Abstract
Campylobacter species infections have been associated with malnutrition and intestinal inflammation among children in low-resource settings. However, it remains unclear whether that association is specific to Campylobacter jejuni/coli. The aim of this study was to assess the association between both all Campylobacter species infections and Campylobacter jejuni/coli infections on growth and enteric inflammation in children aged 1–24 months. We analyzed data from 1715 children followed from birth until 24 months of age in the MAL-ED birth cohort study, including detection of Campylobacter species by enzyme immunoassay and Campylobacter jejuni/coli by quantitative PCR in stool samples. Myeloperoxidase (MPO) concentration in stool, used as a quantitative index of enteric inflammation, was measured. The incidence rate per 100 child-months of infections with Campylobacter jejuni/coli and Campylobacter species during 1–24 month follow up were 17.7 and 29.6 respectively. Female sex of child, shorter duration of exclusive breastfeeding, lower maternal age, mother having less than 3 living children, maternal educational level of <6 years, lack of routine treatment of drinking water, and unimproved sanitation were associated with Campylobacter jejuni/coli infection. The cumulative burden of both Campylobacter jejuni/coli infections and Campylobacter species were associated with poor growth and increased intestinal inflammation.
Subject terms: Biological techniques, Infection
Introduction
Campylobacter species are curved, gram-negative bacterial enteropathogens with diverse human and animal reservoirs, which have been associated with linear growth shortfalls in children in low-resource settings1–3. There are more than 25 species of Campylobacter, of which the thermotolerant variants such as Campylobacter jejuni and Campylobacter coli are thought to most commonly infect humans4,5. There are multiple microbiologic approaches for detection of Campylobacter species, including bacterial culture, enzyme immunoassay (EIA), and PCR. While both culture and PCR assays can target Campylobacter jejuni and Campylobacter coli, PCR is substantially more sensitive. The most commonly used EIA tests have been shown to detect a broader range of Campylobacter species6.
Campylobacter infections in young children have been associated with dysentery, diarrhea, and malnutrition1,5,7. Environmental enteric dysfunction is a subclinical intestinal disorder which is highly prevalent in low-resource settings and characterized by intestinal inflammation and alteration in gut structure and function8–10. Myeloperoxidase (MPO) concentration in the stool can be used as a quantitative index of enteric inflammation11 and previous studies suggests MPO as is a simple, noninvasive, and a direct marker of inflammation1,12–14. MPO, an enzyme found in granulocytes, is involved with the release of hypochlorous acid. It induces oxidative tissue damage of host tissue following extracellular phagocytic activation at the inflammatory site, resulting in microbial destruction15,16. The increase in mucosal MPO levels can be used as a biomarker in human patients with inflammatory bowel disease17,18.
The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) study is a birth cohort performed at 8 sites in South America, sub-Saharan Africa, and Asia19. Campylobacter species were originally detected by EIA in this study, and a previous analysis showed a strong association between Campylobacter species infection and growth1. However, the degree to which this association was specific to Campylobacter jejuni and Campylobacter coli, other Campylobacter species, or both remains unclear. Here, we sought to identify risk factors for Campylobacter jejuni/coli infection and assess the association with enteric inflammation and linear growth in children and compare these associations with the burden of infestation by Campylobacter species by EIA.
Results
General characteristics
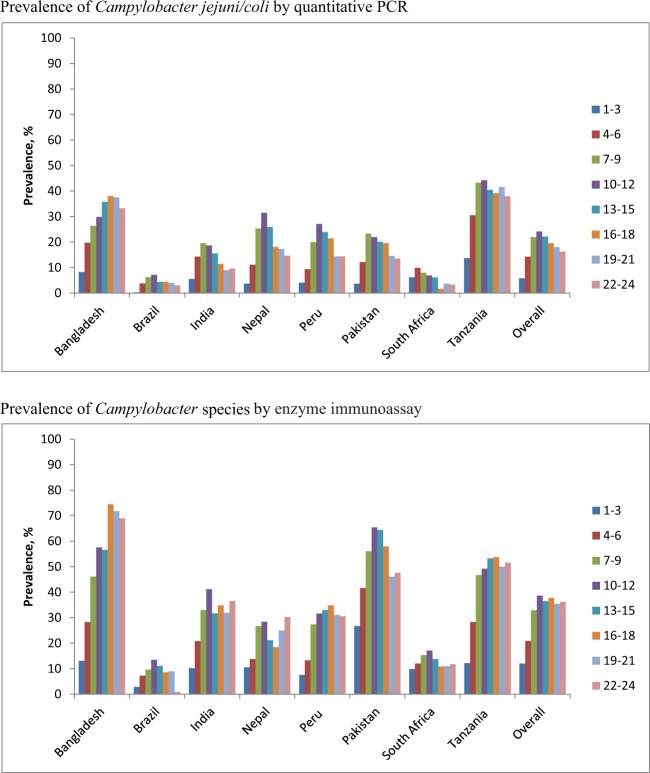
A total of 1715 participants who completed follow-up to 24 months contributed 34,622 surveillance stool samples tested for Campylobacter jejuni/coli by quantitative PCR whereas Campylobacter species done by EIA was tested during 1–12, 15, 18, 21, 24 months on 22,614 surveillance stool samples. The demographic characteristics of the study participants are presented in Table 1. The prevalence of Campylobacter jejuni/coli and Campylobacter species in surveillance stool samples during 1–24 months by sites is shown in Fig. 1. The overall prevalence of Campylobacter species infections was approximately twice that of C. jejuni/coli infections. Both peaked at approximately one year of age and then subsequently declined for C. jejuni/coli and was stable for Campylobacter species. The burden was highest in children at the Bangladesh and Tanzania sites.
Table 1.
General characteristics of the study subjects (n = 1715).
| Characteristics, n (%) | Bangladesh | Brazil | India | Nepal | Peru | Pakistan | South Africa | Tanzania | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Male sex | 108 (51.4) | 89 (53.9) | 105 (46.3) | 122 (53.7) | 105 (54.1) | 120 (48.8) | 120 (50.6) | 105 (50.2) | 874 (51.0) |
| Days of exclusive breastfeeding† | 143.2 ± 42.7 | 93.7 ± 57.8 | 105.4 ± 42.9 | 92.5 ± 54.5 | 89.5 ± 61.3 | 19.9 ± 22.7 | 38.6 ± 26.3 | 62.2 ± 35 | 78.6 ± 57.7 |
| Birth weight (kg)† | 2.8 ± 0.4 | 3.4 ± 0.5 | 2.9 ± 0.4 | 3 ± 0.4 | 3.1 ± 0.4 | 2.7 ± 0.4 | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.0 ± 0.5 |
| Weight for age z-score at Enrollment† | −1.3 ± 0.9 | −0.2 ± 1 | −1.3 ± 1 | −0.9 ± 1 | −0.6 ± 0.9 | −1.4 ± 1 | −0.4 ± 1 | −0.1 ± 0.9 | −0.8 ± 1.1 |
| Length for age z−score at Enrollment† | −0.96 ± 1 | −0.8 ± 1.1 | −1 ± 1.1 | −0.7 ± 1 | −0.9 ± 1 | −1.3 ± 1.1 | −0.7 ± 1 | −1 ± 1.1 | −0.9 ± 1.1 |
| Length for age z-score at 24 month† | −2.0 ± 0.9 | 0 ± 1.1 | −1.9 ± 1 | −1.3 ± 0.9 | −1.9 ± 0.9 | N/A | −1.7 ± 1.1 | −2.7 ± 1 | −1.7 ± 1.2 |
| Maternal age (years)† | 25.0 ± 5.0 | 25.4 ± 5.6 | 23.9 ± 4.2 | 26.6 ± 3.7 | 24.8 ± 6.3 | 28.1 ± 5.9 | 27 ± 7.2 | 29.1 ± 6.5 | 26.3 ± 5.9 |
| Maternal weight (kg) | 49.7 ± 8.5 | 62 ± 11.5 | 50.3 ± 9.3 | 56.2 ± 8.3 | 56.3 ± 9.6 | 50.7 ± 9.6 | 68 ± 15.3 | 55.7 ± 8.8 | 55.9 ± 12 |
| Maternal height (cm)† | 149.0 ± 5.0 | 155.1 ± 6.7 | 151.1 ± 5.2 | 149.7 ± 5.3 | 150.2 ± 5.5 | 153.4 ± 5.7 | 158.7 ± 6.6 | 155.9 ± 5.9 | 152.9 ± 6.6 |
| Maternal educational level < 6 y | 133 (63.3) | 22 (13.3) | 80 (35.2) | 59 (26) | 44 (22.7) | 202 (82.1) | 5 (2.1) | 75 (35.9) | 620 (36.2) |
| Mother has less than 3 living children | 160 (76.2) | 113 (68.5) | 157 (69.8) | 199 (87.7) | 111 (57.2) | 105 (42.7) | 141 (59.5) | 58 (27.8) | 1044 (61) |
| Ownership of chickens/ducks | 3 (1.4) | 1 (0.6) | 14 (6.2) | 73 (32.2) | 75 (38.7) | 144 (62.3) | 87 (37.2) | 204 (97.6) | 601 (35.4) |
| Ownership of cows/bulls | 1 (0.5) | 0 (0) | 5 (2.2) | 3 (1.3) | 0 (0) | 146 (59.4) | 33 (13.9) | 157 (75.1) | 345 (20.1) |
| Routine treatment of drinking water | 130 (61.9) | 10 (6.1) | 7 (3.1) | 98 (43.2) | 32 (16.5) | 0 (0) | 12 (5.1) | 12 (5.7) | 301 (17.6) |
| Improved drinking water source | 210 (100) | 165 (100) | 227 (100) | 227 (100) | 184 (94.9) | 246 (100) | 196 (82.7) | 89 (42.6) | 1544 (90.0) |
| Improved latrine | 210 (100) | 165 (100) | 121 (53.3) | 227 (100) | 66 (34) | 197 (80.1) | 232 (97.9) | 19 (9.1) | 1237 (72.1) |
| Improved floor | 204 (97.1) | 165 (100) | 222 (97.8) | 109 (48) | 69 (35.6) | 81 (32.9) | 231 (97.5) | 13 (6.2) | 1094 (63.8) |
| Monthly income < $150 | 69 (32.9) | 161 (97.6) | 19 (8.4) | 106 (46.7) | 58 (29.9) | 115 (46.8) | 179 (75.5) | 0 (0) | 707 (41.2) |
†Mean ± Standard deviation.
Figure 1.
Prevalence of Campylobacter jejuni/coli and Campylobacter species in stool during 1–24 months by age group.
Incidence and incidence rate of Campylobacter infection
The cumulative incidences of Campylobacter jejuni/coli and Campylobacter species were 86.1% and 90.0% respectively. The incidence rate per 100 child-months of infections with Campylobacter jejuni/coli and Campylobacter species during 1–24 month follow up were 17.7 (95% CI: 17.0, 18.5) and 29.6 (95% CI: 28.16, 30.3) respectively. The incidence and incidence rate were highest for Bangladesh and Tanzania sites (Table 2). We identified factors associated with Campylobacter jejuni/coli and Campylobacter species detection using negative binomial regression in surveillance stool samples across all sites (Table 3). The incidence rate for infection of Campylobacter jejuni/coli in female children was 7% higher [IRR: 1.07 (95% CI: 1.07, 1.14); p = 0.048] than in male children. Shorter duration of exclusive breastfeeding [IRR: 0.98 per additional month (95% CI: 0.95, 0.99); p = 0.035], lower maternal age in years [IRR: 0.99 per year (95% CI: 0.97, 0.99); p < 0.001], mother having no less than 3 living children [IRR: 1.15 (95% CI: 1.05, 1.26); p = 0.002], maternal education not greater than or equal to 6 years [IRR: 1.09 (95% CI: 1.01, 1.17); p = 0.021], lack of treatment of drinking water [IRR: 1.26 (95% CI: 1.14, 1.40); p = 0.002], and unimproved sanitation [IRR: 1.11 (95% CI: 1.00, 1.23); p = 0.043] were associated with infection with Campylobacter jejuni/coli. Furthermore, female children, shorter duration of exclusive breastfeeding, enrollment weight-for-age z-score (WAZ), lower maternal age, mother having no less than 3 living children, maternal education not greater than or equal to 6 years, no treatment of drinking water, unimproved sanitation, and household ownership of cattle/poultry were also found to be more strongly associated with Campylobacter species as detected by EIA compared to Campylobacter jejuni/coli as detected by qPCR. The incidence rate ratio for the sites of Brazil (BR), India (IN), Nepal (NP), Peru (PE), Pakistan (PK), and South Africa (SA) were lower compared to the Bangladesh site.
Table 2.
Incidence rate per 100 Child-months and cumulative incidence of Campylobacter jejuni/coli and Campylobacter species infection by site.
| Sites | Campylobacter jejuni/coli (PCR) | Campylobacter species (EIA) | ||
|---|---|---|---|---|
| Incidence rate per 100 Child-months (95% CI) | Cumulative incidence | Incidence rate per 100 Child-months (95% CI) | Cumulative incidence | |
| Bangladesh | 28.3 (26.2, 30.6) | 99.5 | 44.1 (41.6, 46.8) | 100.0 |
| Brazil | 4.2 (3.4, 5.1) | 47.9 | 8.2 (6.8, 10) | 54.6 |
| India | 12.9 (11.7, 14.4) | 89.0 | 28.4 (25.9, 31.3) | 90.3 |
| Nepal | 18.8 (17.4, 20.2) | 96.5 | 21.2 (19.5, 23.1) | 93.0 |
| Peru | 16.9 (15.5, 18.3) | 94.9 | 23.2 (21, 25.5) | 93.3 |
| Pakistan | 16.3 (14.9, 17.7) | 92.3 | 49.7 (47, 52.5) | 99.2 |
| South Africa | 5.7 (5.0, 6.5) | 62.5 | 13.3 (12.1, 14.6) | 83.5 |
| Tanzania | 36.8 (34.2, 39.6) | 100.0 | 39.8 (37.3, 42.4) | 99.0 |
| Overall | 17.7 (17.0, 18.5) | 86.1 | 29.2 (28.1, 30.3) | 90.2 |
Incidence rate per was calculated using negative binomial regression where outcome variables were the number of infection of Campylobacter jejuni/coli and Campylobacter species infection and offset variables were log of number of follow up.
Table 3.
Risk factors for Campylobacter detection in monthly surveillance stool samples.
| Risk Factors by Category | Campylobacter jejuni/coli (PCR) | Campylobacter species (EIA) | ||
|---|---|---|---|---|
| IRR (95% CI) | p-value | IRR (95% CI) | p-value | |
| Sex of child | ||||
| Male | Reference | Reference | ||
| Female | 1.07 (1.00, 1.14) | 0.048 | 1.07 (1.01, 1.13) | 0.014 |
| Duration of EBF (month) | 0.98 (0.95, 0.99) | 0.035 | 0.97 (0.95, 0.99) | 0.004 |
| Enrollment WAZ | 1.00 (0.97, 1.04) | 0.814 | 1.03 (1.01, 1.06) | 0.015 |
| Maternal age in years | 0.99 (0.98, 0.99) | <0.001 | 0.98 (0.98, 0.99) | <0.001 |
| Maternal educational level < 6 y | ||||
| No | Reference | Reference | ||
| Yes | 1.09 (1.01, 1.17) | 0.021 | 1.12 (1.05, 1.19) | 0.001 |
| Mother has less than 3 living children | ||||
| Yes | Reference | Reference | ||
| No | 1.15 (1.05, 1.26) | 0.002 | 1.25 (1.16, 1.34) | <0.001 |
| Routine treatment of drinking water | ||||
| Yes | Reference | Reference | ||
| No | 1.26 (1.14, 1.4) | 0 < 0.001 | 1.39 (1.27, 1.53) | <0.001 |
| Improved sanitation | ||||
| Yes | Reference | Reference | ||
| No | 1.11 (1.00, 1.23) | 0.043 | 1.16 (1.07, 1.26) | <0.001 |
| Household ownership of cattle/poultry | ||||
| No | Reference | Reference | ||
| Yes | 1.07 (0.97, 1.17) | 0.167 | 1.12 (1.04, 1.21) | 0.003 |
| alpha (α) | 0.13 (0.11, 0.16) | <0.001 | 0.05 (0.03, 0.08) | <0.001 |
Model: Negative binomial regression; Dependent variable: Number of infection during follow up (1–24 m); Offset: Log of total number of follow up; alpha (α): dispersion parameter; Adjusted for site and all variables included in multivariable model.
Association of Campylobacter infections with growth and enteric inflammation
The cumulative burden of both Campylobacter jejuni/coli infections [−0.18 difference in 24-month length-for-age z-score (LAZ) for children with high vs. low burden of infection (95% CI: −0.30, −0.06), p = 0.004] and Campylobacter species [−0.31 difference in 24-month LAZ (95% CI: −0.46, −0.15), p < 0.001] were associated with poor growth, with a stronger association seen for Campylobacter species both overall and for the majority of sites (Table 4). Meanwhile, after controlling for infection with enteroaggregative E. coli (EAEC), heat-labile enterotoxin-producing E. coli (LT-ETEC), heat-stable enterotoxin-producing E. coli (ST-ETEC), Shigella/enteroinvasive E. coli (Shigella/EIEC), both Campylobacter species and Campylobacter jejuni/coli infections were also clearly and consistently associated with increased enteric inflammation as measured by MPO, with a stronger association seen for Campylobacter jejuni/coli (Table 5).
Table 4.
Association of Campylobacter jejuni/coli and Campylobacter species infection burden on children growth at 24 months.
| Sites | Campylobacter jejuni/coli (PCR) | Campylobacter species (EIA) | ||
|---|---|---|---|---|
| Coef. (95% CI) | p-value | Coef. (95% CI) | p-value | |
| Bangladesh | −0.33 (−0.58, −0.09) | 0.008 | −0.51 (−0.84, −0.18) | 0.002 |
| Brazil | −0.39 (−1.27, 0.48) | 0.374 | −0.25 (−1.07, 0.57) | 0.543 |
| India | −0.28 (−0.61, 0.05) | 0.096 | −0.17 (−0.49, 0.14) | 0.284 |
| Nepal | −0.30 (−0.59, −0.01) | 0.042 | −0.40 (−0.83, 0.02) | 0.062 |
| Peru | −0.07 (−0.40, 0.27) | 0.692 | −0.40 (−0.77, −0.03) | 0.035 |
| South Africa | −0.62 (−1.20, −0.05) | 0.034 | −0.13 (−0.85, 0.59) | 0.719 |
| Tanzania | 0.13 (−0.10, 0.36) | 0.276 | −0.24 (−0.58, 0.11) | 0.180 |
| Overall | −0.18 (−0.30, −0.06) | 0.004 | −0.31 (−0.46, −0.15) | <0.001 |
Adjusted in linear regression model for sex, WAMI Index (water/sanitation, assets, maternal education, and income); enrollment length-for-age z score; maternal height; poultry/cattle in house, mother has less than 3 living children and site for overall estimate; Dependent variable: length-for-age z score at 24 m; Independent variables: Campylobacter burden.
Table 5.
Association between Campylobacter jejuni/coli and enteric inflammation (stool myeloperoxidase).
| Sites | Stool myeloperoxidase (MPO) concentration | |||
|---|---|---|---|---|
| Campylobacter jejuni/coli (PCR) | Campylobacter species (EIA) | |||
| Coef. (95% CI) | p-value | Coef. (95% CI) | p-value | |
| Bangladesh | 0.20 (0.08, 0.31) | 0.001 | 0.23 (0.13, 0.33) | <0.001 |
| Brazil | 0.54 (0.19, 0.90) | 0.003 | 0.49 (0.24, 0.74) | <0.001 |
| India | 0.40 (0.27, 0.53) | <0.001 | 0.24 (0.14, 0.33) | <0.001 |
| Nepal | 0.34 (0.23, 0.45) | <0.001 | 0.24 (0.13, 0.34) | <0.001 |
| Peru | 0.26 (0.12, 0.41) | <0.001 | 0.22 (0.09, 0.35) | 0.001 |
| Pakistan | 0.28 (0.15, 0.41) | <0.001 | 0.20 (0.10, 0.30) | <0.001 |
| South Africa | 0.37 (0.19, 0.55) | <0.001 | 0.17 (0.05, 0.30) | 0.007 |
| Tanzania | 0.24 (0.13, 0.34) | <0.001 | 0.09 (−0.01, 0.19) | 0.088 |
| Overall | 0.29 (0.24, 0.34) | <0.001 | 0.20 (0.16, 0.24) | <0.001 |
Adjusted in the in GEE model for sex, age, WAMI Index (water/sanitation, assets, maternal education, and income); enrollment length-for-age z score; maternal height; number of children, poultry/cattle in house, seasonality, site for overall estimate, some alternative pathogens (EAEC, LT-ETEC, ST-ETEC, Shigella/EIEC), and age as time variable. Dependent variable was log(MPO); Independent variables: presence of Campylobacter at each months.
Discussion
In this prospective multisite birth cohort study, we documented a high burden of Campylobacter infections, with most of the children having Campylobacter detected in a monthly surveillance stool sample by one year of age at seven of the eight sites. The burden was highest in Bangladesh and Tanzania and consistent with prior studies20,21. Our study also shows the incidence rates are more among Bangladesh and Tanzania than other sites (for instance, Brazil, India, Nepal, Peru, Pakistan, South Africa). Overall, the incidence of Campylobacter species infections is approximately 65% higher than that of Campylobacter jejuni/coli alone. In keeping with data from previous study by Amour et al., showed that promotion of exclusive breastfeeding, drinking water treatment, improved latrines, and targeted antibiotic treatment may reduce the burden of Campylobacter species infection [1], our study found that Campylobacter infections were significantly associated with female sex, shorter duration of exclusive breastfeeding, lower maternal age, less maternal education, lack of treatment of drinking water, and unimproved sanitation. Birth weight-for-age is a marginal predictor for Campylobacter species whereas the presence of Campylobacter species is associated with growth shortfalls1. Among malnourished children from a case control study where the cases comprised of children with weight-for-age z score (WAZ) < −2 aged 6–23 months in Dhaka, prevalence of Campylobacter was high compared to healthy (control) children [weight-for-age z score (WAZ) > −1] but the adjusted effect size was not statistically significant20.
The association of Campylobacter jejuni/coli infection with nutritional status and fecal MPO concentrations of children less than 2, after controlling for seasonality and potential confounders including socio-economic and demographic factors, suggests that Campylobacter jejuni/coli have influence on childhood malnutrition and intestinal inflammation20. This finding suggests that Campylobacter can drive intestinal inflammation, which is partly due to altering of the composition of the intestinal microbiota, impairing the intestinal barrier, and priming the intestine for chronic inflammatory responses and is consistent with results of other studies1,7. Meanwhile, the association with growth was stronger for Campylobacter species than with Campylobacter jejuni/coli, which might suggest that non-jejuni/coli species are more strongly associated with poor growth. However, the association with inflammation is stronger for Campylobacter jejuni/coli. Further elucidation of the prevalence, clinical relevance, and mechanisms for association with poor growth are needed for diverse Campylobacter species.
There were some limitations in this paper. As an observational cohort study, the causality of the associations between Campylobacter infections and both intestinal inflammation and linear growth cannot be confirmed but can be inferred based on a number of criteria, including the appropriate adjustment of the models for potential confounders, the strength and consistency of the associations, and the biological plausibility. We have not established a temporal relationship between infections and the outcomes, which would require structured longitudinal models, however previous analyses using such approaches found consistent results for the association between Campylobacter infections and linear growth2. These findings suggest that Campylobacter species other than Campylobacter jejuni/coli may be more strongly associated with child growth shortfalls. Further work is needed to directly assess the epidemiology and impact of individual Campylobacter species. Secondly, interventions that reduce exposure to these diverse Campylobacter species need to be identified, and the impact of these interventions on child growth need to be assessed. We compared the associations of Campylobacter species and Campylobacter jejuni/coli infections with growth and found that other non-jejuni/coli species were also associated with poor growth, however, the absence of direct microbiologic assays for specific non-jejuni/coli Campylobacter species limited our ability to understand what species or group of species are driving these associations.
In conclusion, children harboring risk factors such as female sex, shorter duration of exclusive breastfeeding, lower maternal age in years, maternal education not greater than or equal to 6 years, mother having less than 3 living children, lack of routine treatment of drinking water, unimproved sanitation, and ownership of cattle/poultry were more prone to Campylobacter infection and thereby have compromised nutritional status; such infection was higher in Bangladesh and Tanzania compared to other sites. The burden of Campylobacter was associated with increased enteric inflammation among children in the first 2 years of life. Campylobacter species had a stronger association with growth whereas the association with inflammation was strongest for Campylobacter jejuni/coli.
Method
Study design and participants
The MAL-ED study design and methodology have been previously described19. Briefly, children were enrolled November, 2009 to February, 2012 from the community within 17 days of birth at eight locations: Dhaka, Bangladesh; Vellore, India; Bhaktapur, Nepal; Naushero Feroze, Pakistan; Venda, South Africa; Haydom, Tanzania; Fortaleza, Brazil; and Loreto, Peru. Children were included if the maternal age was 16 years or older, their family intended to remain in the study area for at least 6 months from enrolment, they were from a singleton pregnancy, and they had no other siblings enrolled in the study. Children with a birthweight or enrolment weight of less than 1500 gm and children diagnosed with congenital disease or severe neonatal disease were excluded. The study was approved by the Research Review Committee and the Ethical Review Committee of icddr,b (Bangladesh), the Local Institutional Review Board at the Federal Universisty of Ceará and the national IRB Conselho Nacional de Ética em Pesquisa (Brazil), the Christian Medical College Institutional Review Board and the Emory University Institutional Review Board (India), the Nepal Health Research Council and Walter Reed Institute of Research (Nepal), the Ethics Committee of Asociacion Benefica PRISMA, the Regional Health Directorate of Loreto and the IRB of Johns Hopkins Bloomberg School of Public Health (Peru), the Ethical Review Committee of Aga Khan University (Pakistan), the Institutional Review Boards at the University of Venda (South Africa), the National Institute for Medical Research (Tanzania), and the Institutional Review Board of the University of Virginia (UAS). Written informed consent was obtained from the parents or legal guardian of every child19,22. All methods were performed in accordance with the relevant guidelines and regulations.
Data collection
Household demographics, presence of siblings, maternal characteristics, and other data on the child’s birth and anthropometry were obtained at enrollment19. The socioeconomic status (SES) of families was assessed at 6, 12, 18, and 24 months. SES score, the water/sanitation, assets, maternal education and income (WAMI) index was developed using composite indicators including the variables such as access to improved water and sanitation, eight selected assets, maternal education, and household income23. Improved water and sanitation were defined following World Health Organization guidelines24. Treatment of drinking water was defined as filtering, boiling, or adding bleach1. Anthropometric measurements and vaccination history were collected monthly. Details of illness and child feeding practices were collected during twice-weekly household visits25. Stool samples were collected monthly and were preserved, transported, and processed at all sites using harmonized protocols26. Child anthropometry was measured using standard scales (seca gmbh & co. kg., Hamburg, Germany). Length-for-age Z score (LAZ) was calculated through the use of the 2006 WHO standards for children27. The Z-score scale, calculated as (observed value - average value of the reference population)/standard deviation value of reference population, is linear and therefore a fixed interval of Z-scores has a fixed length difference in cm for all children of the same age. Z-scores are also sex-independent, thus permitting the evaluation of children’s growth status by combining sex and age groups28.
Laboratory testing
Stool samples were collected without fixative by field workers and raw stool aliquots were kept at −80 °C before nucleic acid extraction. All lab testing was done at the site specific laboratories11,14. Stool samples were assayed for Campylobacter species by enzyme immunoassay (ProSpecT, Remel, Lenexa, KS, USA). In addition, myeloperoxidase (MPO) (Alpco, Salem, New Hampshire) was measured using commercially-available Enzyme Linked Immunosorbent Assay (ELISA) kits following the instructions of the manufacturers1,8. Campylobacter jejuni/coli were detected in the stool samples by quantitative PCR targeting the cadF gene using the TaqMan Array Card (TAC) platform, a compartmentalized probe-based real-time PCR assays for detecting enteropathogens in fecal samples, as previously described22,29. The analytic cutoff of each pathogen was a quantification cycle (Cq) of 35; thus, a Cq < 35 was considered positive20,30.
Statistical methods
All statistical tests were performed in STATA 14 (Stata Corporation, College Station, TX). Campylobacter burden was defined as the number of pathogens detected divided by the number of stools collected and was scaled divided by (10th vs 90th percentile). Descriptive statistics such as proportion, mean and standard deviation for symmetric data, and median with inter‐quartile range (IQR) for asymmetric quantitative variables were used to summarize the data. Chi-square and proportion test was used to see the association between two categorical variables and t-test was used to see the mean difference between two groups for symmetric distribution. Cumulative incidence of Campylobacter jejuni/coli and Campylobacter species was defined as the proportion of subjects who were infected at least once during the study period. Incidence rates and risk factors associated with Campylobacter detection in surveillance stool samples were calculated using negative binomial regression models due to over dispersion. In the final multiple negative binomial regression model, the following variables were considered for inclusion using stepwise forward selection: child sex, duration of exclusive breastfeeding in months, enrollment weight for age z-score, maternal age in years, maternal education greater than or equal to 6 years, mother having less than 3 living children, routine treatment of drinking water, improved sanitation, and household ownership of cattle/poultry. The MPO values were log‐transformed before the analysis. We excluded children from the Pakistan site for growth analysis, owing to bias noted in a subset of length measurements at this site. Seasonality was calculated via the terms sin(2mπ/12) + cos(2mπ/12), where “m” is the calendar month1,31. Associations between Campylobacter infection and inflammation was estimated using generalized estimating equations to fit regression models after adjusting for seasonality, sex, age, water/sanitation, assets, maternal education, and income (WAMI) index; enrollment length-for-age; maternal height; poultry/cattle in house, some alternative pathogens which were significantly associated with log(MPO) such as enteroaggregative E. coli (EAEC), heat-labile enterotoxin-producing E. coli (LT-ETEC), heat-stable enterotoxin-producing E. coli (ST-ETEC), Shigella/enteroinvasive E. coli (Shigella/EIEC), and site for overall estimate and age in month as time variable32. The Gaussian family with identity link was used for the continuous outcome of log(MPO). To access and compare the associations of Campylobacter jejuni/coli and Campylobacter species infection burden on growth at 24 months of age, we used multi-variable linear regression after adjusting for site and the necessary covariates.
Acknowledgements
We thank the staff and participants of the MAL-ED Network for their important contributions. We acknowledge with gratitude the commitment of the Government of the People’s Republic of Bangladesh to icddr,b’s research strength. We also acknowledge the following donors for providing unrestricted support to icddr,b’s effort and advancement to its strategic plan: Canada (DFATD), Sweden (SIDA), and the United Kingdom (DFID). The study was funded by University of Virginia with support from MAL-ED Network Investigators in the Foundation of National Institute of Health, Fogarty International Centre (FIC) with overall support from the Bill & Melinda Gates Foundation. We also acknowledge all the research assistants who conducted the interviews during data collection.
Author contributions
E.R.H., Z.A.H., D.L., R.L.G., S.K.S., A.A.M.L., M.N.K., G.K., P.B., L.B., T.A. and E.M. originated the idea for the study and led the protocol design. M.M., A.S., S.K.S., A.H., S.S., E.M., M.N.K. and G.K. conducted the study and supervised the sample and data collection. M.A.H., J.A.P.M. and T.A. conceptualized the manuscript. J.A.P.M. contributed on pathogen data handling, supervised this work, oversaw the statistical analysis and suggested necessary improvements from the statistical point of view. M.A.H. performed statistical analysis and drafted the manuscript. S.S., M.A.A., J.A.P.M., T.A. and M.A.H. interpreted the results. J.A.P.M., S.S., A.S., T.A. and M.M. critically reviewed and provided feedback to revise the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amour C, et al. Epidemiology and Impact of Campylobacter Infection in Children in 8 Low-Resource Settings: Results From the MAL-ED Study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;63:1171–1179. doi: 10.1093/cid/ciw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogawski Elizabeth T, Liu Jie, Platts-Mills James A, Kabir Furqan, Lertsethtakarn Paphavee, Siguas Mery, Khan Shaila S, Praharaj Ira, Murei Arinao, Nshama Rosemary, Mujaga Buliga, Havt Alexandre, Maciel Irene A, Operario Darwin J, Taniuchi Mami, Gratz Jean, Stroup Suzanne E, Roberts James H, Kalam Adil, Aziz Fatima, Qureshi Shahida, Islam M Ohedul, Sakpaisal Pimmada, Silapong Sasikorn, Yori Pablo P, Rajendiran Revathi, Benny Blossom, McGrath Monica, Seidman Jessica C, Lang Dennis, Gottlieb Michael, Guerrant Richard L, Lima Aldo A M, Leite Jose Paulo, Samie Amidou, Bessong Pascal O, Page Nicola, Bodhidatta Ladaporn, Mason Carl, Shrestha Sanjaya, Kiwelu Ireen, Mduma Estomih R, Iqbal Najeeha T, Bhutta Zulfiqar A, Ahmed Tahmeed, Haque Rashidul, Kang Gagandeep, Kosek Margaret N, Houpt Eric R, Acosta Angel Mendez, Rios de Burga Rosa, Chavez Cesar Banda, Flores Julian Torres, Olotegui Maribel Paredes, Pinedo Silvia Rengifo, Trigoso Dixner Rengifo, Vasquez Angel Orbe, Ahmed Imran, Alam Didar, Ali Asad, Rasheed Muneera, Soofi Sajid, Turab Ali, Yousafzai Aisha, Zaidi Anita KM, Shrestha Binob, Rayamajhi Bishnu Bahadur, Strand Tor, Ammu Geetha, Babji Sudhir, Bose Anuradha, George Ajila T, Hariraju Dinesh, Jennifer M. Steffi, John Sushil, Kaki Shiny, Karunakaran Priyadarshani, Koshy Beena, Lazarus Robin P, Muliyil Jayaprakash, Ragasudha Preethi, Raghava Mohan Venkata, Raju Sophy, Ramachandran Anup, Ramadas Rakhi, Ramanujam Karthikeyan, Rose Anuradha, Roshan Reeba, Sharma Srujan L, Sundaram Shanmuga, Thomas Rahul J, Pan William K, Ambikapathi Ramya, Carreon J Daniel, Doan Viyada, Hoest Christel, Knobler Stacey, Miller Mark A, Psaki Stephanie, Rasmussen Zeba, Richard Stephanie A, Tountas Karen H, Svensen Erling, Amour Caroline, Bayyo Eliwaza, Mvungi Regisiana, Pascal John, Yarrot Ladislaus, Barrett Leah, Dillingham Rebecca, Petri William A, Scharf Rebecca, Ahmed AM Shamsir, Alam Md Ashraful, Haque Umma, Hossain Md Iqbal, Islam Munirul, Mahfuz Mustafa, Mondal Dinesh, Nahar Baitun, Tofail Fahmida, Chandyo Ram Krishna, Shrestha Prakash Sunder, Shrestha Rita, Ulak Manjeswori, Bauck Aubrey, Black Robert, Caulfield Laura, Checkley William, Lee Gwenyth, Schulze Kerry, Scott Samuel, Murray-Kolb Laura E, Ross A Catharine, Schaefer Barbara, Simons Suzanne, Pendergast Laura, Abreu Cláudia B, Costa Hilda, Di Moura Alessandra, Filho José Quirino, Leite Álvaro M, Lima Noélia L, Lima Ila F, Maciel Bruna LL, Medeiros Pedro HQS, Moraes Milena, Mota Francisco S, Oriá Reinaldo B, Quetz Josiane, Soares Alberto M, Mota Rosa MS, Patil Crystal L, Mahopo Cloupas, Maphula Angelina, Nyathi Emanuel. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. The Lancet Global Health. 2018;6(12):e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee G, et al. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS neglected tropical diseases. 2013;7:e2036. doi: 10.1371/journal.pntd.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global Epidemiology of Campylobacter. Infection. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francois R, et al. The other Campylobacters: Not innocent bystanders in endemic diarrhea and dysentery in children in low-income settings. PLoS neglected tropical diseases. 2018;12:e0006200. doi: 10.1371/journal.pntd.0006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platts-Mills JA, et al. Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. Journal of clinical microbiology. 2014;52:1074–1080. doi: 10.1128/JCM.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nature reviews. Microbiology. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 8.Fahim Shah Mohammad, Das Subhasish, Gazi Md. Amran, Mahfuz Mustafa, Ahmed Tahmeed. Association of intestinal pathogens with faecal markers of environmental enteric dysfunction among slum-dwelling children in the first 2 years of life in Bangladesh. Tropical Medicine & International Health. 2018;23(11):1242–1250. doi: 10.1111/tmi.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet (London, England) 2009;374:1032–1035. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 10.Owino V, et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics. 2016;138:e20160641. doi: 10.1542/peds.2016-0641. [DOI] [PubMed] [Google Scholar]
- 11.Kosek M, et al. Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59(Suppl 4):S239–247. doi: 10.1093/cid/ciu457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosek M, et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. The American journal of tropical medicine and hygiene. 2013;88:390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saiki T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. The Kurume medical journal. 1998;45:69–73. doi: 10.2739/kurumemedj.45.69. [DOI] [PubMed] [Google Scholar]
- 14.Kosek MN. Causal Pathways from Enteropathogens to Environmental Enteropathy: Findings from the MAL-ED Birth Cohort Study. EBioMedicine. 2017;18:109–117. doi: 10.1016/j.ebiom.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanifeh M, et al. S100A12 concentrations and myeloperoxidase activities are increased in the intestinal mucosa of dogs with chronic enteropathies. BMC veterinary research. 2018;14:125. doi: 10.1186/s12917-018-1441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roncucci L, et al. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:2291–2297. doi: 10.1158/1055-9965.EPI-08-0224. [DOI] [PubMed] [Google Scholar]
- 17.Kruidenier L, et al. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. The Journal of pathology. 2003;201:17–27. doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- 18.Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World journal of gastroenterology. 2010;16:4145–4151. doi: 10.3748/wjg.v16.i33.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MAL-ED Network Investigators The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59(Suppl 4):S193–206. doi: 10.1093/cid/ciu653. [DOI] [PubMed] [Google Scholar]
- 20.Platts-Mills JA, et al. Association between enteropathogens and malnutrition in children aged 6–23 mo in Bangladesh: a case-control study. The American journal of clinical nutrition. 2017;105:1132–1138. doi: 10.3945/ajcn.116.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platts-Mills JA, et al. Association between stool enteropathogen quantity and disease in Tanzanian children using TaqMan array cards: a nested case-control study. The American journal of tropical medicine and hygiene. 2014;90:133–138. doi: 10.4269/ajtmh.13-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platts-Mills James A, Liu Jie, Rogawski Elizabeth T, Kabir Furqan, Lertsethtakarn Paphavee, Siguas Mery, Khan Shaila S, Praharaj Ira, Murei Arinao, Nshama Rosemary, Mujaga Buliga, Havt Alexandre, Maciel Irene A, McMurry Timothy L, Operario Darwin J, Taniuchi Mami, Gratz Jean, Stroup Suzanne E, Roberts James H, Kalam Adil, Aziz Fatima, Qureshi Shahida, Islam M Ohedul, Sakpaisal Pimmada, Silapong Sasikorn, Yori Pablo P, Rajendiran Revathi, Benny Blossom, McGrath Monica, McCormick Benjamin J J, Seidman Jessica C, Lang Dennis, Gottlieb Michael, Guerrant Richard L, Lima Aldo A M, Leite Jose Paulo, Samie Amidou, Bessong Pascal O, Page Nicola, Bodhidatta Ladaporn, Mason Carl, Shrestha Sanjaya, Kiwelu Ireen, Mduma Estomih R, Iqbal Najeeha T, Bhutta Zulfiqar A, Ahmed Tahmeed, Haque Rashidul, Kang Gagandeep, Kosek Margaret N, Houpt Eric R, Acosta Angel Mendez, Rios de Burga Rosa, Chavez Cesar Banda, Flores Julian Torres, Olotegui Maribel Paredes, Pinedo Silvia Rengifo, Trigoso Dixner Rengifo, Vasquez Angel Orbe, Ahmed Imran, Alam Didar, Ali Asad, Rasheed Muneera, Soofi Sajid, Turab Ali, Yousafzai Aisha, Zaidi Anita KM, Shrestha Binob, Rayamajhi Bishnu Bahadur, Strand Tor, Ammu Geetha, Babji Sudhir, Bose Anuradha, George Ajila T, Hariraju Dinesh, Jennifer M. Steffi, John Sushil, Kaki Shiny, Karunakaran Priyadarshani, Koshy Beena, Lazarus Robin P, Muliyil Jayaprakash, Ragasudha Preethi, Raghava Mohan Venkata, Raju Sophy, Ramachandran Anup, Ramadas Rakhi, Ramanujam Karthikeyan, Rose Anuradha, Roshan Reeba, Sharma Srujan L, Sundaram Shanmuga, Thomas Rahul J, Pan William K, Ambikapathi Ramya, Carreon J Daniel, Doan Viyada, Hoest Christel, Knobler Stacey, Miller Mark A, Psaki Stephanie, Rasmussen Zeba, Richard Stephanie A, Tountas Karen H, Svensen Erling, Amour Caroline, Bayyo Eliwaza, Mvungi Regisiana, Pascal John, Yarrot Ladislaus, Barrett Leah, Dillingham Rebecca, Petri William A, Scharf Rebecca, Ahmed AM Shamsir, Alam Md Ashraful, Haque Umma, Hossain Md Iqbal, Islam Munirul, Mahfuz Mustafa, Mondal Dinesh, Nahar Baitun, Tofail Fahmida, Chandyo Ram Krishna, Shrestha Prakash Sunder, Shrestha Rita, Ulak Manjeswori, Bauck Aubrey, Black Robert, Caulfield Laura, Checkley William, Lee Gwenyth, Schulze Kerry, Scott Samuel, Murray-Kolb Laura E, Ross A Catharine, Schaefer Barbara, Simons Suzanne, Pendergast Laura, Abreu Cláudia B, Costa Hilda, Di Moura Alessandra, Filho José Quirino, Leite Álvaro M, Lima Noélia L, Lima Ila F, Maciel Bruna LL, Medeiros Pedro HQS, Moraes Milena, Mota Francisco S, Oriá Reinaldo B, Quetz Josiane, Soares Alberto M, Mota Rosa MS, Patil Crystal L, Mahopo Cloupas, Maphula Angelina, Nyathi Emanuel. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. The Lancet Global Health. 2018;6(12):e1309–e1318. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psaki SR, et al. Measuring socioeconomic status in multicountry studies: results from the eight-country MAL-ED study. Population health metrics. 2014;12:8. doi: 10.1186/1478-7954-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. Progress on drinking water and sanitation: 2012 update. (Accessed 14 April 2016).
- 25.Richard SA, Barrett LJ, Guerrant RL, Checkley W, Miller MA. Disease surveillance methods used in the 8-site MAL-ED cohort study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59(Suppl 4):S220–224. doi: 10.1093/cid/ciu435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houpt E, et al. Microbiologic methods utilized in the MAL-ED cohort study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59(Suppl 4):S225–232. doi: 10.1093/cid/ciu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Onis M, et al. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Global database on child growth and malnutrition. (New York, 2017). [DOI] [PubMed]
- 29.Taniuchi M, et al. Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine. 2016;34:3068–3075. doi: 10.1016/j.vaccine.2016.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. The Lancet. Infectious diseases. 2014;14:716–724. doi: 10.1016/S1473-3099(14)70808-4. [DOI] [PubMed] [Google Scholar]
- 31.Stolwijk AM, Straatman H, Zielhuis GA. Studying seasonality by using sine and cosine functions in regression analysis. Journal of epidemiology and community health. 1999;53:235–238. doi: 10.1136/jech.53.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanin KI, et al. Micronutrient adequacy is poor, but not associated with stunting between 12-24 months of age: A cohort study findings from a slum area of Bangladesh. PloS one. 2018;13:e0195072. doi: 10.1371/journal.pone.0195072. [DOI] [PMC free article] [PubMed] [Google Scholar]