Abstract
目的
探讨苦参碱对人肝癌HepG2和Huh7细胞增殖活性、细胞干性、β-catenin转录活性以及AKT/GSK3β/β-catenin信号通路的影响。
方法
应用MTT法检测细胞增殖活性,平板克隆形成实验检测0 g/mL、200 g/mL、400 μg/mL、800 μg/mL苦参碱对人肝癌HepG2和Huh7细胞的克隆形成能力,荧光定量PCR分析0 g/mL、200 g/mL、400 μg/mL、800 μg/mL苦参碱对人肝癌HepG2和Huh7细胞干性基因CD90、EpCAM(上皮细胞粘附分子)和CD133 mRNA的表达水平,双荧光素酶报告基因检测0 g/mL、200 g/mL、400 μg/mL、800 μg/mL苦参碱对人肝癌HepG2和Huh7细胞内β-catenin转录活性,Western blotting检测0 g/mL、400 μg/mL、800 μg/mL苦参碱对人肝癌HepG2和Huh7细胞内AKT(蛋白激酶B)、GSK-3β和β-catenin及其相应磷酸化蛋白的表达水平。
结果
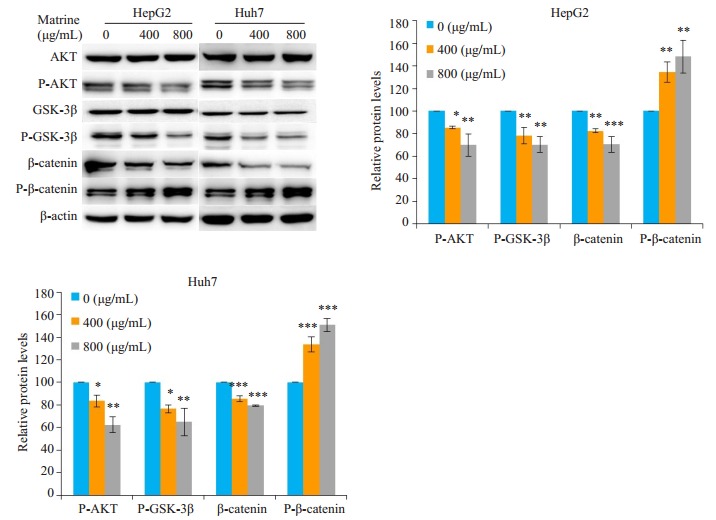
MTT实验结果显示,苦参碱呈时间-浓度依赖性地抑制肝癌HepG2和Huh7细胞的增殖,处理24、48、72 h对HepG2细胞的半数抑制浓度依次为2369、1565、909.1 μg/mL,对Huh7细胞的半数抑制浓度依次为1355、781.8、612.8 μg/mL。苦参碱浓度依赖性地抑制肝癌细胞的克隆形成能力,400 μg/mL、800 μg/mL苦参碱能够显著抑制HepG2细胞的克隆形成能力(P < 0.01,P < 0.001),200 g/mL、400 μg/mL、800 μg/mL苦参碱能够显著抑制Huh7细胞的克隆形成能力(P < 0.05,P < 0.01,P < 0.001)。苦参碱能够显著肝癌细胞内CD90、EpCAM和CD133等干细胞标志物mRNA的表达,其中400 μg/mL、800 μg/mL苦参碱能够显著抑制HepG2细胞中CD90(P < 0.01,P < 0.001)、EpCAM(P < 0.05,P < 0.01)和CD133(P < 0.01,P < 0.01)mRNA的表达,400 μg/mL、800 μg/mL苦参碱能够显著抑制Huh7细胞中CD90(P < 0.01,P < 0.01)、EpCAM(P < 0.05,P < 0.01)和CD133(P < 0.01,P < 0.01)mRNA的表达。同时400 μg/mL、800 μg/mL苦参碱显著抑制HepG2细胞(P < 0.001,P < 0.001)和Huh7细胞(P < 0.01,P < 0.001)内β-catenin的转录活性。研究结果显示400 μg/mL、800 μg/mL苦参碱能够显著降低HepG2和Huh7细胞内β-catenin和磷酸化的AKT和GSK-3β蛋白水平,增加β-catenin的磷酸化水平。
结论
苦参碱可抑制肝癌HepG2和Huh7细胞的增殖、克隆形成以及肝癌干性基因CD90、EpCAM和CD133的表达,其机制可能与抑制AKT/GSK3β/β-catenin信号通路,从而降低β-catenin的转录活性有关。
Keywords: 苦参碱, 肝细胞癌, 肿瘤干性, β-catenin
Abstract
Objective
To explore the effects of matrine on the proliferation, tumor cell stemness, β-catenin transcriptional activity and AKT/GSK3β/β-catenin signaling pathway in human hepatocellular carcinoma (HCC) HepG2 and Huh7 cells.
Methods
The proliferation and colony formation ability of HepG2 and Huh7 cells treated with 200, 400, and 800 μg/mL matrine were evaluated with MTT assay and colony formation assay, respectively. Real-time quantitative PCR was performed to detect the mRNA expressions of CD90, epithelial cell adhesion molecule (EpCAM) and CD133, and dual-luciferase assay was used to detect the transcriptional activity of β-catenin in the treated cells. The effects of matrine on the expressions of protein kinase B (AKT), P-AKT, GSK-3β, P-GSK-3β, P-β-catenin and β-catenin proteins in the Wnt/β-catenin signaling pathway were assessed using Western blotting.
Results
Matrine inhibited the proliferation of the two HCC cell lines in a time- and concentration-dependent manner. The half-inhibitory concentrations of matrine were 2369, 1565 and 909.1 μg/mL at 24, 48 and 72 h in HepG2 cells, respectively, and were 1355, 781.8, and 612.8 μg/mL in Huh7 cells, respectively. Matrine concentrationdependently suppressed colony formation of the HCC cells, producing significant inhibitory effects at 400 μg/mL P < 0.01) and 800 μg/mL P < 0.001) in HepG2 cells and at 200 μg/mL P < 0.05), 400 μg/mL P < 0.01), and 800 μg/mL P < 0.001) in Huh7 cells. Matrine at 400 and 800 μg/mL significantly inhibited the mRNA expression of CD90, EpCAM and CD133 and the transcriptional level of β-catenin in both HepG2 and Huh7 cells P < 0.05 or 0.01). Matrine at 400 and 800 μg/mL also significantly decreased the protein levels of β-catenin, P-AKT and P-GSK-3β and increased the phosphorylation level of β-catenin in both of the cell lines.
Conclusion
Matrine inhibits the proliferation, colony formation, and the expressions of tumor stem cell markers CD90, EpCAM and CD133 in both HepG2 and Huh7 cells probably by inhibiting Wnt/β-catenin signaling pathway and the transcriptional activity ofβ-catenin.
Keywords: matrine, hepatocellular carcinoma, tumor cell stemness, β-catenin
肝细胞癌(HCC)是恶性程度最高的消化系统肿瘤之一,其发病率和死亡率均位居恶性肿瘤前列[1]。研究发现,包括肝癌在内的大部分肿瘤都具有异质性,肿瘤中部分细胞亚群具有干细胞样特征——较强的自我更新能力、侵袭能力、耐药性和可能存在的多潜能分化能力。这群细胞被称为肿瘤干细胞(CSCs)或肿瘤起始细胞(TICs)[2]。肿瘤干细胞与肿瘤复发、转移以及耐药密切相关。在肝癌中分离到表达干性标志物CD90、EpCAM(上皮细胞粘附分子)和CD133的肝癌干细胞,这些干性基因的表达受到上游Wnt/β-catenin等信号通路的调控[3]。苦参碱是从天然豆科植物苦参根中分离提取的生物碱[4],具有抗病毒、免疫抑制、抗肿瘤、抗肝纤维化以及调节心律失常等药理作用[5-9]。近年来,临床上将苦参碱与其它化疗药物联合试用于肿瘤的治疗,并取得一定的疗效[10-11]。有研究报道,苦参碱抑制肿瘤细胞增殖、诱导凋亡和自噬,抗肿瘤血管生成等[12-14]。但其具体作用机制尚未明确。我们以往的研究发现,苦参碱具有抑制肝癌Hep3B细胞生长和β-catenin转录活性的作用,本研究旨在探讨苦参碱对肝癌细胞干性的影响,并从Wnt/β-catenin信号通路探讨其作用机制,为临床应用苦参碱防治肝癌转移复发提供实验和理论依据。
1. 材料和方法
1.1. 材料
1.1.1. 细胞株
人肝癌HepG2细胞株购自美国典型培养物中心(ATCC),人肝癌Huh7细胞株购买于中国科学院细胞库。
1.1.2. 药物与试剂
苦参碱注射液(广州白云山明兴制药有限公司);DMEM高糖细胞培养基(Gibco);MTT(Sigma);PBS(博士德);二甲基亚砜(Sigma);4%多聚甲醛(Biosharp);结晶紫(远航化工厂);RIPA裂解液(康为世纪);蛋白酶抑制剂(康为世纪),磷酸酶抑制剂(康为世纪),Pierce BCA Protein Assay Kit试剂盒(Thermo Scientific);一抗AKT,P- AKT,GSK3β,P- GSK3β,β- catenin,P-β- catenin(Cell signaling technology);二抗(碧云天);Immobilon ECL发光液(弗德),荧光素酶质粒TOPFlash和FOPFlash(Upstate),pRL-TK(Promega),双荧光素酶报告系统(Promega),Lipofectamin 2000(Invitrogen),SYBR® Premix Ex TaqTM II试剂盒(Takara),RT-PCR逆转录试剂盒(Takara)。
1.2. 方法
1.2.1. MTT法检测细胞增殖活性
取生长状态良好并处于对数生长期的人肝癌HepG2和Huh7细胞,吸弃培养液,用胰酶消化后制备单细胞悬液,根据2.5×103/孔的细胞密度接种于96孔板,各组设置3个复孔。待细胞过夜贴壁稳定生长后,吸弃培养基,孔内加入含有不同浓度梯度苦参碱(0、200、400、800、1 600 μg/mL)的培养液。并于苦参碱处理24、48、72 h后加入终浓度为0.5 mg/mL MTT溶液,放入CO2培养箱继续孵育4 h。吸弃培养液,加入150 μL二甲基亚砜,震荡10 min至甲臢结晶完全溶解,在多功能酶标仪检测吸光度A490 nm。并根据公式计算苦参碱对肝癌细胞生长的抑制率,并用Prism 5.0软件计算IC50。细胞生长抑制率按以下公式计算:抑制率%=(1- A处理组/A对照组)×100%。
1.2.2. 克隆形成实验检测细胞克隆形成能力
取生长状态良好并处于对数生长期的人肝癌HepG2和Huh7细胞,吸弃培养液,用胰酶消化后制备单细胞悬液,以3× 102/孔的细胞密度接种于6孔板中,呈十字型摇晃均匀。待细胞过夜贴壁稳定生长后,吸弃培养基,孔内加入含有不同浓度梯度苦参碱(0、200、400、800 μg/mL)的培养液。待苦参碱处理48 h后,换成不含药的完全培养液,继续培养至出现肉眼可见克隆斑。吸弃板中培养液,用PBS清洗2次后加入1 mL 4%多聚甲醛固定细胞,10 min后吸弃固定液,加入1 mL 0.5%结晶紫染色10 min后流水冲洗多余染液,晾干后对板内克隆斑进行拍照和计数。根据如下公式计算各组的细胞克隆形成率:克隆形成率%=(克隆形成数/接种细胞数)×100%。
1.2.3. qRT-PCR检测肝癌细胞干性标志基因表达
取生长状态良好并处于对数生长期的人肝癌HepG2和Huh7细胞制备单细胞悬液,以1×105/孔的细胞密度接种于6孔板中,待细胞过夜贴壁稳定生长后,吸弃培养基,孔内加入含有不同浓度梯度苦参碱(0、200、400、800 μg/mL)的培养液。苦参碱处理48 h根据总RNA提取试剂盒对肝癌细胞内总RNA提取,采用逆转录试剂盒对提取的RNA样本进行逆转录成cDNA,逆转录结束后,运用实时荧光定量PCR反应对cDNA进行扩增检测目的基因的mRNA表达水平。引物:CD133:F:5'- TTCTATGCTGTGTCCTGGGGC-3';R:5'-TTGTTG GTGCAAGCTCTTCAAGGT-3';CD90:F:5'-AAGTG TCACTGGCATGTATACAC-3';R:5'-GGTCCTTTTCA CCAGCAA-3';EpCAM:F:5'-GTGCTGGTGTGTGAA CACTG-3';R:5'-AGCCACATCAGCTATGTCCA-3'。根据qRT-PCR检测结果,采用2-△△Ct方法对数据进行定量,它表示处理中目的基因表达水平是对照组的2-△△Ct倍。
△△Ct=(Ct目的基因-Ct内参基因)处理组-(Ct目的基因-Ct内参基因)对照组。
1.2.4. 双荧光素酶报告基因系统检测转录活性
取生长状态良好并处于对数生长期的人肝癌HepG2和Huh7细胞制备单细胞悬液,以3×103/孔的细胞密度接种于96孔板中,每组设置3个副孔。待细胞过夜贴壁稳定生长后,使用Lipofectamin 2000将荧光素酶质粒TOPFlash,FOPFlash和pRL-TK(双荧光素酶报告系统内参)共转染进人肝癌HepG2和Huh7细胞内。孵育24 h后,分别加入含浓度梯度苦参碱(0、400、800 μg/mL)继续孵育24 h,根据双荧光素酶系统操作说明裂解细胞,并在多功能酶标仪下测定发光值。实验数据在经内参校正后采用TOP/FOP数值表征β-catenin的转录活性。以对照组均值为100%计,其他处理组与对照组的比值为相对转录活性。
1.2.5. Western blotting法检测蛋白表达水平
人肝癌HepG2和Huh7细胞经不同浓度梯度的含苦参碱(0、400、800 μg/mL)培养液处理48 h后,于冰上用RIPA裂解液进行裂解,12 000 r/min 4 ℃离心15 min后吸取上清液。采用Pierce BCA Protein Assay Kit试剂盒检测蛋白浓度,蛋白样品浓度采用上样缓冲液(5×)和稀释液将蛋白调整至所需浓度,接着将蛋白样品置于100 ℃金属浴中变性5 min。待蛋白样品冷却经10 % SDSPAGE凝胶电泳分离后转至PVDF膜。采用5 %BSA封闭1 h后,置于一抗中4 ℃孵育至12 h。用TBST洗膜3次后,在室温下用二抗孵育1 h,经TBST将二抗清洗后加ECL化学发光液于多功能成像系统下采集曝光图片。
1.3. 统计学方法
采用GraphPad Prism 5.0软件对实验结果进行统计分析并作图,多组间比较采用One way-ANOVA,数据以均值±标准差表示,P < 0.05认为差异具有统计学意义。
2. 结果
2.1. 苦参碱对人肝癌HepG2和Huh7细胞增殖活性的抑制作用
不同浓度药物(0、200、400、800和1600 μg/mL)处理24、48、72 h后,苦参碱呈时间-浓度依赖性地抑制人肝癌HepG2和Huh7细胞的增殖活性(图 1)。在人肝癌HepG2和Huh7细胞中,浓度为400 μg/mL苦参碱作用48 h后即呈现显著的抗增殖作用,抑制率分别为(16.63±2.27)%和(23.27±1.11)%,苦参碱在24、48、72 h对HepG2细胞的半数抑制浓度依次为2369、1565和909.1 μg/mL,对Huh7细胞的半数浓度依次为1355、781.8、612.8 μg/mL(表 1)。
1.
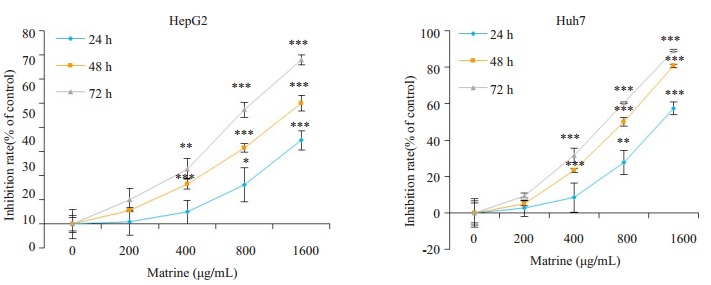
苦参碱对肝癌HepG2和Huh7细胞增殖的影响
Effects of matrine on proliferation of HepG2 and Huh7 cells (Mean±SD, n=3). Compared with control, *P < 0.05, **P < 0.01, ***P < 0.001
1.
苦参碱抑制肝癌细胞增殖的IC50
IC50 of matrine in HCC cells
| Incubation time with matine Time (h) |
IC50 (μg/mL) | |
| HepG2 | Huh7 | |
| 24 | 2369 | 1355 |
| 48 | 1565 | 781.8 |
| 72 | 909.1 | 612.8 |
2.2. 苦参碱显著抑制肝癌HepG2和Huh7细胞克隆形成
MTT实验结果主要表征细胞活力,而细胞克隆形成实验则更准确表征细胞增殖能力和细胞干性。肿瘤干细胞往往具有较强的克隆形成能力。图 2显示,克隆斑的形成数量随苦参碱药物浓度增加而呈浓度依赖性减少,在HepG2细胞中,400 μg/mL和800 μg/mL苦参碱处理组的克隆形成率分别为(50.51 ± 5.736)%,(38.89±13.66)%,与0 μg/mL组相比较均具有统计学差异。在Huh7细胞内,200、400、800 μg/mL苦参碱处理组的克隆形成率分别为(64.20 ± 5.685)%,(53.09 ± 13.01)%,(16.05±8.553)%,与0 μg/mL组相比较均具有统计学差异。
2.
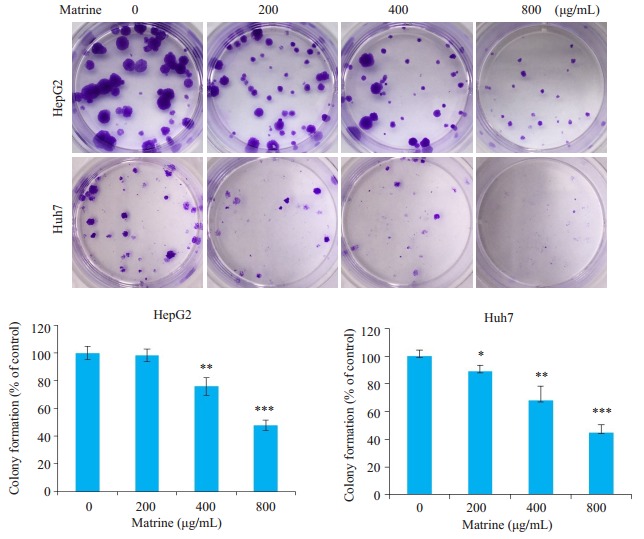
苦参碱对肝癌HepG2和Huh7细胞克隆形成能力的影响镜下观察(上)及统计学分析(下)
Effects of matrine on colony formation of HepG2 and Huh7 cells. Upper panel: Microscopic observation; Lower panel: Statistical analysis (Mean±SD, n=3). Compared with control, *P < 0.05, **P < 0.01, ***P < 0.001
2.3. 苦参碱显著抑制肝癌HepG2和Huh7细胞内干性标志物CD90、EpCAM和CD133 mRNA的表达
实时荧光定量PCR检测结果显示,在肝癌HepG2和Huh7细胞,苦参碱能够呈浓度依赖性抑制肝癌细胞的中CD90、EpCAM和CD133等肿瘤干性标志物的表达(图 3)。其中400、800 μg/mL苦参碱能够显著抑制HepG2细胞中CD90(P < 0.01,P < 0.001)、EpCAM(P < 0.05,P < 0.01)和CD133(P < 0.01,P < 0.01)mRNA的表达,400、800 μg/mL苦参碱能够显著抑制Huh7细胞中CD90(P < 0.01,P < 0.01)、EpCAM(P < 0.05,P < 0.01)和CD133(P < 0.01,P < 0.01)mRNA的表达。
3.
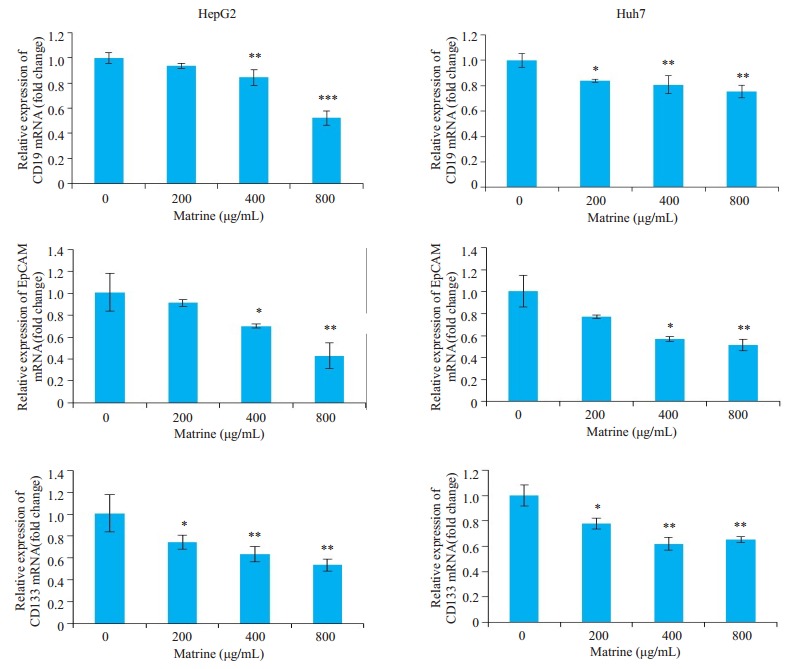
苦参碱对肝癌HepG2和Huh7细胞中CD90、EpCAM和CD133 mRNA表达水平的影响
Effects of matrine on expressions of CD90, EpCAM and CD133 mRNA in HepG2 and Huh7 cells (Mean±SD, n=3).Compared with control, *P < 0.05, **P < 0.01, ***P < 0.001
2.4. 苦参碱显著抑制肝癌HepG2和Huh7细胞内β-catenin的转录活性
为进一步探讨苦参碱抑制肝癌细胞干性的作用机制,采用双荧光素酶报告系统检测苦参碱对肝癌细胞β-catenin的转录活性的影响。在肝癌HepG2和Huh7细胞,苦参碱能够呈浓度依赖性抑制肝癌细胞内的β- catenin的转录活性,400 μg/mL的苦参碱对HepG2和Huh7细胞的抑制率分别为(24.74±4.682)%和(32.98± 3.871)%。800 μg/mL药物浓度处理时,HepG2和Huh7细胞内β-catenin的转录活性抑制率分别为(51.42 ± 5.256)%和(53.04±5.939)%(图 4)。
4.
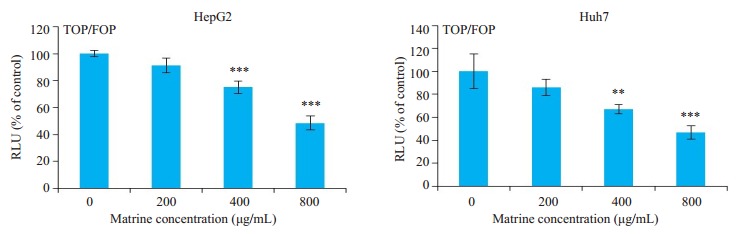
苦参碱对肝癌HepG2和Huh7细胞内β-catenin转录活性的影响
Effects of matrine on transcriptional activity of β-catenin in HepG2 and Huh7 cells (Mean±SD, n=3). Compared with control, **P < 0.01, ***P < 0.001
2.5. 苦参碱对肝癌细胞内Wnt/β-catenin信号通路的影响
研究发现,400 μg/mL和800 μg/mL苦参碱处理48 h后,肝癌HepG2和Huh7细胞中磷酸化AKT(P-AKT)、磷酸化GSK-3β(P-GSK-3β)和β-catenin蛋白水平显著降低,同时磷酸化β-catenin(P-β-catenin)蛋白表达增加。
5.
苦参碱对肝癌HepG2和Huh7细胞中CD90、EpCAM和CD133 mRNA表达水平的影响
Effects of matrine on expressions of CD90, EpCAM and CD133 mRNA in HepG2 and Huh7 cells (Mean±SD, n=3).Compared with control, *P < 0.05, **P < 0.01, ***P < 0.001
3. 讨论
肝细胞癌是复发率和死亡率较高的恶性肿瘤之一,易于转移,对传统的化疗药和靶向药耐药,导致预后较差。研究发现,肝癌中存在着一群具有自我更新、多向分化、无限增殖潜能、及致瘤性等生物学特性的干细胞,特异性地表达CD90、EpCAM、CD133等干性基因,介导肿瘤细胞的异常增殖,转移和耐药[15]。因此,从天然产物中筛选特异性抑制肝癌细胞干性的药物,应用于临床肝癌的治疗意义重大。苦参碱是从传统中药苦参根中提取出的主要生物碱,临床上具有抗肿瘤,抗炎等作用[16-17]。研究报道,苦参碱能够抑制肿瘤细胞的增殖,促进肿瘤细胞凋亡,抑制肿瘤的耐药等[18]。我们的研究结果显示,苦参碱能够呈浓度-时间依赖性地抑制肝癌HepG2和Huh7细胞的增殖活性,并且显著抑制肝癌细胞的克隆形成能力,表明苦参碱能够抑制肝癌细胞无限增殖的干性,进而发挥抗肿瘤作用。CD90、EpCAM、CD133是肝癌干细胞的特异性标志物,CD90是一细胞表面糖蛋白,附着在细胞膜的胞浆面,参与肿瘤干细胞的增殖分化、机体免疫反应、细胞因子释放等过程, 与肝癌干细胞的增殖分化,克隆形成密切相关[19]。EpCAM是黏附分子家族成员之一,属于上皮细胞间黏附分子,在细胞发生癌变过程中发挥着关键作用,能够调控肿瘤干细胞的侵袭、增殖、分化等功能,也是一类特异性的肝癌干细胞标记物,研究发现EpCAM可以通过促进细胞增殖、免疫逃逸等机制促进肿瘤的发生发展[20-21]。CD133也是肝癌干细胞的特异性表面标记物之一,在肝癌干细胞的增殖,侵袭和分化方面发挥重要作用[22]。本研究发现苦参碱能够显著抑制CD90、EpCAM、CD133三个肝癌干性标志物的mRNA的表达,进一步提示苦参碱具有抑制肝癌细胞干性的作用。
Wnt/β-catenin信号通路被发现在大于40%的肝癌细胞中被激活,参与调控肿瘤细胞增殖、分化、转移及维持肝癌细胞干性[23]。活化的β-catenin转录入核,能够促进CD90、EpCAM、CD133等干性基因的表达,继而诱导肿瘤细胞的转移和无限增殖[24-27]。我们以往的研究曾发现,苦参碱抑制肝癌Hep3B细胞生长和β-catenin的转录活性,因此我们推测苦参碱抗肝癌细胞干性的作用与Wnt/β-catenin信号通路相关。在β-catenin信号通路未被激活时,GSK3β通常与APC、Axin形成复合物,将胞浆中的β-catenin进行磷酸化,从而促进其泛素化降解。而在生长旺盛的肝癌细胞中,GSK-3β可被上游活化的蛋白激酶AKT磷酸化,失去磷酸化β-catenin的作用,积累的β-catenin发生核转位[28]。本研究显示,苦参碱能够显著抑制AKT和GSK-3β的磷酸化,同时增加β-catenin的磷酸化水平,降低β-catenin蛋白水平,表明苦参碱通过抑制AKT和GSK-3β磷酸化后,使GSK3β恢复磷酸化β-catenin的能力,从而促进β-catenin的磷酸化使其发生降解。荧光素酶报告基因结果显示苦参碱能够显著抑制肝癌细胞内β-catenin的转录活性,进一步表明苦参碱能够通过抑制肝癌细胞内β-catenin信号通路的激活,进而抑制β-catenin的转录活性和下游肝癌细胞干性基因的表达。那么,苦参碱作用的靶分子是什么?通过何种方式抑制AKT的磷酸化呢?AKT上游信号主要涉及磷脂酰肌醇-3激酶的激活和雷帕霉素靶蛋白的活化两条途径[29],文献报道和我们实验曾发现苦参碱具有诱导肝癌细胞自噬的作用,提示药物可能抑制雷帕霉素靶蛋白的活化。在下一步的工作中,我们拟采用RNA干扰等实验方法进一步研究β-catenin信号通路对肝癌细胞干性的调控作用,阐明苦参碱的作用靶点。
综上所述,苦参碱通过抑制Wnt/β-catenin信号通路的活化,继而干扰CD90、EpCAM、CD133等干性标志物的表达,从而削弱肝癌细胞无限增殖、分化等恶性生物学特性,发挥抗肿瘤作用。研究结果对临床应用苦参碱治疗肝细胞癌具有重要的意义。
Biography
戴美琴,硕士研究生,E-mail: 1191571995@qq.com
Funding Statement
广东省自然科学基金(2016A030313595);广州市科技计划项目(201707010074)
Contributor Information
戴 美琴 (Meiqin DAI), Email: 1191571995@qq.com.
郭 丹 (Dan GUO), Email: gdan_2007@126.com.
References
- 1.Costentin CC. Hepatocellular carcinoma surveillance. Presse Med. 2017;46(4):381–5. doi: 10.1016/j.lpm.2016.11.006. [Costentin CC. Hepatocellular carcinoma surveillance[J]. Presse Med, 2017, 46(4): 381-5.] [DOI] [PubMed] [Google Scholar]
- 2.Zhang PY, Yang YJ, Xue Y, et al. Cancer stem cells: targeting tumors at the source. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3327048. Eur Rev Med Pharmacol Sci. 2015;19(10):1821–8. [Zhang PY, Yang YJ, Xue Y, et al. Cancer stem cells: targeting tumors at the source[J]. Eur Rev Med Pharmacol Sci, 2015, 19(10): 1821-8.] [PubMed] [Google Scholar]
- 3.Ji JF, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012;39(4):461–72. doi: 10.1053/j.seminoncol.2012.05.011. [Ji JF, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma[J]. Semin Oncol, 2012, 39(4): 461-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun J, Mao J, Liu X, et al. Separation and mechanism elucidation for six structure-like matrine-type alkaloids by micellar liquid chromatography. J Sep Sci. 2009;32(12):2043–50. doi: 10.1002/jssc.200900066. [Sun J, Mao J, Liu X, et al. Separation and mechanism elucidation for six structure-like matrine-type alkaloids by micellar liquid chromatography[J]. J Sep Sci, 2009, 32(12): 2043-50.] [DOI] [PubMed] [Google Scholar]
- 5.Guzman JR, Koo JS, Goldsmith JR, et al. Oxymatrine prevents NFkappa B nuclear translocation and ameliorates acute intestinal inflammation. http://cn.bing.com/academic/profile?id=ea025b9e3c8dbba82bb0ee5e8d38efee&encoded=0&v=paper_preview&mkt=zh-cn. Sci Rep. 2013;32(1):1629. doi: 10.1038/srep01629. [Guzman JR, Koo JS, Goldsmith JR, et al. Oxymatrine prevents NFkappa B nuclear translocation and ameliorates acute intestinal inflammation[J]. Sci Rep, 2013, 32(1): 1629.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, Liu ZY, Li YY, et al. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur J Pharm Sci. 2011;44(5):573–9. doi: 10.1016/j.ejps.2011.09.020. [Zhang B, Liu ZY, Li YY, et al. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice[J]. Eur J Pharm Sci, 2011, 44(5): 573-9.] [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Xiu J, Zhang X, et al. Antiviral effect of matrine against human enterovirus 71. Molecules. 2012;17(9):10370–6. doi: 10.3390/molecules170910370. [Yang Y, Xiu J, Zhang X, et al. Antiviral effect of matrine against human enterovirus 71[J]. Molecules, 2012, 17(9): 10370-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai NL, Fu Q, Shi H, et al. Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells. World J Gastroenterol. 2012;18(31):4199–206. doi: 10.3748/wjg.v18.i31.4199. [Chai NL, Fu Q, Shi H, et al. Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells[J]. World J Gastroenterol, 2012, 18(31): 4199-206.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao YG, Jing S, Li L, et al. Antiarrhythmic effects and Ionic mechanisms of oxymatrine from Sophora flavescens. Phytother Res. 2010;24(12):1844–9. doi: 10.1002/ptr.3206. [Cao YG, Jing S, Li L, et al. Antiarrhythmic effects and Ionic mechanisms of oxymatrine from Sophora flavescens[J]. Phytother Res, 2010, 24(12): 1844-9.] [DOI] [PubMed] [Google Scholar]
- 10.何 成邦, 孙 成芝. 复方苦参注射液治疗中晚期肝癌30例疗效观察. 山东医药. 2010;50(25):23. doi: 10.3969/j.issn.1002-266X.2010.25.047. [何成邦, 孙成芝.复方苦参注射液治疗中晚期肝癌30例疗效观察[J].山东医药, 2010, 50(25): 23.] [DOI] [Google Scholar]
- 11.Yang CL, Liu SS, Ma YG, et al. The influence of intraoperative pleural perfusion with matrine-cisplatin or cisplatin on stromal cellderived factor-1 in non-small cell lung cancer patients with subclinical pleural metastasis. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=30fc19a759da7b59c609e25c4f8b9173. Med Oncol. 2012;29(2):574–81. doi: 10.1007/s12032-011-9849-4. [Yang CL, Liu SS, Ma YG, et al. The influence of intraoperative pleural perfusion with matrine-cisplatin or cisplatin on stromal cellderived factor-1 in non-small cell lung cancer patients with subclinical pleural metastasis[J]. Med Oncol, 2012, 29(2): 574-81.] [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Zhuo XB, Hu YP, et al. A novel matrine derivative WM622 inhibits hepatocellular carcinoma by inhibiting PI3K/AKT signaling pathways. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b8c0f766a4f27578539b6e4781305f8a. Mol Cell Biochem. 2018;449(1/2):47–54. doi: 10.1007/s11010-018-3341-9. [Sun X, Zhuo XB, Hu YP, et al. A novel matrine derivative WM622 inhibits hepatocellular carcinoma by inhibiting PI3K/AKT signaling pathways[J]. Mol Cell Biochem, 2018, 449(1/2): 47-54.] [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Xu J, Wang H, et al. Effect and mechanism of sophoridine to suppress hepatocellular carcinoma in vitro and vivo. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=526d1cf9ca321441d04adab10f5ae5d9. Biomed Pharmacother. 2017;95(3):324–30. doi: 10.1016/j.biopha.2017.08.029. [Wang B, Xu J, Wang H, et al. Effect and mechanism of sophoridine to suppress hepatocellular carcinoma in vitro and vivo[J]. Biomed Pharmacother, 2017, 95(3): 324-30.] [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhang S, Liu J, et al. Matrine inhibits the invasive and migratory properties of human hepatocellular carcinoma by regulating epithelial-mesenchymal transition. http://cn.bing.com/academic/profile?id=ffa3e22728ae06942fa2c71f9afddc47&encoded=0&v=paper_preview&mkt=zh-cn. Mol Med Rep. 2018;18(1):911–9. doi: 10.3892/mmr.2018.9023. [Wang Y, Zhang S, Liu J, et al. Matrine inhibits the invasive and migratory properties of human hepatocellular carcinoma by regulating epithelial-mesenchymal transition[J]. Mol Med Rep, 2018, 18(1): 911-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiba T, Iwama A, Yokosuka O. Cancer stem cells in hepatocellular carcinoma: Therapeutic implications based on stem cell biology. Hepatol Res. 2016;46(1):50–7. doi: 10.1111/hepr.12548. [Chiba T, Iwama A, Yokosuka O. Cancer stem cells in hepatocellular carcinoma: Therapeutic implications based on stem cell biology[J]. Hepatol Res, 2016, 46(1): 50-7.] [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Du T, Xu GB, et al. Matrine suppresses invasion of castration-resistant prostate cancer cells by downregulating MMP-2/ 9 via NF-kappa B signaling pathway. Int J Oncol. 2017;50(2):640–8. doi: 10.3892/ijo.2016.3805. [Huang H, Du T, Xu GB, et al. Matrine suppresses invasion of castration-resistant prostate cancer cells by downregulating MMP-2/ 9 via NF-kappa B signaling pathway[J]. Int J Oncol, 2017, 50(2): 640-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Lin H, Zhang R. The clinical efficacy and adverse effects of interferon combined with matrine in chronic hepatitis B: A systematic review and Meta-Analysis. Phytother Res. 2017;31(6):849–57. doi: 10.1002/ptr.5808. [Wang X, Lin H, Zhang R. The clinical efficacy and adverse effects of interferon combined with matrine in chronic hepatitis B: A systematic review and Meta-Analysis[J]. Phytother Res, 2017, 31 (6): 849-57.] [DOI] [PubMed] [Google Scholar]
- 18.Zhou BG, Wei CS, Zhang S, et al. Matrine reversed multidrug resistance of breast cancer MCF-7/ADR cells through PI3K/AKT signaling pathway. J Cell Biochem. 2018;119(5):3885–91. doi: 10.1002/jcb.26502. [Zhou BG, Wei CS, Zhang S, et al. Matrine reversed multidrug resistance of breast cancer MCF-7/ADR cells through PI3K/AKT signaling pathway[J]. J Cell Biochem, 2018, 119(5): 3885-91.] [DOI] [PubMed] [Google Scholar]
- 19.Zhang XL, Jia Q, Lv L, et al. Tumorspheres derived from HCC cells are enriched with cancer stem cell-like cells and present high chemoresistance dependent on the Akt pathway. Anticancer Agents Med Chem. 2015;15(6):755–63. doi: 10.2174/1871520615666150202111721. [Zhang XL, Jia Q, Lv L, et al. Tumorspheres derived from HCC cells are enriched with cancer stem cell-like cells and present high chemoresistance dependent on the Akt pathway[J]. Anticancer Agents Med Chem, 2015, 15(6): 755-63.] [DOI] [PubMed] [Google Scholar]
- 20.Dolle L, Theise ND, Schmelzer E, et al. EpCAM and the biology of hepatic stem/progenitor cells. Am J Physiol Gastrointest Liver Physiol. 2015;308(4):G233–50. doi: 10.1152/ajpgi.00069.2014. [Dolle L, Theise ND, Schmelzer E, et al. EpCAM and the biology of hepatic stem/progenitor cells[J]. Am J Physiol Gastrointest Liver Physiol, 2015, 308(4): G233-50.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroepil F, Dulian A, Vallböhmer D, et al. High EpCAM expression is linked to proliferation and lauren classification in gastric cancer. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3724596. BMC Res Notes. 2013;6(2):253. doi: 10.1186/1756-0500-6-253. [Kroepil F, Dulian A, Vallböhmer D, et al. High EpCAM expression is linked to proliferation and lauren classification in gastric cancer [J]. BMC Res Notes, 2013, 6(2): 253.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerra-Rebollo M, Garrido C, Sánchez-Cid L, et al. Targeting of replicating CD133 and OCT4/SOX2 expressing glioma stem cells selects a cell population that reinitiates tumors upon release of therapeutic pressure. Sci Rep. 2019;9(1):9549. doi: 10.1038/s41598-019-46014-0. [Guerra-Rebollo M, Garrido C, Sánchez-Cid L, et al. Targeting of replicating CD133 and OCT4/SOX2 expressing glioma stem cells selects a cell population that reinitiates tumors upon release of therapeutic pressure[J]. Sci Rep, 2019, 9(1): 9549.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. http://cn.bing.com/academic/profile?id=e998b1afe66773ad6057faae6e186dd4&encoded=0&v=paper_preview&mkt=zh-cn. Science. 2014;346(625):1248012. doi: 10.1126/science.1248012. [Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control[J]. Science, 2014, 346(625): 1248012.] [DOI] [PubMed] [Google Scholar]
- 24.Yamashita T, Budhu A, Forgues M, et al. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67(22):10831–9. doi: 10.1158/0008-5472.CAN-07-0908. [Yamashita T, Budhu A, Forgues M, et al. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma[J]. Cancer Res, 2007, 67(22): 10831-9.] [DOI] [PubMed] [Google Scholar]
- 25.Zhou FQ, Qi YM, Xu H, et al. Expression of EpCAM and Wnt/ β- catenin in human colon cancer. Genet Mol Res. 2015;14(2):4485–94. doi: 10.4238/2015.May.4.6. [Zhou FQ, Qi YM, Xu H, et al. Expression of EpCAM and Wnt/ β- catenin in human colon cancer[J]. Genet Mol Res, 2015, 14(2): 4485-94.] [DOI] [PubMed] [Google Scholar]
- 26.Skowron MA, Niegisch G, Fritz G, et al. Phenotype plasticity rather than repopulation from CD90/CK14 + cancer stem cells leads to cisplatin resistance of urothelial carcinoma cell lines. http://cn.bing.com/academic/profile?id=5f95fa5a9f789698c728c4030153392a&encoded=0&v=paper_preview&mkt=zh-cn. J Exp Clin Cancer Res. 2015;34(8):144. doi: 10.1186/s13046-015-0259-x. [Skowron MA, Niegisch G, Fritz G, et al. Phenotype plasticity rather than repopulation from CD90/CK14 + cancer stem cells leads to cisplatin resistance of urothelial carcinoma cell lines[J]. J Exp Clin Cancer Res, 2015, 34(8): 144.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shevchenko V, Arnotskaya N, Korneyko M, et al. Proteins of the Wnt signaling pathway as targets for the regulation of CD133 + cancer stem cells in glioblastoma. http://cn.bing.com/academic/profile?id=1fa9b71b85020b4d0cd767190e386cf2&encoded=0&v=paper_preview&mkt=zh-cn. Oncol Rep. 2019;41(5):3080–8. doi: 10.3892/or.2019.7043. [Shevchenko V, Arnotskaya N, Korneyko M, et al. Proteins of the Wnt signaling pathway as targets for the regulation of CD133 + cancer stem cells in glioblastoma[J]. Oncol Rep, 2019, 41(5): 3080-8.] [DOI] [PubMed] [Google Scholar]
- 28.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–7. doi: 10.1126/science.1094291. [Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways[J]. Science, 2004, 303(5663): 1483-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23(10):1515–27. doi: 10.1016/j.cellsig.2011.05.004. [Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease [J]. Cell Signal, 2011, 23(10): 1515-27.] [DOI] [PubMed] [Google Scholar]


