Abstract
目的
探讨白术多糖(PAM)对肝癌细胞增殖和侵袭能力的影响及其机制。
方法
体外培养肝癌细胞HepG2分别给予不同浓度的PAM处理,通过CCK-8和Transwell实验检测肝癌细胞的增殖、侵袭能力。采用免疫荧光技术检测肝癌细胞中β-catenin蛋白表达水平,采用RT-PCR技术和免疫印迹法检测AKT、GSK-3β的基因和蛋白表达水平及其磷酸化表达,检测MMP-2的基因和蛋白表达水平。HepG2细胞给予LiCl/PAM/LiCl联合PAM处理,检测细胞增殖、侵袭能力改变,检测GSK-3β磷酸化和MMP2的蛋白水平。
结果
与空白对照组相比,PAM处理后肝癌细胞体外增殖能力下降、侵袭能力下降(P < 0.05);PAM处理可以下调β-catenin蛋白表达、下调MMP-2基因与蛋白表达水平,同时抑制AKT与GSK-3β的磷酸化(P < 0.05)。PAM对肝癌细胞体外增殖、侵袭及AKT/GSK-3β/β-catenin通路的影响呈浓度依赖性:PAM的浓度越高,对肝癌细胞体外增殖、侵袭的抑制作用越强;对AKT与GSK-3β的磷酸化的抑制作用越强,对β-catenin表达的抑制作用也越强。LiCl可以逆转PAM对肝癌细胞增殖、侵袭的抑制作用,同时可以逆转PAM对GSK-3β磷酸化和MMP2的蛋白表达的抑制。
结论
PAM对肝癌细胞的体外增殖和侵袭能力具有抑制作用,PAM可能通过影响Wnt/β-catenin信号通路发挥作用。
Keywords: 白术多糖, 肝癌细胞, 增殖, 侵袭, Wnt/β-catenin信号通路
Abstract
Objective
To investigate the inhibitory effect of polysaccharide of Atractylodes macrocephala (PAM) on the proliferation and invasion of hepatocellular carcinoma cells and the underlying mechanism.
Methods
Hepatocellular carcinoma HepG2 cells were treated with different concentrations of PAM, and their proliferation and invasive ability were examined using CCK-8 assay and Transwell assay. Immunofluorescence assay was performed to detect the expression level of β-catenin, and real-time PCR and Western blotting were used to detect the mRNA and protein expressions of AKT, GSK-3β and MMP-2 in the cells. The changes in the proliferation, invasiveness and the expressions of pGSK-3β and MMP2 were examined in the cells following treatment with LiCl/PAM/LiCl plus PAM.
Results
PAM treatment significantly reduced the cell viability, the number of migration cells, and the expression levels of β-catenin and MMP-2 (P < 0.05), and obviously inhibited the phosphorylation of AKT and GSK-3β in the cells (P < 0.05) in a dose-dependent manner. The rescue experiment showed that LiCl reversed the inhibition of cell proliferation, invasiveness, and the Wnt/β-catenin pathway induced by PAM.
Conclusion
PAM can inhibit the proliferation and invasion of hepatocellular carcinoma cells in vitro possibly by inhibiting the Wnt/β-catenin signaling pathway.
Keywords: polysaccharide of Atractylodes macrocephala, hepatocellular carcinoma cells, proliferation, invasion, Wnt/β-catenin
肝细胞癌发病率位列我国所有肿瘤的第2位,且发病率逐年上升[1]。虽然目前针对肝癌存在多种治疗手段,如肝移植、局部切除、介入治疗、微创治疗及分子靶向治疗等,但均无法完全抑制肝癌细胞的生长和转移[2-3]。因此,探索肝癌的新的治疗药物具有重要的临床意义。近年来,探讨中草药的活性成分的抗肿瘤作用及其机制已经成为抗肿瘤药物研究领域的热点。白术为多年生草本植物,属传统中草药,有健脾益气、燥湿利尿,止汗安胎的作用,现代药理研究表明白术有着多种药理作用,如抗氧化、保肝、抗炎、抗过敏、抗血栓、抗病毒等[4-8]。白术同时具有抗肿瘤作用,已有研究提示白术可抑制胃癌、肺癌、胆管癌等多种肿瘤细胞的增殖,并诱导其凋亡[9-11]。白术多糖(PAM)是白术的有效活性成分之一,主要作用集中在消化系统、免疫系统,可发挥降糖、保护胃肠道黏膜及免疫调节等作用[12]。研究还发现,PAM具有抗肿瘤作用,体外研究表明,PAM可以促进人胃癌细胞和神经胶质瘤细胞的凋亡[13-14]。
目前对于白术及其有效成分的抗肝癌作用的研究较少。既往研究发现,在小鼠肝癌细胞H22的荷瘤小鼠模型中,白术可以抑制皮下移植瘤的生长和转移[15]。另有研究发现PAM可以抑制H22细胞在小鼠体内的增殖,其机制可能与增强机体免疫功能相关[16]。然而以上研究所用细胞均为小鼠肝癌细胞,且对白术及PAM的抗肝癌的具体机制并未进行深入探讨。PAM对人肝癌细胞的增殖和侵袭是否有影响仍需进一步的研究确定。本研究拟探讨PAM对HepG2肝癌细胞的增殖和侵袭能力的影响,并进一步探究其相关机制,以期为PAM的临床应用提供理论依据。
1. 材料和方法
1.1. 实验试剂
CCK8试剂盒(凯基);氯化锂(LiCl)(鼎国);AKT、AKT磷酸化蛋白(pSer 473),GSk-3β、GSK-3β (pSer 9)磷酸化蛋白、β-catenin抗体(Cell Signaling Technology);MMP-2抗体(Santa Cruz);Transwell小室(康宁);基质胶(BD);培养基、胎牛血清(GIBICO);cDNA合成试剂盒、PCR试剂盒(GeneCopoeia)。
1.2. PAM的制备
称取白术粉1000 g,匀浆,用氯仿-乙醇(1:1,v/V)进行连续萃取,使用丙酮去除油脂和有色杂质,冷却至室温后过滤。上清液在50 ℃减压离心,加95%的乙醇至终浓度为80%进行冲洗,4 ℃过夜,10 000×g离心20 min。用无水乙醇、丙酮反复冲洗,离心,去溶液取沉淀,即PAM。进一步配制成体积分数为4%的PAM溶液,经灭菌处理后4 ℃保存备用。
1.3. 肝癌细胞培养和处理
人肝癌细胞株HepG2,购自中国科学院典型培养物保藏委员会细胞库。用含10%小牛血清DMEM培养基在37 ℃,5% CO2,饱和湿度的孵箱内常规培养HepG2细胞。细胞处理:(1)空白对照组,给予对照溶剂;低浓度组(L),给予30 mg/mL PAM;中浓度组(M),给予60 mg/mL PAM;高浓度组(H),给予120 mg/mL PAM。(2)空白对照组,给予对照溶剂;PAM组,给予120 mg/mL PAM;LiCl组,给予4 mmol/L LiCl;PAM+LiCl组,给予120 mg/mL PAM+4 mmol/L LiCl。
1.4. 细胞增殖能力检测
将位于对数生长期的肝癌细胞以1×105/mL接种于96孔板,每孔200 μL培养液。具体实验方法参照文献[17]。实验重复3次。
1.5. 细胞侵袭能力检测
Transwell小室上室面铺Matrigel。HepG2细胞分别予对应处理,48 h后吸去培养液,调整细胞密度为4× 105/mL,以200 μL/孔移入Transwell小室的上室,下室加入500 μL含5%FBS的DMEM。常规培养24 h后,用棉签轻轻刮去膜上室面的细胞,4%多聚甲醛固定-结晶紫染色,每个小室随机取5个高倍视野,在显微镜下计数细胞数目。
1.6. 免疫荧光检测β-catenin蛋白表达
取前述方法处理48 h后的各组HepG2细胞,将细胞按4×105/cm2的密度种在有无菌盖玻片的6孔板中,置于10%胎牛血清培养基中培养。24 h后PBS洗涤盖玻片3次,每次5 min。用4%多聚甲醛室温固定30 min,小心取出盖玻片,放入封闭液,37 ℃孵育1 h;5%的PBS清洗3次,加入一抗(1:1000)后4 ℃孵育过夜,加入二抗(1:3000)避光室温1 h,用5%的PBS清洗3次,DAPI染色后用50%的甘油封片,荧光显微镜下观察照相。
1.7. RT-PCR检测AKT、GSK-3β和MMP-2的mRNA水平
取前述方法处理48 h后的各组HepG2细胞,按照试剂盒的要求用Trizol法提取细胞总RNA,所提RNA经吸光度(A)测定,A260 nm/A280 nm比值均在1.8~2.0。检测方法参见文献[18]。以GAPDH为内参基因。引物序列见表 1。
1.
引物序列
Sequences of the primers for real-time PCR
| Gene | Forward | Reverse |
| MMP2 | 5'TGCTGGAGACAAATTCTGGA 3' | 5'GAAGAAGTAGCTGTGACCGC 3' |
| AKT | 5'GGAGGTTTTTGGGCTTGCG 3' | 5'CTCTGATGCACCAGCTGACA 3' |
| GSK3 | 5'TGGACTAAGGATTCGTCAGGA 3' | 5'TGAATCCGAGCATGAGGAGG 3' |
| GAPDH | 5'CTTCATTGACCTCAACTACA 3' | 5'AGGGGCCATCCACAGTCTTC 3' |
1.8. 蛋白质免疫印迹法检测MMP-2、AKT、GSK-3β、pAKT和p-GSK-3β的蛋白水平
肝癌细胞分别予前述方法处理60 min及48 h,弃去上清液,加入100 μL的裂解液,4 ℃ 12 000 r/min,离心15 min,收集上清液待用。Western blot的具体方法参照文献[19]。
1.9. 统计学分析
采用SPSS 20.0软件处理数据。计量资料以均数±标准差表示,实验数据组间比较采用单因素方差分析法,P < 0.05为差异有统计学意义。
2. 结果
2.1. PAM对肝癌细胞增殖能力的影响
CCK8法检测肝癌细胞的增殖水平,各组肝癌细胞存活率结果显示(图 1),PAM组细胞增殖水平明显低于空白对照组(P < 0.05),且H组 < M组 < L组(P < 0.05)。在同一浓度下,PAM干预时间越久,对细胞增殖的抑制作用越强。
1.
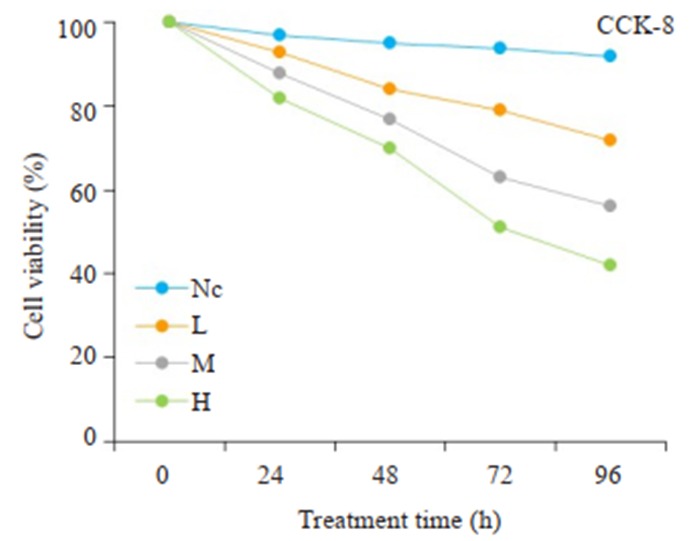
CCK-8细胞实验检测PAM对肝癌细胞增殖能力的影响
CCK-8 assay of proliferation of HepG2 cells treated with different doses of PAM. NC: Negative control group; L: Low-dose group; M: Medium-dose group; H: High-dose group.
2.2. PAM对肝癌细胞侵袭能力的影响
Transwell实验检测PAM对肝癌细胞的侵袭能力的影响,结果显示(图 2),L组、M组、H组的穿膜细胞数目低于空白对照组(P < 0.05),且H组 < M组 < L组(P < 0.05)。
2.
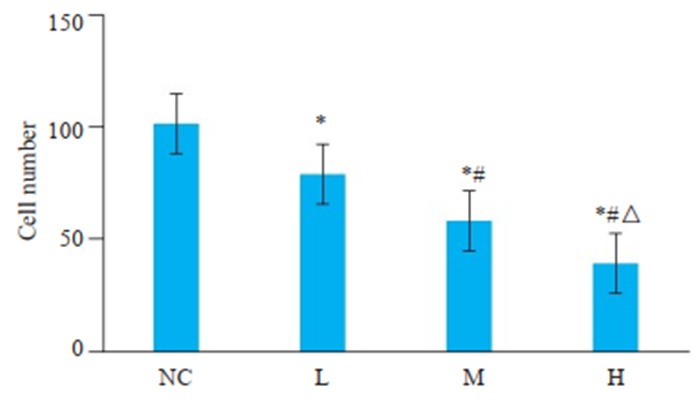
Transwell侵袭实验检测PAM对肝癌细胞侵袭能力的影响
Transwell assay of invasion ability of HepG2 cells treated with different doses of PAM. *P < 0.05 vs NC group; #P < 0.05 vs L group; ΔP < 0.05 vs M group.
2.3. PAM对肝癌细胞β-catenin蛋白表达的影响
免疫荧光检测PAM对肝癌细胞HepG2中β-catenin的影响,结果显示,绿色荧光为β-catenin蛋白,荧光的强弱表示β-catenin的蛋白表达水平的高低。与空白对照组相比,PAM处理组肝癌细胞β-catenin蛋白的荧光强度明显降低,且荧光强度随着PAM的浓度增加而降低(图 3),呈现剂量依赖性。
3.
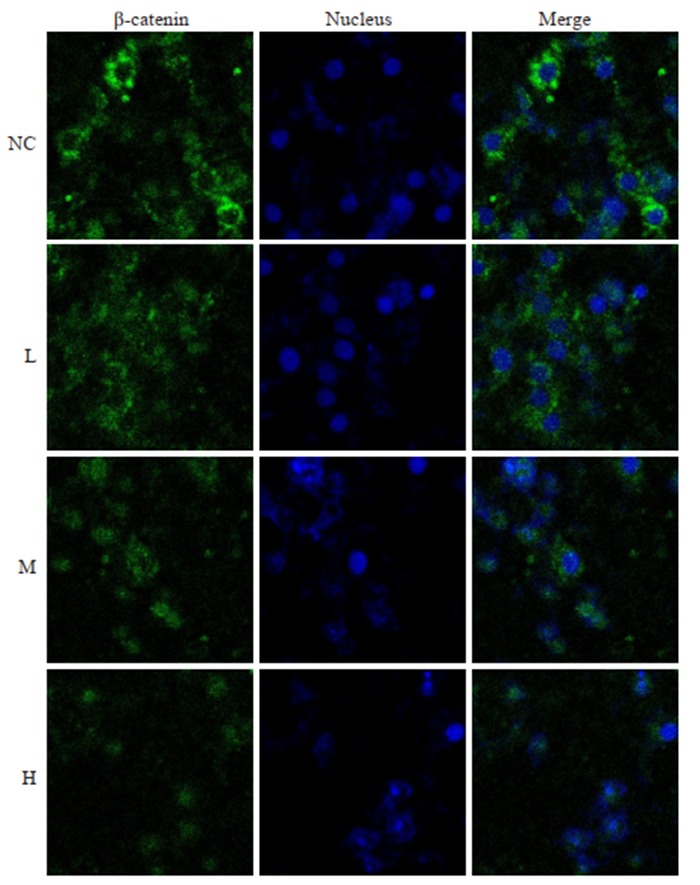
免疫荧光实验检测PAM对肝癌细胞β-catenin蛋白表达的影响
Immunofluorescence detection of β-catenin in HepG2 cells in different groups.
2.4. PAM对肝癌细胞MMP-2 mRNA和蛋白表达水平的影响
分别用RT-PCR和蛋白质免疫印迹法法检测PAM对肝癌细胞中MMP-2 mRNA和蛋白表达的影响(图 4A、B),结果显示,L组、M组、H组中MMP-2的mRNA及蛋白的表达水平相较于空白对照组显著下调(P < 0.05),且H组 < M组 < L组。
4.
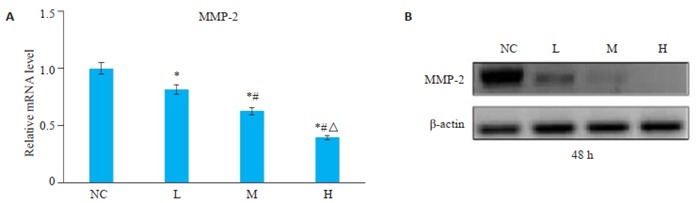
Western blot和RT-PCR检测PAM对肝癌细胞MMP-2基因和蛋白表达的影响
Western blotting and RT-PCR for mRNA and protein expressions of MMP-2 in HepG2 cells in different groups. *P < 0.05 vs NC group; #P < 0.05 vs L group; ΔP < 0.05 vs M group.
2.5. PAM对肝癌细胞AKT、GSK-3β基因转录和蛋白表达的影响
与空白对照组相比,L组、M组、H组的AKT、GSK-3β的mRNA和蛋白表达差异无统计学意义(图 5A、B、D)。与空白对照组相比,L组、M组、H组磷酸化AKT、磷酸化GSK-3β蛋白的表达水平显著下调(P < 0.05,图 5C),且磷酸化AKT和磷酸化GSK-3β的水平H组 < M组 < L组。
5.
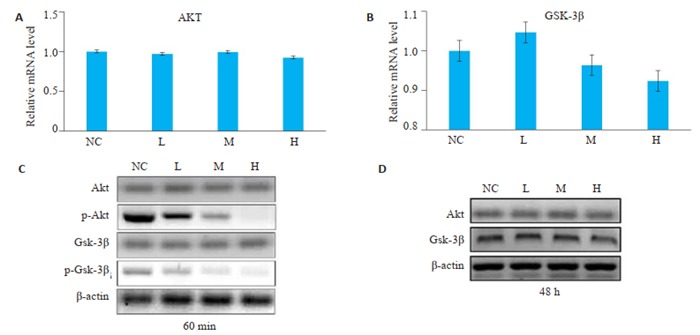
Western blot和RT-PCR检测PAM对肝癌细胞AKT、GSK-3β基因和蛋白表达的影响
Western blotting and RT-PCR of the mRNA and protein expressions of AKT and GSK-3β in HepG2 cells in different groups. A, B, D: No significant difference in the expressions of AKT and GSK-3β mRNA and protein is found among the 4 groups; C: The expressions of phosphorylated AKT and phosphorylated GSK-3 are decreased significantly in groups L, M and H.
2.6. PAM通过Wnt/β-catenin调控肝癌细胞的增殖和侵袭
与PAM处理组相比,LiCl与PAM联用组肝癌细胞的增殖和侵袭能力增强(图 6A、B)。Western blot结果显示,LiCl可以减弱PAM对GSK-3β的磷酸化的抑制作用(图 6C);同时,LiCl联合PAM处理后,MMP2的蛋白水平较单用PAM处理组升高(图 6D)。
6.
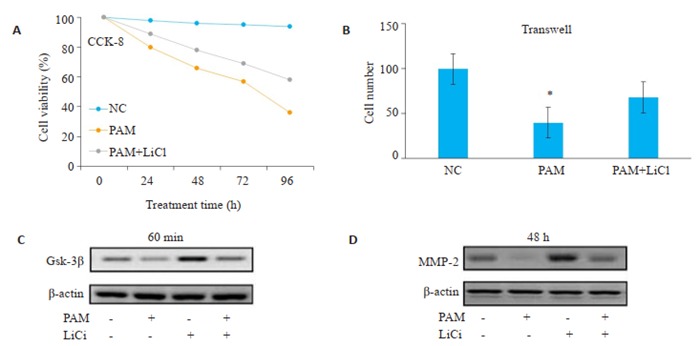
Wnt/β-catenin激活剂可以逆转PAM对肝癌细胞增殖和侵袭的抑制作用
Wnt/β-catenin activator rescues PAM-induced inhibition of HCC cell proliferation and invasion. A, B: LiCl combined with PAM promotes proliferation and invasion of HCC cells; C: LiCl attenuates the inhibitory effect of PAM on the phosphorylation of Gsk-3β; D: LiCl combined with PAM increases the protein level of MMP-2 compared with PAM treatment alone.
3. 讨论
本研究选取具有高转移性的人肝癌细胞HepG2作为研究对象,探究PAM对肝癌细胞体外增殖和侵袭能力的影响,并进一步探讨其机制,结果发现PAM对HepG2细胞的增殖能力具有明显的抑制作用,且其抑制作用具有浓度依赖性;PAM可以显著降低肝癌细胞的侵袭能力,且其抑制作用与PAM的浓度呈正相关。Wnt/β-catenin信号通路在肿瘤发生及侵袭转移过程中发挥重要作用[20-21]。β-catenin蛋白是Wnt信号通路的关键成员,可调控转录活性,介导细胞间粘附及相关信号的转导[22]。已有研究表明,β-catenin在肝癌细胞中呈高表达,且其表达水平与肝癌细胞的增殖和侵袭能力正相关[23-24]。本实验结果表明,PAM对肝癌细胞中β-catenin蛋白的表达有显著抑制作用,且这种抑制作用与PAM浓度呈正相关。这表明β-catenin蛋白可能是PAM的一个特异性作用位点。接下来,本研究继续探讨了PAM对β-catenin上游及下游通路的影响。AKT/GSK-3β是Wnt通路中β-catenin上游的调控因子[25]。Wnt信号通路被活化时,AKT被磷酸化后形成磷酸化AKT,后者具有激酶活性,可使GSK-3β磷酸化形成磷酸化GSK-3β而失活,引起胞内β-catenin的降解减少[26]。而当Wnt信号缺失时,GSK-3β活化可使胞质内的β-catenin被磷酸化后泛素化降解[25]。本实验结果显示,PAM并不直接抑制AKT和GSK-3β的mRNA和蛋白的表达,而对AKT和GSK-3β蛋白的磷酸化有显著的抑制作用。
基质金属蛋白酶是Wnt/β-catenin信号通路的直接下游靶分子,参与肿瘤细胞的迁移[27-28]。MMP家族参与细胞外基质及基底膜降解,能够水解基底膜的LN、FN和Ⅳ型胶原,破坏细胞外基质及基底膜结构[29]。在MMP家族成员中,MMP-2是在肝癌转移中起主导作用的分子[30-31]。研究表明,MMP-2的过表达与肝癌的转移密切相关,高水平表达的MMP-2可以作为肝癌预后不良的指标[32]。本研究发现,PAM可以显著抑制MMP-2的基因转录和蛋白表达。这提示PAM可能通过影响Wnt/βcatenin信号通路来调控MMP-2的表达,最终抑制肝癌细胞的侵袭转移。LiCl是经典的Wnt/β-catenin通路的激动剂,在肿瘤研究中被广泛使用[33]。在体外培养的肝癌细胞中,LiCl可以促进GSK-3β磷酸化,抑制GSK-3β的活性,抑制β-catenin的降解。本研究表明,LiCl可以减弱PAM对肝癌细胞的增殖和侵袭能力的抑制作用;减弱PAM对GSK-3β的磷酸化和MMP2表达的抑制作用。这进一步表明,PAM是通过抑制Wnt/β-catenin信号通路抑制肝癌细胞的增殖和侵袭。
综上所述,本研究发现PAM可以抑制肝癌细胞的体外增殖和侵袭。其机制可能与PAM调控Wnt/β- catenin信号通路相关。PAM通过抑制AKT/GSK-3β的磷酸化,降低β-catenin蛋白在肝癌细胞中的积累,从而抑制其下游靶分子-MMP2的基因转录和蛋白表达。本研究表明,PAM可能作为肝癌潜在治疗药物。
Biography
朱云,博士,E-mail: zyfreemail@126.com
Funding Statement
国家自然科学基金(81802339, 81572797);广东省自然科学基金(2016A030310254, 2016A030311015)
Supported by National Natural Science Foundation of China (81802339, 81572797)
Contributor Information
朱 云 (Yun ZHU), Email: zyfreemail@126.com.
程 旸 (Yang CHENG), Email: chengyang2872@sina.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424.] [DOI] [PubMed] [Google Scholar]
- 2.Burkhart RA, Ronnekleiv SM, Pawlik TM. Personalized therapy in hepatocellular carcinoma: molecular markers of prognosis and therapeutic response. Surg Oncol. 2017;26(2):138–45. doi: 10.1016/j.suronc.2017.01.009. [Burkhart RA, Ronnekleiv SM, Pawlik TM. Personalized therapy in hepatocellular carcinoma: molecular markers of prognosis and therapeutic response[J]. Surg Oncol, 2017, 26(2): 138-45.] [DOI] [PubMed] [Google Scholar]
- 3.Zhang SH, Yue M, Shu RH, et al. Recent advances in the management of hepatocellular carcinoma. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3496873. J Balkan Union Oncol. 2016;21(2):307–11. [Zhang SH, Yue M, Shu RH, et al. Recent advances in the management of hepatocellular carcinoma[J]. J Balkan Union Oncol, 2016, 21(2): 307-11.] [PubMed] [Google Scholar]
- 4.Xu K, Yang YN, Feng ZM, et al. Six new compounds from atractylodes lancea and their hepatoprotective activities. Bioorg Med Chem Lett. 2016;26(21):5187–92. doi: 10.1016/j.bmcl.2016.09.069. [Xu K, Yang YN, Feng ZM, et al. Six new compounds from atractylodes lancea and their hepatoprotective activities[J]. Bioorg Med Chem Lett, 2016, 26(21): 5187-92.] [DOI] [PubMed] [Google Scholar]
- 5.Li CQ, He LC, Dong HY, et al. Screening for the anti-inflammatory activity of fractions and compounds from atractylodes macrocephala koidz. J Ethnopharmacol. 2007;114(2):212–7. doi: 10.1016/j.jep.2007.08.002. [Li CQ, He LC, Dong HY, et al. Screening for the anti-inflammatory activity of fractions and compounds from atractylodes macrocephala koidz[J]. J Ethnopharmacol, 2007, 114(2): 212-7.] [DOI] [PubMed] [Google Scholar]
- 6.Kim CK, Kim M, Oh SD, et al. Effects of atractylodes macrocephala koidzumi rhizome on 3T3-L1 adipogenesis and an animal model of obesity. J Ethnopharmacol. 2011;137(1):396–402. doi: 10.1016/j.jep.2011.05.036. [Kim CK, Kim M, Oh SD, et al. Effects of atractylodes macrocephala koidzumi rhizome on 3T3-L1 adipogenesis and an animal model of obesity[J]. J Ethnopharmacol, 2011, 137(1): 396-402.] [DOI] [PubMed] [Google Scholar]
- 7.Zheng F, Chen L, Gao JM, et al. Identification of autotoxic compounds from atractylodes macrocephala koidz and preliminary investigations of their influences on immune system. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ece68cfe8d97b897e1577aa672453519. J Plant Physiol. 2018;230(2):33–9. doi: 10.1016/j.jplph.2018.08.006. [Zheng F, Chen L, Gao JM, et al. Identification of autotoxic compounds from atractylodes macrocephala koidz and preliminary investigations of their influences on immune system[J]. J Plant Physiol, 2018, 230(2): 33-9.] [DOI] [PubMed] [Google Scholar]
- 8.Deng P, Zheng S, Yun W, et al. A visual and sensitive Hg (2+) detection strategy based on split DNAzyme amplification and peroxidase-like activity of hemin-graphene composites. Spectrochim Act Mol Biomol Spectrosc. 2019;5(210):335–40. doi: 10.1016/j.saa.2018.11.048. [Deng P, Zheng S, Yun W, et al. A visual and sensitive Hg (2+) detection strategy based on split DNAzyme amplification and peroxidase-like activity of hemin-graphene composites[J]. Spectrochim Act Mol Biomol Spectrosc, 2019, 5(210): 335-40.] [DOI] [PubMed] [Google Scholar]
- 9.Zhao M, Wang Q, Ouyang Z, et al. Selective fraction of atractylodes lancea (Thunb.) DC. and its growth inhibitory effect on human gastric cancer cells. Cytotechnology. 2014;66(2):201–8. doi: 10.1007/s10616-013-9559-1. [Zhao M, Wang Q, Ouyang Z, et al. Selective fraction of atractylodes lancea (Thunb.) DC. and its growth inhibitory effect on human gastric cancer cells[J]. Cytotechnology, 2014, 66(2): 201-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu HY, Zhu YJ, Zhang T, et al. Anti-Tumor effects of atractylenolide Ⅰ isolated from atractylodes macrocephala in human lung carcinoma cell lines. Molecules. 2013;18(11):13357–68. doi: 10.3390/molecules181113357. [Liu HY, Zhu YJ, Zhang T, et al. Anti-Tumor effects of atractylenolide Ⅰ isolated from atractylodes macrocephala in human lung carcinoma cell lines[J]. Molecules, 2013, 18(11): 13357-68.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plengsuriyakarn T, Matsuda N, Karbwang J, et al. Anticancer activity of atractylodes lancea (thunb.) DC in a hamster model and application of PET-CT for early detection and monitoring progression of cholangiocarcinoma. Asian Pac J Cancer Prev. 2015;16(15):6279–84. doi: 10.7314/APJCP.2015.16.15.6279. [Plengsuriyakarn T, Matsuda N, Karbwang J, et al. Anticancer activity of atractylodes lancea (thunb.) DC in a hamster model and application of PET-CT for early detection and monitoring progression of cholangiocarcinoma[J]. Asian Pac J Cancer Prev, 2015, 16(15): 6279-84.] [DOI] [PubMed] [Google Scholar]
- 12.Zhu B, Zhang QL, Hua JW, et al. The traditional uses, phytochemistry, and pharmacology of atractylodes macrocephala koidz: a review. J Ethnopharmacol. 2018;226(6):143–67. doi: 10.1016/j.jep.2018.08.023. [Zhu B, Zhang QL, Hua JW, et al. The traditional uses, phytochemistry, and pharmacology of atractylodes macrocephala koidz: a review[J]. J Ethnopharmacol, 2018, 226(6): 143-67.] [DOI] [PubMed] [Google Scholar]
- 13.赵 艳, 金 鑫, 李 楠, et al. ALDH1、CXCR4、E-cadherin在胃癌组织中的表达及与淋巴结转移的关系. http://www.j-smu.com/oa/DArticle.aspx?type=view&id=2016101390. 南方医科大学学报. 2016;36(10):1390–5. doi: 10.3969/j.issn.1673-4254.2016.10.16. [赵艳, 金鑫, 李楠, 等. ALDH1、CXCR4、E-cadherin在胃癌组织中的表达及与淋巴结转移的关系[J].南方医科大学学报, 2016, 36(10): 1390-5.] [DOI] [PubMed] [Google Scholar]
- 14.Li XE, Liu F, Li Z, et al. Atractylodes macrocephala polysaccharides induces mitochondrial-mediated apoptosis in glioma C6 cells. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f99fe61bbebbcb080a8ec1c7022e7f67. Int J Biol Macromol. 2014;66(3):108–12. doi: 10.1016/j.ijbiomac.2014.02.019. [Li XE, Liu F, Li Z, et al. Atractylodes macrocephala polysaccharides induces mitochondrial-mediated apoptosis in glioma C6 cells[J]. Int J Biol Macromol, 2014, 66(3): 108-12.] [DOI] [PubMed] [Google Scholar]
- 15.董 小玉, 刘 秀敏, 刘张 苑珠, et al. 胃肠道肿瘤的多光子成像与光学活检. 分子影像学杂志. 2017;40(3):344–9. doi: 10.3969/j.issn.1674-4500.2017.03.26. [董小玉, 刘秀敏, 刘张苑珠, 等.胃肠道肿瘤的多光子成像与光学活检[J].分子影像学杂志, 2017, 40(3): 344-9.] [DOI] [Google Scholar]
- 16.周 剑, 苏 德春, 宋 国权. 白术多糖对H22肝癌小鼠抗肿瘤作用实验研究. http://d.old.wanfangdata.com.cn/Periodical/ytctyy201517004. 亚太传统医药. 2015;11(17):9–10. [周剑, 苏德春, 宋国权.白术多糖对H22肝癌小鼠抗肿瘤作用实验研究[J].亚太传统医药, 2015, 11(17): 9-10.] [Google Scholar]
- 17.Cheng Y, Zhu Y, Xu JJ, et al. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumor-associated macrophages via regulating DUSP6-Erk1/2 pathway. Mol Cancer. 2018;17(1):13–25. doi: 10.1186/s12943-017-0747-z. [Cheng Y, Zhu Y, Xu JJ, et al. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumor-associated macrophages via regulating DUSP6-Erk1/2 pathway[J]. Mol Cancer, 2018, 17(1): 13-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Cheng Y, Guo YB, et al. Protein kinase D2 contributes to TNF-alpha-induced epithelial mesenchymal transition and invasion via the PI3K/GSK-3 beta/beta-catenin pathway in hepatocellular carcinoma. Oncotarget. 2016;7(5):5327–41. doi: 10.18632/oncotarget.6633. [Zhu Y, Cheng Y, Guo YB, et al. Protein kinase D2 contributes to TNF-alpha-induced epithelial mesenchymal transition and invasion via the PI3K/GSK-3 beta/beta-catenin pathway in hepatocellular carcinoma[J]. Oncotarget, 2016, 7(5): 5327-41.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y, Zheng H, Wang B, et al. Sorafenib and fluvastatin synergistically alleviate hepatic fibrosis via inhibiting the TGF beta 1/Smad3 pathway. Dig Liver Dis. 2018;50(4):381–8. doi: 10.1016/j.dld.2017.12.015. [Cheng Y, Zheng H, Wang B, et al. Sorafenib and fluvastatin synergistically alleviate hepatic fibrosis via inhibiting the TGF beta 1/Smad3 pathway[J]. Dig Liver Dis, 2018, 50(4): 381-8.] [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Wang X, Zhang L, et al. Wnt/beta-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review) Molecul Med Reports. 2017;16(2):1007–13. doi: 10.3892/mmr.2017.6718. [Zhang Z, Wang X, Zhang L, et al. Wnt/beta-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (Review)[J]. Molecul Med Reports, 2017, 16(2): 1007-13.] [DOI] [PubMed] [Google Scholar]
- 21.Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101–13. doi: 10.1186/s13045-017-0471-6. [Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: modulating anticancer immune response[J]. J Hematol Oncol, 2017, 10(1): 101-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh M, Katoh M. Molecular genetics and targeted therapy of WNT-related human diseases (Review) http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=6a008355-7a3a-4985-a5ab-399031bb82ec. Intern J Molecul Med. 2017;40(3):587–606. doi: 10.3892/ijmm.2017.3071. [Katoh M, Katoh M. Molecular genetics and targeted therapy of WNT-related human diseases (Review)[J]. Intern J Molecul Med, 2017, 40(3): 587-606.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang TS, Li L, Moalim-Nour L, et al. A regulatory network involving beta-catenin, E-Cadherin, PI3K/Akt, and slug balances self-renewal and differentiation of human pluripotent stem cells in response to Wnt signaling. Stem Cells. 2015;33(5):1419–33. doi: 10.1002/stem.1944. [Huang TS, Li L, Moalim-Nour L, et al. A regulatory network involving beta-catenin, E-Cadherin, PI3K/Akt, and slug balances self-renewal and differentiation of human pluripotent stem cells in response to Wnt signaling[J]. Stem Cells, 2015, 33(5): 1419-33.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell JO, Monga SP. Wnt/beta-catenin signaling in liver development, homeostasis and pathobiology. Annu Rev Pathol. 2018;24(13):351–78. doi: 10.1146/annurev-pathol-020117-044010. [Russell JO, Monga SP. Wnt/beta-catenin signaling in liver development, homeostasis and pathobiology[J]. Annu Rev Pathol, 2018, 24(13): 351-78.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain S, Ghanghas P, Rana C, et al. Role of GSK-3 beta in regulation of canonical Wnt/beta-catenin signaling and PI3-K/Akt oncogenic pathway in colon cancer. Cancer Invest. 2017;35(7):473–83. doi: 10.1080/07357907.2017.1337783. [Jain S, Ghanghas P, Rana C, et al. Role of GSK-3 beta in regulation of canonical Wnt/beta-catenin signaling and PI3-K/Akt oncogenic pathway in colon cancer[J]. Cancer Invest, 2017, 35(7): 473-83.] [DOI] [PubMed] [Google Scholar]
- 26.Mccubrey JA, Rakus D, Gizak A, et al. Effects of mutations in Wnt/beta-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-diverse effects on cell growth, metabolism and cancer. Biochim Biophys Acta. 2016;1863(12):2942–76. doi: 10.1016/j.bbamcr.2016.09.004. [Mccubrey JA, Rakus D, Gizak A, et al. Effects of mutations in Wnt/beta-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-diverse effects on cell growth, metabolism and cancer[J]. Biochim Biophys Acta, 2016, 1863(12): 2942-76.] [DOI] [PubMed] [Google Scholar]
- 27.Royer PJ, Henrio K, Pain M, et al. TLR3 promotes MMP-9 production in primary human airway epithelial cells through Wnt/beta-catenin signaling. Respir Res. 2017;18(1):208–19. doi: 10.1186/s12931-017-0690-y. [Royer PJ, Henrio K, Pain M, et al. TLR3 promotes MMP-9 production in primary human airway epithelial cells through Wnt/beta-catenin signaling[J]. Respir Res, 2017, 18(1): 208-19.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Zhang Z, Pan S, et al. Interaction between the Wnt/-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis. J Periodontal Res. 2018;53(5):842–52. doi: 10.1111/jre.12574. [Liu X, Zhang Z, Pan S, et al. Interaction between the Wnt/-catenin signaling pathway and the EMMPRIN/MMP-2, 9 route in periodontitis[J]. J Periodontal Res, 2018, 53(5): 842-52.] [DOI] [PubMed] [Google Scholar]
- 29.Arii S, Mise M, Harada T, et al. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. 1996;24(2):316–22. doi: 10.1002/hep.510240206. [Arii S, Mise M, Harada T, et al. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential[J]. Hepatology, 1996, 24(2): 316-22.] [DOI] [PubMed] [Google Scholar]
- 30.Liu JW, Li XC, Huang JZ, et al. Matrix metalloproteinase 2 knockdown suppresses the proliferation of HepG2 and Huh7 cells and enhances the cisplatin effect. Open Med. 2019;14(1):384–91. doi: 10.1515/med-2019-0039. [Liu JW, Li XC, Huang JZ, et al. Matrix metalloproteinase 2 knockdown suppresses the proliferation of HepG2 and Huh7 cells and enhances the cisplatin effect[J]. Open Med, 2019, 14(1): 384-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou CY, Cao CJ, Wang Z, et al. EFEMP1 inhibits migration of hepatocellular carcinoma by regulating MMP2 and MMP9 via ERK1/2 activity. Oncol Rep. 2016;35(6):3489–95. doi: 10.3892/or.2016.4733. [Dou CY, Cao CJ, Wang Z, et al. EFEMP1 inhibits migration of hepatocellular carcinoma by regulating MMP2 and MMP9 via ERK1/2 activity[J]. Oncol Rep, 2016, 35(6): 3489-95.] [DOI] [PubMed] [Google Scholar]
- 32.Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and-9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie. 2005;87(3):287–97. doi: 10.1016/j.biochi.2005.01.014. [Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and-9) and their natural inhibitors as prognostic indicators in solid cancers[J]. Biochimie, 2005, 87(3): 287-97.] [DOI] [PubMed] [Google Scholar]
- 33.Cheng L, Zhao Y, Qi D, et al. Wnt/beta-catenin pathway promotes acute lung injury induced by LPS through driving the Th17 response in mice. Biochem Biophys Res Commun. 2018;495(2):1890–5. doi: 10.1016/j.bbrc.2017.12.058. [Cheng L, Zhao Y, Qi D, et al. Wnt/beta-catenin pathway promotes acute lung injury induced by LPS through driving the Th17 response in mice[J]. Biochem Biophys Res Commun, 2018, 495(2): 1890-5.] [DOI] [PubMed] [Google Scholar]


