Abstract
Background
Pain following brain surgery can compromise recovery. Several pharmacological interventions have been used to prevent pain after craniotomy; however, there is currently a lack of evidence regarding which interventions are most effective.
Objectives
The objectives are to assess the effectiveness of pharmacological interventions for prevention of acute postoperative pain in adults undergoing brain surgery; compare them in terms of additional analgesic requirements, incidence of chronic headache, sedative effects, length of hospital stay and adverse events; and determine whether these characteristics are different for certain subgroups.
Search methods
We searched MEDLINE, Embase, CINAHL, CENTRAL, Web of Science and two trial registries together with reference checking and citation searching on 28th of November 2018.
Selection criteria
We included blinded and non‐blinded, randomized controlled trials evaluating pharmacological interventions for the prevention of acute postoperative pain in adults undergoing neurosurgery, which had at least one validated pain score outcome measure.
Data collection and analysis
We used standard Cochrane methodological procedures. We calculated mean differences for the primary outcome of pain intensity; any pain scores reported on a 0 to 100 scale were converted to a 0 to 10 scale.
Main results
We included 42 completed studies (3548 participants) and identified one ongoing study.
Nonsteroidal anti‐inflammatories (NSAIDs)
Nonsteroidal anti‐inflammatories (NSAIDs) reduce pain up to 24 hours (0 to 6 hours, MD −1.16, 95% CI −1.57 to −0.76; 12 hours, MD −0.62, 95% CI −1.11 to −0.14; 24 hours, MD −0.66, 95% CI −1.18 to −0.13; 6 studies, 742 participants; all high‐quality evidence). Results for other outcomes were imprecise (additional analgesic requirements: MD 1.29 mg, 95% CI −5.0 to 2.46, 4 studies, 265 participants; nausea and vomiting RR 1.34, 95% CI 0.30 to 5.94, 2 studies, 345 participants; both low‐quality evidence).
Dexmedetomidine reduces pain up to 12 hours (0 to 6 hours, MD −0.89, 95% CI −1.27 to −0.51, moderate‐quality evidence; 12 hours, MD −0.81, 95% CI −1.21 to −0.42, low‐quality evidence). It did not show efficacy at 24 hours (MD −0.08, 95% CI −0.32 to 0.16; 2 studies, 128 participants; low‐quality evidence). Dexmedetomidine may decrease additional analgesic requirements (MD −21.36 mg, 95% CI −34.63 to −8.1 mg, 2 studies, 128 participants, low‐quality evidence). Results for other outcomes were imprecise (nausea and vomiting RR −0.43, 95% CI 0.06 to 3.08, 3 studies, 261 participants; hypotension RR 0.5, 95% CI 0.05 to 5.28, 3 studies, 184 participants; both low‐quality evidence).
Scalp blocks may reduce pain up to 48 hours (0 to 6 hours, MD −0.98, 95% CI −1.66 to −0.3, 10 studies, 414 participants; 12 hours, MD −0.95, 95% CI −1.53 to −0.37, 8 studies, 294 participants; 24 hours, MD −0.78, 95% CI −1.52 to −0.05, 9 studies, 433 participants, all low‐quality evidence; 48 hours, MD −1.34, 95% CI −2.57 to −0.11, 4 studies, 135 participants, very low‐quality evidence. When studies with high risk of bias were excluded, significance remained at 12 hours only. Scalp blocks may decrease additional analgesia requirements (SMD −1.11, 95% CI −1.97 to −0.25, 7 studies, 314 participants). Results for other outcomes were imprecise (nausea and vomiting RR 0.66, 95% CI 0.33 to 1.32, 4 studies, 165 participants, very low‐quality evidence).
Scalp Infiltration may reduce pain postoperatively but efficacy was inconsistent, with a significant effect at 12 and 48 hours only (12 hours, MD −0.71, 95% CI −1.34 to −0.08, 7 studies, 309 participants, low‐quality evidence; 48 hours, MD ‐ 1.09, 95% CI ‐2.13 to ‐ 0.06, 3 studies, 128 participants, moderate‐quality evidence). No benefit was observed at other times (0 to 6 hours, MD −0.64, 95% CI −1.28 to −0.00, 9 studies, 475 participants, moderate‐quality evidence; 24 hours, MD −0.39, 95% CI −1.06 to 0.27,6 studies, 260 participants, low‐quality evidence. Scalp infiltration may reduce additional analgesia requirements MD −9.56 mg, 95% CI −15.64 to −3.49, 6 studies, 345 participants, very low‐quality evidence). When studies with high risk of bias were excluded, scalp infiltration lost the pain benefit at 12 hours and effects on additional analgesia requirements, but retained the pain‐reducing benefit at 48 hours (MD −0.56, 95% CI −1.20 to ‐0.32, 2 studies, 100 participants, very low‐quality evidence). Results for other outcomes were imprecise (nausea and vomiting, RR 0.74, 95% CI 0.48 to 1.41, 4 studies, 318 participants, low‐quality evidence).
Pregabalin or gabapentin may reduce pain up to 6 hours (2 studies, 202 participants), MD ‐1.15,95% CI −1.66 to −0.6, 2 studies, 202 participants, low‐quality evidence). One study examined analgesic efficacy at 12 hours showing significant benefit. No analgesia efficacy was shown at later times (24 hours, MD ‐0.29, 95% CI ‐0.78 to ‐0.19; 48 hours, MD ‐ 0.06, 95% CI ‐0.86 to 0.77, 2 studies, 202 participants, low‐quality evidence). Additional analgesia requirements were not significantly less (MD −0.37 (95% CI −1.10 to 0.35, 3 studies, 234 participants, low‐quality evidence). Risk of nausea and vomiting was significantly reduced (RR 0.51, 95% CI 0.29 to 0.89, 3 studies, 273 participants, low‐quality evidence). Results for other outcomes were imprecise (additional analgesia requirements: MD −0.37, 95% CI −1.10 to 0.35, 3 studies, 234 participants, low‐quality evidence).
Acetaminophen did not show analgesic benefit (0 to 6 hours, MD −0.35, 95% CI −1.00 to 0.30; 12 hours, MD −0.51, 95% CI −1.04 to 0.03, 3 studies, 332 participants, moderate‐quality evidence; 24 hours, MD ‐0.34, 95% CI ‐1.20 to 0.52, 4 studies, 439 participants, high‐quality evidence). Results for other outcomes remained imprecise (additional analgesia requirements, MD 0.07, 95% CI −0.86 to 0.99, 4 studies, 459 participants, high‐quality evidence; length of hospitalizations, MD −3.71, 95% CI −14.12 to 6.7, 2 studies, 335 participants, moderate‐quality evidence).
Authors' conclusions
There is high‐quality evidence that NSAIDs reduce pain up to 24 hours postoperatively. The evidence for reductions in pain with dexmedetomidine, pregabalin or gabapentin, scalp blocks, and scalp infiltration is less certain and of very low to moderate quality. There is low‐quality evidence that scalp blocks and dexmedetomidine may reduce additional analgesics requirements. There is low‐quality evidence that gabapentin or pregabalin may decrease nausea and vomiting, with the caveat that the total number of events for this comparison was low.
Plain language summary
Preventing pain after brain surgery
The problem
There is increasing evidence that people who have undergone brain surgery experience significant pain. This pain can have serious consequences including raised blood pressure, agitation, prolonged recovery time and an increased risk of long‐term headaches. Research studies have looked at different drugs in an attempt to reduce the risk of pain for these people. There is now more evidence about pain reduction options for adults undergoing brain surgery but there remains uncertainty as to which options work best.
The question
This review aimed to determine which drugs provide the best chance of reducing pain for adults undergoing brain surgery, by collecting and combining the results of studies that looked at pain‐relieving drugs for this patient group. To provide an accurate answer to this question, only studies conducted in accordance with an approved high standard were included. Studies published in different languages and countries were included in order to obtain as much information as possible.
In addition to determining which drugs were best at preventing or reducing pain after brain surgery, this review attempted to determine additional information such as how much additional pain‐relieving treatment was required in addition to the treatment under study; whether participants' pain was adequately controlled or not; how drowsy the participants were; what side effects they experienced; and how long they needed to stay in intensive care and in hospital. This review also considered whether some treatments worked better when given before or after surgery or for people undergoing different approaches to brain surgery.
The results
A total of 43 eligible studies, (42 complete and one still in progress), were found. Of the 42 completed studies (3548 participants), 10 studied injections of local anaesthetic into the scalp, 12 studied injection of local anaesthetic around specific scalp nerves, 8 studied nonsteroidal anti‐inflammatory drugs (NSAIDs), 4 studied dexmedetomidine, 4 studied acetaminophen aka paracetamol), 2 studied opioid drugs, 3 studied gabapentin or pregabalin (anti‐seizure drugs that can also be used for pain relief) together with 1 study each of local anaesthetic injected into the veins, local anaesthetic injected into the jaw and the drug flupirtine.
Sufficient information was abstracted to calculate the overall pain‐preventing effects of the following: local anaesthetic injections around the surgical wound, local anaesthetic injections around specific scalp nerves, NSAIDs, acetaminophen, dexmedetomidine and pregabalin or gabapentin. When only high‐quality studies were examined: NSAIDs reduced pain up to 24 hours after surgery, dexmedetomidine and local anaesthetics injected around specific scalp nerves reduced pain in the first 12 hours after surgery, pregabalin or gabapentin reduced pain in the first 6 hours after surgery and local anaesthetic injections around the surgical wound significantly reduced pain 48 hours after surgery, but did not affect pain at earlier time points.
When the timing of injection of local anaesthetics was examined, local anaesthetics injected around specific scalp nerves provided better early pain relief (first 6 hours) when injected after surgery and better late pain relief (12 and 24 hours) when injected before surgery.
The following interventions were also found to reduce the need for additional pain‐relieving drugs: local anaesthetics injected around specific scalp nerves and dexmedetomidine. Gabapentin or pregabalin was found to reduce the risk of nausea and vomiting after surgery.
Acetaminophen was not found to prevent pain after brain surgery or reduce the need for additional pain‐relieving drugs.
Insufficient evidence was found to determine whether any of these drugs made the participants more or less drowsy, affected how long they needed to stay in intensive care or whether different drugs worked better for adults undergoing different approaches to brain surgery.
The overall quality of the evidence that contributed to the results of this review was assessed and judged to be 'high' for pain‐reducing effects of NSAIDs, 'moderate' to 'low' for pain‐reducing effects of dexmedetomidine, acetaminophen, pregabalin and gabapentin and local anaesthetics injected around specific scalp nerves and ' low' to ' very low' for pain‐reducing effects of local anaesthetic injections around the surgical wound, additional pain relief requirements and risk of nausea and vomiting after surgery .
Summary of findings
Summary of findings for the main comparison. Nonsteroidal anti‐inflammatory drugs (NSAIDs) compared with control or placebo medications for prevention of pain in adults undergoing brain surgery.
| NSAIDs compared with control or placebo medications for prevention of pain in adults undergoing brain surgery | ||||||
|
Patient or population: adults undergoing brain surgery Settings: hospitals, countries: Australia, Hungary, Turkey and India Intervention: NSAIDs Comparison: control or placebo medications | ||||||
| Outcomes | Absolute Effects (95% CI) | Relative Effect, Risk Ratio (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
|
Acute postoperative pain 0 to 6 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 1.5 to 4.4 | Mean difference in pain intensity was 1.11 points lower in those who received NSAIDS when compared with those who received control or placebo medications (1.64 points lower to 0.58 points lower) | Not applicable | 742 (6) |
⊕⊕⊕⊕ high | |
|
Acute postoperative pain at 12 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 1.5 to 4.4 | Mean difference in pain intensity was 0.74 points lower in those who received NSAIDS when compared with those who received control or placebo medications (1.22 points lower to 0.26 points lower) | Not applicable | 742 (6) |
⊕⊕⊕⊕ high | |
|
Acute postoperative pain at 24 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 1.16 to 5.6 | Mean difference in pain intensity was 0.70 points lower in those who received NSAIDS when compared with those who received control or placebo medications (1.26 points lower to 0.14 points lower) | Not applicable | 742 (6) |
⊕⊕⊕⊕ high | |
|
Acute postoperative pain at 48 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain score in the control group was 1.0 | The mean pain score in the treatment group was 1.0, the same as the mean pain score in the control group so there was no mean difference in pain intensity between the two groups | Not applicable | 149 (1) |
⊕⊝⊝⊝ very low1 | Only 1 study reported this outcome |
|
Additional analgesia requirements 0 to 24 hours (Milligrams) |
Mean analgesia requirement in the control group ranged from 16 to 28.4 mg | Mean difference in additional analgesia requirements in the first 24 hours after surgery 1.07 mg less in those who received NSAIDS when compared with those who received control or placebo medications (4.88 mg less to 2.72 mg more) | Not applicable | 265 (4) |
⊕⊕⊝⊝ low2 | |
| Analgesic Success | 27 percent of patients in the control group had no worse than mild pain at 12 hours | 48 percent of patients in the treatment group had no worse than mild pain at 12 hours | Not applicable | Not applicable | ⊕⊝⊝⊝ very low1 | Only 1 study reported this outcome |
| Sedation | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Chronic Headache | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Length of critical care stay (hours) | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Length of hospital stay (hours) | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
|
Adverse event nausea and vomiting (0 to 24 hours) |
17 per 1000 | 23 per 1000 | Risk of nausea and vomiting was 1.34 times greater in those who received NSAIDS when compared with those who received control or placebo medications (0.30 to 5.94) | 345 (2) |
⊕⊕⊝ low 3 | |
| CI: confidence interval; RR: risk ratio; VAS: visual analogue scale; NRS: numerical rating scale; NSAIDS: nonsteroidal anti‐inflammatory drugs | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
- The evidence was downgraded three levels due to the fact that all the evidence came from one small study.
- The evidence was downgraded two levels due to a small pooled sample size and imprecision as the 95% CI for the effect estimate was wide and included the possibility of either no benefit or increased analgesic requirements in those who received NSAIDs.
- The evidence was downgraded two levels due to imprecision of results i.e. a low number of total events and a wide 95% confidence that included the possibility of less, equal or greater risk of nausea and vomiting in those who received NSAIDS when compared with those who received control or placebo medication
Summary of findings 2. Dexmedetomidine compared with control or placebo medications for prevention of pain in adults undergoing brain surgery.
| Dexmedetomidine compared with control or placebo medications for prevention of pain in adults undergoing brain surgery | ||||||
|
Patient or population: adults undergoing brain surgery Settings: hospitals, countries: China, USA Intervention: dexmedetomidine Comparison: control or placebo medications | ||||||
| Outcomes | Absolute Effects (95% CI) | Relative effect, Risk Ratio (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
|
Acute postoperative pain 0 to 6 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 3.0 to 3.6 |
Mean difference in pain intensity was 0.89 points lower in those who received dexmedetomidine when compared with those who received control or placebo medication (1.27 points lower to 0.51 points lower) | Not applicable | 128 (2) |
⊕⊕⊕⊝ moderate1 |
|
|
Acute postoperative pain at 12 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 3.0 to 3.1 |
Mean difference in pain intensity was 0.81 points lower in those who received dexmedetomidine when compared with those who received control or placebo medication (1.21 points lower to 0.42 points lower) | Not applicable | 128 (2) |
⊕⊕⊝⊝ low2 | |
|
Acute postoperative pain at 24 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 2.0 to 2.7 | Mean difference in pain intensity was 0.08 points lower in those who received dexmedetomidine when compared with those who received control or placebo medication (0.32 points lower to 0.16 points greater) | Not applicable | 128 (2) |
⊕⊕⊝⊝ low 3 | |
| Acute postoperative pain at 48 hours | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No study reported this outcome |
|
Additional analgesia requirements 0 to 24 hours (Milligrams) |
Mean analgesia requirement in the control group ranged from 52 to 170 mg | Mean difference in additional analgesia requirements in the first 24 hours after surgery 21.36 mg less in those who received dexmedetomidine when compared with those who received control or placebo medication (34 mg less to 8.1 mg less) | Not applicable | 128 (2) |
⊕⊕⊝⊝ low2 | |
| Analgesic Success | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Sedation | Mean sedation score at 24 hours was 2.2 in the control group | Mean sedation score at 24 hours was 2.4 in the treatment group | Not applicable | 52 (1) |
⊕⊝⊝⊝ very low4 | Only one eligible study addressed this outcome |
| Chronic Headache | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Length of hospital stay (hours) | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
|
Adverse event nausea and vomiting (0 to 24 hours) |
152 per 1000 | 67 per 1000 | Risk of nausea and vomiting was 0.43 times less in those who received dexmedetomidine when compared with those who received control or placebo medication (0.06 to 3.08) | 261 (3) |
⊕⊕⊝⊝ low 5 |
|
|
Adverse event hypotension (0 to 24 hours) |
22 per 1000 | 11 per 1000 | Risk of hypotension was 0.5 times less in those who received dexmedetomidine when compared with those who received control or placebo medication (0.05 to 5.28 | 184 (3) |
⊕⊕⊝⊝ low6 | |
| CI: Confidence interval; RR: Risk Ratio; VAS: Visual Analogue Scale; NRS: Numerical Rating Scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
- The evidence was downgraded by one level due to imprecision due a small pooled sample size i.e. 128 participants.
- The evidence was downgraded by two levels due to imprecision due to a small pooled sample size and inconsistency of results in the form of unexplained important heterogeneity.
- The evidence was downgraded two levels due to imprecision due to a small pooled sample size and a wide 95% CI which included the possibility of either no effect or greater pain intensity in those who received dexmedetomidine.
- The evidence was downgraded three levels as it came from one small study.
- The evidence was downgraded by two levels due imprecision due to a small total number of events and a wide 95% CI which included the possibility of less, equal or greater risk of nausea and vomiting in those who received dexmedetomidine when compared with those who received control or placebo medication.
- The evidence was downgraded two levels due to imprecision due to a small total number of events and a wide 95% CI which included the possibility of less, equal or greater risk of hypotension in those who received dexmedetomidine when compared with those who received control or placebo medication.
Summary of findings 3. Pregabalin or Gabapentin compared with control or placebo medications for prevention of pain in adults undergoing brain surgery.
| 0.9 Pregabalin or gabapentin compared with control or placebo medications for prevention of pain in adults undergoing brain surgery | ||||||
|
Patient or population: adults undergoing brain surgery Settings: hospitals, countries: Israel, India Intervention: gabapentin or pregabalin Comparison: control or placebo medication | ||||||
| Outcomes | Absolute Effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
|
Acute postoperative Pain 0 to 6 hours |
The mean pain scores in the control group ranged from 2.9 to 3.9 |
Mean difference in pain intensity was 1.15 points lower in those who received gabapentin or pregabalin when compared to those who received control or placebo medication (1.66 points lower to 0.6 points lower) * | Not applicable | 202 (2) |
⊕⊕⊝⊝ low1 | * These results were measured as standardized mean differences and re‐expressed as mean differences |
| Acute postoperative pain at 12 hours | The mean pain score in the control group was 2.26 | Mean pain score in those who received pregabalin was 1.5 which was 1.1 times lower than the mean score in the control group | Not calculated | 100 (1) |
⊕⊝⊝⊝ very low2 | Only 1 study reported this outcome |
| Acute postoperative pain at 24 hours | The mean pain scores in the control group ranged from 1.47 to 3.0 |
Mean difference in pain intensity was 0.29 points lower in those who received gabapentin or pregabalin when compared to those who received control or placebo medication (0.78 points lower to 0.19 points lower) * | Not applicable | 202 (2) |
⊕⊕⊝⊝ low1 | * These results were measured as standardized mean differences and re‐expressed as mean differences |
| Acute postoperative pain at 48 hours | The mean pain scores in the control group ranged from 1.13 to 2.0 |
Mean difference in pain intensity was 0.06 points lower in those who received gabapentin or pregabalin when compared to those who received control or placebo medication (0.86 points lower to 0.77 points higher) * | Not applicable | 202 (2) |
⊕⊕⊝⊝ low1 | * These results were measured as standardized mean differences and re‐expressed as mean differences |
| Additional analgesia requirements at 0 to 24 hours | Mean additional analgesia requirement in the control group ranged from 0.34 to 9.40 mg with agents used being fentanyl and morphine | Standardized mean difference in additional analgesia requirements in the first 24 hours after surgery 0.37 less in those who received gabapentin or pregabalin when compared with those who received control or placebo medications (1.10 less to 0.35 more) | Not applicable | 234 (3) |
⊕⊕⊝⊝ low1 | Using Cohens rule of thumb: an effect size of 0.37 represents a small, non‐significant effect size |
| Analgesic Success | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Sedation | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Chronic Headache | Mean pain score at 3 months of 1.51 in the control group | Mean pain score at 3 months of 1.28 in the control group | Not applicable | 54 [1) |
⊕⊝⊝⊝ very low2 | Only one study addressed this outcome |
| Length of critical care stay (hours) | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Length of hospital stay (hours) | Mean length of stay in hospital in the control group was 8.3 days | Mean length of stay in hospital in those who received pregabalin group was 7.9 days | Not applicable | 100 (1) |
⊕⊝⊝⊝ very low2 | Only one study reported this outcome |
|
Adverse event nausea and vomiting (0 to 24 hours) |
379 per 1000 | 203 per 1000 | Risk of nausea and vomiting was 0.51 times less in those who received gabapentin or pregabalin when compared with those who received control or placebo medications (0.29 to 0.89) | 273 (3) |
⊕⊕⊝⊝ low3 | |
| CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
- The evidence was downgraded by two levels due to small pooled sample size and possible indirectness of effect as the two drugs studied (pregabalin and gabapentin) differ somewhat in their pharmacological properties.
- The evidence was downgraded three levels due to the fact that all the evidence came from one small study.
- The evidence was downgraded by two levels due to imprecision as the number of total events were small and indirectness as the two drugs differ somewhat in their pharmacological properties.
.
Summary of findings 4. Acetaminophen compared with control or placebo medications for prevention of pain in adults undergoing brain surgery.
| Acetaminophen compared with control or placebo medications for prevention of pain in adults undergoing brain surgery | ||||||
|
Patient or population: adults undergoing brain surgery Settings: hospitals, countries: Turkey, India, United States of America Intervention: acetaminophen Comparison: control or placebo medication | ||||||
| Outcomes | Absolute Effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
|
Acute postoperative pain 0 to 6 hours |
The mean pain scores in the control group ranged from 1.5 to 5,6 | Mean difference in pain intensity was 0.35 points lower in those who received acetaminophen when compared to those who received control or placebo medication (1.00 points lower to 0.30 points higher) | Not applicable | 332 (3) |
⊕⊕⊕⊝ moderate1 |
|
| Acute postoperative pain at 12 hours | The mean pain scores in the control group ranged from 2.0 to 5.8 | Mean difference in pain intensity was 0.51 points lower in those who received acetaminophen when compared to those who received control or placebo medication (1.04 points lower to 0.03 points higher) | Not applicable | 332 (3) |
⊕⊕⊕⊝ moderate 1 |
|
| Acute postoperative pain at 24 hours | The mean pain scores in the control group ranged from 1.16 to 5.4 | Mean difference in pain intensity was 0.34 points lower in those who received acetaminophen when compared with those who received control or placebo medication (1.20 points lower to 0.52 points higher) | Not applicable | 459 (4) |
⊕⊕⊕⊕ high | |
| Acute postoperative pain at 48 hours | The mean pain scores in the control group was 5.5 | The mean pain scores in the control group was 5.5, with no significant difference between the groups | Not applicable | 202 (1) |
⊕⊝⊝⊝ very low2 | Only 1 study reported this outcome |
|
Additional analgesia requirements 0 to 24 hours (milligrams) |
Mean additional analgesia requirement in the control group ranged from 1.75 mg to 85.5 mg | Mean difference in additional analgesia requirements in the first 24 hours after surgery 0.07 mg less in those who received acetaminophen when compared with those who received control or placebo medication (0.86 mg less to 0.99 mg more) | Not applicable | 459 (4) |
⊕⊕⊕⊕ high | |
| Analgesic Success | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
|
Sedation score at 24 hours (Richmond Agitation Sedation scale) |
Mean sedation score in the control group was zero | Mean sedation score in the acetaminophen group was zero | Not applicable | 131 (1) |
⊕⊝⊝⊝ very low2 | Only 1 study reported this outcome |
| Chronic headache | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Length of critical care stay (hours) | The median length of stay in the control group was 28 hours | The median length of stay in the acetaminophen group was 26 hours | Not applicable | 131 (1) |
⊕⊝⊝⊝ very low2 | Only 1 study reported this outcome |
| Length of hospital stay (hours) | Mean length of stay in hospital in the control group ranged from 75.5 to 137 days | Mean difference in length of stay in hospital of 3.71 hours less in those who received acetaminophen when compared with those who received control or placebo medication (14.12 hours less to 6.7 hours more) | Not applicable | 335 (2) |
⊕⊕⊕⊝ moderate1 |
|
| Adverse events | Not calculated | Not calculated | Not calculated | Not applicable | Not applicable | No two studies reported comparable adverse events |
| CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
- The evidence was downgraded by one level due to a small pooled sample size.
- The evidence was downgraded three levels due to the fact that all the evidence came from one small study.
Summary of findings 5. Scalp infiltration compared with control or placebo intervention for prevention of pain in adults undergoing brain surgery.
| Scalp infiltration compared with control or placebo intervention for prevention of pain in adults undergoing brain surgery | |||||||
|
Patient or population: adults undergoing brain surgery Settings: hospitals, countries: France, India, USA, Saudi Arabia, Greece, Thailand and China Intervention: scalp Infiltration Comparison: control or placebo Intervention |
|||||||
| Outcomes | Absolute Effect (95% CI) | Relative Effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Asuumed Risk | Corresponding Risk | ||||||
|
Acute postoperative pain 0 to 6 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 2.0 to 5.4 | Mean difference in pain intensity was 0.64 points lower in those who received scalp infiltration when compared with those who received control or placebo interventions (1.28 points lower to 0.00 points lower) | Not applicable | 475 (9) |
⊕⊕⊕⊝ moderate 1 | ||
|
Acute postoperative pain at 12 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 1.6 to 5.0 | Mean difference in pain intensity was 0.71 points lower in those who received scalp infiltration when compared with those who received control or placebo interventions (1.34 points lower to 0.08 points lower) | Not applicable | 309 (7) |
⊕⊕⊝⊝ low 2 | ||
|
Acute postoperative pain at 24 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 1.1 to 5.0 | Mean difference in pain intensity was 0.39 points lower in those who received scalp infiltration when compared with those who received control or placebo interventions (1.06 points lower to 0.27 points higher) | Not applicable | 260 (6) |
⊕⊕⊕⊝ moderate 1 | ||
| Acute postoperative pain at 48 hours (score 0 to 10, VAS or NRS scale) | The mean pain scores in the control group ranged from 2.3 to 3.8 | Mean difference in pain intensity was 1.09 points lower in those who received scalp infiltration when compared with those who received control or placebo interventions (2.13 points lower to 0.06 points lower) | Not applicable | 128 (3) |
⊕⊕⊕⊝ moderate 3 | ||
|
Additional analgesia requirements 0 to 24 hours (milligrams) |
Mean additional analgesia requirement in the control group ranged from 13 mg to 58 mg | Mean difference in additional analgesia requirements in the first 24 hours after surgery 9.56 mg less in those who received scalp infiltration when compared with those who received control or placebo interventions (15.64 mg less to 3.49 mg less) | Not applicable | 345 (6) |
⊕⊝⊝⊝ very low 4 | ||
| Analgesic Success | 8 percent of patients in the control group were pain‐free at 6 hours | 4 percent of patients in the treatment group were pain‐free at 6 hours | Not applicable | 49 (1) |
⊕⊝⊝⊝ very low 5 | Only one study addressed this outcome | |
| Sedation | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome | |
| Chronic headache | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome | |
| Length of critical care stay (hours) | Not calculated | Not calculated | Not calculated | Not applicable | Not applicable | No eligible study addressed this outcome | |
| Length of hospital stay (hours) | Not calculated | Not calculated | Not calculated | Not applicable | Not applicable | No eligible study addressed this outcome | |
|
Adverse event nausea and vomiting (0 to 24 hours) |
236 per 1000 | 174 per 1000 | Risk of nausea and vomiting was 0.74 times less in those who received scalp infiltration when compared with those who received control or placebo interventions (0.48 to 1.41) | 318 (4) |
⊕⊕⊝⊝ low 6 | ||
| CI: Confidence interval; RR: Risk Ratio; VAS: Visual Analogue Scale; NRS: Numerical Rating Scale | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
- Consistency, precision, directness was rated high. The evidence was downgraded by one level due to inconsistency in the form of unexplained important heterogeneity.
- The evidence was downgraded by two levels due to imprecision due to a small pooled sample size and loss of significance of results on sensitivity analysis.
- The evidence was downgraded by one level due to a small pooled sample size.
- The evidence was downgraded three levels due to imprecision due to a small pooled sample size, inconsistency in the form of unexplained important heterogeneity and loss of significance of results on sensitivity analysis.
- The evidence was downgraded by three levels as the results came from one small study.
- The evidence was downgraded two levels level due to imprecision i.e. a small number of total events and a wide 95% confidence that included the possibility of either no effect or increased nausea and vomiting in the intervention group.
Summary of findings 6. Scalp block compared with control or placebo intervention for prevention of pain in adults undergoing brain surgery.
| Scalp block compared with control or placebo intervention for prevention of pain in adults undergoing brain surgery | ||||||
|
Patient or population: adults undergoing brain surgery Settings: hospitals, countries: USA, India, Korea, Canada, Thailand, China and Spain Intervention: scalp block Comparison: control or placebo intervention | ||||||
| Outcomes | Absolute Effect (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | |||||
|
Acute postoperative pain 0 to 6 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 2.0 to 5.7 | Mean difference in pain intensity was 0.98 points lower in those who received scalp block when compared with those who received control or placebo interventions (1.66 points lower to 0.39 points lower) | Not applicable | 414 (10) |
⊕⊕⊝⊝ low 1 | |
|
Acute postoperative pain at 12 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 2.3 to 5.0 | Mean difference in pain intensity was 0.95 points lower in those who received scalp block when compared with those who received control or placebo interventions (1.53 points lower to 0.37 points lower) | Not applicable | 294 (8) |
⊕⊕⊝⊝ low 2 | |
|
Acute postoperative pain at 24 hours (Score 0 to 10, VAS or NRS Scale) |
The mean pain scores in the control group ranged from 1.7 to 4.2 | Mean difference in pain intensity was 0.78 points lower in those who received scalp block when compared with those who received control or placebo interventions (1.52 points lower to 0.05 points lower) | Not applicable | 433 (9) |
⊕⊕⊝⊝ low 1 | |
| Acute postoperative pain at 48 hours (Score 0 to 10, VAS or NRS Scale) | The mean pain scores in the control group ranged from 1.6 to 4.0 | Mean difference in pain intensity was 1.34 points lower in those who received scalp block when compared with those who received control or placebo interventions (2.57 points lower to 0.11 points lower) | Not applicable | 135 (4) |
⊕⊝⊝⊝ very low 3 | |
| Additional analgesia requirements 0 to 24 hours | The mean additional analgesia requirement in the control group was 0.3 mg to 15 mg with agents used being fentanyl and morphine | Standardized mean difference in additional analgesia requirements in the first 24 hours after surgery 1.11 less in those who received scalp block when compared with those who received control or placebo interventions (1.97 less to 0.25 less) | Not applicable | 314 (7) |
⊕⊕⊝⊝ low 2 | Using Cohens rule of thumb: an effect size of 1.11 represents a large effect size |
| Analgesic success | 10 percent of patients in the control group were pain‐free at 12 hours after surgery | 15 percent of patients who had scalp blocks were pain‐free at 12 hours after surgery | Not applicable | 40 (1) |
⊕⊕⊝⊝ very low 4 | Only one eligible study addressed this outcome |
| Sedation | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No eligible study addressed this outcome |
| Chronic headache | Not calculated | Not calculated | Not applicable | Not applicable | Not applicable | No study addressed pain at 3 months |
| Length of critical care stay (hours) | Not calculated | Not calculated | Not calculated | Not applicable | Not applicable | No eligible study addressed this outcome |
| Length of hospital stay (hours) | Not calculated | Not calculated | Not calculated | Not applicable | Not applicable | No eligible study addressed this outcome |
|
Adverse event nausea and vomiting (0 to 24 hours) |
514 per 1000 | 308 per 1000 | Risk of nausea and vomiting was 0.66 times less in those who received scalp block when compared with those who received control or placebo interventions (0.33 to 1.32) | 165 (4) |
⊕⊕⊝⊝ very low 4 | |
| CI: Confidence interval; RR: Risk Ratio; VAS: Visual Analogue Scale; NRS: Numerical Rating Scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
- The evidence was downgraded two levels due to inconsistency in the form of unexplained important heterogeneity and failure to retain significance on sensitivity analysis.
- The evidence was downgraded two levels due to imprecision due to a small pooled sample size and inconsistency in the form of unexplained important heterogeneity.
- The evidence was downgraded three levels as there was imprecision due to a sample pooled sample size, inconsistency in the form of unexplained important heterogeneity and failure to retain significance on sensitivity analysis and small pooled sample size.
- The evidence was downgraded three levels as it came from one small study.
- The evidence was downgraded three levels as there was inconsistency in the form of unexplained important heterogeneity and imprecision due to a small number of total events and a wide 95% CI which included the possibility of either no benefit or increased nausea and vomiting in the intervention group.
Background
Description of the condition
Craniotomy (brain surgery) was previously considered less painful than other surgical procedures because the brain tissue lacks pain receptors and the relative immobility of the soft tissues of the head protects against pain due to tension and traction in the postoperative period (Dunbar 1999). Concern regarding the side effects of pain medication in this population, that is, impaired postoperative neurological assessment with opioids and increased risk of bleeding with nonsteroidal medication, also contributed to limited use of analgesics in those undergoing brain surgery.
Evidence now suggests that these patients experience significant postoperative pain. A prospective study of 256 participants undergoing elective craniotomy showed that 87% of patients reported pain in the first 24 hours after surgery with 55% reporting moderate to severe pain (Mordhorst 2009). Other similar studies showed high rates of postoperative pain in this population (De Oliveria Riberio 2013; Hansen 2013). Aside from the obvious patient discomfort, pain can delay recovery and increase length of hospital stay. It can also increase the risk of postoperative complications including hypertension, agitation and vomiting (Molnar 2014). These complications can be particularly problematic in neurosurgical populations as they can mimic, obscure and increase the risk of other neurosurgical complications such as raised intracranial pressure (pressure in the brain) and intracranial haemorrhage (bleeding into the brain).
Acute postoperative pain may also play a role in central sensitization and up‐regulation of pain receptors, factors implicated in the development of chronic post‐craniotomy headache. While the incidence of chronic headache varies with type of brain surgery, it can be as high as 23% to 34% at three months and 12% to 16% at one year after surgery (Harner 1993; Schaller 2003). For those who develop it, it is a debilitating and difficult‐to‐treat condition that can significantly impair quality of life and social functioning (Imayev 2013; Molnar 2014). Much of the data about post‐craniotomy headache relates more to its epidemiology and treatment once established, than to the efficacy of interventions aimed at its prevention, making it difficult to elucidate the benefit of any particular analgesic strategy in reducing the incidence of chronic post‐craniotomy headache. However, the fact that chronic post‐craniotomy headache becomes evident as failure of resolution of postoperative headache rather than the de novo appearance of a new condition, supports a common etiologic pathway with acute post‐craniotomy pain and hence a reasonable likelihood that interventions aimed at preventing acute headache may also be beneficial in reducing the risk of chronic headache (De Gray 2005). Evidence from wider surgical populations suggests that local and regional anaesthetic techniques rather than conventional analgesics may help reduce the incidence of chronic postoperative pain: whether this is true for patients undergoing brain surgery remains unknown as none of the studies included in that systematic review were conducted in this population (Weinstein 2018).
Description of the intervention
Several pharmacological interventions are currently available for the management of pain following craniotomy, although evidence suggests that opioid derivatives and nonsteroidal anti‐inflammatory drugs (NSAIDs) are the most commonly used (De Oliveria Riberio 2013; Kotak 2009). In line with increasing awareness of the problem of post‐craniotomy pain, there has been a sharp increase in the number of studies of interventions aimed at its prevention (Hwang 2015; Morad 2009; Williams 2011). A wide variety of strategies have been evaluated including various NSAIDs, scalp infiltration, regional scalp block and novel agents (Hwang 2015; Morad 2009; Williams 2011; Yadav 2014). While these interventions work through different mechanisms, the timing of their administration may be a factor in determining both the incidence and intensity of pain experienced after surgery.
The concept of 'pre‐emptive analgesia'' was first proposed in the 1980s (Wall 1988), and centres on the theory that analgesia given before pain becomes established may ameliorate the mechanisms involved in the development of both acute and chronic postoperative pain. Pre‐emptive, as opposed to rescue analgesia, may be particularly relevant to those undergoing brain surgery for the following reasons: the very nature of the surgery itself can impede the patient's ability to report pain, making it more difficult to achieve effective postoperative analgesia in this population (Hansen 2013; Kotak 2009); the need for accurate postoperative neurological assessment limits the analgesic options suitable for the relief of pain in the postoperative period in these patients (Gottschalk 2009; Molnar 2014); non‐sedating analgesic options including scalp blocks and local anaesthetic infiltration may be technically easier and more tolerable for the patient when performed before the end of the operation and hence before pain is reported; systemic consequences of established pain including hypertension, vomiting and haemorrhage can be particularly undesirable in those who have had recent brain surgery and so prevention of acute pain in the postoperative period may help to reduce the risk of these problems (Basali 2000; Molnar 2014). Finally, prevention of acute pain after brain surgery might help to decrease the risk of chronic headache (De Gray 2005).
How the intervention might work
Pharmacological interventions used in the management of craniotomy pain act through different mechanisms.
Opioids
Opioids mediate their analgesic effects through central opioid receptors, blocking neurotransmitter (chemical) release and nociceptive (pain‐transmitting) pathways (Martin 1983). While they are currently a mainstay of analgesia for craniotomy, their popularity is based more on tradition and familiarity than on evidence. A recent survey showed that 70% of neurosurgical units used codeine as the first‐line opioid in the management of craniotomy pain (Kotak 2009). While codeine is not commonly used for postoperative pain in other forms of surgery, its popularity in neurosurgery centres on its minimal sedative properties in comparison to stronger opioids like morphine (Molnar 2014). Concerns about morphine's sedating properties and consequent impediment of neurological assessment have limited its use. Tramadol is generally used as a third‐ or fourth‐line agent but has some important side effects including nausea, vomiting and reduction in the seizure threshold (Kotak 2009).
Acetaminophen
Acetaminophen inhibits cyclo‐oxygenase and prostaglandin production. It is rarely used as a sole agent for the prevention of post‐craniotomy pain as it is generally not adequate alone (Molnar 2014). However, it has been shown to reduce morphine requirements by 20% in the postoperative period (Remy 2005).
Nonsteroidal anti‐inflammatory drugs (NSAIDs)
Nonsteroidal anti‐inflammatory drugs (NSAIDs) inhibit cyclo‐oxygenase and consequently prostaglandin production with resultant analgesic and anti‐inflammatory effects (Higgs 1980). While NSAIDs have been shown to be effective for the relief of headache, their usefulness has been limited by their antiplatelet (platelet‐inhibiting) action with concerns about an increased risk of intracerebral bleeding in neurosurgical patients (Imayev 2013). This led to an increasing interest in selective cyclo‐oxygenase inhibitors, which are free of antiplatelet effects (Williams 2011).
Local anaesthetics‐scalp infiltration and scalp blocks
Local anaesthetics produce a localized reversible block of pain fibres preventing propagation of the pain impulse (Becker 2012), and have been shown to provide effective analgesia through several different routes of administration and across a wide range of surgical populations (Tayeb 2017; Weinstein 2018), although their efficacy is yet not proven when given by more novel routes of administration (i.e. intravenous) (Weibel 2018). Common routes of administration of local anaesthetics in patients undergoing brain surgery are scalp infiltration and scalp block. Scalp infiltration addresses no specific sensory pathways, while regional scalp block involves infiltration of local anaesthetic at well‐defined anatomical sites targeting the major sensory innervation (nerve) pathways of the scalp. Early results of regional scalp block are promising but inconclusive (Gilfoyle 2012).
Flupirtine
Flupirtine is a novel, centrally‐acting analgesic that has N‐methyl‐D‐aspartate receptor antagonist (opposing) properties. It has not yet been studied extensively in patients undergoing brain surgery but at least one study reported it to be as effective as diclofenac sodium for postoperative pain reduction in this population (Yadav 2014).
Dexmedetomidine
This is a highly selective alpha 2‐agonist with no central respiratory depressive effects, anti‐delirium and analgesic properties making it an increasingly popular choice for sedation of patients at high risk of respiratory compromise or delirium and for anaesthesia for awake brain surgery. While it has very few side effects, it is known to increase the risk of bradycardia (abnormally slow heart rate) and hypotension (abnormally low blood pressure) (Dunn 2016). Recently its analgesic effects in those undergoing brain surgery are being explored (Peng 2015; Song 2016).
Pregabalin or Gabapentin
These anti‐convulsant medications inhibit central neurotransmitter release, reducing pain perception. They have found a role both in the management of acute postoperative pain and relief of chronic pain. There are concerns, however, that they may be associated with sedating effects and delayed extubation (removal of breathing tube) (Haldar 2015).
Why it is important to do this review
Postoperative pain relief is frequently suboptimal in this population (Hansen 2013). This is likely due to a number of factors. Firstly, the need for prompt and accurate neurologic assessment following brain surgery means healthcare providers are reluctant to use sedating analgesics which may impede that assessment. Secondly, despite the availability of a wide variety of analgesic options, the lack of robust evidence of the superiority of one over another contributes to a reliance on traditional and perhaps less efficacious forms of pain relief. Thirdly, there is still a lack of appreciation among healthcare providers of the frequency and severity of post‐craniotomy pain (Ribeiro 2012). Finally, many of these patients may not be able to express their pain verbally and require a more proactive approach to pain evaluation and treatment than other surgical populations.
Given the challenges in achieving adequate postoperative pain relief and the particularly undesirable systemic consequences of pain in this population, studies have been increasingly focusing on the effectiveness of analgesia administered either before emergence from anaesthesia or before pain has become established (Hwang 2015; Williams 2011; Yadav 2014). Such an approach is supported by evidence of the role of pre‐emptive analgesia in postoperative pain prevention in other surgical populations (Inanoglu 2007), and in children undergoing craniotomy in whom scheduled analgesia achieved significantly lower acute postoperative pain scores than 'as required' analgesia (Smyth 2004). However, pre‐emptive analgesia carries its own risks, including exposure to analgesia‐related adverse events and over‐treatment of those who may otherwise have developed mild pain at worst.
Combining data from individual studies and addressing patient‐relevant outcomes is key to establishing the relative efficacy and risk/benefit balance of pain prevention measures (McQuay 1995). While reviews of the effectiveness of some of these pharmacological interventions exist for neurosurgical patients, they are either confined to single interventions or studies which were published in English and for which the full‐text papers were readily available (Gilfoyle 2012; Hansen 2011). As yet, there is no comprehensive review that attempts to quantify and synthesize the effectiveness and safety profiles of all the evaluated interventions. Without this process, we do not know which pharmacological interventions are most effective and how additional analgesic requirements and adverse effects compare between interventions. This review attempts to determine the current overall state of knowledge in this regard.
There is an increasing realization that patients consider good pain control to be the achievement of 'no worse than mild pain' (Moore 2013). This outcome measure has yet to be widely adopted in trials of analgesia for prevention of pain following brain surgery, however, where the data permits, this review will attempt to address this outcome, using the same definitions of 'no worse than mild pain' used in a recent Cochrane review of oral morphine for the relief of cancer pain (Wiffen 2016).
Objectives
The objectives of this review are to assess the effectiveness of pharmacological interventions for prevention of acute postoperative pain in adults undergoing brain surgery; compare them in terms of additional analgesic requirements, incidence of chronic headache, sedative effects, length of hospital stay and adverse events; and determine whether these characteristics are different for certain subgroups
Methods
Criteria for considering studies for this review
Types of studies
We included blinded and non‐blinded, controlled, randomized trials evaluating the effectiveness of any pharmacological drug or technique for the prevention of acute postoperative pain in adults undergoing neurosurgery, which have at least one validated pain score as an outcome measure.
We excluded review articles, observational studies, case reports, case series, non‐randomized studies and studies that had no control groups. We also excluded studies that investigated the use of agents with analgesic potential for non‐analgesic purposes. The rationale for this decision was based on a high likelihood of important differences — in inclusion and exclusion criteria, dosages, timing, ancillary analgesic usage and attributable side effects — between studies that investigated these agents for their analgesic efficacy and studies that investigated them for their non‐analgesic effects.
Types of participants
We included adults (defined as more than or equal to 18 years of age at the time of study enrolment), undergoing either supratentorial or infratentorial craniotomy or craniectomy either as an elective or emergency procedure. We excluded those undergoing neurosurgical procedures that did not involve accessing the brain such as spinal operations.
Types of interventions
We included any pharmacological drug or pharmacological technique evaluated against a control for the prevention of acute postoperative pain in adults undergoing neurosurgery. We excluded interventions that were specifically given for the relief of established acute pain after brain surgery as opposed to those given before pain had become established.
Types of outcome measures
Primary outcomes
-
Mean differences in validated measures of acute postoperative pain intensity measured at the following times:
anytime in the first six hours postoperatively;
12 hours postoperatively
24 hours postoperatively;
48 hours postoperatively
Secondary outcomes
Analgesic success as measured by achievement of 'no worse than mild pain' with 'no worse then mild pain' being defined as a score of ≤ 30/100 mm on a visual analogue scale or ≤ 3/10 on a numerical rating scale.
Mean difference in additional analgesia requirement at the same time points.
Mean difference in validated measures of sedation at the same time points.
Mean difference in incidence of chronic post‐craniotomy headache with chronic post‐craniotomy headache being defined as headache persisting three months or more after surgery.
Mean difference in length of critical care unit stay.
Mean difference in length of hospital stay.
Rate of the adverse events in the perioperative period (intraoperatively until four days postoperatively) including, but not confined to, the following: respiratory depression, hypercapnia, elevated intracranial pressure, hypotension, nausea, vomiting, gastrointestinal bleeding, haematoma formation, nerve injury, local anaesthetic toxicity, local or systemic infection and death from any cause.
To capture all reported adverse events, we did not predefine each event but instead provided information in the review regarding how included studies defined these events and how those definitions varied in their wording and application between studies.
Our primary and secondary outcomes differed somewhat from those stated in the original protocol (Galvin 2015). Those differences and the reasons for those differences are detailed in the section entitled Differences between protocol and review.
Search methods for identification of studies
The search strategy used in this review was based on both Joanna Briggs Institute 2011, and the Institute of Medicine of the National Academics, and involved three steps. An initial search of MEDLINE was developed by a librarian (Amanda Ross, Bracken Health Sciences Library, Queens University, Kingston), in collaboration with the lead author (IMG) and sent for feedback from all authors. We included any changes suggested by the authors along with a text‐word and index term analysis to better refine the search and ensure a more complete recall. In this second stage, we conducted a search, using all identified keywords and index terms, in MEDLINE, Embase, CINAHL, CENTRAL, and Web of Science’s Citation Index. We completed the initial search in the week of 12 September 2016. We conducted an updated search using the same search strategy on 24 October 2017 and a further updated search on 28 November 2018.
In the third search stage, we searched the reference lists of all identified reports and articles for additional studies. We placed no language limits on the search. The initial MeSH terms we used were: craniotomy, decompressive craniectomy, trephining, brain neoplasms, narcotics, analgesics, local anaesthetics, local anaesthetics, aminopyridines, flupirtine, acetaminophen, morphine, tramadol, codeine, paracetamol, postoperative pain, pain, acute pain, headache, and slit ventricle syndrome. We also used the Controlled Clinical Trials hedge outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Our full strategy can be found in Appendix 1. We then uploaded the results to Covidence, systematic review software, for review by the authors as to relevance and whether a priori criteria were met.
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011 ). We did not apply restrictions to language or publication status. We searched the following databases for relevant trials:
Cochrane Central Register of Controlled Trials (CENTRAL; Issue 9 2017);
MEDLINE (Ovid SP, 1966 to 28 November 2018 );
Embase (Ovid SP, 1988 to 28 November 2018);
CINAHL (Ovid SP,1982 to 28 November2018);
Web of Science (1990 to 28 November 2018).
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other databases listed. Where appropriate, the search strategy was expanded with search terms for identifying RCTs. All search strategies can be found in Appendix 1.
Searching other resources
We scanned the following trials registries for ongoing and unpublished trials (28 November 2018).
The World Health Organization International Clinical Trials Registry Platform (WHOICTRP) (http://apps.who.int/trialsearch/).
ClinicalTrials.gov.
We scanned the reference lists and citations of included trials and any relevant systematic reviews identified for further references to additional trials.
We searched conference abstracts to identify unpublished or ongoing studies.
When necessary, we contacted trial authors for additional information.
Data collection and analysis
Selection of studies
We uploaded the search results to Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia (Covidence), from where two authors (IMG, RL), independently screened the citations. This was done in two stages.
In the first stage, the two review authors (IMG, RL) independently examined the abstracts of all studies arising from the literature search and voted 'Yes', 'No', or 'Maybe' using the Covidence blinded voting system. We excluded studies which were clearly ineligible (e.g. in vitro studies, animal studies, studies in children, case reports) at this stage.
In the second stage, the same two review authors (IMG and RL) independently examined the full‐text version of the studies selected in the first stage, and, where applicable, completed the study selection form for each study to determine its eligibility (Appendix 2). We resolved any conflicts identified by Covidence by discussion between both authors. At this stage in the screening process, any studies found by both authors (IMG and RL) to be eligible for inclusion, proceeded to the data extraction stage as described in the next section (Data extraction and management), while those found to be ineligible for inclusion were listed in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (IMG and RL) independently extracted data from the studies selected above using a comprehensive data extraction form (Appendix 3). We resolved any conflicts by discussion between both authors.
Assessment of risk of bias in included studies
Two authors (IMG, RL), independently assessed the risk of bias in included studies using Cochrane's tool for assessing risk of bias as described in the Cochrane Handbook of Systematic Reviews for Interventions (Higgins 2011). We resolved any discrepancies by discussion. For each included primary study, we assessed bias in the following seven domains:
random sequence generation;
allocation concealment;
performance bias;
detection bias;
attrition bias;
reporting bias; and
other bias.
For each domain, we determined the risk of bias as low, unclear or high, according to methods used to ensure the minimization of each form of bias. In general, we categorized the level of risk of bias as follows. See 'Sensitivity analysis' for further information on the approach used for making overall risk of bias judgements.
Low risk: where information was available that clearly demonstrated that efforts were made to ensure minimal bias in that domain and the described methods were robust enough to have a high likelihood of being effective.
Unclear risk: when the information available was insufficient to be confident that the method used to minimize bias was robust enough to be effective.
High risk: when the study did not report any method to minimize bias in that domain.
We choose to include unblinded, single‐blinded and double‐blinded studies in this review to provide a comprehensive summary of the overall available evidence. In doing so, we accepted a greater level of overall 'performance' bias than if we had included only double‐blinded studies.
Measures of treatment effect
We measured pooled estimates of effect for primary and secondary outcomes, providing these were reported at the relevant time points by two or more studies of any eligible intervention.
Where no more than one study of an eligible intervention reported an outcome of interest, we did not calculate a pooled estimate of effect.
For continuous outcomes, we calculated the mean differences (MDs), and standardized mean differences (SMDs), where studies used the same and different scales of measurement, respectively. For the primary outcome of pain intensity, we rescaled any pain scores that were reported on a 0 to 100 scale to a 0 to 10 scale and for studies where standardized mean differences were used to calculate pain outcomes, we re‐expressed these as mean differences using the methods described in section 12 of the Cochrane Handbook (Higgins 2011). For the secondary outcome of additional analgesia requirement, we calculated MDs for analyses in which all included studies used additional analgesics solely in either milligram or microgram amounts, and we calculated SMDs for analyses including studies which used additional analgesics in both milligram and microgram amounts. Where standardized mean differences were used to measure additional analgesia requirements, we used Cohen's rule of thumb to provide an indication of effect size, where a standard mean difference of 0.2 to 0.49 represents a small effect size, 0.5 to 0.79, a moderate effect size and 0.8 or greater, a large effect size. (Higgins 2011).
For dichotomous outcomes, we calculated the risk ratios (RRs).
We presented all pooled estimates of effect with their respective P values and 95% confidence intervals (CIs).
Unit of analysis issues
Due to the high possibility of carry‐over effects, we did not include cross‐over studies in this review. For studies with more than one treatment arm, we included only the relevant arms, i.e. arms where a pharmacological analgesic intervention was assessed for its efficacy in terms of prevention of acute postoperative pain after brain surgery. Where more than one treatment arm in any one trial was eligible for inclusion in the same meta‐analysis, then we divided the control group equally between the two arms, to avoid double counting. For example, for a trial evaluating 'Drug A' versus placebo versus 'Drug B' versus placebo for the prevention of acute postoperative pain after craniotomy, we divided the placebo control group equally between the group assigned to 'Drug A' and the group assigned to 'Drug B' for the meta‐analysis of each outcome for which both groups were eligible.
Dealing with missing data
We handled missing data as follows:
Missing pain intensity outcome data
For missing pain scores, we planned to impute missing data using the last observation carried forward method. No missing pain scores were identified (among studies eligible for inclusion in the meta‐analysis for this outcome), so we did not have to employ this technique. However, since the vast majority of studies reporting pain intensity outcomes, reported these at discrete time points as opposed to over time periods, we amended the way in which we analysed and reported the primary outcome 'pain intensity', to reflect the time points reported in the included studies. Further details of this amendment and its rationale are provided in the sections Types of outcome measures and Differences between protocol and review. The timing of 'pain intensity' measurements were not reported by two studies (which would otherwise have been eligible for inclusion in the meta‐analysis for this outcome) (Rahimi 2006; Rahimi 2010). We excluded these two studies from the main comparison for this outcome as it was impossible to determine with any certainty when these measurements were made. No absolute values for pain scores were reported by another study (Ryan 2005), and so we excluded this study from the analysis as there were no data to base any imputed value on.
Missing additional analgesia consumption data
We excluded one study, which would otherwise have been eligible for inclusion in the meta‐analysis for this outcome, as the time period over which the outcome was measured was not reported (Rahimi 2006).
Missing sedation scores
For missing sedation scores, we planned to impute missing data using the last observation carried forward method. Sedation scores were not widely measured in the included studies and, where absolute values were not reported, the authors did report that no significant difference was observed, therefore no imputation was required.
Missing adverse event data
For missing adverse event outcome data, we planned to analyse the data based on a worst and best case scenario (and present both analyses in the review), assuming that all and none of those whose data were missing developed the adverse event in question. No missing results for reported adverse events were identified. Some studies did not report absolute values for certain adverse events but reported that no significant difference was observed (Batoz 2009; Dilmen 2016; Jones 2009; Rigamonti 2013). These studies were not included in the calculation of the pooled estimate of effect for this outcome.
Missing standard deviations
Where standard deviations (SDs) were missing, they were calculated, where possible, from CIs and standard errors (SEs) as described in the Cochrane Handbook of Systematic Reviews for Interventions (Higgins 2011). Where P values only were reported, standard deviations were calculated using the method described in the Cochrane Handbook of Systematic Reviews for Interventions section 7.7.3.3 (Higgins 2011). Where none of the above data were available, standard deviations were imputed using the mean of SDs for the same continuous outcome measure, measured at the same time point for studies of the same intervention in this review.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the following factors between studies: participants, setting, surgical techniques, intervention types, timing and dosages, outcomes assessed and ancillary treatments.
We assessed methodological heterogeneity by comparing the risk of bias in the included studies.
We assessed statistical heterogeneity by visual inspection of forest plots, the Chi 2 test, and calculation of the I2 statistic. We considered a P value < 0.1 in the Chi 2 test and an I2 statistic > 50% as indicative of significant statistical heterogeneity.
Assessment of reporting biases
To determine the presence or absence of reporting bias, we planned to examine funnel plots for each meta‐analysis that included 10 or more studies to determine the degree of symmetry. However, no meta‐analysis in this review included 10 or more studies. As the majority of studies eligible for inclusion in this review were small studies (typically including fewer than 100 participants), we cannot be confident that publication bias was insignificant. However, by conducting a robust and comprehensive search for all eligible studies and by applying no language restrictions, we hope to have reduced the likelihood of not including studies whose results were not reported in the mainstream literature.
Data synthesis
We calculated pooled estimates of effect for the above outcomes and subgroups if all of the following conditions were met:
absence of substantial clinical or methodological heterogeneity between included studies;
inclusion of at least two eligible studies deemed to have either a low or unclear risk of bias;
absence of substantial publication bias.
Where significant statistical heterogeneity was present, we presented the pooled estimate of effect with subsequent discussion as to the likely impact of heterogeneity on the accuracy and quality of the estimate in the 'quality of evidence' section (Quality of the evidence).
We performed meta‐analysis using Cochrane statistical software, (Review Manager 2014). We used a random‐effects model to best represent the differences in treatment effects across studies (Results).
Subgroup analysis and investigation of heterogeneity
Where sufficient numbers of eligible studies (two or more) reporting relevant data were identified, we planned to perform the following subgroup analyses to determine the efficacy and safety of each evaluated intervention.
Participants undergoing infratentorial versus supratentorial craniotomy. The basis for this subgroup analysis was that the infratentorial approach is associated with higher postoperative pain scores (De Gray 2005).
Participants in whom the intervention was administered pre or intraoperatively versus postoperatively based on the rationale that early administration of analgesia may have greater preventative potential.
Participants who received inhalational versus total intravenous anaesthesia based on current controversy regarding the benefit of one form of anaesthesia over the other with some evidence showing a higher intensity of post‐craniotomy pain in those who received inhalation anaesthesia (Mordhorst 2009), while other evidence suggesting no significant difference (Prabhakar 2016).
Participants who received steroids in the perioperative period based on evidence showing that the intraoperative administration of steroids reduced pain intensity after craniotomy (Mordhorst 2009).
Sensitivity analysis
Where appropriate, we performed the following sensitivity analyses:
Analysis excluding trials with a high risk of bias. A study was judged to have an overall high risk of bias if it had a high risk of bias in four or more of the seven domains of bias or a high risk of bias in three or more of the seven domains of bias with an unclear risk of bias in one or more domain. The rationale for choosing these criteria were based on author agreement that high or high and unclear risks of bias across multiple domains raised doubt about the overall methodological rigour of that study and was likely a reasonable way to determine an overall high risk of bias in a consistent manner for studies in a review of interventions that were given at different times relative to surgery and anaesthesia and by different routes of administration.
Analysis excluding studies with missing data considered to be missing for reasons likely related to either the intervention or outcomes studied.
'Summary of findings' table and GRADE
We constructed a 'Summary of findings' table for each evaluated pharmacological intervention:
NSAIDs (Table 1);
dexmedetomidine (Table 2);
gabapentin or pregabalin (Table 3);
acetaminophen (Table 4);
scalp infiltration (Table 5); and
scalp blocks (Table 6);
using GRADEpro GDT. For each comparison, we used the principles of the GRADE system to assess the quality of the body of evidence associated with the following outcomes (Guyatt 2008):
acute postoperative pain intensity during the first six hours;
acute postoperative pain intensity at 12 hours;
acute postoperative pain intensity at 24 hours;
acute postoperative pain intensity at 48 hours;
additional analgesia requirement from 0 to 24 hours postoperatively;
adverse events.
We took the following factors into account when evaluating the quality of the evidence: risk of bias among studies contributing to each outcome measure, inconsistency, imprecision, indirectness of results and the likelihood of publication bias.
We used the criteria below to downgrade evidence for a particular outcome:
no serious limitation in any of the above factors: evidence was not downgraded;
serious limitation in any of the above factors: evidence was downgraded by one point;
serious limitation in two or more of the above factors or very serious limitation in any one of the above factors: evidence was downgraded by two points.
One review author (IG) initially applied the GRADE system and then discussed the quality of evidence ratings for each outcome with a second author (RL). We reached final decisions on the ratings through discussion and consensus. It is important to note that the choice of outcomes included in our 'Summary of Findings' tables differed from those stated in our original protocol (Galvin 2015). The differences and the rationale for these differences are explained in the Differences between protocol and review.
Results
Description of studies
Results of the search
The initial search conducted on 12 September 2016 returned 1149 articles and another 4 articles were identified from searching the reference list of other published reviews. An updated search conducted on 24 October 2017 identified a further 62 articles and a further updated search on 28 November 2018 identified 502 articles making a total of 1717 articles. The identification and selection of eligible studies are described in the next paragraph and detailed in Figure 1.
1.
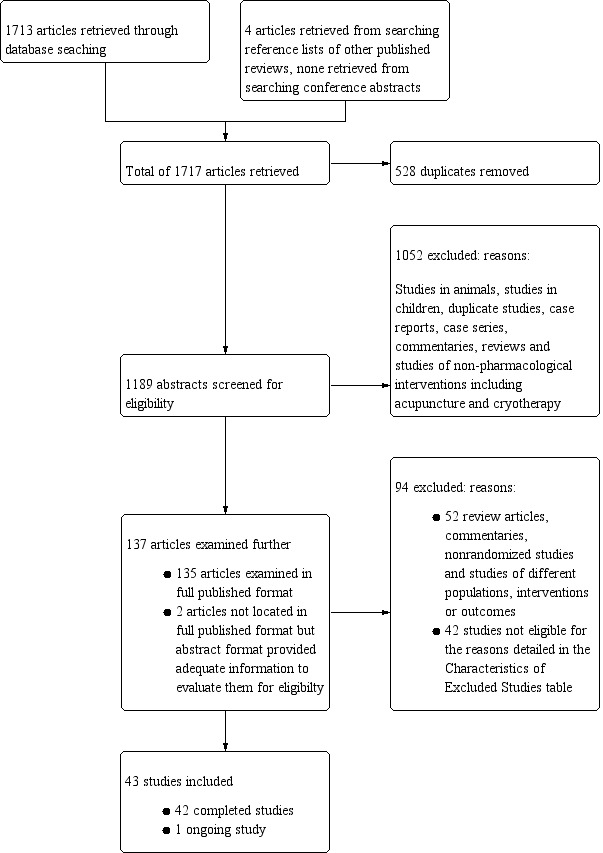
Search results
Of the1717 articles retrieved: 528 were found to be duplicates, leaving 1189 articles, of which 1052 were excluded on initial screening. Excluded studies at this stage included animal studies, studies conducted in those < 18 years of age, case reports, commentaries, narrative reviews, duplicate reports etc.
Of the remaining 137 articles: 135 were available in full‐text format and 2 were only available in abstract format but there was still enough information in the abstract to evaluate them for eligibility. We excluded 94 of these 137 articles, 52 having been found to be either review articles, commentaries or non‐randomized studies and 42 having been found to be studies which were ineligible for the reasons detailed in the Characteristics of excluded studies table. Of the 43 eligible studies we found, 42 of these were completed studies and 1 was an ongoing study (see: Characteristics of ongoing studies).
Included studies
A summary of the included studies is provided in the characteristics of included studies table (Characteristics of included studies).
Participants
All participants were adults (≥ 18 years of age), ASA classification I to ASA classification III who were undergoing elective craniotomy. Not all studies provided details of the indication for surgery but among those that did, the commonest indication was for resection of intracranial tumour. The vast majority of completed studies included only participants who were undergoing supratentorial craniotomy, 14 studies either did not specify surgical approach or included participants undergoing either supra or infratentorial craniotomy (Batoz 2009; Bekker 2008; Can 2017; Choi 2009; Cokay 2013; Greenberg 2017; Jones 2009; Misra 2013; Molnár 2015; Rahimi 2010; Ryan 2005; Shimony 2016; Sivakumar 2018; Yardav 2014), and three studies included only those undergoing infratentorial surgery (Akcil 2017; Jellish 2006; Zeng 2019). Common exclusion criteria included an inability to understand the pain scoring system, decreased level of consciousness, known or suspected allergies to study medications, previous scalp incisions and pre‐existing long‐term opioid usage or chronic pain.
Interventions
Scalp infiltration
Ten studies examined the efficacy of infiltration versus saline placebo or no intervention in preventing pain after craniotomy (Akcil 2017; Batoz 2009; Biswaz 2003; Bloomfield 1998; El‐Dawlatly 2007; Kiskira 2006; Law‐Koune 2005; Saringcarinkul 2015; Zhang 2003; Zhou 2016). The local anaesthetics used to infiltrate around the surgical wound site included: bupivacaine 0.5% (Akcil 2017), bupivacaine 0.25% (Biswaz 2003; El‐Dawlatly 2007), bupivacaine 0.25% with added epinephrine (Bloomfield 1998; Kiskira 2006), bupivacaine 0.375% with added epinephrine (Law‐Koune 2005), bupivacaine 0.5% with added epinephrine (Saringcarinkul 2015), ropivacaine 0.75% (Batoz 2009; Zhang 2003), and ropivacaine 0.5% (Zhou 2016).
In four studies, scalp infiltration was performed before surgical incision (Akcil 2017; Biswaz 2003; El‐Dawlatly 2007; Zhou 2016). In four, it was performed at the end of surgery before skin closure (Batoz 2009; Law‐Koune 2005; Saringcarinkul 2015; Zhang 2003), and in two, it was performed pre‐ and again post‐incision (Bloomfield 1998; Kiskira 2006).
Scalp block
Twelve included studies examined scalp block versus either saline placebo or no intervention (Akcil 2017; Bala 2006; Can 2017; Choi 2009; Cokay 2013; Ganzoni 2008; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Rigamonti 2013; Tucinda 2010; Zhang 2003). Local anaesthetics used included bupivacaine 0.25% with added epinephrine (Hernández Palazón 2007; Tucinda 2010), bupivacaine 0.5% without added epinephrine (Akcil 2017; Can 2017; Cokay 2013; Rigamonti 2013), and with added epinephrine (Bala 2006; Tucinda 2010), bupivacaine 0.75% with added epinephrine (Hwang 2015), ropivacaine 0.5% (Ganzoni 2008), ropivacaine 0.75% ( Choi 2009; Nguygen 2001; Zhang 2003), and levo‐bupivacaine 0.5% (Can 2017) .
Ten studies provided details of the nerves blocked and in these studies the nerves targeted were the supraorbital, supratrochlear, zygomaticotemporal, auriculotemporal, postauricular branches of the greater auricular nerves and the greater, lesser and third occipital nerves (Akcil 2017; Bala 2006; Can 2017; Choi 2009; Ganzoni 2008; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Rigamonti 2013; Tucinda 2010). Two studies did not provide specific details of the nerves blocked (Cokay 2013; Zhang 2003).
In six studies, scalp block was performed before surgical incision (Akcil 2017; Can 2017; Cokay 2013; Ganzoni 2008; Rigamonti 2013; Tucinda 2010), and in six, it was performed at the end of surgery (Bala 2006; Choi 2009; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Zhang 2003).
Pregabalin or Gabapentin
Two studies addressed gabapentin (Misra 2013: Zeng 2019), and one addressed pregabalin (Shimony 2016). The dosages of gabapentin used were 600 mg given the night before and again on the morning of surgery (Zeng 2019), and 600 mg given two hours prior to surgery (Misra 2013). The dosage of pregabalin was 150 mg given the evening prior to surgery, 90 minutes before surgery, two hours after surgery and every 12 hours thereafter until 72 hours postoperatively (Shimony 2016).
NSAIDs
Eight studies addressed the role of NSAIDs in this population (Dilmen 2016; Jones 2009; Molnár 2015; Rahimi 2006; Ryan 2005; Shepherd 2018; Willams 2011; Yardav 2014).
The agents studied included:
parecoxib 40 mg versus saline placebo, given orally at dural closure (Jones 2009; Willams 2011). In the study by Williams and colleagues, all participants in both the intervention and control group received scalp infiltration as well (Willams 2011);
rofecoxib 50 mg versus placebo, given orally one hour before surgery (Ryan 2005);
COX 2 Inhibitor 25 mg orally twice daily started postoperatively. No placebo medication was used in the control group (Rahimi 2006);
diclofenac 50 mg orally versus placebo, every eight hours from the second postoperative day, until 48 hours postoperatively (Yardav 2014);
diclofenac 100 mg orally one hour before surgery. No placebo medication was used in the control group (Molnár 2015);
dexketoprofen 50 mg intravenously versus placebo, given at skin closure and every 8 hours thereafter (Dilmen 2016);
metamizole 1 gram intravenously versus placebo, given at skin closure and every six hours thereafter (Dilmen 2016);
ibuprofen 800 mg intravenously every 8 hours with the first dose given intraoperatively (Shepherd 2018).
Opioids
Two studies addressed the role of opioids in this context (Jellish 2006; Rahimi 2010). Jellish and colleagues looked at the role of morphine patient‐controlled analgesia with or without added ondansetron versus placebo in reducing the incidence of pain in those undergoing skull base surgery (Jellish 2006). Rahimi and colleagues addressed the role of tramadol versus no tramadol in reducing the intensity of pain after elective craniotomy for vascular lesions, tumour resection or epilepsy surgery (Rahimi 2010).
Dexmedetomidine
Four studies looked at dexmedetomidine versus placebo (Bekker 2008; Peng 2015; Song 2016; Yun 2016). Intravenous infusion dosages ranged from 0.5 to 0.8 mcg/kg/hr with one study including a 1 mcg/kg bolus loading dose (Bekker 2008). The timing and duration of infusions varied from after induction of anaesthesia until the start of skin closure (Bekker 2008; Peng 2015; Song 2016), to a brief infusion for 10 minutes, one hour before surgery ended (Bekker 2008).
Acetaminophen
Four studies addressed acetaminophen versus placebo (Artime 2018; Dilmen 2016; Greenberg 2017; Sivakumar 2018). The dosage used in all studies was one gram given intravenously. In Artime 2018, the first dose was given before skin incision. In the other three studies, it was given after surgery (Dilmen 2016; Greenberg 2017; Sivakumar 2018). In Sivakumar 2018, it was repeated every 8 hours postoperatively for a total of 24 hours. In Dilmen 2016, it was repeated every six hours postoperatively. In Greenberg 2017, it was repeated every 6 hours until 18 hours after surgery, and in Artime 2018, it was repeated every 6 hours postoperatively for a total of 24 hours.
Lidocaine
Only one study addressed intravenous lidocaine infusion versus placebo as a potential agent in the prevention of postoperative pain in this population (Peng 2016). A bolus of 1.5 mg/kg was given after induction of anaesthesia, followed by an infusion of 2 mcg/kg/hr.
Flupirtine
One study addressed this medication versus placebo (Yardav 2014). The dose used was 100 mg and it was given orally every eight hours from the second postoperative day until 48 hours postoperatively.
Sphenopalatine ganglion blocks
There was one eligible study of the role of sphenopalatine ganglion blockade versus placebo in reducing postoperative pain in those undergoing endoscopic transnasal resection of pituitary tumours (Ali 2010). Bilateral blocks were performed with 0.5% bupivacaine after induction of anaesthesia.
Outcomes
Acute postoperative pain intensity
Forty studies measured postoperative pain intensity. The 10 cm (100 mm) visual analogue scale was the most commonly used tool to measure pain intensity, being used in 30 studies (Akcil 2017; Ali 2010; Artime 2018; Batoz 2009; Biswaz 2003; Bloomfield 1998; Can 2017; Choi 2009; Cokay 2013; Dilmen 2016; El‐Dawlatly 2007; Ganzoni 2008; Greenberg 2017; Hernández Palazón 2007; Jones 2009; Kiskira 2006; Law‐Koune 2005; Molnár 2015; Nguygen 2001; Rahimi 2006; Rahimi 2010; Rigamonti 2013; Ryan 2005; Shepherd 2018; Sivakumar 2018; Tucinda 2010; Yardav 2014; Zeng 2019; Zhang 2003). The 0 to 10 or 0 to 100 numerical rating scale was used in nine studies (Bala 2006; Hwang 2015; Jellish 2006; Peng 2015; Peng 2016; Saringcarinkul 2015; Shimony 2016; Song 2016; Willams 2011), with one study using a pain rating between 0 and 3 (Misra 2013).
Seven studies measured pain beyond 48 hours postoperatively (Batoz 2009; Hwang 2015; Misra 2013; Molnár 2015; Rigamonti 2013; Shimony 2016; Zhou 2016). Of these, there were only four studies that measured pain anytime between 48 hours and one month postoperatively (Hwang 2015; Misra 2013; Molnár 2015; Rigamonti 2013).
Two studies reported no timing of their pain intensity measurements (Rahimi 2006; Rahimi 2010)
While most studies that measured pain intensity reported this outcome in terms of absolute numbers, four did not (Cokay 2013; Misra 2013; Peng 2016; Ryan 2005); of these, pain intensity was either reported as being above or below a threshold value (Misra 2013; Peng 2016), in terms of the overall statistical significance of the results (Cokay 2013), or not reported at all (Ryan 2005).
Analgesic success
This outcome was not widely reported with only six studies measuring it (Bala 2006; Jellish 2006; Misra 2013; Molnár 2015; Peng 2016; Saringcarinkul 2015). it was reported as numbers free of pain or with no worse than mild pain at various time points or it was possible to calculate from reports of those experiencing moderate or severe pain.
Additional analgesic requirements
Thirty‐one studies reported this outcome (Akcil 2017; Artime 2018; Batoz 2009; Biswaz 2003; Can 2017; Choi 2009; Dilmen 2016; Ganzoni 2008; Greenberg 2017; Hernández Palazón 2007; Hwang 2015; Jellish 2006; Jones 2009; Kiskira 2006; Law‐Koune 2005; Misra 2013; Nguygen 2001; Peng 2015; Rahimi 2006; Rahimi 2010; Rigamonti 2013; Ryan 2005; Saringcarinkul 2015; Shepherd 2018; Shimony 2016; Sivakumar 2018; Song 2016; Tucinda 2010; Willams 2011; Zeng 2019; Zhou 2016). All of these studies measured additional analgesia consumption in terms of quantity of analgesic required, with the exception of one study which measured it in terms of the number of patients requiring additional analgesia (Can 2017). Of rescue analgesic consumption, opioids were the most commonly measured agents including morphine, hydromorphone, fentanyl, tramadol and nalbuphine. Non‐opioid analgesics used included acetaminophen and diclofenac.
Sedation
Sixteen studies measured the level of postoperative sedation (Artime 2018; Batoz 2009; Greenberg 2017; Hernández Palazón 2007; Hwang 2015; Jones 2009; Law‐Koune 2005; Peng 2015; Saringcarinkul 2015; Shepherd 2018; Song 2016; Tucinda 2010; Willams 2011; Yardav 2014; Zeng 2019; Zhou 2016). The scales and methods used to measure sedation varied with four studies using the Ramsey sedation scale (Greenberg 2017; Peng 2015; Yardav 2014; Zeng 2019), with the remainder using either 4 or 5‐point scales or patient‐reported levels of drowsiness.
Chronic headache
This outcome was reported by only three studies with much variation in the time points used. It was measured as persistent pain at three months by one study (Shimony 2016), and persistent pain at two months by two studies (Batoz 2009; Rigamonti 2013).
Length of stay in critical care or hospital
Two studies measured length of stay in critical care (Greenberg 2017; Sivakumar 2018), but only one reported their results (Greenberg 2017). Six studies measured length of stay in hospital (Greenberg 2017; Rahimi 2006; Rahimi 2010; Shepherd 2018; Shimony 2016; Sivakumar 2018).
Adverse events
The commonest adverse event measured was the incidence of nausea and vomiting, being reported by 25 studies (Akcil 2017; Ali 2010; Artime 2018; Batoz 2009; Can 2017; Dilmen 2016; El‐Dawlatly 2007; Ganzoni 2008; Hernández Palazón 2007; Hwang 2015; Jellish 2006; Jones 2009; Law‐Koune 2005; Misra 2013; Peng 2015; Rigamonti 2013; Saringcarinkul 2015; Shimony 2016; Song 2016; Tucinda 2010; Willams 2011; Yardav 2014; Yun 2016; Zhou 2016; Zeng 2019). Other less commonly measured adverse events measured included hypotension, hypertension, bleeding, delirium, visual disturbances, agitation, respiratory depression, pruritis, diarrhoea and constipation. Few studies provided definitions for adverse events or measures of their severity.
Subgroups
Infratentorial versus supratentorial craniotomy
Only one eligible study (Molnár 2015), analysed pain outcomes separately in those undergoing supra versus infratentorial craniotomy and only one measured pain outcomes in those undergoing supratentorial versus supra and infratentorial craniotomy (Greenberg 2017).
Intervention timing
Of the 42 included completed studies, 21 commenced or completed the intervention before skin incision (Akcil 2017; Ali 2010; Artime 2018; Biswaz 2003; Bloomfield 1998; Can 2017; Cokay 2013; El‐Dawlatly 2007; Ganzoni 2008; Misra 2013; Molnár 2015; Peng 2015; Peng 2016; Rigamonti 2013; Ryan 2005; Saringcarinkul 2015; Shimony 2016; Song 2016; Tucinda 2010; Yun 2016; Zeng 2019), and 21 after skin incision (Bala 2006; Batoz 2009; Bekker 2008; Choi 2009; Dilmen 2016; Greenberg 2017; Hernández Palazón 2007; Hwang 2015; Jellish 2006; Jones 2009; Kiskira 2006; Law‐Koune 2005; Nguygen 2001; Rahimi 2006; Rahimi 2010; Shepherd 2018; Sivakumar 2018; Willams 2011; Yardav 2014; Zhang 2003; Zhou 2016).
Inhalation versus total intravenous anaesthesia
Only four studies of four different interventions used an exclusively total intravenous anaesthetic technique (Batoz 2009; Can 2017; Song 2016; Willams 2011).
Preoperative steroids
Only two studies of two different interventions included participants who had received preoperative steroids (Bloomfield 1998; Misra 2013).
Excluded studies
We excluded 42 studies.
The reasons for exclusion were as follows:
no postoperative pain outcome: three studies (Bajaj 2017; Bishnoi 2016; Doumiri 2015). These studies used agents that can also be used for analgesia i.e. clonidine, dexmedetomidine and lidocaine, however, the focus of these studies was on their efficacy for nonanalgesic outcomes including operating conditions and intraoperative haemodynamics. Although these agents have analgesic potential, these studies were excluded on the basis that the agents investigated were not used with analgesic intent or investigated for their analgesic potential or side effect profile in the context of use as analgesic agents.
no distinction between intraoperative and postoperative pain outcomes: one study (Soliman 2011);
no control group: 32 studies (Ackil 2018; Ayoub 2006; Citerio 2012; Domenech 2006; Dudko 2014; El Dahab 2009; Ferber 2000; Girard 2010; Goldsack 1996; Graham 1999; Hassani 2015; Honnma 2002; Imaev 2008; Imaev 2010; Jayaram 2016; Jeffrey 1999; Jose 2017; Luo 2014; Mohamed 2018; Morad 2009; Na 2011; Palazón 2006; Rajan 2016; Reddy 2018; Simon 2012; Stoneham 1996; Sudheer 2007; Tanskanen 1999; Theerth 2018; Ture 2009; Vallapu 2018; Verchere 2002);
different patient populations studied: four studies (Lu 2009; Venkatraghavan 2016; Wu 2014; Zhao 2013);
cross‐over trial (Stone 2018).
Of the 42 excluded studies, most were published in English, one was published in French (Doumiri 2015), two were published in Chinese (Lu 2009; Luo 2014), one in Japanese (Honnma 2002), one in Spanish (Palazón 2006), and one in Russian (Imaev 2010).
Forty‐one of the excluded studies had been completed, and one was an ongoing study (Wu 2014).
Details are provided in the Characteristics of excluded studies table.
Ongoing studies
We identified one ongoing study ( KCT0000274). Details of this study are provided in the table, Characteristics of ongoing studies.
Studies awaiting classification
There are no studies awaiting classification.
Risk of bias in included studies
For each included study, a detailed 'Risk of bias' assessment is provided in the Characteristics of Included Studies table (Characteristics of included studies). A summary of the risk of bias among included studies is provided in (Figure 2), and a graphical representation of overall risk of bias in each domain for all included studies is provided in (Figure 3).
2.
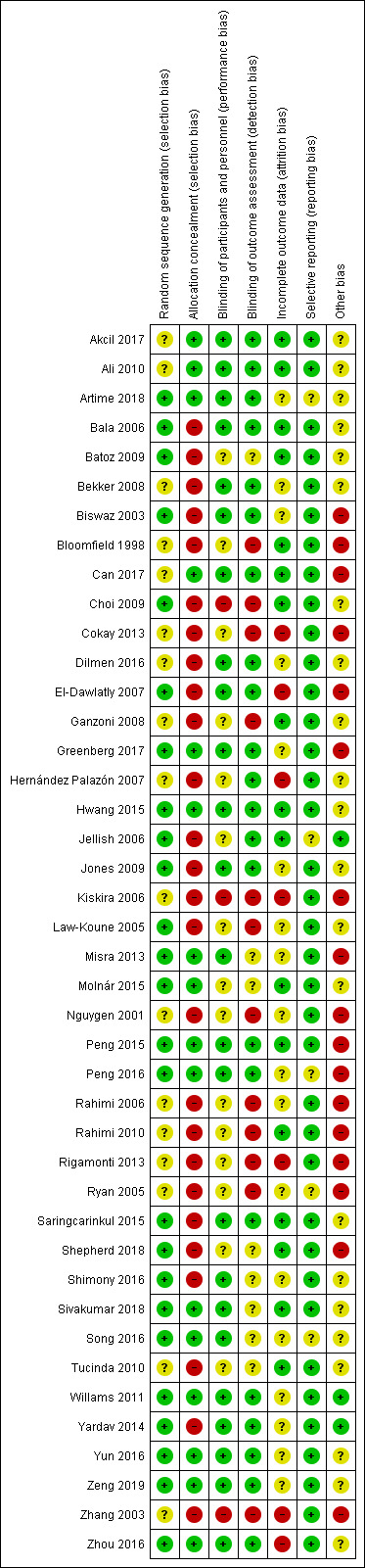
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
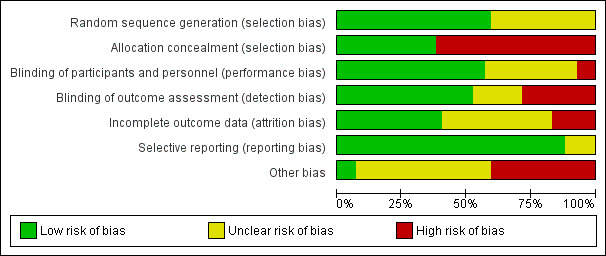
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Of the 42 completed studies included in the review, 25 were deemed to have a low risk, 17 were deemed to have an unclear risk and none were deemed to have a high risk of bias in this domain. Where authors reported the trial as randomized and provided detail regarding the method of randomization used, the study was judged to have a low risk of bias in that domain. Where authors reported the trial as randomized but did not describe the method of randomization used, the study was judged to have an unclear risk of bias in that domain.
Allocation concealment
Sixteen of the 42 completed studies which were included in the review were judged to have a low risk of bias in this domain, the remaining 26 being judged to have a high risk of bias. Studies assigned to the low risk category included those that provided details of the methods used to prevent those enrolling participants from guessing upcoming assignments; these methods included the use of sealed envelopes to conceal the treatment allocation (Akcil 2017; Ali 2010; Can 2017; Greenberg 2017; Misra 2013; Molnár 2015; Willams 2011; Yun 2016; Zhao 2013), and the use of coded vials which were assigned according to the randomization table (Peng 2016). A low risk judgement was also given to those studies reporting the performance of randomization, and study drug preparation by personnel who were not involved in treatment allocation (Hwang 2015; Peng 2015; Sivakumar 2018; Song 2016). While this method alone is not guarantee that those assigning the treatments were always unaware of the upcoming assignment, it does imply that methods were taken to conceal both the randomization sequence and the treatment being allocated. A high risk judgement was applied to all studies which did not describe any method of allocation concealment, or described a method that allowed those assigning interventions to predict which participant would receive which intervention.
Blinding
Performance bias
Blinding of participants and study personnel to the treatment administered was judged separately.
Blinding of participants
For the subjective outcome of pain, blinding of participants to treatment received is vital to validity. Of the 17 included completed studies which provided either 'no' or an 'inadequate' description of the methods used to blind participants, in 15 of these studies, the impact on validity was lessened significantly by the use of placebo medications or interventions or the intraoperative timing of the procedure, or both (Bekker 2008; Choi 2009; Cokay 2013; Ganzoni 2008; Hernández Palazón 2007; Jellish 2006; Kiskira 2006; Law‐Koune 2005; Nguygen 2001; Rigamonti 2013; Ryan 2005; Shepherd 2018; Tucinda 2010; Zhang 2003). Three studies that did not use placebo medications and did not administer the study medication under anaesthesia, reported that they were blinded but provided inadequate details about the blinding method used. (Molnár 2015; Rahimi 2006; Rahimi 2010), These studies were judged to have an unclear risk of bias in this domain due to the possibility that patients may have reported pain outcomes differently based on the knowledge that they had or had not received the active treatment.
Blinding of study personnel
Of the 42 completed included studies, nine provided either 'no' or 'inadequate' details of the methods used to blind those administering scalp infiltration or scalp block (Batoz 2009; Choi 2009; Cokay 2013; Ganzoni 2008; Hernández Palazón 2007; Rigamonti 2013; Shepherd 2018; Tucinda 2010; Zhang 2003). While inadequate blinding of those administering pain medications or interventions is unlikely to have a significant effect on the way patients report pain outcomes or the way in which additional analgesic consumption is measured, it may have implications for the adequacy of performance of a scalp block or scalp infiltration even if a saline placebo is used. An operator who knows that they are blocking nerves or infiltrating the scalp with an inert saline placebo may be less rigorous in their attention to detail than one who knows that they are using an active medication, with important implications for intervention efficacy and study validity.
Detection bias
Twenty of the 42 included studies provided either 'no' or 'inadequate' details regarding how those assessing pain outcomes were blinded to treatment received (Batoz 2009; Bloomfield 1998; Choi 2009; Cokay 2013; Ganzoni 2008; Kiskira 2006; Law‐Koune 2005; Misra 2013; Molnár 2015; Nguygen 2001; Rahimi 2006; Rahimi 2010; Rigamonti 2013; Ryan 2005; Shepherd 2018; Shimony 2016; Sivakumar 2018; Song 2016; Tucinda 2010; Zhang 2003). For the outcomes of patient‐rated pain using validated measuring tools, this is unlikely to have had a serious impact on the effect estimates. Similarly, for defined adverse events, length of stay in hospital and incidence of patient‐reported chronic headache, the impact of this bias is likely to be minimal.
Incomplete outcome data
Bias due to incomplete outcome data was judged to be 'serious' for seven studies (Cokay 2013; El‐Dawlatly 2007; Hernández Palazón 2007; Kiskira 2006; Rigamonti 2013; Zhang 2003; Zhou 2016). For six of the studies, the reason for this judgement was on the basis of a lack of information in the study reports regarding numbers followed up and numbers included in the final analysis (Cokay 2013; El‐Dawlatly 2007; Hernández Palazón 2007; Kiskira 2006; Rigamonti 2013; Zhang 2003), while for one (Zhao 2013), it was due to a very high proportion (31%) of enrolled participants being lost to follow‐up for the primary outcome of postoperative analgesic consumption, without the subsequent performance of an intention‐to‐treat analysis.
A large number of studies (17) were judged to be at unclear risk of attrition bias due to losses to follow‐up of up to 22% of enrolled participants without a subsequent intention‐to‐treat analysis (Artime 2018; Bekker 2008; Biswaz 2003; Dilmen 2016; Greenberg 2017; Jones 2009; Law‐Koune 2005; Misra 2013; Nguygen 2001; Peng 2016; Rahimi 2010; Ryan 2005; Song 2016; Willams 2011; Yardav 2014; Yun 2016; Zeng 2019). In only one study, in which 12 of the recruited participants were lost to follow‐up, was an intention‐to‐treat analysis conducted (Shimony 2016). Often no reasons were provided for losses to follow‐up but, where they were provided, they most commonly included the need for ongoing postoperative intubation and inability to communicate after surgery due to reduced level of consciousness. While these postoperative problems are common in patients undergoing brain surgery and while assessing patient‐reported pain in these circumstances is virtually impossible, the exclusion of these participants from the analysis, makes it difficult to judge the efficacy of any pain‐preventing intervention accurately.
Selective reporting
Five of the 42 included completed studies were judged to be at 'unclear risk' of selective reporting bias (Artime 2018; Jellish 2006; Peng 2016; Ryan 2005; Song 2016) .
The reasons for an unclear risk rating included lack of clarity regarding outcome priorities, with two studies failing to define which of their reported outcomes were primary and which were secondary (Peng 2016; Song 2016). In Song 2016, four outcomes were reported (postoperative pain, morphine consumption, sedation scores and adverse events), however, the authors did not report which outcome was primary although their sample size calculation implied that it was' morphine consumption'. In Peng 2016, several outcomes were reported (differences in physiological parameters, pain scores, dysphoria, nausea and vomiting), again with no definition of which outcome was primary and unfortunately no sample size calculation to assist the author in determining what the primary outcome may have been.
An unclear risk rating was also applied to a study which provided no absolute figures for its primary outcome of 'postoperative pain', reporting only those of its secondary outcome of 'morphine consumption' and to studies that gave greater priority in their reports to statistically significant secondary outcomes rather than statistically insignificant primary outcomes (Artime 2018; Jellish 2006; Ryan 2005).
The remaining studies were judged to be at low risk as outcomes were reported in the order specified in the methods section of their reports, so overall, bias due to selective reporting of outcomes was unlikely to have had a significant effect on the findings of this review.
Other potential sources of bias
Twenty‐three studies were judged to be at unclear risk of other sources of bias. These included small studies with total enrolled numbers of fewer than 100 participants, studies that did not prespecify their subgroup analyses, studies that did not achieve target sample size, studies that did not adequately adjust for multiple data testing and those funded by pharmaceutical companies (Akcil 2017; Ali 2010; Artime 2018; Bala 2006; Batoz 2009; Bekker 2008; Choi 2009; Dilmen 2016; Ganzoni 2008; Hernández Palazón 2007; Hwang 2015; Jones 2009; Law‐Koune 2005; Molnár 2015; Saringcarinkul 2015; Shepherd 2018; Shimony 2016; Sivakumar 2018; Song 2016; Tucinda 2010; Yun 2016; Zeng 2019; Zhou 2016).
Sixteen studies were judged to be at high risk of other sources of bias. These included studies reported in abstract format only where there was an overall lack of information regarding methods and analysis, making it difficult for the reader to judge the rigour of their methodology (Cokay 2013; Kiskira 2006; Rigamonti 2013; Ryan 2005), studies which provided either none or an unclear sample size calculation, making it difficult to determine whether they were adequately powered for their primary outcomes (Biswaz 2003; Bloomfield 1998; El‐Dawlatly 2007; Misra 2013; Nguygen 2001; Peng 2015; Peng 2016; Rahimi 2006; Rahimi 2010; Zhang 2003), and studies with a long duration between completion and publication (Can 2017; Greenberg 2017).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
1. NSAIDs
Primary outcome
Acute postoperative pain intensity
We included six studies (742 participants) in the meta‐analysis for this outcome (Dilmen 2016; Jones 2009; Molnár 2015; Shepherd 2018; Willams 2011; Yardav 2014). Five different NSAIDs (diclofenac, parecoxib, dexketoprofen, methimazole and ibuprofen) were included. We excluded two relevant studies from the analysis, as one did not provide any timing for the pain outcome measures (Rahimi 2006), and the other study provided no absolute figures in its reported results (Ryan 2005).
0 to 12 hours
The pooled estimate of effect for MD in pain intensity was −1.11 (95% CI −1.64 to −0.58, P < 0.0001), in the first six hours postoperatively (Analysis 1.1) and −0.74 (95% CI −1.22 to −0.26, P = 0.02) at 12 hours postoperatively (Analysis 1.2). We judged the quality of the evidence to be high.
1.1. Analysis.
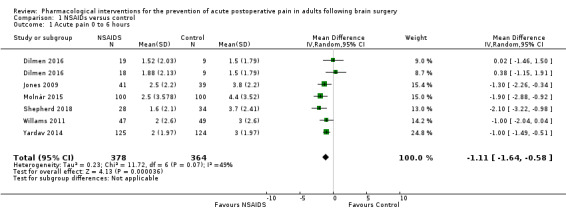
Comparison 1 NSAIDs versus control, Outcome 1 Acute pain 0 to 6 hours.
1.2. Analysis.
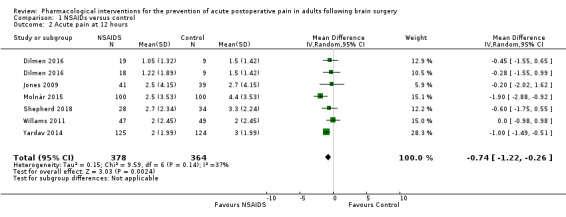
Comparison 1 NSAIDs versus control, Outcome 2 Acute pain at 12 hours.
24 to 48 hours
The pooled estimate of effect for the MD in pain intensity at 24 hours was −0.70 (95% CI −1.26 to − 0.14, P = 0.01, Figure 4). Again, we judged the quality of the evidence to be high. Only one study measured pain at 48 hours and reported a mean pain score of 1 in both groups at 48 hours (Yardav 2014). As this was the only study that reported pain at 48 hours, we did not calculate a pooled estimate of effect for this time point (Yardav 2014). The quality of the evidence was judged to be very low on the basis of these results coming from a single small study.
4.
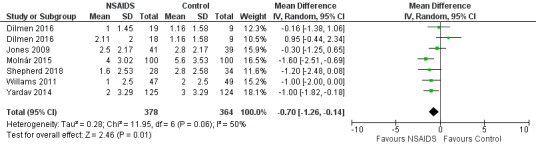
Forest plot of comparison: 1 NSAIDs versus control, outcome: 1.3 Acute pain at 24 hours.
Secondary outcomes
1. Analgesic success as measured by achievement of 'no worse than mild pain' with 'no worse then mild pain' being defined as a score of ≤ 30/100 mm on a visual analogue scale or ≤ 3/10 on a numerical rating scale
Only one eligible study addressed this outcome (Molnár 2015), and so we did not calculate a pooled estimate of effect. This study reported a significant difference in numbers of patients who experienced no worse than mild pain 12 hours after surgery with 48 percent of patients who received diclofenac having either no or mild pain versus 27 percent of patients who received the control.
2. Additional analgesia requirements
We included four studies (265 participants) in the pooled estimate of effect for this outcome (Dilmen 2016; Jones 2009; Ryan 2005; Willams 2011). We included one study twice (with the control group divided between both arms) as it studied this outcome for two different NSAIDs (Dilmen 2016). All of the studies used morphine or morphine equivalents measured in milligrams. The pooled estimate of effect for the MD in additional analgesic requirements was −1.07 (95% CI −4.85 to 2.72, P = 0.58, Analysis 1.4). We judged the quality of the evidence to be low. This was due to imprecision as the pooled sample size was less than 400, and the 95% CI for the effect estimate was wide and included the possibility of either no benefit or increased analgesic requirements in those who received NSAIDs.
1.4. Analysis.
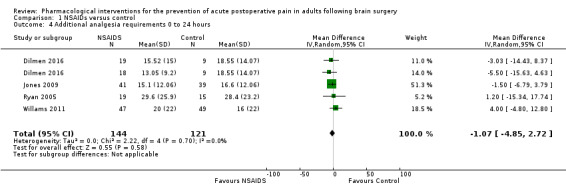
Comparison 1 NSAIDs versus control, Outcome 4 Additional analgesia requirements 0 to 24 hours.
3. Sedation
Of the four eligible studies that measured postoperative sedation (Jones 2009; Shepherd 2018; Willams 2011; Yardav 2014), none measured it using comparable scales and at relevant comparable time points, so we did not calculate a pooled estimate of effect.
4. Chronic headache
No eligible study reported this outcome.
5. Length of stay in a critical care unit
No eligible study reported this outcome.
6. Length of stay in hospital
Two studies addressed this outcome but only one reported P values and standard deviations (Shepherd 2018); the other reported neither a P value nor a standard deviation, so a pooled estimate of effect was not calculated for this outcome (Rahimi 2006).
7. Adverse events
Nausea and vomiting
Two studies (345 participants) compared the incidence of nausea and vomiting in those given NSAIDs versus control medication (Willams 2011; Yardav 2014). The pooled estimate of effect for the risk ratio for nausea and vomiting was 1.34 (95% CI 0.30 to 5.94, P = 0.70, Analysis 1.5), with 70% of the weight coming from the study by Yardav and colleagues (Yardav 2014). We judged the quality of the evidence to be low due to imprecision of the results, i.e. a small number of total events and a wide 95% CI that included the possibility of decreased, equivocal or increased risk of nausea or vomiting in those who received NSAIDs.
1.5. Analysis.
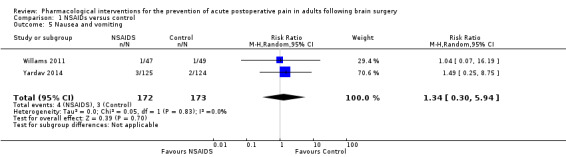
Comparison 1 NSAIDs versus control, Outcome 5 Nausea and vomiting.
Subgroup analyses
Infratentorial versus supratentorial craniotomy
As only one eligible study analysed pain outcomes separately in those undergoing supra versus infratentorial craniotomy we did not perform this subgroup analysis (Molnár 2015).
Intervention timing: pre‐ versus post‐incision
In two studies, NSAIDs were administered prior to surgical skin incision (Molnár 2015; Ryan 2005). In five studies, they were given some time after surgical skin incision (Dilmen 2016; Jones 2009; Shepherd 2018; Willams 2011; Yardav 2014;). Subgroup analysis was not performed as only one study of pre‐incision NSAIDs reported pain outcome figures (Molnár 2015).
Inhalation versus total intravenous anaesthesia
As only one eligible study used an exclusively total intravenous anaesthetic technique, there were not enough eligible studies to enable us to calculate a pooled estimate for the effect for this subgroup analysis (Willams 2011).
Preoperative steroids
No eligible studies addressed this outcome.
Sensitivity analyses
We did not conduct sensitivity analysis. This was because no eligible studies were judged to be either at high risk of bias or to have missing data considered to be missing for reasons likely related to either the intervention or the outcomes studied.
2. Dexmedetomidine
Primary outcomes
Acute postoperative pain intensity
We included two studies (128 participants) measuring postoperative pain intensity in the meta‐analysis for this outcome (Peng 2015; Song 2016). Both studies used dexmedetomidine in similar ways, using infusions of up to 0.5 mcg/kg/hr or placebo infusions intraoperatively.
0 to12 hours
The pooled estimate of effect for the MD in pain intensity was −0.89 (95% CI −1.27 to −0.51, P < 0.00001, Analysis 2.1), during the first six hours postoperatively and −0.81 (95% CI −1.21 to −0.42, P = 0.0004 at 12 hours postoperatively; Analysis 2.2, Figure 5). We downgraded the quality of the evidence by one level for pain intensity at 0 to 6 hours, to a final grade of moderate quality, as the total number of studies and participants was small. We downgraded the quality of the evidence by two levels for pain intensity at 12 hours, to a final grade of low quality, due to the small total number of participants and inconsistency in the form of unexplained important heterogeneity.
2.1. Analysis.
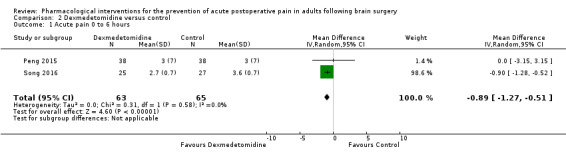
Comparison 2 Dexmedetomidine versus control, Outcome 1 Acute pain 0 to 6 hours.
2.2. Analysis.
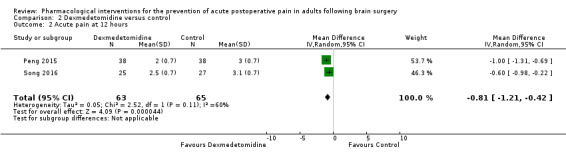
Comparison 2 Dexmedetomidine versus control, Outcome 2 Acute pain at 12 hours.
5.
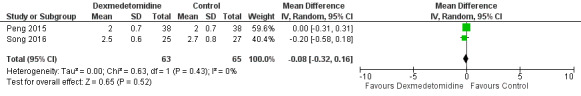
Forest plot of comparison: 2 Dexmedetomidine versus control, outcome: 2.3 Acute pain at 24 hours.
24 to 48 hours
The pooled estimate of effect for the MD in pain intensity at 24 hours was −0.08 (95% CI −0.32 to 0.16, P = 0.52, Analysis 2.3), which was not statistically significant. We downgraded the evidence by two levels, to a final grade of low quality, due to the small total number of participants and due to imprecision, i.e. the 95 % CI was wide, including the possibility of either lesser, equivocal or greater pain intensity in those who received dexmedetomidine.
2.3. Analysis.
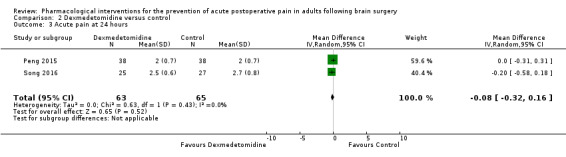
Comparison 2 Dexmedetomidine versus control, Outcome 3 Acute pain at 24 hours.
No relevant studies addressed pain intensity beyond 24 hours.
Secondary outcomes
1. Analgesic success as measured by achievement of 'no worse than mild pain' with 'no worse then mild pain' being defined as a score of ≤ 30/100 mm on a visual analogue scale or ≤ 3/10 on a numerical rating scale.
No eligible studies addressed this outcome.
2. Additional analgesia requirements
Two studies (128 participants) contributed to this outcome (Peng 2015; Song 2016). Both studies used morphine to provide additional analgesia. The pooled estimate of effect for the MD in additional analgesia requirement was −21.36 (95% CI −34.63 to −8.1, P = 0.002, Analysis 2.4), with 65% of the weight coming from Song 2016. We downgraded the quality of the evidence by two levels to a final grade of low quality, due to the small total number of participants and due to unexplained important heterogeneity.
2.4. Analysis.
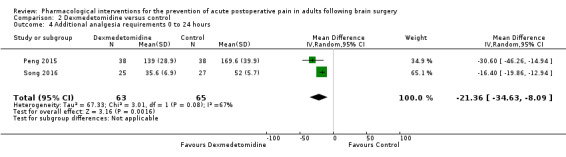
Comparison 2 Dexmedetomidine versus control, Outcome 4 Additional analgesia requirements 0 to 24 hours.
3. Sedation
Only one eligible study addressed this outcome and so no pooled estimate of effect was calculated (Song 2016). This study reported a mean Ramsey Sedation score of 2.4 in the treatment group and 2.2 in the control group at 24 hours with no significant differences between the groups.
4. Chronic headache
No eligible studies reported this outcome.
5. Length of stay in a critical care unit
No eligible studies reported this outcome.
6. Length of stay in hospital
No eligible studies reported this outcome.
7. Adverse events
Nausea and vomiting
Three studies (261 participants) were included in the pooled estimate of effect for this outcome (Peng 2015; Song 2016; Yun 2016). The risk ratio for nausea and vomiting in those receiving dexmedetomidine versus control was 0.43 (95% CI 0.06 to 3.08, P = 0.40, Analysis 2.5). We judged the quality of the evidence to be low due to imprecision due to the small number of total events and a wide 95% CI that included the possibility of less, equal or greater risk of nausea and vomiting in those who received dexmedetomidine.
2.5. Analysis.
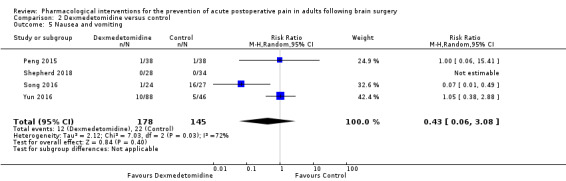
Comparison 2 Dexmedetomidine versus control, Outcome 5 Nausea and vomiting.
Hypotension
Three studies (184 participants) were included in the pooled estimate of effect for this outcome (Bekker 2008; Peng 2015; Song 2016). The risk ratio for hypotension in those receiving dexmedetomidine versus control was 0.50 (95% CI 0.05 to 5.28, P = 0.56, Analysis 2.6), with all the events occurring in only one study (Peng 2015). We judged the quality of the evidence to be low on the basis of a small number of total participants and a wide 95% CI that included the possibility of less, equal or greater risk of hypotension in those who received dexmedetomidine.
2.6. Analysis.
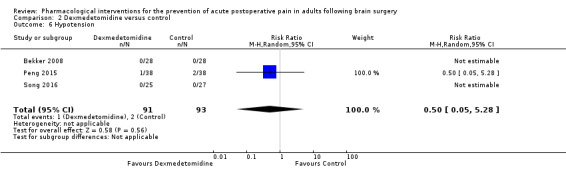
Comparison 2 Dexmedetomidine versus control, Outcome 6 Hypotension.
Subgroup analyses
We did not conduct subgroup analyses. This was because no eligible studies addressed the effects of either intervention timing, surgical approach or preoperative steroids on the relevant outcomes, and only one eligible study used an exclusively intravenous anaesthetic technique (Song 2016).
Sensitivity analyses
We did not conduct sensitivity analysis. This was because no eligible studies were judged to be either at high risk of bias or to have missing data considered to be missing for reasons likely related to either the intervention or outcomes studied.
3. Pregabalin or Gabapentin
Primary outcomes
Acute postoperative pain intensity
Two studies (202 participants) addressed this outcome (Shimony 2016; Zeng 2019) using the numerical rating scale and the visual analogue scale, respectively. One study examined the efficacy of gabapentin (Zeng 2019), while the other examined the efficacy of pregabalin (Shimony 2016).
0 to 6 hours
The pooled estimate of effect was a SMD in pain intensity of −0.62 (95% CI −0.90 to −0.34, P < 0.0001, Analysis 3.1). When re‐expressed as the mean difference in pain scores, these values were as follows; MD ‐1.15 (95% CI −1.66 to −0.6). The quality of the evidence was downgraded by two levels to a final level of low, due to a small pooled sample size and possible indirectness of effect as the two drugs studied (pregabalin and gabapentin) differ somewhat in their pharmacological properties.
3.1. Analysis.
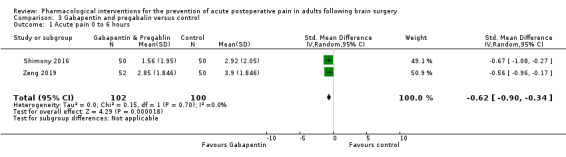
Comparison 3 Gabapentin and pregabalin versus control, Outcome 1 Acute pain 0 to 6 hours.
12 hours
Only one study reported this outcome (Shimony 2016), so a pooled estimate of effect was not calculated. The study found a significant difference in pain at 12 hours in those who received pregabalin versus those who did not, with a mean score of 1.5 in the pregabalin group and mean score of 2.26 in the control group, with a P value of < 0.01.
24 hours
The pooled estimate of effect was a SMD in pain intensity of −0.78 (95% CI −2.06 to −0.51), P = 0.24, Analysis 3.2). When re‐expressed as the mean difference in pain scores, these values were as follows; MD −0.29 (95% CI −0.78 to −0.19). The quality of the evidence was downgraded by two levels to a final level of low, due to a small pooled sample size and possible indirectness of effect as the two drugs studied (pregabalin and gabapentin) differ somewhat in their pharmacological properties.
3.2. Analysis.
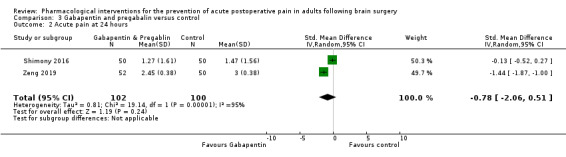
Comparison 3 Gabapentin and pregabalin versus control, Outcome 2 Acute pain at 24 hours.
48 hours
The pooled estimate of effect was a SMD in pain intensity of −0.02 (95% CI −0.29 to 0.26, P value 0.91, Analysis 3.3). When re‐expressed as the mean difference in pain scores, these values were as follows; MD −0.06 (95% CI −0.86 to 0.77). The quality of the evidence was downgraded by two levels to a final level of low, due to a small pooled sample size and possible indirectness of effect as the two drugs studied (pregabalin and gabapentin) differ somewhat in their pharmacological properties
3.3. Analysis.
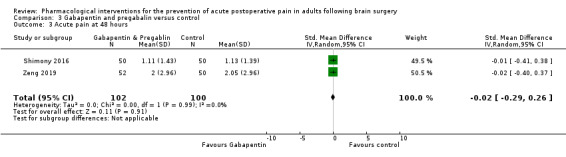
Comparison 3 Gabapentin and pregabalin versus control, Outcome 3 Acute pain at 48 hours.
Secondary outcomes
1. Analgesic success as measured by achievement of 'no worse than mild pain' with 'no worse then mild pain' being defined as a score of ≤ 30/100 mm on a visual analogue scale or ≤ 3/10 on a numerical rating scale
Only one eligible study addressed this outcome, so no pooled estimate of effect was calculated (Misra 2013).
2. Additional analgesia requirements
Three studies including 235 participants addressed this outcome: one study of pregabalin (Shimony 2016), and two studies of gabapentin (Misra 2013; Zeng 2019). Agents used were morphine and fentanyl. The pooled estimate of effect for the SMD in additional analgesia requirement was −0.37 (95% CI −1.10 to −0.35, P = 0.31, Analysis 3.4). Using Cohen's rule of thumb, this represents a small, non‐significant effect size. The quality of the evidence was downgraded by two levels to a final level of low, due to a small pooled sample size and possible indirectness of effect as the two drugs studied (pregabalin and gabapentin) differ somewhat in their pharmacological properties
3.4. Analysis.
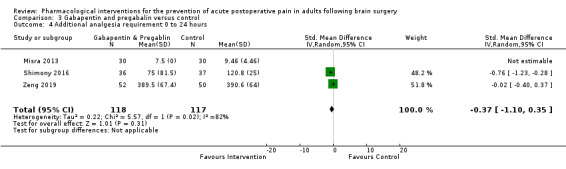
Comparison 3 Gabapentin and pregabalin versus control, Outcome 4 Additional analgesia requirement 0 to 24 hours.
3. Sedation
No eligible study addressed this outcome.
4. Chronic headache
Only one eligible study reported this outcome and so no pooled estimate of effect was calculated (Shimony 2016). That study found a mean pain score three months after surgery of 1.28 in the treatment group and 1.51 in the placebo group, with no statistical difference between groups.
5. Length of stay in a critical care unit
No eligible study reported this outcome.
6. Length of stay in hospital
Only one eligible study reported this outcome and so no pooled estimate of effect was calculated (Shimony 2016). This one study reported a non‐significant difference in the number of days spent in hospital, with those who received pregabalin spending a mean of 7.9 days in hospital and those in the control group spending a mean of 8.3 days in hospital.
7. Adverse events
Nausea and vomiting
Three studies (275 participants) were included in the pooled estimate of effect for this outcome (Misra 2013; Shimony 2016; Zeng 2019). The risk ratio for nausea and vomiting was found to be significantly less in those treated with either gabapentin or pregabalin versus control interventions, risk ratio 0.51 (95% CI 0.29 to 0.89, P = 0.02, Analysis 3.5). The quality of the evidence was judged to be low due to imprecision as the number of total events was small and due to indirectness as the two medications differ somewhat in their pharmacologic properties.
3.5. Analysis.
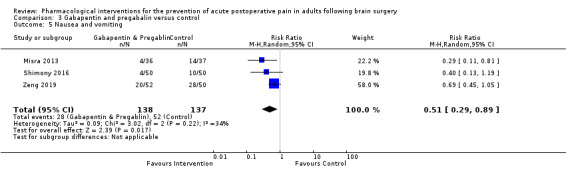
Comparison 3 Gabapentin and pregabalin versus control, Outcome 5 Nausea and vomiting.
Subgroup analyses
No subgroup analyses were conducted as no eligible studies addressed the effects of either intervention timing, surgical approach, preoperative steroids or anaesthetic technique on the relevant outcomes.
Sensitivity analyses
No sensitivity analyses were conducted as no eligible studies were judged to be either at high risk of bias or to have missing data considered to be missing for reasons likely related to either the intervention or outcomes studied.
4. Acetaminophen
Primary outcomes
Acute postoperative pain intensity
0 to 6 hours
Three studies (332 participants) contributed to a pooled estimate of effect for the MD in acute pain intensity in the first six hours after surgery, of −0.35 (95% CI −1.00 to 0.30, P = 0.29, Analysis 4.1) (Artime 2018; Dilmen 2016; Sivakumar 2018). The quality of the evidence was judged to be moderate due to a small pooled sample size.
4.1. Analysis.
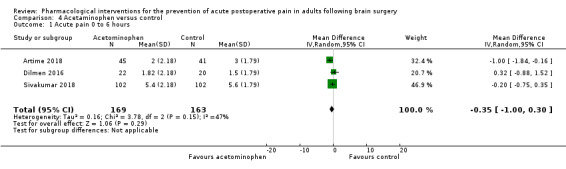
Comparison 4 Acetaminophen versus control, Outcome 1 Acute pain 0 to 6 hours.
12 hours
Three studies (332 participants) contributed to a pooled estimate of effect for the MD in acute pain intensity at 12 hours, of −0.51 (95% CI −1.04 to 0.03, P = 0.06, Analysis 4.2) (Artime 2018; Dilmen 2016; Sivakumar 2018). The quality of the evidence was judged to be moderate due to a small pooled sample size.
4.2. Analysis.
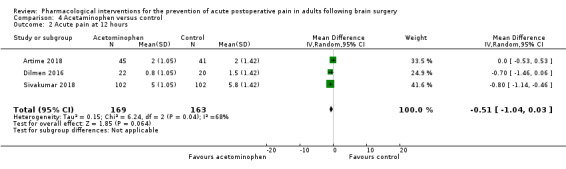
Comparison 4 Acetaminophen versus control, Outcome 2 Acute pain at 12 hours.
24 hours
Four studies (439 participants) contributed to a pooled estimate of effect for MD in acute pain intensity at 24 hours, of 0.34 (95% CI −1.20 to 0.52, P = 0.44, Analysis 4.3) (Artime 2018; Dilmen 2016; Greenberg 2017; Sivakumar 2018). The quality of the evidence was judged to be high.
4.3. Analysis.
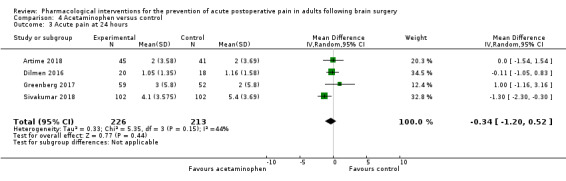
Comparison 4 Acetaminophen versus control, Outcome 3 Acute pain at 24 hours.
48 hours
Only one study addressed this outcome (Sivakumar 2018) and showed a mean pain score of 5.5 in the control group and 4.5 in the treatment group, with no significant differences between the groups.
Secondary outcomes
1. Analgesic Success. Analgesic success as measured by achievement of 'no worse than mild pain' with 'no worse then mild pain' being defined as a score of ≤ 30/100 mm on a visual analogue scale or ≤ 3/10 on a numerical rating scale
No eligible study addressed this outcome.
2. Additional analgesia requirements
Four studies (459 participants) contributed to a pooled estimate effect for MD in additional analgesia requirement, of −0.07 (95% CI −0.86 to 0.99, P = 0.89, Analysis 4.4), (Artime 2018; Dilmen 2016; Greenberg 2017; Sivakumar 2018). The quality of the evidence was judged to be high.
4.4. Analysis.
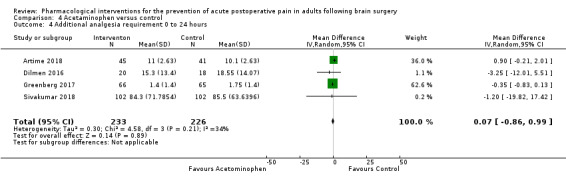
Comparison 4 Acetaminophen versus control, Outcome 4 Additional analgesia requirement 0 to 24 hours.
3. Sedation
Only one eligible study addressed this outcome using a validated scale, and so no pooled estimate of effect was calculated (Greenberg 2017). That study reported a Richmond Agitation Sedation score of zero in both groups at 24 hours.
4. Chronic headache
No eligible studies addressed this outcome.
5. Length of stay in a critical care unit
Only one eligible study reported this outcome, and so no pooled estimate of effect was calculated (Greenberg 2017). That study reported a median length of stay in critical care of 28 hours in the control group and 26 hours in the acetaminophen group with no significant differences between the groups.
6. Length of stay in hospital
Two studies (335 participants) contributed to a pooled estimate of effect for MD in length of hospital stay of −3.71 hours (95% CI −14.12 to 6.7, P 0.48, Analysis 4.5) (Greenberg 2017; Sivakumar 2018). The quality of the evidence was judged to be moderate, being downgraded one level due to a small pooled sample size.
4.5. Analysis.
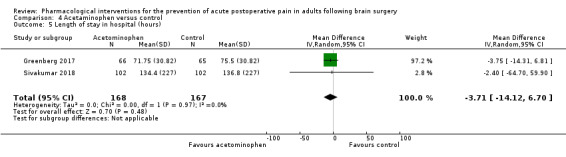
Comparison 4 Acetaminophen versus control, Outcome 5 Length of stay in hospital (hours).
7. Adverse events
No two studies addressed comparable adverse events, and so a pooled estimate of effect was not calculated.
Subgroup analyses
We did not conduct subgroup analyses as only one study addressed pain outcomes separately in those undergoing supratentorial craniotomy (Greenberg 2017), and no eligible studies addressed the effects of intervention timing, preoperative steroids or anaesthetic technique on the relevant outcomes.
Sensitivity analyses
No studies were judged to be at a high risk of bias.
5. Scalp infiltration
Primary outcomes
Acute postoperative pain intensity
0 to12 hours
Pain in the first six hours: nine studies, including a total of 475 participants, contributed to this estimate (Akcil 2017: Biswaz 2003; Bloomfield 1998; El‐Dawlatly 2007; Kiskira 2006; Law‐Koune 2005; Saringcarinkul 2015; Zhang 2003; Zhou 2016). The effect estimate for the MD in pain intensity was ‐ 0.64 (95% CI −1.28 to −0.00, P = 0.05) supporting a non‐statistically significant benefit of scalp infiltration in terms of reduction in early postoperative pain intensity (Analysis 5.1). We downgraded the evidence by one level to a final grade of moderate quality, due to unexplained important heterogeneity.
5.1. Analysis.
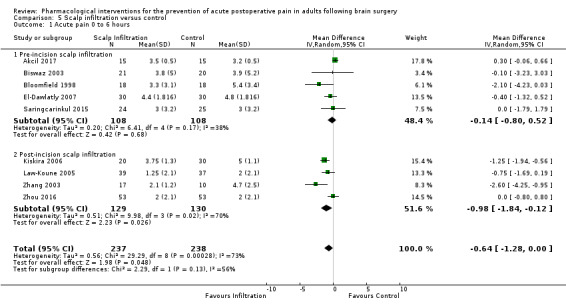
Comparison 5 Scalp infiltration versus control, Outcome 1 Acute pain 0 to 6 hours.
Pain at 12 hours: seven studies, including 309 participants, measured pain at 12 hours, producing a pooled effect estimate for SMD in pain intensity of −0.71 (95% CI −1.34 to −0.08, P = 0.03, Analysis 5.3) with non‐significant statistical heterogeneity (Akcil 2017; Batoz 2009; Biswaz 2003; El‐Dawlatly 2007; Kiskira 2006; Saringcarinkul 2015; Zhang 2003). We downgraded the evidence by two levels to a final grade of low quality, due to a small pooled sample size and loss of significance of results on sensitivity analysis.
5.3. Analysis.
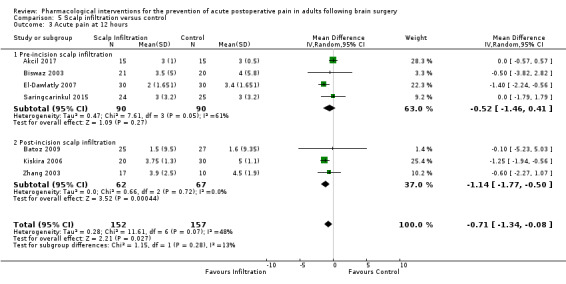
Comparison 5 Scalp infiltration versus control, Outcome 3 Acute pain at 12 hours.
24 to 48 hours
Pain at 24 hours: six studies including 260 participants, measured pain at 24 hours using comparable pain scales, producing a pooled estimate of effect for MD in pain intensity of −0.39 (95% CI −1.06 to 0.27, P = 0.24, Analysis 5.5), which was not statistically significant (Akcil 2017; Batoz 2009; Biswaz 2003; El‐Dawlatly 2007; Kiskira 2006; Zhang 2003). We downgraded the evidence two levels to a final grade of low quality, due to a small pooled sample size and unexplained important heterogeneity.
5.5. Analysis.
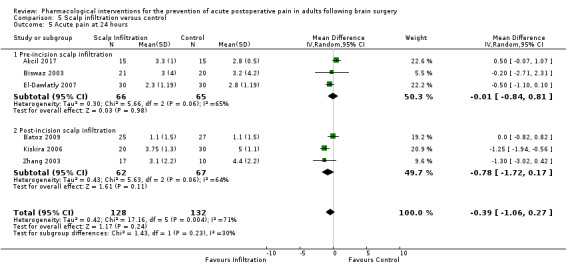
Comparison 5 Scalp infiltration versus control, Outcome 5 Acute pain at 24 hours.
Pain at 48 hours: only three studies (128 participants) contributed to this outcome (Biswaz 2003; El‐Dawlatly 2007; Zhang 2003). The effect estimate for the MD in pain intensity was −1.09 (95% CI −2.13 to ‐0.06, P = 0.04, Analysis 5.7). We downgraded the evidence by one level, to a final grade of moderate quality, due to a small pooled sample size.
5.7. Analysis.
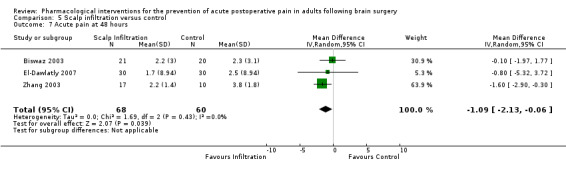
Comparison 5 Scalp infiltration versus control, Outcome 7 Acute pain at 48 hours.
Secondary outcomes
1. Analgesic success as measured by achievement of 'no worse than mild pain' with 'no worse then mild pain' being defined as a score of ≤ 30/100 mm on a visual analogue scale or ≤ 3/10 on a numerical rating scale
Only one eligible study addressed this outcome, so no pooled estimate of effect was calculated (Saringcarinkul 2015). That study showed no significant difference in the numbers of patients who were pain‐free six hours after surgery with 4% of patients who received scalp infiltration being free of pain versus 8% of patient who received control.
2. Additional analgesia requirements
Six studies (345 participants) measured this outcome (Akcil 2017; Batoz 2009; Biswaz 2003; Kiskira 2006; Law‐Koune 2005; Zhou 2016). The agents used to provide supplementary analgesia included diclofenac, morphine and nalbuphine. Dosages were all calculated in milligrams and so MD was used to calculate the pooled estimate of the effect of −9.56 (95% CI −15.64 to −3.49, P = 0.002, Analysis 5.10). There was moderate statistical heterogeneity which was unexplained, a small pooled sample size and the result lost significance when studies were included in a sensitivity analysis, so we downgraded the evidence by three levels to a final grade of very low quality.
5.10. Analysis.
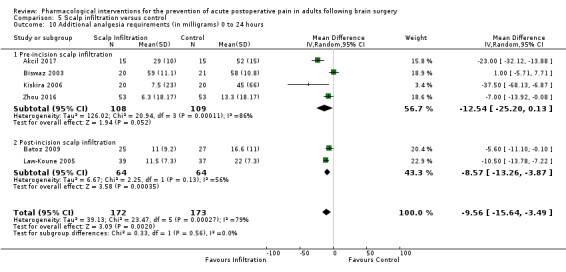
Comparison 5 Scalp infiltration versus control, Outcome 10 Additional analgesia requirements (in milligrams) 0 to 24 hours.
3. Sedation
Of the four studies (337 participants) of scalp infiltration that measured postoperative sedation, none measured it using comparable scales and at relevant comparable time points so no pooled estimate of effect was calculated (Batoz 2009; Law‐Koune 2005; Saringcarinkul 2015; Zhou 2016).
4. Chronic headache
Only two studies reported this outcome (Batoz 2009; Zhou 2016); as neither reported it at the same time point, no pooled estimate of effect was calculated.
5. Length of stay in a critical care unit
No eligible study reported this outcome.
6. Length of stay in hospital
No eligible study reported this outcome.
7. Adverse events
Nausea and vomiting
Four studies (318 participants) reported this outcome (El‐Dawlatly 2007; Law‐Koune 2005; Saringcarinkul 2015; Zhou 2016). The pooled estimate of effect for the risk ratio for nausea and vomiting was 0.74 (95% CI 0.48 to 1.14, P = 0.17, Analysis 5.9). We downgraded the evidence by two levels to a final grade of low quality, due to imprecision, i.e. a small total number of events, and a wide 95% CI that included the possibility of either less, equal or greater risk of nausea and vomiting in those who received scalp infiltration.
5.9. Analysis.
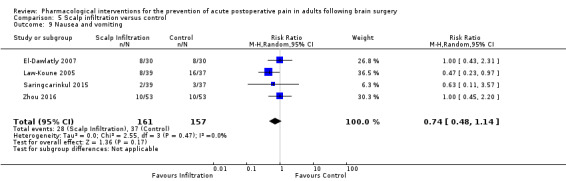
Comparison 5 Scalp infiltration versus control, Outcome 9 Nausea and vomiting.
Subgroup analyses
Infratentorial versus supratentorial craniotomy
No eligible studies addressed these subgroups.
Intervention timing: pre‐ versus post‐incision
Pooled estimates of effect were calculated for acute postoperative pain intensity and additional analgesic consumption for those who received scalp infiltration before surgical incision and for those who received scalp infiltration some time after surgery had commenced. The results were as follows:
Mean difference in pain intensity in the first 6 hours was −0.14 (95% CI −0.08 to 0.52, P = 0.68) for those who received pre‐incision scalp infiltration (5 studies, 216 participants) (Akcil 2017; Biswaz 2003; Bloomfield 1998; El‐Dawlatly 2007; Saringcarinkul 2015), and −0.98 (95% CI −1.84 to −0.12, P = 0.03) for those who received post‐incision scalp infiltration (4 studies, 259 participants) (Kiskira 2006; Law‐Koune 2005; Zhang 2003; Zhou 2016), (Analysis 5.1).
Mean difference in pain intensity at 12 hours was −0.52 (95% CI −1.46 to 0.41, P = 0.27) for those who received pre‐incision scalp infiltration (4 studies, 180 participants) (Akcil 2017;Biswaz 2003; El‐Dawlatly 2007; Saringcarinkul 2015), and −1.14 (95% CI −1.77 to −0.50, P = 0.004) for those who received post‐incision scalp infiltration (3 studies, 129 participants) (Batoz 2009; Kiskira 2006; Zhang 2003), (Analysis 5.3).
Mean difference in pain intensity at 24 hours was −0.01 (95% CI −0.84 to 0.81, P = 0.98) for those who received pre‐incision scalp infiltration (3 studies, 131 participants) (Akcil 2017;Biswaz 2003; El‐Dawlatly 2007), and −1.78 (95% CI −1.72 to 0.17, P = 0.11) for those who received post‐incision scalp infiltration (3 studies, 129 participants) (Batoz 2009; Kiskira 2006; Zhang 2003), (Analysis 5.5).
Mean difference in additional analgesia requirement was −12.54 (95% CI −25.20 to 0.13, P = 0.05) for those who received pre‐incision scalp infiltration (4 studies, 217 participants) (Akcil 2017; Biswaz 2003; Kiskira 2006; Zhou 2016), and −8.57 (95% CI −13.26 to −3.87, P = 0.0003) for those who received post‐incision scalp infiltration (2 studies, 128 participants) (Batoz 2009; Law‐Koune 2005) (Analysis 5.10).
Inhalation versus total intravenous anaesthesia
This subgroup analysis was not conducted as only one eligible study used an exclusively intravenous anaesthetic technique (Batoz 2009).
Preoperative steroids
Only one eligible study addressed this outcome so no subgroup analysis was conducted (Bloomfield 1998).
Sensitivity analyses
Excluding studies with a high risk of bias
We determined a study to have an overall 'high risk' of bias if it was judged to have a high risk of bias in four or more of the seven domains of bias or a high risk of bias in three or more of the seven domains of bias with an unclear risk of bias in one or more domain (Figure 2). Three studies of scalp infiltration fulfilled these criteria (Bloomfield 1998; Kiskira 2006; Zhang 2003), and so sensitivity analysis excluding these studies was conducted for the following outcomes:
Acute postoperative pain intensity
Exclusion of these studies changed the pooled estimate of effect for the MD in acute pain intensity as follows:
Mean difference in acute pain intensity in the first 6 hours became −0.04 (95% CI −0.43 to 0.35, P = 0.85, Analysis 5.2). Mean difference in pain intensity became 0.20 (95% CI −0.13 to 0.52, P = 0.24) for those who received scalp infiltration pre‐surgical incision and −0.39 (95% CI −1.22 to 0.44, P = 0.36) for those who received it post‐incision.
5.2. Analysis.
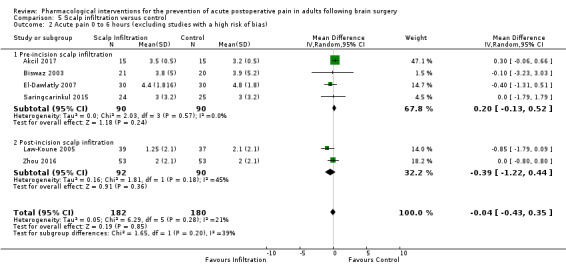
Comparison 5 Scalp infiltration versus control, Outcome 2 Acute pain 0 to 6 hours (excluding studies with a high risk of bias).
Mean difference in acute pain intensity at 12 hours became −0.35 (95% CI −1.31 to 0.61, P = 0.48, Analysis 5.4), with insufficient studies to analyse the effects of pre‐ versus post‐incision scalp infiltration.
5.4. Analysis.
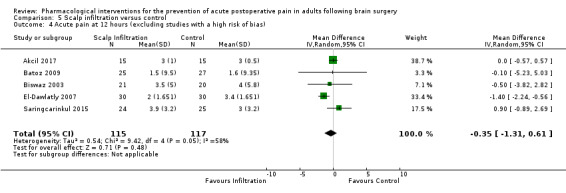
Comparison 5 Scalp infiltration versus control, Outcome 4 Acute pain at 12 hours (excluding studies with a high risk of bias).
Mean difference in acute pain intensity at 24 hours became −0.01 (95% CI −0.75 to 0.73, P = 0.99, Figure 6), with insufficient studies to analyse the effects of pre‐ versus post‐incision scalp infiltration.
6.
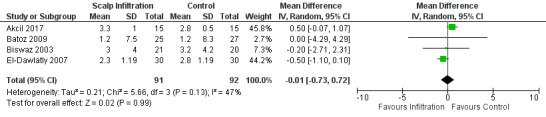
Forest plot of comparison: 5 Scalp infiltration versus control, outcome: 5.6 Acute Pain at 24 hours (Excluding Studies with a High Risk of Bias).
Mean difference in acute pain intensity at 48 hours became −0.76 (95% CI −1.20 to −0.32, P = 0.0007, Analysis 5.8), with insufficient studies to analyse the effects of pre‐ versus post‐incision scalp infiltration.
5.8. Analysis.
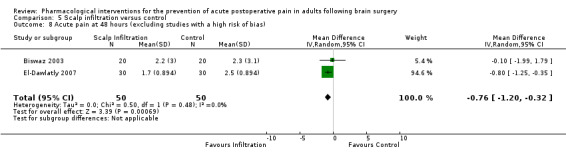
Comparison 5 Scalp infiltration versus control, Outcome 8 Acute pain at 48 hours (excluding studies with a high risk of bias).
For the 12‐hour time period, the exclusion of studies with a high risk of bias changed the initially significant result to non‐significant and for the 48‐hour time period, the exclusion of studies with a high risk of bias changed the initially non‐significant result to significant, with virtually all the weight for the pooled estimate of effect coming from one study (El‐Dawlatly 2007). For the 0‐ to 6‐hour and 12‐hour time periods, sensitivity analysis did not change the significance of the results.
Additional analgesia requirements
Sensitivity analysis changed the effect estimate to −8.16 (95% CI −16.5 to 0.18, P = 0.06), making the initial statistically significant result insignificant (Analysis 5.11).
5.11. Analysis.
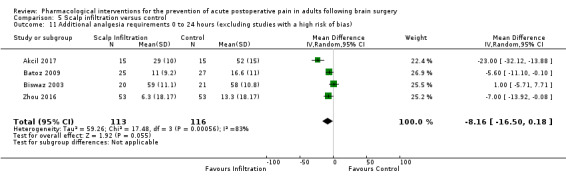
Comparison 5 Scalp infiltration versus control, Outcome 11 Additional analgesia requirements 0 to 24 hours (excluding studies with a high risk of bias).
Excluding studies with missing data considered to be missing for reasons likely related to either the intervention or outcomes studied
No studies were found where missing data was thought to be missing for reasons related to the intervention or outcomes being measured, so this analysis was not conducted.
6. Scalp blocks
Primary outcomes
Acute postoperative pain intensity
0 to 12 hours
Pain in the first 6 hours: 10 studies (414 participants) used comparable pain scales to measure pain in the first 6 hours, producing a pooled estimate of effect for MD in pain intensity of −0.98 (95% CI −1.66 to −0.30, P = 0.005, Analysis 6.1), in favour of scalp block producing a statistically significant reduction in pain intensity (Akcil 2017; Bala 2006; Can 2017; Choi 2009; Ganzoni 2008; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Tucinda 2010; Zhang 2003). We judged the quality of the evidence to be low due failure to retain significance when studies with a high risk of bias were excluded and important unexplained heterogeneity.
6.1. Analysis.
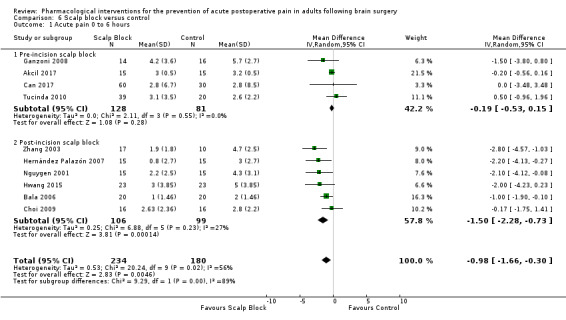
Comparison 6 Scalp block versus control, Outcome 1 Acute pain 0 to 6 hours.
Pain at 12 hours: 8 studies (294 participants) contributed to a pooled estimate of effect of for MD in pain intensity of −0.95 (95% CI −1.53 to −0.37, P = 0.001, Analysis 6.3), again in favour of scalp block producing a statistically significant reduction in pain intensity but with the limitation, important unexplained heterogeneity and a small pooled sample size (Akcil 2017; Batoz 2009; Choi 2009; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Tucinda 2010; Zhang 2003). We judged the quality of the evidence to be low, because of imprecision due to a small pooled sample size and inconsistency due to unexplained important heterogeneity.
6.3. Analysis.
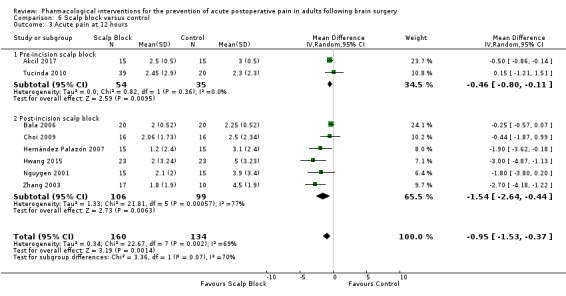
Comparison 6 Scalp block versus control, Outcome 3 Acute pain at 12 hours.
24 to 48 hours
Pain at 24 hours: 9 studies (433 participants) contributed to a pooled estimate of effect for MD in pain intensity of −0.78 (95% CI −1.52 to −0.05, P = 0.04, Analysis 6.5), in favour of scalp block producing a statistically significant reduction in pain intensity but with the limitation of significant statistical heterogeneity (Akcil 2017; Can 2017; Choi 2009; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Rigamonti 2013; Tucinda 2010; Zhang 2003). We downgraded the evidence by two levels to a final grading of low quality, due to failure to retain significance when studies with a high risk of bias were excluded and due to important unexplained heterogeneity.
6.5. Analysis.
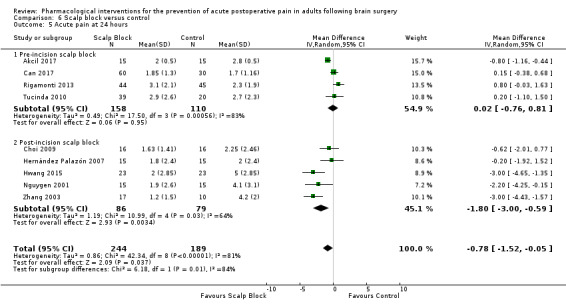
Comparison 6 Scalp block versus control, Outcome 5 Acute pain at 24 hours.
Pain at 48 hours: 4 studies (135 participants) contributed to a pooled estimate of effect for SMD in pain intensity of −1.34 (95% CI −2.57 to −0.11, P = 0.03, Analysis 6.7), in favour of scalp block producing a statistically significant reduction in pain intensity (Choi 2009; Hwang 2015; Nguygen 2001; Zhang 2003 ). We downgraded the evidence by three levels to a final grade of very low quality, as there was inconsistency in the form of important unexplained heterogeneity, the beneficial effect of scalp block on postoperative pain intensity as 48 hours was not sustained when studies deemed to have a high overall risk of bias were excluded, and the pooled sample size was small.
6.7. Analysis.
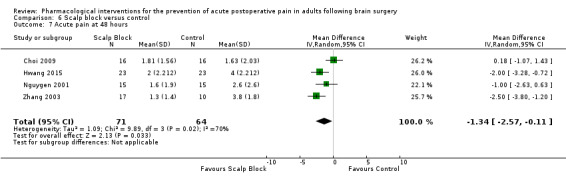
Comparison 6 Scalp block versus control, Outcome 7 Acute pain at 48 hours.
Secondary outcomes
1. Analgesic success as measured by achievement of 'no worse than mild pain' with 'no worse then mild pain' being defined as a score of ≤ 30/100 mm on a visual analogue scale or ≤ 3/10 on a numerical rating scale
Only one eligible study addressed this outcome, so no pooled estimate of effect was calculated (Bala 2006).
2. Additional analgesia requirements
Seven studies (314 participants) contributed to this outcome ( Akcil 2017; Ganzoni 2008; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Rigamonti 2013; Tucinda 2010). The medications used to provide additional analgesia included morphine, morphine equivalents and codeine which were measured in milligrams, and fentanyl which was measured in micrograms. The pooled estimate of effect for the SMD in additional analgesic requirement was −1.11 (95% CI −1.97 to −0.25, P = 0.01, Analysis 6.9). Using Cohen's rule of thumb, this represents a large effect size. We downgraded the evidence by two levels to a final grade of low quality, due to a small pooled sample size and important unexplained heterogeneity.
6.9. Analysis.
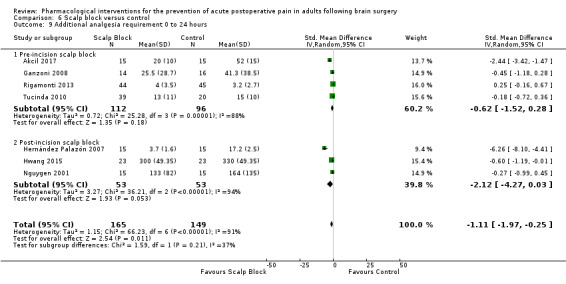
Comparison 6 Scalp block versus control, Outcome 9 Additional analgesia requirement 0 to 24 hours.
3. Sedation
Of the three studies of scalp blocks that measured postoperative sedation (142 participants), none measured it using comparable scales and at relevant comparable time points, so no pooled estimate of effect was calculated (Hernández Palazón 2007; Hwang 2015; Tucinda 2010;).
4. Chronic headache
Only one study of scalp blocks reported pain at 2 months (Rigamonti 2013) and none reported pain at 3 months.
5. Length of stay in a critical care unit
No eligible study reported this outcome.
6. Length of stay in hospital
No eligible study reported this outcome.
7. Adverse events
Nausea and vomiting
Four studies (165 participants) contributed to a pooled estimate of effect for the risk ratio of nausea and vomiting of 0.66 (95% CI 0.33 to 1.32, P = 0.24, Analysis 6.10), (Ganzoni 2008; Hernández Palazón 2007; Hwang 2015; Tucinda 2010). We downgraded the evidence by three levels to a final grade of very low quality, as there was inconsistency in the form of important unexplained heterogeneity and imprecision, i.e. a wide 95% CI that included the possibility of either no benefit or increased nausea and vomiting in the intervention group and a small number of total events.
6.10. Analysis.
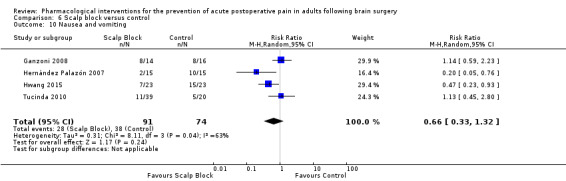
Comparison 6 Scalp block versus control, Outcome 10 Nausea and vomiting.
Subgroup analyses
Infratentorial versus supratentorial craniotomy
No eligible studies addressed these subgroups.
Intervention timing: pre‐ versus post‐incision
We calculated pooled estimates of effect for acute pain intensity for those who received scalp blocks before surgical incision and for those who received scalp blocks some time after surgery had commenced. The results were as follows:
Mean difference in acute pain intensity in the first 6 hours was −0.19 (95% CI −0.53 to 0.15, P = 0.28) for those who received pre‐incision scalp block (4 studies, 209 participants) (Akcil 2017; Can 2017; Ganzoni 2008; Tucinda 2010), and −1.92 (95% CI −3.08 to −0.76, P = 0.001) for those who received post‐incision scalp block (6 studies, 205 participants) (Bala 2006; Choi 2009; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Zhang 2003) (Analysis 6.1).
Mean difference in acute pain intensity at 12 hours was −0.46 (95% CI −0.8 to −0.11, P = 0.01) for those who received pre‐incision scalp block (2 studies, 89 participants) (Akcil 2017; Tucinda 2010), and −1.54 (95% CI −2.64 to −0.44, P = 0.006) for those who received post‐incision scalp block (6 studies, 205 participants) (Bala 2006; Choi 2009; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Zhang 2003), (Analysis 6.3).
Mean difference in acute pain intensity at 24 hours was 0.01 (95% CI −1.07 to 1.09, P = 0.99) for those who received pre‐incision scalp blocks (4 studies, 268 participants) (Akcil 2017; Can 2017; Rigamonti 2013; Tucinda 2010), and −1.80 (95% CI −3.00 to −0.59, P = 0.003) for those who received post‐incision scalp infiltration (5 studies, 165 participants) (Choi 2009; Hernández Palazón 2007; Hwang 2015; Nguygen 2001; Zhang 2003), (Analysis 6.5).
Pooled estimates of effect were calculated for additional analgesia requirements in those who received pre‐ versus post‐incision scalp blocks. The results were as follows:
Standardized mean difference in additional analgesia requirements in the first 24 hours postoperatively were −0.62 (95% CI −1.52 to 0.28, P = 0.18) for those who received pre‐incision scalp blocks (4 studies, 208 participants) (Akcil 2017; Ganzoni 2008; Rigamonti 2013; Tucinda 2010), and −2.12 (95% CI −4.27 to 0.03, P = 0.05) for those who received post‐incision scalp blocks (3 studies, 106 participants) (Hwang 2015; Hernández Palazón 2007; Nguygen 2001), (Analysis 6.9). Using Cohen's rule of thumb, this represents a moderate and large effect size respectively; however, neither achieved statistical significance.
Inhalation versus total intravenous anaesthesia
No subgroup analysis was conducted as only one eligible study used an intravenous anaesthetic technique (Can 2017).
Preoperative steroids
No subgroup analysis was conducted as no eligible studies were found.
Sensitivity analyses
Excluding studies with a high risk of bias
We determined a study to have an overall high risk of bias if it was judged to have a high risk of bias in four or more of the seven domains of bias or a high risk of bias in three or more of the seven domains of bias with an unclear risk of bias in one or more domain (Figure 2). Five studies of scalp blocks fulfilled these criteria (Choi 2009; Cokay 2013; Rigamonti 2013; Nguygen 2001; Zhang 2003).
Sensitivity analyses (excluding these studies) were therefore performed for the relevant following outcomes:
Acute postoperative pain intensity
Exclusion of studies with a high risk of bias changed the pooled estimate of effect for the MD in acute pain intensity as follows:
Mean difference in acute pain intensity in the first 6 hours became −0.97 (95% CI −1.98 to 0.05, P = 0.06, Analysis 6.2). Mean difference in acute pain intensity in the first 6 hours became −0.19 (95% CI −0.54 to 0.15, P = 0.27) for those who received pre‐incision scalp block and −1.71 (95% CI −2.44 to −0.98, P < 0.00001) for those who received post‐incision scalp block.
6.2. Analysis.
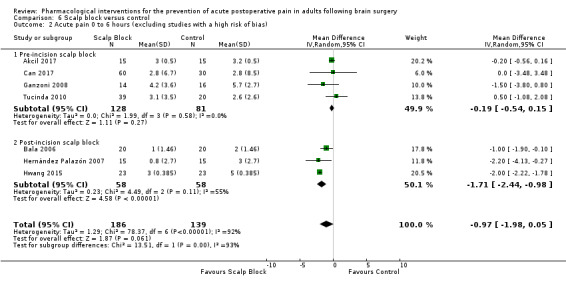
Comparison 6 Scalp block versus control, Outcome 2 Acute pain 0 to 6 hours (excluding studies with a high risk of bias).
Mean difference in acute pain intensity at 12 hours became −0.64 (95% CI −1.21 to −0.07, P = 0.03, Analysis 6.4). Mean difference in acute pain intensity at 12 hours became −0.46 (95% CI −8.0 to −0.11, P = 0.01) for those who received pre‐incision scalp block and −1.54 (95% CI −3.33 to 0.26, P = 0.09) for those who received post‐incision scalp block.
6.4. Analysis.
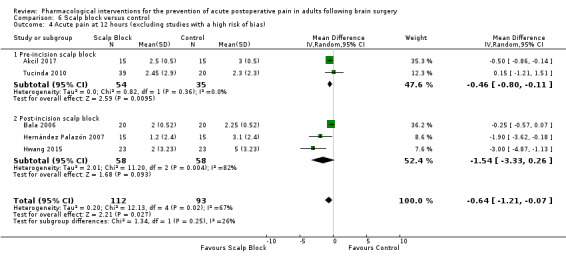
Comparison 6 Scalp block versus control, Outcome 4 Acute pain at 12 hours (excluding studies with a high risk of bias).
Mean difference in acute pain intensity at 24 hours became −0.86 (95% CI −1.84 to 0.12, P = 0.08, Figure 7). Mean difference in acute pain intensity at 24 hours became −0.63 (95% CI −1.14 to −0.22, P = 0.004) for those who received pre‐incision scalp block and −1.61 (95% CI ‐4.35 to 1.14, P = 0.25) for those who received post‐incision scalp block.
7.
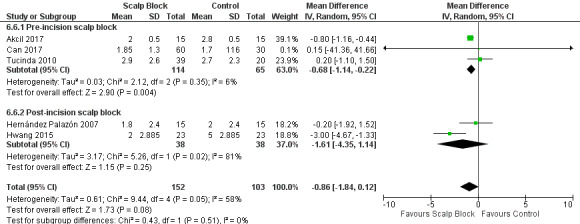
Forest plot of comparison: 6 Scalp block versus control, outcome: 6.6 Acute pain at 24 hours (excluding studies with a high risk of bias).
Mean difference in acute pain intensity at 48 hours became −0.91 (95% CI −3.04 to 1.25, P = 0.41, Analysis 6.8).
6.8. Analysis.
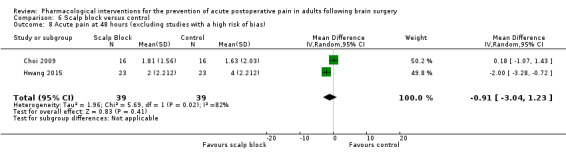
Comparison 6 Scalp block versus control, Outcome 8 Acute pain at 48 hours (excluding studies with a high risk of bias).
Additional analgesia requirements
Exclusion of studies with a high risk of bias, changed the pooled estimate of effect to −1.71 (95% CI −2.95 to −0.46, P = 0.007, Analysis 6.11). Using Cohen's rule of thumb, this represents a large effect size. Neither pre‐ or post‐incision scalp blocks showed superiority.
6.11. Analysis.
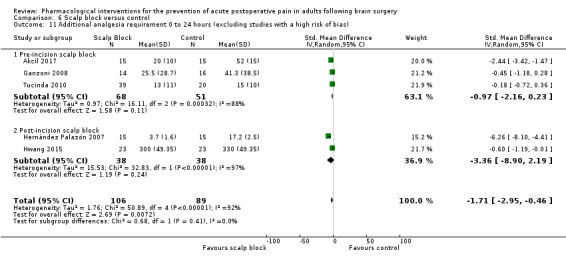
Comparison 6 Scalp block versus control, Outcome 11 Additional analgesia requirement 0 to 24 hours (excluding studies with a high risk of bias).
Excluding studies with missing data considered to be missing for reasons likely related to either the intervention or outcomes studied
No studies were found where missing data was thought to be missing for reasons related to the intervention or outcomes being measured, so this analysis was not conducted.
Other pharmacological interventions
No other pharmacological interventions were found to have two or more eligible studies addressing relevant comparable outcomes so no pooled estimates of effect could be calculated for studies of opioids, flupirtine, intravenous lidocaine or sphenopalatine ganglion blocks.
Discussion
Summary of main results
Pain intensity
For the primary outcome of postoperative pain intensity, nonsteroidal anti‐inflammatories (NSAIDs) were beneficial up to 24 hours, dexmedetomidine was effective in the first 12 hours and pregabalin or gabapentin were effective in the first six hours after surgery.
When studies with a high risk of bias were excluded, scalp blocks were effective at 12 hours and scalp infiltration at 48 hours but not at earlier time points.
Acetaminophen did not show any benefit.
Additional analgesia requirements
In the first 24 hours after surgery, dexmedetomidine and scalp blocks significantly reduced additional analgesia requirements with the limitation of the quality of the evidence being low to very low. Nonsteroidal anti‐inflammatories (NSAIDs), scalp infiltration, gabapentin or pregabalin and acetaminophen did not show any benefit.
Intervention timing
When studies with a high risk of bias were excluded, post‐incision scalp blocks were effective at reducing early acute postoperative pain (0 to 6 hours), and pre‐incision scalp blocks were effective at reducing postoperative pain at 12 and 24 hours.
Adverse events
The only significant difference detected was low‐quality evidence for a lower risk of nausea and vomiting in those treated with pregabalin or gabapentin.
Length of hospital stay
Acetaminophen did not alter length of stay in hospital (this was the only intervention in which this outcome was studied),
Other outcomes and interventions
There were insufficient data to:
make accurate conclusions regarding the effects of the included interventions on overall analgesic success, sedative effects, the incidence of chronic headache or length of stay in critical care;
determine the effect of the following interventions on the intensity of postoperative pain: opioids, flupirtine, intravenous lidocaine and sphenopalatine ganglion blocks.
Overall completeness and applicability of evidence
The evidence obtained from this review provides a reasonably comprehensive picture of the potential role of several common pharmacological interventions in the prevention of post‐craniotomy pain up to 48 hours postoperatively, helping clinicians to choose which interventions may provide the best analgesic benefit for patients. The strengths of this review include its broad search strategy without language restriction, the inclusion of unblinded, as well as blinded trials, the capture of a wide range of pharmacological interventions, and the focus on patient‐centred outcomes.
There are, however, some very important limitations to the completeness of this evidence, which in turn limit its clinical applicability. These include data quality, data quantity and heterogeneity, as detailed below.
Data quantity
There were not enough data to provide an overall measure of the effect in this population for the following outcomes:
prevention of postoperative pain: opioids, flupirtine, intravenous lidocaine, sphenopalatine ganglion blocks;
analgesic success;
additional analgesic requirement: opioids, flupirtine, intravenous lidocaine, sphenopalatine ganglion blocks;
sedation;
chronic headache;
length of stay in critical care;
adverse events, other than hypotension and nausea and vomiting;
effects of site of surgery, steroids and anaesthetic technique on the pain prevention effects of any interventions or medications.
Data quality
This is discussed in the next section (Quality of the evidence).
Heterogeneity
While several pharmacological interventions (scalp infiltration, scalp blocks, acetaminophen and dexmedetomidine) were relatively clinically homogenous in terms of their components and methods of administration, some interventions (NSAIDs, gabapentin and pregabalin) were not. Several different medications (diclofenac, ibuprofen, parecoxib, dexketoprofen and metamizole) were grouped together to provide an estimate of the overall efficacy of NSAIDs; while these drugs all belong to the same pharmacological group, they may well differ in their clinical efficacy. They were also administered at different time points relative to surgery making it more difficult to judge their overall effect as a group. Furthermore, there were not enough data to separate out their efficacy based on the timing of their administration relative to surgery.
The pooling of gabapentin and pregabalin provided an estimate for the efficacy of two very similar GABA‐like (gamma‐aminobutyric acid) drugs, that while they share a similar mechanism of action, they have several pharmacokinetic and pharmacodynamic differences which may affect their efficacy. Several of the effect estimates were also limited by significant unexplained important heterogeneity.
Quality of the evidence
Acute postoperative pain intensity
For the primary outcome of acute postoperative pain intensity, the quality of the evidence varied across interventions.
For NSAIDs, the overall quality of the evidence was judged to be high (Table 1).
For dexmedetomidine, the evidence was also judged to be of moderate quality for the pooled estimate of effect for acute postoperative pain intensity in the first six hours after surgery, after having been being downgraded one level due to a small pooled sample size. For postoperative pain intensity at 12 hours and at 24 hours, the quality of the evidence was judged to be low, due to a small pooled sample size in addition to unexplained important heterogeneity and imprecision respectively (Table 2).
For pregabalin or gabapentin, the evidence was judged to be of low quality at all time points due to small pooled sample sizes and possible indirectness of effect due to difference in pharmacological properties between the two drugs (Table 3).
For acetaminophen, the quality of the evidence for acute postoperative pain intensity in the first 6 hours and at 12 hours was judged to be moderate, being downgraded one level due to a small pooled sample size. The quality of the evidence for pain intensity at 24 hours was judged to be high (Table 4).
For scalp infiltration, the overall quality of the evidence was judged to be moderate for the pooled estimates of effect for acute postoperative pain intensity in the first 6, 24 and 48 hours after surgery after having been being downgraded one level due to unexplained important heterogeneity or small sample sizes. For acute postoperative pain intensity at 12 hours, the quality of the evidence was judged to be low due to a small pooled sample size and loss of significance of results on sensitivity analysis (Table 5).
For scalp block, the overall quality of the evidence was judged to be low for the pooled estimates of effect for acute postoperative pain intensity in the first 6 hours, at 12 hours and at 24 hours after surgery, after having been downgraded two levels due to unexplained important heterogeneity and failure to retain significance of results on sensitivity analysis. For acute postoperative pain intensity at 48 hours, the quality of the evidence was judged to be very low, due to unexplained important heterogeneity, small pooled sample size and failure to retain the initial beneficial effect with sensitivity analysis (Table 6).
Additional analgesia requirement
For the outcome of additional analgesia requirement in the first 24 hours postoperatively, a low quality rating was assigned to the pooled estimates of effect for NSAIDs (due to a small pooled sample size and a wide 95% CI), dexmedetomidine and scalp block (due to a small pooled sample size and unexplained important heterogeneity) (Table 1; Table 2; Table 6), and gabapentin and pregabalin due to a small pooled sample size and indirectness of effect (Table 3). A high quality rating was assigned to the pooled estimate of effect for acetaminophen (Table 4).
A very low‐quality rating was also assigned to the effect estimate for scalp infiltration due to a small pooled sample size, a wide 95% CI and unexplained important heterogeneity (Table 5).
A high‐quality rating was assigned to the effect estimate for acetaminophen (Table 4).
Length of hospital stay (hours)
For the one intervention (acetaminophen) that measured this outcome, the quality of the evidence was judged to be moderate, being downgraded one level due to a small pooled sample size (Table 4).
Adverse event: nausea and vomiting
The quality of the evidence for this outcome was judged to low for NSAIDs, dexmedetomidine and scalp infiltration, being downgraded two levels due to a small number of total events and wide 95% CIs (Table 1; Table 2; Table 5). The quality of the evidence for this outcome for gabapentin or pregabalin was also judged to be low due to a small number of total events and indirectness, as the two drugs differ somewhat in their pharmacological properties (Table 3). The quality of the evidence for this outcome for scalp blocks was judged to be very low on the basis of a small number of total events, unexplained important heterogeneity and a wide 95% CI (Table 6).
Potential biases in the review process
Differences between the protocol and review
Due to the way in which data were reported in the included studies, it was necessary to make several deviations from the protocol in an effort to make the best use of available data (see Differences between protocol and review). The change from the intended measure of pain intensity over time periods to the actual measure of pain intensity at discrete times compromises the accuracy of any inference regarding the analgesic efficacy of included interventions at time points other than those analysed. Similarly, the change in the measure of additional analgesia consumption over the intended four time periods to the single 0 to 24‐hour period (which was again made to reflect the way in which studies reported this outcome), means that inferences about time periods outside 0 to 24 hours postoperatively are subject to inaccuracy.
We chose to exclude studies that investigated the use of agents with analgesic potential for non‐analgesic purposes. The rationale for this decision was based on a high likelihood of important differences in inclusion and exclusion criteria, dosages, timing, ancillary analgesic usage, and attributable side effects between studies that investigated these agents for their analgesic efficacy and studies that investigated them for their non‐analgesic effects. While this approach meant that potential outcomes of interest were not captured when these agents were investigated for their other non‐analgesic effects, it provided a more accurate estimate of the effects and side effects of those agents, when used with analgesic intent.
Approach to overall risk of bias judgements
To determine whether a study had an overall high risk of bias, we choose the following definition: a high risk of bias in four or more of the seven domains of bias, or a high risk of bias in three or more of the seven domains of bias with an unclear risk of bias in one or more domain. This approach is more liberal than the Cochrane guidance (Higgins 2011), which recommends classifying a study at an overall high risk of bias if it is deemed to be at high risk of bias in one or more domains or raises concerns across multiple domains. We choose a more liberal definition on the basis of the following considerations.
Firstly, our review examined a discrete population with higher short‐term morbidity and mortality than many other study populations. Clinical studies in this population often have to be conducted with smaller sample sizes and are subject to early losses to follow‐up due to a higher incidence of postoperative complications, need for advanced life support and relatively high postoperative mortality. Classifying studies as having an overall high risk of bias on the basis of high loss to follow‐up alone would have incurred the risk of undervaluing a sizeable amount of data, and limiting external validity by giving greater credence to studies that may have included participants with lower perioperative mortality and morbidity than the average patient undergoing brain surgery. While small sample sizes are common in clinical studies of discrete populations, and while we chose not to use this criterion in isolation to judge a study as being at high risk of bias, we did downgrade the quality of evidence for effect estimates where the pooled sample size was small.
Secondly, many of our included studies were deemed to be at high of bias due to lack of allocation concealment. While allocation concealment is an important measure to prevent selection bias, classifying studies that did not report it as having an overall high risk of bias would likely overestimate the true occurrence and impact of selection bias for trials that were conducted in a discrete group of patients with similar baseline characteristics who were undergoing similar surgery and whose postoperative outcomes would be assessed over similar time periods. While allocation concealment would be the ideal measure to ensure minimization of selection bias, its absence does not necessarily imply that research personal would have either the inclination or opportunity to preselect patients from an already predefined cohort to achieve a particular result or that the probability of achieving that result would be significantly altered by their actions. The complex reality of pain physiology with psychosocial, genetic, biochemical and, as yet, unknown mechanisms all feeding into individual pain perception and pain reporting make predicting individual postoperative pain outcomes among otherwise similar groups of patients very difficult and not likely to be easily predicted or influenced by research personnel.
Thirdly, although blinding is important for validity, for patient‐reported pain outcomes, the impact of lack of blinding on study validity is likely to be greater when the study participants are not blinded then when the study personnel are not blinded. A lack of blinding of study personnel would be much more likely to compromise validity where study participants are not blinded either. All three studies that we deemed to have a high risk of bias due to lack of blinding of study participants, (Choi 2009; Kiskira 2006; Zhang 2003), were also at high risk of bias due to lack of blinding of study personnel and all three studies were deemed to have an overall high risk of bias based on our criteria.
Our more liberal definition seemed a sensible approach to estimating the overall risk of bias for studies of the efficacy of different interventions for preventing patient‐reported pain in those undergoing brain surgery. However, it may have underestimated the overall risk of bias for studies where a high risk or unclear risk of bias in any particular domain may have had a particular influence on study validity. What effect these factors have in isolation on overall study validity are difficult to estimate but it is probably fair to say they are more likely to compromise it significantly if other efforts to reduce risk of bias are not robust.
Scope of the review
Another important limitation of this review is that it addressed only pharmacological interventions aimed at preventing postoperative pain and did not address other approaches to pain prevention, including acupuncture, hypnosis or psychological techniques, some of which have been shown to be effective in other surgical populations (Powell 2016).
Agreements and disagreements with other studies or reviews
We found three other systematic reviews of pain relief after craniotomy.
The study by Hansen and colleagues, (Hansen 2011), included only double‐blind trials in which the results were published in English. Four interventions were included: scalp block, scalp infiltration, morphine and parecoxib, but no pooled estimates of effect were calculated. They concluded that scalp block may provide analgesia up to six hours postoperatively, scalp infiltration may provide analgesia adequate analgesia for the first few hours after surgery, morphine may reduce additional analgesia consumption, with little evidence to support the use of parecoxib.
A systematic review and meta‐analysis of regional scalp block after craniotomy including seven RCTs (Gilfoyle 2012), found a pooled mean reduction in pain score (measured on a scale of 0 to 10) at one hour postoperatively of −1.61 (95% CI −2.06 to −1.15; P < 0.001). Subgroup analysis showed that preoperative scalp block reduced pain significantly in the first four hours after surgery while postoperative scalp block reduced pain significantly up to 12 hours postoperatively. There was also an overall reduction in the opioid requirements over the first 24 hours postoperatively. The authors concluded scalp block had a role in the reduction of pain after craniotomy.
A recently published review of 19 RCTs showed that opioids provided superior pain relief over other analgesics (Tsaousi 2016), however there were some important limitations to their findings: four of the five included studies examining the role of opioids for pain relief after craniotomy used an active comparator rather than a control with the comparison being between different opioid regimens rather than an evaluation of the efficacy of opioids alone; no pooled estimate of effect was calculated; and no studies published in a language other than English or published prior to 2011 were included. The same study evaluated scalp infiltration and scalp blocks together and only three RCTs were included. The results of these studies were presented separately with no overall assessment of effect, making it difficult to draw any conclusion regarding overall effectiveness. The authors found some evidence to support the use of diclofenac and dexmedetomidine with the caveat that the number of included studies for each intervention was small (three RCTs each).
When comparing our results to these three published reviews, it is important to note these comparisons are limited by the methodologic differences between our review and these published studies. Overall, our review captures a broader view of the available evidence as it is neither language‐restricted or intervention‐specific. It also differs from the study by Tsaosi in that only interventions evaluated against a control or placebo were included.
Both Hansen and colleagues (Hansen 2011), and Gilfoyle and colleagues (Gilfoyle 2012) reported beneficial effects of scalp blocks in reducing early postoperative pain. When we excluded studies with a high risk of bias, we did not find benefit in the very early postoperative period but did find that scalp block reduced pain at 12 hours. Interestingly, our subgroup analysis results differed from those of Gilfoyle, in that we found that scalp blocks performed before surgical incision reduced pain at 12 and 24 hours postoperatively, while those performed after the surgical incision, reduced pain in the first 6 hours. These effects were only seen when we excluded studies with a high risk of bias which may in part explain why our findings differed from those of Gilfoyle. When we did not exclude studies with a high risk of bias, we found the post‐incision scalp block produced significant pain relief at all time points up to and including 24 hours.
Unlike the study by Hansen (Hansen 2011), we did not find that scalp infiltration reduced early postoperative pain but we did find that it reduced pain at 48 hours.
Similar to the study by Tsaousi (Tsaousi 2016), we found that both NSAIDs and dexmedetomidine may have a role in the reduction of post‐craniotomy pain.
Regarding the role of opioids, we did not find an adequate number of eligible studies to calculate a pooled estimate of effect for their effect on postoperative pain intensity or additional analgesia consumption, and so can neither refute nor confirm their role in either regard.
Comparing our results to systematic reviews of postoperative analgesia in wider surgical populations, there are some commonalities.
Doleman and colleagues (Doleman 2018), addressed the role of pre versus post‐incision opioids in adults undergoing all types of surgery and did not find any significant difference in analgesic efficacy with the important caveat that their findings were severely limited by both the quantity and quality of evidence. We encountered a lack of evidence to guide intervention timing, with scalp block being the only intervention with enough high‐quality studies to provide robust information regarding the efficacy of pre‐ versus post‐incision blocks.
Jessen Lundoff and colleagues (Jessen Lundorf 2016,) examined the role of dexmedetomidine in pain reduction after abdominal surgery and found that it seemed to have an opioid‐sparing effect but did not, in general, reduce acute postoperative abdominal pain. Their results contrast with our findings of a reduction in pain in the first 12 hours after brain surgery in patients receiving dexmedetomidine, but are concordant with our findings of reduced additional analgesia requirements in those who received dexmedetomidine. However, it is important to note that the quality of the evidence that we found for the postoperative analgesic effect of dexmedetomidine was generally low. The only moderate‐quality evidence available was for its analgesic potential in the first six hours after surgery.
It makes physiological sense for a reduction in additional analgesia requirement to be related to analgesic benefit and so it is reasonable to assume that an effective analgesic would lessen the need for other analgesics. However, like Lundoff, our review did not find the two effects were consistently linked. We found, for instance, that NSAIDs provided effective pain relief for up to 24 hours after brain surgery, but did significantly result in reduced additional analgesia requirements in the same time period. The reason for this dichotomy is uncertain, with the quality and quantity of the evidence being a confounding factor when determining whether it is a true finding or not.
Authors' conclusions
Implications for practice.
There is high‐quality evidence that NSAIDs reduce pain up to 24 hours postoperatively. The evidence for reductions in pain with dexmedetomidine, pregabalin or gabapentin, scalp blocks, and scalp infiltration is less certain and of generally low quality. There is low‐quality evidence that scalp blocks and dexmedetomidine may reduce additional analgesics requirements. There is evidence that gabapentin or pregabalin may decrease nausea and vomiting, with the caveat that the total number of events for this comparison was low.
Implications for research.
Future studies addressing this research question would benefit from focusing on some of the limitations we encountered with current evidence.
Specifically:
Most existing data arise from studies of pain measured at discrete time points, making the assessment of pain as a true continuous outcome very difficult. To get an accurate picture of postoperative pain, future studies would benefit from measuring it over time periods rather than at discrete time points.
We found most eligible studies did not address pain or additional analgesia requirements beyond the first 24 hours postoperatively; in order to get a more complete picture of the effect of any pain‐reducing intervention, future studies should consider addressing pain outcomes beyond the first 24 hours.
There is currently a paucity of data regarding chronic headache after craniotomy and the role of pain‐reducing interventions in its incidence.
Aside from nausea and vomiting, other adverse events were not generally widely studied or well defined; future studies should ensure that clearly defined adverse events are included in their outcomes.
Some of our findings were limited by the methodologic quality of the included studies; as with all research questions, methodologic rigour should be a priority to provide an accurate answer to the question being addressed.
Given the limitations we encountered, our study did provide some potentially interesting information that may inform future research in this area:
NSAIDs showed significant analgesic efficacy up to 24 hours postoperatively. However, one should bear in mind their potential side effects which are particularly relevant in this population;
We found it somewhat surprising that while NSAIDs produced a reduction in pain intensity; this did not translate into a significant reduction in additional analgesia requirements and this would be worth investigating further;
Gabapentin or pregabalin reduced pain up to six hours postoperatively, with the limitation of this finding being based on low‐quality evidence;
Dexmedetomidine is a relative newcomer to the postoperative analgesia scene and may have a role in the reduction of early postoperative pain after craniotomy. Further studies addressing its effects in this population would help establish whether the benefit we observed is real or not. The generally low quality of the evidence we found for its postoperative analgesic potential in this population underscores the uncertainty regarding its effectiveness;
Similar to previous reviews, we found scalp block to be beneficial for acute postoperative pain but, in contrast to previous reviews, we found that scalp blocks performed before surgery might provide more prolonged pain relief while those performed after surgery might provide more prompt pain relief;
While scalp infiltration reduced pain 48 hours after surgery, we did not find that it was effective in the reduction of pain at any time prior to this.
Acknowledgements
We wish to thank Laurie Scott (Librarian, Bracken Health Sciences Library, Queen's University) for her assistance with the retrieval of studies relevant to the preparation of the protocol. We wish to thank Amanda Ross (Librarian, Bracken Health Sciences Library, Queen's University) for conducting the search for the completed review. We would also like to thank Janne Vendt, Cochrane Information Specialist for her expertise and refinement of our most recent search.
We would like to thank Javier Eslava‐Schmalbach (Content Editor), Vibeke E Horstmann and Susanne Schmitz, (Statistical Editors), Andrew Moore, Federico Bilotta, Keith Ruskin (Peer Reviewers), Roy Buffery (Consumer Referee), Teo Quay (Managing Editor), and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review. We would also like to thank Jane Cracknell (Managing Editor of Cochrane Anaesthesia during conduct of this review) for her assistance with the preparation of this review.
Appendices
Appendix 1. Search Strategy
MEDLINE (R) ALL Ovid
1 Craniotomy/
2 Decompressive Craniectomy/
3 Trephining/
4 craniotomy.ab,ti.
5 craniectomy.ab,ti.
6 (brain adj3 (surg* or operat*)).ab,ti.
7 (post?craniotom* or post craniotom*).ab,ti.
8 (post?craniectom* or post craniectom*).ab,ti.
9 exp Brain Neoplasms/su [Surgery]
10 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
11 exp Narcotics/
12 exp Analgesics/
13 exp Anesthetics, Local/
14 exp Aminopyridines/
15 flupirtine.ab,ti.
16 acetominophen.ab,ti.
17 acetaminophen.ab,ti.
18 morphine.ab,ti.
19 tramadol.ab,ti.
20 codeine.ab,ti.
21 paracetemol.ab,ti.
22 paracetamol.ab,ti.
23 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24 Pain, Postoperative/
25 exp Pain/ or pain.ti,ab.
26 Acute Pain/
27 headache/ or slit ventricle syndrome/
28 24 or 25 or 26 or 27
29 10 and 28
30 randomised controlled trial.pt.
31 controlled clinical trial.pt.
32 randomi?ed.ab.
33 placebo.ab.
34 drug therapy.fs.
35 randomly.ab.
36 trial.ab.
37 groups.ab.
38 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39 exp animals/ not humans.sh.
40 38 not 39
41 10 and 40
42 23 or 28
43 41 and 42
44 limit 43 to "all adult (19 plus years)"
45 limit 43 to "all child (0 to 18 years)"
46 43 not 45
47 44 or 46
Embase Ovid
1 craniotomy/
2 skull surgery/
3 cranioplasty/
4 craniectomy/
5 decompressive craniectomy/
6 exp brain surgery/
7 exp brain tumor/su
8 craniotomy.ab,ti.
9 craniectomy.ab,ti.
10 (post?craniotom* or post craniotom*).ab,ti.
11 (post?craniectom* or post craniectom*).ab,ti.
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
13 exp narcotic agent/
14 exp analgesic agent/
15 exp local anesthetic agent/
16 aminopyridine derivative/
17 flupirtine/
18 paracetamol/
19 exp morphine/
20 tramadol/
21 exp codeine/
22 flupirtine.ab,ti.
23 acetominophen.ab,ti.
24 acetaminophen.ab,ti.
25 morphine.ab,ti.
26 tramadol.ab,ti.
27 codeine.ab,ti.
28 paracetemol.ab,ti.
29 paracetamol.ab,ti.
30 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31 postoperative pain/
32 pain/
33 exp "headache and facial pain"/
34 pain.ab,ti.
35 31 or 32 or 33 or 34
36 12 and 30 and 35
37 crossover procedure/
38 double blind procedure/
39 randomised controlled trial/
40 single blind procedure/
41 random$.mp.
42 factorial$.mp.
43 crossover$.mp.
44 cross‐over$.mp.
45 cross over$.mp.
46 placebo$.mp.
47 (doubl$ adj blind$).mp.
48 (singl$ adj blind$).mp.
49 assign$.mp.
50 allocat$.mp.
51 volunteer$.mp.
52 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51
53 36 and 52
54 12 and 35 and 52
55 exp animal/ not exp human/
56 exp child/ not exp adult/
57 54 not 55
58 57 not 56
Central
#1 Craniotomy or craniectomy or trephining
#2 Pain, Postoperative or Pain or Acute Pain or headache or “slit ventricle syndrome”
#3 #1 and #2
Web of Science
#1 TS=(Craniotomy OR Craniectomy OR Trephining OR “brain surg*” OR postcraniotomy OR postcraniectomy) AND TS=(Pain OR “postoperative pain” OR headache* OR “acute pain” OR “slit ventricle syndrome”)
#2 TS=(“randomised controlled trial” or “controlled clinical trial” or placebo” or “drug therapy” or random* or trial or groups)
#3 #2 AND #1
CINAHL
S1 (MH "Craniotomy") OR (MH "Decompressive Craniectomy") OR (MH "Brain Surgery")
S2 "Trephining" Expanders ‐ Apply related words
S3 (MH "Brain Neoplasms/SU")
S4 AB craniotomy Expanders ‐ Apply related words
S5 TI craniotomy Expanders ‐ Apply related words
S6 AB craniectomy Expanders ‐ Apply related words
S7 TI craniectomy Expanders ‐ Apply related words
S8 AB post?craniotomy Expanders ‐ Apply related words
S9 AB postcraniotomy Expanders ‐ Apply related words
S10 AB post‐craniotomy Expanders ‐ Apply related words
S11 TI postcraniotomy Expanders ‐ Apply related words
S12 TI post‐craniotomy Expanders ‐ Apply related words
S13 AN postcraniectomy Expanders ‐ Apply related words
S14 AN postcraniectomy Expanders ‐ Apply related words
S15 AB postcraniectomy Expanders ‐ Apply related words
S16 AB post‐craniectomy Expanders ‐ Apply related words
S17 TI postcraniectomy Expanders ‐ Apply related words
S18 TI post‐craniectomy Expanders ‐ Apply related words
S19 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18
S20 (MH "Pain+") Expanders ‐ Apply related words
S21 (MH "Postoperative Pain") Expanders ‐ Apply related words
S22 (MH "Headache+") Expanders ‐ Apply related words
S23 TI pain Expanders ‐ Apply related words
S24 AB pain Expanders ‐ Apply related words
S25 S20 OR S21 OR S22 OR S23 OR S24
S26 S19 AND S25
Appendix 2. Study selection form
| STUDY TITLE: | ||
| AUTHORS: | ||
| SOURCE: | ||
| STUDY CHARACTERISTICS | YES (Please tick as appropriate) | NO (Please tick as appropriate) |
| Section 1. Design | ||
| Prospective study | ||
| Controlled trial | ||
| Randomized | ||
| Control | ||
|
YES to all – complete section 2. NO to 1 or more – exclude | ||
| Section 2. Participants | ||
| Participants ≥ 18 yrs | ||
| Craniotomy or craniectomy | ||
|
YES to all – complete section 3. NO to 1 or more – exclude | ||
| Section 3. Interventions | ||
| Pharmacological intervention | ||
| For prevention as opposed to relief of established pain after craniotomy | ||
| Compared against placebo control | ||
|
YES to all – complete section 4. NO to 1 or more – exclude | ||
| Section 4. Outcomes | ||
| Validated measure of pain intensity in the first 4 days postoperatively | ||
| Additional analgesia consumption in the first 4 days postoperatively | ||
| Validated measure of depth of sedation in the same time period | ||
| Any adverse event in the first 4 days postoperatively | ||
|
YES to any ‐ include NO to all ‐ exclude | ||
Appendix 3. Data extraction form
| Review ID |
CARG 308 Interventions for the prevention of acute postoperative pain in adults following brain surgery |
| Review Author ID | |
| Study | |
| Study ID | |
| Citation | |
| Source | |
| Methods | |
| Study design | |
| Study start date | |
| Study end date | |
| Study duration | |
| Randomization method | |
| Sequence generation | |
| Allocation concealment method | |
| Blinded | Yes / No |
| Blinding method | |
| Blinding adequacy | |
| Participants | |
| Number | |
| Mean age | |
| No. of male participants | |
| Setting | |
Surgical procedure type
|
|
Anaesthetic type
|
|
| Inclusion criteria | |
| Exclusion criteria | |
| Interventions | |
| No of arms | |
| Intervention type | |
|
Intervention timing Pre‐, intra‐, postoperatively |
|
| Route of administration | |
| Dosage | |
| Duration | |
| Intervention Integrity | |
| Ancillary treatment | |
| Adjunctive therapy | |
| Outcomes | |
| Primary | |
| Secondary | |
| Adverse events | |
| Reported events | |
| Definitions used for each event | |
| Severity | |
| Seriousness | |
| Association with intervention | |
| Results | |
| No. of participants allocated to each treatment arm | |
| No. who received each treatment arm | |
| No. who did not receive intended treatment and why | |
| Number followed up | |
| Number lost to follow‐up and why | |
| Number included in the final analysis | |
| Analysis | |
| Method | |
| Sample size details | |
| Reported effect size | |
| Authors conclusions | |
| Funding source | |
| Reviewer comments | |
Data and analyses
Comparison 1. NSAIDs versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute pain 0 to 6 hours | 6 | 742 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.64, ‐0.58] |
| 2 Acute pain at 12 hours | 6 | 742 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.22, ‐0.26] |
| 3 Acute pain at 24 hours | 6 | 742 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.26, ‐0.14] |
| 4 Additional analgesia requirements 0 to 24 hours | 4 | 265 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐4.85, 2.72] |
| 5 Nausea and vomiting | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.30, 5.94] |
1.3. Analysis.
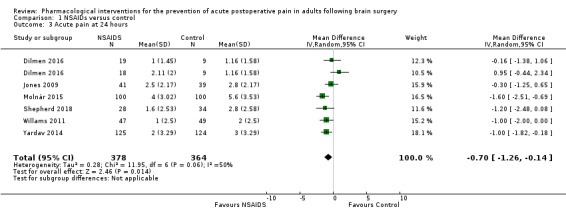
Comparison 1 NSAIDs versus control, Outcome 3 Acute pain at 24 hours.
Comparison 2. Dexmedetomidine versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute pain 0 to 6 hours | 2 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.27, ‐0.51] |
| 2 Acute pain at 12 hours | 2 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.21, ‐0.42] |
| 3 Acute pain at 24 hours | 2 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.32, 0.16] |
| 4 Additional analgesia requirements 0 to 24 hours | 2 | 128 | Mean Difference (IV, Random, 95% CI) | ‐21.36 [‐34.63, ‐8.09] |
| 5 Nausea and vomiting | 4 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.06, 3.08] |
| 6 Hypotension | 3 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.28] |
Comparison 3. Gabapentin and pregabalin versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute pain 0 to 6 hours | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.90, ‐0.34] |
| 2 Acute pain at 24 hours | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐2.06, 0.51] |
| 3 Acute pain at 48 hours | 2 | 202 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.29, 0.26] |
| 4 Additional analgesia requirement 0 to 24 hours | 3 | 235 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.10, 0.35] |
| 5 Nausea and vomiting | 3 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.29, 0.89] |
Comparison 4. Acetaminophen versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute pain 0 to 6 hours | 3 | 332 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.00, 0.30] |
| 2 Acute pain at 12 hours | 3 | 332 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.04, 0.03] |
| 3 Acute pain at 24 hours | 4 | 439 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.20, 0.52] |
| 4 Additional analgesia requirement 0 to 24 hours | 4 | 459 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.86, 0.99] |
| 5 Length of stay in hospital (hours) | 2 | 335 | Mean Difference (IV, Random, 95% CI) | ‐3.71 [‐14.12, 6.70] |
Comparison 5. Scalp infiltration versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute pain 0 to 6 hours | 9 | 475 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.28, ‐0.00] |
| 1.1 Pre‐incision scalp infiltration | 5 | 216 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.80, 0.52] |
| 1.2 Post‐incision scalp infiltration | 4 | 259 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.84, ‐0.12] |
| 2 Acute pain 0 to 6 hours (excluding studies with a high risk of bias) | 6 | 362 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.43, 0.35] |
| 2.1 Pre‐incision scalp infiltration | 4 | 180 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.13, 0.52] |
| 2.2 Post‐incision scalp infitration | 2 | 182 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.22, 0.44] |
| 3 Acute pain at 12 hours | 7 | 309 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.34, ‐0.08] |
| 3.1 Pre‐incision scalp infiltration | 4 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.46, 0.41] |
| 3.2 Post‐incision scalp infiltration | 3 | 129 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.77, ‐0.50] |
| 4 Acute pain at 12 hours (excluding studies with a high risk of bias) | 5 | 232 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.31, 0.61] |
| 5 Acute pain at 24 hours | 6 | 260 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.06, 0.27] |
| 5.1 Pre‐incision scalp infiltration | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.84, 0.81] |
| 5.2 Post‐incision scalp infiltration | 3 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.72, 0.17] |
| 6 Acute pain at 24 hours (excluding studies with a high risk of bias) | 4 | 183 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.73, 0.72] |
| 7 Acute pain at 48 hours | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐2.13, ‐0.06] |
| 8 Acute pain at 48 hours (excluding studies with a high risk of bias) | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.20, ‐0.32] |
| 9 Nausea and vomiting | 4 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.48, 1.14] |
| 10 Additional analgesia requirements (in milligrams) 0 to 24 hours | 6 | 345 | Mean Difference (IV, Random, 95% CI) | ‐9.56 [‐15.64, ‐3.49] |
| 10.1 Pre‐incision scalp infiltration | 4 | 217 | Mean Difference (IV, Random, 95% CI) | ‐12.54 [‐25.20, 0.13] |
| 10.2 Post‐incision scalp infiltration | 2 | 128 | Mean Difference (IV, Random, 95% CI) | ‐8.57 [‐13.26, ‐3.87] |
| 11 Additional analgesia requirements 0 to 24 hours (excluding studies with a high risk of bias) | 4 | 229 | Mean Difference (IV, Random, 95% CI) | ‐8.16 [‐16.50, 0.18] |
5.6. Analysis.
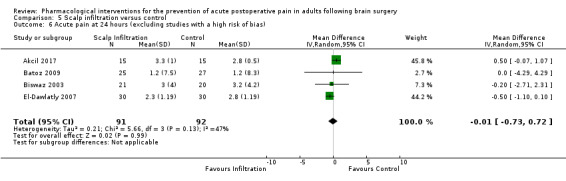
Comparison 5 Scalp infiltration versus control, Outcome 6 Acute pain at 24 hours (excluding studies with a high risk of bias).
Comparison 6. Scalp block versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute pain 0 to 6 hours | 10 | 414 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.66, ‐0.30] |
| 1.1 Pre‐incision scalp block | 4 | 209 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.53, 0.15] |
| 1.2 Post‐incision scalp block | 6 | 205 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐2.28, ‐0.73] |
| 2 Acute pain 0 to 6 hours (excluding studies with a high risk of bias) | 7 | 325 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.98, 0.05] |
| 2.1 Pre‐incision scalp block | 4 | 209 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.54, 0.15] |
| 2.2 Post‐incision scalp block | 3 | 116 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐2.44, ‐0.98] |
| 3 Acute pain at 12 hours | 8 | 294 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.53, ‐0.37] |
| 3.1 Pre‐incision scalp block | 2 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.80, ‐0.11] |
| 3.2 Post‐incision scalp block | 6 | 205 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐2.64, ‐0.44] |
| 4 Acute pain at 12 hours (excluding studies with a high risk of bias) | 5 | 205 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.21, ‐0.07] |
| 4.1 Pre‐incision scalp block | 2 | 89 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.80, ‐0.11] |
| 4.2 Post‐incision scalp block | 3 | 116 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐3.33, 0.26] |
| 5 Acute pain at 24 hours | 9 | 433 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.52, ‐0.05] |
| 5.1 Pre‐incision scalp block | 4 | 268 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.76, 0.81] |
| 5.2 Post‐incision scalp block | 5 | 165 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐1.00, ‐0.59] |
| 6 Acute pain at 24 hours (excluding studies with a high risk of bias) | 5 | 255 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.84, 0.12] |
| 6.1 Pre‐incision scalp block | 3 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.14, ‐0.22] |
| 6.2 Post‐incision scalp block | 2 | 76 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐4.35, 1.14] |
| 7 Acute pain at 48 hours | 4 | 135 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐2.57, ‐0.11] |
| 8 Acute pain at 48 hours (excluding studies with a high risk of bias) | 2 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐3.04, 1.23] |
| 9 Additional analgesia requirement 0 to 24 hours | 7 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.97, ‐0.25] |
| 9.1 Pre‐incision scalp block | 4 | 208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.52, 0.28] |
| 9.2 Post‐incision scalp block | 3 | 106 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐4.27, 0.03] |
| 10 Nausea and vomiting | 4 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.33, 1.32] |
| 11 Additional analgesia requirement 0 to 24 hours (excluding studies with a high risk of bias) | 5 | 195 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐2.95, ‐0.46] |
| 11.1 Pre‐incision scalp block | 3 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.16, 0.23] |
| 11.2 Post‐incision scalp block | 2 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.36 [‐8.90, 2.19] |
6.6. Analysis.
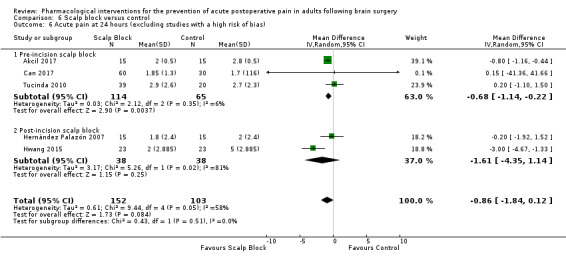
Comparison 6 Scalp block versus control, Outcome 6 Acute pain at 24 hours (excluding studies with a high risk of bias).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akcil 2017.
| Methods | Study design: randomized, placebo controlled study (3 arms) Study duration: May 2014 to December 2016 Study setting: hospital, single centre, Turkey |
|
| Participants | Adults undergoing elective infratentorial craniotomy (n = 45) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote ''Patients were randomized to one of three groups using a closed envelope technique.'' The authors describe the study as randomized, providing details about how allocations were concealed. However, they do not describe how random assignment was ensured |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''The patient and the anesthesiologist who recorded postoperative pain scores were blinded in every case. But the anesthesiologist who applied the scalp block and followed the haemodynamic response to pin fixation and skin incision were sometimes same person''. This implies that study personnel blinding was not consistent for their primary outcome of intraoperative haemodynamic response to pin insertion but was consistent for the their secondary postoperative pain outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''the patient and the anesthesiologist who recorded postoperative pain scores were blinded''. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/45 patients were excluded, 1 due to intraoperative blood loss and 1 due to a low level of consciousness at the end of surgery. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified |
| Other bias | Unclear risk | Small study |
Ali 2010.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: Febuary 2007 to September 2008 Study setting: hospital, single centre, Egypt |
|
| Participants | Adults undergoing elective trans‐nasal resection of pituitary tumours Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 1.5 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomized but method not reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study solutions were prepared by an investigator who was not otherwise involved in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study solutions were prepared by an investigator who was not otherwise involved in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Small study |
Artime 2018.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: 2013 to 2016 Study setting: hospital, single centre, USA |
|
| Participants |
Inclusion criteria
Exclusion criteria.
Mean age (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage As above |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding The study was sponsored by Mallinckrodt Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Randomization was performed by the hospital investigational pharmacy based on computer based random list generator''. |
| Allocation concealment (selection bias) | Low risk | Quote: ''Patients and all study personnel including research assistants, anaesthesiologists, neurosurgeons, and intensivists were blinded to group allocation''. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''Patients and all study personnel including research assistants, anaesthesiologists, neurosurgeons, and intensives were blinded to group allocation''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote ''This blind remained closed until after collection of data and analysis by the biostatistician''. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 patients in the intervention group were not included in the analysis (2 had mental status changes that precluded assessment, 2 had redo procedures and 1 had a different procedure). 9 patients in th control group were not included in the analysis (1 had a missing data sheet, 5 had complications, 3 withdrew from the study). |
| Selective reporting (reporting bias) | Unclear risk | The title focused on a positive secondary outcome while the primary outcome itself was not statistically significant. |
| Other bias | Unclear risk | Funded by a pharmaceutical company After losses to follow‐up the study was underpowered for its primary outcome. |
Bala 2006.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, India |
|
| Participants | Adult patients undergoing elective supratentorial craniotomy for brain tumours (n = 40) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp block of the following nerves with 0.5% bupivacaine and adrenaline 1:400,000 adrenaline
versus scalp block, with saline and adrenaline 1:400,000, at the end of surgery Dosage 20 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''patients were randomly divided into two groups using a computer generated random number chart''. |
| Allocation concealment (selection bias) | High risk | Authors did not provide any details regarding how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''the neurosurgeon, the anaesthetist performing the scalp block and the patients were blinded to the drug being administered''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''the anaesthetist performing the block did not participate in the postoperative pain assessment''. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants were followed up for outcomes. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Small study |
Batoz 2009.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: June 2006 to April 2007 Study setting: hospital, single centre, France |
|
| Participants | Adults undergoing elective craniotomy for tumour resection (n = 53) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage Not reported |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported that a computer‐generated randomization method was used. |
| Allocation concealment (selection bias) | High risk | Authors did not provide any details regarding how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The participants were blinded but the method and its adequacy was not described and no mention was made of blinding those who administered the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Those assessing outcomes were blinded to treatment received but again the method and its adequacy were not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were relatively few losses to follow‐up and clear reasons were provided for these: 52 of the 53 enrolled participants were followed up for the primary outcome, 1 participant being lost to follow‐up as their data were mislaid. 48 participants were followed up for pain outcomes at 2 months, 3 had died and 1 participant had moved away. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the priority in which they were specified. |
| Other bias | Unclear risk | Small study |
Bekker 2008.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, USA |
|
| Participants | Adults undergoing elective craniotomy (n = 72) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ''patients scheduled for elective craniotomy were randomly assigned to receive either sevoflurane–opioid or sevoflurane–opioid–DEX anaesthesia''. However, the method of randomization was not described. |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''the anaesthetic was managed by experienced neuroanaesthesiologists blinded to DEX or placebo regimen''. There was no mention of blinding participants, however as the infusion was started after the participants were anaesthetized and stopped before they were woken up, the lack of patient blinding is very unlikely to have had a significant impact on the results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''anesthesiologist and nurses who were unaware of anaesthetic technique managed postoperative recovery of the study patients''. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 participants were recruited but not included in the final analysis. Of these, 1 from each of the study groups was removed from analysis, 1 because of bleeding and the other because they remained intubated after surgery. The remaining 14 recruited participants were not included in the final analysis as technical problems precluded recovery of their data. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Small study |
Biswaz 2003.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, India |
|
| Participants | Adults undergoing elective supratentorial craniotomy for tumour resection (n = 50) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 25 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported that a computer‐generated randomization method was used. |
| Allocation concealment (selection bias) | High risk | Authors did not provide any details regarding how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''one investigator prepared 25 mL of bupivacaine (0.25%) without adrenaline for the treatment group and a 25‐mL solution of normal saline for the control group and handed it to the assisting nursing staff for scalp infiltration by the neurosurgeon. All solutions were prepared in identical syringes, and everyone was blinded to the assignment except the second author''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: '' each patient was coded by the second author. The code was broken only after all the data were collected and analysed by the first author''. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Nine participants were excluded from the analysis as they required postoperative ventilation: 5 from the intervention group: 4 from control group. The subsequent lack of an intention‐to‐treat analysis makes the effects of their exclusion on the measured outcomes difficult to determine. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the priority in which they were specified. |
| Other bias | High risk | Small study and no sample size calculation provided |
Bloomfield 1998.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, USA |
|
| Participants | Adults undergoing elective craniotomy (n = 36) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage Max 2 mg/kg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomized but no details were provided regarding the method used |
| Allocation concealment (selection bias) | High risk | Authors did not provide any details regarding how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: ''the anaesthesiologist, surgeon, and patient were blinded to the solution''. However no details were provided regarding the method used or how or if its adequacy was assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details were provided as to whether or not those assessing outcomes were blinded to treatments received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up and all 36 participants were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | High risk | Small study and no sample size calculation provided |
Can 2017.
| Methods | Study design: randomized, placebo controlled study (3 arms) Study duration: March 2008 to April 2009 Study setting: hospital, single centre, Turkey |
|
| Participants | Adults undergoing elective craniotomy (n = 90) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp block of the following nerves:
5 minutes prior to pinning, using either 20 mL of 0.5% bupivacaine, 20 mL of 0.5% levo‐bupivacaine or saline |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote 'The patients were randomly divided into three groups using a sealed‐enveloped technique'' The authors describe allocation concealment but do not provide details regarding how random allocation was ensured |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Block solutions were prepared and numbered by a blinded assistant. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Those assessing outcomes were unaware of the treatment allocations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | High risk | Long time between study conduct and publication and small study |
Choi 2009.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, China |
|
| Participants | Adults undergoing elective craniotomy (n = 32) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp block of the following nerves:
with 0.75% ropivacaine versus scalp block with saline, at the end of surgery Dosage 2 to 3 mL per nerve |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Published in Chinese only
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization table was used. |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. All participants were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Small study |
Cokay 2013.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Turkey |
|
| Participants | Adults undergoing elective craniotomy (n = 60) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 20 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Published in abstract format only
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomized but method used was not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as double‐blinded but method or adequacy not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | High risk | A lot of data missing so difficult to determine overall methodologic rigour |
Dilmen 2016.
| Methods | Study design: randomized controlled trial (4 arms) Study duration: June 2013 to January 2015 Study setting: hospital, single centre, Turkey |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 83) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ''patients were randomised to one of four groups using opaque envelopes.'' However, it was not clear how the envelopes were selected to ensure random allocation. |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''the study drugs that were dissolved in 100 mL 0.9% saline solution were prepared by a nurse and administered by another nurse whereas postoperative data were collected by a blinded anaesthesiologist''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperative data was collected by a blinded anaesthesiologist. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 participants were excluded from the analysis. 1 participant: could not be extubated at the end of surgery (did not state which group he or she was in) 2 participants: did not regain consciousness at the end of surgery (both in the metamizol group) 3 participants: suffered seizures (1 in the dexketoprofen group and 2 in the paracetamol group) 2 participants: required merperidine for postoperative shivering (both in the saline control group) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Small study |
El‐Dawlatly 2007.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Saudi Arabia |
|
| Participants | Adults undergoing elective supratentorial craniotomy for tumour resection (n = 60) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender Not reported |
|
| Interventions |
Technique and timing
Dosage 10 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''randomisation was performed by a computer generated form''. |
| Allocation concealment (selection bias) | High risk | Authors did not provide any details regarding how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''bupivacaine 0.25% without adrenaline or saline was prepared in a 10 mL identical syringe, by the second author. It was then given to the surgeon to infiltrate in a sterilized manner. Everyone was blind about the study drug except the second author. The code was broken only after all the data were collected and analysed by the first author''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: '' the code was broken only after all the data were collected and analysed by the first author''. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The numbers who received each treatment as intended, the numbers lost to follow‐up and the numbers included in the final analysis were not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the priority in which they were specified. |
| Other bias | High risk | Small study and no sample size calculation was reported making it difficult to determine if the study was adequately powered for the primary outcome. |
Ganzoni 2008.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, USA |
|
| Participants | Adults undergoing elective supratentorial craniotomy for tumours (n = 30) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp block of the following nerves:
with 0.5% ropivacaine versus no scalp block, after induction of anaesthesia Dosage Maximum of 30 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomized study but method of randomization not described |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as blinded but method used not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants were followed up for outcomes. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | Unclear risk | Small study |
Greenberg 2017.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: Febuary 2012 to September 2015 Study setting: hospital, single centre, USA |
|
| Participants | Adults undergoing craniotomy of > 2 hours duration (n = 140) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Immediately upon the beginning of closure (time 0), 1000 mg of IV acetaminophen (in 100 mL) or 100 mL of IV placebo (normal saline) was administered. The study drug or placebo was then administered every 6 hours thereafter (for a total of 3 additional doses, at 6, 12, and 18 hours). Dosage 1000 mg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding Funding in the amount of USD 9000 was provided by Mallinckrodt Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomized to receive either placebo (saline) or acetaminophen using a computer‐generated randomization code. |
| Allocation concealment (selection bias) | Low risk | Quote: ''individual group assignments were concealed in opaque envelopes''. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''the clinical providers administering placebo or IV acetaminophen were blinded to the group to which patients were assigned''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Those assessing outcomes were not aware of treatment received. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants in the acetaminophen group and 5 participants in the control group did not receive the intervention as intended and were excluded from the final analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | High risk | Study likely not adequately powered, long study duration and multiple outcomes |
Hernández Palazón 2007.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Spain |
|
| Participants | Adults undergoing elective supratentorial craniotomy for resection of brain tumours (n = 30) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp block of the following nerves:
with 0.25% bupivacaine with adrenaline 1:200,000 versus scalp block with saline, at the end of surgery Dosage 20 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Paper published in Spanish only Paper published in Spanish only, translation software used but translation errors possible Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomized but method not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as double‐blinded but method or adequacy not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Anaesthesiologist performing postoperative pain assessment did not participate in scalp block. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses to follow‐up and numbers included in final analysis were not reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Small study |
Hwang 2015.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Korea |
|
| Participants | Adults undergoing elective supratentorial craniotomy for clipping of an unruptured cerebral aneurysm (n = 52) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp block of the following nerves
with 0.75% bupivacaine with adrenaline 1:200,000 versus scalp block with saline, at the end of surgery Dosage 7 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number chart was used for randomization. |
| Allocation concealment (selection bias) | Low risk | An independent anaesthesiologist was responsible for patient allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''syringes containing the same volume (7 mL) of normal saline (group C) or 0.75% levo bupivacaine with epinephrine (group L) were prepared by an anaesthetic nurse not involved in the study. The anaesthesiologist performing the scalp block, patients, and investigators were blinded to group assignments''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''the anaesthesiologist performing the scalp block, patients, and investigators were blinded to group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 46 of the original 52 participants were included in the final analysis. 3 participants from the intervention group were too sedated to assess outcomes. 3 participants from the control group were not included due to delayed extubation. As the losses were equal in both groups and the reasons for the losses were clinically similar, their omission was unlikely to have had a significant impact on the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the priority in which they were specified. |
| Other bias | Unclear risk | Small study |
Jellish 2006.
| Methods | Study design: randomized controlled trial (3 arms) Study duration: not reported Study setting: hospital, single centre, USA |
|
| Participants | Adults undergoing elective skull base surgery (n = 120) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization table was used. |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: ''all study drugs were prepared in the pharmacy", implying investigators were blinded. Not clear if patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''all study drugs were prepared in the pharmacy. The PCA container held the same volume of solution and was blinded to all individuals who collected the data''. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed up and included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | The secondary outcome of pain was given greater reporting priority than the primary outcome of nausea and vomiting. |
| Other bias | Low risk | No other significant biases were identified. |
Jones 2009.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Australia |
|
| Participants | Adults undergoing elective craniotomy (n = 82) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 40 mg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding Vincents research grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permuted block |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs were prepared by a third party and labelled ‘study drug’. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment received. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants were excluded as surgery was cancelled. An intention‐to‐treat analysis was not performed and the groups to which these participants were initially assigned was not reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | Unclear risk | Small study |
Kiskira 2006.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Greece |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 40) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 30 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Published in abstract format only
Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomized but method of randomization not reported |
| Allocation concealment (selection bias) | High risk | Authors did not provide any details regarding how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details provided as to whether or how the study was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details provided as to whether or how the study was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details provided regarding numbers allocated to each treatment arm, numbers who received each treatment, numbers followed up or numbers analysed |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | High risk | A lot of unreported data, so difficult to determine how robust the methodology was |
Law‐Koune 2005.
| Methods | Study design: randomized controlled trial (3 arms) Study duration: not reported Study setting: hospital, single centre, France |
|
| Participants | Adults undergoing elective supratentorial craniotomy for tumour resection (n = 80) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 20 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used for randomization |
| Allocation concealment (selection bias) | High risk | Authors did not provide any details regarding how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: ''the anaesthesiologist, surgeon, and patient were blinded to the solution''. However no details were provided regaining the method used or how or if its adequacy was assessed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details were provided as to whether or not those assessing outcomes were blinded to treatments received. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants were excluded from the final analysis due to postoperative complications: 3 in the control group (2 due to unspecified neurological complications and 1 due to excessive sedation) 1 in the ropivacaine group (due to an unspecified neurological complication) The lack of an intention‐to‐treat analysis made the effect of their exclusion difficult to determine. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | Unclear risk | Small study |
Misra 2013.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, India |
|
| Participants | Adults undergoing elective craniotomy for tumour resection who were receiving preoperative intravenous (IV) dexamethasone for at least 48 hours (n = 79) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 600 mg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''randomisation was done by means of a computer‐generated random number scheme.'' Patients were allocated to receive either placebo (vitamin B‐complex capsule) (group D) or 600 mg of gabapentin (group GD), administered orally, 2 hours before the induction of anaesthesia by means of a sealed envelope. |
| Allocation concealment (selection bias) | Low risk | Quote: ''patients were allocated to receive either placebo (vitamin B‐complex capsule) (group D) or 600 mg of gabapentin (group GD), administered orally, 2 hours before the induction of anaesthesia by means of a sealed envelope''. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo tablets were used to blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 participants were lost to follow‐up: 3 in each group due to delayed extubation. An intention‐to‐treat analysis was not used. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | High risk | Small study and no sample size calculation provided |
Molnár 2015.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Hungary |
|
| Participants | Adults undergoing elective craniotomy (n = 200) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 100 mg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding
Other methodologic issues
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: ''neither the physician performing the anaesthesia, nor the physicians obtaining post‐operative VAS scores were aware of patient assignments; the study was thus entirely double‐blinded''. However, the blinding method or its adequacy was not described, a factor which is especially relevant since there was no placebo medication used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: ''neither the physician performing the anaesthesia, nor the physicians obtaining post‐operative VAS scores were aware of patient assignments; the study was thus entirely double‐blinded''. However, the blinding method or its adequacy was not described, a factor which is especially relevant since there was no placebo medication used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants were followed up and included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Subgroup analysis was not prespecified. |
Nguygen 2001.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Canada |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 30) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp block of the following nerves:
with 0.75% ropivacaine versus scalp block with saline, at the end of surgery Dosage 20 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding
Other methodologic issues
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomized but method not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear, the solution for the block was prepared by the attending anaesthesiologist and administered by the principal investigator but the authors did not provide information as to whether or how the solution was presented in a way to disguise its true contents. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | For primary outcome – the numbers followed up was not stated. Quote: ‘'only data obtained from patients who were oriented with regard to person, place, and time and with a Glasgow coma score of at least 14 (they would open their eyes to speech) were considered for statistical analysis’' suggesting that some participants were excluded. For the secondary outcome, from the numbers presented in the results, it appeared that all 30 participants were followed up. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | High risk | Small study and no sample size calculation provided |
Peng 2015.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, China |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 80) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 0.5 mcg/kg/hr |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding
Other methodologic issues
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table was used. |
| Allocation concealment (selection bias) | Low risk | Randomization and study drug preparation were done by a research assistant who was not otherwise involved in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''all patients, anaesthesiologists, surgeons, and postoperative observers were blinded to the group allocation''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''all patients, anaesthesiologists, surgeons, and postoperative observers were blinded to the group allocation''. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants, 2 from each group were excluded from the analysis as they required re‐operation. As the numbers excluded and reasons for exclusion were the same in both groups, the impact on the effect estimate was likely not significant. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | High risk | The study was likely underpowered for the primary outcome. |
Peng 2016.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: 2009 to 2012 Study setting: hospital, single centre, China |
|
| Participants | Adults undergoing elective supratentorial craniotomy for tumour resection (n = 94) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage As above |
|
| Outcomes |
Not clear which outcomes were primary and which were secondary The authors reported outcomes for:
|
|
| Notes |
Funding None Other methodologic issues No sample size calculation reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table |
| Allocation concealment (selection bias) | Low risk | Coded vials |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''a research nurse gave the participants an equal volume of lidocaine or saline from a coded vial according to the randomised control table". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''the research team that collected and analysed the data was blinded to the treatment allocation''. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14 participants were excluded from the analysis. Group intervention: 6 excluded (2 due to delayed extubation, 4 not alert enough to assess pain score) Group control: 8 excluded (3 due to delayed extubation, 4 not alert enough to assess pain score, 1 due to dysphoria) |
| Selective reporting (reporting bias) | Unclear risk | Unclear outcome priority |
| Other bias | High risk | No sample size calculation |
Rahimi 2006.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, USA |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 27) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 25 mg per dose |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None Other methodologic issues Lack of details regarding several aspects of study design and methodology including randomization, blinding methods, sample size and timing of outcome measurements |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated that it was randomized but no details were provided. |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors stated that it was single‐blinded but did not report how or whom was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants from the intervention group were not included in the analysis for the primary outcome. No reason was provided by the authors. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | High risk | General lack of detail about study design, sample size, timing of outcome measures |
Rahimi 2010.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, USA |
|
| Participants | Adults undergoing elective craniotomy (n = 50) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage As above |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None Other methodologic issues Lack of details regarding several aspects of study design and methodology including randomization, blinding methods, sample size and timing of outcome measurements |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomized but method not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as blinded but authors did not report who was blinded or how they were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed up and included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | High risk | Lack of details regarding several aspects of study design and methodology including randomization, blinding methods, sample size and timing of outcome measurements |
Rigamonti 2013.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre,Canada |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 89) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 20 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Published in abstract format only Paper published in abstract format only so many details not reported Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as 'double‐blinded' but method or adequacy not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | High risk | Lots of unreported data, so difficult to determine overall methodologic rigour |
Ryan 2005.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, United Kingdom |
|
| Participants | Adults undergoing elective craniotomy (n = 42) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Oral rofecoxib verus placebo given 1 hr prior to surgery Dosage 50 mg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Published in abstract format only Paper published in abstract format only so many details not reported Funding No funding source reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomized but no details reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as 'double‐blinded' but no details provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 participants were lost to follow‐up. No reasons were provided. |
| Selective reporting (reporting bias) | Unclear risk | Absolute figures were only provided for 'morphine consumption', with no figures provided for the primary outcome. |
| Other bias | High risk | Lots of unreported data |
Saringcarinkul 2015.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: 2006 to 2009 Study setting: hospital, single centre, Thailand |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 50) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 20 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding Chiang Mai University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number chart was used. |
| Allocation concealment (selection bias) | High risk | Authors did not provide any details regarding how the allocation sequence was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''both solutions were prepared by the scrub nurse, who did not participate in the postoperative pain assessment. The neuro‐surgeon performing the infiltration, anaesthesiologist and patient were blinded to the drug being administered''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The nurse performing the outcome assessments was blinded to treatment received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was excluded from the final analysis due to decreased level of consciousness. Although an intention‐to‐treat analysis was not performed, the omission of 1 participant only is unlikely to have had a significant impact on the results. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | Unclear risk | Small study |
Shepherd 2018.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: 2015 to 2016 Study setting: hospital, single centre, USA |
|
| Participants | Adults participants undergoing transsphenoidal surgery for resection of pituitary tumours Exclusion criteria
Mean age (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 800 mg every 8 hours with the first dose given intraoperatively |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:''Patients were randomised in a 1:1 ratio with blinded treatment assignment. The patients were randomised using a computer‐generated list of random numbers from www.random.org.'' |
| Allocation concealment (selection bias) | High risk | Quotes: ''The randomised list was placed with an ordered list of numbers from 1 to 100. Odd numbers were assigned to Group 1 and even numbers to Group 2.'' ''The research nurse generated the random number sequence and performed the blinded assignment''. This implied that treatment allocation was predictable to the research nurse before the moment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: ''Patients, family members, bedside nurses, and providers were blinded to treatment assignment. The treatment assignment was known by the research nurse and a research pharmacist''. Blinding was neither well described nor complete. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the report who exactly assessed outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized patients were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | High risk | The study was substantially underpowered for its primary outcome. Quote:''Fifty treated patients in each group were required to detect a 2‐point MD on the 11‐point (0–10) VAS, with a standard deviation of 3.2 for the placebo group and 3.5 for the treatment group, with α set at 0.05 and 90% power.'' |
Shimony 2016.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Israel |
|
| Participants | Adults undergoing elective craniotomy for tumour resection (n = 100) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 150 mg per dose |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It was clear how participants were blinded. Quote: ''patients in the placebo group were given identical capsules containing 500 mg of starch at the same time points''. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial was reported as 'double‐blinded' but it was not clear how investigations were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12 participants were lost to follow‐up, 5 in the intervention group and 7 in the control group. The reasons were not clearly explained in the report with the CONSORT flowchart mentioning these as having (quote): ''dropped out''. However, the authors did perform an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Massive number of comparisons were made with no statistical adjustment for multiple testing. |
Sivakumar 2018.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: 2013 to 2015 Study setting: hospital, single centre, USA |
|
| Participants | Adults undergoing elective craniotomy (n = 212) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 1000 mg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding Hospital |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''Randomization was performed with the use of a block‐of‐4 randomisation scheme. Randomization was performed by the investigational drug service at the university hospital, thereby maintaining the double‐blind aspect of the study (patients and study personnel/investigators). Patient study identification numbers were recorded in an Excel database, randomized permuted blocks of 4 were created, and patients were allotted to either the acetaminophen or placebo group by the pharmacy.'' |
| Allocation concealment (selection bias) | Low risk | Drugs were prepared by pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method not well described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 participants (4 in each group) were excluded after randomization due to untimely administration of the study drug or patient transfer . As equal numbers were lost from both groups, it was unlikely to have a significant impact on the results. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | The study was slightly underpowered with the power calculation being based on a total of 210 patients. |
Song 2016.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: 2006 to 2009 Study setting: hospital, single centre, China |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 60) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Intravenous infusion of dexmedetomidine 0.5 mcg/kg/hr for 10 minutes before induction of anaesthesia, then 0.2 to 0.5 mcg/kg/hr until skin closure versus placebo Dosage As above |
|
| Outcomes |
Not clear from the report, which outcomes were primary and which were secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Quote: ''an anaesthesia nurse prepared the syringe according to the computer‐generated random number and was the only person who knew whether the active drug or placebo was administered''. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''an anaesthesia nurse prepared the syringe according to the computer‐generated random number and was the only person who knew whether the active drug or placebo was administered''. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear if the anaesthesia nurse who prepared the study drugs was involved in assessing outcomes or not |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants (1 in the intervention group and 2 in the control group) did not receive their allocated treatments as their surgery was cancelled. 5 participants were lost to follow‐up after receiving their allocated treatments as they were not extubated after surgery (4 in the invention group and 1 in the control group). An intention‐to‐treat analysis was not performed. |
| Selective reporting (reporting bias) | Unclear risk | The lack of clarity regarding outcome priorities makes it difficult to exclude reporting bias. |
| Other bias | Unclear risk | Small study |
Tucinda 2010.
| Methods | Study design: randomized controlled trial (3 arms) Study duration: 2006 to 2007 Study setting: hospital, single centre, Thailand |
|
| Participants | Patients undergoing elective supratentorial craniotomy (n = 60) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp block of the following nerves:
with 0.5% bupivacaine with 1:200,000 adrenaline (Group 1) versus 0.25% bupivacaine with 1:200,000 adrenaline (Group 2) versus saline with 1:200,000 adrenaline (Group 3), before surgery Dosage Max 3 mg/kg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding Chulalongkorn University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Anaesthesiologist performing the block was blinded but the method or its adequacy were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant from group 2 was excluded after he/she developed a postoperative intracranial haematoma; 59/60 participants were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Small study |
Willams 2011.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, Australia |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 100) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosage 40 mg |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization list was used. |
| Allocation concealment (selection bias) | Low risk | Quote: ''computer‐generated randomisation results were concealed in opaque envelopes until consent had been obtained. The randomisation was stratified by gender''. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''the study medication was prepared by an anaesthetist who was not involved with the case. The patients, attending anaesthetists, surgeons, and postoperative observers were blind to group allocation''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants were lost to follow‐up after enrolment. 1 was excluded as surgery was cancelled and 2 participants in the intervention group and 1 in the control group withdrew consent after surgery. These were not included in the final analysis. The lack of an intention‐to‐treat analysis makes it difficult to accurately estimate the impact of these losses on the effect estimate. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | Low risk | No other significant biases were identified. |
Yardav 2014.
| Methods | Study design: randomized controlled trial (3 arms) Study duration: 2010 to 2013 Study setting: hospital, single centre, India |
|
| Participants | Adults undergoing elective craniotomy (n = 390) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
given orally every 8 hours, beginning on the second postoperative day and continued for a total of 48 hours Dosages As above |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization table |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''a nurse, who was not part of the study, administered 1 tablet and 1 capsule of similar shape to all the patients 8 hourly, on second postoperative day for 48 hours. Neither patients nor the observer was aware of the type of medications''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''a nurse, who was not part of the study, administered 1 tablet and 1 capsule of similar shape to all the patients 8 hourly, on second postoperative day for 48 hours. Neither patients nor the observer was aware of the type of medications''. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 participants were excluded from the analysis (6 in group 1, 5 in group 2 and 8 in group 3); the authors did not provide details of the reasons or the stage in the study at which these patients were excluded. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Low risk | No other significant biases were identified. |
Yun 2016.
| Methods | Study design: randomized controlled trial (3 arms) Study duration: not reported Study setting: hospital, single centre, China |
|
| Participants | Adults undergoing elective supratentorial craniotomy (n = 150) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Group 1: dexmedetomidine infusion 0.4 mcg/kg Group 2: dexmedetomidine infusion 0.8 mcg/kg Group 3: control,saline infusion Dosages As above |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote. ''using computer‐generated random numbers and a sealed‐envelop technique, patients were allocated randomly into 1 of 3 groups: small‐dose DEX (0.4 mg/kg), median‐dose DEX (0.8 mg/kg), or vehicle control (an equivalent volume of normal saline)." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote. "the attending anaesthesiologists were unaware of the grouping'', implying that those administering the infusion were unaware of the contents of the syringe''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''the attending anaesthesiologists were unaware of the grouping, and the measurements were recorded by 1 nurse, who was also blinded''. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17 participants were excluded from the final analysis as follows: 8 participants did not receive the allocated intervention. Group 1: 3 excluded (1 had prolonged surgery and 2 had blood loss > 1400 mL) Group 2: 2 excluded (1 had a seizure and 1 had blood loss > 1400 mL) Group 3: 2 excluded (1 had a prolonged surgery and 1 had blood loss > 1400 mL) 9 participants were lost to follow‐up after receiving their allocated intervention. Group 1: 2 excluded (1 had an intracranial bleed and 1 had a seizure requiring sedation) Group 2: 5 excluded (1 had a weight < 45 kg, 1 had an intracranial bleed, 1 had prolonged surgery, 1 had delayed recovery and I had an unclear reason) Group 3: 2 excluded (1 had an intracranial bleed and 1 had a low level of consciousness) The relatively large numbers excluded and the inequality of both numbers and reasons across groups together with the lack of an intention‐to‐treat analysis, made it difficult to measure the true effect estimate accurately. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | Unclear risk | Small study |
Zeng 2019.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, China |
|
| Participants | Adults undergoing elective subtemporal or suboccipital craniotomy (n = 150) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Group 1: Placebo Vitamin B capsules Group 2: Gabapentin 600 mg orally the night before surgery and again 2 hours before induction of anaesthesia Dosages As above |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding Youth Programme Funding of Beijing Tiantan Hospital, Capital Medical University (number: YQN201210) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ''A computer‐generated randomisation table prepared by an investigator with no involvement in the trial was used.'' |
| Allocation concealment (selection bias) | Low risk | Quote: ''An individual not involved in the enrolment handled the randomisation list to guarantee allocation concealment''. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''The nurse, anesthesiologists, patients, and outcome assessors were all blinded to the grouping.'' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''The nurse, anesthesiologists, patients, and outcome assessors were all blinded to the grouping.'' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 patients in the placebo group and 9 patients in the treatment group were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | Unclear risk | Analysis was not intention‐to‐treat. |
Zhang 2003.
| Methods | Randomized controlled trial (4 arms) | |
| Participants | Adults undergoing elective craniotomy (n = 60) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing
Dosages
|
|
| Outcomes |
Primary
Secondary None |
|
| Notes | Paper only available in Chinese so some translation errors possible Funding None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | High risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as specified |
| Other bias | High risk | Small study and analysis method not completely described |
Zhou 2016.
| Methods | Study design: randomized controlled trial (2 arms) Study duration: not reported Study setting: hospital, single centre, China |
|
| Participants | Adults undergoing elective craniotomy (n = 154) Inclusion criteria
Exclusion criteria
Mean age, range (years)
Numbers allocated to each arm
Male gender
|
|
| Interventions |
Technique and timing Scalp infiltration with 0.5% ropivacaine versus scalp infiltration with saline before surgery Dosage 10 mL |
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
Funding Study was funded by a pharmaceutical company Astra Zeneca. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random number chart was used. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ''on the day of surgery, according to the random code of the patient, an anaesthetic nurse prepared the solution of normal saline or ropivacaine. All solutions were prepared in identical syringes. The random code, patient’s information and group name were enclosed in the sealed opaque envelope. The anaesthetists who performed the anaesthesia and recorded the intraoperative data, the neurosurgeons who performed the scalp infiltration, and patients were all blinded to the group assignment. The envelope was opened only if emergency un blinding was required''. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ''all the follow‐up procedures were conducted by another nurse who was also blind to the treatment group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 48 participants were lost to primary outcome follow‐up, after randomization: 7 due to cancelled surgery 3 due to requiring admission to intensive care 16 due to inability to communicate 12 due to requiring analgesia other than morphine 5 more participants were lost to follow‐up for outcomes measured at 3 months. These participants were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified. |
| Other bias | Unclear risk | Study was funded by a pharmaceutical company Astra Zeneca. |
ASA: American Anesthesiology Society Classification BMI: body mass index COX: cyclo‐oxygenase DEX: dexmedetomidine GCS: Glasgow coma score Hr: hour HR: heart rate Hrs: hours ICP: intra cranial pressure IV: intravenous kg: kilograms mcg: micrograms mg: milligrams mL: millilitres mmHg: millimetres of mercury n: number NRS: numerical pain score NSAID: non steroidal anti inflammatories PCA: patient controlled analgesia VAS: visual analogue pain score Vit: vitamin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackil 2018 | No control group ‐ morphine v dexketoprofen |
| Ayoub 2006 | No control group: morphine v scalp block |
| Bajaj 2017 | Outcomes of Interest not addressed This study was conducted to establish the efficacy and safety of clonidine in reducing intraoperative oozing and improving operating conditions in pituitary adenoma surgery. The purpose of the study was not to establish its analgesic potential efficacy or safety in the context of use for as an analgesic. |
| Bishnoi 2016 | Outcomes of Interest not addressed This study was conducted to establish it dexmedetomidine reduced unwanted intraoperative patient movement and patient and surgical satisfaction. The purpose of the study was not to establish its analgesic potential efficacy or safety in the context of use as an analgesia. |
| Citerio 2012 | No control group: all 3 groups received opioid medication. |
| Dolmatova 2009 | No control group: scheduled v as‐needed lornoxicam |
| Domenech 2006 | No control group: parecoxib v paracetamol |
| Doumiri 2015 | No postoperative pain outcomes. The aim of this study was to establish the intraoperative haemodynamic stability profile of a lidocaine scalp block. It was not conducted to address either its analgesic potential or side effect profile in the context of use for analgesic purposes. |
| Dudko 2014 | No control group: bupivacaine v paracetamol and ketoprofen |
| El Dahab 2009 | No control group: skull block v fentanyl |
| Ferber 2000 | No control group: 2 different doses of tramadol |
| Girard 2010 | No control group: morphine v codeine |
| Goldsack 1996 | No control group: superficial cervical plexus block v morphine |
| Graham 1999 | No control group: tramadol v codeine phosphate |
| Hassani 2015 | No control group – sufentanil v paracetamol v morphine |
| Honnma 2002 | No control group and no randomization |
| Imaev 2008 | No control group: lornoxicam v ropivacaine v fentanyl |
| Imaev 2010 | No control group: xefocam v ropivacaine v durogesic |
| Jayaram 2016 | No control group: maxillary block v scalp block |
| Jeffrey 1999 | No control group: codeine v tramadol |
| Jose 2017 | No control group: local anaesthetic with added steroid v local anaesthetic alone |
| Lu 2009 | Different patient population: participants already had established postoperative pain No control group: morphine/acetaminophen v rotunidine |
| Luo 2014 | No control group: lidocaine v procaine |
| Mohamed 2018 | No control group ‐ scalp block v scalp block with hyaluronidase |
| Morad 2009 | No control group: fentanyl as required v fentanyl PCA |
| Na 2011 | No control group: fentanyl and ketorolac as required v fentanyl and ketorolac PCA |
| Palazón 2006 | No control group – sevoflurane v propofol |
| Rajan 2016 | No control group: dexmedetomidine infusion versus remifentanil infusion |
| Reddy 2018 | No control group ‐ scalp block v local anaesthetic infiltration |
| Simon 2012 | No true control group – intervention group were enrolled prospectively and the historical controls were randomly selected from a database |
| Soliman 2011 | No distinction between intraoperative and postoperative pain outcomes |
| Stone 2018 | Cross‐over study of acetaminophen versus placebo in patients undergoing bilateral craniotomies for Moyamoya disease. Excluded due to the high potential for carry‐over effects and period effects |
| Stoneham 1996 | No control group – morphine v codeine phosphate |
| Sudheer 2007 | No control group – morphine v tramadol v codeine phosphate |
| Tanskanen 1999 | No control group – paracetamol v ketoprofen |
| Theerth 2018 | Bo control group ‐ scalp block v local anaesthetic infiltration |
| Ture 2009 | No control group – gabapentin v phenytoin |
| Vallapu 2018 | No control group ‐ scalp infiltration v scalp block |
| Venkatraghavan 2016 | Different patient population – patients already had established postoperative pain. |
| Verchere 2002 | No control group – paracetamol v tramadol v nalbuphine |
| Wu 2014 | Ongoing study. Different patient population: participants admitted to ICU with delayed extubation after craniotomy |
| Zhao 2013 | Different patient population: participants admitted to ICU with delayed extubation after craniotomy |
ICU: intensive care unit PCA: patient controlled analgesia V: versus
Characteristics of ongoing studies [ordered by study ID]
KCT0000274.
| Trial name or title | Scalp blocks with levo‐bupivacaine reduced postoperative pain and the requirement of anti‐hypertensive agent after craniotomy for aneurysmal clipping |
| Methods | Randomized controlled double‐blinded trial, target sample size 52, single centre, South Korea |
| Participants | Adult patients undergoing craniotomy for elective cerebral aneurysm clipping Exclusion criteria:
|
| Interventions | Scalp block with levo‐bupivacaine versus scalp block with saline |
| Outcomes | Primary
Secondary
|
| Starting date | Registered Nov 2011, anticipated completion date was May 2012 but no further details available regarding progress |
| Contact information | Junghee Ryu, Seoul National University Bundang Hospital |
| Notes |
|
PCA: patient controlled analgesia
VAS: visual analogue pain score
Differences between protocol and review
We made the following changes to the published protocol (Galvin 2015).
1. Title
The protocol title was 'Interventions for the prevention of acute postoperative pain in adults following brain surgery' with the objectives of determining the effectiveness of pharmacological interventions for the prevention of acute postoperative pain in this population. On the recommendations of the CEU screening criteria and editors' plan of action to harmonise the pharmacological focus of the review throughout the review, the title was amended to 'Pharmacological interventions for the prevention of acute postoperative pain in adults following brain surgery'.
2. Background section. How the intervention might work
We added information regarding two other interventions, 'dexmedetomidine' and 'gabapentin and pregabalin'. We did this as both of these interventions were found on literature search to have studies that were eligible for inclusion in the review.
3. Exclusion Criteria
As specified a priori in our protocol, we excluded review articles, observational studies, case reports, case series, non‐randomized studies and studies that had no control groups. We also excluded studies that investigated the use of agents with analgesic potential for non‐analgesic purposes. The rationale for this decision was based on a high likelihood of important differences in inclusion and exclusion criteria, dosages, timing, ancillary analgesic usage and attributable side effects between studies that investigated these agents for their analgesic efficacy and studies that investigated them for their non‐analgesic effects. While this approach meant that potential outcomes of interest were not captured when these agents were investigated for their other non‐analgesic effects, it provided a more accurate estimate of the effects and side effects of those agents, when used with analgesic intent. A total of three studies were excluded on this basis (Bajaj 2017; Bishnoi 2016; Doumiri 2015); These studies used agents that have analgesia potential i.e. clonidine, dexmedetomidine and lidocaine; however, the focus of these studies was on their efficacy in a non‐analgesic context and including them would have run the risk of .misrepresenting these agents efficacy and side effect profile when used with analgesic intent.
4. Outcomes
Primary outcome: pain intensity
Initially, we planned to produce pooled estimates of effect for the following outcomes:
Mean difference in validated measures of pain intensity in the following acute postoperative periods:
total (0 to four days);
early (0 to 12 hours);
intermediate (13 to 24 hours);
late (25 hours to four days).
We changed this to:
Mean differences in validated measures of pain intensity at:
Anytime in the first six hours postoperatively;
12 hours postoperatively;
24 hours postoperatively;
48 hours postoperatively.
We did this because:
a) The use of discrete time points rather than time periods best reflected the way in which the vast majority of included studies reported this outcome.
b) We wanted to ensure accuracy of reported and calculated standard deviations, having found no accurate method of calculating standard deviations over time periods when the data were not reported in this way.
Secondary outcome: analgesic requirement
We measured this at '0 to 24 hours' only rather than over the four time periods stated in the protocol. We chose this approach as this was the most common time period over which the included studies reported this outcome, allowing the most accurate pooled estimate for each intervention and the best comparison between interventions.
5. 'Summary of findings' tables
Originally we intended to produce a 'Summary of findings' table for each comparison, addressing the following outcomes:
mean differences in validated measures of pain intensity in the total (0 to 4 days) postoperative period;
mean differences in validated measures of sedation in the total (0 to 4 days) postoperative period;
mean difference in additional analgesia requirement in the total (0 to 4 days) postoperative period;
mean difference in analgesic success in the total (0 to 4 days) postoperative period;
mean difference in length of stay in the critical care unit;
mean difference in length of stay in hospital;
mean difference in the incidence of headache persisting three months or more after surgery.
Instead, we produced a main 'Summary of findings' table for each comparison addressing the following outcomes:
Acute pain Intensity during the first six hours postoperatively;
Acute pain Intensity at 12 hours postoperatively;
Acute pain Intensity at 24 hours postoperatively;
Additional analgesia requirement from 0 to24 hours postoperatively;
Adverse events.
We did this because:
a) For the primary outcome of pain intensity, it allowed a simple summary comparison of pooled differences in pain scores at different time points for each intervention.
b) For secondary outcomes, it made the best use of the available data given that several of our prespecified secondary outcomes were not reported.
Contributions of authors
Imelda M Galvin (IMG), Ron Levy (RL), Andrew G Day (AGD), Ian Gilron (IG)
Conceiving the review: IMG, IG, RL
Co‐ordinating the review: IMG
Undertaking manual searches: IMG, RL
Screening search results: IMG, RL
Organizing retrieval of papers: IMG, RL
Screening retrieved papers against inclusion criteria: IMG, RL
Appraising quality of papers: IMG, RL
Abstracting data from papers: IMG, RL
Writing to authors of papers for additional information: IMG
Providing additional data about papers: IMG, RL
Obtaining and screening data on unpublished studies: IMG, RL
Data management for the review: IMG, RL
Entering data into Review Manager 2014: IMG RL
RevMan statistical data: IMG, AGD
Other statistical analysis not using Review Manager 2014: IMG, AGD
Interpretation of data: IMG, RL, IG, AGD
Statistical inferences: IMG, AGD
Writing the review: IMG, RL, IG, AGD
Guarantor for the review (one author): IMG
Person responsible for reading and checking review before submission: IMG
Sources of support
Internal sources
None, Canada.
External sources
None, Canada.
Declarations of interest
Imelda M Galvin: none known
Ron Levy: none known
Andrew G Day: none known
Ian Gilron: in the past five years (2012 to 2017), IG has received consulting fees or honorarium (advisory board: Eli Lilly, Astra Zeneca; Johnson & Johnson, Pfizer); board membership: (Chair, Data Safety & Monitoring Board: Wex pharmaceuticals; Data Safety & Monitoring Board: Taris, Adynxx); consulted for various pharmaceutical companies (Johnson & Johnson, Astra Zeneca, Pfizer, Eli Lilly); received lecture fees from pharmaceutical companies that market analgesics and other healthcare interventions; received research support from government (Canadian Institutes of Health Research) and industry sources (Adynxx, Taris Biomedical, Wex Pharmaceuticals); grants/grants pending (research support 'in‐kind' in the form of clinical trial study drug provision: Apotex, Ethypharm, Novopharm and Pfizer; industry‐partnered research salary award (together with Canadian Institutes of Health Research): Pfizer) but no such support was received for this work.
New
References
References to studies included in this review
Akcil 2017 {published data only}
- Akcil EF, Dilmen OK, Vehid H, Ibısoglu LS, Tunali Y. Which one is more effective for analgesia in infratentorial craniotomy? The scalp block or local anesthetic infiltration. Clinical Neurology and Neurosurgery 2017;154:98‐103. [DOI: ; PUBMED: 28183036 ] [DOI] [PubMed] [Google Scholar]
Ali 2010 {published data only}
- Ali AR, Sakr SA, Rahman AS. Bilateral sphenopalatine ganglion block as adjuvant to general anaesthesia during endoscopic transnasal resection of pituitary adenoma. Egyptian Journal of Anaesthesia 2010;26(4):273‐80. [DOI: 10.1016/j.egja.2010.05.002] [DOI] [Google Scholar]
Artime 2018 {published data only}
- Artime CA, Aijazi H, Zhang H, Syed T, Cai C, Gumbert SD, et al. Scheduled intravenous acetaminophen improves patient satisfaction with postcraniotomy pain management: a prospective, randomized, placebo‐controlled, double‐blind study. Journal of Neurosurgical Anesthesiology 2018;30:231‐6. [PUBMED: 29117012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bala 2006 {published data only}
- Bala I, Gupta B, Bhardwaj N, Ghai B, Khosia VK. Effect of scalp block on postoperative pain relief in craniotomy patients. Anesthesia and Intensive Care 2006;34(2):224‐7. [PUBMED: 16617645] [DOI] [PubMed] [Google Scholar]
Batoz 2009 {published data only}
- Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesthesia and Analgesia 2009;109(1):240‐4. [PUBMED: 19535716 ] [DOI] [PubMed] [Google Scholar]
Bekker 2008 {published data only}
- Bekker AI, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesthesia and Analgesia 2008;107(4):1340‐7. [PUBMED: 18806050] [DOI] [PubMed] [Google Scholar]
Biswaz 2003 {published data only}
- Biswas BK, Bithal PK. Preincision 0.25% bupivacaine scalp infiltration and postcraniotomy pain: a randomized double‐blind, placebo‐controlled study. Journal of Neurosurgical Anesthesiololgy 2003;15(3):234‐9. [PUBMED: 12826971] [DOI] [PubMed] [Google Scholar]
Bloomfield 1998 {published data only}
- Bloomfield EL, Schubert A, Secic M, Barnett G, Shutway F, Ebrahim ZY. The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesthesia and Analgesia 1998;87(3):579‐82. [PUBMED: 9728832] [DOI] [PubMed] [Google Scholar]
Can 2017 {published data only}
- Can BO, Bilgin H. Effects of scalp block with bupivacaine versus levobupivacaine on haemodynamic response to head pinning and comparative efficacies in postoperative analgesia: a randomized controlled trial. Journal of International Medical Research 2017;45(2):439‐50. [PUBMED: 28415943] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2009 {published data only}
- Choi EM, Choi S‐H, Lee NH, Min KT. The effect of scalp nerve block on pain after craniectomy. Anesthesia and Pain Medicine 2009;4(2):142‐5. [Google Scholar]
Cokay 2013 {published data only}
- Co KY, Can OS, Kecik Y. Effects of scalp block with levobupivacaine on hemodynamic and bispectral index parameters and postoperative in adult patients undergoing craniotomy: a randomized clinical trial. European Surgical Research ‐ 48th Congress of the European Society for Surgical Research 2013;OP87:45. [DOI: 10.1159/000351978] [DOI] [Google Scholar]
Dilmen 2016 {published data only}
- Dilmen OK, Akcil EF, Tunali Y, Karabulut ES, Bahar M, Altindas F, et al. Postoperative analgesia for supratentorial craniotomy. Clinical Neurology and Neurosurgery 2016;146:90‐5. [PUBMED: 27164511 ] [DOI] [PubMed] [Google Scholar]
El‐Dawlatly 2007 {published data only}
- El‐Dawlatly A, Abbas S, Turkistani A, Elwatidy S, Elgamal E, Jamjoom Z, et al. Use of tenoxicam for post craniotomy pain relief with or without bupivacaine scalp infiltration: a randomized study. Internet Journal of Anesthesiology 2007;15(2):1‐6. [http://ispub.com/IJA/15/2/5523] [Google Scholar]
Ganzoni 2008 {published data only}
- Gazoni FM, Pouratian N, Nemergut EC. Effect of ropivacaine skull block on perioperative outcomes in patients with supratentorial brain tumors and comparison with remifentanil: a pilot study. Journal of Neurosurgery 2008;109(1):44‐9. [PUBMED: 18590431] [DOI] [PubMed] [Google Scholar]
Greenberg 2017 {published data only}
- Greenberg S, Murphy GS, Avram MJ, Shear T, Benson J, Parikh KN, et al. Postoperative intravenous acetaminophen for craniotomy patients: a randomized controlled trial. World Neurosurgery 2017;14:14. [PUBMED: 29042333] [DOI] [PubMed] [Google Scholar]
Hernández Palazón 2007 {published data only}
- Hernández Palazón J, Doménech Asensi P, Burguillos López S, Pérez Bautista F, Sánchez Amador A, Clavel Claver N. Cranial nerve block with bupivacaine for postoperative analgesia following supratentorial craniotomy. Revista Espanola de Anestesiologia Reanimacion 2007;54(2):274‐8. [PUBMED: 17598717] [PubMed] [Google Scholar]
Hwang 2015 {published data only}
- Hwang JY, Bang JS, Oh CW, Joo JD, Park SJ, Do SH, et al. Effect of scalp blocks with levobupivacaine on recovery profiles after craniotomy for aneurysm clipping: a randomized, double‐blind, and controlled study. World Neurosurgery 2015;83(1):108‐13. [PUBMED: 23743219 ] [DOI] [PubMed] [Google Scholar]
Jellish 2006 {published data only}
- Jellish WS, Leonetti JP, Sawicki K, Anderson D, Origitano TC. Morphine/ondansetron PCA for postoperative pain, nausea, and vomiting after skull base surgery. Otolaryngology Head and Neck Surgery 2006;135(2):175‐81. [PUBMED: 16890064] [DOI] [PubMed] [Google Scholar]
Jones 2009 {published data only}
- Jones SJ, Cormack J, Murphy MA, Scott DA. Parecoxib for analgesia after craniotomy. British Journal of Anaesthesia 2009;102(1):76‐9. [PUBMED: 19022794] [DOI] [PubMed] [Google Scholar]
Kiskira 2006 {published data only}
- Kiskira O, Tsitsopoulos P, Kolotoura A, Emexidis T, Kyrallidou A, Pouliou A. The efficacy of levobupivacaine plus epinephrine scalp infiltration in reducing pain severity and analgesic requirements after supratentorial craniotomy: A‐868. European Journal of Anaesthesiology 2006;23:224‐5. [http://journals.lww.com/ejanaesthesiology/Citation/2006/06001/The_efficacy_of_levobupivacaine_plus_epinephrine.806.aspx] [Google Scholar]
Law‐Koune 2005 {published data only}
- Law‐Koune JD, Szekely B, Fermanian C, Peuch C, Liu N, Fischler M. Scalp infiltration with bupivacaine plus epinephrine or plain ropivacaine reduces postoperative pain after supratentorial craniotomy. Journal of Neurosurgical Anesthesiology 2005;17(3):139‐43. [PUBMED: 16037734] [DOI] [PubMed] [Google Scholar]
Misra 2013 {published data only}
- Misra S, Parthasarathi G, Vilanilam GC. The effect of gabapentin premedication on postoperative nausea, vomiting, and pain in patients on preoperative dexamethasone undergoing craniotomy for intracranial tumors. Journal of Neurosurgical Anesthesiology 2013;25(4):386‐91. [PUBMED: 23603887] [DOI] [PubMed] [Google Scholar]
Molnár 2015 {published data only}
- Molnar C, Simon E, Kazup A, Gal J, Molnar L, Novak L, et al. A single preoperative dose of diclofenac reduces the intensity of acute postcraniotomy headache and decreases analgesic requirements over five postoperative days in adults: a single center, randomized, blinded trial. Journal of Neurological Sciences 2015;353(1‐2):70‐3. [PUBMED: 25899314] [DOI] [PubMed] [Google Scholar]
Nguygen 2001 {published data only}
- Nguyen A, Girard F, Boudreault D, Fugère F, Ruel M, Moumdjian R, et al. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesthesia and Analgesia 2001;93(5):1272‐6. [PUBMED: 11682413] [DOI] [PubMed] [Google Scholar]
Peng 2015 {published data only}
- Peng K, Jin XH, Liu SL, Ji FH. Effect of intraoperative dexmedetomidine on post‐craniotomy pain. Clinical Therapeutics 2015;37(5):1114‐21. [PUBMED: 25769614] [DOI] [PubMed] [Google Scholar]
Peng 2016 {published data only}
- Peng Y, Zhang W, Kass IS, Han R. Lidocaine reduces acute postoperative pain after supratentorial tumor surgery in the PACU: a secondary finding from a randomized, controlled trial. Journal of Neurosugical Anesthesiology 2016;28(4):309‐15. [PUBMED: 26397235] [DOI] [PubMed] [Google Scholar]
Rahimi 2006 {published data only}
- Rahimi SY, Vender JR, Macomson SD, French A, Smith JR, Alleyne CH Jr. Postoperative pain management after craniotomy: evaluation and cost analysis. Neurosurgery 2006;59(4):852‐7. [PUBMED: 17038949] [DOI] [PubMed] [Google Scholar]
Rahimi 2010 {published data only}
- Rahimi SY, Alleyne CH, Vernier E, Witcher MR, Vender JR. Postoperative pain management with tramadol after craniotomy: evaluation and cost analysis. Journal of Neurosurgery 2010;112(2):268‐72. [PUBMED: 19630495] [DOI] [PubMed] [Google Scholar]
Rigamonti 2013 {published data only}
- Rigamonti A, Garavaglai M, Hanlon J, Cusimano MD, MacDonald RL, O'Hare GMT, et al. Scalp blocks for postoperative supratentorial craniotomy analgesia. ASA Abstracts 2013;NA:A 462. [Google Scholar]
Ryan 2005 {published data only}
- Ryan MF, Andrzejowski JC, Power L. Pre‐operative analgesic use of a selective COX‐2 inhibitor (rofecoxib) in elective craniotomy: A‐715. European Journal of Anaesthesiology 2005;22:184. [http://journals.lww.com/ejanaesthesiology/Fulltext/2005/05001/Pre_operative_analgesic_use_of_a_selective_COX_2.666.aspx] [Google Scholar]
Saringcarinkul 2015 {published data only}
- Saringcarinkul A, Boonsri S. Effect of scalp infiltration on postoperative pain relief in elective supratentorial craniotomy with 0.5% bupivacaine with adrenaline 1:400,000. Journal of the Medical Association of Thailand 2008;91(10):1518‐23. [PUBMED: 18972894] [PubMed] [Google Scholar]
Shepherd 2018 {published data only}
- Shepherd DM, Jahnke H, White WL, Little AS. Randomized, double‐blinded, placebo‐controlled trial comparing two multimodal opioid‐minimizing pain management regimens following transsphenoidal surgery. Journal of Neurosurgery 2018;128(2):444‐51. [PUBMED: 28298041] [DOI] [PubMed] [Google Scholar]
Shimony 2016 {published data only}
- Shimony N, Amit U, Minz B, Grossman R, Dany MA, Gonen L, et al. Perioperative pregabalin for reducing pain, analgesic consumption, and anxiety and enhancing sleep quality in elective neurosurgical patients: a prospective, randomized, double‐blind, and controlled clinical study. Journal of Neurosurgery 2016;125(6):1513‐22. [PUBMED: 26871201 ] [DOI] [PubMed] [Google Scholar]
Sivakumar 2018 {published data only}
- Sivakumar W, Jensen M, Martinez J, Tanana M, Duncan N, Hoesch R, et al. Intravenous acetaminophen for postoperative supratentorial craniotomy pain ‐ a randomized, double‐blinded, placebo‐controlled trial. Journal of Neurosurgery 2019;130(3):766‐72. [DOI: 10.3171/2017.10.JNS171464; PUBMED: 29676689] [DOI] [PubMed] [Google Scholar]
Song 2016 {published data only}
- Song J, Ji Q, Sun Q, Gao T, Liu K, Li L. The opioid‐sparing effect of intraoperative dexmedetomidine infusion after craniotomy. Journal of Neurosurgical Anesthesiology 2016;28(1):14‐20. [PUBMED: 25955866] [DOI] [PubMed] [Google Scholar]
Tucinda 2010 {published data only}
- Tucinda L, Soboonviboon W, Supbornsug K, Worathongchai S, Limutaitip S. Bupivacaine scalp nerve block: hemodynamic response during craniotomy, intraoperative and post‐operative analgesia. Asian Biomedicine 2010;4(2):243‐51. [http://imsear.li.mahidol.ac.th/bitstream/123456789/129984/1/abm2010v4n2p243.pdf] [Google Scholar]
Willams 2011 {published data only}
- Williams DL, Pemberton E, Leslie K. Effect of intravenous parecoxib on post‐craniotomy pain. British Journal of Anaesthesia 2011;107(3):398‐403. [PUBMED: 21841050] [DOI] [PubMed] [Google Scholar]
Yardav 2014 {published data only}
- Yadav G, Choupoo S, Das SK, Das SK, Behera SS, Khuba S, et al. Evaluating the role of flupirtine for postcraniotomy pain and compare it with diclofenac sodium: a prospective, randomized, double blind, placebo‐controlled study. Journal of Neurosurgical Anesthesiology 2014;26(1):32‐6. [PUBMED: 23764718] [DOI] [PubMed] [Google Scholar]
Yun 2016 {published data only}
- Yun Y, Wang J, Tang RR, Yin XR, Zhou H, Pei L. Effects of an intraoperative dexmedetomidine bolus on the postoperative blood pressure and pain subsequent to craniotomy for supratentorial tumors. Journal of Neurosurgical Anesthesiology 2017;29(3):211‐8. [PUBMED: 26859547] [DOI] [PubMed] [Google Scholar]
Zeng 2019 {published data only}
- Zeng M, Dong J, Lin N, Zhang W, Zhang K, Peng K, et al. Preoperative gabapentin administration improves acute postoperative analgesia in patients undergoing craniotomy: a randomized controlled trial. Journal of Neurosurgical Anesthesiology 2019;31(4):392‐8. [PUBMED: 30134301] [DOI] [PubMed] [Google Scholar]
Zhang 2003 {published data only}
- Zhang XP, Ren YG. The comparison of three methods of the scalp block, wound infiltration and superficial cervical plexus block for decreasing postoperative pain after craniotomy. Chinese Journal of Misdiagnostics 2003;8:1148‐50. [http://en.cnki.com.cn/Article_en/CJFDTOTAL‐ZWZX200308015.htm] [Google Scholar]
Zhou 2016 {published data only}
- Zhou H, Ou M, Yang Y, Ruan Q, Pan Y, Li Y. Effect of skin infiltration with ropivacaine on postoperative pain in patients undergoing craniotomy. Springerplus 2016;5(1):1180. [PUBMED: 27512639] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ackil 2018 {published data only}
- Akcil EF, Korkmaz Dilmen O, Ertem Vehid H, Yentur E, Tunali Y. The role of "Integrated Pulmonary Index" monitoring during morphine‐based intravenous patient‐controlled analgesia administration following supratentorial craniotomies: a prospective, randomized, double‐blind controlled study. Current Medical Research and Opinion 2018;34:2009‐14. [DOI: 10.1080/03007995.2018.1501352] [DOI] [PubMed] [Google Scholar]
Ayoub 2006 {published data only}
- Ayoub C, Girard F, Boudreault D, Chouinard P, Ruel M, Moumdjian R. A comparison between scalp nerve block and morphine for transitional analgesia after remifentanil‐based anesthesia in neurosurgery. Anesthesia and Analgesia 2006;103(5):1237‐40. [PUBMED: 17056961] [DOI] [PubMed] [Google Scholar]
Bajaj 2017 {published data only}
- Bajaj J, Mittal RS, Sharma, A. Preoperative clonidine use in trans‐sphenoidal pituitary adenoma surgeries ‐ a randomized controlled trial. British Journal of Neurosurgery 2017;31:2‐4. [PUBMED: 27535352] [DOI] [PubMed] [Google Scholar]
Bishnoi 2016 {published data only}
- Bishnoi V, Kumar B, Bhagat H, Salunke P, Bishnoi S. Comparison of dexmedetomidine versus midazolam‐fentanyl combination for monitored anesthesia care during burr‐hole surgery for chronic subdural hematoma. Journal of Neurosurgical Anesthesiology 2016;28(2):141‐6. [PUBMED: 26018670] [DOI] [PubMed] [Google Scholar]
Citerio 2012 {published data only}
- Citerio G, Pesenti A, Latini R, Masson S, Barlera S, Gaspari F, et al. NeuroMorfeo Study Group. A multicentre, randomised, open‐label, controlled trial evaluating equivalence of inhalational and intravenous anaesthesia during elective craniotomy. European Journal of Anesthesiology 2012;29(8):371‐9. [PUBMED: 22569025 ] [DOI] [PubMed] [Google Scholar]
Dolmatova 2009 {published data only}
- Dolmatova EV, Imaev AA, Lubnin AY. 'Scheduled' dosing of lornoxicam provides analgesia superior to that provided by 'on request' dosing following craniotomy. European Journal of Anaesthesiology 2009;26(8):633‐7. [PUBMED: 19593886] [DOI] [PubMed] [Google Scholar]
Domenech 2006 {published data only}
- Doménech P, Hernández‐Palazón J, Segura B. A randomized, double‐blind comparison between parecoxib sodium and paracetamol for postoperative analgesia after intracranial surgery. European Journal of Anesthesiology 2006;23:A‐398. [Google Scholar]
Doumiri 2015 {published data only}
- Doumiri M, Motiaa Y, Razine R, Amor M, Moussaoui A, Kabbaj S, et al. Should we continue to infiltrate the scalp with a local anesthetic for a craniotomy?. Pan African Medical Journal 2015;3(22):2. [PUBMED: 26759693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dudko 2014 {published data only}
- Dudko J, Juske M, Banevicius G. Postoperative pain management after craniotomy: 14AP9‐11. European Journal of Anesthesiology 2014;31:241. [EMBASE: 71638557] [Google Scholar]
El Dahab 2009 {published data only}
- Dahab HA. General anesthesia combined with skull block versus conventional general anesthesia with fentanyl during supratentorial craniotomies in geriatric patients: a comparative study. Egyptian Journal of Anaesthesia 2009;25(4):439‐51. [Google Scholar]
Ferber 2000 {published data only}
- Ferber J, Juniewicz H, Głogowska E, Wroński J, Abraszko R, Mierzwa J. Tramadol for postoperative analgesia in intracranial surgery. Its effect on ICP and CPP. Neurologia i Neurochirurgia Polska 2000;34(6 suppl):70‐9. [PUBMED: 11452859] [PubMed] [Google Scholar]
Girard 2010 {published data only}
- Girard F, Quentin C, Charbonneau S, Ayoub C, Boudreault D, Chouinard P, et al. Superficial cervical plexus block for transitional analgesia in infratentorial and occipital craniotomy: a randomized trial. Canadian Journal of Anesthesiology 2010;57(12):1065‐70. [PUBMED: 20878375] [DOI] [PubMed] [Google Scholar]
Goldsack 1996 {published data only}
- Goldsack C, Scuplak SM, Smith M. A double‐blind comparison of codeine and morphine for postoperative analgesia following intracranial surgery. Anaesthesia 1996;51(11):1029‐32. [PUBMED: 8943593] [DOI] [PubMed] [Google Scholar]
Graham 1999 {published data only}
- Graham AC, Reid MM, Andrews PJD. Perception of pain experienced and adequacy of analgesia following elective craniotomy. Anaesthesia 1999;54(8):814‐5. [PUBMED: 10460707] [DOI] [PubMed] [Google Scholar]
Hassani 2015 {published data only}
- Hassani E, Mahoori A, Sane S, Tolumehr A. Comparison the effects of paracetamol with sufentanil infusion on postoperative pain control after craniotomy in patients with brain tumor. Advanced Biomedical Research 2015;4(1):64. [PUBMED: 25821764 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Honnma 2002 {published data only}
- Honnma T, Imaizumi T, Chiba M, Niwa J. Preemptive analgesia for postoperative pain after frontotemporal craniotomy. No Shinkei Geka 2002;30(2):171‐4. [PUBMED: 11857941] [PubMed] [Google Scholar]
Imaev 2008 {published data only}
- Imaev AA, Dolmotova EV, Lubnin AI. Preemptive analgesia after craniotomy using lornoxicam: 7AP3‐4. European Journal of Anesthesiology 2008;25:97. [Google Scholar]
Imaev 2010 {published data only}
- Imaev AA, Dolmotova EV, Lubnin AI. Comparative evaluation of preventive analgesia with xefocam, ropivacaine, and transdermal drug delivery system of durogesic in patients after craniotomy. Anesteziologiia i Reanimatologiia 2010;4:15‐9. [PUBMED: 20922845] [PubMed] [Google Scholar]
Jayaram 2016 {published data only}
- Jayaram K, Srilata M, Kulkarni D, Ramachandran G. Regional anesthesia to scalp for craniotomy: innovation with innervation. Journal of Neurosurgical Anesthesiology 2016;28(1):32‐7. [PUBMED: 26083426] [DOI] [PubMed] [Google Scholar]
Jeffrey 1999 {published data only}
- Jeffrey HM, Charlton P, Mellor DJ, Moss E, Vucevic M. Analgesia after intracranial surgery: a double‐blind, prospective comparison of codeine and tramadol. British Journal of Anaesthesia 1999;83(2):245‐9. [PUBMED: 10618937] [DOI] [PubMed] [Google Scholar]
Jose 2017 {published data only}
- Jose R, Chakravarthy K, Nair S, Joseph M, Jeyaseelan V, Korula G. A randomized controlled trial studying the role of dexamethasone in scalp nerve blocks for supratentorial craniotomy. Journal of Neurosurgical Anesthesiology 2017;29(2):150‐6. [PUBMED: 26756502] [DOI] [PubMed] [Google Scholar]
Lu 2009 {published data only}
- Lu JJ, Yu S, Qian K. Effect of multimodal analgesia on incision of scalp pain after craniotomy. Chinese Journal of Rehabilitation and Practice 2009;15(7):619‐20. [Google Scholar]
Luo 2014 {published data only}
- Luo F, Liu Y, Jiang JY. Effect of compound lidocaine hydrochloride on postoperative pain after craniotomy. Chinese Journal of Rehabilitation Theory Practice 2007;13(8):757‐8. [Google Scholar]
Mohamed 2018 {published data only}
- Mohamed AA, Radwan TA, Mohamed MM, Mohamed HA, Mohamed Elemady MF, Osman SH, et al. Safety and efficacy of addition of hyaluronidase to a mixture of lidocaine and bupivacaine in scalp nerves block in elective craniotomy operations; comparative study. BMC Anesthesiology 2018;18(1):129. [PUBMED: 30219027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morad 2009 {published data only}
- Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE, et al. Efficacy of intravenous patient‐controlled analgesia after supratentorial intracranial surgery: a prospective randomized controlled trial. Journal of Neurosurgery 2009;111(2):343‐50. [PUBMED: 19249923 ] [DOI] [PubMed] [Google Scholar]
Na 2011 {published data only}
- Na HS, An SB, Park HP, Lim YJ, Hwang JW, Jeon YT, et al. Intravenous patient‐controlled analgesia to manage the postoperative pain in patients undergoing craniotomy. Korean Journal of Anesthesology 2011;60(1):30‐5. [PUBMED: 21359078] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palazón 2006 {published data only}
- Hernández‐Palazón J, Doménech‐Asensi P, Burguillos‐López S, Segura‐Postigo B, Sánchez‐Ródenas L, López‐Hernández F. Comparison of anesthetic maintenance and recovery with propofol versus sevofluranecombined with remifentanil in craniotomy for supratentorial neoplasm. Revista Espanola Anesthesiolologica Reanimacion 2006;53(2):88‐94. [PUBMED: 16553341] [PubMed] [Google Scholar]
Rajan 2016 {published data only}
- Rajan S, Hutcherson MT, Sessler D, Kurz A, Yang D, Ghobrial M, et al. The effects of dexmedetomidine and remifentanil on hemodynamic stability and analgesic requirement after craniotomy: a randomized controlled trial. Journal of Neurosurgical Anesthesiology 2016;28:282‐90. [PUBMED: 26325514 ] [DOI] [PubMed] [Google Scholar]
Reddy 2018 {published data only}
- Reddy M, Theerth KA, Kamath S. Comparison of scalp block and local anesthetic skin infiltration for craniotomy on analgesia nociceptive index‐guided fentanyl consumption: a randomized controlled trial. Journal of Neurosurgical Anesthesiology 2018;30:100. [DOI: ] [Google Scholar]
Simon 2012 {published data only}
- Simon E, Bánk J, Gál J, Siró P, Novák L, Fülesdi B, et al. Administration of preemptive analgesia by diclofenac to prevent acute postcraniotomy headache. Ideggyogy Sz 2012;65(9‐10):302‐6. [PUBMED: 23126214] [PubMed] [Google Scholar]
Soliman 2011 {published data only}
- Soliman RN, Hassan AR, Rashwan AM, Omar AM. Prospective, randomized study to assess the role of dexmedetomidine in patients with supratentorial tumors undergoing craniotomy under general anaesthesia. Middle East Journal of Anesthesiology 2011;21(3):325‐34. [PUBMED: 22428485] [PubMed] [Google Scholar]
Stone 2018 {published data only}
- Stone S, Burbridge MA, Jaffe R. Acetaminophen does not reduce postoperative opiate consumption in patients undergoing craniotomy for cerebral revascularization: a randomized control trial. Journal of Neurosugical Anesthesiololgy 2018;30:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoneham 1996 {published data only}
- Stoneham MD, Cooper R, Quiney NF, Walters FJ. Pain following craniotomy: a preliminary study comparing PCA morphine with intramuscular codeine phosphate. Anaesthesia 1996;51(12):1176‐8. [PUBMED: 9038464] [DOI] [PubMed] [Google Scholar]
Sudheer 2007 {published data only}
- Sudheer PS, Logan SW, Terblanche C, Ateleanu B, Hall JE. Comparison of the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy. Anaesthesia 2007;62(6):555‐60. [PUBMED: 17506732] [DOI] [PubMed] [Google Scholar]
Tanskanen 1999 {published data only}
- Tanskanen P, Kyttä J, Randell T. Patient‐controlled analgesia with oxycodone in the treatment of postcraniotomy pain. Acta Anaesthesiologica Scandinavica 1999;43(1):42‐5. [PUBMED: 9926187] [DOI] [PubMed] [Google Scholar]
Theerth 2018 {published data only}
- Theerth KA, Sriganesh K, Reddy KM, Chakrabarti D, Umamaheswara Rao GS. ANI‐guided intraoperative fentanyl consumption and postoperative analgesia in patients receiving scalp block versus incision‐site infiltration for craniotomy. Minerva Anestesiologica 2018;84(12):1361‐8. [PUBMED: 29991223 ] [DOI] [PubMed] [Google Scholar]
Ture 2009 {published data only}
- Türe H, Sayin M, Karlikaya G, Bingol CA, Aykac B, Türe U. The analgesic effect of gabapentin as a prophylactic anticonvulsant drug on postcraniotomy pain: a prospective randomized study. Anesthesia and Analgesia 2009;109(5):1625‐31. [PUBMED: 19713257 ] [DOI] [PubMed] [Google Scholar]
Vallapu 2018 {published data only}
- Vallapu S, Panda NB, Samagh N, Bharti N. Efficacy of dexmedetomidine as an adjuvant to local anesthetic agent in scalp block and scalp Infiltration to control postcraniotomy pain: a double‐blind randomized trial. Journal of Neurosciences in Rural Practice 2018;9(1):73‐9. [PUBMED: 29456348 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venkatraghavan 2016 {published data only}
- Venkatraghavan L, Li L, Bailey T, Manninen PH, Tymianski M. Sumatriptan improves postoperative quality of recovery and reduces postcraniotomy headache after cranial nerve decompression. British Journal of Anaesthesia 2016;117(1):73‐9. [PUBMED: 27317706 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verchere 2002 {published data only}
- Verchère E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. Journal of Neurosurgical Anesthesiology 2002;14(2):96‐101. [PUBMED: 11907388] [DOI] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu Y‐X, Chen H, Zhou J‐X. Short‐term use of remifentanil during endotracheal extubation for prophylactic analgesia in neurosurgical patients after craniotomy (SURE after Craniotomy Study): a study protocol and statistical analysis plan for a randomised controlled trial. BMJ Open 2014;4(9):e005635. [PUBMED: 25270857] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2013 {published data only}
- Zhao LH, Shi ZH, Yin NN, Zhou JX. Use of dexmedetomidine for prophylactic analgesia and sedation in delayed extubation patients after craniotomy: a study protocol and statistical analysis plan for a randomized controlled trial. Trials 2013;13(14):251. [PUBMED: 23941549] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
KCT0000274 {published data only}
- KCT0000274. Scalp blocks with levobupivacaine reduced postoperative pain and the requirement of anti‐hypertensive agent after craniotomy for aneurysmal clipping. cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=2082 (first received 13 October 2013).
Additional references
Basali 2000
- Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology 2000;93(1):48‐54. [PUBMED: 10861145] [DOI] [PubMed] [Google Scholar]
Becker 2012
- Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesthesia Progress 2012;59(2):90‐101. [PUBMED: 22822998] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritals Health Innovation. Covidence. Version accessed 10 January 2016. Melbourne, Australia: Veritals Health Innovation.
De Gray 2005
- Gray LC, Matta BF. Acute and chronic pain following craniotomy: a review. Anaesthesia 2005;60:693‐704. [PUBMED: 15960721] [DOI] [PubMed] [Google Scholar]
De Oliveria Riberio 2013
- Oliveria Riberio MC, Pereira CU, Sallum A, Martins‐ Filho RS, Santana JM, Dilva Nunes M, et al. Immediate post‐craniotomy headache. Cephalalgia 2013;33(11):897‐905. [PUBMED: 23482724] [DOI] [PubMed] [Google Scholar]
Doleman 2018
- Doleman B, Leonardi‐Bee J, Heinink TP, Lund J, Williams JP. Pre‐emptive and preventive opioids for postoperative pain in adults undergoing all types of surgery. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD012624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunbar 1999
- Dunbar PJ, Visco E, Lam AM. Craniotomy procedures are associated with less analgesic requirements than other surgical procedures. Anesthesia and Analgesia 1999;88(2):335‐40. [PUBMED: 9972752] [DOI] [PubMed] [Google Scholar]
Dunn 2016
- Dunn LK, Naik BI, Nemergut EC, Durieux ME. Post‐craniotomy pain management: beyond opioids. Current Neurology and Neuroscience Reports 2016;16(10):93. [PUBMED: 27604271] [DOI] [PubMed] [Google Scholar]
Gilfoyle 2012
- Gilfoyle MR, Helmy A, Duane D, Hutchinson PJ. Regional scalp block for postcraniotomy analgesia: a systematic review and meta‐analysis. Anesthesia and Analgesia 2013;116(5):1093‐102. [PUBMED: 23477962] [DOI] [PubMed] [Google Scholar]
Gottschalk 2009
- Gottschalk A, Yaster M. The perioperative management of pain from intracranial surgery. Neurocritical Care 2009;10(3):387‐402. [PUBMED: 18830699] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, et al. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haldar 2015
- Haldar R, Kashaul A, Gupta D, Srivastva S, Singh P. Pain following craniotomy: reassessment of the available options. BioMed Research International 2015;2015:509164. [PUBMED: 26495298] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2011
- Hansen MS, Brennum J, Moltke FB, Dahl J. Pain treatment after craniotomy: where is the procedure specific evidence? A qualitative systematic review. European Journal of Anesthesiology 2011;28(12):821‐9. [PUBMED: 21971206] [DOI] [PubMed] [Google Scholar]
Hansen 2013
- Hansen MS, Breenum J, Moltke FB, Dahl JB. Suboptimal pain treatment after craniotomy. Danish Medical Journal 2013;60(2):A4569. [PUBMED: 23461986 ] [PubMed] [Google Scholar]
Harner 1993
- Harner SG, Beatty CW, Ebersold MJ. Headache after acoustic neuroma excision. American Journal of Otology 1993;14(6):552‐5. [PUBMED: 8296857 ] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgs 1980
- Higgs GA. Arachidonic acid metabolism, pain and hyperalgesia: the mode of action of non‐steroid mild analgesics. British Journal of Clinical Pharmacology 1980;10 Suppl 2:233S‐5S. [PUBMED: 7002184] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imayev 2013
- Imayev AA, Dolmatova EV, Lubnin AYU. Management of postoperative analgesia in patients after craniotomy. Burdenko's Journal of Neurosurgery 2013;77(3):54‐61. [PUBMED: 23866579] [PubMed] [Google Scholar]
Inanoglu 2007
- Inanoglu K, Gorur S, Akkort CO, Guven OE, Karamaraz A. The analgesic efficacy of preoperative versus postoperative lornoxicam in variocele repair. Journal of Clinical Anesthesia 2007;19(8):587‐90. [PUBMED: 18083471] [DOI] [PubMed] [Google Scholar]
Institute of Medicine of the National Academics
- Institute of Medicine of the National Academics. Finding What Works in Health Care: Standards for Systematic Reviews. Washington: Institute of Medicine of the National Academics, 2011. [PubMed] [Google Scholar]
Jessen Lundorf 2016
- Jessen Lundorf L, Korvenius Nedergaard H, Møller AM. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD010358] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joanna Briggs Institute 2011
- Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual. Adelaide: Joanna Briggs Institute, 2011. [Google Scholar]
Kotak 2009
- Kotak D, Cheserem B, Solth A. A survey of post‐craniotomy analgesia in British neurosurgical centres: time for perceptions and prescribing to change?. British Journal of Neurosugery 2009;23(5):538‐42. [PUBMED: 19863401] [DOI] [PubMed] [Google Scholar]
Martin 1983
- Martin WR. Pharmacology of opioids. Pharmacological Reviews 1983;35(4):283‐323. [PUBMED: 6144112] [PubMed] [Google Scholar]
McQuay 1995
- McQuay H. Pre‐emptive analgesia: a systematic review of clinical studies. Annals of Medicine 1995;27(2):249‐56. [PUBMED: 7632422] [DOI] [PubMed] [Google Scholar]
Molnar 2014
- Molnar L, Simon E, Nemes R, Fulesdi B, Molnar C. Postcraniotomy headache. Journal of Anesthesia 2014;28(1):102‐11. [PUBMED: 23846599] [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anesthesia 2013;68(4):400‐12. [PUBMED: 23347230] [DOI] [PubMed] [Google Scholar]
Mordhorst 2009
- Mordhorst C, Latz B, Kerz T, Wisser G, Schmidt A, Schneider A, et al. Prospective assessment of postoperative pain after craniotomy. Journal of Neurosurgical Anesthesiology 2010;22(3):202‐6. [PUBMED: 20479664] [DOI] [PubMed] [Google Scholar]
Powell 2016
- Powell R, Scott NW, Manyande A, Bruce J, Vögele C, Byrne‐Davis LMT, et al. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD008646] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prabhakar 2016
- Prabhakar H, Singh GP, Mahajan C, Kapoor I, Kalaivani M, Anand V. Intravenous versus inhalational techniques for rapid emergence from anaesthesia in patients undergoing brain tumour surgery. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD010467] [DOI] [PMC free article] [PubMed] [Google Scholar]
Remy 2005
- Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side effects and consumption after major surgery: meta‐analysis of randomized controlled trials. British Journal of Anaesthesia 2005;94(4):505‐13. [PUBMED: 15681586] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ribeiro 2012
- Riberio MCO, Pereira CU, Sallum AMC, Albuquerque MF, Fujishima PK. Knowledge of doctors and nurses on pain in patients undergoing craniotomy. Revista Latino‐Americana de Enfermagem 2012;20(6):1057‐63. [PUBMED: 23258718] [DOI] [PubMed] [Google Scholar]
Schaller 2003
- Schaller B, Baumann A. Headache after removal of vestibular schwannoma via the retrosigmoid approach: a long tern follow‐up study. Otolaryngology 2003;128(3):387‐95. [PUBMED: 12646842] [DOI] [PubMed] [Google Scholar]
Smyth 2004
- Smyth M, Banks J, Tubbs R, Wellens JC, Oakes WJ. Efficacy of scheduled nonnarcotic analgesic medications in children after suboccipital craniotomy. Journal of Neurosurgery 2004;100(2 suppl.):183‐6. [PUBMED: 14758947] [DOI] [PubMed] [Google Scholar]
Tayeb 2017
- Tayeb BO, Eidelman A, Eidelman CL, McNicol ED, Carr DB. Topical anaesthetics for pain control during repair of dermal laceration. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD005364.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsaousi 2016
- Tsaousi GG, Logan SW, Bilotta F. Postoperative pain control following craniotomy: a systematic review of recent clinical literature. Pain Practice 2016;17(7):968‐81. [PUBMED: 27996204] [DOI] [PubMed] [Google Scholar]
Wall 1988
- Wall PD. The prevention of postoperative pain. Pain 1988;33(3):289‐90. [PUBMED: 3419835] [DOI] [PubMed] [Google Scholar]
Weibel 2018
- Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LHJ, Hahnenkamp K, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD009642] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinstein 2018
- Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, Johnson M, et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD007105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2016
- Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD003868.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2011
- Williams DL, Pemberton E, Leslie K. Effects of intravenous parecoxib on post craniotomy pain. British Journal of Anesthesia 2011;107(3):398‐403. [PUBMED: 21841050] [DOI] [PubMed] [Google Scholar]
Yadav 2014
- Yadav G, Choupoob S, Das SK, Das SK, Behera SS, Khuba S, et al. Evaluating the role of flupirtine for postcraniotomy pain and compare it with diclofenac sodium: a prospective, randomized, double blind, placebo‐controlled study. Journal of Neurosurgical Anesthesiology 2014;26(1):32‐6. [PUBMED: 23764718] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Galvin 2015
- Galvin IM, Levy R, Day AG, Gilron I. Interventions for the prevention of acute postoperative pain in adults following brain surgery. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011931] [DOI] [PMC free article] [PubMed] [Google Scholar]


