Abstract
Background
Prospective, randomized trials are needed to determine optimal treatment approaches for palliative care problems such as malignant bowel obstruction (MBO). Randomization poses unique issues for such studies, especially with divergent treatment approaches and varying levels of equipoise. We report our experience accruing randomized patients to the Prospective Comparative Effectiveness Trial for Malignant Bowel Obstruction (SWOG S1316) study, comparing surgical and non-surgical management of MBO.
Methods
Patients with MBO who were surgical candidates and had treatment equipoise were accrued and offered randomization to surgical or non-surgical management. Patients choosing non-randomization were offered prospective observation. Trial details are listed on www.clinicaltrials.gov (NCT #). An accrual algorithm was developed to enhance enrollment.
Results
Accrual is ongoing with 176 patients enrolled. Most (89%) patients chose non-randomization, opting for non-surgical management. Of 25 sites that have accrued to this study, six enrolled patients on the randomization arm. Approximately 59% (20/34) of the randomization accrual goal has been achieved. Patient-related factors and clinician bias have been the most prevalent reasons for lack of randomization. An algorithm was developed from clinician experience to aid randomization. Using principles in this tool, repeated physician conversations discussing treatment options and goals of care, and a supportive team-approach has helped increase accrual.
Conclusions
Experience gained from the S1316 can aid future palliative care trials. While difficult, it is possible to randomize patients to palliative studies by giving clinicians clear recommendations utilizing an algorithm of conversation, allotment of necessary time to discuss the trial, and encouragement to overcome internal bias.
Keywords: Malignant Bowel Obstruction, Equipoise, S1316, Randomization, Surgery, Palliative
Introduction
Intestinal obstruction is a common late occurrence among many patients with disseminated abdominal and pelvic malignancies. Malignant bowel obstruction (MBO) is estimated to occur in 2% of all advanced malignancies, up to 28% of colorectal cancers and 40% of ovarian malignancies.1,2 The management of an MBO and the decision of when and how to intervene surgically has been primarily left to individual clinical judgment. Several systematic reviews have documented the benefits of palliative surgery in the care of MBO, although this comes with the risk of substantial morbidity and mortality.3–5 Less invasive alternatives, including somatostatin analogues, have also been shown to reduce nausea and vomiting, although the level of evidence is mixed.6–10 The challenge for a clinical trial is that it mandates the physicians not inject pre-conceived notions into the decision-making process and to focus on clinical equipoise rather than personal equipoise.11
The principle of equipoise provides the ethical basis for medical research that involves assigning patients to different treatment arms where there is uncertainty over whether a particular treatment is of clear benefit.12 Physicians often have strong convictions about the superiority of a treatment option, making it difficult to claim individual equipoise.13 The variability of opinion regarding equipoise is particularly challenging in studies involving complex cases and multi-disciplinary treatments.13 Equipoise has been identified as one of the reasons for low numbers of randomized controlled trials (RCTs) in the field of surgery14 and difficulties in recruitment.13 Those enrolling patients into RCTs have often not received much or any formal training and misunderstand key aspects of trial design, specifically the concept of equipoise.15 This is exacerbated by the nature of the surgical practice which instills strong opinions, sometimes based on uncertain or contradictory evidence.16 An example of precisely this situation was reported related to a prostate cancer surgical trial wherein these strongly held opinions caused multiple issues with design of and accrual to the trial.17
There are similar difficult issues facing the accrual of patients to RCTs in palliative care. In a study of healthcare professionals, few demonstrated a willingness to refer cancer patients to palliative care trials.18 A systematic review of gatekeeping in palliative care research identified five groups of potential gatekeepers: healthcare professionals, research ethics committees, clinical and/or research, patient families, and clinical investigators.19 The fear of burdening vulnerable patients was the most reported reason. Other reasons for gatekeeping included difficulty disclosing current health status to patients, fear of burdening the patient’s families, doubts about the importance or quality of the palliative care study, general attitudes toward research, and overall logistic challenges.19 Randomized controlled trials with surgical versus non-surgical treatment options represent additional complexities as surgeons must consider the risk to benefit ratio of potential surgical complications with quality of life goals.
We report accrual and randomization challenges for an ongoing clinical trial for patients with malignant small intestine obstruction. The purpose of this report is to describe modalities we have found beneficial in accruing patients to such a trial, and the specific challenges to be addressed when discussing randomization.
Methods
SWOG’s Prospective Comparative Effectiveness Trial for Malignant Bowel Obstruction (S1316) is an RCT evaluating the impact of surgical or non-surgical treatment of small intestinal MBO. The definition of an MBO for this study includes: 1) clinical evidence of a bowel obstruction via history/physical/radiographic examination; 2) bowel obstruction beyond the ligament of Treitz; and 3) intra-abdominal primary cancer with incurable disease. Non-intra-abdominal primary cancer with clear intraperitoneal disease are excluded. The primary outcome is days alive and out of the hospital within the first three months in order to capture what one might colloquially refer to as “good days”. The study uses a hybrid design that incorporates both a randomized and an observational component (Figure 1).
Figure 1.
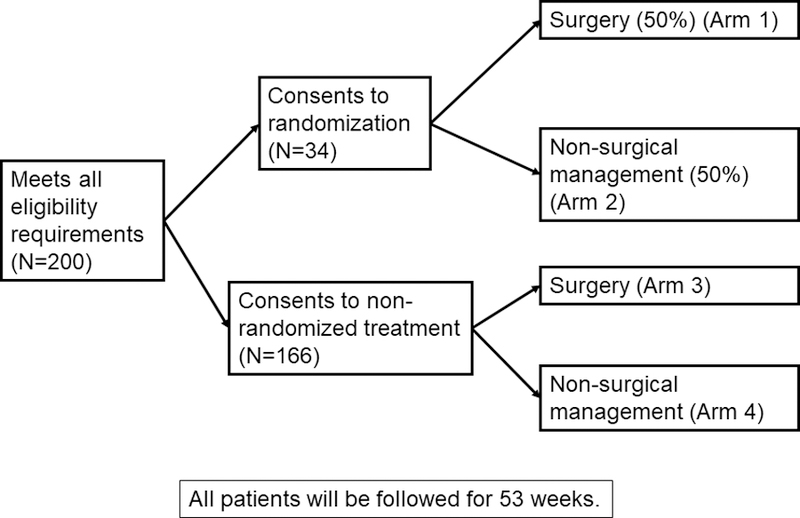
SWOG S1316 Malignant Bowel Obstruction Randomization Schema
Eligibility is defined identically in both components, so that all patients must be considered “randomizable”. Eligible patients are offered randomization to surgical vs. non-surgical management; those who consent are then randomized to either surgical management or non-surgical management (best supportive non-surgical palliative care) of their MBO. If the patient agrees to enroll but does not consent to randomization, then they are registered to the observational arm of their choice, either to receive best medical and supportive care or an operation. For patients who do not choose randomization, the choice of care will be determined by the patient and his/her treating physician at the time of registration, and is considered their initial care plan for the analyses. For all patients, regardless of their initial care plan or randomization status, ongoing care will be determined by the clinical team. Alternatives to surgical and/or non-surgical management of each patient’s MBO and to S1316 participation are discussed with patients at the time of initial evaluation by study personnel and throughout the consent process.
S1316 is an international, multicenter study within the National Clinical Trials Network framework (www.clinicaltrials.gov, NCT #02270450). Currently, there are 31 sites participating in this trial. All sites underwent approval by their Human Subjects Committees. Eligible patients include adults who are admitted to the hospital with a small bowel obstruction due to intra-abdominal cancer. All eligible patients must be surgical candidates (able to tolerate an operation and an operation could offer clinical benefit) whose clinical teams believe there is equipoise regarding surgical and non-surgical treatments; this ensures that all patients are randomizable. After patients are deemed eligible and equipoise is documented, patients must be registered (and randomized, if possible) within three days. MBO treatment (surgery or non-surgical management) must be initiated within 48 hours after registration. The study-specified treatment plans do not differ by randomization status. The non-surgical arm recommends but does not mandate a somatostatin analogue (typically octreotide) due to difficulty obtaining this medication in some institutions. Subsequent treatment on the non-surgical arm may include surgical management, if clinically appropriate. Comparisons between surgical and non-surgical management will be evaluated on a pseudo intent-to-treat basis. Patients are followed for one year or until death.
To examine recruiting barriers in a systematic way, sites were requested for monthly updates on recruiting attempts and outcomes. In addition, monthly conference calls were held with research coordinators to discuss accrual and barriers to recruitment and randomization. Finally, serial conversations were had with site investigators to gain further insight into recruitment issues. This process was used to modify recruitment procedures, and we present in the results the primary themes of these discussions regarding barriers to and facilitators of randomization.
Results
Currently, there have been 176 registrants to the study, 156 to the non-randomized arm and 20 to the randomized arm. Of 25 centers which have accrued patients to this study, six successfully enrolled patients to the randomized arm. The results described and discussion that follows are an observational perspective on a challenging aspect of this trial which has affected accrual to the randomized treatment arm.
Patient-related factors were a commonly reported reason for lack of randomization. Many centers have had open dialogue with patients who are strongly considering randomization, but who eventually want to decide their own treatment and ultimately choose non-randomization. This has been the most frequently encountered reason for lack of randomization cited by many high-accruing centers during monthly conference calls.
Clinician bias has also been a significant barrier to overcome when considering randomization for this trial. It has been the experience of many centers that the site Principal Investigator or study coordinator who is consulted to evaluate a patient with an MBO is often one of many clinicians caring for these particular patients. By the time of initial consultation, regardless of the specific circumstances, many patients have been counseled to seek comfort measures or pursue hospice care. Therefore, attempts were made to involve as many varying medical specialists as possible at each clinical site. Addressing these biases with the clinicians while educating patients and the personnel involved with their care is one method used to overcome this barrier and possibly allow randomization.
Lastly, centers that successfully randomized patients have generally had the study open longer and have had more experience enrolling patients to either the randomized or non-randomized treatment arms. The common trend has been that these centers have successfully enrolled many patients to the non-randomized treatment arm prior to enrolling a patient to the randomized treatment arm. Although prior enrollment to the non-randomized portion is not necessary to successfully randomize patients, the experience gained in communicating study goals, procedures, risk and benefits to patients who entered the non-randomized component was useful towards developing a successful recruitment approach. From this, we developed recommendations to guide these early patient interactions. Due to the difficulty of accruing patients to the study, especially to the randomized arm, therefore, we developed an accrual algorithm for physicians to use when discussing the trial with patients. (Figure 2)
Figure 2.



SWOG S1316 Randomization Recommendations
The purpose of these recommendations is to help with the accrual process, especially in introducing the concept of randomization in patients with advanced cancer. They were developed by study clinical investigators who successfully randomized patients, based on their experience with the accrual process. In conversation with site-investigators, this communication aid has been felt to be helpful to initiate these difficult conversations and attempt to enroll patients to this study. The instructions contain helpful information such as gently introducing the concept of randomization to patients after a relationship of trust has developed, and avoid using terms such as “trial” and instead refer to S1316 as a “study.” This document is not intended for patients but has been circulated to the site-investigator team members who will discuss the trial with patients in an effort to aid in discussion of treatment options and consideration for randomization.
Discussion
Patients with advanced intra-abdominal malignancies, especially those who have received prior multimodality treatment, are complex cases, and can develop an intestinal obstruction due to disease progression or related to surgical interventions. Due to the conflicting evidence guiding management, there should be equipoise for most clinicians treating patients who present with an MBO. These views are often based on intimate relationships developed between cancer surgeons and their patients during the treatment process. Surgeons may not be able to predict how aggressive a person with advanced illness wants to be and the choices that person may make regarding trial participation.20 Investigators may also preferentially offer trial participation to patients who have previously chosen surgery. However, past choices may not necessarily predict future behavior, as patients who initially undergo curative intent surgery are not any more likely to choose aggressive palliative care treatments compared to those who did not.20
The S1316 trial attempts to answer the question of the most appropriate therapy (surgical or non-surgical) for patients and clinicians facing these difficult issues. Participation provides the opportunity for patients to be altruistic, providing additional dietary and quality of life information that may impact future patients with MBO. Participation in the randomized treatment arm has proven challenging, as most patients elect to choose their treatment.
The randomization scheme of SWOG S1316 has evolved in response to these pragmatic challenges and to our knowledge never has been attempted in a palliative care setting. The novel hybrid randomization design of a smaller randomized component within a larger non-randomized patient choice comparison of the same treatment options is based on the premise that most patients would want to be actively involved in the decision-making process of their MBO management, especially when the treatment arms are so divergent. Therefore, we assumed that most patients who participated would elect to choose their treatment, and this has been observed with the disproportionate numbers of non-randomized accruals. The randomized component of S1316, however, is equally as critical to the success of this trial as randomization represents the gold standard for clinical trials.21 Randomization provides comparable groups so that the outcomes (days alive and out of the hospital, symptom and quality of life scores, and/or nutritional intake) can be directly attributable to the intervention (surgical versus non-surgical management), thereby minimizing the possibility of confounding and further augments the observational arms.
The advantages of this hybrid design are that it uses the strengths of the RCT to give an unbiased estimate of treatment differences while allowing us to accrue more and a broader range of MBO patients who are eligible for surgery but unwilling to be randomized. By allowing more patients to participate, we hope to improve power and potentially expand the inference to a more clinically relevant population who are treated according to usual practice patterns. These results will have better external validity than a stand-alone RCT through their inclusion of broader and more generalizable patient and physician populations.22,23
Due to difficulty accruing to the randomized arm, the trial authors reduced the size of the randomized component and developed the recommendations shown in Figure 2. These guidelines were developed by study personnel who successfully enrolled multiple patients to the randomized treatment arm. The study investigators found the reasons for low randomization are multifactorial but most notably include the perceived lower risk of a non-surgical approach and potential morbidity associated with a surgical approach. Patients often have difficulty coming to terms with such disparate treatment options, especially in the setting of limited survival and end of life discussions. Similarly, clinicians may be unlikely to recommend to their patients a particular treatment dependent upon their inherent bias to which treatment is better. In developing this study, it became clear that detailed communication with site key personnel throughout the duration of the study was imperative. Physicians are often risk averse, and thus are more likely to perform interventions on lower-risk patients and to avoid intervention on potentially higher-risk patients.24 Clinicians have rational but differing opinions or preferences as to the most appropriate treatment for an MBO. Bias, or limiting one’s bias, plays a large role in the willingness and ability to randomize to S1316, or even to accrue patients to the trial at all. Prior anecdotal experience likely also plays a role in clinician participation and willingness to offer randomization in this trial.
It is important to also recognize patients and their support system (e.g. family, caregivers, clergy) also play a significant role in participation in RCTs for advanced cancer.25 Patients have a desire for meaning, comfort and direction in what remains of their life.25 Factors such as family support, marital status and caregiver preferences also impact how and where patients choose to die.26 The reasons patients participate but do not agree to randomize are also likely multi-factorial and difficult to quantify. Their surgeon’s opinion and biases can be a major determining factor in the decision.27 Some patients may feel that by agreeing to participate on a study that they are likely to receive experimental (non-standard of care) treatment and therefore find randomization disturbing.28 This highlights the importance of the physician-patient relationship, the basis of which is built on confidence and trust.13 Patients who are considering randomization want to know that the clinician’s interest in their participation is not limited to the study and the end result of the trial, but also includes their well-being and maximizing their long-term quality of life. They want to know that their surgeon cares.
Conversations with patients regarding participation in SWOG S1316 are often lengthy and performed over multiple interactions over several days. Several factors must be considered when introducing the concept of randomization for an MBO, including 1) the patient’s prior oncologic history and understanding of their MBO diagnosis and prognosis, 2) patient symptoms and performance status, and 3) patient and clinician perceived biases to a particular treatment. Introducing randomization as a concept to patients and their families requires a thoughtful, focused discussion predicated on the understanding of the patient’s and families intended wishes and goals of care. It is incumbent upon the researcher introducing the study to be introspective whether a patient would be appropriately and justifiably treated with either (operative or non-operative). If equipoise is fulfilled, patients are offered study participation and asked to consider randomization. In situations where the researcher feels one particular treatment modality would be superior, equipoise is not present and these patients would not be asked to participate. Indeed, many centers evaluated patients for potential participation but because of lack of equipoise were unable to successfully enroll them.
Centers that have had success in randomizing patients have observed that patients appreciate open, honest dialogue, with a detailed discussion of the potential benefits and risks of each treatment modality and to also declare any personal preference as to their opinion of the most appropriate therapy. Re-assurance that surgical and non-surgical management are clinically acceptable standards of care for their diagnosis of a malignant bowel obstruction has been one method to improve patient understanding of such divergent treatment arms, and potential willingness to consider randomization. It has been the experience of the authors that frequent, repeated conversations with patients and their family members over multiple days has been proven effective by those investigators who have had success randomizing patients. Offering re-assurance that irrespective of the treatment chosen during the randomization process, the patient will continue to be treated by the clinician and will continue to be followed after discharge from the hospital is important. These reassurances are often comforting to patients under consideration for enrollment, many of whom are struggling with their diagnosis and abrupt loss of independence and sense of control.
There are several limitations to this analysis. Clinician discussions were not standardized, although the methods outlined in this manuscript were encouraged as more experience was gained. Site investigators often discussed barriers to randomization, and successful methods were relayed to other investigators. While no formal qualitative methodology was used, these frequent interactions allowed clear accrual recommendations to emerge.
Conclusion
Accrual to RCTs is always difficult in the setting of advanced cancer, especially when the study is focused primarily on maximizing quality of life and not primarily on extending survival. Divergent treatment arms, clinician bias, and patient-related factors are potential barriers to accrual and randomization. Lessons learned from S1316 can help future palliative care trials for cancer patients. Caring, sometimes lengthy conversations, overcoming internal bias, and a supportive team-approach allow for randomization and accrual to these trials.
Acknowledgments
Funding
This work was supported under grant awards by the Agency for Healthcare Research and Quality (HS021491) and the National Cancer Institute, Division of Cancer Prevention, NCORP Research Base (UG1CA189974). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures/Conflict of Interest
All authors declare no conflicts of interest.
This work has not been previously presented or published in any format. This work has been approved by all authors and is not under consideration for publication elsewhere.
References
- 1.Ripamonti C, De Conno F, Ventafridda V, Rossi B, Baines MJ. Management of bowel obstruction in advanced and terminal cancer patients. Ann Oncol 1993; 4: 15–21. [DOI] [PubMed] [Google Scholar]
- 2.Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res 2012; 4: 159–169. doi: 10.2147/CMAR.S29297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul Olson TJ, Pinkerton C, Brasel KJ, Schwarze ML. Palliative surgery for malignant bowel obstruction from carcinomatosis: a systematic review. JAMA Surg 2014; 149: 383–392. doi: 10.1001/jamasurg.2013.4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santangelo ML, Grifasi C, Criscitiello C, et al. Bowel obstruction and peritoneal carcinomatosis in the elderly. A systematic review. Aging Clin Exp Res 2017; 29: 73–78. doi: 10.1007/s40520-016-0656-9 [DOI] [PubMed] [Google Scholar]
- 5.Shariat-Madar B, Jayakrishnan TT, Gamblin TC, Turaga KK. Surgical management of bowel obstruction in patients with peritoneal carcinomatosis. J Surg Oncol 2014; 110: 666–669. doi: 10.1002/jso.23707 [DOI] [PubMed] [Google Scholar]
- 6.Currow DC, Quinn S, Agar M et al. Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manage 2015; 49: 814–821. doi: 10.1016/j.jpainsymman.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 7.Peng X, Wang P, Li S, Zhang G, Hu S. Randomized clinical trial comparing octreotide and scopolamine butylbromide in symptom control of patients with inoperable bowel obstruction due to advanced ovarian cancer. World J Surg Oncol 2015; 13: 50. doi: 10.1186/s12957-015-0455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007; 33: 217–223. [DOI] [PubMed] [Google Scholar]
- 9.Laval G, Rousselot H, Toussaint-Martel S et al. SALTO: a randomized, multicenter study assessing octreotide LAR in inoperable bowel obstruction. Bull Cancer 2012; 99: E1–9. doi: 10.1684/bdc.2011.1535 [DOI] [PubMed] [Google Scholar]
- 10.Mariani P, Blumberg J, Landau A et al. Symptomatic treatment with lanreotide microparticles in inoperable bowel obstruction resulting from peritoneal carcinomatosis: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2012; 30: 4337–4343. doi: 10.1200/JCO.2011.40.5712 [DOI] [PubMed] [Google Scholar]
- 11.Cook C, Sheets C. Clinical equipoise and personal equipoise: two necessary ingredients for reducing bias in manual therapy trials. J Man Manip Ther 2011; 19: 55–57. doi: 10.1179/106698111X12899036752014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adibe OO, St Peter SD. Equipoise, ethics, and the necessity of randomized trials in surgery. Arch Surg 2012; 147: 899–900. doi: 10.1001/archsurg.2012.1796 [DOI] [PubMed] [Google Scholar]
- 13.Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol 2014; 67: 912–920. doi: 10.1016/j.jclinepi.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014; 15: 5. doi: 10.1186/1745-6215-15-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziebland S, Featherstone K, Snowdon C, et al. Does it matter if clinicians recruiting for a trial don’t understand what the trial is really about? Qualitative study of surgeons’ experiences of participation in a pragmatic multi-centre RCT. Trials 2007; 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joffe S, Miller FG. Equipoise: asking the right questions for clinical trial design. Nat Rev Clin Oncol 2012; 9: 230–235. doi: 10.1038/nrclinonc.2011.211 [DOI] [PubMed] [Google Scholar]
- 17.Elliott D, Hamdy FC, Leslie TA, et al. Overcoming difficulties with equipoise to enable recruitment to a randomised controlled trial of partial ablation vs radical prostatectomy for unilateral localised prostate cancer. BJU Int 2018; 122: 970–977. doi: 10.1111/bju.14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White C, Gilshenan K, Hardy J. A survey of the views of palliative care healthcare professionals towards referring cancer patients to participate in randomized controlled trials in palliative care. Support Care Cancer 2008; 16: 1397–1405. doi: 10.1007/s00520-008-0441-1 [DOI] [PubMed] [Google Scholar]
- 19.Kars MC, van Thiel GJ, van der Graaf R, et al. A systematic review of reasons for gatekeeping in palliative care research. Palliat Med 2016; 30: 533–548. doi: 10.1177/0269216315616759 [DOI] [PubMed] [Google Scholar]
- 20.Schubart JR, Green MJ, Van Scoy LJ, et al. Advanced Cancer and End-of-Life Preferences: Curative Intent Surgery Versus Noncurative Intent Treatment. J Palliat Med 2015; 18: 1015–1018. doi: 10.1089/jpm.2015.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacks H, Chalmers TC, Smith H Jr. Randomized versus historical controls for clinical trials. Am J Med 1982; 72: 233–240. [DOI] [PubMed] [Google Scholar]
- 22.McKee M, Britton A, Black N, et al. Methods in health services research. Interpreting the evidence: choosing between randomised and non-randomised studies. BMJ 1999; 319: 312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Concato J, Horwitz RI. Beyond randomised versus observational studies. Lancet 2004; 363: 1660–1661. [DOI] [PubMed] [Google Scholar]
- 24.Stukel TA, Fisher ES, Wennberg DE et al. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 2007; 297: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaldjian LC, Curtis AE, Shinkunas LA, Cannon KT. Goals of care toward the end of life: a structured literature review. Am J Hosp Palliat Care 2008; 25: 501–511. doi: 10.1177/1049909108328256 [DOI] [PubMed] [Google Scholar]
- 26.Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ 2006; 332: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet 2001; 358: 1772–1777. [DOI] [PubMed] [Google Scholar]
- 28.Tobias JS, Souhami RL. Fully informed consent can be needlessly cruel. BMJ 1993; 307: 1199–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]


