The physiopathology of malaria, one of the most deadly human parasitic diseases worldwide, is complex, as it is a systemic disease involving multiple parasitic stages and hosts and leads to the activation of numerous immune cells and release of inflammatory mediators.
KEYWORDS: B1 B cells, GM-CSF, IL-3, IRA B cells, interferon gamma, Plasmodium, malaria, plasmablasts, type I interferon
ABSTRACT
The physiopathology of malaria, one of the most deadly human parasitic diseases worldwide, is complex, as it is a systemic disease involving multiple parasitic stages and hosts and leads to the activation of numerous immune cells and release of inflammatory mediators. While some cytokines increased in the blood of patients infected with Plasmodium falciparum have been extensively studied, others, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3), have not received much attention. GM-CSF and IL-3 belong to the β common (βc/CD131) chain family of cytokines, which exhibit pleiotropic functions, including the regulation of myeloid cell growth, differentiation, and activation. GM-CSF can be secreted by multiple cell types, whereas IL-3 is mostly restricted to T cells, yet innate response activator (IRA) B cells, a subset of innate B1 B cells, also produce significant amounts of these cytokines during bacterial sepsis via Toll-like receptor 4 (TLR4)/MyD88 sensing of lipopolysaccharides. Herein, using murine models of malaria, we report a sustained production of GM-CSF and IL-3 from IgM+ and IgM−/IgG+ CD138+ Blimp-1+ innate B1b B cell plasmablasts. IgM+ B1b B cells include IRA-like and non-IRA B cells and express higher levels of both cytokines than do their IgG+ counterparts. Interestingly, as infection progresses, the relative proportion of IgM+ B1 B cells decreases while that of IgG+ plasmablasts increases, correlating with potential isotype switching of GM-CSF- and IL-3-producing IgM+ B1 B cells. GM-CSF/IL-3+ B1 B cells originate in the spleen of infected mice and are partially dependent on type I and type II interferon signaling to produce both cytokines. These data reveal that GM-CSF and IL-3 are produced during malaria infections, initially from IgM+ and then from IgG+ B1b B cell plasmablasts, which may represent important emergency cellular sources of these cytokines. These results further highlight the phenotypic heterogeneity of innate B1 B cell subsets and of their possible fates in a relevant murine model of parasitic infection in vivo.
INTRODUCTION
Malaria is caused by the protozoan of the genus Plasmodium, among which P. falciparum is the deadliest species. Malaria remains prevalent worldwide, with 216 million cases and 445,000 deaths in 2016, primarily in children (1). Immunity against malaria involves both humoral and cell-mediated immune mechanisms that target the liver and blood stages of the infection (2), and multiple immune cell subsets contribute to either improve or worsen clinical symptoms (3). The onset of acute blood-stage malaria and severe clinical symptoms are associated with increased blood levels of inflammatory mediators and immune cell activation in human patients, as well as in mouse models (4–8). Levels of the proinflammatory cytokines tumor necrosis factor (TNF), gamma interferon (IFN-γ), interleukin-6 (IL-6), IL-8, IL-12, IL-1β, and IL-18 are augmented and correlate with the control of parasite growth but at the cost of infection severity (6, 9, 10). TNF and IFN-γ can promote phagocyte activation to clear infected red blood cells and effectively kill parasites, yet they might also contribute to deleterious inflammation (11–14). As immunity is gained upon recurrent exposure, anti-inflammatory regulatory cytokines, like IL-10 and transforming growth factor beta (TGF-β), are reported to be generally increased, allowing for a less inflammatory and more controlled antiparasitic immune response (7, 15). While the prior cytokines have been investigated across many studies, some reports have also measured in the blood of Plasmodium falciparum-infected patients the presence of the granulocyte-macrophage colony-stimulating factor (GM-CSF) (16) and IL-3 (17), which both belong to the beta common chain (βc/CD131) family of cytokines (18). Further evidence using mice genetically deficient for GM-CSF infected with chronic Plasmodium chabaudi suggested that GM-CSF contributes to the control of parasite growth and rebounds (19). Interestingly, mice lacking IL-3 better resisted Plasmodium berghei-induced cerebral malaria, suggesting that IL-3 may impair mechanisms of protective immunity in this model (20). GM-CSF and IL-3 exhibit pleiotropic functions at steady state, as well as during inflammatory processes and immune defenses, which include promoting monocyte/neutrophil and megakaryocyte/mast cell/basophil production and activation (18, 21). While GM-CSF can be secreted by multiple distinct cell types, the production of IL-3 seems more restricted to T cells. Yet, a series of relatively recent reports have identified a subset of innate B1a B cells, the innate response activator (IRA) B cells, as an important source of GM-CSF and IL-3 during bacterial sepsis, pneumonia, and atherosclerosis (22–25). IRA B cells contribute to host responses by enhancing inflammation, producing polyreactive IgM, and promoting extramedullary hematopoiesis. However, whether such a B cell population exists and plays a role in other microbial infections or pathological processes remains unknown. More generally, B1 B cells arise early during development and can differentiate into natural IgM antibody (Ab)-secreting cells (ASCs) and cytokine-producing B cells (26–28). They represent the major producers of natural IgM which play a critical role in microbial pathogen protection and in preventing autoimmunity. Peritoneal and pleural cavities are enriched for B1 B cells; however, spleen and bone marrow represent by far the major sites for natural IgM production by the B1 B cells (28). There is an important heterogeneity within the B1 B cell-derived ASCs, with some expressing classical markers of B1 B cells while others express those of terminally differentiated plasma cells exhibiting high levels of cell surface CD138 and intracellular Blimp-1 (27, 29, 30).
Here, using a surrogate murine model of acute blood-stage malaria infection, we report that IgM+ and IgM− CD138+ innate B1b B cell plasmablasts that include IRA-like and non-IRA B cells exhibit sustained production of GM-CSF and IL-3 during blood parasitemia. We found that the production of GM-CSF by the B1 B cell plasmablasts is partially dependent on both cell-extrinsic type I (IFN-α/β) and cell-intrinsic type II (IFN-γ) interferon signaling but is independent of MyD88 signaling, a key cytosolic adaptor of most TLR signaling. Given that GM-CSF and IL-3 are reported to play opposite roles in malaria infection outcomes in mouse models (19, 20), we speculate that GM-CSF and IL-3 from these subsets of B1 B cell plasmablasts could represent important contributors to clinical malaria in humans.
RESULTS
B cells produce GM-CSF and IL-3 in the course of malaria infections.
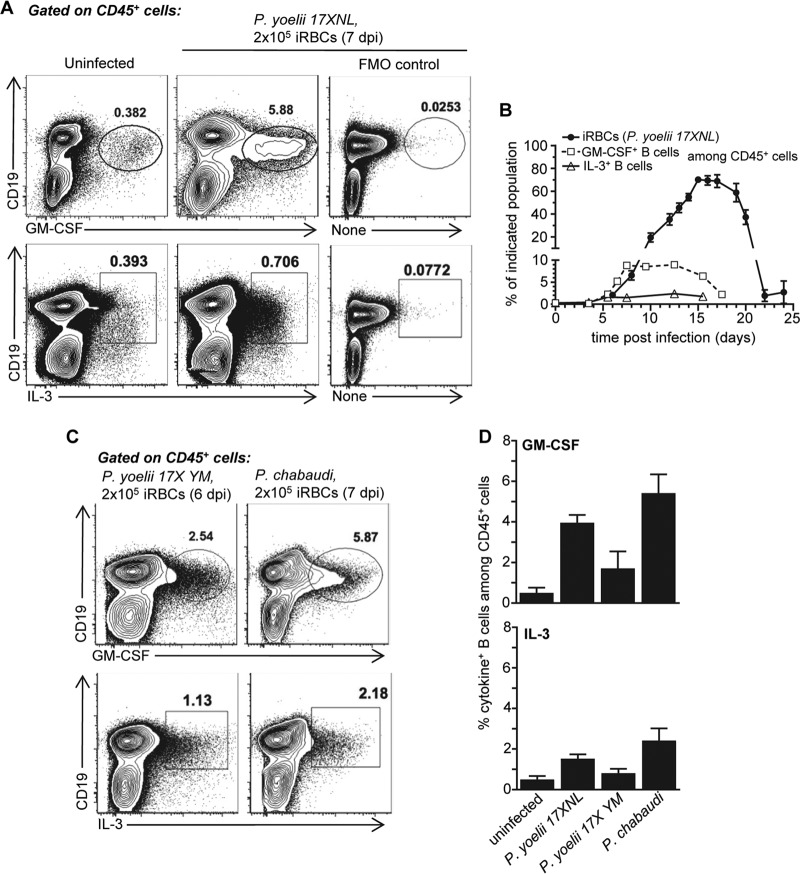
To investigate if GM-CSF and IL-3 are produced during malaria and whether B cells are a source of these cytokines, we inoculated wild-type (WT) C57BL/6 (B6) mice with the nonlethal rodent strain of Plasmodium, Plasmodium yoelii 17XNL, and monitored the production of both cytokines by splenic B cells during the course of the infection (Fig. 1A and B). While GM-CSF- and IL-3-producing B cells could be detected in the spleens of uninfected mice, the frequency of GM-CSF- and IL-3-producing splenic B cells increases up to 20 times and reaches peak production ∼6 to 7 days postinfection; at that time infection progresses with infected red blood cell (iRBC) proportion over 2% before undergoing a decline at 12 to 15 days postinfection. Both of these cytokines are detected from B cells ex vivo upon direct intracellular staining with no need of further restimulation or in vitro incubation, suggesting steady and sustained production by the B cells. The peak production of GM-CSF+ and IL-3+ B cells occurs prior to the peak of parasitemia and diminishes after blood parasitemia starts decreasing, suggesting a correlation with blood parasite elimination kinetics.
FIG 1.
GM-CSF and IL-3 are produced by B cells during malaria infections. Wild-type (WT) C57BL/6 (B6) mice were inoculated with infected red blood cells (iRBCs) of the indicated murine Plasmodium strain (P. yoelii 17XNL, P. yoelii 17X YM, or P. chabaudi) or injected with the same numbers of uninfected RBCs (uninfected). At indicated times postinfection, spleen cells were harvested and stained for cell surface CD45 and CD19 and intracellular GM-CSF or IL-3. (A) Intracellular GM-CSF or IL-3 staining among CD19+ B cells, gated on CD45+ live cells in the different experimental groups. Fluorescence minus 1 (FMO) is shown for the intracellular cytokine staining. (B) Kinetics of GM-CSF and IL-3 production by CD19+ B cells and blood parasitemia following P. yoelii infection. (C) GM-CSF and IL-3 production from B cells after infection with indicated Plasmodium strains. (D) Average proportion of GM-CSF- or IL-3-producing B cells among CD45+ splenic cells 6 (P. yoelii 17X YM) or 7 (P. yoelii 17XNL, P. chabaudi) days postinfection (dpi) across experiments (n = 3 to 12 mice). In all experiments, representative FACS dot plots of 2 to 5 independent replicate experiments with at least 3 mice are presented. Graphs show the average of the results from each experiment along with the standard error of the mean (SEM).
We next asked whether B cells also produced these cytokines after infection with other rodent strains of Plasmodium, namely, P. yoelii 17X YM and P. chabaudi chabaudi AS, which induce lethal and short chronic infections, respectively (Fig. 1C and D; see also Fig. S1 in the supplemental material). Similar to P. yoelii 17XNL infection, we found substantially increased proportions of GM-CSF- and IL-3-producing B cells in the spleen at peak parasitemia in both of these malaria infections, extending our observations to other models of murine malaria.
GM-CSF+ and IL-3+ B cells induced during malaria infections are IgM+ and IgM−/IgG+ CD138+ Blimp-1+ B1 B cell plasmablasts.
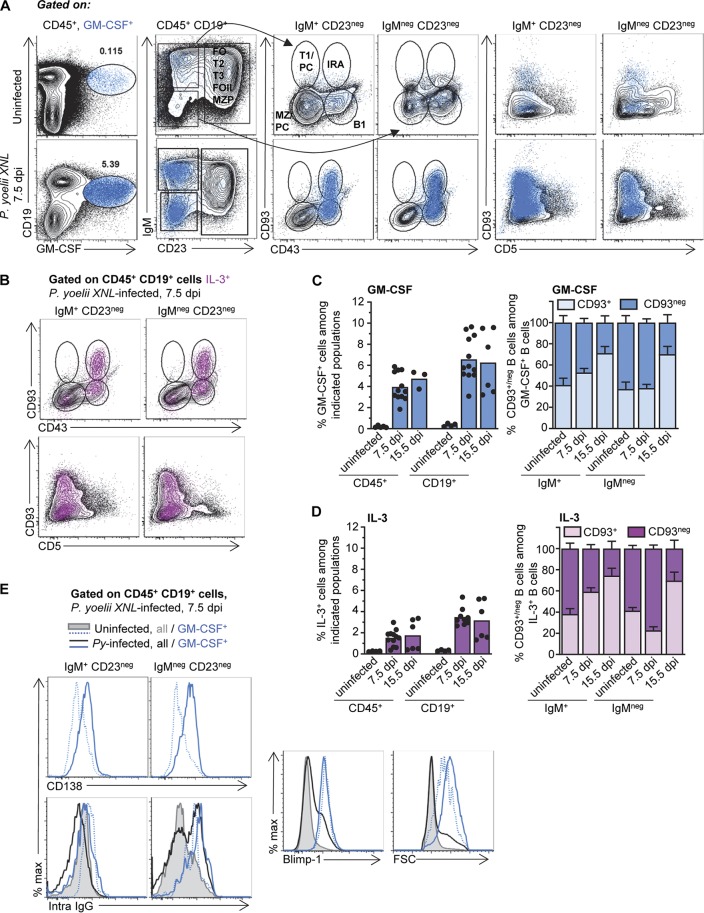
To determine if GM-CSF and IL-3 are produced by specific subsets of B cells, we conducted an extensive flow cytometry characterization of the B cells producing these cytokines in uninfected versus Plasmodium yoelii 17XNL-infected mice after staining for cell surface CD19, IgM, CD93, CD23, CD43, and CD5 and intracellular GM-CSF, IL-3, IgG, and Blimp-1 (Fig. 2 and S2). GM-CSF+ and IL-3+ cells are CD23− CD93+ (or CD93−) CD43+ CD5low, identifying them as B1b B cells (Fig. 2A and B and S2A). GM-CSF+ and IL-3+ B1 B cells, respectively, represent between ∼0.2% (naive) and 1.5 to 4% (P. yoelii-infected) of hematopoietic (CD45+) cells, which account for ∼0.35% (naive) and 3.5 to 6.5% (infected) of the B (CD19+) cells (Fig. 2B to D). Approximately half of the GM-CSF+ or IL-3+ cells in naive or infected mice express cell surface IgM, while the other half do not. Within the cytokine-producing IgM+ B cells, 50 to 70% express CD93, reminiscent of the IRA B cell phenotype (25) (Fig. 2A to D and S2A). At the peak of parasitemia (day 15), CD93+ cells represented the main producers (>70%) of GM-CSF and IL-3, regardless of IgM expression (Fig. 2C and D). Interestingly, both IgM+ and IgM− GM-CSF- and IL-3-producing counterparts express high levels of cell surface CD138 and the Blimp-1 transcription factor, consistent with plasmablast features (Fig. 2E and S2B). Of note, GM-CSF+ and IL-3+ IgM− plasmablasts expressed high levels of intracellular IgG. These cells are also enlarged (forward scatter [FSC]) in infected compared to uninfected mice and all B cells. Thus, these results establish that a population of B1b B cells producing GM-CSF and IL-3 is found in the spleen of naive WT B6 mice, and they can expand ∼10 to 20 times upon malaria infection and acquire phenotypic features of plasmablasts.
FIG 2.
B1b B cell plasmablasts produce GM-CSF and IL-3 during P. yoelii infection. WT B6 mice were inoculated with P. yoelii 17XNL iRBCs or injected with the same numbers of uninfected RBCs (uninfected). (A to C) Spleens from uninfected or day 7.5 P. yoelii-infected mice were harvested and the cells stained for cell surface CD45, CD19, IgM, CD23, CD43, CD93, and CD5 and intracellular GM-CSF or IL-3. (A and B) Dot plots are shown with the successive gating strategy used (FO, follicular; T1/T2/T3, transitional 1/2/3; MZP, marginal zone precursor; MZ, marginal zone; PC, plasma cell; IRA, innate response activator). GM-CSF+ (blue) or IL-3+ (fuchsia) cells are overlaid on the indicated populations. (C and D) Bar graphs summarize the frequency of GM-CSF-producing (C) or IL-3-producing (D) cells among indicated cell populations (left) and the proportion of GM-CSF+ or IL-3+ IgM+/− cells that express CD93 or not (right) in uninfected versus P. yoelii-infected mice. (E) Spleen cells from uninfected or day 7.5 P. yoelii-infected mice were stained for cell surface CD45, CD19, IgM, CD23, and CD138 and intracellular GM-CSF, IgG, and Blimp-1. In all experiments, representative FACS dot plots of 1 mouse across 5 replicate experiments are shown (n = 8 to 14). Graphs show the average of the results from each experiment, with each dot representing 1 individual mouse or with the mean ± SEM. max, maximum.
Proportions of IgM−/IgG+ GM-CSF+ and IL-3+ B1b B cell plasmablasts increase while that of the IgM+ counterpart decreases as infection progresses.
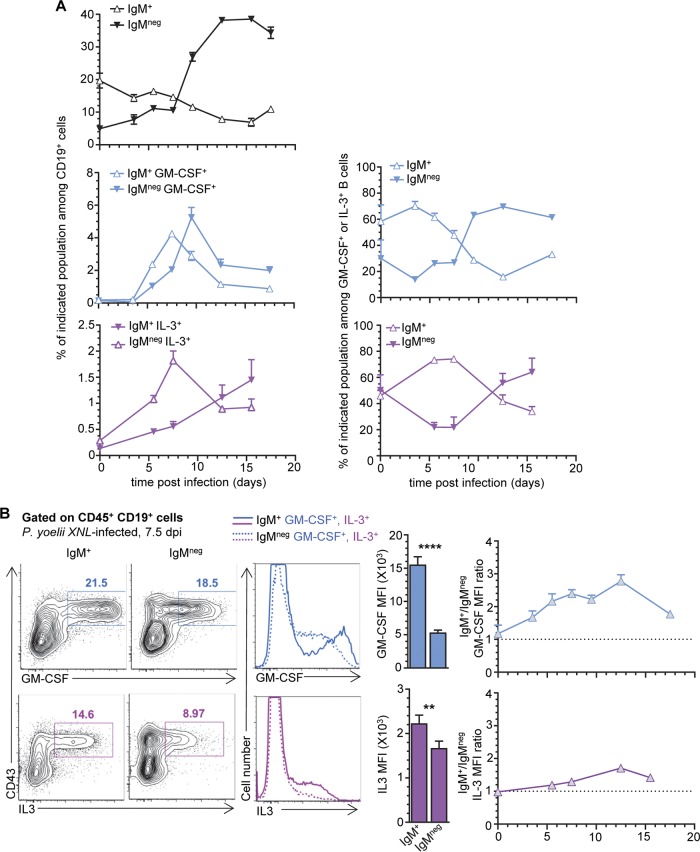
Since in both naive and P. yoelii-infected mice, about half of the GM-CSF+ or IL-3+ splenic B cells are IgM+ while the other half are not, we next asked if the IgM+ subset may convert into the IgM−/IgG+ subset or simply expand from those found at steady state (Fig. 3). We monitored the kinetics of IgM+ and IgM− GM-CSF- and IL-3- or noncytokine-producing B cell expansion after P. yoelii infection, hypothesizing that the proportion of IgM− GM-CSF/IL-3+ B cells should increase concomitantly to the decrease of IgM+ counterparts if IgM+ converts to IgM−/IgG+ B cells (Fig. 3A). As reported in prior studies using both P. yoelii and other murine models of malaria, the overall proportion of all splenic IgM+ B cells decreases (factor of ∼2), while that of IgM− (IgG+) increases (factor of ∼10) as infection progresses and robust parasite-specific germinal center reactions and IgG responses are taking place (31, 32). Interestingly, in our model, the frequencies of IgM+ GM-CSF+ and IL-3+ B cells among B cells increase to peak by ∼7 to 8 days postinfection and then steadily diminish. IgM−/IgG+ GM-CSF+ B cells then augment comparatively to reach a plateau before contracting with kinetics similar to that of total splenic IgM− B cells (Fig. 3A). Thus, this finding correlates with the idea that IgM+ GM-CSF+ and IL-3+ B1 B cell plasmablasts could undergo isotype switching during the infection. Levels of cell surface CD19 are also lower in IgM− but not IgM+ GM-CSF+ and IL-3+ B cells from infected spleens, consistent with a possible long-lived plasma cell fate of switched cells (Fig. S2C). We also note that GM-CSF and IL-3 staining ratios in the IgM+ fraction are, respectively, ∼3- and 1.2-fold higher than that of the IgM− plasmablasts, and their ratios remain sustained over time, supporting the idea that GM-CSF+ and IL-3+ IgM+ and IgM− B1 B cells are functionally different (Fig. 3B). Collectively, our results show that in the course of malaria infection, as GM-CSF- and IL-3-producing IgM+ splenic B1 B cell proportions decrease, that of the IgM−/IgG+ counterpart increases, correlating with possible isotype switching and plasma cell differentiation as they expand in the spleen of P. yoelii-infected mice.
FIG 3.
Expansion and contraction of IgM+ GM-CSF/IL-3+ B1b B cell plasmablasts inversely correlate with those of the IgM−/IgG+ counterparts. WT B6 mice were inoculated with P. yoelii iRBCs or injected with the same amount of uninfected RBCs (uninfected). Spleens from uninfected or P. yoelii-infected mice were harvested at the indicated days, and cells were stained for cell surface CD19, IgM, CD23, CD43, and CD93 and intracellular GM-CSF or IL-3 in some but not all experiments. (A) Top left graph shows the proportion of indicated IgM+ or IgM− CD19+ B cells over time postinfection. The middle and lower graphs on the left show the proportions of the indicated IgM+/− GM-CSF+ or IL-3+ cell populations among B cells over time postinfection. Right graphs show the relative proportions of IgM+ versus IgM− GM-CSF+ or IL-3+ B cells. (B) Representative dot plots of intracellular GM-CSF or IL-3 staining of IgM+ or IgM− B cells from spleen of day 7.5 P. yoelii-infected mice. Bar graphs show the corresponding average mean fluorescence intensity (MFI) for indicated intracellular cytokine. The right graphs depict the ratios of GM-CSF or IL-3 MFI in IgM+ versus IgM− B cells over time after infection. Two to 5 independent replicate experiments with at least 3 mice are presented. Graphs show the average of the results from each experiment along with the SEM. P values are indicated when applicable.
GM-CSF+ and IL-3+ B cells originate from the spleen but not the peritoneal cavity.
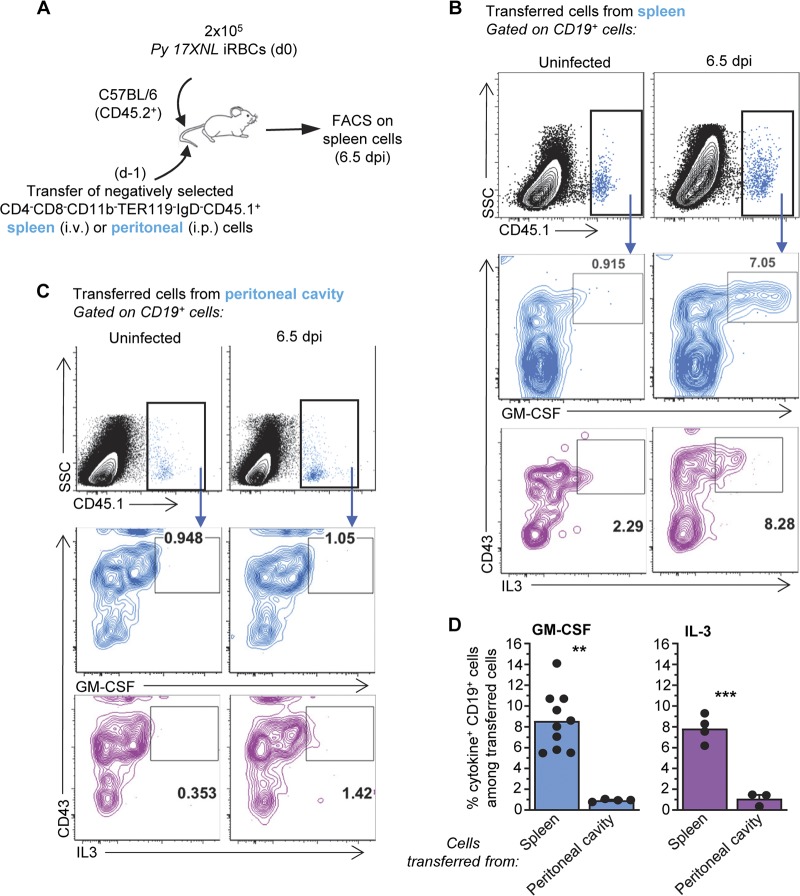
While we would expect GM-CSF/IL-3+ plasmablasts to originate in the spleen, IRA B cells are reported to come from peritoneal B1a B cells during bacterial sepsis (25). To determine whether GM-CSF/IL-3+ B1 B cells originate from the spleen or the peritoneal cavity of P. yoelii-infected mice, we isolated CD4− CD8− CD11b− TER119− IgD− cells from either the spleen or the peritoneal cavity of WT CD45.1+ mice and adoptively transferred them to WT CD45.2+ recipient mice that we further inoculated with P. yoelii-iRBCs the next day (Fig. 4A). Six and a half days later, spleens were harvested and cells stained to track GM-CSF- and IL-3-producing transferred B cells (Fig. 4B to D). We detected in the transferred B cells whether they originated from the spleen or the peritoneal cavity of donor mice, yet only those originating from the spleen but not from the peritoneal cavity stained positive for GM-CSF and IL-3. Thus, in contrast to what is reported during bacterial sepsis, in the course of malaria infection, GM-CSF- and IL-3-producing B1 B cells seem to expand from a local splenic progenitor.
FIG 4.
GM-CSF- and IL-3-producing B cells originate from the spleen of P. yoelii-infected mice. (A) Schematic of experimental design. i.v., intravenous. (B and C) Spleen cells from P. yoelii-infected or uninfected mice were stained for cell surface CD45.1, CD19, and CD43 and intracellular GM-CSF or IL-3. Data show a representative FACS dot plot of GM-CSF+ or IL-3+ cells after gating on transferred CD45.1+ cells isolated from the spleen (B) or peritoneal cavity (C). SSC, side scatter. (D) Average proportion of GM-CSF- or IL-3-producing CD19+ B cells among transferred cells across two independent replicate experiments, with each dot featuring an individual mouse (n = 3 to 10 mice). Graphs shown the average of the results from each experiment along with the SEM. P values are indicated when applicable.
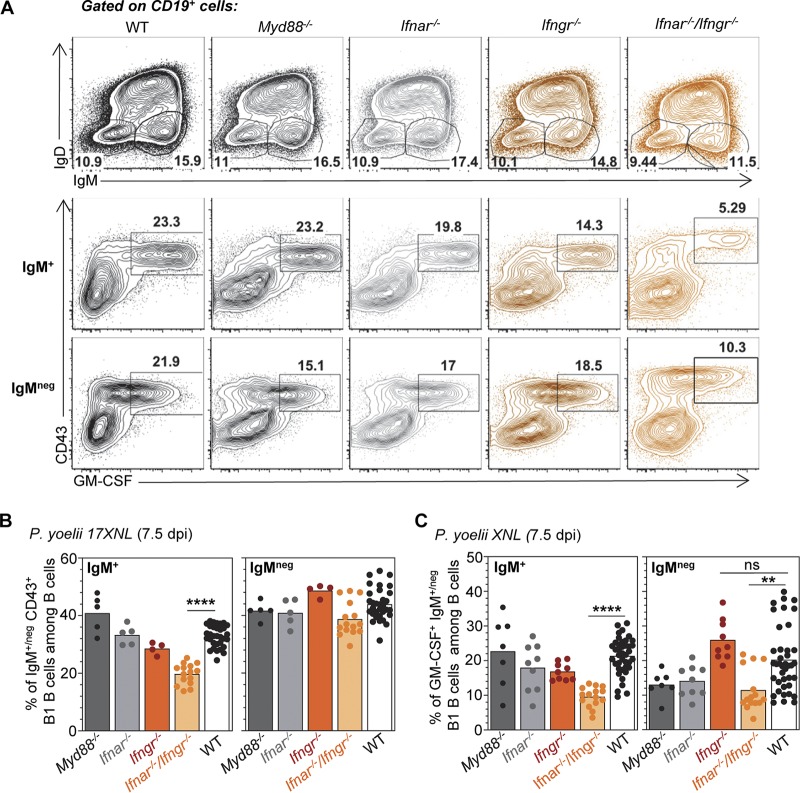
Generation of B1 B cell plasmablasts and their production of GM-CSF are partially dependent upon interferon signaling.
We next assessed if major innate sensing or cytokine signaling pathways controlled (i) the generation of splenic B1 B cell plasmablasts and (ii) their induced production of GM-CSF and IL-3. Mice lacking the major intracellular sensing adaptor MyD88 (Myd88−/−), the type I interferon (Ifnar−/−) receptor, the type II interferon (Ifngr−/−) receptor, both interferon receptors (Ifnar−/− Ifngr−/−), or control WT counterparts were inoculated with P. yoelii iRBCs, and we monitored GM-CSF and IL-3 production from CD19+ cells 7.5 days later (Fig. 5). Only the combined lack of both type I and type II interferon signaling, but not MyD88, significantly decreases the generation of IgM+ CD43+ B1 B cells among B cells, but there are no significant differences in producing the IgM− plasmablasts (Fig. 5A and B). Interestingly, the induction of GM-CSF in both IgM+ and IgM− plasmablasts is impaired when type I and type II interferon signaling is disrupted (Fig. 5A and C). Altogether, these results show that while only the generation of IgM+ but not IgM− B1 B cell plasmablasts is enhanced by type I and type II interferons, their GM-CSF-producing capacity is promoted by interferon signaling in both subsets of plasmablasts, suggesting that these processes are regulated through distinct yet overlapping mechanisms.
FIG 5.
Type I and II interferon signaling enhances the generation of B1 B cell plasmablasts and their production of GM-CSF during malaria infection. Indicated knockout or WT mice (all B6 background) received 2 × 105 P. yoelii 17XNL-infected iRBCs, and 7.5 days later, spleens were harvested and stained for cell surface CD45, CD19, IgM, IgD, and CD43 and intracellular GM-CSF. (A) Representative FACS dot plots across 2 to 3 independent replicate experiments are shown. (B and C) The average result across all experiments is shown, with each dot representing an individual mouse (n = 4 to 19 for knockout [KO] mice, n = 44 for WT mice).
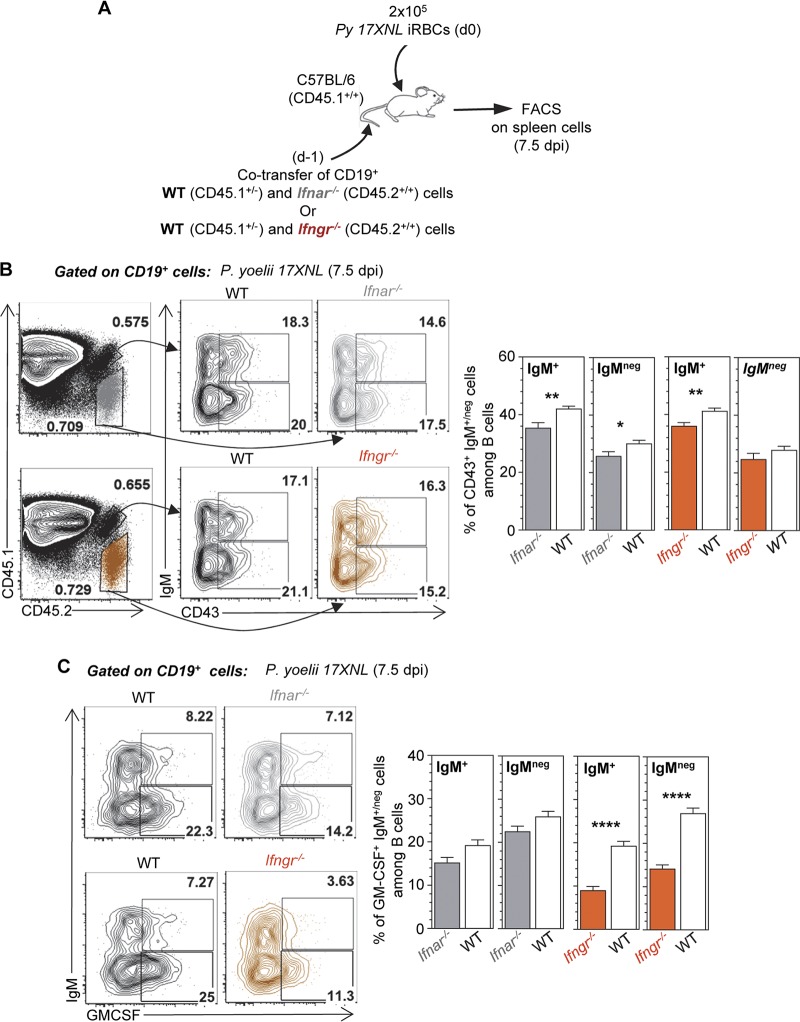
To further determine whether cell-intrinsic or cell-extrinsic interferon signaling accounted for our observations, we adoptively cotransferred splenocytes from Ifnar−/− or Ifngr−/− and control WT mice into congenically distinct WT recipient mice, which we subsequently inoculated with P. yoelii and tracked the differentiation and GM-CSF/IL-3 production of transferred B cells 7.5 days postinfection (Fig. 6A). We found that the generation of CD43+ IgM+ B1 B cells is impaired by the lack of direct, cell-intrinsic type I or II interferon signaling, while that of the IgM− counterparts is less affected (Fig. 6B), consistent with results in the full-knockout mice (Fig. 5A and B). Interestingly, we also reveal that disrupting cell-intrinsic IFN-γ signaling significantly decreases GM-CSF production by both IgM+ and IgM− B1 B cells (Fig. 6C). In contrast, we observed only a mild impact in B cells lacking type I IFN signaling, suggesting that type I interferon largely promotes GM-CSF production through cell-extrinsic mechanisms. In conclusion, both type I interferon and IFN-γ signaling enhance the generation of CD43+ IgM+, but not IgM−/IgG+, B1 B cells, largely through direct, cell-intrinsic mechanisms. Moreover, cell-intrinsic IFN-γ and cell-extrinsic type I interferon signaling contributes to enhancing the production of GM-CSF by IgM+ and IgM− B1 B cells.
FIG 6.
Cell-intrinsic interferon signaling enhances the generation of B1 B cell plasmablasts, while production of GM-CSF is promoted by both cell-intrinsic IFN-γ and cell-extrinsic type I interferon signaling during P. yoelii infection. (A) Schematic of experimental design. (B and C) Spleen cells from P. yoelii-infected (day 7.5) or uninfected mice were stained for cell surface CD45.1, CD45.2, CD19, IgM, and CD43 and intracellular GM-CSF. Data show a representative FACS dot plot of CD43+ (B) or GM-CSF+ (C) CD19+ B cells after gating on transferred cells. Graphs show the average of the results from one experiment with 5 mice along with the SEM. P values are indicated when applicable.
DISCUSSION
The current study characterizes a population of splenic innate B1 B cells that exhibits sustained production of the myeloid trophic and activating cytokines GM-CSF and IL-3 during murine malaria infection. These cells are B1b B cells (CD23− CD43+ CD5−) that express markers of plasmablasts (CD138high Blimp-1high). Interestingly, while the majority of these cells express surface IgM early on, as infection progresses, a population of IgM− IgG+ B1b B cell plasmablasts increases as IgM+ counterparts are lost, suggesting that IgM+ could be giving rise to the IgM−/IgG+ subset after isotype switching. Whether the GM-CSF/IL-3-producing IgG+ B1 B cells arise from the IgM+ subset or expand from a preexisting population, the two subsets appear to be functionally different; for instance, IgG+ plasmablasts exhibit lower intracellular staining for GM-CSF and IL-3 than do their IgM+ counterparts. Our results also establish that type I interferon signaling and type II interferon signaling are essential in the generation of IgM+ but not IgM− B1 B cell plasmablasts, via B cell-intrinsic mechanisms, and in the induction of GM-CSF production, via B cell intrinsic for IFN-γ and cell extrinsic for type I IFN signaling, from both IgM+ and IgM− B1 B cell plasmablasts during malaria infection.
Prior work has reported the characterization of a population of splenic GM-CSF- and IL-3-producing innate B1 B cells, the IRA B cells, in models of bacterial sepsis (24, 25) and pneumonia (23) and during atherosclerosis (22). In our malaria infection model, we observed that about 50% of GM-CSF- and IL-3-producing IgM+ B1 B cells are phenotypically identical to the IRA B cells (CD23− CD43+ CD93+ CD138+). However, these cells also show greater heterogeneity since half of them do not express the stem cell marker CD93, which is reported to be functionally important in antibody secretion and plasma cell maintenance in bone marrow (33). In addition, GM-CSF and IL-3 production in these cells is independent of MyD88, and they originate from a splenic B1b B cell precursor, all features that are distinct from that of IRA B cells, which are reported to (i) require MyD88 to initiate the production of GM-CSF and IL-3 and (ii) arise from B1a B cells residing in serosal sites (peritoneal or pleural cavity). Our data also show a clear role of cell-intrinsic IFN-γ and cell-extrinsic type I interferon signaling in enhancing GM-CSF and IL-3 secretion from these innate B1 B cells during malaria infection. Thus, despite a phenotypic heterogeneity, their common splenic origin and comparable mechanism of GM-CSF and IL-3 cytokine induction support the idea that the GM-CSF- and IL-3-producing B1 B cells in malaria infection have functional requirements distinct from those of the IRA B cells described previously. It is worth noting, however, that IRA B cells were discovered in an acute, short-term model of microbial sepsis in the peritoneal cavity, and the long-term fate of IRA B cells was not analyzed further. The kinetics and tropism of Plasmodium infection are clearly very different from those of acute sepsis and may well be accounting for the distinct origin, fates, phenotype, and mechanism of activation of these GM-CSF- and IL-3-producing B1 B cells.
The fact that the GM-CSF- and IL-3-producing B1 B cells we describe undergo functional maturation into plasma cells and could possibly also be undergoing isotype switching has also not been reported to happen for IRA B cells, though this has been suggested to occur in B1 B cells (30, 34). Their expression of the plasma cell surface marker CD138 and the transcription factor Blimp-1 is a feature consistent with plasma cell functional differentiation. While our kinetic analysis is consistent with the possibility that, as infection develops, IgM+ B1 B cells become IgM−/IgG+, it still is possible that the IgM−/IgG+ population is specifically expanded and has a competitive edge on the IgM+ counterpart, and that no progeny relationship exists between these IgM+ and IgM− B1 B cells. Along these lines, B1 B cells can secrete natural IgG3 antibodies (Abs) (29) and may selectively expand during this parasitic infection. In patients, IgG3 Abs against the merozoite-derived protein MSP3 of Plasmodium falciparum have been strongly associated with long-term clinical protection (35) and opsonization (36), although the origin of the Plasmodium falciparum-specific IgG3 Abs was not investigated. The IgM−/IgG+ plasmablasts we characterize express high levels of the Blimp-1 transcription factor, which suggests that they are acquiring features of switched plasma cells and are different (or represent a more differentiated stage) from the previously described natural IgG3-producing B1 B cells. A tempting hypothesis given the current results is that infection-induced GM-CSF+- and IL-3+ IgM+-producing B1 B cell-derived plasmablasts contribute to a first wave of innate immune defense that is sustained through either the production of a progeny of IgM− or P. yoelii-specific IgG-secreting plasmablasts or the expansion of IgM− natural IgG3-producing plasmablasts. A recent study in human malaria proposed an interesting link of an IgG3 polymorphism in the neonatal Fc receptor that increases Plasmodium falciparum-specific IgG3 transplacental transfer and clinical protection in infants (37).
Similarly to bacterial sepsis, in which IRA B cells are reported to either promote (via IL-3) or protect (via GM-CSF) the host, a lack of GM-CSF or IL-3 has opposite outcomes during malaria infection, with GM-CSF-deficient mice exhibiting lower resistance to chronic P. chabaudi infection (19), while IL-3-deficient mice resisted better P. berghei-induced experimental cerebral malaria (ECM) (20). IRA B cell-derived GM-CSF increases resistance to bacterial sepsis through secreted IgM and subsequent activation of innate myeloid cell phagocytic capacity and bacterial clearance. In contrast, IRA B cell-derived IL-3 enhances extramedullary emergency hematopoiesis, the production and activation of phagocytes and their progenitors, and the subsequent release of inflammatory cytokines, such as TNF, IL-1β, and IL-6, all of which also take place during blood-stage malaria and may account for IL-3-deficient mouse-augmented resistance to ECM. However, none of the prior reports analyzing mice lacking GM-CSF or IL-3 explored if one or several cell subsets producing either of these cytokines account for the observed susceptibility or resistance phenotypes. Given the sustained production of both cytokines we found in innate B1 B cells in the course of murine malaria infections, it is possible that these cells indeed represent key actors of resistance to this significant parasitic disease. Along these lines, human subjects with genetic polymorphisms in the IL-3 gene exhibit increased resistance to malaria (38). While the cell surface phenotypes of the cells producing GM-CSF and IL-3 during malaria infection appear to be identical, despite multiple attempts, we have not been able to determine whether the same cells express both cytokines, largely due to technical limitations associated with the available reagents and associated fluorochromes. The proportion of GM-CSF+ B1 B cells is 3 to 5 times higher than that of IL-3 during the course of malaria infection; thus, it may be that GM-CSF+ versus IL-3+ B1 B cells represent a functional subspecialization, consistent with their presumably distinct functional roles described in the various infection models. Also, it is unclear what the contribution of other cell subsets that may produce GM-CSF and IL-3 is during malaria, though we have not detected significant de novo sources other than that of the B1 B cells. T cells and innate lymphoid cells (ILCs), for instance, are known to be able to secrete both of these cytokines, but it is likely that specific conditions of ex vivo restimulation would have to be further tested. It is worth noting that a population of GM-CSF-producing B cells has been reported in human patients suffering from multiple sclerosis (39), yet these cells belong to the B2 B cell lineage, express markers of memory B cells, and are induced through CD40L- and T cell-derived cytokines.
In conclusion, this work and current literature on GM-CSF- and IL-3-producing B cells further underline the important heterogeneity in their origin, phenotypes, and functions. Many questions remain to be explored in order to further define (i) both the cell-intrinsic and -extrinsic cues that drive their differentiation into GM-CSF- and/or IL-3-producing cells, depending on their origin, (ii) their functional fates (whether they do undergo isotype switching and, if yes, of which predominant isotype), and (iii) at least for malaria, if and how they may contribute to resolving or promoting the pathogenesis of this deadly infection.
MATERIALS AND METHODS
Mice.
Wild-type (WT) C57BL/6J (B6), CD45.1+/+ (strain 2014; Jackson Laboratory), Myd88−/− (strain 009088; Jackson Laboratory), Ifnar−/− (kind gift of Jake Kohlmeier, Emory Vaccine Center), Irf3−/− (kind gift of Thomas Moran, Mount Sinai School of Medicine), and Ifngr−/− (strain 003288; Jackson Laboratory) mice were housed and bred in our specific-pathogen-free (SPF) animal facility for all experiments. The mice used for all experiments were 6 to 12 weeks old. This study was carried out in strict accordance with the recommendations by the Institutional Animal Care and Use Committee (IACUC) at the Albert Einstein College of Medicine (protocol number 20171113), which adheres to the regulations and guidelines set by the National Research Council. The Albert Einstein College of Medicine is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care.
Plasmodium infections and blood parasitemia.
(i) Infections. Plasmodium yoelii (17XNL([1.1]) parasites (stock MRA-593), Plasmodium yoelii (17X) clone YM parasites (stock MRA-755), and Plasmodium chabaudi chabaudi AS parasites (stock MRA-748) were obtained from the Malaria Research and Reference Reagent Resource Center as part of the BEI Resources Repository (NIAID, NIH, Manassas, VA; deposited by D. Walliker). P. yoelii-infected red blood cells (iRBCs) from a frozen stock (stored in −80°C in Alsever’s solution, 10% glycerol) were intraperitoneally (i.p.) injected into a WT B6 mouse and grown for ∼4 days. When parasitemia reached 2 to 5%, P. yoelii iRBCs were diluted to appropriate concentrations with 1× phosphate-buffered saline (PBS) and injected intravenously into each experimental mouse, unless otherwise indicated.
(ii) Parasitemia. Blood parasitemia was determined by flow cytometry on 1 μl of blood obtained by cutting the tip of the tail with a sterile razor. Blood was fixed in 200 μl of 0.025% glutaraldehyde in 1× PBS containing 1 mM EDTA before being washed and permeabilized with 0.25% Triton X-100 in 1× PBS for 5 min. RBCs then were incubated in 1 mg/ml RNase A (Sigma) for 30 min at room temperature (RT), stained with 0.5 μM YOYO-1 dye (Invitrogen) for 30 min at RT, and directly analyzed on a BD FACSCanto II (Becton, Dickinson, CA). RBCs were gated based on forward and side scatter, and parasitemia was determined as the frequency of YOYO-1+ cells among all cells. Eye counting of Giemsa-stained blood smears by microscopy confirmed that the determination of parasitemia using the above-described method gave comparable results.
Cell suspensions for flow cytometry and adoptive transfer.
Spleens were dissociated on nylon meshes (100-μm pore size) and incubated at 37°C for 20 min in Hanks’ balanced salt solution (HBSS) medium containing 4,000 U/ml collagenase I (Gibco) and 0.1 mg/ml DNase I (Roche), and RBCs were lysed with NH4Cl buffer (0.83% [vol/vol]). Cell suspensions were resuspended in fluorescence-activated cell sorting (FACS) buffer (PBS, 1% fetal calf serum [FCS], 2 mM EDTA, 0.02% sodium azide) and used for the different analyses (see below).
Cell staining for FACS analysis.
Cell suspensions were incubated with 2.4G2 Fc block for 15 min at 4°C and then stained with specified antibody cocktail (Table S1) in FACS buffer. For intracellular GM-CSF and IL-3 de novo staining, cells were fixed in intracellular (IC) fixation buffer (eBioscience) for 15 min at 4°C after extracellular staining and incubated with intracellular antibodies for 30 min in 1× Perm/Wash buffer (eBioscience). Stained cells were collected on either a FACS BD LSR II or a FACSAria III. Data were analyzed using FlowJo version 9 (TriStar).
Statistics.
Statistical significance was calculated using an unpaired Student's t test with GraphPad Prism software, and two-tailed P values are given as *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; and ns, P > 0.05. All P values of 0.05 or less were considered significant and are referred to as such in the text.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Einstein FACS core facility and Matty Scharff for critical discussions.
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grants AI122801 and AI128735 to G.L. and grant AI139297 to J.C. L.C. received fellowships from ARC, Fondation Bettencourt-Schuller, and the American Association of Immunology (AAI). S.S.C. was supported by NIH training grant T32A170117. Core resources for FACS were supported by the Einstein Cancer Center (National Cancer Institute [NCI] cancer center support grant 2P30CA013330).
L.C. and S.S.C. designed, performed, and interpreted the experiments with G.L. S.S.C. and G.L. designed and assembled the figures. G.L. wrote the paper, with critical reading by L.C., S.S.C., and J.C.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00482-19.
REFERENCES
- 1.Chaussabel D, Pajak B, Vercruysse V, Bisseye C, Garze V, Habib M, Goldman M, Moser M, Vray B. 2003. Alteration of migration and maturation of dendritic cells and T-cell depletion in the course of experimental Trypanosoma cruzi infection. Lab Invest 83:1373–1382. doi: 10.1097/01.LAB.0000087587.93781.6F. [DOI] [PubMed] [Google Scholar]
- 2.Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK. 2014. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol 32:157–187. doi: 10.1146/annurev-immunol-032713-120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb TJ, Brown DE, Potocnik AJ, Langhorne J. 2006. Insights into the immunopathogenesis of malaria using mouse models. Expert Rev Mol Med 8:1–22. doi: 10.1017/S1462399406010581. [DOI] [PubMed] [Google Scholar]
- 4.Spaulding E, Fooksman D, Moore JM, Saidi A, Feintuch CM, Reizis B, Chorro L, Daily J, Lauvau G. 2016. STING-licensed macrophages prime type I IFN production by plasmacytoid dendritic cells in the bone marrow during severe Plasmodium yoelii malaria. PLoS Pathog 12:e1005975. doi: 10.1371/journal.ppat.1005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning L, Laman M, Davis WA, Davis TM. 2014. Clinical features and outcome in children with severe Plasmodium falciparum malaria: a meta-analysis. PLoS One 9:e86737. doi: 10.1371/journal.pone.0086737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. 2004. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayimba E, Hegewald J, Segbena AY, Gantin RG, Lechner CJ, Agosssou A, Banla M, Soboslay PT. 2011. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin Exp Immunol 166:218–226. doi: 10.1111/j.1365-2249.2011.04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chimma P, Roussilhon C, Sratongno P, Ruangveerayuth R, Pattanapanyasat K, Perignon JL, Roberts DJ, Druilhe P. 2009. A distinct peripheral blood monocyte phenotype is associated with parasite inhibitory activity in acute uncomplicated Plasmodium falciparum malaria. PLoS Pathog 5:e1000631. doi: 10.1371/journal.ppat.1000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, Lambert PH. 1989. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 10.Walther M, Woodruff J, Edele F, Jeffries D, Tongren JE, King E, Andrews L, Bejon P, Gilbert SC, De Souza JB, Sinden R, Hill AV, Riley EM. 2006. Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol 177:5736–5745. doi: 10.4049/jimmunol.177.8.5736. [DOI] [PubMed] [Google Scholar]
- 11.Randall LM, Engwerda CR. 2010. TNF family members and malaria: old observations, new insights and future directions. Exp Parasitol 126:326–331. doi: 10.1016/j.exppara.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. 1987. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 13.Meding SJ, Cheng SC, Simon-Haarhaus B, Langhorne J. 1990. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun 58:3671–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gun SY, Claser C, Tan KS, Renia L. 2014. Interferons and interferon regulatory factors in malaria. Mediators Inflamm 2014:243713. doi: 10.1155/2014/243713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portugal S, Moebius J, Skinner J, Doumbo S, Doumtabe D, Kone Y, Dia S, Kanakabandi K, Sturdevant DE, Virtaneva K, Porcella SF, Li S, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD. 2014. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog 10:e1004079. doi: 10.1371/journal.ppat.1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ringwald P, Peyron F, Vuillez JP, Touze JE, Le Bras J, Deloron P. 1991. Levels of cytokines in plasma during Plasmodium falciparum malaria attacks. J Clin Microbiol 29:2076–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgmann H, Looareesuwan S, Wiesinger EC, Winter W, Graninger W. 1997. Levels of stem cell factor and interleukin-3 in serum in acute Plasmodium falciparum malaria. Clin Diagn Lab Immunol 4:226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hercus TR, Kan WLT, Broughton SE, Tvorogov D, Ramshaw HS, Sandow JJ, Nero TL, Dhagat U, Thompson EJ, Shing K, McKenzie DR, Wilson NJ, Owczarek CM, Vairo G, Nash AD, Tergaonkar V, Hughes T, Ekert PG, Samuel MS, Bonder CS, Grimbaldeston MA, Parker MW, Lopez AF. 2018. Role of the β common (βc) family of cytokines in health and disease. Cold Spring Harb Perspect Biol 10:a028514. doi: 10.1101/cshperspect.a028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riopel J, Tam M, Mohan K, Marino MW, Stevenson MM. 2001. Granulocyte-macrophage colony-stimulating factor-deficient mice have impaired resistance to blood-stage malaria. Infect Immun 69:129–136. doi: 10.1128/IAI.69.1.129-136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auclair SR, Roth KE, Saunders BL, Ogborn KM, Sheikh AA, Naples J, Young AM, Boisen DK, Tavangar AT, Welch JE, Lantz CS. 2014. Interleukin-3-deficient mice have increased resistance to blood-stage malaria. Infect Immun 82:1308–1314. doi: 10.1128/IAI.01140-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, Lopez AF, Parker MW. 2012. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev 250:277–302. doi: 10.1111/j.1600-065X.2012.01164.x. [DOI] [PubMed] [Google Scholar]
- 22.Hilgendorf I, Theurl I, Gerhardt LM, Robbins CS, Weber GF, Gonen A, Iwamoto Y, Degousee N, Holderried TA, Winter C, Zirlik A, Lin HY, Sukhova GK, Butany J, Rubin BB, Witztum JL, Libby P, Nahrendorf M, Weissleder R, Swirski FK. 2014. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation 129:1677–1687. doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber GF, Chousterman BG, Hilgendorf I, Robbins CS, Theurl I, Gerhardt LM, Iwamoto Y, Quach TD, Ali M, Chen JW, Rothstein TL, Nahrendorf M, Weissleder R, Swirski FK. 2014. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J Exp Med 211:1243–1256. doi: 10.1084/jem.20131471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, Brenner T, Uhle F, Iwamoto Y, Robbins CS, Noiret L, Maier SL, Zonnchen T, Rahbari NN, Scholch S, Klotzsche-von Ameln A, Chavakis T, Weitz J, Hofer S, Weigand MA, Nahrendorf M, Weissleder R, Swirski FK. 2015. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 347:1260–1265. doi: 10.1126/science.aaa4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. 2012. Innate response activator B cells protect against microbial sepsis. Science 335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumgarth N. 2016. B-1 cell heterogeneity and the regulation of natural and antigen-induced IgM Production. Front Immunol 7:324. doi: 10.3389/fimmu.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgarth N. 2017. A hard(y) look at B-1 cell development and function. J Immunol 199:3387–3394. doi: 10.4049/jimmunol.1700943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage HP, Baumgarth N. 2015. Characteristics of natural antibody-secreting cells. Ann N Y Acad Sci 1362:132–142. doi: 10.1111/nyas.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, Baumgarth N. 2017. Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med 214:2777–2794. doi: 10.1084/jem.20161122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarlinton DM, McLean M, Nossal GJ. 1995. B1 and B2 cells differ in their potential to switch immunoglobulin isotype. Eur J Immunol 25:3388–3393. doi: 10.1002/eji.1830251228. [DOI] [PubMed] [Google Scholar]
- 31.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. 2011. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achtman AH, Stephens R, Cadman ET, Harrison V, Langhorne J. 2007. Malaria-specific antibody responses and parasite persistence after infection of mice with Plasmodium chabaudi chabaudi. Parasite Immunol 29:435–444. doi: 10.1111/j.1365-3024.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 33.Chevrier S, Genton C, Kallies A, Karnowski A, Otten LA, Malissen B, Malissen M, Botto M, Corcoran LM, Nutt SL, Acha-Orbea H. 2009. CD93 is required for maintenance of antibody secretion and persistence of plasma cells in the bone marrow niche. Proc Natl Acad Sci U S A 106:3895–3900. doi: 10.1073/pnas.0809736106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy B, Brennecke AM, Agarwal S, Krey M, Duber S, Weiss S. 2013. An intrinsic propensity of murine peritoneal B1b cells to switch to IgA in presence of TGF-beta and retinoic acid. PLoS One 8:e82121. doi: 10.1371/journal.pone.0082121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roussilhon C, Oeuvray C, Müller-Graf C, Tall A, Rogier C, Trape J-F, Theisen M, Balde A, Pérignon J-L, Druilhe P. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med 4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kana IH, Adu B, Tiendrebeogo RW, Singh SK, Dodoo D, Theisen M. 2017. Naturally acquired antibodies target the glutamate-rich protein on intact merozoites and predict protection against febrile malaria. J Infect Dis 215:623–630. doi: 10.1093/infdis/jiw617. [DOI] [PubMed] [Google Scholar]
- 37.Dechavanne C, Dechavanne S, Sadissou I, Lokossou AG, Alvarado F, Dambrun M, Moutairou K, Courtin D, Nuel G, Garcia A, Migot-Nabias F, King CL. 2017. Associations between an IgG3 polymorphism in the binding domain for FcRn, transplacental transfer of malaria-specific IgG3, and protection against Plasmodium falciparum malaria during infancy: a birth cohort study in Benin. PLoS Med 14:e1002403. doi: 10.1371/journal.pmed.1002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer CG, Calixto Fernandes MH, Intemann CD, Kreuels B, Kobbe R, Kreuzberg C, Ayim M, Ruether A, Loag W, Ehmen C, Adjei S, Adjei O, Horstmann RD, May J. 2011. IL3 variant on chromosomal region 5q31-33 and protection from recurrent malaria attacks. Hum Mol Genet 20:1173–1181. doi: 10.1093/hmg/ddq562. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, Moore CS, Michel L, Althekair F, Rajasekharan S, Gommerman JL, Prat A, Fillatreau S, Bar-Or A, BciMST C. 2015. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 7:310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.