Abstract
Recent basic studies have clarified that aneurysmal wall inflammation plays an important role in the pathophysiology of intracranial aneurysms. However, it remains an interdisciplinary challenge to visualize aneurysm wall status in vivo. MR-vessel wall imaging (VWI) is a current topic of advanced imaging techniques since it could provide an additional value for unruptured intracranial aneurysms (UIAs) risk stratification. With regard to ruptured intracranial aneurysms, VWI could identify a ruptured aneurysm in patients with multiple intracranial aneurysms. Intraluminal thrombus could be a clue to interpret aneurysm wall enhancement on VWI in ruptured intracranial aneurysms. The interpretation of VWI findings in UIAs would require much caution. Actually aneurysm wall enhancement in VWI was significantly associated with consensus morphologic risk factors. However, aneurysmal wall with contrast enhancement oftentimes associated with atherosclerotic, degenerated and thickened wall structure. It remains ill defined if thin wall without wall enhancement (oftentimes invisible in VWI) could be actually safe or look over wall vulnerability. We reviewed currently available studies, especially focusing on VWI for intracranial aneurysms and discussed the clinical utility of VWI.
Keywords: intracranial aneurysm, vessel wall imaging, histopathology, inflammation
Introduction
An unruptured intracranial aneurysm (UIA) is a common vascular lesion with a prevalence of around 3–5% in the general population.1) The pathophysiology of UIA leads to a dilemma in clinical practice since the majority of aneurysms never bleed, with an annual rupture rate of around 1%, but hazardous bleeding and poor outcomes can occur after rupture.1,2) Microsurgical and/or endovascular treatment are considered the preventive treatments of choice, although procedural morbidity and mortality of around 2–5% is not negligible.1) Establishing a reliable method of risk stratification for aneurysms has been an immense task. The PHASES score is used to predict the 5-year risk of aneurysm rupture, based on patient demographic features and aneurysm morphology.3) However, there has been some criticism of this score because it is inadequate for evaluation of small aneurysms and does not consider geometrical factors or patient-related factors such as smoking.4) Recent investigations have clarified that aneurysm wall inflammation is associated with aneurysmal instability.5–7) MR-vessel wall imaging (VWI) is a novel technique for demonstrating vessel wall structures that is expected to be applied clinically for detection of unstable aneurysms prone to rupture.8–10) In this article, we reviewed previous studies on VWI of ruptured and unruptured intracranial aneurysms and discuss its clinical implications.
The Concept of Vessel Wall Imaging of Intracranial Aneurysms
Angiography only characterizes the luminal features of intracranial vessels, and it has been a challenge to depict wall structures. Recent advances in MR imaging have allowed assessment of the vessel wall architecture by a specific imaging technique, so called VWI. It is expected that VWI can be used to clarify the pathophysiology of intracranial vasculopathies, to decide treatment options, and to investigate the response to medication.8,9)
Vessel wall imaging suppresses signals of vessel lumen and cerebrospinal fluid and can image detectable wall signals. Diseased vessels accompany with wall thickening (i.e. atherosclerosis, vasculitis or thrombosis), conspicuity of wall structure could be increased in voxel dimensions. Using contrast agents could enhance the lesion presumably due to inflammation and neovascularization. Although some studies showed conventional VWI (1.5 T) detectable diseased wall structures as well, high signal-to-noise ratio with stronger magnetic field (>3 T) would be recommended.
Currently applied sequences for VWI is T1 black blood method based on three-dimensional variable-flip-angle fast spin echo sequence, named as CUBE (GE Healthcare, Milwaukee, WI, USA), volume isotropic turbo spin-echo acquisition (Philips Medical Systems, Eindhoven, Netherlands), sampling perfection with application-optimized contrasts by using different flip angle evolutions (Siemens Healthcare GmbH, Erlangen, Germany) and multiplanar voxel (CANON MEDICAL SYSTEMS CORPORATION, Ohtawara, Tochigi, Japan). There are two options to obtain VWI using multiplanar 2D or 3D sequence. Considering total scanning time, spatial resolution and need to find lesions in a saccular structure, 3D volumetric sequence would be much advantageous. Multiphasic scanning would be recommended to avoid motion artifact and create fusion images.
VWI of Ruptured Intracranial Aneurysms
Since Matouk et al.11) showed that contrast-enhanced VWI can be used to identify the culprit aneurysm causing subarachnoid hemorrhage in patients with multiple aneurysms, many reports were published on this imaging technique for ruptured intracranial aneurysms. Aneurysm wall enhancement (AWE) is found more frequently in ruptured IAs than in UIAs,12–15) and meta-analysis has shown that detection of the culprit aneurysm can be achieved clinically with high sensitivity and specificity using AWE.16,17) At present, no international stroke guidelines recommend routine use of MRI in patients with aneurysmal subarachnoid hemorrhage (SAH). However, as noted by expert opinion, VWI could be considered for patients with ruptured IA in the following situations: (1) when a patient had multiple aneurysms, (2) patients with SAH of unknown origin.9,10)
What does AWE of ruptured intracranial aneurysms potentially tell us?
The most critical point regarding VWI in ruptured IAs is qualitative assessment of whether wall enhancement is partial/focal or circumferential. The correlation between VWI findings and histopathology has rarely been investigated, and the mechanism underlying AWE is not well defined.18,19) Ishikawa et al. explored the mechanism of hemostasis in ruptured IAs.20,21) When assessing the VWI features of ruptured IAs, the influence of intraluminal thrombus should be considered. The wall of the IA is extremely thin (approximately 20 μm) at the site of rupture, and it is arguable whether such a thin wall can be reliably visualized by MRI since it is assumed to be far beyond the resolution of this modality. We stress that focal wall enhancement is suggestive of intraluminal thrombus at the actual rupture site, whereas we have no explanation for the underlying mechanism of circumferential wall enhancement.19) Actually specimens of ruptured IAs with circumferential wall enhancement revealed abundant inflammation and neovascularization of the aneurysm wall. It remains arguable when such circumferential AWE started on. Further investigation will be required to determine whether the VWI findings of ruptured IAs are the same as those of UIAs.
VWI of ruptured IAs in clinical practice
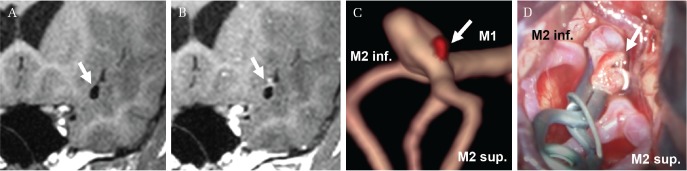
Besides finding a culprit aneurysm in multiple aneurysms, VWI is expected to provide useful information for clinical management. Detection of focal AWE can be informative for microsurgical clipping or endovascular coiling, especially if the aneurysm is irregular or multi-lobular. While the majority of aneurysms bleed from the fundus, some can bleed from the neck (Fig. 1). Therefore, anatomical information from VWI could assist with meticulous handling of the aneurysm, whether microsurgical clipping or endovascular treatment is selected (Fig. 2). We consider performing VWI when optimal treatment depends on precisely identifying the site of rupture.
Fig. 1.
A 53-year-old woman with multiple intracranial aneurysms including the left middle cerebral artery and the left internal carotid artery–anterior choroidal artery. Vessel wall imaging (VWI) shows partial wall enhancement from the middle cerebral artery aneurysms (arrow) (A: native, B: contrast-enhanced imaging). The fusion image between time-of-flight magnetic resonance angiography and VWI demonstrates the wall enhancement (colored red) around the aneurysmal neck (arrow) (C). Intraoperative view demonstrates actual rupture point around the neck (arrow) (D). Lt. M1 and M2 indicate the left middle cerebral artery first and second segment, respectively. Sup. and Inf. indicate superior and inferior, respectively. MR-VWI is acquired on a standard strength 1.5 T Scanner (GE) with 8-channel head coil. Scanning sequence and parameter comprise a T1 black blood method (T1 CUBE; TE/TR: 10/550, matrix: 256 × 256, field of view: 240 × 240 mm,2) slice thickness: 1.0 mm, flip angle: variable refocusing flip angle, acquisition time: 3 min 39 s).
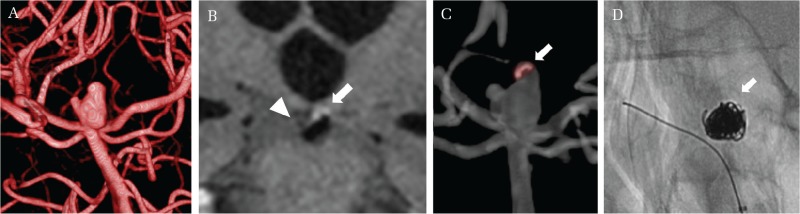
Fig. 2.
A 69-year-old woman with a ruptured intracranial aneurysm of the basilar artery. Preoperative three-dimensional digital subtraction angiography suggests an irregular multilobular aneurysm at the basilar bifurcation (A). Vessel wall imaging demonstrates focal wall enhancement in a main sac (arrow), whereas no wall enhancement in a smaller daughter sac (arrow head) (B). Fusion image created from time-of-flight magnetic resonance angiography and contrast-enhanced vessel wall imaging suggests the sites of aneurysm wall enhancement, indicating the rupture point (arrow) (C). The aneurysm is tightly packed without coils into the ruptured component (D).
VWI of Unruptured Intracranial Aneurysms
With regard to UIAs, the clinical relevance of VWI is a current topic if it could identify unstable UIAs prone to rupture. To date, a number of studies have been published on VWI of unruptured intracranial aneurysms, but there have been some differences among the findings. When the correlation between AWE and aneurysm instability is discussed, arguments about the definition of “instability” arise. Some studies have classified ruptured IAs or symptomatic UIAs as unstable aneurysms.18,22–24) We stress that the definition of “unstable aneurysm” should be carefully considered and the clinical relevance of AWE in UIAs needs to be interpreted with caution. Symptomatic UIAs are often large, so surgical or interventional treatment needs to be considered, which is perhaps why recent VWI studies have tended to focus on asymptomatic UIAs.25–29) Symptomatic aneurysms are often large, hence these aneurysms should be treated if we consider previous prospective results.2) Smaller aneurysms (<7 mm) are still a concern.
Factors correlating with AWE in unruptured intracranial aneurysms
We have summarized studies on VWI of unruptured intracranial aneurysms and our series in Table 1. Reviewing these studies indicates that the prevalence of wall enhancement ranges widely from 29% to 74%. In the majority of studies, the strongest correlation was noted between AWE and aneurysm size, followed by irregular aneurysm shape and aneurysm location. We reviewed a relatively large series of 157 consecutive VWI studies of UIAs at a single center (Table 2). AWE was detected in 33% of UIAs in our series. There was a significant relationship between AWE and morphological factors, including aneurysm size and shape (P <0.001). Considering the results of studies of aneurysm behavior and histopathological analysis, AWE in larger aneurysms could be explained by degeneration of the lesion.5–7)
Table 1.
Studies on vessel wall imaging of unruptured intracranial aneurysms
| Authors (year) | Aneurysms (n) | AWE (%) | Valuables associated with AWE by univariate analysis | |||
|---|---|---|---|---|---|---|
| Liu et al. (2016) | 61 | 54 | Size | |||
| Bakes et al. (2017) | 89 | 29 | Size | Location (PCoA and MCA) | ||
| Lv et al. (2018) | 140 | 59 | Size | Irregular shape | Location | PHASES |
| Hartman et al. (2019) | 65 | 65 | PHASES > 3 | |||
| Wang et al. (2019) | 88 | 74 | Size | Irregular shape | High aspect ratio | |
| Our series | 157 | 32 | Size | Irregular shape | PHASES | |
AWE: aneurysm wall enhancement, MCA: middle cerebral artery, PCoA: posterior communicating artery.
Table 2.
Unruptured intracranial aneurysms. Correlations between aneurysm wall enhancement and patient demographics or aneurysm location/morphology
| Total (n = 157) | AWE (+) (n = 52) | AWE (−) (n = 105) | P-value | |
|---|---|---|---|---|
| Patient demographics | ||||
| Age, mean ± SE | 70 ± 10.9 | 71 ± 1.5 | 69 ± 1.2 | 0.75 |
| Female sex, n (%) | 113 (72) | 43 (83) | 70 (67) | 0.04 |
| Aneurysm location | 0.05 | |||
| ICA (paraclinoid), n (%) | 16 (10) | 3 (19) | 13 (81) | |
| ICA (Ach or PCoA), n (%) | 31 (20) | 10 (32) | 21 (68) | |
| ACA, n (%) | 35 (22) | 11 (31) | 24 (69) | |
| MCA, n (%) | 76 (42) | 21 (32) | 45 (68) | |
| Posterior circulation, n (%) | 9 (6) | 7 (78) | 2 (22) | |
| Aneurysm morphology | ||||
| Maxim diameter, mean ± SE (mm) | 5.1 ± 0.2 | 6.5 ± 0.6 | 4.5 ± 0.1 | <0.001 |
| Aspect ratio, mean ± SE | 1.2 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 0.13 |
| Irregularity, n (%) | 75 (48) | 37 (71) | 38 (36) | <0.001 |
| PHASES | 7.4 ± 0.2 | 8.3 ± 0.3 | 7.0 ± 0.2 | 0.002 |
AWE: aneurysm wall enhancement, ACA: anterior cerebral artery, Aspect ratio: dome diameter/neck diameter, ICA: internal cerebral artery, MCA: middle cerebral artery, PCoA: posterior communicating artery, SE: standard error.
What does AWE of unruptured intracranial aneurysms tell us?
Recent basic studies have clarified that wall inflammation plays an important role in the pathophysiology of aneurysm rupture.5–7) Exploring the correlations between VWI findings and clinicopathological data on aneurysm wall architecture could help us to understand the clinical significance of VWI in UIAs. However, only a few histopathological studies (including nanoparticle imaging) have assessed the association between aneurysm wall findings on imaging and mural inflammation.18,30–33) It has been hypothesized that a strong correlation exists between atherosclerotic factors and mural degeneration in unstable aneurysms.34,35) We have found that inflammation and the vasa vasorum in the thickened aneurysm wall are associated with AWE.32) Of course, not all aneurysm walls can be visualized, even by ultra-high field 7 T MRI, mainly due to the limited spatial resolution of imaging.36) The clinical significance of a thin aneurysm wall remains controversial and has been debated in the literature.30,31) Some studies have investigated the relation between wall thinning and hemodynamics.37) Enhancement might imply fragility of the aneurysm wall that leads to remodeling, thinning, and daughter sac formation.
Can VWI serve as a possible imaging biomarker of unstable UIAs?
Latest studies showed supportive results that aneurysm wall enhancement on VWI could identify unstable aneurysms based on a short-term prospective observational study or the finding that AWE is associated with currently recognized clinical risk factors for rupture.22,38,39) It is noteworthy that the majority of UIAs with unstable growth (i.e., daughter sac formation) showed AWE.22,30) Although these studies did not include prospective data, the findings were of interest. Omodaka et al.14,15,22) quantitatively measured the signal intensity ratio of enhanced wall structures relative to the pituitary stalk on VWI. They found a significant difference between stable aneurysms, unstable aneurysms (growing or symptomatic), and ruptured aneurysms, with the signal intensity ratio of the enhanced area seeming to increase parallel to the wall instability.22)
It is still arguable as to whether this novel technique could change current clinical practice, with further research being needed to provide additional information about identifying unstable aneurysms with VWI. Further discussion of the role of wall fragility is required, especially in UIAs without wall enhancement. We consider that comprehensive assessment of UIAs using VWI, computational fluid dynamics, and histopathological examination is required.
VWI of unruptured intracranial aneurysms in clinical practice
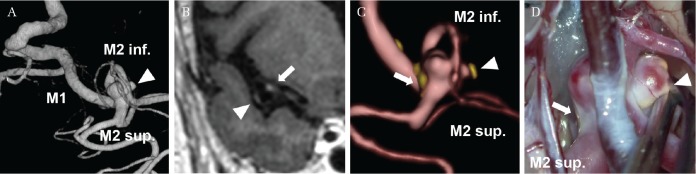
Among the clinicopathological insights provided by VWI of UIAs, this technique should be useful for predicting atherosclerosis in UIAs.40) In case of large or giant aneurysms, atherosclerosis around the aneurysmal neck was observed. Since atherosclerotic aneurysms are associated with ischemic complications when microsurgical clipping is done, VWI could be useful for preoperative simulation and for deciding treatment options to ensure optimal management (Fig. 3).
Fig. 3.
A 73-year-old woman with an unruptured intracranial aneurysm of the middle cerebral artery. Preoperative three-dimensional digital subtraction angiography suggests an irregular aneurysm with a daughter sac (arrow head) (A). Vessel wall imaging (VWI) demonstrates focal wall enhancement in a daughter sac (arrow head) and aneurysm neck (arrow) (B). Fusion image created from time-of-flight magnetic resonance angiography and contrast-enhanced VWI suggests the sites of aneurysm wall enhancement (C). Intraoperative inspection demonstrates atherosclerotic wall feature of the daughter sac (arrow head) and of the aneurysmal neck (arrow) (D), which correspond well to VWI.
VWI of Thrombosed Aneurysms
Large or giant UIAs frequently contain organized intraluminal thrombus. The critical issue is that some of this minor group can become life-threatening due to perifocal edema, especially those in the posterior circulation. Iihara et al.41) reported a patient who showed development of thrombosed aneurysms even after parental vessel occlusion, and they suggested that progressive growth of the vasa vasorum and mural inflammation could be possible explanations for such unexpected expansion. Histopathological studies performed by Nagahiro et al.42) have determined that thrombosed aneurysms expand due to repeated hemorrhage of fragile recruited vessels along with organization of intraluminal thrombus. Thus, neovascularization seems to be a clue to the malignant behavior of large or giant aneurysms. Recent studies have suggested that ultra-high field MR-VWI can identify very fine wall structures and might be able to precisely visualize neovascularization.43,44) Further investigation could help to improve our understanding of the pathophysiological basis of aneurysm expansion with neovascularization and edema formation, as well as the optimal timing of treatment.
Limitations
So far, no reports have explained the clinical significance of AWE in small UIAs, which are treated conservatively. Accordingly, these aneurysms should be followed up carefully. Second, VWI was conducted with different MR units and/or scanning parameters in previous studies. Optimal customization of VWI for each MR unit could achieve better image quality. Further discussion of the correct timing for performing VWI after injection of contrast material is also required. Finally, histopathological correlations with VWI were only investigated in four studies. The complete aneurysmal sac could not be harvested after microsurgical clipping due to the need to achieve hemostasis. Therefore, the significance of circumferential wall enhancement should be carefully interpreted.
Conclusion
Vessel wall imaging can provide various new insights into IAs, although interpretation of VWI findings might differ between ruptured IAs and UIAs. With regard to ruptured IAs, AWE is very informative for detecting the culprit lesion in patients with multiples IAs and the specific pattern of AWE can be used to identify intraluminal thrombus suggesting the site of rupture. Approximately one-third of UIAs showed AWE, which was associated with wall thickening that corresponded to histological neovascularization and mural inflammation. Monitoring patients who have UIAs with or without AWE could be expected to provide further information for risk stratification based on aneurysm wall inflammation.
Acknowledgments
We thank the MRI Team (M. Takeuchi, T. Fujimoto, S. Yokota, Y. Okada, and S. Honji) at Asa City Hiroshima Citizens Hospital for special effort when performing VWI.
Footnotes
Conflicts of Interest Disclosure
None.
References
- 1).Etminan N, Rinkel GJ: Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol 12: 699–713, 2016 [DOI] [PubMed] [Google Scholar]
- 2).UCAS Japan Investigators. Morita A, Kirino T, et al. : The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366: 2474–2482, 2012 [DOI] [PubMed] [Google Scholar]
- 3).Greving JP, Wermer MJ, Brown RD, Jr, et al. : Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13: 59–66, 2014 [DOI] [PubMed] [Google Scholar]
- 4).Bijlenga P, Gondar R, Schilling S, et al. : PHASES score for the management of intracranial aneurysm: a cross-sectional population-based retrospective study. Stroke 48: 2105–2112, 2017 [DOI] [PubMed] [Google Scholar]
- 5).Chalouhi N, Ali MS, Jabbour PM, et al. : Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab 32: 1659–1676, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Frösen J, Piippo A, Paetau A, et al. : Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke 35: 2287–2293, 2004 [DOI] [PubMed] [Google Scholar]
- 7).Tulamo R, Frösen J, Hernesniemi J, Niemelä M: Inflammatory changes in the aneurysm wall: a review. J Neurointerv Surg 10: i58–i67, 2018 [DOI] [PubMed] [Google Scholar]
- 8).Alexander MD, Yuan C, Rutman A, et al. : High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry 87: 589–597, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Mandell DM, Mossa-Basha M, Qiao Y, et al. : Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 38: 218–229, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Lehman VT, Brinjikji W, Mossa-Basha M, et al. : Conventional and high-resolution vessel wall MRI of intracranial aneurysms: current concepts and new horizons. J Neurosurg 128: 969–981, 2017 [DOI] [PubMed] [Google Scholar]
- 11).Matouk CC, Mandell DM, Günel M: Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery 72: 492–496; discussion 496, 2013 [DOI] [PubMed] [Google Scholar]
- 12).Edjlali M, Gentric JC, Régent-Rodriguez C, et al. : Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke 45: 3704–3706, 2014 [DOI] [PubMed] [Google Scholar]
- 13).Nagahata S, Nagahata M, Obara M, et al. : Wall enhancement of the intracranial aneurysms revealed by magnetic resonance vessel wall imaging using three-dimensional turbo spin-echo sequence with motion-sensitized driven-equilibrium: a sign of ruptured aneurysm? Clin Neuroradiol 26: 277–283, 2016 [DOI] [PubMed] [Google Scholar]
- 14).Omodaka S, Endo H, Niizuma K, et al. : Quantitative assessment of circumferential enhancement along the wall of cerebral aneurysms using MR imaging. AJNR Am J Neuroradiol 37: 1262–1266, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Omodaka S, Endo H, Niizuma K, et al. : Circumferential wall enhancement on magnetic resonance imaging is useful to identify rupture site in patients with multiple cerebral aneurysms. Neurosurgery 82: 638–644, 2018 [DOI] [PubMed] [Google Scholar]
- 16).Texakalidis P, Hilditch CA, Lehman V, Lanzino G, Pereira VM, Brinjikji W: Vessel wall imaging of intracranial aneurysms: systematic review and meta-analysis. World Neurosurg 117: 453–458.e451, 2018 [DOI] [PubMed] [Google Scholar]
- 17).Wang X, Zhu C, Leng Y, Degnan AJ, Lu J: Intracranial aneurysm wall enhancement associated with aneurysm rupture: a systematic review and meta-analysis. Acad Radiol 26: 664–673, 2019 [DOI] [PubMed] [Google Scholar]
- 18).Hu P, Yang Q, Wang DD, Guan SC, Zhang HQ: Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiology 58: 979–985, 2016 [DOI] [PubMed] [Google Scholar]
- 19).Matsushige T, Shimonaga K, Mizoue T, et al. : Focal aneurysm wall enhancement on MRI indicates intraluminal thrombus and the rupture point. World Neurosurg 127: e578–e584, 2019 [DOI] [PubMed] [Google Scholar]
- 20).Ishikawa T, Nakayama N, Yoshimoto T, et al. : How does spontaneous hemostasis occur in ruptured cerebral aneurysms? Preliminary investigation on 247 clipping surgeries. Surg Neurol 66: 269–275; discussion 275–276, 2006 [DOI] [PubMed] [Google Scholar]
- 21).Ishikawa T, Miyata H, Moroi J, et al. : Pathological consideration for hemostatic clot on ruptured cerebral aneurysms. Surg Cereb Stroke (Jpn) 40: 223–228, 2012 [Google Scholar]
- 22).Omodaka S, Endo H, Niizuma K, et al. : Circumferential wall enhancement in evolving intracranial aneurysms on magnetic resonance vessel wall imaging. J Neurosurg 2018, in press [DOI] [PubMed] [Google Scholar]
- 23).Edjlali M, Guédon A, Ben Hassen W, et al. : Circumferential thick enhancement at vessel wall MRI has high specificity for intracranial aneurysm instability. Radiology 289: 181–187, 2018 [DOI] [PubMed] [Google Scholar]
- 24).Wang GX, Gong MF, Zhang D, et al. : Wall enhancement ratio determined by vessel wall MRI associated with symptomatic intracranial aneurysms. Eur J Radiol 112: 88–92, 2019 [DOI] [PubMed] [Google Scholar]
- 25).Backes D, Hendrikse J, van der Schaaf I, et al. : Determinants of gadolinium-enhancement of the aneurysm wall in unruptured intracranial aneurysms. Neurosurgery 83: 719–725, 2018 [DOI] [PubMed] [Google Scholar]
- 26).Hartman JB, Watase H, Sun J, et al. : Intracranial aneurysms at higher clinical risk for rupture demonstrate increased wall enhancement and thinning on multicontrast 3D vessel wall MRI. Br J Radiol 92: 20180950, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Liu P, Qi H, Liu A, et al. : Relationship between aneurysm wall enhancement and conventional risk factors in patients with unruptured intracranial aneurysms: a black-blood MRI study. Interv Neuroradiol 22: 501–505, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Lv N, Karmonik C, Chen S, et al. : Relationship between aneurysm wall enhancement in vessel wall magnetic resonance imaging and rupture risk of unruptured intracranial aneurysms. Neurosurgery 84: E385–E391, 2019 [DOI] [PubMed] [Google Scholar]
- 29).Wang GX, Li W, Lei S, Ge XD, Yin JB, Zhang D: Relationships between aneurysmal wall enhancement and conventional risk factors in patients with intracranial aneurysm: a high-resolution MRI study. J Neuroradiol 46: 25–28, 2019 [DOI] [PubMed] [Google Scholar]
- 30).Hudson JS, Zanaty M, Nakagawa D, et al. : Magnetic resonance vessel wall imaging in human intracranial aneurysms. Stroke 50: e1, 2019 [DOI] [PubMed] [Google Scholar]
- 31).Larsen N, von der Brelie C, Trick D, et al. : Vessel wall enhancement in unruptured intracranial aneurysms: an indicator for higher risk of rupture? High-resolution MR imaging and correlated histologic findings. AJNR Am J Neuroradiol 39: 1617–1621, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Shimonaga K, Matsushige T, Ishii D, et al. : Clinicopathological insights from vessel wall imaging of unruptured intracranial aneurysms. Stroke 49: 2516–2519, 2018 [DOI] [PubMed] [Google Scholar]
- 33).Hasan DM, Mahaney KB, Magnotta VA, et al. : Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol 32: 1032–1038, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Frösen J, Tulamo R, Heikura T, et al. : Lipid accumulation, lipid oxidation, and low plasma levels of acquired antibodies against oxidized lipids associate with degeneration and rupture of the intracranial aneurysm wall. Acta Neuropathol Commun 1: 71, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Kosierkiewicz TA, Factor SM, Dickson DW: Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J Neuropathol Exp Neurol 53: 399–406, 1994 [DOI] [PubMed] [Google Scholar]
- 36).Kleinloog R, Korkmaz E, Zwanenburg JJ, et al. : Visualization of the aneurysm wall: a 7.0-tesla magnetic resonance imaging study. Neurosurgery 75: 614–622; discussion 622, 2014 [DOI] [PubMed] [Google Scholar]
- 37).Kadasi LM, Dent WC, Malek AM: Colocalization of thin-walled dome regions with low hemodynamic wall shear stress in unruptured cerebral aneurysms. J Neurosurg 119:172–179, 2013 [DOI] [PubMed] [Google Scholar]
- 38).Matsushige T, Shimonaga K, Ishii D, et al. : Vessel wall imaging of evolving unruptured intracranial aneurysms. Stroke 50: 1891–1894, 2019 [DOI] [PubMed] [Google Scholar]
- 39).Vergouwen MDI, Backes D, van der Schaaf IC, et al. : Gadolinium enhancement of the aneurysm wall in unruptured intracranial aneurysms is associated with an increased risk of aneurysm instability: a follow-up study. AJNR Am J Neuroradiol 40: 1112–1116, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Hashimoto Y, Matsushige T, Shimonaga K, et al. : Vessel wall imaging predicts the presence of atherosclerotic lesions in unruptured intracranial aneurysms. World Neurosurg 2019, in press [DOI] [PubMed] [Google Scholar]
- 41).Iihara K, Murao K, Sakai N, et al. : Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum. Case report. J Neurosurg 98: 407–413, 2003 [DOI] [PubMed] [Google Scholar]
- 42).Nagahiro S, Takada A, Goto S, Kai Y, Ushio Y: Thrombosed growing giant aneurysms of the vertebral artery: growth mechanism and management. J Neurosurg 82: 796–801, 1995 [DOI] [PubMed] [Google Scholar]
- 43).Matsushige T, Chen B, Ringelstein A, et al. : Giant intracranial aneurysms at 7T MRI. AJNR Am J Neuroradiol 37: 636–641, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Sato T, Matsushige T, Chen B, et al. : Wall contrast enhancement of thrombosed intracranial aneurysms at 7T MRI. AJNR Am J Neuroradiol 40: 1106–1111, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]