Schistosomiasis is a parasitic helminth disease that can cause severe inflammatory pathology, leading to organ damage, in humans. During a schistosomal infection, the eggs are trapped in the host liver, and products derived from eggs induce a polarized Th2 cell response, resulting in granuloma formation and eventually fibrosis.
KEYWORDS: S. japonicum, inflammasome, liver injury, pyroptosis, taurine
ABSTRACT
Schistosomiasis is a parasitic helminth disease that can cause severe inflammatory pathology, leading to organ damage, in humans. During a schistosomal infection, the eggs are trapped in the host liver, and products derived from eggs induce a polarized Th2 cell response, resulting in granuloma formation and eventually fibrosis. Previous studies indicated that the nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing protein 3 (NLRP3) inflammasome is involved in schistosomiasis-associated liver fibrosis and that taurine could ameliorate hepatic granulomas and fibrosis caused by Schistosoma japonicum infection. Nevertheless, the precise role and molecular mechanism of the NLRP3 inflammasome and the protective effects of taurine in S. japonicum infection have not been extensively studied. In this study, we investigated the role of the NLRP3 inflammasome and the hepatoprotective mechanism of taurine in schistosoma-induced liver injury in mice. NLRP3 deficiency ameliorated S. japonicum-infection-induced hepatosplenomegaly, liver dysfunction, and hepatic granulomas and fibrosis; it also reduced NLRP3-dependent liver pyroptosis. Furthermore, taurine suppressed hepatic thioredoxin-interacting protein (TXNIP)/NLRP3 inflammasome activation in mice with S. japonicum infections, thereby inhibiting the activation of downstream inflammatory mediators such as interleukin-1β and subsequent pyroptosis. Our results suggest that the TXNIP/NLRP3 inflammasome pathway and mediating pyroptosis are involved in S. japonicum-induced liver injury and may be a potential therapeutic target for schistosomiasis treatment. In addition, taurine may be useful to alleviate or to prevent the occurrence of schistosomiasis-associated liver fibrosis.
INTRODUCTION
Schistosomiasis is a parasitic disease that seriously harms human health. The disease affects 200 million people worldwide, with many more at risk (1). Schistosoma japonicum is one of the three main species of schistosome responsible for human infections and is the major causative agent of infections in Southeast Asia and China. After the schistosomal cercariae infect humans or animals, they develop into adult worms in the host portal vein and mesenteric venous system. The eggs produced by female worms are mostly deposited in tissues of the liver and intestines. The characteristics of liver injury associated with S. japonicum infection include pronounced immunological and inflammatory responses caused by the soluble egg antigen released by miracidia within eggs, inducing granuloma formation and subsequent fibrosis, i.e., schistosomiasis-associated liver fibrosis. Hepatic fibrosis is the principal cause of morbidity and death in humans with schistosomal infections.
After the pathogens are eliminated by efficacious schistosomicidal treatment, the development of hepatic fibrosis cannot be completely reversed or prevented, which may be due to a sustained pathological process such as chronic inflammation. To date, the precise mechanisms that mediate this perpetual activation of inflammation around egg granulomas in the liver during S. japonicum infection remain poorly understood.
Cell death and inflammation are two crucial elements in the development of liver fibrosis. Accumulating evidence indicates that inflammation plays pivotal roles in S. japonicum-associated liver injury (2, 3). Inflammasomes are intracellular multiprotein complexes that are expressed in both hepatocytes and nonparenchymal cells in the liver and are key regulators of inflammation and cell fate (4–6). The nucleotide-binding oligomerization domain (NOD)-, leucine-rich repeat (LRR)-, and pyrin domain-containing protein 3 (NLRP3) inflammasome, the most well-studied inflammasome, consists of the NOD-like receptor family member NLRP3, proinflammatory caspase-1, and the adaptor protein apoptosis-related spot-like protein containing a caspase activation and recruitment domain (CARD), and can be activated by different stimulus signals, including pathogen-associated molecular patterns and damage-associated molecular patterns (7). When activated, the NLRP3 inflammasome triggers the cleavage of pro-caspase-1 to active caspase-1. The activated caspase-1 fragment cleaves pro-interleukin-18 (IL-18) and pro-IL-1β and then promotes IL-18 and IL-1β maturation and secretion. IL-1β could promote the activation of hepatic stellate cells (HSCs), which play a central role in the progression of liver fibrosis (8). In addition, IL-1β could recruit a large number of inflammatory factors (9). IL-18 mainly promotes the activation of T cells and macrophages and promotes inflammatory responses (10).
Previous studies have shown that the NLRP3 inflammasome is markedly activated in mouse HSCs, both in vivo and in vitro, during S. japonicum infection (11). However, the effector mechanism of NLRP3 activation in schistosomal infection is still unclear. In addition, previous studies have indicated that, under oxidative stress, thioredoxin-interacting protein (TXNIP) could dissociate from thioredoxin and activate the NLRP3 inflammasome directly in liver disease (12). TXNIP deficiency could impair the activation of the NLRP3 inflammasome and subsequent secretion of IL-1β (13). Nevertheless, we have little evidence for the effect of TXNIP on schistosomal infection.
Pyroptosis, which is distinct from other cell death forms, is defined as caspase-1- or caspase-11-dependent proinflammatory programmed cell death (14). During pyroptosis, gasdermin D (GSDMD), the pore-forming effector protein, is cleaved by caspase-1; its N terminus (GSDMD-N) inserts into the cell membranes, resulting in rapid cell death and release of proinflammatory intracellular contents (15). Therefore, pyroptosis is accompanied by plasma membrane rupture, cytoplasmic swelling, osmotic lysis, DNA cleavage, NLRP3 inflammasome activation, and the release of proinflammatory cellular contents. Increasing evidence has indicated that hepatocyte pyroptosis has an important role in various inflammation-related liver diseases, including alcoholic hepatitis (16) and steatohepatitis (17). However, whether pyroptosis occurs and is involved in S. japonicum-induced liver injury has not been investigated.
Taurine, a sulfur-containing β-amino acid, is a major free intracellular amino acid that is present in many human and animal tissues. Taurine has various physiological functions and protective properties, including protection against various types of hepatic damage. In addition, taurine possesses anti-inflammatory and immune regulatory properties (18). Exogenous supplementation with taurine can prevent liver injury caused by different harmful substances and also can inhibit extracellular matrix (ECM) deposition in the damaged liver and prevent liver fibrosis (19). Our previous studies indicated that taurine supplementation could reduce hepatic lesions and restrain granuloma formation and resulting fibrosis in S. japonicum-infected mice (20). However, the exact target of taurine during this process and its mechanism still need further investigation.
Here, we investigated the role of the NLRP3 inflammasome and the protective mechanism of taurine in hepatic injury during S. japonicum infection. We found that the TXNIP/NLRP3 inflammasome signal pathway was involved in schistosomal pathogenesis and NLRP3 deficiency could ameliorate S. japonicum-associated liver injury. S. japonicum infection could induce NLRP3 inflammasome-dependent pyroptosis. Furthermore, taurine suppressed hepatic TXNIP/NLRP3 inflammasome activation in mice with S. japonicum infection, thereby inhibiting the activation of downstream inflammatory mediators (such as IL-1β) and subsequent pyroptosis.
RESULTS
The NLRP3 inflammasome has a crucial role in schistosoma-induced liver injury.
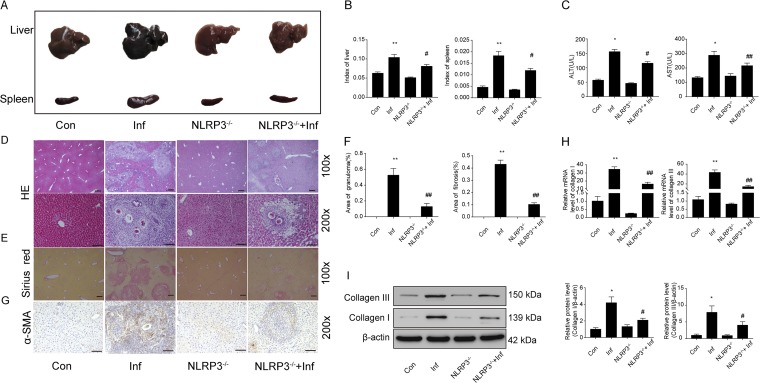
The NLRP3 inflammasome was activated in the livers of mice with S. japonicum infections, and the protein levels of NLRP3, activated caspase-1, and IL-1β were significantly enhanced in infected livers (see Fig. S1 in the supplemental material). To verify the effects of the NLRP3 inflammasome in schistosoma-induced liver injury, we infected NLRP3−/− and wild-type mice with S. japonicum. At 6 weeks postinfection, we analyzed the pathological lesions in the liver by biochemical analysis, hematoxylin and eosin (H&E) staining, Sirius red staining, and real-time-PCR. The gross appearance, including white spots of granuloma nodules and hepatosplenomegaly, was greatly improved in infected NLRP3−/− mice, compared with infected wild-type mice (Fig. 1A). In addition, liver and spleen indexes were increased in infected wild-type mice because of weight loss and hepatosplenomegaly, but infected NLRP3−/− mice had lower liver and spleen indexes (Fig. 1B). Serum alanine transaminase (ALT) and aspartate transaminase (AST) levels indicated that NLRP3 deficiency alleviated liver function injury caused by schistosomiasis (Fig. 1C).
FIG 1.
The NLRP3 inflammasome has a crucial role in schistosoma-induced liver injury. Wild-type and NLRP3−/− mice were percutaneously treated with or without 30 ± 2 S. japonicum cercaria. At 6 weeks postinfection, hepatic pathological lesions were analyzed. (A) Gross appearance of the liver and spleen of control (Con), infected (Inf), NLRP3−/−, and NLRP3−/− infected (NLRP3−/−+Inf) mice. (B) Liver and spleen indexes. (C) Serum levels of ALT and AST measured with a biochemical analyzer. (D) H&E staining of liver sections. Original magnification, ×100 or ×200; scale bars, 125 μm or 250 μm, respectively. The granulomatous area as a percentage of the total area was measured by computer-assisted morphometric analysis. (E) Sirius red staining for collagen content and distribution. Original magnification, ×100; scale bars, 125 μm. (F) Quantitative changes in granulomatous and fibrotic areas measured by computer-assisted morphometric analysis. (G) Representative immunohistochemistry images of α-SMA in liver tissue. Original magnification, ×200; scale bars, 250 μm. (H and I) Quantification of collagen I and collagen III mRNA and protein levels. Data are mean ± SEM of 6 mice/group. *, P < 0.05 versus control; **, P < 0.01 versus control; #, P < 0.05 versus infected; ##, P < 0.01 versus infected.
Hepatic granulomas and fibrosis are the pathological hallmarks of schistosomal infection. The granuloma form is centered on the eggs, with infiltration of a large number of immune cells and inflammatory factors. The granulomatous area was smaller in infected NLRP3−/− mice than in infected wild-type mice (Fig. 1D and F). Moreover, NLRP3 deficiency reduced the area of fibrosis (Fig. 1E and F) and the expression of alpha smooth muscle actin (α-SMA), a hallmark of activated HSCs (Fig. 1G). In addition, NLRP3 deficiency decreased the mRNA levels and protein levels of collagen I and III (Fig. 1H and I). Hence, NLRP3 deficiency attenuated hepatic injury caused by S. japonicum infection.
S. japonicum infection can induce NLRP3 inflammasome-dependent pyroptosis.
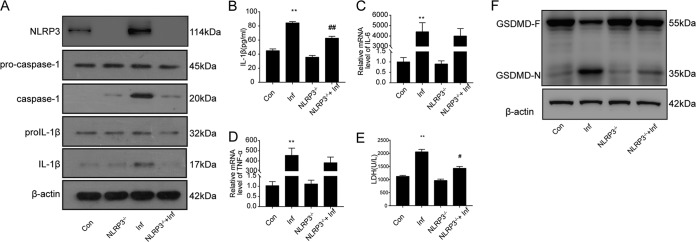
Inflammasomes modulate host defense responses by producing eicosanoids (21) and through other mechanisms (22), but the induction of pyroptosis and the secretion of proinflammatory IL-1β and IL-18 are considered the prominent outcomes of inflammasome signaling (23). Therefore, we determined whether S. japonicum-infected mice showed pyroptosis and whether NLRP3 affected the release of proinflammatory factors and pyroptosis. The hepatic protein levels of caspase-1 and IL-1β were markedly higher in S. japonicum-infected mice than in noninfected mice, but NLRP3 deficiency reversed this increase (Fig. 2A). Moreover, serum IL-1β level findings were consistent with these changes (Fig. 2B). However, NLRP3 deficiency did not affect the expression of IL-6 or tumor necrosis factor alpha (TNF-α) (Fig. 2C and D). Furthermore, the serum levels of lactate dehydrogenase (LDH) were greatly elevated in infected mice, which suggests that liver cells died after infection with S. japonicum (Fig. 2E).
FIG 2.
S. japonicum infection can induce NLRP3-inflammasome-dependent pyroptosis. Wild-type and NLRP3−/− mice were percutaneously treated with or without 30 ± 2 S. japonicum cercaria. At 6 weeks postinfection, liver tissue and serum were collected to analyze pyroptosis-associated protein and proinflammatory cytokine levels. (A) Western blot analysis of NLRP3, caspase-1, and IL-1β protein levels in liver. Con, control group; Inf, infected group; NLRP3−/−, NLRP3 deficiency group; NLRP3−/−+Inf, NLRP3−/− infected group. (B) ELISA of serum levels of IL-1β. (C and D) Quantification of IL-6 (C) and TNF-α (D) mRNA levels. (E) Serum levels of LDH measured with a biochemical analyzer. (F) Western blot analysis of GSDMD protein levels in liver. Data are mean ± SEM of 6 mice/group. **, P < 0.01 versus control; #, P < 0.05 versus infected; ##, P < 0.01 versus infected.
We further explored whether GSDMD-N, the effector for pyroptosis, was expressed in mouse liver with S. japonicum infection. In Western blot analyses, livers from infected mice but not noninfected controls showed that GSDMD was sheared to produce a GSDMD-N fragment. However, compared with wild-type infected livers, NLRP3 deficiency blocked the production of GSDMD-N (Fig. 2F). These data indicate that schistosomal infection could cause NLRP3-dependent pyroptosis in the liver.
TXNIP expression increases and interacts with NLRP3 in the livers of mice with S. japonicum infections.
TXNIP plays an important role in NLRP3 inflammasome activation, and TXNIP/NLRP3 activation is required for the inflammatory response (24). In this study, we detected the expression of TXNIP in livers of mice with S. japonicum infections. TXNIP levels were markedly increased in infected versus noninfected livers (Fig. 3A and B). Furthermore, immunoprecipitation assays indicated that TXNIP interacted with NLRP3 after S. japonicum infection (Fig. 3C).
FIG 3.
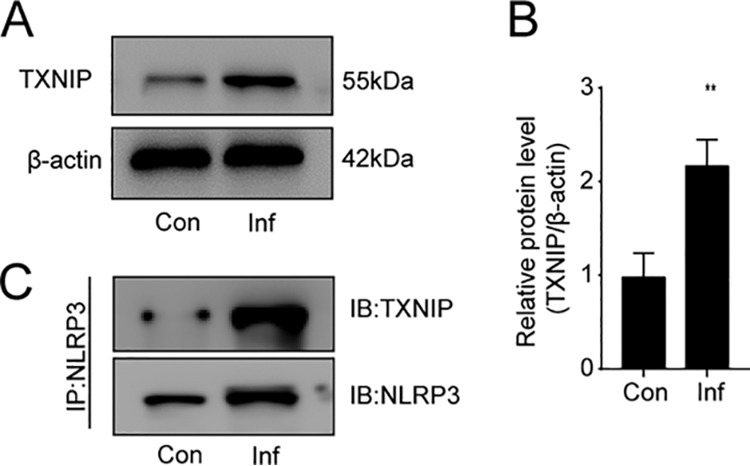
TXNIP interacts with NLRP3 in the livers of mice with S. japonicum infections. Wild-type mice were percutaneously treated with or without 30 ± 2 S. japonicum cercaria. At 6 weeks postinfection, liver tissue was collected to analyze TXNIP protein levels and the interactions of TXNIP and NLRP3. (A and B) Western blot analysis (A) and quantification (B) of TXNIP protein levels in liver. Con, control group; Inf, infected group. Data are mean ± SEM (n = 6 in each group) of three independent experiments. (C) Immunoprecipitation (IP) and immunoblot (IB) analyses of the interactions between TXNIP and NLRP3 in liver tissues. **, P < 0.01 versus control.
Taurine protects against S. japonicum-induced hepatic injury.
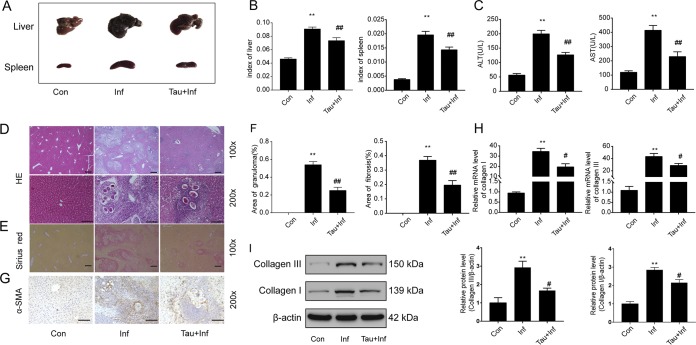
To evaluate the potential protective effect of taurine against S. japonicum-induced hepatic pathological lesions, 1.5% taurine in drinking water was administered to mice with S. japonicum infection. Taurine treatment markedly improved the gross appearance, hepatosplenomegaly, and liver and spleen indexes of mice with S. japonicum infections (Fig. 4A and B). In accordance with these findings, taurine significantly reduced the serum levels of ALT and AST in infected mice (Fig. 4C) and greatly alleviated the hepatic granulomas and area of fibrosis, as well as the expression of α-SMA, caused by S. japonicum infection (Fig. 4D to G). In addition, taurine reduced the mRNA levels and protein levels of collagen I and III (Fig. 4H and I). Collectively, these results indicate that taurine supplementation protected against S. japonicum-induced liver injury.
FIG 4.
Taurine improves S. japonicum infection-associated liver injury. Wild-type mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1.5% taurine in drinking water for 6 weeks postinfection. (A) Gross appearance of the liver and spleen of control (Con), infected (Inf), and taurine-treated infected (Tau+Inf) mice. (B) Liver and spleen indexes. (C) Serum levels of ALT and AST measured with a biochemical analyzer. (D) H&E staining of liver sections. Original magnification, ×100 or ×200; scale bars, 125 μm or 250 μm, respectively. The granulomatous area as a percentage of the total area was measured by computer-assisted morphometric analysis. (E) Sirius red staining for collagen content and distribution. Original magnification, ×100; scale bars, 125 μm. (F) Quantitative changes in granulomatous and fibrotic areas measured by computer-assisted morphometric analysis. (G) Representative immunohistochemistry images of α-SMA in liver tissue. Original magnification, ×200; scale bars, 250 μm. (H and I) Quantification of collagen I and collagen III mRNA and protein levels. Data are mean ± SEM of 6 mice/group. **, P < 0.01 versus control; #, P < 0.05 versus infected; ##, P < 0.01 versus infected.
Taurine alleviates schistosoma-induced liver injury by inhibiting TXNIP/NLRP3 inflammasome signaling and pyroptosis.
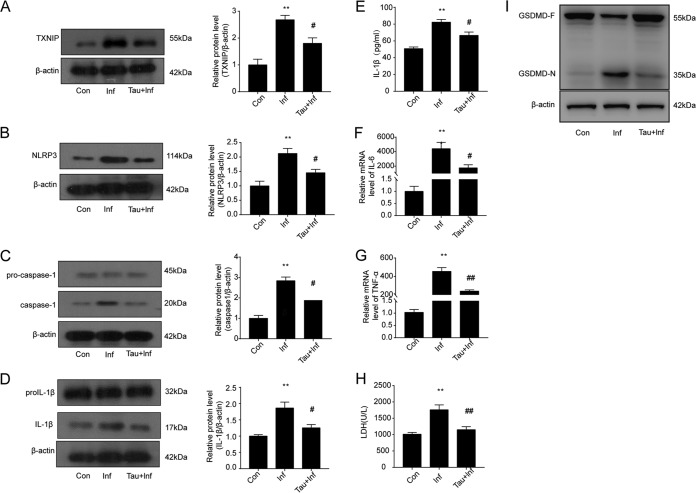
To further investigate the protective roles of taurine against hepatic injury caused by S. japonicum infection, we examined the effects of taurine on the NLRP3 inflammasome signal pathway and pyroptosis. Taurine treatment efficiently inhibited the S. japonicum infection-increased protein levels of TXNIP, NLRP3, caspase-1, and IL-1β (Fig. 5A to D) and reduced the serum levels of the proinflammatory cytokine IL-1β (Fig. 5E). In addition, taurine markedly suppressed the increased mRNA levels of IL-6 and TNF-α (Fig. 5F and G) and decreased serum LDH levels and liver tissue levels of GSDMD-N, the pyroptosis effector (Fig. 5H and I). Thus, taurine inhibited the TXNIP/NLRP3 inflammasome pathway and its effector action, pyroptosis, in hepatic injury caused by S. japonicum infection.
FIG 5.
Taurine alleviates schistosoma-induced liver injury by inhibiting TXNIP/NLRP3 inflammasome signaling and pyroptosis. Wild-type mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1.5% taurine in drinking water for 6 weeks postinfection. (A to D) Western blot analyses of protein levels of TXNIP (A), NLRP3 (B), caspase-1 (C), and IL-1β (D) in liver. Con, control group; Inf, infected group; Tau+Inf, taurine-treated infected group. (E) ELISA of serum levels of IL-1β. (F and G) Quantification of IL-6 (F) and TNF-α (G) levels. (H) Serum levels of LDH measured with a biochemical analyzer. (I) Western blot analysis of GSDMD protein levels in liver. Data are mean ± SEM of 6 mice/group. **, P < 0.01 versus control; #, P < 0.05 versus infected; ##, P < 0.01 versus infected.
DISCUSSION
Inflammasomes are a group of protein complexes that are activated by various pathogen infections or cellular and physiological stresses that provoke a rapid release of proinflammatory cytokines for recruitment of native immune cells for defense against intruders (25). In addition, inflammasome activation can induce a form of cell death called pyroptosis (26). In this study, we found that the NLRP3 inflammasome was activated in the livers of mice with S. japonicum infections. Moreover, for the first time we found that pyroptosis was involved in S. japonicum-infection-induced liver injury. NLRP3 deficiency could ameliorate the S. japonicum-infection-associated liver injury and pyroptosis. Furthermore, we confirmed that taurine alleviated schistosoma-induced liver injury by inhibiting TXNIP/NLRP3 inflammasome signaling and pyroptosis.
The pathological basis of liver injury caused by schistosomiasis is the host’s immune response to sustained antigenic stimulation from the trapped eggs. Inflammatory cytokines and immune cells are sequentially recruited to the sites of infection, which leads to the formation of periovular granulomas and chronic fibrosis (27). Recent evidence has revealed NLRP3 inflammasome activation and IL-1β production associated with several chronic liver diseases, including fibrosis development (28–30). IL-1β is a vital inflammatory cytokine that plays an important role in regulating the downstream inflammatory response (31). Mature IL-1β generation and secretion are based on NF-κB and NLRP3 inflammasome activation (32).
First, our study focused on the effect of NLRP3 on schistosoma-induced liver injury. We found that NLRP3 and the downstream targets caspase-1 and IL-1β were upregulated during S. japonicum infection. Next, we compared the hepatic injury in wild-type and NLRP3-deficient mice with S. japonicum infections. NLRP3 deficiency ameliorated S. japonicum-infection-induced liver injury and hepatosplenomegaly and also attenuated S. japonicum-infection-associated pathological lesions, liver granulomas, and fibrosis. These data confirm that the NLRP3 inflammasome has a crucial role in schistosoma-induced liver injury, which is consistent with previous studies (33–35).
In addition to being a potent proinflammatory factor, the NLRP3 inflammasome can induce inflammatory programmed cell death (pyroptosis), which is an essential and fundamental role in the development and survival of the host in the body’s defense against infections (36). Similar to apoptotic cells, pyroptotic cells have a shrunken nucleus. Also, like necrotic cells, pyroptotic cells swell, burst, release their contents, and cause inflammatory reactions. Recently, GSDMD has been found to form a pore and act as the effector for pyroptosis. In fact, expression of the GSDMD-N domain of GSDMD in mammalian cells is sufficient to induce pyroptosis. Full-length GSDMD is inactive because of inhibitory binding to GSDMD-N by its GSDMD-C domain. Activated caspase-1, a component of the NLRP3 inflammasome, efficiently cleaves GSDMD at an aspartate site within the linking loop (36). However, GSDMD is insensitive to apoptotic caspases.
Extensive studies have found that pyroptosis is involved in various liver diseases (37, 38). Wree et al. found that the NLRP3 inflammasome induced pyroptosis in hepatocytes; after pyroptosis of hepatocytes, a large number of inflammatory substances, such as IL-18 and IL-1β, were released to activate HSCs to produce large amounts of ECM to promote fibrosis (39). When liver tissue is damaged, necrotic hepatocytes can recruit eosinophils to secrete IL-1β and IL-18, and even induce pyroptosis, while further inducing hepatocyte death, surrounding tissue inflammation, and the development of liver fibrosis. This process could be counteracted by caspase-1 inhibitors, which suggests that infiltrating eosinophils affect hepatocytes via cell pyroptosis and promote liver fibrosis (40). Therefore, we determined whether S. japonicum infection could induce pyroptosis of liver cells in mice. We found increased protein levels of cleaved GSDMD-N in S. japonicum-infected mouse livers. Because the pyroptosis was downstream of the inflammasome, many studies indicated that NLRP3 inflammasome activation can influence the severity of liver disease by regulating pyroptosis (37, 38). However, it is unclear whether the NLRP3 inflammasome can regulate the progression of pyroptosis to affect schistosomiasis-associated liver fibrosis. Our results showed that NLRP3 deficiency inhibited the increased protein levels of GSDMD-N; therefore, S. japonicum-induced pyroptosis is at least partly dependent on the NLRP3 inflammasome. However, which cells in the liver show pyroptosis and how fibrosis is promoted need further investigation.
TXNIP is known to participate in inflammatory signaling upon oxidative stress. In resting cells, thioredoxin binds to TXNIP and keeps it in an inactive state. In the event of oxidative stress and increased levels of reactive oxygen species, TXNIP is released from thioredoxin and binds to NLRP3, thus activating the inflammasome to participate in inflammatory signaling (41). TXNIP could induce IL-1β production by activating the NLRP3 inflammasome and IL-1β mRNA transcription in β cells (42). Cao et al. found that repression of the TXNIP/NLRP3 inflammasome pathway could prevent liver inflammation and hepatocyte death (43). Liu et al. found that SjCa8, an 8-kDa calcium-binding protein derived from S. japonicum that is specifically expressed in cercariae and skin-stage schistosomula but is silenced in other stages and eggs, could downregulate Txnip gene expression during the process of skin penetration (44). However, there was no study to investigate the role of TXNIP in hepatic injury during S. japonicum infection. In the present study, TXNIP levels were markedly increased during S. japonicum infection in mice, and a coimmunoprecipitation assay further demonstrated the interaction of TXNIP and NLRP3 in vivo. These results indicated that S. japonicum infection induced NLRP3 inflammasome activation accompanied by TXNIP signal initiation. The TXNIP/NLRP3 axis might be a promising therapeutic target for schistosomiasis treatment.
Taurine is the most abundant free amino acid in humans and plays an important role in several essential biological processes, such as bile acid conjugation, maintenance of calcium homeostasis, osmoregulation, and membrane stabilization. Taurine relieves nonalcoholic steatohepatitis caused by chronic arsenic exposure by inhibiting the inflammasome (45). Our previous study indicated that taurine could alleviate schistosoma-induced liver injury. The present results confirmed this conclusion. However, the exact targets of taurine during this process remain unclear. In this study, we found that taurine blocked TXNIP/NLRP3 signaling and inhibited proinflammatory cytokine release, eventually suppressing pyroptosis. Taurine alleviated schistosoma-induced liver injury by inhibiting the TXNIP/NLRP3 inflammasome signal pathway and its role in pyroptosis.
In conclusion, our study revealed that S. japonicum infection initiated the TXNIP/NLRP3 inflammasome signal pathway, which resulted in proinflammatory IL-1β secretion and pyroptosis, eventually leading to schistosoma-induced liver injury. Taurine alleviated schistosoma-induced liver injury in part by inhibiting the TXNIP/NLRP3 inflammasome signal pathway and its role in pyroptosis (Fig. 6). These data suggest that the NLRP3 inflammasome may be a potential therapeutic target for treating schistosomiasis, and taurine may be of great benefit in alleviating or preventing the occurrence of schistosomiasis-associated liver fibrosis.
FIG 6.
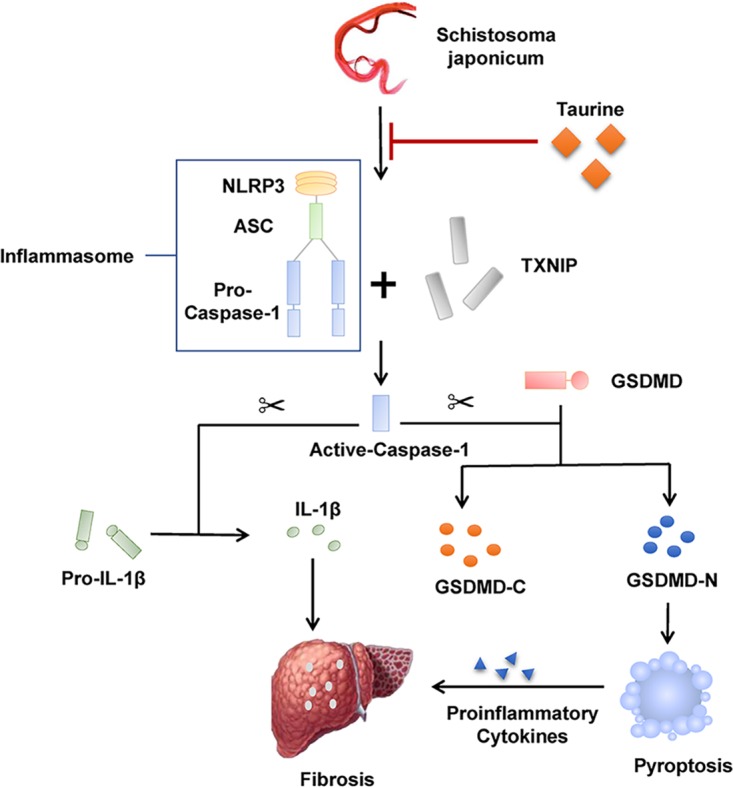
Proposed schematic diagram of the protective effect of taurine in alleviating schistosoma-induced liver injury by inhibiting the TXNIP/NLRP3 inflammasome pathway and pyroptosis. ASC, apoptosis-related spot-like protein containing a CARD.
MATERIALS AND METHODS
Ethics statement.
All animal care and experimental protocols complied with the Guide for the Care and Use of Laboratory Animals (46) and were approved by the Animal Care Committee of Peking University Health Science Center (protocol number LA2014109).
Animals, parasites, and infection.
Male C57BL/6 mice (6 to 8 weeks of age) were purchased from the Department of Laboratory Animal Science of Peking University Health Science Center (Beijing, China). NLRP3−/− mice were obtained from Peking Union Medical College Hospital (Beijing, China). S. japonicum (Chinese mainland strain)-infected Oncomelania hupensis snails were purchased from the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Shanghai, China).
Anesthetized mice were percutaneously infected with 30 ± 2 cercariae of S. japonicum freshly shed from snails. For taurine supplementation, mice were fed standard chow and 1.5% (wt/vol) taurine in drinking water. At 6 weeks postinfection, all mice were anesthetized with ether, the body weight of each mouse was determined, serum was separated from blood taken from the mouse eye socket, the mice were killed by cervical dislocation, and liver tissue and serum samples were collected for analysis.
Serum biochemical analysis and inflammatory cytokine assays.
Blood samples were centrifuged at 3,000 × g for 15 min at 4°C to obtain serum samples. Serum levels of ALT, AST, and LDH were detected by using an Olympus AU5800 automatic biochemical analyzer (Olympus, Tokyo, Japan). Serum levels of IL-1β were measured by using a mouse enzyme-linked immunosorbent assay (ELISA) kit (Boster, Wuhan, China), according to the manufacturer’s instruction.
Liver and spleen indexes.
Liver and spleen tissues were weighed. Liver and spleen indexes were calculated as ratios of liver weight to body weight and spleen weight to body weight, respectively.
Histology and histopathology of liver sections.
Liver samples were immediately fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) and then embedded in paraffin. For histology, according to standard procedures, liver sections (5 μm) were stained with H&E to assess liver granulomas and with Sirius red to evaluate the extent of hepatic fibrosis. Granulomas and areas of fibrosis were analyzed under a Leica DMI 300B fluorescence microscope. Formaldehyde-fixed paraffin sections were incubated with primary antibody to α-SMA (1:100 dilution) overnight and then with secondary antibody for 1 h at room temperature. The nucleus was stained with hematoxylin and then treated with diaminobenzidine. Negative controls contained no antibody. The granulomatous and fibrotic areas as a percentage of the total area for each side were measured by using computer-assisted morphometric software (Leica Application Suite 4.1). For each specimen, ≥3 noncontiguous slides were measured and the mean values from 6 mice in each group were used for statistical analyses.
Isolation of total RNA and real-time PCR.
Total RNA was extracted from the liver tissue of each mouse by using TRIzol reagent (Applygen Technologies, Beijing, China). In total, 1 μg RNA was reverse transcribed to cDNA by using the PrimeScript reverse transcription reagent kit (TaKaRa Biotechnology, Dalian, China). Real-time PCR experiments were performed in triplicate with SYBR Premix Ex Taq II (TaKaRa Biotechnology) on an ABI 7500 fast real-time PCR system (Applied Biosystems, Fullerton, CA). The PCR primers were designed by using the Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast). Primers for real-time PCR are presented in Table 1. Relative mRNA expression was calculated by the 2−ΔΔCt method and normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression.
TABLE 1.
Primers used for real-time reverse transcription-PCR assays
| Target | Sequence |
|---|---|
| GAPDH | |
| Sense | 5′-ACTTTGTCAAGCTCATTTCC-3′ |
| Antisense | 5′-TGCAGCGAACTTTATTGATG-3′ |
| IL-6 | |
| Sense | 5′-TAGTCCTTCCTACCCCAATTTCC-3′ |
| Antisense | 5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| TNF-α | |
| Sense | 5′-CCCTCACACTCAGATCATCTTCT-3′ |
| Antisense | 5′-GCTACGACGTGGGCTACAG-3′ |
| Collagen I | |
| Sense | 5′-ATCCTGCCGATGTCGCTAT-3′ |
| Antisense | 5′-CCACAAGCGTGCTGTAGGT-3′ |
| Collagen III | |
| Sense | 5′-CATGACTGTCCCACGTAAGC-3′ |
| Antisense | 5′-ATTCGCCTTCATTTGATCCCA-3′ |
Western blot analysis.
Liver tissues were homogenized in RIPA buffer (Beyotime Biotechnology, Nanjing, China) containing a cocktail of protease inhibitors (Roche, Basel, Switzerland) and were centrifuged at 12,000 × g for 15 min. The supernatant was then harvested, and protein concentrations were quantified by using a bicinchoninic acid (BCA) protein assay kit (Beyotime). Protein samples (60 μg/sample) were mixed with loading buffer, boiled for 15 min at 95°C, electrophoresed in an 8%, 12%, or 15% gel via SDS-PAGE, and transferred to a polyvinylidene fluoride membrane (Bio-Rad) for 2 h with transfer buffer. The membranes were blocked for 1 h in 5% nonfat powdered milk dissolved in Tris-buffered saline with Tween (TBST) (20 mmol/liter Tris-HCl [pH 7.6], 150 mmol/liter NaCl, and 0.02% Tween 20) and then were incubated overnight at 4°C with antibodies against NLRP3 (1:400; Santa Cruz Biotechnology), IL-1β (1:600; Santa Cruz Biotechnology), caspase-1 (1:400; Sigma), TXNIP (1:800; Cell Signaling Technology), GSDMD (1:1,000; Abcam), collagen I (1:1,000; Abcam), collagen III (1:1,000; Abcam), and β-actin (1:2,000; Santa Cruz Biotechnology). After three washes for 5 min each in TBST, membranes were incubated for 1 h at room temperature with appropriate horseradish peroxidase-conjugated secondary antibodies (1:2,000 or 1:4,000). The proteins were detected by enhanced chemiluminescence (Applygen Technologies). Protein contents were analyzed by using NIH ImageJ and were normalized to β-actin expression. All experiments were repeated at least three times.
Immunoprecipitation.
Liver samples were lysed on ice with immunoprecipitation lysis buffer (Beyotime) with protease inhibitor cocktail (Beyotime). Samples were centrifuged at 15,000 × g for 15 min, and 200 μl supernatant was transferred to a new tube as the input. Approximately 500 to 1,000 μg protein was incubated with 1 μg antibody overnight at 4°C. In total, 30 μl protein A/G-agarose beads (Beyotime) was then added to each sample for another 2 h at 4°C the next day. The beads were then washed three times with immunoprecipitation lysis buffer. After a final centrifugation, the beads were boiled for 5 min with 60 μl SDS loading buffer (Beyotime), and samples underwent Western blot analysis with standard protocols.
Statistical analysis.
Data are presented as mean ± standard error of the mean (SEM). Statistical analysis involved the use of GraphPad Prism 5 for Windows (GraphPad Software, San Diego, CA, USA). Student's t test was used to evaluate the differences between two groups. One-way or two-way analysis of variance was used to detect significant differences among multiple groups. P values of <0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Qiang Shen and Ya-Bo Zhou for technical assistance.
This work was supported by the Major Research Plan of the National Natural Science Foundation of China for Regulation Mechanism of Vascular Homeostasis and Remodeling (grant 91339203 to Y.-F.Q.) and the National Natural Science Foundation of China (grant 30901247 to Y.-R.Y. and grant 81670434 to Y.-F.Q.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00732-19.
REFERENCES
- 1.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, Utzinger J. 2013. Time to set the agenda for schistosomiasis elimination. Acta Trop 128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Zhang L, Wang J, Chen J, Zhu D, Shen P, He X, Pan J, Peng W, Duan Y. 2015. Schistosoma japonicum protein SjP40 inhibits TGF-β1-induced activation of hepatic stellate cells. Parasitol Res 114:4251–4257. doi: 10.1007/s00436-015-4663-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhan T, Zhang T, Wang Y, Wang X, Lin C, Ma H, Duan Z, Li C, Xu J, Xia C. 2017. Dynamics of Th9 cells and their potential role in immunopathogenesis of murine schistosomiasis. Parasit Vectors 10:305. doi: 10.1186/s13071-017-2242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. 2017. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 66:1037–1046. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang S, Zhang Y, Zheng JH, Li X, Yao YL, Wu YL, Song SZ, Sun P, Nan JX, Lian LH. 2017. Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation. Pharmacol Res 117:82–93. doi: 10.1016/j.phrs.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Kim HY, Kim SJ, Lee SM. 2015. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J 282:259–270. doi: 10.1111/febs.13123. [DOI] [PubMed] [Google Scholar]
- 7.Savage CD, Lopez-Castejon G, Denes A, Brough D. 2012. NLRP3-inflammasome activating DAMPs stimulate an inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol 3:288. doi: 10.3389/fimmu.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaping Z, Ying W, Luqin D, Ning T, Xuemei A, Xixian Y. 2014. Mechanism of interleukin-1β-induced proliferation in rat hepatic stellate cells from different levels of signal transduction. APMIS 122:392–398. doi: 10.1111/apm.12155. [DOI] [PubMed] [Google Scholar]
- 9.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, van den Bosch MWM, Quinn SR, Domingo-Fernandez R, Johnston DGW, Jiang JK, Israelsen WJ, Keane J, Thomas C, Clish C, Vander Heiden M, Xavier RJ, O’Neill L. 2015. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab 21:347. doi: 10.1016/j.cmet.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Qiu G, Fang B, Dai X, Cai J. 2018. Deficiency of IL-18 aggravates esophageal carcinoma through inhibiting IFN-γ production by CD8+ T cells and NK cells. Inflammation 41:667–676. doi: 10.1007/s10753-017-0721-3. [DOI] [PubMed] [Google Scholar]
- 11.Lu YQ, Zhong S, Meng N, Fan YP, Tang WX. 2017. NLRP3 inflammasome activation results in liver inflammation and fibrosis in mice infected with Schistosoma japonicum in a Syk-dependent manner. Sci Rep 7:8120. doi: 10.1038/s41598-017-08689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian D, Dai L, Xie Z, Zhou X, Liu X, Zhang Y, Huang Y, Chen Y. 2018. Periodontal ligament fibroblasts migration injury via ROS/TXNIP/Nlrp3 inflammasome pathway with porphyromonas gingivalis lipopolysaccharide. Mol Immunol 103:209–219. doi: 10.1016/j.molimm.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 14.Lacey CA, Mitchell WJ, Dadelahi AS, Skyberg J. 2018. Caspase-1 and caspase-11 mediate pyroptosis, inflammation, and control of Brucella joint infection. Infect Immun 86:e00361-18. doi: 10.1128/IAI.00361-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou L, Yang Z, Wang Z, Zhang X, Zhao Y, Yang H, Zheng B, Tian W, Wang S, He Z, Wang X. 2018. NLRP3/ASC-mediated alveolar macrophage pyroptosis enhances HMGB1 secretion in acute lung injury induced by cardiopulmonary bypass. Lab Invest 98:1052–1064. doi: 10.1038/s41374-018-0073-0. [DOI] [PubMed] [Google Scholar]
- 16.Khanova E, Wu R, Wang W, Yan R, Chen Y, French SW, Llorente C, Pan SQ, Yang Q, Li Y, Lazaro R, Ansong C, Smith RD, Bataller R, Morgan T, Schnabl B, Tsukamoto H. 2018. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis. Hepatology 67:1737–1753. doi: 10.1002/hep.29645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun W, Zeng C, Liu S, Fu J, Hu L, Shi Z, Yue D, Ren Z, Zhong Z, Zuo Z, Cao S, Peng G, Deng J, Hu Y. 2018. Ageratina adenophora induces mice hepatotoxicity via ROS-NLRP3-mediated pyroptosis. Sci Rep 8:16032. doi: 10.1038/s41598-018-34492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos CO, Campos KKD, Costa GP, Cangussú SD, Talvani A, Bezerra FS. 2018. Taurine treatment decreases inflammation and oxidative stress in lungs of adult mice exposed to cigarette smoke. Regul Toxicol Pharmacol 98:50–57. doi: 10.1016/j.yrtph.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Li F, Zhang L, Wu J, Wang Y, Yu H. 2017. Taurine alleviates lipopolysaccharide induced liver injury by anti-inflammation and antioxidants in rats. Mol Med Rep 16:6512–6517. doi: 10.3892/mmr.2017.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu YR, Ni XQ, Huang J, Zhu YH, Qi YF. 2016. Taurine drinking ameliorates hepatic granuloma and fibrosis in mice infected with Schistosoma japonicum. Int J Parasitol Drugs Drug Resist 6:35–43. doi: 10.1016/j.ijpddr.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norris PC, Gosselin D, Reichart D, Glass CK, Dennis EA. 2014. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci U S A 111:12746–12751. doi: 10.1073/pnas.1404372111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, Soriani FM, Silveira TN, Cunha LD, Zamboni DS, Quesniaux V, Peres RS, Cunha TM, Cunha FQ, Ryffel B, Souza DG, Teixeira MM. 2012. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B4 in a murine model of gout. Arthritis Rheum 64:474–484. doi: 10.1002/art.33355. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen I, Lopez JP, Laufer SA, Miao EA. 2016. IL-1β, IL-18, and eicosanoids promote neutrophil recruitment to pore-induced intracellular traps following pyroptosis. Eur J Immunol 46:2761–2766. doi: 10.1002/eji.201646647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed IN, Hafez SS, Fairaq A, Ergul A, Imig JD, El-Remessy AB. 2014. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia 57:413–423. doi: 10.1007/s00125-013-3101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moossavi M, Parsamanesh N, Bahrami A, Atkin SL, Sahebkar A. 2018. Role of the NLRP3 inflammasome in cancer. Mol Cancer 17:158. doi: 10.1186/s12943-018-0900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuah C, Jones MK, Burke ML, McManus DP, Gobert GN. 2014. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol 30:141–150. doi: 10.1016/j.pt.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Molyvdas A, Georgopoulou U, Lazaridis N, Hytiroglou P, Dimitriadis A, Foka P, Vassiliadis T, Loli G, Phillipidis A, Zebekakis P, Germenis AE, Speletas M, Germanidis G. 2018. The role of the NLRP3 inflammasome and the activation of IL-1β in the pathogenesis of chronic viral hepatic inflammation. Cytokine 110:389–396. doi: 10.1016/j.cyto.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed IN, Sarhan NR, Eladl MA, El-Remessy AB, El-Sherbiny M. 2018. Deletion of thioredoxin-interacting protein ameliorates high fat diet-induced non-alcoholic steatohepatitis through modulation of Toll-like receptor 2-NLRP3-inflammasome axis: histological and immunohistochemical study. Acta Histochem 120:242–254. doi: 10.1016/j.acthis.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Han CY, Rho HS, Kim A, Kim TH, Jang K, Jun DW, Kim JW, Kim B, Kim SG. 2018. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury. Cell Rep 24:2985–2999. doi: 10.1016/j.celrep.2018.07.068. [DOI] [PubMed] [Google Scholar]
- 30.Sadatomo A, Inoue Y, Ito H, Karasawa T, Kimura H, Watanabe S, Mizushina Y, Nakamura J, Kamata R, Kasahara T, Horie H, Sata N, Takahashi M. 2017. Interaction of neutrophils with macrophages promotes IL-1β maturation and contributes to hepatic ischemia-reperfusion injury. J Immunol 199:3306–3315. doi: 10.4049/jimmunol.1700717. [DOI] [PubMed] [Google Scholar]
- 31.Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H. 2016. Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circ Res 118:1525–1539. doi: 10.1161/CIRCRESAHA.116.308501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu F, Ji Q, Zhang J, Huang W, Cao Z, Li Y. 2018. AlCl3 inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production through suppressing NF-κB signaling pathway in murine peritoneal macrophages. Chemosphere 209:972–980. doi: 10.1016/j.chemosphere.2018.06.171. [DOI] [PubMed] [Google Scholar]
- 33.Ritter M, Gross O, Kays S, Ruland J, Nimmerjahn F, Saijo S, Tschopp J, Layland LE, Prazeres da Costa C. 2010. Schistosoma mansoni triggers dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc Natl Acad Sci U S A 107:20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng N, Xia M, Lu YQ, Wang M, Boini KM, Li PL, Tang WX. 2016. Activation of NLRP3 inflammasomes in mouse hepatic stellate cells during Schistosoma J. infection. Oncotarget 7:39316–39331. doi: 10.18632/oncotarget.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang WJ, Fang ZM, Liu WQ. 2019. NLRP3 inflammasome activation from Kupffer cells is involved in liver fibrosis of Schistosoma japonicum-infected mice via NF-κB. Parasit Vectors 12:29. doi: 10.1186/s13071-018-3223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 37.Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, Becerril S, Catalán V, Gómez-Ambrosi J, Silva C, Salvador J, Colina I, Malagón MM, Rodríguez A. 2019. Ghrelin reduces TNF-α-induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metab 104:21–37. doi: 10.1210/jc.2018-01171. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Huang H, Alam A, Chen Q, Suen KC, Cui J, Sun Q, Ologunde R, Zhang W, Lian Q, Ma D. 2018. VEGF mitigates histone-induced pyroptosis in the remote liver injury associated with renal allograft ischemia-reperfusion injury in rats. Am J Transplant 18:1890–1903. doi: 10.1111/ajt.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE. 2014. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palacios-Macapagal D, Connor J, Mustelin T, Ramalingam TR, Wynn TA, Davidson TS. 2017. Cutting edge: eosinophils undergo caspase-1-mediated pyroptosis in response to necrotic liver cells. J Immunol 199:847–853. doi: 10.4049/jimmunol.1601162. [DOI] [PubMed] [Google Scholar]
- 41.Lu L, Lu Q, Chen W, Li J, Li C, Zheng Z. 2018. Vitamin D3 protects against diabetic retinopathy by inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome pathway. J Diabetes Res 2018:8193523. doi: 10.1155/2018/8193523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oslowski CM, Hara T, O'Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F. 2012. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab 16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao Z, Fang Y, Lu Y, Tan D, Du C, Li Y, Ma Q, Yu J, Chen M, Zhou C, Pei L, Zhang L, Ran H, He M, Yu Z, Zhou Z. 2017. Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J Pineal Res 62:e12389. doi: 10.1111/jpi.12389. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Pan T, You X, Xu Y, Liang J, Limpanont Y, Sun X, Okanurak K, Zheng H, Wu Z, Lv Z. 2015. SjCa8, a calcium-binding protein from Schistosoma japonicum, inhibits cell migration and suppresses nitric oxide release of RAW264.7 macrophages. Parasit Vectors 8:513. doi: 10.1186/s13071-015-1119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, Bai J, Yang G, Gao N, Yang L, Qi S, Yan R, Liu X, Sun X. 2018. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis 9:946. doi: 10.1038/s41419-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Institutes of Health. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.