Abstract
For many years, oncology phase I trials have been referred to as ‘toxicity trials’ and have been believed to have low clinical utility other than that of establishing the adverse event profile of novel therapeutic agents. The traditional distinction of clinical trials into three phases has been challenged in the past few years by the introduction of targeted therapies and immunotherapies into the routine management of patients with cancer. This transformation has especially affected early phase trials, leading to the current situation in which response rates are increasingly reported from phase I trials. In this Perspectives, we highlight key elements of phase I trials and discuss how each one of them contributes to a new paradigm whereby preliminary measurements of the clinical benefit from a novel treatment can be obtained in current phase I trials, which can therefore be considered to have a therapeutic intent.
Traditional drug development in oncology involves three sequential phases of clinical trials: phase I trials encompass the first exploration of new therapies in humans (typically in a limited number of patients); phase II studies involve the first formal evaluation of therapeutic activity in a small cohort of patients; and phase III trials are randomized comparative studies of the efficacy of the experimental therapy relative to that of standard-of-care treatment1–3. In the past few years, these definitions have become less rigid, and early phase trials are increasingly including efficacy end points and large expansion cohorts (for example, KEYNOTE-001 () or CheckMate 032 ()); however, the paradigmatic division of clinical testing into three phases prevails in oncology.
Phase I studies are the cornerstone of translating findings from preclinical research into clinical practice and are used by clinical investigators to determine the recommended dose and schedule of an experimental therapeutic compound4. Thus, phase I trials have been historically referred to as ‘toxicity trials’ and are widely viewed as studies aimed at elucidating the adverse event (AE) and pharmacokinetic profiles of experimental agents, with limited or no therapeutic intent. Determining the safety of an agent remains the mainstay of phase I clinical trials; however, with the increasing availability of molecularly targeted agents associated with biomarkers that enable refined patient selection, the majority of phase I trials could have a therapeutic aim, even in the first-in-human setting. Furthermore, phase I studies are increasingly incorporating phase II extensions to demonstrate efficacy and, since the beginning of this millennium, the era of phase I trials with small and unselected cohorts of patients has begun to pass5. Instead, drug development in oncology is being transformed by the arrival of agents with well-defined mechanisms of action and targets, and hence with potential efficacy that can be unveiled rapidly via innovatively designed phase I trials enrolling hundreds of patients6. Accordingly, in the past few years, the FDA has approved investigational drugs on the basis of results from phase I studies. Examples include ceritinib, which was approved for patients with ALK-rearranged non-small-cell lung cancer (NSCLC) on the basis of an overall response rate (ORR) of 58% reported in a phase I study;7,8 and the immune-checkpoint inhibitor (ICI) pembrolizumab, which was approved for patients with melanoma on the basis of a 38% ORR observed in a dose-expansion cohort of a first-in-human phase I trial9,10. Regardless, whether or not trials of experimental drugs in early stages of clinical development should be presented to patients as offering the opportunity of therapeutic benefit remains controversial11.
The concept that, in addition to providing the first data on the toxicity profile of experimental agents, phase I trials can also provide a preliminary indication of the therapeutic value of such agents has been advanced by a deep understanding of the molecular and immune underpinnings of cancer, the development of highly specific molecularly targeted agents, and the increasing use of biomarkers for patient selection, even in the phase I setting. Nevertheless, although the outcomes of phase I trials incorporating these principles are generally more favourable than those of traditional phase I trials, the oncology community should acknowledge that phase I oncology trials encompass a variety of designs and test agents with distinct modes of action, and thus outcomes can differ depending on whether the therapies tested involve combinations including approved agents versus non-approved agents as monotherapy and whether or not large expansion cohorts (sometimes involving hundreds of patients) are included12. The ORRs observed in phase I trials conducted in the 1970s and 1980s were <5%, rose to ~11% in the 1990s and are now almost 20%, or even higher (~42%) when a genomic biomarker is used for patient selection13–19 (TABLE 1). For comparison, the ORRs of approved oncology drugs as monotherapy are often >20%20. Of note, an analysis of drug approvals has revealed that an ORR of >30% with a monotherapy is likely to lead to accelerated approval by the FDA20. Indeed, the positive predictive power of an ORR of >15% for regulatory approval of an anticancer drug is 76% and rises to 89% if the ORR is >30%20. Furthermore, the incidence of grade 5 (fatal) AEs that are at least possibly drug-related is typically low in phase I trials: ~0.5% for agents tested as monotherapy15,21.
Table 1 |.
Response rates observed in selected oncology phase I trials
| Series | Period covered | Trials included (n) | Patients (n) | Agents tested (n) | ORR | Grade 5 AEs at least possibly related to drug | Ref. |
|---|---|---|---|---|---|---|---|
| Estey et al. (1986) | 1974–1982 | 187 | NR | 54 | 4.2% | NR | 13 |
| Decoster et al. (1990) | 1972–1987 | 211 | 6,639 | 87 | 4.5% | 0.5% | 14 |
| Horstmann et al. (2005) | 1991–2002 | 460 | 11,935 | NR | 10.6% | 0.49%; | 15 |
| Roberts et al. (2004) | 1991–2002 | 213 | 6,474 | 149 | 3.8% | 0.54% | 16 |
| Schwaederle et al. (2016) | 2011–2013 | Biomarker-driven trials of targeted agents: 57 | Biomarker-driven trials: 2,655 | NR | 31.1% (42% in the case of genomic biomarkers) | 1.9% | 17 |
| Non-biomarker-driven trials of targeted agents: n = 177 | Non-biomarker-driven trials: n = 10,548 | 5.1% | NR | ||||
| Non-biomarker-driven trials of cytotoxic agents: n = 116 | Non-biomarker-driven trials of cytotoxic agents: 4.7% | Non-biomarker-driven trials of cytotoxic agents: 2.2% | |||||
| Waligora et al. (2018) | 2004–2015 | 170 | 4,604 | NR | 10.29% | 2.09% | 18 |
| Chakiba et al. (2018) | 2014–2015 | 224 | NR | 224 | 19.8% | NR | 19 |
AE, adverse event; NR, not reported; ORR, overall response rate
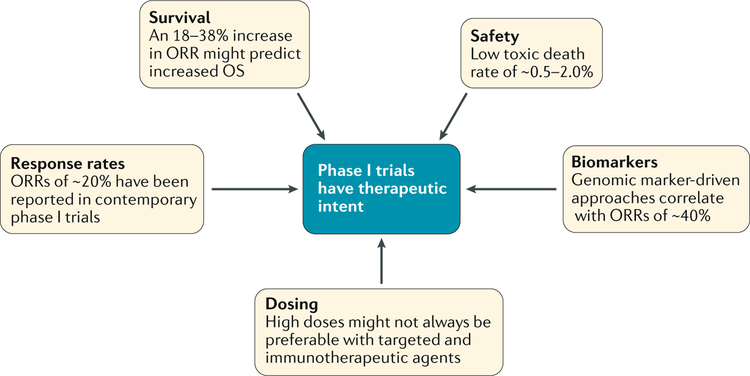
In this Perspectives, we propose that, in addition to providing the first evaluation of the toxicity profile of a therapeutic agent, important information on the antitumour activity of such agents can be obtained in contemporary phase I trials. We aim to dissect the ethical dilemmas surrounding the therapeutic intent of phase I trials, and to provide evidence of how these trials have evolved in the past few years and how this evolution has contributed to the concept that current phase I trials can be a therapeutic option for patients with cancer (FIG. 1).
Fig. 1 |. Phase I trials as valid therapeutic options.
The design adaptations made in phase I trials in the past few years (in aspects including dosing63, biomarkers8,9,17,30,56,70, safety14, survival71 and responses18) have helped them to become valid therapeutic options. We propose that researchers can anticipate therapeutic responses in contemporary phase I trials and therefore these trials can be considered to have therapeutic intent.
Ethical considerations
Phase I trials in oncology involve the administration of experimental therapies with unproven effects to patients with terminal disease, and thus the ethics of such studies remain the subject of intense debate, especially in regard to therapeutic intent11. Nevertheless, ASCO has released a policy statement asserting the importance of phase I clinical trials as a treatment modality with potential clinical benefits for patients with advanced-stage malignancies22. The position of ASCO is that investigators can and should present phase I trial enrolment as providing the “prospect of a direct medical benefit”22. Kimmelman has refuted this stance by stating that “the therapeutic position is equivalent to saying that the [risk:benefit ratio] for receiving experimental drugs in phase I trials is consistent with and possibly superior to standard of care”11 and that participation in phase I studies could be considered as an approach consistent with competent medical care but that the administration of an experimental drug should be looked at as a research procedure “akin to a research biopsy”11. Other authors have stated that these trials have a “paucity of benefits with substantial risks”12 and that “interview surveys indicate that substantial proportions of trial participants do not understand the purpose of these trials”23. Some authors have contrary views. For instance, Saad et al.24 have stated that patients participate in phase I trials because they expect to derive clinical benefit; these authors built on this premise to make a case for randomly assigning patients to different interventions as early as phase I trials.
In our experience, some of these statements offer a distorted view of the therapeutic intent of phase I trials, for several reasons. First, in regard to the claim regarding the risk:benefit ratio of agents in phase I trials11, in order to be eligible for a phase I trial, most patients must have shown tumour progression after receiving standard-of-care treatments, although patients can also seek to be enrolled in a phase I trial before having exhausted all approved treatment options available to them25. Second, data derived from phase I studies conducted in the 1980s and 1990s indicate that the ORRs were ~5%13,16,26, but higher response rates (~20%) have been observed in contemporary phase I trials19.
Another reason posited against the potential therapeutic intent of phase I trial participation is that the results of meta-analyses of clinical trials are unreliable because of problems such as ‘natural regression’11, which is defined as improvement in the radiological response of patients with cancer after receiving placebo in randomized controlled trials27, and has been documented to occur in 2.7% of patients on the basis of a systematic review of data from 47 randomized trials27. However, our decades-long clinical experience suggests that spontaneous responses actually occur in <1% of patients with treatment-refractory metastatic cancers.
A final argument against the therapeutic intent of phase I trials is that only approximately one in ten agents entering phase I trials achieve FDA approval; therefore, by definition, studies designed to determine the therapeutic benefit from the remaining 90% of agents are not worth pursuing because the agents are not active11. This assumption is false; therapeutic agents fail to progress to FDA approval for multiple reasons other than lack of efficacy28. For example, sometimes, an agent tested in a phase I trial fails to gain approval because the patient populations involved in early phase studies represented a large share of the potential market for an investigational drug, rather than being enriched with those patients who would have been most likely to respond to the drug as was seen in ECHO-301/KEYNOTE-25229. Furthermore, the cost of phase II and III studies can exceed US$100 million, and thus pharmaceutical companies might not pursue further stages of drug development for financial reasons.
Statistical considerations
New study designs attenuate some of the aspects of phase I clinical trials that might have limited the possibility of demonstrating clinical benefit. Perhaps the most striking example is the inclusion of biomarker measurements in the trial eligibility criteria in order to select those patients most likely to benefit from a given treatment; as a corollary, outcomes are increasingly analysed in subgroups of patients harbouring a particular feature and receiving treatment, rather than in all screened patients. The impact of this study design on ORRs is perhaps best exemplified by the study of larotrectinib in patients with NTRK fusion-positive cancers, which resulted in an ORR of 75% in the phase I portion of the trial30, yet NTRK fusions are found in less than 1% of the cancer population31. This model can also be exploited more broadly in platform trials that include multiple substudies, wherein each patient can be genotyped and allocated to receive a treatment that is rationally matched to the genomic alterations detected in their tumour (if actionable)32,33. Additionally, the use of Bayesian statistical modelling tools to minimize the number of patients receiving inactive doses of therapeutic agents is also meant to maximize the potential for beneficial effects in the phase I setting34. One such example is the study of the combination of lenvatinib and everolimus in patients with metastatic renal cell carcinoma (E7080-G000–218 ()), in which patients are randomly assigned to receive either a high or a low dose of lenvatinib with flexibility for dose adjustments on the basis of responses and/or toxicities. Newer designs that enable intra-patient dose escalation and, hence, rapid titration of new combinations of drugs could also improve the chances of achieving clinically meaningful antitumour activity in phase I trials35. Finally, phase I study designs that incorporate dose-expansion cohorts and even randomization24 are additional strategies to improve the statistical power and potentially enhance the readout of potential therapeutic efficacy associated with phase I trials (for example, KEYNOTE-001, CheckMate 032 and SHRINK ()).
End point considerations
Another reason that has been stated as an argument for caution in assuming that clinical benefit can be obtained in phase I trials relates to the lack of consensus on whether or not a favourable ORR translates into a survival advantage; the promising ORRs observed in many phase II trials are not predictive of survival benefits in subsequent phase III studies36,37. Nevertheless, extensive evidence is also available in the literature indicating that ORRs and progression-free survival (PFS) can be valuable surrogate end points, especially when threshold values are used38,39. Johnson et al.39 defined the thresholds that enable reliable prediction of an extension of overall survival to be either an 18–38% increase in ORR or an incremental PFS gain of 1.8–3.3 months. Nevertheless, analyses of patient data conducted by Buyse et al.40, Burzykowski et al.41 and Blumenthal et al.42 in the settings of advanced-stage colorectal cancer, breast cancer and NSCLC, respectively, led to conclusions contrary to those found by Johnson et al.39; these studies found no significant correlations. These analyses involved patients receiving cytotoxic chemotherapy and, thus, the results might not apply to newer agents with a specific mechanism of action that, accordingly, would be expected to have higher levels of antitumour activity. Regardless, an assessment of the 31 anticancer agents approved by the FDA between 1973 and 2006 on the basis of ORR or PFS data from single-arm trials demonstrated that these compounds have positive long-term safety and efficacy outcomes and remain recommended for the indication for which they were initially approved43. With the increasing number of trials of immunotherapy approaches, the use of immune response evaluation criteria in solid tumours (iRECIST)44, rather than RECIST, might also help attenuate potential errors in radiological evaluation and thereby improve the assessment of therapeutic responses. The DART trial () is an example of a trial in which iRECIST is used in addition to RECIST to better evaluate responses in patients. Furthermore, biomarker assessment using baseline and serial follow-up assays can improve the evaluation of trial outcomes. One such example is the analysis of circulating tumour DNA (ctDNA) in plasma for patient selection (at baseline) and follow-up samples, which show decreasing or increasing levels of ctDNA in plasma (reflecting therapeutic response or disease progression, respectively)45,46.
Policy and access to treatment
Importantly, one of the most problematic aspects of proposing that phase I trials can have a therapeutic intent relates to the fact that such a position has policy implications. In particular, if an agent is considered potentially therapeutic on the basis of preclinical and early phase clinical evidence alone, why should we restrict access to that agent only to patients enrolled in early phase clinical trials? This question is a reasonable one. However, implicit in countering this question is the general agreement that restricting access is appropriate owing to inadequate therapeutic benefit. Yet, a widespread effort across the USA in promoting access to medicines has resulted in the federal Right to Try law (referring to experimental drugs), which reflects legislative disagreement (admittedly still controversial) with the current situation in which access to experimental medicines is limited47,48.
In this regard, Kimmelman11 states that compassionate-use policies restrict access until after phase I studies are complete, indicating that “drugs should not be considered therapeutic until after phase I studies are completed”11. However, the FDA does not restrict the compassionate use of medicines to those that have been tested in completed phase I trials. Indeed, compassionate use has been granted before completion of early phase trials49. The lack of restrictions from the FDA in this regard could, by extension, be construed as evidence that these drugs are indeed considered therapeutic in the phase I setting. Additionally, the authors of a study assessing the safety and efficacy of the 208 treatments approved under the Right to Try law for compassionate use in 179 patients between 2012 and 2017 found that the ORR was 20.1%50.
Another important issue to consider is the fairness argument: declaring that phase I trials have therapeutic intent raises profound questions of fairness because such studies tend to disproportionately involve affluent patients in urban areas. This fairness argument implies that the participation in phase I trials as a therapeutic option will create a bias against less-affluent and/or remote populations11. However, the desire to state that we are fair and ethical is not, and cannot be, a rationale for claiming that a drug is or is not therapeutic. This claim would suggest that expensive anticancer drugs that are approved cannot be considered therapeutic if people in low-income and middle-income countries or those in high-income countries without health insurance cannot access them. Importantly, we must acknowledge that not all treatments are easily affordable and that issue needs to be addressed for many patients worldwide; however, arguing that lack of access to certain drugs reflects a lack of activity of those drugs is not a viable stance. Another important ethical consideration is that the informed consent process ensures that patients are presented with all the potential risks and benefits of entering a trial, including an accurate portrayal of the known activity of the drug. This mechanism protects patients and maintains their autonomy in the patient-decision-making process51.
Eligibility criteria
The large number of eligibility criteria required in phase I trials can be seen to support the position that phase I trials do not have a therapeutic intent11. According to these claims, if these drugs provide a real therapeutic benefit, the trials should not have so many restrictive eligibility criteria. For example, some patients are excluded from these trials because of extenuating circumstances, such as having lesions that cannot be biopsied or requiring a repeat biopsy for trial participation52; excluding a patient from a study with therapeutic intent owing, for example, to their tumour being inaccessible for biopsy would be unethical because such a procedure serves only to investigate a scientific question. In addition, the variable availability of openings for trial participation can make the enrolment of patients with certain disease subtypes difficult53. While we agree that these reasons for excluding patients from phase I clinical trials are not fair if the drug involved provides clinical benefit, we do not agree with the view that the existence of exclusions means that no or inadequate therapeutic benefit exists. Again, our desire to be perceived as ethical cannot be the deciding factor in determining whether or not a drug is therapeutic. While the ethical dilemma about patient inclusion is important, the number of eligibility criteria is not related to the activity or lack thereof of a drug. Eligibility criteria must be put in place in order to protect patients (in case of comorbidities that might make them vulnerable to toxicities) and because one of the high priorities of a clinical trial is to prove activity to regulatory agencies, an aim that is best achieved by enrolling participants with a better performance status.
Indeed, some of these restrictive eligibility criteria might be questionable, especially when accurate biomarkers are used to carefully select patients, thereby potentially improving the therapeutic index. For example, the availability of genomic tests to detect EGFR or ALK alterations, each of which is found in a small percentage of patients with NSCLC (11–16% and 3–6%, respectively, in Western countries)54, or NTRK fusions (a very rare alteration across various cancer types)31, has enabled the enrolment of patients who are likely to benefit from molecularly targeted agents matched to these aberrations. In these populations, high response rates (>50%) were found in phase I trials and confirmed in subsequent studies8,30,55. Other illustrative cases abound. For example, agents with high specificity for specific molecular alterations, such as inhibitors of RET fusion proteins, have led to remarkable ORRs across tumour subtypes56. In patients with acquired RET fusions and resistance to the EGFR inhibitor osimertinib, treatment with the novel RET inhibitor BLU-667 induced a rapid radiographic response in two patients who received the drugs but would not have met the eligibility criteria for enrollment in a trial because they harboured EGFR mutations57. The examples presented herein related to highly efficacious drugs with limited toxicities in the current era of phase I clinical trials; the need for special eligibility criteria in trials of these drugs must be acknowledged.
Incidence of toxicities
The issue of handling an unknown toxicity profile is also presented as an argument against the therapeutic intent of phase I trials and, indeed, ~70% of clinically relevant toxicities are identified in phase I trials58. Of note, the possibility of encountering catastrophic AEs, such as those derived from treatment with TGN141259, always exists, but such events are uncommon. Overall, death that is at least possibly related to drug toxicity occurs in 0.5–2% of patients enrolled in phase I trials (TABLE 1).
With regard to ICI, the authors of a study of 25 trials of anti-programmed cell death 1 (PD-1) or anti-programmed cell death 1 ligand 1 (PD-L1) antibodies including 6,473 patients found that, in trials with >118 patients, the incidence of toxicities reported in early phase trials was consistent with that observed for the same agent in later phase trials (P = 0.048)60. The only common toxicity that was reported more often in later-phase studies was colitis (P = 0.045)60.
Along these lines, a 3.6% frequency of grade ≥3 AEs at least possibly related to treatment was observed in the iPREDICT study in patients who received agents with a high matching score for their tumour genomic profile (including de novo combinations) compared with 15.6% in patients who received agents with a low matching score35. Taken together, these data suggest that early phase trials have acceptable safety profiles. Furthermore, important toxicities can also be discovered after an agent is approved by the FDA and in post-approval surveillance analyses61,62, and do not detract from viewing that drug as a therapeutic agent.
Effect on drug development
The potential therapeutic intent of phase I trials has additionally been posited to be incompatible with practices that are important for viable efforts in drug development11. For example, problems with dose escalation are pertinent, with the assumption of a linear relationship between dose and efficacy. In such scenarios, giving low doses of therapeutic agents to patients in the context of dose-escalation schemes in phase I trials seems hard to justify. The supposition that higher doses always yield better outcomes, however, does not necessarily apply to all targeted agents nor, in particular, to immunotherapies63. Nevertheless, doses beyond those offered to the initial dose-escalation group, at which antitumour activity is detected and that do not exceed the maximum-tolerated dose, are those with the most favourable prospect of clinical benefit in phase I trials63,64. Using study designs such as intrapatient dose escalations or n-of-1 initial cohorts enables expedient dose escalation and dose-finding studies and can help minimize the potential for underdosing65.
Importantly, some ICIs do not act in a linear fashion in terms of the efficacy to toxicity relationship. For example, antibodies against cytotoxic T lymphocyte antigen 4 (CTLA-4) reach a maximal point of efficacy after which increasing the dose only elevates the risk of toxicities66, and no well-defined association between efficacy and toxicity exists for anti-PD-1 antibodies67,68. In summary, considering an experimental drug as therapeutic does not have to detract from the efforts to establish the optimal dose.
Effect on later phases of development
The final issue raised against a therapeutic intent for phase I trials is perhaps the most problematic: if a therapeutic benefit is derived from an agent tested in a phase I study, why would investigators authorize subsequent research efforts (such as randomized trials) that are crucial for generating reliable proof of clinical efficacy, but deprive one half of the patients enrolled from receiving the therapeutic agent?11 Some randomized trials, however, might no longer meet the equipoise standard38 that is the main ethical consideration in justifying a randomized controlled trial. Equipoise is based on the concept that randomization is only ethically justifiable if a state of genuine uncertainty about the relative merits of each arm of the study exists (for example, the interventions in all arms are equally likely to be beneficial) and if all arms are consistent with reasonable medical care. Furthermore, the fact that clinicians are unsure about which agent is associated with the better outcome in a certain setting does not mean that only one of the drugs should be considered clinically beneficial. Indeed, both agents can have therapeutic value and yet one might have advantages over the other in certain situations. In this scenario, a randomized trial would help optimize the use of each drug.
Conclusions
In summary, phase I trials have undergone a substantial transformation over the past decade owing to advances in preclinical research that have yielded a profound understanding of the molecular and immunological basis of cancer, and to the development of innovative technologies, such as next-generation sequencing, that enable increasingly accurate patient selection. Historically, presenting phase I trial participation as having potential therapeutic intent has been questioned, but nowadays many of the arguments against this position can be refuted. Indeed, therapeutic intent cannot be predicated upon issues of fair access to medicines or the need for studies to accurately establish dosing and efficacy in comparison with standard-of-care treatment. In phase I trials, therapeutic benefit should be measured according to the ORRs and their durability, despite potential disparities between this surrogate and survival. In the current era of drug development, the data show that ORRs of ~20% on average can be reported in phase I trials19, a value well within the range of ORRs of several drugs approved by the FDA — although important differences in ORRs exist depending on the type of trial. For example, biomarker-based patient selection is associated with an increase in ORR to 31% (and even 42% if a genomic biomarker is used)17. Lower response rates have been reported in some phase I studies, such as those of targeted agents that are given without a biomarker (~5%)17. Furthermore, some large-scale analyses of phase I studies might be biased by the inclusion of a large number of studies with expansion cohorts or combination trials19. Trials of combination therapies might enable synergy of drugs that would otherwise be less effective if used as monotherapy or combinations of agents targeting more than one subgroup of patients, thus increasing response rates.
An analysis of phase I trials has shown that possibly drug-related mortality occurs in <1% receiving experimental agents given as monotherapies15. In addition, many patients (~33% in one study21) are able to enrol in subsequent phase I trials after their tumour progresses during the initial phase I trial. Furthermore, in patients with advanced-stage cancers, the available choices might include palliative care only, off-label administration of agents (without well-established evidence of efficacy) or the administration of approved drugs (albeit after many lines of prior therapy), when benefit from treatment is marginal or uncertain. Therefore, the term ‘investigational drug’ does not necessarily contradict the term ‘therapeutic option’.
Finally, we now know that the data obtained during phase I clinical trials are fundamental to the development of the agent being tested. Trial protocols are developed to maximize clinical benefit, and the investigational nature of phase I trials should not undermine their capacity to benefit individuals who are included in them. Nonetheless, patient expectations might not be congruent with reality, and physicians should do their best to inform patients of the expected outcomes from the intervention tested and of the likelihood that enrolling in a specific trial might extend their life expectancy69. In the current evolving landscape of phase I clinical trials, which includes biomarker-based patient selection, the advent of potent immunotherapies and the potential for FDA approval on the basis of phase I results, and appropriate consent language that emphasizes the uncertainty of an individual trial, these studies can be viewed as valid therapeutic options.
Acknowledgements
The work of R.K. is funded in part by the Joan and Irwin Jacobs Fund and NIH P30 grant CA023100.
Footnotes
Competing interests
P.M.L. is an advisory board member for Agenus, Cyrexa, CytomX and Genentech; a data safety monitoring board or committee member for Agios, FivePrime and Halozyme; an imCORE Alliance member for Roche; and a consultant for SOTIO. D.S.H. receives research and/or grant support from AbbVie, Adaptimmune, Amgen, Astra-Zeneca, Bayer, BMS, Daiichi-Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa, Lilly, LOXO, Merck, MedImmune, Mirati, MiRNA, Molecular Templates, Mologen, NCI–CTEP, Novartis, Pfizer, Seattle Genetics and Takeda; travel and accommodation support from AACR, ASCO, Genmab, LOXO, MiRNA and SITC; is a consultant or adviser for Alpha Insights, Axiom, Adaptimmune, Baxter, Bayer, Genentech, GLG, Group H, Guidepoint Global, Infinity, Janssen, Merrimack, Medscape, Molecular Match, Numab, Presagia, Pfizer, Seattle Genetics, Takeda, Trieza Therapeutics and WebMD; and is a founder of OncoResponse. R.K. owns stock and has other equity interests in CureMatch, IDbyDNA and Soluventis; is a consultant or adviser for Actuate Therapeutic, Gaido, LOXO, NeoMed, Roche, Soluventis and X-Biotech; has received speaker’s fees from Roche; is a board member of CureMatch; and her institution receives research support from Foundation Medicine, Genentech, Grifols, Guardant Health, Incyte, Konica Minolta Merck Serono, OmniSeq, Pfizer and Sequenom. J.J.A. declares no competing interests.
Disclaimer
P.M.L., D.S.H. and R.K. have developed their medical career as experts in phase I trials.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
RELATED LINKS
References
- 1.Storer BE Design and analysis of phase I clinical trials. Biometrics 45, 925–937 (1989). [PubMed] [Google Scholar]
- 2.Simon R Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials 10, 1–10 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Carter SK Clinical trials in cancer chemotherapy. Cancer 40, 544–557 (1977). [DOI] [PubMed] [Google Scholar]
- 4.Cook N et al. Early phase clinical trials to identify optimal dosing and safety. Mol. Oncol 9, 997–1007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manji A et al. Evolution of clinical trial design in early drug development: systematic review of expansion cohort use in single-agent phase I cancer trials. J. Clin. Oncol 31, 4260–4267 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Postel-Vinay S & Soria JC Phase I trials in oncology: a new era has started. Ann. Oncol 26, 7–9 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Chabner BA Approval after phase I: ceritinib runs the three-minute mile. Oncologist 19, 577–578 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med 370, 1189–1197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuk MK et al. FDA approval summary: accelerated approval of pembrolizumab for second-line treatment of metastatic melanoma. Clin Cancer Res 23, 5666–5670 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Chen C et al. Pembrolizumab KEYNOTE-001: an adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann. Oncol 28, 1388–1398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmelman J Is participation in cancer phase I trials really therapeutic? J. Clin. Oncol 35, 135–138 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal M & Emanuel EJ Ethics of phase 1 oncology studies: reexamining the arguments and data. JAMA 290, 1075–1082 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Estey E et al. Therapeutic response in phase I trials of antineoplastic agents. Cancer Treat. Rep 70, 1105–1115 (1986). [PubMed] [Google Scholar]
- 14.Decoster G, Stein G & Holdener EE Responses and toxic deaths in phase I clinical trials. Ann. Oncol 1, 175–181 (1990). [DOI] [PubMed] [Google Scholar]
- 15.Horstmann E et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N. Engl. J. Med 352, 895–904 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Roberts TG Jr. et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA 292, 2130–2140 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Schwaederle M et al. Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: a meta-analysis. JAMA Oncol 2, 1452–1459 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Waligora M et al. Risk and surrogate benefit for pediatric phase I trials in oncology: a systematic review with meta-analysis. PLoS Med 15, e1002505 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakiba C et al. Encouraging trends in modern phase 1 oncology trials. N. Engl. J. Med 378, 2242–2243 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Oxnard GR et al. Response rate as a regulatory end point in single-arm studies of advanced solid tumors. JAMA. Oncol 2, 772–779 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurzrock R & Benjamin RS Risks and benefits of phase 1 oncology trials, revisited. N. Engl. J. Med 352, 930–932 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Weber JS et al. American Society of Clinical Oncology policy statement update: the critical role of phase I trials in cancer research and treatment. J. Clin. Oncol 33, 278–284 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller FG & Joffe S Phase 1 oncology trials and informed consent. J. Med. Ethics 39, 761–764 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Saad ED et al. Precision medicine needs randomized clinical trials. Nat. Rev. Clin. Oncol 14, 317–323 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Weber JS et al. Reaffirming and clarifying the American Society of Clinical Oncology’s policy statement on the critical role of phase I trials in cancer research and treatment. J. Clin. Oncol 35, 139–140 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunwald HW Ethical and design issues of phase I clinical trials in cancer patients. Cancer Invest 25, 124–126 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Chvetzoff G & Tannock IF Placebo effects in oncology. J. Natl Cancer Inst 95, 19–29 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Jardim DL et al. Factors associated with failure of oncology drugs in late-stage clinical development: a systematic review. Cancer Treat. Rev 52, 12–21 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Long GV et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol 20, 1083–1097 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Drilon A et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med 378, 731–739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura R et al. Analysis of NTRK alterations in pan-cancer adult and pediatric malignancies: implications for NTRK-targeted therapeutics. JCO Precis. Oncol 10.1200/PO.18.00183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangat PK et al. Rationale and design of the targeted agent and profiling utilization registry study. JCO Precis. Oncol 10.1200/PO.18.00122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Severson TM et al. The BRCA1ness signature is associated significantly with response to PARP inhibitor treatment versus control in the I-SPY 2 randomized neoadjuvant setting. Breast Cancer Res 19, 99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asakawa T, Hirakawa A & Hamada C Bayesian model averaging continual reassessment method for bivariate binary efficacy and toxicity outcomes in phase I oncology trials. J. Biopharm. Stat 24, 310–325 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Sicklick JK et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med 25, 744–750 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zia MI et al. Comparison of outcomes of phase II studies and subsequent randomized control studies using identical chemotherapeutic regimens. J. Clin. Oncol 23, 6982–6991 (2005). [DOI] [PubMed] [Google Scholar]
- 37.De Ridder F Predicting the outcome of phase III trials using phase II data: a case study of clinical trial simulation in late stage drug development. Basic Clin. Pharmacol. Toxicol 96, 235–241 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Kurzrock R & Stewart DJ Equipoise abandoned? Randomization and clinical trials. Ann. Oncol 24, 2471–2474 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Johnson KR et al. Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: a meta-analysis. Lancet Oncol 7, 741–746 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Buyse M et al. Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Meta-Analysis Group in Cancer. Lancet 356, 373–378 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Burzykowski T et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J. Clin. Oncol 26, 1987–1992 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Blumenthal GM et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J. Clin. Oncol 33, 1008–1014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsimberidou AM et al. Ultimate fate of oncology drugs approved by the US Food and Drug Administration without a randomized trial. J. Clin. Oncol 27, 6243–6250 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Seymour L et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18, e143–e152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen TJ et al. Genome-wide sequencing of cell-free DNA identifies copy-number alterations that can be used for monitoring response to immunotherapy in cancer patients. Mol. Cancer Ther 18, 448–458 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Rothwell DG et al. Utility of ctDNA to support patient selection for early phase clinical trials: the TARGET study. Nat. Med 25, 738–743 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Cohen-Kurzrock BA, Cohen PR & Kurzrock R Health policy: the right to try is embodied in the right to die. Nat. Rev. Clin. Oncol 13, 399–400 (2016). [DOI] [PubMed] [Google Scholar]
- 48.US Congress S.204 - Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017 Congress.govhttps://www.congress.gov/bill/115th-congress/senate-bill/204/text (2018).
- 49.Puthumana J, Miller JE, Kim J & Ross JS Availability of investigational medicines through the US Food and Drug Administration’s expanded access and compassionate use programs. JAMA Netw. Open 1, e180283 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feit NZ et al. Use, safety, and efficacy of single-patient use of the US Food and Drug Administration expanded access program. JAMA Oncol 5, 570–572 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenfeld EH et al. Use of standardized visual aids improves informed consent for appendectomy in children: a randomized control trial. Am. J. Surg 216, 730–735 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Lim C Patients with advanced non-small cell lung cancer: are research biopsies a barrier to participation in clinical trials? J. Thorac. Oncol 11, 79–84 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Prasad V & Goldstein JA Clinical trial spots for cancer patients by tumour type: the cancer trials portfolio at clinicaltrials.gov Eur. J. Cancer 51, 2718–2723 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Recondo G et al. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat. Rev. Clin. Oncol 15, 694–708 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Herbst RS et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J. Clin. Oncol 20, 3815–3825 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Drilon AE et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J. Clin. Oncol 36, 102–102 (2018). [Google Scholar]
- 57.Piotrowska Z et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and blu-667 for acquired RET fusion. Cancer Discov 8, 1529–1539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jardim DL et al. Predictive value of phase I trials for safety in later trials and final approved dose: analysis of 61 approved cancer drugs. Clin. Cancer Res 20, 281–288 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Suntharalingam G et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med 355, 1018–1028 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Costa R et al. Analyses of selected safety endpoints in phase 1 and late-phase clinical trials of anti-PD-1 and PD-L1 inhibitors: prediction of immune-related toxicities. Oncotarget 8, 67782–67789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chhabra P, Chen X & Weiss SR Adverse event reporting patterns of newly approved drugs in the USA in 2006: an analysis of FDA Adverse Event Reporting System data. Drug Saf 36, 1117–1123 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Gliklich RE, Dreyer NA, Leavy MB Registries for evaluating patient outcomes: a user’s guide (ed 3rd). (Agency for Healthcare Research and Quality, 2014). [PubMed] [Google Scholar]
- 63.Jain RK et al. Phase I oncology studies: evidence that in the era of targeted therapies patients on lower doses do not fare worse. Clin. Cancer Res 16, 1289–1297 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta S et al. Meta-analysis of the relationship between dose and benefit in phase I targeted agent trials. J. Natl Cancer Inst 104, 1860–1866 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Le Tourneau C, Lee JJ & Siu LL Dose escalation methods in phase I cancer clinical trials. J. Natl Cancer Inst 101, 708–720 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber JS, Kahler KC & Hauschild A Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol 30, 2691–2697 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Topalian SL et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med 366, 2443–2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brahmer JR et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med 366, 2455–2465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dolly SO et al. A study of motivations and expectations of patients seen in phase 1 oncology clinics. Cancer 122, 3501–3508 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soria JC et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med 378, 113–125 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Wheler J et al. Survival of patients in a phase 1 clinic: the M. D. Anderson Cancer Center experience. Cancer 115, 1091–1099 (2009). [DOI] [PubMed] [Google Scholar]