Abstract
The capability model of alpha asymmetries posits that state emotional manipulations are a more powerful detector of depression-related motivational deficits than alpha activity at rest. The present study used a time-frequency approach to investigate the temporal dynamics of event-related changes in alpha power during passive viewing of emotional pictures in individuals with dysphoria (n = 23) and in individuals without dysphoria (n = 24). In the whole group, the processing of pleasant and unpleasant compared to neutral pictures was associated with a decrease in event-related alpha power (i.e., alpha desynchronization) at centro-parietal and parietal scalp sites in the 538–1400 ms post-stimulus. The group with dysphoria revealed a smaller alpha desynchronization than the group without dysphoria in response to pleasant, but not neutral and unpleasant, stimuli at frontal, fronto-central and centro-parietal sites. Interestingly, at central and centro-parietal scalp sites, the difference between groups in response to pleasant stimuli was lateralized to the right hemisphere, whereas no clear lateralization was observed at frontal and fronto-central scalp sites. These findings suggest that decreased cortical activity (i.e., reduced alpha desynchronization) in a network involving bilateral frontal and right-lateralized parietal regions may provide a specific measure of deficits in approach-related motivation in depression.
Subject terms: Emotion, Human behaviour, Depression
Introduction
Depression is characterized by excessive and persistent negative mood, and/or by anhedonia and loss of pleasure in daily activities1. It has been proposed that both emergence and maintenance of depressive symptoms are accounted for by a preferential processing bias for mood-congruent (i.e., negative) information (for a review see Clark & Beck2). Such bias is supposed to produce a dominance of negative or threat-related thoughts, images and interpretations, in line with the idea that negative mood potentiates like-valenced or matching emotions3, known as the negative potentiation hypothesis.
Despite the empirical support to this conceptualization4–6, recent evidence suggests, on the contrary, that depressive symptoms emerge mostly as a consequence of reduced emotional response to positively valenced and rewarding stimuli7,8, possibly indicating a dysregulation of the approach-related motivational system in the brain9,10. Importantly, reduced approach-related motivation constitutes an important risk factor for clinical depression11 and – despite its scarce consideration in clinical practice – it may account for core symptoms such as anhedonia, apathy and loss of interests (for a review see Admon & Pizzagalli12). This model, known as the positive attenuation hypothesis, has been recently extended by a third alternative that postulates that depression is characterized by blunted reactivity to all emotional stimuli regardless of their valence, the so-called emotional context insensitivity (ECI)13,14. Specifically, the ECI model holds that individuals with depression exhibit reduced reactivity in response to both pleasant and, in contrast with the negative potentiation hypothesis, unpleasant stimuli, as a result of underactivation of the appetitive and the defensive motivational systems, respectively.
Reduced approach-related motivation in subclinical15,16 and clinical depression17,18, as well as in euthymic participants with a history of depression19,20, has been associated with hypoactivity of the left frontal lobe compared to the right. These findings support a renowned conceptual model which proposed that left frontal activity subtends the propensity to approach or engage a stimulus, whether reduced left vs. right frontal activity indicates a reduction in approach behaviors and increased withdrawal motivation21–24. Unbalanced cortical activity between the frontal areas of the two hemispheres is typically measured as asymmetry in the alpha band, a brain rhythm associated with cortical inhibition25. Accordingly, a recent EEG study confirmed that alpha asymmetry measured from the scalp correlated with asymmetry in the activation of lateral mid-frontal regions of the brain, and that participants with a history of depressive episodes were characterized by less left relative to right cortical activity in these regions26.
So far, the vast majority of studies on frontal alpha asymmetry in depression have investigated reduced approach-related motivation at rest. This is consistent with a dispositional model of motivation and affective style, which proposes that individuals have a trait-like tendency to respond with either approach or withdrawal, irrespective of the specific demands of the situation23. Nonetheless, inconsistent results have emerged, raising criticisms about the effective value of resting frontal alpha asymmetry as a potential biomarker for depression (for a recent meta-analysis see van der Vinne et al.27). Following these concerns, a capability model has been proposed, which states that individuals differ in their emotion regulatory abilities in situations with specific emotional charges28. In other words, reduced approach-related motivation in depression is thought to be more evident in response to emotional stimuli than at rest, since the emotional demands of the context highlight the motivational deficit, and reduce undesirable variance associated with resting states25,28,29.
To date, only few studies have started investigating alpha asymmetry in depression in emotion- or reward-related tasks9,29–31. Moreover, two methodological shortcomings ought to be mentioned. First, EEG activity has typically been averaged over several seconds, providing no information regarding the time course of the response to emotional stimuli. This is surprising considering that emotional responding and regulation occur within a few hundred of millisecond, and considering that alpha asymmetry has been reported to burst transiently also at rest32. Furthermore, recent studies have analyzed alpha activity only at anterior scalp sites, even if asymmetry in the alpha band in depression has been reported also at posterior scalp sites, despite some exception33. In particular, depression (current or remitted) and familiarity for depression are characterized by a right temporo-parietal dysfunction, as indicated by increased right relative to left parietal alpha activity19,33,34. Decreased right parietal activity is thought to reflect reduced arousal and impaired processing of emotional stimuli33,35–37, and may thus concur to emotional dysregulation in depression.
A time-frequency analysis of the EEG response to emotional stimuli allows to overcome most of these limitations. Importantly, frontal alpha response to emotional stimuli can be evaluated over time, overcoming the “static picture” provided by conventional fast Fourier transform (FFT) spectral analysis based on averaging procedures. Interestingly, this method has been proven successful in detecting transient motivational responses to phobic pictures in specific phobia38, as indicated by alpha power at frontal sites. More specifically, the time-frequency approach allows to assess how depression-related motivational disposition affects alpha power in response to emotional stimuli with an excellent temporal resolution (in the millisecond range).
The goal of the present study was to investigate motivational deficits in depression through the analysis of the time-frequency changes in response to emotional stimuli from the International Affective Picture System (IAPS) library39, according to the capability model of alpha asymmetries. So far, affective pictures processing in a passive viewing task has been assessed mostly with regard to distinct components of the event-related potentials (ERPs). In particular, compared with low-arousing neutral stimuli, high-arousing pleasant and unpleasant stimuli typically elicit larger P3 and late positive potential (LPP) amplitudes over the centro-parietal regions in the 300–700 ms time window, which are thought to reflect attentional processing of emotional stimuli40–42. In the present study, pictures (pleasant, neutral and unpleasant) were selected to elicit robust P3/LPP complex, serving as an experimental manipulation check. The group with dysphoria was expected to show a smaller decrease in event-related alpha power (i.e., a reduced alpha desynchronization) in the left frontal and the right posterior regions in response to pleasant (but not neutral and unpleasant) pictures compared to controls, as a correlate of reduced approach-related motivation. Given that the negative potentiation model and the ECI model make two opposite predictions with respect to depression-related emotional reactivity in response to negative stimuli, no a priori hypothesis was formulated regarding the direction of changes in event-related alpha power in response to unpleasant pictures.
Methods
Participants
The method used to recruit participants was based on that described in a previous study by Messerotti Benvenuti et al.10 Specifically, in order to preliminary identify participants with dysphoria, 197 undergraduate students from the University of Padua completed an online version of the Beck Depression Inventory-II (BDI-II43; Italian version by Ghisi et al.44). The BDI-II is a reliable and valid self-report questionnaire that evaluates the severity of symptoms of depression in the past two weeks. Answers are given on a four-point (0–3) Likert scale and scores range from 0 to 63, with the higher scores indicating more severe depressive symptoms. In the Italian version, a score of 12 has been reported as the optimal cut-off score to discriminate individuals with and without depressive symptoms44. Participants scoring equal to or greater than 12 on the online version of BDI-II (n = 77) were invited to participate in the study and were administered a paper-and-pencil version of the BDI-II and the mood episode module (module A) of the Structured Clinical Interview for the DSM-IV Axis I (SCID-I45; Italian version by Mazzi et al.46) approximately one week after the initial screening. The module A of the SCID-I was administered to confirm the presence of dysphoria and to exclude individuals with major depression, dysthymia or bipolar disorder. The module A of the SCID-I was administered by a trained psychologist who had previous experience with administering structured clinical interviews. Twenty-three participants [22 females and 1 male; age, mean (M) = 21.9, standard deviation (SD) = 2.2; BDI-II score, M = 17.3, SD = 4.4], who scored equal to or greater than 12 on both versions of the BDI-II and had at least two current depressive symptoms, at least two weeks in duration, without meeting the diagnostic criteria for major depression, dysthymia or bipolar disorder, were assigned to the group with dysphoria. In order to ensure separation between groups with and without dysphoria, we selected 24 individuals without dysphoria [23 female and 1 male; age, M = 22.0, SD = 1.9; BDI-II score, M = 2.6, SD = 1.9] with an online BDI-II score ≤8 (corresponding to the 45° percentile) and confirmed in the subsequent administration of the paper-and-pencil version of the BDI-II. Participants who scored between 9 and 11 either on the online or the paper-and-pencil BDI-II, or had at least one depressive symptom as evaluated by the SCID-I interview were excluded from the present study.
All the participants enrolled in the present study met the following inclusion criteria as assessed by an ad-hoc interview: 1) being medically healthy, and 2) being free of psychotropic medications. With respect to demographic variables, the two groups (with dysphoria, without dysphoria) did not differ in terms of gender (Fisher’s exact test, p = 0.99) and age, F(1,45) = 0.05, p = 0.83, η2p = 0.00. The group with dysphoria showed significantly higher BDI-II scores than the group without dysphoria, F(1,45) = 226.69, p < 0.001, η2p = 0.83.
Ethics statement and informed consent
The present study was conducted with the adequate understanding and written consent of the participants in accordance with the Declaration of Helsinki. The study was approved by the local Ethics Committee, University of Padua (prot. No. 2101), and written informed consent was obtained from each participant enrolled in the study.
Stimuli and procedure
Participants were presented seventy-two pictures selected from the IAPS39, divided into three categories: 24 pleasant (e.g., erotic scenes, sports), 24 neutral (e.g., neutral faces, household objects), and 24 unpleasant (e.g., attacking humans and animals). The pictures were selected on the basis of their standardized ratings of affective arousal and valence. The mean (SD) normative valence ratings were 7.0 (0.5), 4.9 (0.3) and 2.9 (0.7) for pleasant, neutral and unpleasant pictures, respectively. The mean (SD) normative arousal ratings were 6.5 (0.4), 2.9 (0.7) and 6.5 (0.5) for pleasant, neutral and unpleasant pictures, respectively. Pleasant and unpleasant stimuli were matched for arousal (p = 0.92). The IAPS picture numbers were 1050, 1114, 1120, 1300, 1302, 1930, 1932, 3500, 4611, 4647, 4651, 4652, 4660, 4664, 4670, 4680, 4683, 4690, 4695, 4810, 6200, 6210, 6230, 6242, 6243, 6244, 6250, 6260, 6312, 6313, 6370, 6510, 6540, 6550, 6560, 7000, 7002, 7004, 7009, 7010, 7020, 7035, 7036, 7041, 7050, 7056, 7059, 7130, 7175, 7224, 7233, 7242, 7491, 7500, 7547, 7560, 7595, 7700, 7950, 8030, 8031, 8034, 8080, 8161, 8180, 8185, 8186, 8200, 8370, 8400, 8490, 9425.
Pictures were presented for 6,000 ms each in a semi-randomized sequence (i.e., no more than one stimulus in the same emotional condition had to be shown consecutively). Each picture was preceded by a 3,000-ms gray interval with a white fixation-cross placed centrally on the screen. In order to ensure that participants processed each picture’s content, they were required to look at the central fixation-cross and keep their gaze on the center of the screen. An acoustic startle probe was presented at one of four intervals (i.e., 300, 1500, 3500 or 4500 ms after picture onset) on each trial, thus providing 6 data points for each time condition within each emotional category. The data analysis did not include trials on which a startle probe was delivered at 300 ms after pictures onset. Therefore, six stimuli for each emotional condition were excluded from the analysis. The startle reflex (and heart rate) data are not presented here. The inter-stimulus interval was randomly varied between 6,000 and 8,000 ms. The task was presented by a Pentium IV computer on a 19-in. computer screen, using E-prime 2.0 presentation software (Psychology Software Tools, Pittsburgh, PA, USA).
According to the procedure reported in a previous study by Messerotti Benvenuti et al.10, upon arrival at the laboratory, the participants were first administered a paper-and-pencil version of the BDI-II and the mood episode module (module A) of the SCID-I interview. Then, participants were seated 100 cm away from the computer monitor, in a dimly lit, sound-attenuated room. After the sensors were attached, six practice trials including two pleasant, two neutral, and two unpleasant pictures were provided. Then, each participant performed the emotional passive viewing task.
At the end of the passive viewing task, 36 pictures (12 for each emotional category) were presented again in a randomized sequence, and ratings of emotional valence and arousal were obtained using a two computerized 9-point Self-Assessment Manikin (SAM) scales47. The SAM uses manikin figures for both valence and arousal dimensions. On the valence dimension, the SAM figures range from a frowning-unhappy figure (1, very unpleasant) to a smiling-happy figure (9, very pleasant). On the arousal dimension, the SAM figures range from a static-eyes-closed figure (1, very calm) to an active-wide-eyed figure (9, very aroused). Following completion of the self-evaluation of emotional valence and arousal, the participants were fully debriefed. The entire procedure lasted approximately 90 min.
Electroencephalographic recordings
The EEG was recorded from 32 scalp sites using an elastic cap with tin electrodes (Waveguard EEG cap, ANT Neuro, Enschede, Netherlands). The EEG sites were FP1, FPz, FP2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, POz, O1, Oz, O2, A1 (left mastoid) and A2 (right mastoid), all referenced online to CPz. To control for eye movements and eye blinks, vertical and horizontal electrooculograms (EOGs) were recorded using bipolar montages. Electrode pairs were placed at the supra- and suborbital right eye and at the external canthi of the eyes. Electrode impedance was kept below 10 kΩ. The EEG and EOG signals were amplified with eego amplifier (ANT Neuro, Enschede, Netherlands), bandpass filtered (0.3–40 Hz), and digitized at 1000 Hz.
Data preprocessing
The EEG signal was downsampled to 500 Hz and re-referenced offline to a linked mastoids montage. The EEG was filtered offline with a low-pass filter at 30 Hz and manually corrected for blink artifacts using independent component analysis (ICA) as implemented in EEGLAB48. Further processing was conducted in Brainstorm49. The EEG was then segmented into 4,000 epochs, from 2,000 ms before to 2,000 ms after stimulus onset, in order to prevent boundary effects. Each epoch was baseline-corrected by subtracting the mean pre-stimulus voltage between −252 ms and −52 ms. Then, segments containing residual artifacts exceeding ±70 μV (peak-to-peak) were excluded. The artifact rejection led to an average (SD) acceptance for the ERP and for the time-frequency analyses of 17.0 (1.2) pleasant trials, 17.0 (1.1) neutral trials and 17.1 (1.0) unpleasant trials in the group with dysphoria, and of 16.6 (1.1) pleasant trials, 16.8 (1.2) neutral trials and 17.0 (0.9) unpleasant trials in the group without dysphoria. No significant differences between groups or among emotional conditions in the average acceptance of pleasant, neutral and unpleasant trials were noted (all ps > 0.25).
Event-related potentials (ERPs)
ERPs were calculated by averaging EEG epochs in the time domain separately for each participant and emotional condition.
Time-frequency analysis
With respect to the time-frequency analysis, Morlet wavelet transformation on individual trials was applied for each 1 Hz frequency bin between 1 and 20 Hz, using a mother wavelet at 1 Hz with 2-s time resolution (as calculated by the full width at half maximum; FWHM). Time-frequency decompositions were then averaged for each subject and emotional condition, and the event-related spectral perturbation (ERSP) was computed as the change in power expressed in decibels (dB) relative to the baseline (−500 to −52 ms) in each frequency bin at each time point. Then, data were grand averaged across participants with dysphoria and across participants without dysphoria for each emotional condition.
Statistical analysis
Self-report data
Separate mixed analyses of variance (ANOVAs) with Group (with dysphoria, without dysphoria) as a between-subjects factor, and Category (pleasant, neutral, unpleasant) as a within-subjects factor, were conducted on self-reported valence and arousal. The corrected p-values for effects involving within-subjects variables with more than two levels are reported together with the Greenhouse-Geisser epsilon (ε) and the uncorrected degrees of freedom. Significant main effects and/or interactions (p < 0.05) were followed by Tukey HSD post-hoc tests in order to correct for multiple comparisons. Cohen’s d (absolute value) for relevant comparisons was calculated as a measure of the effect size. All effect sizes, corrected for the sample bias50, are reported with 95% confidence intervals (CIs) and were considered significant if the CIs did not overlap zero.
EEG data: general method
In order to perform statistical analysis on EEG data, a cluster-based approach has been conducted to control over the type I error rate arising from multiple comparisons across electrodes and time points51. Statistical tests were run across electrodes and time points; the resulting values were thresholded and the differences among emotional conditions or groups were shuffled pseudo-randomly 2000 times. The maximal cluster-level statistics (i.e., the sum of values across contiguously significant electrodes and time points at the threshold level) were extracted for each shuffle to compute a ‘null’ distribution of effect sizes. For each significant cluster in the original (non-shuffled) data, it was computed the proportion of clusters in the null distribution whose statistics exceeded the one obtained for the cluster in question, corresponding to its cluster-corrected p-value. Clusters with a pcorr < 0.05 were considered statistically significant.
ERP data: Repeated measures ANOVAs over all electrodes and time-points in the −100 to 700 ms interval were employed to test differences in ERP amplitudes among emotional conditions (Category: pleasant, neutral, unpleasant), with the group variable collapsed. An initial conservative alpha of 0.001 was employed to threshold the matrices due to the expected large effect of emotional category on P3/LPP complex, in order to highlight the electrodes and time points where the difference was more prominent (note that this value does not affect the false alarm rate of the statistical test at the cluster-level51).
When the time window was identified, a second cluster-based analysis was run to test the differences between groups within each emotion category. In this analysis, a two-tailed unpaired t-test on the ERP amplitude averaged over the significant time window was conducted across electrodes for each emotional condition.
Time-frequency data: A similar cluster-based analysis was conducted on event-related alpha power (8–13 Hz), with a −100–1400 ms time window and a pthresh = 0.05. Then, in order to perform analysis at the group level, the same cluster-based approach (statistic = one-tailed unpaired t-test) on the event-related alpha power averaged over the significant time points was conducted across electrodes for each emotional category. One-tailed t-test was used based on an a priori hypothesis about the direction of the difference between groups in event-related alpha power in response to pleasant stimuli.
In order to control for the specificity of the effects on alpha, the same statistical approach was conducted on event-related changes in delta (1–3 Hz), theta (4–7 Hz), and beta (14–20 Hz) power, using a two-tailed unpaired t-test.
Results
Self-report data
The mixed ANOVA on valence ratings yielded a significant main effect for Category, F(2,90) = 280.76, p < 0.001, ε = 0.85, ηp2 = 0.86. Unpleasant pictures were evaluated as significantly more unpleasant than neutral (p < 0.001; d = 3.36, 95% CI = 2.73, 3.99) and pleasant (p < 0.001; d = 4.11, 95% CI = 3.40, 4.82) pictures. Pleasant stimuli were rated as significantly more pleasant than neutral ones (p < 0.001; d = 1.49, 95% CI = 1.04, 1.95). No significant main effect for Group or Group × Category interaction was found (all ps > 0.11). Similarly, the ANOVA on arousal ratings revealed a significant main effect for Category F(2,90) = 114.89, p < 0.001, ε = 0.83, ηp2 = 0.72. Specifically, arousal ratings were higher for both pleasant and unpleasant pictures compared to neutral ones (pleasant vs. neutral: p < 0.001; d = 1.46, 95% CI = 1.01, 1.92; unpleasant vs. neutral: p < 0.001; d = 1.96, 95% CI = 1.47, 2.46). Unpleasant pictures were rated as more arousing than pleasant stimuli (p < 0.001; d = 0.54, 95% CI = 0.12, 0.95). However, it is important to note that this effect was driven by participants with dysphoria (d = 0.84, 95% CI = 0.24, 1.44) instead of controls, in which the effect size of the difference between the pleasant and unpleasant stimuli was small (d = 0.31, 95% CI = −0.26, 0.88). No significant main effect for Group or Group × Category interaction was found (all ps > 0.18). The descriptive statistics of self-report measures are reported in Table 1.
Table 1.
Ratings of each self-report measure in the group with dysphoria and in the group without dysphoria.
| Self-report measure | Group with dysphoria (n = 23) | Group without dysphoria (n = 24) | ||||
|---|---|---|---|---|---|---|
| Pleasant | Neutral | Unpleasant | Pleasant | Neutral | Unpleasant | |
| Valence | 6.3 (1.0) | 5.3 (0.5) | 2.7 (1.2) | 6.7 (0.8) | 5.3 (0.7) | 2.4 (0.8) |
| Arousal | 5.1 (1.4) | 2.8 (1.7) | 6.4 (1.5) | 4.9 (1.8) | 2.6 (1.5) | 5.5 (1.8) |
Note. Data are M (SD).
ERP data
Differences among emotional categories
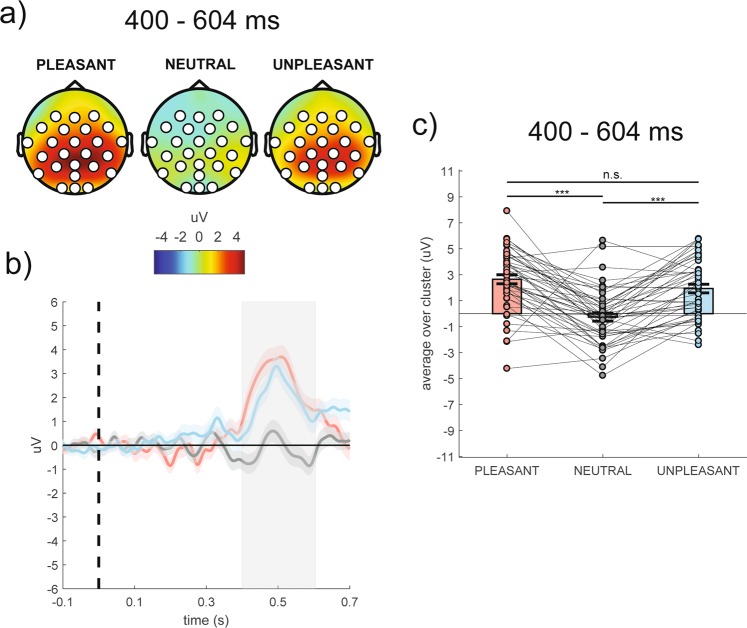
The cluster-based analysis on ERP data showed a significant positive fronto-centro-parieto-occipital cluster (cluster F-valuemax = 63009.06, pcorr < 0.001, time window = 400–604 ms, electrodes = F3, Fz, F4, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, PZ, P4, P8, POz, O1, Oz, O2), as shown in Fig. 1 (panel a). Specifically, the whole group revealed a significantly larger P3/LPP complex in response to pleasant and unpleasant stimuli than neutral ones (all ps < 0.001; Fig. 1, panels b,c), especially at central, centro-parietal and parietal scalp sites (Fig. 1, panel a).
Figure 1.
(Panel a) Topography of the mean ERP amplitude (μV) averaged over the significant time points (400–604 ms time window) for pleasant, neutral and unpleasant conditions. (Panel b) Time course of grand-average ERP waveforms averaged over the significant electrodes for pleasant (red line), neutral (grey line) and unpleasant (light blue line) conditions. Shaded areas represent ± standard error of the mean (SEM); the colored frame represents the significant time window (400–604 ms). (Panel c) Mean ERP amplitude of each participant averaged over the significant electrodes and time points for pleasant, neutral and unpleasant conditions. Each circle represents one participant; colored frames represent the mean ERP amplitude across all participants and the solid black lines represent ± SEM. ***p < 0.001.
Differences between groups for each emotional category
Unpaired t-test conducted on the P3/LPP amplitude averaged over the 400–604 ms time window, where the effect of emotion emerged in the previous analysis, did not reveal any significant cluster for the difference between the groups within each emotional condition.
Time-frequency data
Differences among emotional categories in event-related alpha power
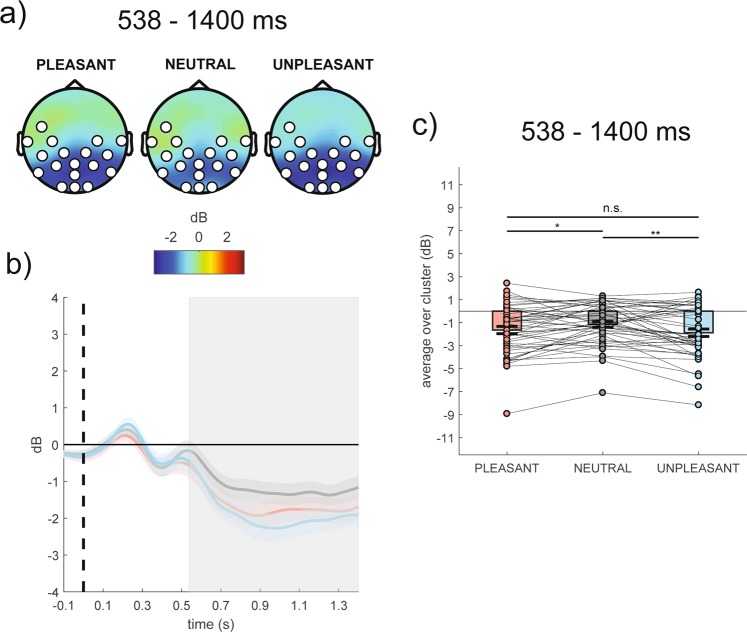
In the whole group, the cluster-based analysis on event-related alpha power revealed a significant positive centro-parieto-occipital cluster (cluster F-valuemax = 37097.61, pcorr = 0.01, time window = 538–1400 ms, electrodes = FC5, T7, C3, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, POz, O1, Oz, O2), as shown in Fig. 2 (panel a). In particular, reduced event-related alpha power was evident in response to pleasant and unpleasant stimuli compared to neutral ones (all ps < 0.05), as shown in Fig. 2 (panels b,c).
Figure 2.
(Panel a) Topography of the mean event-related alpha power (dB) averaged over the significant time points (538–1400 ms time window) for pleasant, neutral and unpleasant conditions. (Panel b) Time course of grand-average event-related alpha power averaged over the significant electrodes for pleasant (red line), neutral (grey line) and unpleasant (light blue line) conditions. Shaded areas represent ± standard error of the mean (SEM); the colored frame represents the significant time window (538–1400 ms). (Panel c) Mean event-related alpha power of each participant averaged over the significant electrodes and time points for pleasant, neutral and unpleasant conditions. Each circle represents one participant; colored frames represent the mean event-related alpha power across all participants and the solid black lines represent ± SEM. *p < 0.05; **p < 0.01.
Differences between groups in event-related alpha power for each emotional category
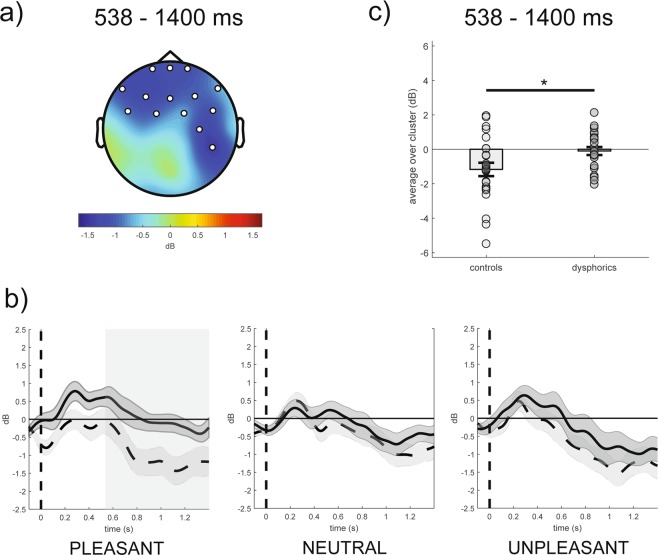
With respect to the differences between groups for each emotional condition in the 538–1400 ms time window, the cluster-based analysis on event-related alpha power showed a significant negative fronto-centro-parietal cluster in the pleasant condition (cluster t-valuemax = −29.24, pcorr = 0.02, electrodes = FP1, FPz, FP2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, C4, CP6), as shown in Fig. 3 (panel a). Specifically, the group without dysphoria revealed a larger decrease in event-related alpha power in response to pleasant stimuli than the group with dysphoria (Fig. 3, panels b,c). It is intriguing to note that at central and centro-parietal scalp sites, the difference between groups in response to pleasant stimuli was lateralized to the right hemisphere, whereas no lateralization was observed at frontal and fronto-central scalp sites (Fig. 3, panel a). It is also worth noting that no significant differences between groups in response to neutral and unpleasant stimuli were noted (Fig. 3, panel b).
Figure 3.
(Panel a) Topography of the mean difference between groups in event-related alpha power (dB; group without dysphoria minus group with dysphoria) averaged over the significant time points (538–1400 ms time window) for the pleasant condition. (Panel b) Time course of grand-average event-related alpha power averaged over the significant electrodes for pleasant, neutral and unpleasant conditions in the group with dysphoria (solid line) and in the group without dysphoria (dashed line). Shaded areas represent ± standard error of the mean (SEM); the colored frame represents the significant time window (538–1400 ms). (Panel c) Mean event-related alpha power of each participant in the group with dysphoria and in the group without dysphoria (i.e., controls) averaged over the significant electrodes and time points for the pleasant condition. Each circle represents one participant; the frames represent the mean event-related alpha power across all participants in the group with dysphoria and in the group without dysphoria and the solid black lines represent ± SEM. *p < 0.05.
Differences between groups in event-related delta, theta and beta power for each emotional category
Unpaired t-test conducted on event-related power of other EEG frequency bands averaged over the significant time windows (delta: 100–972 ms; theta: 186–724 ms; beta: 750–1064 ms), where the effect of emotion emerged in the ANOVAs, did not reveal any significant cluster for the difference between the groups within each emotional condition.
Discussion
The present study investigated motivational deficits in depression during the passive viewing of emotional pictures, according to the capability model of alpha asymmetries. A time-frequency approach was used to examine event-related changes in alpha power in individuals with dysphoria vs. healthy controls with high temporal resolution. Based on previous literature reporting reduced approach-related motivation in depression10–12,33,35,37, individuals with dysphoria were expected to show less alpha desynchronization in the left frontal and the right posterior regions in response to pleasant pictures compared to controls.
In line with our hypothesis, individuals with dysphoria showed less alpha desynchronization in response to pleasant stimuli than the group without dysphoria. The effect was evident between 538–1400 ms post-stimulus and was seen at frontal, fronto-central, central and centro-parietal scalp sites. It is important to note that event-related changes in alpha power in response to unpleasant and neutral stimuli were comparable between the two groups. In addition, no difference between groups emerged for delta, theta and beta power, indicating that the present findings were specific for alpha power. Accordingly, these results suggest that depression is characterized by reduced activation of the appetitive (approach) motivational system8,10,11.
However, only partially in line with our hypothesis, the difference between groups in response to pleasant stimuli was lateralized to the right hemisphere at centro-parietal scalp sites, whereas no evident lateralization was observed at frontal and fronto-central scalp sites. The present result reflects a reduced cortical activation over bilateral anterior and right-lateralized centro-parietal regions during the processing of pleasant stimuli in individuals with dysphoria as compared to controls. In turn, this suggests that a decreased cortical activation in a network involving bilateral frontal and right-lateralized parietal regions may provide a specific measure of deficits in the Approach Motivation construct within the Positive Valence Systems proposed by the NIMH Research Domain Criteria (RDoC)52.
Along the same line of reasoning, the present results provide support for the capability model of individual differences in alpha asymmetry at posterior, but not at anterior scalp sites. It can be suggested that lateralized event-related alpha power at central and centro-parietal scalp sites is more likely to reflect depression-related deficits in the processing of motivationally relevant stimuli than frontal alpha asymmetry. However, the latter result is at odds with findings of previous studies reporting that frontal alpha asymmetry discriminates individuals with depression from healthy controls in approach-related and withdrawal-related conditions29,30. An explanation for the discrepant findings may lie in the fact that changes in event-related alpha power were examined in the millisecond range, whereas other studies typically computed alpha activity (and its asymmetries) over longer periods of time29. It is worth noting that the effect reported here was robust because the cluster-based analysis allows to correct for multiple comparisons across electrodes and time points.
In the whole group, the present study showed greater alpha desynchronization in response to high-arousing emotional (pleasant and unpleasant) compared to low-arousing neutral stimuli in the 538–1400 ms time window, suggesting that decrease in event-related alpha power may reflect arousal dimension. This interpretation is consistent with recent findings showing a greater alpha desynchronization in response to high-arousing (i.e., erotic) rather than low-arousing (i.e., romantic) pleasant pictures between 600–1000 ms post-stimulus at anterior and posterior scalp sites53. Similarly, a decrease in event-related alpha power has been reported to be associated with higher arousal for both pleasant and unpleasant stimuli, with the largest alpha desynchronization occurring in response to erotic and mutilation pictures54. It has to be noted that results regarding changes in event-related alpha power in response to emotional stimuli have also reported null findings55 or opposite effects (alpha synchronization56,57). However, these studies varied remarkably in terms of critical methodological aspects such as central or lateralized presentation of the stimuli, data analysis technique to calculate alpha oscillations, limited number of sensors and picture exposure duration.
In addition, in the whole group ERP results showed the presence of the P3/LPP complex, with larger amplitude occurring in response to pleasant and unpleasant than neutral stimuli at centro-parietal and parietal scalp sites in the 400–604 ms time window. Replicating P3/LPP modulations to high-arousing emotional stimuli compared to low-arousing neutral stimuli with predicted polarity, topography and latency confirmed the effectiveness of the experimental manipulation. It is well-established that P3/LPP complex reflects continued allocation of attention to emotional stimuli and facilitated processing and encoding of motivationally relevant stimuli (for a review, see Lang & Bradley40). Our data are in line with those of previous studies showing that LPP and alpha desynchronization may reflect similar processes54. However, it is worth noting that the group with dysphoria and the group without did not differ in P3/LPP amplitude in none of the three emotional conditions. In turn, our data suggest that alpha desynchronization over specific brain regions and at specific latencies may represent a more sensitive measure of depression-related motivational deficits than P3/LPP complex. This suggestion is consistent with the notion that event-related oscillations not only reflect stimulus-evoked oscillations similar to the ERPs but also induced oscillations, which are not phase-locked to the stimulus event. It is therefore possible that event-related oscillations may carry important information about emotional processing, which is not represented in the ERPs58. Further studies are needed to test differences in emotional processing reflected by ERP and time-frequency analyses.
At the subjective level, self-report measures of valence and arousal did not differ between the group with dysphoria and the control group, in line with previous studies in participants with subclinical depressive symptoms59,60 or in patients with clinically significant depression61,62 (but see also Sloan et al.63). An explanation for this null finding is that emotional experience was assessed according to a dimensional model of affective space instead of a discrete emotion model, which may best capture depression-related differences at the subjective level (see Rottenberg et al.3). Otherwise, it can be suggested that decreased bilateral frontal and right-sided posterior activation may precede alterations in subjective reports of emotional experience and therefore provide an early measure of deficits in the appetitive motivational system in individuals with dysphoria.
With respect to clinical implications, the current data show that individuals with dysphoria are characterized by under-engagement of appetitive rather than over-engagement of aversive motivational system7,10,64. In line with this finding, there is recent evidence showing that depressed mood may improve through interventions specifically aimed at increasing appetitive motivation65. It can be also suggested that underactivation of the approach-related motivational system in at-risk individuals (e.g., with dysphoria) may be involved as a risk factor for the development of a full-blown depressive episode. Consistent with this suggestion, the clinical manifestation and the course of depression have been reported to be worsened by underactivation of the appetitive motivational system66. However, longitudinal studies are needed to test whether decreased approach-related motivational drive may play a role in the transition from dysphoria to major depression.
The present study suffers from some methodological shortcomings. First, the sample size included in this study was relatively small and, second, it was composed almost exclusively by females. Therefore, the current findings need to be replicated and extended to males in order to increase their generalizability. Third, whether participants included in the study met the criteria for major depression, dysthymia or bipolar disorder in the past was not investigated. Although having a history of major depression is unlikely to have affected the results obtained in the group with dysphoria because the effect of current and past depression on alpha asymmetry is expected to be the same, it might have partially confounded data obtained in healthy controls. It should be noted, however, that 12-month period prevalence of depression range from 1% to 3% in pre-pubertal children and post-pubertal adolescents67. According to this prevalence rate, and given that only university students were enrolled, the likelihood of having a history of clinically significant depression in participants assigned to healthy control group was very low. Therefore, the potential confounding effect of having a history of major depression was limited in the present study. Lastly, the module A of the SCID-I interview was administered by only one trained psychologist, which prevented us from evaluating the inter-rater reliability. Nonetheless, a high inter-rater reliability has been previously reported for the majority of the disorders assessed by the SCID-I interview68.
To the best of our knowledge, this is the first study investigating motivational deficits in depression using a time-frequency approach, according to the capability model of alpha asymmetries. The excellent temporal resolution of this approach gave us the opportunity to use discrete, short-lasting emotional stimuli needed to elicit a strong activation of the approach- and withdrawal-related motivational systems, as proposed by the capability model. In other words, the time-frequency approach allowed us to go beyond the measurement of a trait-like deficit in approach motivation, detailing how depressed mood affects transient motivational responses.
In conclusion, the results obtained in the present study support the notion that individuals with dysphoria are more likely to be under-engaged in processing approach- than avoidance-related motivationally stimuli rather than the opposite pattern. Most importantly, these novel results add to the existing literature by suggesting that transient reduction in alpha desynchronization involving bilateral frontal and right-lateralized parietal regions may reflect deficits in the approach-related motivational system in depression.
Acknowledgements
The study was supported by a grant from MIUR (Dipartimenti di Eccellenza DM 11/05/2017 n. 262) to the Department of General Psychology, University of Padua.
Author contributions
S.M.B., G.B. and D.P. conceived and designed the study; S.M.B., G.B., R.M. and E.D.B. conducted the study; R.M. contributed to methodological and analytic tools; S.M.B., R.M. and E.D.B. analyzed the data; S.M.B., G.B. and R.M. wrote the paper, and all authors reviewed the manuscript.
Data availability
All data and MATLAB code will be made available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed. (American Psychiatric Association, Arlington, VA, 2013).
- 2.Clark DA, Beck AT. Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends Cogn. Sci. 2010;14:418–424. doi: 10.1016/j.tics.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. J. Abnorm. Psychol. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- 4.Beeney JE, Levy KN, Gatzke-Kopp LM, Hallquist MN. EEG asymmetry in borderline personality disorder and depression following rejection. Personal. Disord. 2014;5:178–85. doi: 10.1037/per0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golin S, Hartman SA, Klatt EN, Munz K, Wolfgang GL. Effects of self-esteem manipulation on arousal and reactions to sad models in depressed and nondepressed college students. J. Abnorm. Psychol. 1977;86:435–439. doi: 10.1037/0021-843X.86.4.435. [DOI] [PubMed] [Google Scholar]
- 6.Lewinsohn PM, Lobitz WC, Wilson S. Sensitivity of depressed individuals to aversive stimuli. J. Abnorm. Psychol. 1973;81:259–263. doi: 10.1037/h0034529. [DOI] [PubMed] [Google Scholar]
- 7.Messerotti Benvenuti S, Mennella R, Buodo G, Palomba D. Dysphoria is associated with reduced cardiac vagal withdrawal during the imagery of pleasant scripts: Evidence for the positive attenuation hypothesis. Biol. Psychol. 2015;106:28–38. doi: 10.1016/j.biopsycho.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Messerotti Benvenuti S, Mennella R, Buodo G, Palomba D. Frontal theta activity as an EEG correlate of mood-related emotional processing in dysphoria. J. Psychopathol. Behav. Assess. 2017;39:241–252. doi: 10.1007/s10862-016-9572-8. [DOI] [Google Scholar]
- 9.Mennella R, Messerotti Benvenuti S, Buodo G, Palomba D. Emotional modulation of alpha asymmetry in dysphoria: Results from an emotional imagery task. Int. J. Psychophysiol. 2015;97:113–119. doi: 10.1016/j.ijpsycho.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Messerotti Benvenuti S, Buodo G, Palomba D. Appetitive and aversive motivation in dysphoria: A time-domain and time-frequency study of response inhibition. Biol. Psychol. 2017;125:12–27. doi: 10.1016/j.biopsycho.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Nusslock R, Walden K, Harmon-Jones E. Asymmetrical frontal cortical activity associated with differential risk for mood and anxiety disorder symptoms: An RDoC perspective. Int. J. Psychophysiol. 2015;98:249–261. doi: 10.1016/j.ijpsycho.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Curr. Opin. Psychol. 2015;4:114–118. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rottenberg J. Mood and emotion in major depression. Curr. Dir. Psychol. Sci. 2005;14:167–170. doi: 10.1111/j.0963-7214.2005.00354.x. [DOI] [Google Scholar]
- 14.Rottenberg, J. Major depressive disorder: Emerging evidence for emotion context insensitivity. In Emotion and psychopathology: Bridging affective and clinical science (eds Rottenberg, J. & Johnson, S. L.) 151–165 (American Psychological Society, Washington DC, 2007).
- 15.Gotlib IH. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cogn. Emot. 1998;12:449–478. doi: 10.1080/026999398379673. [DOI] [Google Scholar]
- 16.Schaffer CE, Davidson RJ, Saron C. Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biol. Psychiatry. 1983;18:753–762. [PubMed] [Google Scholar]
- 17.Allen JJB, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41:269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 18.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J. Abnorm. Psychol. 1991;100:535–545. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- 19.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J. Abnorm. Psychol. 1990;99:22–31. doi: 10.1037//0021-843X.99.1.22. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. J. Abnorm. Psychol. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen JJB, Keune PM, Schönenberg M, Nusslock R. Frontal EEG alpha asymmetry and emotion: From neural underpinnings and methodological considerations to psychopathology and social cognition. Psychophysiology. 2018;55:1–6. doi: 10.1111/psyp.13028. [DOI] [PubMed] [Google Scholar]
- 22.Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 1998;35:607–614. doi: 10.1017/S0048577298000134. [DOI] [PubMed] [Google Scholar]
- 24.Harmon-Jones E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40:838–848. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- 25.Reznik SJ, Allen JJB. Frontal asymmetry as a mediator and moderator of emotion: An updated review. Psychophysiology. 2018;55:e12965. doi: 10.1111/psyp.12965. [DOI] [PubMed] [Google Scholar]
- 26.Smith EE, Cavanagh JF, Allen JJB. Intracranial source activity (eLORETA) related to scalp-level asymmetry scores and depression status. Psychophysiology. 2018;55:e13019. doi: 10.1111/psyp.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Vinne N, Vollebregt MA, van Putten MJAM, Arns M. Frontal alpha asymmetry as a diagnostic marker in depression: Fact or fiction? A meta-analysis. Neuroimage Clin. 2017;16:79–87. doi: 10.1016/j.nicl.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coan JA, Allen JJB, Mcknight PE. A capability model of individual differences in frontal EEG asymmetry. Biol. Psychol. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart JL, Coan JA, Towers DN, Allen JJB. Resting and task-elicited prefrontal EEG alpha asymmetry in depression: Support for the capability model. Psychophysiology. 2014;51:446–455. doi: 10.1111/psyp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson BD, Kessel EM, Klein DN, Shankman SA. Depression symptom dimensions and asymmetrical frontal cortical activity while anticipating reward. Psychophysiology. 2018;55:1–14. doi: 10.1111/psyp.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart JL, Coan JA, Towers DN, Allen JJB. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. J. Affect. Disord. 2011;129:167–174. doi: 10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen JJB, Cohen MX. Deconstructing the “resting” state: Exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression. Front. Hum. Neurosci. 2010;4:232. doi: 10.3389/fnhum.2010.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart JL, Towers DN, Coan JA, Allen JJB. The oft-neglected role of parietal EEG asymmetry and risk for major depressive disorder. Psychophysiology. 2011;48:82–95. doi: 10.1111/j.1469-8986.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruder GE, Tenke CE, Warner V, Nomura Y. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol. Psychiatry. 2005;57:328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Bruder, G. E. Frontal and parietotemporal asymmetries in depressive disorders: Behavioral, electrophysiologic and neuroimaging findings. In The asymmetrical brain (eds Hugdahl, K. & Davidson, R. J.) 719–742 (MIT Press, Cambridge, MA, 2003).
- 36.Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7:476–489. doi: 10.1037/0894-4105.7.4.476. [DOI] [Google Scholar]
- 37.Heller W, Nitscke JB. Regional brain activity in emotion: A framework for understanding cognition in depresion. Cogn. Emot. 1997;11:637–661. doi: 10.1080/026999397379845a. [DOI] [Google Scholar]
- 38.Mennella R, et al. The two faces of avoidance: Time-frequency correlates of motivational disposition in blood phobia. Psychophysiology. 2017;54:1606–1620. doi: 10.1111/psyp.12904. [DOI] [PubMed] [Google Scholar]
- 39.Lang, P. J., Bradley, M. M. & Cuthbert, B. N. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical report A-8. (University of Florida, Gainesville, FL, 2008).
- 40.Lang PJ, Bradley MM. Emotion and the motivational brain. Biol. Psychol. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: Event‐related brain potential studies. Prog. Brain Res. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- 42.Messerotti Benvenuti S, Bianchin M, Angrilli A. Posture affects emotional responses: A Head Down Bed Rest and ERP study. Brain Cogn. 2013;82:313–318. doi: 10.1016/j.bandc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Beck, A. T., Steer, R. A. & Brown, G. K. (1996). Beck depression inventory. Second edition manual. (Psychological Corporation, San Antonio, TX, 1996).
- 44.Ghisi, M., Flebus, G. B., Montano, A., Sanavio, E. & Sica, C. Beck depression inventory-II BDI-II. Manuale. (O.S. Organizzazioni Speciali, Firenze, 2006).
- 45.First, M. B., Spitzer, R. L., Gibbon, M. & Williams, J. B. W. Structured clinical interview for DSM-IV Axis I Disorders (SCID I). Clinical version. (American Psychiatric Press, Washington, DC, 1997).
- 46.Mazzi, F., Morosini, P., De Girolamo, G., Lussetti, M. & Guaraldi, G. P. SCID-I Structured Clinical Interview for DSM-IV Axis I Disorders (Italian version). (O.S. Organizzazioni Speciali, Firenze, 2000).
- 47.Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 48.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: A user-friendly application for MEG/EEG. analysis. Comput. Intell. Neurosci. 2011;2011:879716. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hedges, L. V. & Olkin, I. Statistical methods for meta-analysis. (Academic Press, Orlando, 1985).
- 51.Maris E, Oostenveld R. Nonparametric statistical testing of EEG‐ and MEG‐data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 52.Insel T, et al. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 53.Schubring, D. & Schupp, H. T. Affective picture processing: Alpha‐ and lower beta‐band desynchronization reflects emotional arousal. Psychophysiology e13386, 10.1111/psyp.13386 (2019). [DOI] [PubMed]
- 54.de Cesarei A, Codispoti M. Affective modulation of the LPP and α‐ERD during picture viewing. Psychophysiology. 2011;48:1397–1404. doi: 10.1111/j.1469-8986.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- 55.Baumgartner, T., Esslen, M. & Jäncke, L. From emotion perception to emotion experience: Emotions evoked by pictures and classical music. Int. J. Psychophysiol. 60, 34–43, 10.1016/j.ijpsycho.2005.04.007(2006). [DOI] [PubMed]
- 56.Aftanas LI, Reva NV, Varlamov AA, Pavlov SV, Makhnev VP. Analysis of evoked EEG synchronization and desynchronization in conditions of emotional activation in humans: Temporal and topographic characteristics. Neurosci. Behav. Physiol. 2004;34:859–867. doi: 10.1023/B:NEAB.0000038139.39812.eb. [DOI] [PubMed] [Google Scholar]
- 57.Aftanas LI, Varlamov AA, Pavlov SV, Makhnev VP, Reva NV. Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. Int. J. Psychophysiol. 2002;44:67–82. doi: 10.1016/S0167-8760(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 58.Herrmann CS, Rach S, Vosskuhl J, Strüber D. Time–frequency analysis of event-related potentials: A brief tutorial. Brain Topogr. 2014;27:438–450. doi: 10.1007/s10548-013-0327-5. [DOI] [PubMed] [Google Scholar]
- 59.Mneimne M, McDermut W, Powers AS. Affective ratings and startle modulation in people with nonclinical depression. Emotion. 2008;8:552–559. doi: 10.1037/a0012827. [DOI] [PubMed] [Google Scholar]
- 60.Sloan DM, Sandt AR. Depressed mood and emotional responding. Biol. Psychol. 2010;84:368–374. doi: 10.1016/j.biopsycho.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Allen NB, Trinder J, Brennan C. Affective startle modulation in clinical depression: Preliminary findings. Biol. Psychiatry. 1999;46:542–550. doi: 10.1016/S0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 62.Dichter G, Tomarken A, Shelton R, Sutton S. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41:433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- 63.Sloan DM, Strauss ME, Quirk SW, Sajatovic M. Subjective and expressive emotional responses in depression. J. Affect. Disord. 1997;46:135–141. doi: 10.1016/S0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- 64.Buodo G, Mento G, Sarlo M, Palomba D. Neural correlates of attention to emotional facial expressions in dysphoria. Cogn. Emot. 2015;29:604–620. doi: 10.1080/02699931.2014.926862. [DOI] [PubMed] [Google Scholar]
- 65.Strauman TJ, et al. Microinterventions targeting regulatory focus and regulatory fit selectively reduce dysphoric and anxious mood. Behav. Res. Ther. 2015;72:18–29. doi: 10.1016/j.brat.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J. Abnorm. Psychol. 2002;111:589–597. doi: 10.1037//0021-843X.111.4.589. [DOI] [PubMed] [Google Scholar]
- 67.Angold, A., & Costello, E. J. The epidemiology of depression in children and adolescents. In The depressed child and adolescent (ed. Goodyer, I. M.) 143–178 (Cambridge University Press, New York, 2001).
- 68.Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the structured clinical interview for DSM-III-R Axis I (SCID-I) Acta Psychiatr. Scand. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and MATLAB code will be made available upon request.