Abstract
Background:
Pulmonary exacerbations (PEx) in cystic fibrosis (CF) patients reduce quality of life. Lung function, measured by the percent predicted forced expiratory volume in one second (ppFEV1), is widely used to evaluate PEx treatments. We analyzed the correspondence of ppFEV1 with 8 patient-reported symptom-based questions from the Cystic Fibrosis Respiratory Symptom Diary–Chronic Respiratory Infection Symptom Score (CFRSD-CRISS).
Methods:
Data were derived from the observational Standardized Treatment of Pulmonary Exacerbations (STOP) study. CF patients who had CFRSD-CRISS and ppFEV1 measurements on ≥2 timepoints were included: 1) day of initial PEx, 2) 7 days later, and/or 3) end of PEx. We calculated age-stratified Spearman correlation coefficients and 95% confidence intervals (95% CIs) between the change in ppFEV1 and change in CFRSD-CRISS items from index to day 7 and from index to the end of PEx treatment.
Results:
Lung function and symptom scores improved by the end of treatment; however, correlations between ppFEV1 and the specific CFRSD-CRISS measures were mostly weak to moderate. An exception was that among patients <18, we observed moderately strong correlations between changes in ppFEV1 and cough severity (r=−0.58 (95% CI: −0.80, −0.21)), mucus quantity (r=−0.51 (−0.77, −0.11)), and wheezing (r=−0.53 (−0.78, −0.14)) from index until end of treatment.
Conclusions:
As novel treatments are developed for PEx, it is important to ensure that improvement is measured meaningfully. The generally weak associations between patient-reported symptoms and ppFEV1 that we found suggest that these measures capture different aspects of the disease and both metrics are important when evaluating new treatments.
Keywords: cystic fibrosis, pulmonary exacerbations, lung function, Cystic Fibrosis Respiratory Symptom Diary - Chronic Respiratory Infection Symptom Score
Introduction
Cystic fibrosis (CF) is a complex genetic disorder affecting about 70,000 people worldwide [1]. With the advent of novel treatments, the median predicted survival age in patients has increased dramatically, from about 8 years for patients born in the 1960s [2] to about 46 years for patients born in 2017 [3] and as a result, these people contend with the health effects of CF over long periods of time. The pulmonary symptoms of CF are characterized by abnormal movement of chloride and sodium across epithelial cells in the airways, resulting in decreased clearance of airway secretions which in turn allows chronic endobronchial infections. The immune system response to these infections results in neutrophilic inflammation and when the neutrophils die, they thicken the sputum, leading to further difficulty with airway clearance and thus subsequent pulmonary inflammation and infections. Eventually, this cycle results in irreversible bronchiectasis and respiratory failure [4].
Among the most serious manifestations of CF are recurrent episodes of pulmonary symptoms called pulmonary exacerbations (PEx), which are associated with changes in cough, sputum production, and decreased lung function [5]. As patients get older, they tend to experience PEx more frequently; about 23% of patients per year under age 6 experienced PEx that required intravenous antibiotics compared to 63% of CF patients ≥ 18 years [5]. CF patients can also experience multiple PEx per year, with a recent study reporting that a sample of CF patients had a mean of 2.9 PEx each year [6].
Traditionally, evaluation of the percent predicted forced expiratory volume in one second (ppFEV1) has been used to determine the effectiveness of new PEx treatments [7]. In part because about a quarter of patients who experience PEx fail to return to their baseline lung function [8], emphasis has shifted to evaluation of health status metrics rather than clinical characteristics. These include generic preference-weighted assessments such as the 5-Level EuroQOL-5 Dimensions (EQ-5D-5L) score, which evaluates patients’ general well-being, as well as CF-specific questionnaires like the Cystic Fibrosis Questionnaire-Revised (CFQ-R)[9] and the Cystic Fibrosis Respiratory Symptom Diary-Chronic Respiratory Infection Symptom Score (CFRSD-CRISS) [10], which assess CF symptoms and their impacts on patients’ lives.
Evaluating how strongly biomarker metrics such as lung function are related to patient-reported symptoms is critical for incorporating accurate measures of treatment benefit into the evaluation of new CF treatments. Assessments of both of these types of patient measures are common in clinical trials as well as in contemporary patient care, yet their relationship is not well defined. Furthermore, as people live longer with CF and adapt to the chronic disease, it is possible that the relationship between lung function and patient-reported symptoms may change. The purpose of this paper was to analyze the correspondence of ppFEV1 with 8 symptom-based questions from the CFRSD-CRISS. Understanding the relationship of these measures is necessary for planning intervention trials and interpreting outcome measures when novel treatments for PEx are developed. We hypothesized that a strong negative correlation would exist between the ppFEV1 and symptom measures from the CFRSD-CRISS items of difficulty breathing, chest tightness, wheeze, cough, and amount of mucus coughed up. If these correlations are strong, it suggests that the effectiveness (or lack of effectiveness) of treatments is similarly affecting both ppFEV1 and these CF symptoms. If not, it raises questions about the parameters that should be measured that accurately reflect changes in patients’ health status during the course of pulmonary exacerbation treatment.
Methods
Study Population
Data were derived from Standardized Treatment of Pulmonary Exacerbations (STOP) study, which was designed to determine the feasibility of adopting a standardized protocol for treating PEx [11]. This study collected demographic and clinical characteristics from a total of 220 CF patients who were hospitalized for intravenous (IV) PEx treatment in 11 CF centers throughout the United States from January 2014-January 2015 [11]. Because this was a feasibility study, the treatments that the patients received were not influenced by the study protocols. Institutional Review Board (IRB) approval was obtained from each participating CF center for the parent STOP study and all participants or their guardians provided written informed consent where required; this analysis was approved by the University of Washington IRB (approval number 00000484).
Timing of Assessments and Description of Measures
For these analyses, we used data from 3 time points: 1) the day of the initial pulmonary exacerbation (hereafter termed “index day”); 2) 7 days after initial PEx; and 3) the last day of antibiotic treatment (or day 28 if treatment continued past day 28). At each of these time points, patients completed the 8 items of the CFRSD-CRISS questionnaire, which assessed the following CF-specific symptoms: difficulty breathing, feeling feverish, feeling tired, having chills/sweats, cough severity, mucus production, chest tightness, and wheezing [10, 12]. Rasch analysis [13] was used to transform the ordinal answers to the individual CFRSD-CRISS questions to continuous summary scores ranging from 0 to 100. For both the summary and the specific symptom scores, higher scores indicated greater symptom severity [14]. Spirometry measures were also taken at each of the three time points and the ppFEV1 was calculated using the Global Lung Initiative equations [15]. Although CFRSD-CRISS data were collected every day that patients were participating in the STOP study, we used only CFRSD-CRISS data that were collected on the same day as spirometry measurements. For these analyses, we required that patients had ppFEV1 and CFRSD-CRISS data on at least two of the three time points.
Statistical Analysis
We examined demographic (age, gender, race, ethnicity) and clinical variables (duration of IV antibiotic treatment, whether the patient received any IV therapy after completion of initial therapy (because we theorized that patients who received additional IV therapy would have been expected to have had worse lung function and worse symptoms on their CFRSD-CRISS questionnaires), antibiotic classes used to treat PEx) of the cohort. We also determined whether the patients’ summary CFRSD-CRISS scores improved by a minimally clinically important amount (determined to be ≥11 points [16]) between the index day and the end of PEx treatment. We reported means and standard deviations of continuous variables and counts and percentages for categorical variables. We used t-tests and chi-square tests (or Fisher’s exact test if n<5) to examine the significance of differences between continuous and categorical variables, respectively.
In order to examine trends in measures over the time the PEx were being treated, we created histograms of the ppFEV1 measurements, the summary CFRSD-CRISS scores, and the 8 CRFSD-CRISS symptom-specific questions on the index day, 7 days later, and the end of PEx treatment. Because the CFRSD-CRISS variables were ordinal and the ppFEV1 variable was continuous, we calculated the Spearman correlation coefficients [17] because these do not require any assumptions about the variable distributions [18], and calculated 95% confidence intervals (95% CIs), using the Fisher’s z transformation, which has been reported to calculate confidence limits accurately in populations that do not have high correlations [19]. Correlation coefficients were calculated between the changes in each of the individual CFRSD-CRISS items and the changes in ppFEV1 from 1) index to day 7 and 2) index to the end of PEx treatment. We also calculated the Spearman correlation coefficients and 95% CIs between the change in the summary CFRSD-CRISS score and the change in ppFEV1 in these two time intervals.
We also calculated whether each metric improved, stayed the same (which for ppFEV1 was defined as a change of <1%), or worsened between index and day 7 and between index and the end of PEx treatment. We calculated weighted Cohen’s kappa coefficients [20] to determine agreement between ppFEV1 and each of the individual CFRSD-CRISS items, as well as the summary CFRSD-CRISS score. We also examined the proportions of patients whose summary CFRSD-CRISS scores stayed the same or got worse as well as the proportions whose summary CFRSD-CRISS scores improved from index until day 7 and from index until the end of PEx. We also examined the proportions of those whose ppFEV1 stayed the same or got worse and the proportions whose ppFEV1 improved, again from index until day 7 and from index until the end of PEx.
Because previous research has shown that CF symptoms and ppFEV1 vary by age in CF patients [5, 21], we stratified all analyses into those <18 versus those who were ≥18 years of age. SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina, 2017) was used for all analyses.
Results
A total of 177 (80% of the 220 patients in the STOP study) had measures of the CFRSD-CRISS items and spirometry on at least 2 of the relevant time points and were included in these analyses. Their clinical and demographic characteristics, stratified by age, are shown in Table 1. Most patients were white, and a slight majority of patients in these analyses were female. Almost all patients were treated with agents active against gram positive cocci and gram negative organisms (primarily Pseudomonas); with 81% receiving one or more broad spectrum antibiotics as treatments for their PEx. Most of our population, particularly those <18, had improvement by a clinically significant amount in their CFRSD-CRISS summary scores by the end of treatment.
Table 1.
Clinical and demographic characteristics stratified by age.
| Variable (n (%) unless stated otherwise) | Age <18 (n=32) | Age ≥18 (n=145) |
|---|---|---|
| Age (mean ± std dev) | 15.5 ± 1.8 | 29.2 ± 9.2 |
| Duration of IV treatment (mean ± std dev) | 14.0 ± 4.6 | 16.0 ± 5.8 |
| Any IV therapy after completion of initial therapy | 17 (63%) | 78 (60%) |
| Missing | 5 | 15 |
| Female | 18 (56%) | 81 (56%) |
| Race | ||
| White | 30 (94%) | 138 (95%) |
| Black/African American | <51 | <5 |
| Asian | <5 | <5 |
| Two or more races | <5 | <5 |
| Hispanic | <5 | 8 (6%) |
| Unknown | <5 | 5 |
| What type of insurance did the patient have?2 | ||
| Private | 20 (63%) | 80 (56%) |
| Medicare | <5 | 32 (28%) |
| Medicaid | 16 (50%) | 53 (37%) |
| State special needs | <5 | 11 (8%) |
| Military | <5 | 7 (5%) |
| Other | <5 | 8 (6%) |
| Missing | <5 | <5 |
| Antibiotic Classes received2 | ||
| Gram Positive Cocci | 30 (94%) | 128 (88%) |
| MRSA | 15 (47%) | 77 (53%) |
| Gram Negative | 32 (100%) | 145 (100%) |
| Pseudomonas | 31 (97%) | 74 (100%) |
| Anaerobes | 19 (63%) | 84 (59%) |
| Patient took ≥1 Broad Spectrum Abx | 28 (88%) | 118 (81%) |
| Other meds taken while on antibiotics | ||
| Mucolytic | <5 | <5 |
| Steroid | <5 | <5 |
| CFRSD-CRISS improved by the end of antibiotic therapy | 27 (87%) | 111 (77%) |
In the interest of patient confidentiality, exact numbers are not reported for cells with <5 patients
Patients could have had >1 type of insurance or received >1 antibiotic class
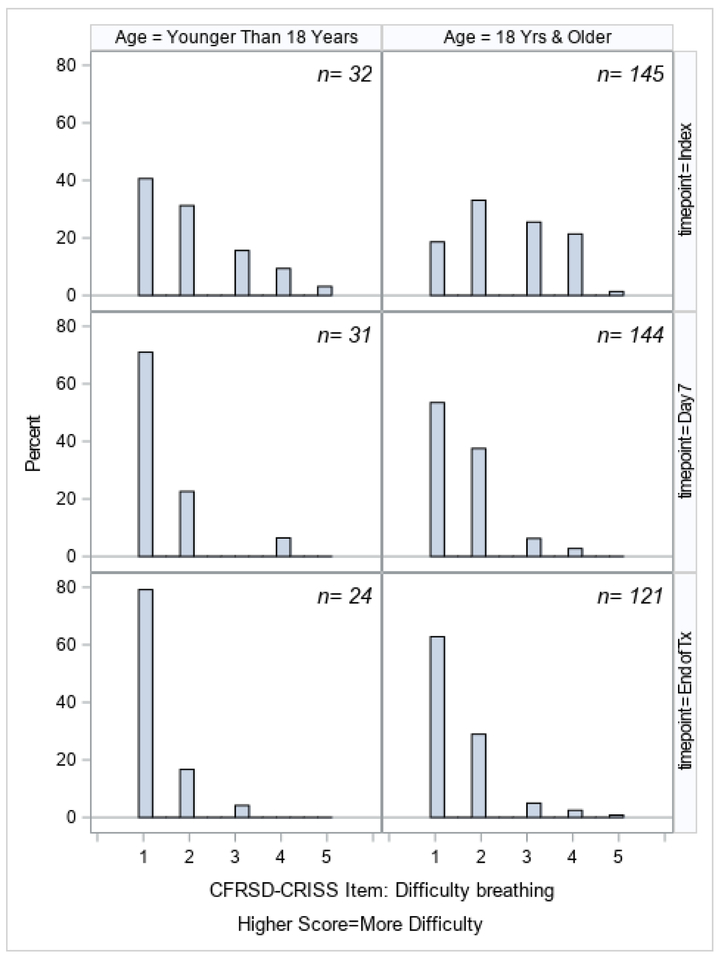
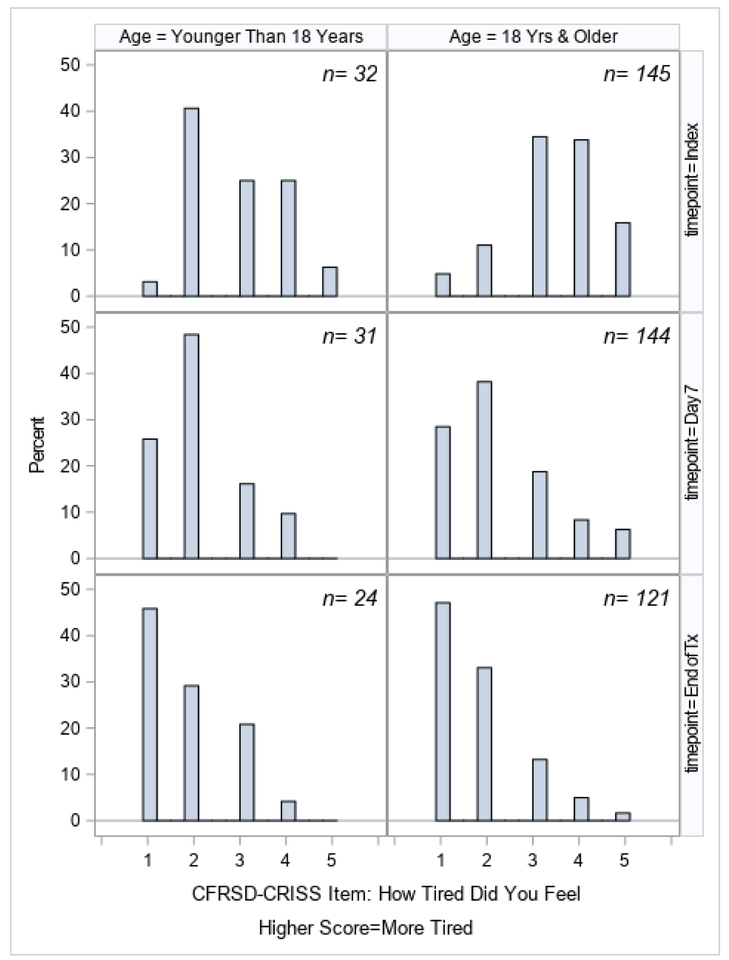
Changes in ppFEV1 from the index day until the end of treatment, stratified by age, are shown in Figure 1. Although ppFEV1 improved in both age categories, patients <18 started with higher ppFEV1 and had greater improvement, with the mean ppFEV1 changing from 69.1% at index to 79.0% by the end of treatment, compared to 47.6% to 55.2% in the group ≥18. Changes in the CFRSD-CRISS summary score and individual symptom scores are shown in Figures 2a–2i. Again, both age groups improved dramatically over time as their exacerbations were treated. The greatest improvements were seen in the symptoms of difficulty breathing, how tired the patients felt, how severe their coughs were, and how much chest tightness they experienced.
Figure 1.
Percent predicted FEV1 (ppFEV1) at index, day 7, and end of treatment, stratified by age category.
Figure 2a.
Summary CFRSD-CRISS score at index, day 7, and end of treatment, stratified by age category.
Figure 2i.
CFRSD-CRISS responses about wheezing at index, day 7, and end of treatment, stratified by age category.
Spearman correlation and Cohen’s kappa coefficients between the changes in the individual and summary CFRSD-CRISS items and ppFEV1 from index to day 7 and index to the end of treatment, stratified by patient age, are shown in Table 2. Correlations were generally weak; however, from index to the end of treatment among patients <18, we did observe moderately strong correlations [22] between ppFEV1 and cough (r=−0.58 (95% CI: −0.80, −0.21)), mucus quantity (r=−0.51 (95% CI: −0.77, −0.11)), and wheezing (r=−0.53 (95% CI: −0.78, −0.14)), along with a weak-to-moderate correlation with difficulty breathing (r=−0.31 (95% CI: −0.65, −0.13)). Also, among patients <18 between index and the end of PEx treatment, we observed moderate agreement (weighted kappa 0.44 (95% CI: 0.10, 0.78)) between cough and ppFEV1 among patients, and fair agreement between ppFEV1 and difficulty breathing, chills, mucus quantity, chest tightness, and the summary CFRSD-CRISS score. There were no statistically significant correlation or kappa coefficients between the change in ppFEV1 and CFRSD-CRISS (items or summary score) in the patients <18 from index to day 7. Among patients ≥18, we observed a moderately strong correlation between ppFEV1 and the summary CFRSD-CRISS score from index to day 7 (r=−0.51 (95% CI: −0.64, −0.35)), as well as statistically significant weak-to-moderate correlations for the difficulty breathing, tired, chills, cough, mucus quantity, chest tightness, and wheezing items. While the correlations were weaker in those patients ≥18 for the changes from index to the end of treatment, they remained statistically significant for difficulty breathing, tired, cough, mucus quantity, and wheezing, with a moderate correlation for the summary CFRSD-CRISS score (r=−0.38 (95% CI: −0.52, −0.21)). Among adults from index to day 7, we observed fair agreement between ppFEV1 and feeling tired, but agreement was poor in all other analyses both between index and day 7 and index to the end of PEx treatment.
Table 2.
Spearman correlation coefficients (ρ),* Cohen’s kappa coefficients (Κ), and 95% confidence intervals between change in ppFEV1 and change in CFRSD-CRISS items.
| Index to Day 7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CRFSD-CRISS Item | |||||||||
| Difficulty Breathing |
Feverish | Tired | Chills | Cough | Mucus
Quantity |
Chest
Tightness |
Wheezing | Summary
CFRSD-CRISS |
|
| Age
<18 N=27 |
|||||||||
| ρ | 0.03 (−0.35, 0.41) | 0.13 (−0.26, 0.49) | 0.18 (−0.21, 0.52) | 0.11 (−0.28, 0.47) | −0.02 (−0.40, 0.36) | −0.20 (−0.54, 0.20) | −0.04 (−0.41, 0.34) | −0.19 (−0.53, 0.20) | 0.21 (−0.19, 0.55) |
| Κ | 0.03 (−0.20, −0.27) | −0.04 (−0.17, 0.09) | 0.06 (−0.28, 0.39) | −0.03 (−0.16, 0.11) | 0.05 (−0.25, 0.35) | 0.03 (−0.39, 0.45) | −0.05 (−0.29, 0.19) | 0.02 (−0.14, 0.18) | 0.14 (−0.16, 0.43) |
| Age ≥18 N=101 |
|||||||||
| ρ | −0.33 (−0.50, −0.15) | −0.19 (−0.38, 0.001) | −0.28 (−0.45, −0.09) | −0.31 (−0.47, −0.12) | −0.40 (−0.55, −0.22) | −0.27 (−0.44, −0.08) | −0.20 (−0.38, −0.01) | −0.32 (−0.48, −0.13) | −0.51 (−0.64, −0.35) |
| Κ | 0.12 (−0.04, 0.28) | 0.04 (−0.06, 0.15) | 0.25 (0.08−0.43) | 0.10 (−0.02, 0.22) | 0.11 (−0.04, 0.25) | 0.14 (−0.05, 0.34) | 0.01 (−0.16, 0.18) | 0.15 (0.002, 0.29) | 0.18 (−0.02, 0.38) |
| Index to End of Treatment | |||||||||
| Age
<18 N=22 | |||||||||
| ρ | −0.31 (−0.65, −0.13) | −0.17 (−0.55, 0.27) | 0.09 (−0.35, 0.49) | 0.27 (−0.17, 0.62) | −0.58 (−0.80, −0.21) | −0.51 (−0.77, −0.11) | −0.38 (−0.69, 0.05) | −0.53 (−0.78, −0.14) | −0.46 (−0.74, −0.05) |
| Κ | 0.21 (−0.11, 0.54) | 0.05 (−0.02, 0.13) | −0.09 (−0.33, 0.16) | −0.26 (−0.50, −0.02) | 0.44 (0.10, 0.78) | 0.30 (−0.04, 0.65) | 0.21 (−0.01, 0.43) | 0.18 (0.04, 0.32) | 0.25 (−0.16, 0.66) |
| Age
≥18 N=118 |
|||||||||
| ρ | −0.33 (−0.48, −0.16) | −0.17 (−0.34, 0.01) | −0.19 (−0.36, −0.01) | −0.09 (−0.27, 0.09) | −0.23 (−0.39, −0.05) | −0.24 (−0.40, −0.06) | −0.17 (−0.34, 0.01) | −0.27 (−0.43, −0.10) | −0.38 (−0.52, −0.21) |
| Κ | 0.18 (0.05, 0.31) | 0.08 (−0.01, 0.16) | 0.07 (−0.06, 0.19) | 0.06 (−0.03, 0.16) | 0.05 (−0.10, 0.20) | 0.09 (−0.07, 0.26) | −0.09 (−0.20, 0.03) | 0.11 (−0.02, 0.23) | 0.08 (−0.09, 0.25) |
Bolded results indicate p<0.05.
The proportions of patients whose ppFEV1 and summary CFRSD-CRISS stayed the same/got worse or improved over the two time periods are shown in Table 3. Across both age groups and in both time periods, most patients (70%−78%) had improvements in both lung function and CF symptoms. While few patients had improvements in ppFEV1 but not summary CFRSD-CRISS scores (ranging from 0–8% of patients), substantial proportions (ranging from 15%−23%) of patients showed improvements in the CFRSD-CRISS summary scores, but their lung function stayed the same or worsened.
Table 3.
Proportions of patients whose summary CFRSD-CRISS scores and ppFEV1 worsened, stayed the same, or improved from index until 1) day 7 or 2) end of PEx treatment.
| Index to Day 7 | |||
|---|---|---|---|
| Age
<18 N=27 |
ppFEV1 Same/Worse at Day 7 | ppFEV1 Improved at Day 7 | Total |
| CFRSD-CRISS Same/Worse at End of Treatment | 1 (4%) | 1 (4%) | 2 (7%) |
| CFRSD-CRISS improved at End of Treatment | 4 (15%) | 21 (78%) | 25 (93%) |
| Total | 5 (19%) | 22 (81%) | 27 |
| Index to Day 7 | |||
| Age
≥18 N=101 |
ppFEV1 Same/Worse at Day 7 | ppFEV1 Improved at Day 7 | Total |
| CFRSD-CRISS Same/Worse at End of Treatment | 5 (5%) | 5 (5%) | 10 (10%) |
| CFRSD-CRISS improved at End of Treatment | 17 (17%) | 74 (73%) | 91 (90%) |
| Total | 22 (22%) | 79 (78%) | 101 |
| Index to End of Treatment | |||
| Age
<18 N=22 |
ppFEV1 Same/Worse at End of Treatment | ppFEV1 Improved at End of Treatment | Total |
| CFRSD-CRISS Same/Worse at End of Treatment | 1 (5%) | 0 | 1 (5%) |
| CFRSD-CRISS improved at End of Treatment | 5 (23%) | 16 (73%) | 21 (95%) |
| Total | 6 (27%) | 16 (73%) | 22 |
| Index to End of Treatment | |||
| Age
≥18 N=118 |
ppFEV1 Same/Worse at End of Treatment | ppFEV1 Improved at End of Treatment | Total |
| CFRSD-CRISS Same/Worse at End of Treatment | 5 (4%) | 9 (8%) | 14 (12%) |
| CFRSD-CRISS improved at End of Treatment | 21 (18%) | 83 (70%) | 104 (88%) |
| Total | 26 (22%) | 92 (78%) | 118 |
Discussion
The results of this study indicate that, although lung function and patient-reported symptoms improve in the days immediately following treatment for PEx, changes between the two metrics were generally weakly correlated. While we did observe some moderately strong correlations among those <18 years in some of the items on the CFRSD-CRISS questionnaire such as cough, mucus quantity, and wheezing, these associations were not as strong as we expected. Among CF patients over 18 years of age, we observed a moderate correlation in the overall symptom score after 7 days of treatment, but only weak to moderate correlations between lung function and the individual patient-reported symptoms at the end of treatment. Furthermore, we identified substantial proportions of patients whose lung functions declined over the course of treatment but reported improvements in their CF symptoms overall. These results indicate that patients’ assessments of their symptoms are important complements to measures of their lung function when evaluating novel treatments for PEx, especially among patients who are over 18 years old.
Other publications have found smaller than expected associations between clinical measures of CF patients and metrics of quality of life. A study by Heltshe at al conducted from 2007–2010 found no association between symptom improvement (measured by the CFRSD-CRISS) and lung function more than 3 months after treatment for PEx [23]. In a study conducted in Portugal, the authors evaluated the relationship between the CFQ-R and pulmonary function and found correlation coefficients ranging from 0.4–0.5 for outcomes such as treatment burden, digestive symptoms, and physical functioning [24]. Similarly weak to moderate correlations were found between pulmonary metrics and the CFQ-R in a Brazilian population [25]. Previous work using the population from the STOP study also found little correlation between the change in EQ-5D-5L and lung function over the time that PEx were being treated [26].
One interesting finding of this study was that, as expected, lung function was much better in children <18 years old compared to adults at all timepoints, but the patients’ responses to the symptom specific CFRSD-CRISS questions were fairly similar between the two age groups in terms of improvement during the course of the exacerbation. This suggests that while CF patients report symptoms consistently during their exacerbations, people who have been living with the disease for longer periods appear to have adapted to poorer lung function and ability to recover post-exacerbation. A recent study examining respiratory muscle strength and lung function evaluated CF patients between age 8 and 33 years old and did not find an association between age and exercise performance, but showed a similar pattern of better lung function in children with CF [27]. However, the impact of exacerbations in adults relative to children likely manifests itself well beyond lung function symptoms, for example in areas such as emotional distress [28]. It is critical to recognize this difference in lung function by age group when assessing the robustness of the relationship between lung function and CF symptoms in order to avoid inaccurate conclusions.
This study assessed a relatively large number of patients who had metrics of both lung function and CF symptoms as they were being treated for PEx. However, we were limited by small numbers of patients <18, and perhaps our results would have been different if we had had more patients in this age group. Furthermore, because this was an observational study, patients received a variety of treatments for their exacerbations and it is possible that these treatments affected their lung function and their reports of their symptoms differently, which could have affected the correlation coefficients. Our results may also have been affected by selection bias in that patients who had more symptoms and/or worse lung function may have been too sick to contribute data at each time point. Finally, although ppFEV1 is a commonly used metric of lung function in CF trials, improvements in CF treatment have slowed the decline in ppFEV1 and its usefulness as an outcome measure for disease progression is decreasing [29]. It is possible that other measures of lung function such as the lung clearance index (LCI) may be more strongly correlated to CF symptoms.
In conclusion, more work is needed to identify instruments that measure PEx outcomes that are relevant both clinically and to CF patients’ quality of life. The fact that we identified only moderately strong correlations between ppFEV1 and symptoms such as cough severity, mucus quantity, and wheezing, and those only among patients <18, indicates that measures of pulmonary function alone do not capture the totality of PEx sequelae that are relevant to patients. Future studies should assess both clinical biomarkers such as lung function and assess patient reports of how their symptoms are affected by PEx treatments.
Figure 2b.
CFRSD-CRISS responses about difficulty breathing at index, day 7, and end of treatment, stratified by age category.
Figure 2c.
CFRSD-CRISS responses about how feverish the patient felt at index, day 7, and end of treatment, stratified by age category.
Figure 2d.
CFRSD-CRISS responses about how tired the patient felt at index, day 7, and end of treatment, stratified by age category.
Figure 2e.
CFRSD-CRISS responses about how bad chills or sweats were at index, day 7, and end of treatment, stratified by age category.
Figure 2f.
CFRSD-CRISS responses about cough at index, day 7, and end of treatment, stratified by age category.
Figure 2g.
CFRSD-CRISS responses about mucus quantity at index, day 7, and end of treatment, stratified by age category.
Figure 2h.
CFRSD-CRISS responses about chest tightness at index, day 7, and end of treatment, stratified by age category.
Acknowledgement:
This work was supported by a Cystic Fibrosis Foundation Therapeutic (CFFT) award: “Cost Effectiveness Analysis and Comparative Effectiveness Research Component of STOP 2.” Award Number: KESSLE17AB0. Drs. Kessler and Goss are both supported by funding from the NIH (P30 DK089507) for the development of outcomes measures in CF.
Abbreviation List
- CF
Cystic fibrosis
- US
United States
- PEx
Pulmonary exacerbations
- EQ-5D-5L
EuroQOL-5 Dimensions
- CFRSD-CRISS
Cystic Fibrosis Respiratory Symptom Diary- Chronic Respiratory Infection Symptom Score
- ppFEV1
Percent predicted of forced expiratory volume in 1 second
- STOP
Standardized treatment of pulmonary exacerbations
- IV
Intravenous
Footnotes
Conflict of Interest Statement:
Laura Gold has no conflicts of interest to report.
Donald Patrick has no conflicts of interest to report.
Ryan Hansen has no conflicts of interest to report.
Christopher Goss has no conflicts of interest to report.
Larry Kessler has no conflicts of interest to report.
References
- [1].Cystic Fibrosis Foundation (CFF). About Cystic Fibrosis. Cystic Fibrosis Foundation 2018. [Google Scholar]
- [2].Reid DW, Blizzard CL, Shugg DM, Flowers C, Cash C, Greville HM. Changes in cystic fibrosis mortality in Australia, 1979–2005. Med J Aust. 2011;195:392–5. [DOI] [PubMed] [Google Scholar]
- [3].Cystic Fibrosis Foundation (CFF). Cystic Fibrosis Foundation Patient Registry: 2017 Annual Data Report. In: (CFF) CFF, editor. Bethesda, MD: 2017. [Google Scholar]
- [4].Yang C, Montgomery M. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. 2018;9:CD001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rubin JL, Thayer S, Watkins A, Wagener JS, Hodgkins PS, Schechter MS. Frequency and costs of pulmonary exacerbations in patients with cystic fibrosis in the United States. Curr Med Res Opin. 2017;33:667–74. [DOI] [PubMed] [Google Scholar]
- [7].Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N Engl J Med. 2017;377:2013–23. [DOI] [PubMed] [Google Scholar]
- [8].Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128:2347–54. [DOI] [PubMed] [Google Scholar]
- [10].Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009;8:245–52. [DOI] [PubMed] [Google Scholar]
- [11].Sanders DB, Solomon GM, Beckett VV, West NE, Daines CL, Heltshe SL, et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: Observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2017;16:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].University of Washington.SEAQOL Instruments. Seattle: University of Washington; 2017. [Google Scholar]
- [13].Boone WJ. Rasch Analysis for Instrument Development: Why, When, and How? CBE Life Sci Educ.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].West NE, Beckett VV, Jain R, Sanders DB, Nick JA, Heltshe SL, et al. Standardized Treatment of Pulmonary Exacerbations (STOP) study: Physician treatment practices and outcomes for individuals with cystic fibrosis with pulmonary Exacerbations. J Cyst Fibros. 2017;16:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stanojevic S GLI-2012 SAS Macro In: resources e-l, editor.2013. [Google Scholar]
- [16].VanDevanter DR, Heltshe SL, Spahr J, Beckett VV, Daines CL, Dasenbrook EC, et al. Rationalizing endpoints for prospective studies of pulmonary exacerbation treatment response in cystic fibrosis. J Cyst Fibros. 2017;16:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spearman C The proof and measurement of association between two things. Am J Psychol. 1904;15:72–101. [PubMed] [Google Scholar]
- [18].Ruscio J Constructing Confidence Intervals for Spearman’s Rank Correlation with Ordinal Data: A Simulation Study Comparing Analytic and Bootstrap Methods Journal of Modern Applied Statistical Methods 2008;7:416–34. [Google Scholar]
- [19].Caruso JC, Cliff N. Empirical size, coverage, and power of confidence intervals for Spearman’s rho. Educ Psychol Meas. 1997;57:637–54. [Google Scholar]
- [20].Cohen J Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–20. [DOI] [PubMed] [Google Scholar]
- [21].Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S–39S. [DOI] [PubMed] [Google Scholar]
- [22].Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- [23].Heltshe SL, Goss CH, Thompson V, Sagel SD, Sanders DB, Marshall BC, et al. Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. Thorax. 2016;71:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ribeiro Moco VJ, Lopes AJ, Vigario Pdos S, de Almeida VP, de Menezes SL, Guimaraes FS. Pulmonary function, functional capacity and quality of life in adults with cystic fibrosis. Rev Port Pneumol (2006). 2015;21:198–202. [DOI] [PubMed] [Google Scholar]
- [25].Gancz DW, Cunha MT, Leone C, Rodrigues JC, Adde FV. Quality of life amongst adolescents and young adults with cystic fibrosis: correlations with clinical outcomes. Clinics (Sao Paulo). 2018;73:e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gold LS, Patrick DL, Hansen RN, Beckett V, Goss CH, Kessler L. Correspondence between symptoms and preference-based health status measures in the STOP study. J Cyst Fibros. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sovtic A, Minic P, Markovic-Sovtic G, Trajkovic GZ. Respiratory Muscle Strength and Exercise Performance in Cystic Fibrosis-A Cross Sectional Study. Front Pediatr. 2018;6:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schmid-Mohler G, Caress AL, Spirig R, Benden C, Yorke J. “Thrust out of normality”-How adults living with cystic fibrosis experience pulmonary exacerbations: A qualitative study. J Clin Nurs. 2019;28:190–200. [DOI] [PubMed] [Google Scholar]
- [29].Sonneveld N, Stanojevic S, Amin R, Aurora P, Davies J, Elborn JS, et al. Lung clearance index in cystic fibrosis subjects treated for pulmonary exacerbations. Eur Respir J. 2015;46:1055–64. [DOI] [PubMed] [Google Scholar]