18. doi: 10.1038/s41522-017-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol 6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 72.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun 63:3373–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsang LH, Cassat JE, Shaw LN, Beenken KE, Smeltzer MS. 2008. Factors contributing to the biofilm-deficient phenotype of Staphylococcus aureus sarA mutants. PLoS One 3:e3361. doi: 10.1371/journal.pone.0003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.