Abstract
Background:
Glucocorticoids and asparaginase, used to treat acute lymphoblastic leukemia (ALL), can cause hypertriglyceridemia. We compared triglyceride levels, risk factors, and associated toxicities in two ALL trials at St. Jude Children’s Research Hospital with identical glucocorticoid regimens, but different asparaginase formulations. In Total XV (TXV), native E. coli L-asparaginase was front-line therapy versus the pegylated formulation (PEG-asparaginase) in Total XVI (TXVI).
Procedure:
Patients enrolled on TXV (n=498) and TXVI (n=598) were assigned to low-risk (LR) or standard/high-risk (SHR) treatment arms (ClinicalTrials.gov identifiers: and ). Triglycerides were measured four times and were evaluable in 925 patients (TXV: n=362; TXVI: n=563). The genetic contribution was assessed using a polygenic risk score (triglyceride-PRS). Osteonecrosis, thrombosis, and pancreatitis were prospectively graded.
Results:
The largest increase in triglycerides occurred in TXVI SHR patients treated with dexamethasone and PEG-asparaginase (4.5-fold-increase; p<1×10−15). SHR patients treated with PEG-asparaginase (TXVI) had more severe hypertriglyceridemia (>1000mg/dL) compared to native L-asparaginase (TXV): 10.5% versus 5.5%, respectively (p=0.007). At week 7, triglycerides did not increase with dexamethasone treatment alone (LR patients) but did increase with dexamethasone plus asparaginase (SHR patients). The variability in triglycerides explained by the triglyceride-PRS was highest at baseline and declined with therapy. Hypertriglyceridemia was associated with osteonecrosis (p=0.0006) and thrombosis (p=0.005), but not pancreatitis (p=0.4).
Conclusion:
Triglycerides were affected more by PEG-asparaginase than native L-asparaginase, by asparaginase more than dexamethasone, and by drug effects more than genetics. It is not clear whether triglycerides contribute to thrombosis and osteonecrosis or are biomarkers of the toxicities.
Keywords: acute lymphoblastic leukemia, asparaginase, hypertriglyceridemia
Introduction:
Glucocorticoids (e.g. dexamethasone and prednisone) and asparaginase are essential for successful treatment of pediatric acute lymphoblastic leukemia (ALL)1–3. However, these agents have been associated with alteration in serum lipids, resulting in hypertriglyceridemia4–6.
The mechanism through which glucocorticoids and asparaginase lead to hypertriglyceridemia is not completely understood. However, glucocorticoids alone increase triglyceride synthesis, cause mobilization of fatty acids, and activate lipoprotein lipase7, the enzyme required for hydrolysis of triglycerides. In contrast, asparaginase inhibits lipoprotein lipase8. Therefore, when glucocorticoids and asparaginase are given together, triglycerides are rapidly formed, but not cleared8. Although there were no overall differences in severe toxicities in a randomized comparison of native E. coli L-asparaginase (L-asparaginase) versus pegylated-asparaginase (PEG-asparaginase), hypertriglyceridemia [defined as grade 4 by National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE); >1000mg/dL] was at 8% versus 4% with PEG-asparaginase versus L-asparaginase9. The relative contribution of glucocorticoids versus asparaginase in the development of hypertriglyceridemia during ALL therapy is not clear.
In healthy children hypertriglyceridemia (triglycerides >500 mg/dL) is a rare event, with an estimated frequency of 0.2%10. The frequency of hypertriglyceridemia in unselected pediatric ALL patients treated with modern therapy is much higher, with reports of severe hypertriglyceridemia (grade 4 by CTCAE; >1000 mg/dL) ranging from 4–19% across protocols following asparaginase and glucocorticoids4–6,9,11,12: the frequency appears comparable in young adults treated with a pediatric ALL drug regimen13. The variability in frequency could be due to varying sample sizes, the timing of measurements relative to chemotherapy, and the asparaginase formulation.
The association between hypertriglyceridemia and other toxicities (thrombosis, osteonecrosis, and pancreatitis) has been previously studied6,14–17. It has been suggested that hypertriglyceridemia may be associated with osteonecrosis and thrombosis, although the associations were not maintained in multivariate analyses6. However, there are few large-scale studies to support hypertriglyceridemia as an independent risk factor. In the general population, triglycerides >1000mg/dL have been associated with pancreatitis18–20, but this has not been confirmed for patients with ALL6,17.
Our aim was to compare triglycerides, risk factors for hypertriglyceridemia, and associated toxicities in two front-line pediatric ALL trials that used identical glucocorticoid regimens but different formulations of asparaginase: Total XV study (native L-asparaginase) and Total XVI study (PEG-asparaginase).
Methods:
Patient information
Newly diagnosed patients with ALL were enrolled on one of two sequential trials at St. Jude Children’s Research Hospital (SJCRH). Total XV Study (TXV: ClinicalTrials.gov ID: )21 enrolled 498 consecutive patients (1 to 18 years of age) from June 2000 to October 2007 and Total XVI Study (TXVI: ClinicalTrials.gov ID: )21–23 enrolled 598 consecutive patients (≤ 18 years of age) from September 2007 to March 2017. TXV and TXVI therapy included remission induction therapy followed by consolidation therapy and 120 weeks of continuation therapy which included two phases of reinduction. Patients were classified for risk adapted therapy after remission induction as either low-risk (LR) or standard/high-risk (SHR)21–23, with SHR patients receiving more intensive treatment. Data on patient characteristics, details of therapy, and adverse events for analyses were obtained from medical records and study databases. Body mass index (BMI) was calculated for children greater than two years of age at consolidation day 15 (± 3.5 days): there was no difference in BMI during the observed course of therapy from day 15 consolidation to week 12/17 of continuation treatment (Supporting Information Figure S1). BMI z-score was derived using the R childsds package24. For children under two years of age, a weight-for-length standardized score was calculated in lieu of a BMI z-score. The studies were approved by the Institutional Review Board. Informed consent was obtained from either the parents or the patient consistent with the Declaration of Helsinki.
Treatment, lipid and asparaginase antibody measurements
Herein, the glucocorticoid and asparaginase drug regimens are described for the observed period of study, ending with the final lipid measurement. The glucocorticoid schedules were identical in TXV and TXVI therapy21: dexamethasone for 5 days/week during weeks 1, 4, and 14 [LR: 8 mg/m2/day, by mouth (PO); SHR: 12 mg/m2/day PO], for 8 days at week 7 (8 mg/m2/day, PO), and for 7 days at week 9 of continuation (8 mg/m2/day, PO). The two protocols differed primarily by asparaginase formulation21–23,25. In TXV, SHR patients received E. coli L-asparaginase (Elspar) weekly during continuation [25,000 U/m2/dose, intramuscular (IM)]; LR patients received E. coli L-asparaginase thrice weekly during continuation weeks 7–9 (reinduction I; 10,000 U/m2/dose, IM). In TXVI, patients were randomized to receive PEG-asparaginase (Oncaspar) 2,500 or 3,500 U/m2/dose [intravenous (IV)] during continuation: SHR weeks 1, 3, 5, 7, 9, 11, 13, and 15; LR weeks 7 and 9 (Supporting Information Figure S2). Unless noted, L-asparaginase refers to the native E. coli formulation. Patients with allergic reaction were switched to Erwinia L-asparaginase (Supporting Information): patients with reaction at or prior to week 7 were considered positive for asparaginase-reaction (ASP-RXN) in triglyceride analyses.
Non-fasting serum triglycerides were measured in a research laboratory by the glycerol phosphate oxidase method, and albumin was measured by the bromcresol green method. Measurements were performed utilizing a Roche Cobas Integra 400+ (Roche Diagnostics, Indianapolis, IN) or an Abbott Architect ci4100 (Abbott Diagnostics, Lake Forest, IL) at four time-points: consolidation day 15 (baseline: >35 days after last dose of asparaginase and >21 days after last dose of glucocorticoid) and day 1 of week 7, 8 (before and after the beginning of reinduction I), and 12 (TXV) or 17 (TXVI) of consolidation (Supporting Information Figure S2). Minimum serum albumin during continuation (week 7, 8 or 12/17) was used as a surrogate for asparaginase (and possibly dexamethasone)26 exposure in statistical modeling26–29. A total of 362 TXV and 563 TXVI patients had at least two triglyceride measurements and were analyzed in time-point specific analyses. 265 TXV and 508 TXVI patients had complete triglyceride data and were analyzed in longitudinal analyses (Supporting Information Figure S3). Anti-asparaginase antibodies against L-asparaginase (TXV) and PEG-asparaginase (TXVI) were measured as reported23; patients were classified as positive or negative for antibodies at or prior to week 7 of continuation.
The triglyceride measurements analyzed herein were obtained for research purposes. Patients may have had clinical testing for serum lipids during the TXV/TXVI protocols, and the decision to initiate therapy for asparaginase-associated hypertriglyceridemia was left to the discretion of the primary attending physician. All patients on asparaginase therapy were encouraged to follow a low-fat diet. Recommendations for treatment of hypertriglyceridemia included a nutritional consult, omega-3 fatty acid supplementation or fenofibrate, depending on severity and duration of hypertriglyceridemia; use of statins was avoided.
Assessment of triglycerides
The maximum triglycerides post-baseline were graded based on CTCAE version 4.0 (v4.0)30. Grade 4 hypertriglyceridemia (>1000mg/dL) was considered “severe hypertriglyceridemia”6,31. The K-means for longitudinal data (KML)32 R package was used for triglyceride cluster analyses.
Adverse event phenotypes
Adverse events were prospectively graded using CTCAE: TXV: Version 2.0 (v2.0)33 and TXVI: Version 3.0: (v3.0)34 [Supporting Information Table S1]. Osteonecrosis was prospectively screened by magnetic resonance imaging (MRI) of the hips/pelvis and knees for all patients in TXV and in older patients (≥9 years of age) in TXVI, with additional MRI scans of patients and/or locations as indicated by symptoms29. Grading of osteonecrosis and thrombosis was identical in CTCAE v2.0 and v3.0. Osteonecrosis was defined as: grade 1 (asymptomatic), grade 2 (symptomatic), grade 3 (symptomatic and intervention indicated), grade 4 (symptomatic or disabling); and thrombosis was defined as: grade 2 [deep vein thrombosis (DVT) or cardiac thrombosis, but intervention not indicated], grade 3 (DVT or cardiac thrombosis and intervention indicated), grade 4 (embolic event or life-threatening thrombus). For pancreatitis, CTCAE v2.0 had only grade 3 (abdominal pain with pancreatic enzyme elevation) and grade 4 (complicated by shock; acute circulatory failure); while CTCAE v3.0 had four grades: grade 1 (asymptomatic), grade 2 (symptomatic, medical intervention indicated), grade 3 (interventional radiology or operative intervention indicated) and grade 4 (life-threatening consequences). Patients were considered positive for an adverse event as follows: symptomatic osteonecrosis was defined as ≥ grade 2; thrombosis was defined ≥ grade 3; comparable definitions of pancreatitis were used between protocols: TXV (≥ grade 3) and TXVI (≥ grade 2). Other patients were considered negative for the adverse event. Only post-remission induction adverse events were analyzed.
Genotyping
Germline DNA from blood was genotyped35 and ancestry was determined using STRUCTURE36,37 (Supporting Information).
Polygenic risk score for elevated triglycerides
Weighted (by allele frequency and effect size) polygenic risk scores for elevated serum triglycerides (triglyceride-PRS) were generated from 16 single nucleotide polymorphisms (SNPs) reported to be associated with increased triglycerides in the general European population38,39 (Supporting Information Table S2).
Statistical analyses
Chi-square tests were used to compare categorical and Wilcoxon tests for continuous variables. Hypertriglyceridemia grade and longitudinal triglyceride cluster group were considered as ordinal variables: univariate and multivariate proportional odds models were used to identify clinical risk factors. A general linear regression model was used to identify risk factors associated with triglyceride levels as a continuous variable. All statistical analyses were conducted using R (version 3.5.0)40. A p-value of less than 0.05 was considered statistically significant and there were no corrections for multiple comparisons.
Data Sharing Statement
For original data, please contact mary.relling@stjude.org.
Results:
Patient characteristics and intrapatient triglyceride changes
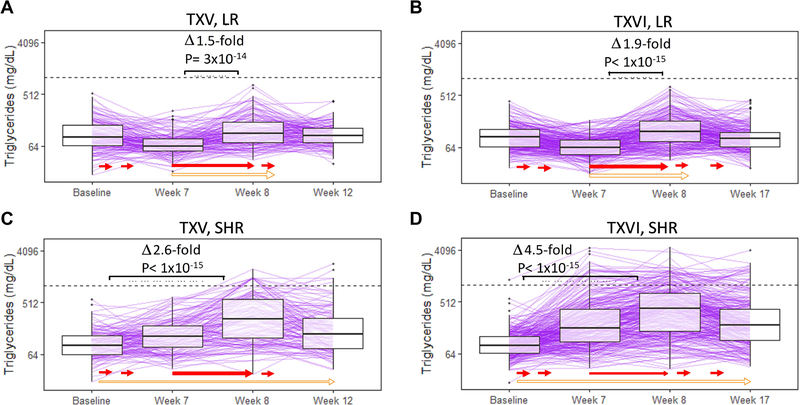
Intrapatient changes in triglycerides were analyzed by protocol and risk-arm (Figure 1). Changes in triglycerides were qualitatively similar within risk-arm between protocols. Among LR patients, triglycerides actually decreased from baseline to week 7 (TXV: p=2×10−8; TXVI: p<1×10−15); because dexamethasone was given during weeks 1 and 4 of continuation (without asparaginase), this suggests no negative effect on triglycerides two weeks post-dexamethasone treatment in LR patients. The largest increase in triglycerides occurred from week 7 to week 8 after both asparaginase and dexamethasone (TXV: p=3×10−14, 1.5-fold increase; TXVI: p<1×10−15, 1.9-fold increase). There was no difference between baseline and the final triglyceride measurement among LR patients (TXV: p=0.7, TXVI: p=0.1; Figs. 1A and 1B; Supporting Information Tables S3A and S3B), after relatively low exposure to asparaginase.
Figure 1: The greatest increase in triglycerides was observed in SHR patients after dexamethasone and asparaginase at week 8.
Intrapatient triglyceride (y-axis) changes shown by protocol and treatment risk-arm. A. TXV, LR (n=142). B. TXVI, LR (n=229). C. TXV, SHR (n=123). D. TXVI, SHR (n=279). Solid red arrows indicate dexamethasone treatment time periods; open orange arrows indicate asparaginase treatment time periods. Details of therapy have been described.23 Grade 4 hypertriglyceridemia (1000 mg/dL) is indicated with a dashed horizontal line. P-values calculated using Wilcoxon tests for paired samples. LR= low risk treatment arm, SHR= standard/high risk treatment arm.
In SHR patients, there were significant changes in triglycerides among all time-points (Figs. 1C and 1D; Supporting Information Tables S3C and S3D). The largest increase in triglycerides occurred from baseline to week 8 of therapy, after both dexamethasone and asparaginase were given, with a larger magnitude of change in TXVI SHR patients: TXV: p<1×10−15, 2.6-fold increase; TXVI: p<1×10−15; 4.5-fold increase, implicating PEG-asparaginase in more pronounced triglyceride elevations versus L-asparaginase. Triglycerides were still raised versus baseline at the final measurement (TXV: p=9×10−9, TXVI: p<1×10−15).
Differences in triglycerides between protocols
There was no difference in triglycerides at baseline between TXV and TXVI protocols for either SHR (p=0.74) or LR patients (p=0.62). However, triglycerides were higher in TXVI SHR versus TXV SHR patients at week 7 (p=8×10−7), week 8 (p=0.0007) and at the final measurement (p=0.008). TXVI had more unique patients with at least one occurrence of grade 4 hypertriglyceridemia compared to TXV: 10.5% (59/563) versus 5.5% (20/362), respectively (p=0.007). In LR patients, the only difference in triglycerides between protocols was at the final measurement, when patients on TXVI had lower levels than those on TXV (p=0.04); this difference could be because the final measurement in TXV (at week 12) was 2-weeks post-L-asparaginase whereas in TXVI (at week 17) was 8-weeks post-PEG-asparaginase (Supporting Information Table S4).
Maximum hypertriglyceridemia grade
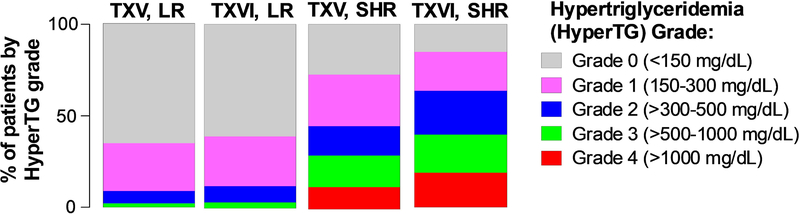
There was no difference in maximum hypertriglyceridemia grade in LR patients between protocols (TXV versus TXVI: p=0.36), whereas SHR patients on the TXVI protocol had higher maximum hypertriglyceridemia grade versus SHR patients on TXV (p=0.0001; Fig. 2; Supporting Information Table S5). Maximum hypertriglyceridemia grade differed by risk-arm: in TXV, 0% (0/189) of LR patients had grade 4 hypertriglyceridemia versus 12% (20/173) of SHR patients (p<1×10−15; Fig. 2; Supporting Information Table S5). Similarly, in TXVI, 0% (0/255) of LR patients versus 19% (59/308) of SHR patients had grade 4 hypertriglyceridemia (p<1×10−15; Fig. 2; Supporting Information Table S5). Only six patients (all on TXVI SHR) had triglycerides over 3000 mg/dL, with the maximum recorded value being 5,234 mg/dL at week 7.
Figure 2: Maximum hypertriglyceridemia grade was significantly higher in TXVI versus TXV SHR arms; there was no difference between TXV versus TXVI for the LR arms.
TXV, LR (n=189); TXVI, LR (n=255); TXV, SHR (n=173); TXVI, SHR (n=308). Only patients with at least two lipid measurements were included in analyses. LR (low risk treatment arm); SHR (standard/high risk treatment arm); hyperTG (hypertriglyceridemia).
In the TXVI cohort, there was no difference in maximum hypertriglyceridemia grade by PEG-asparaginase randomization (2500 versus 3500 U/m2/dose) within patients in the LR arm (p=0.22) or within SHR patients (p=0.44; Supporting Information Figure S4; Supporting Information Table S6). No patients on Erwinia L-asparaginase (n=66) at or prior to week 7 of continuation developed grade 4 hypertriglyceridemia.
In univariate analyses, the risk factors for maximum hypertriglyceridemia grade were older age (p<1×10−15), TXVI protocol (p=3×10−5), SHR therapy (p<1×10−15), lower minimum serum albumin (p<1×10−15), absence of anti-asparaginase antibodies (p=4×10−7), and lack of asparaginase allergy (p=1×10−6) (Table 1). Older age (p=9×10−9), TXVI protocol (p=0.0007), SHR therapy (p=2×10−11), and lower minimum serum albumin (p=1×10−6) remained significant in a multivariate proportional odds model (Table 1). Immunophenotype was not considered in our analysis of risk factors because it is highly confounded with risk-arm, but within SHR groups, there was no impact (p=0.44) of T- versus B-cell ALL on maximum triglyceride level.
Table 1: Univariate and multivariate analysis of risk factors for higher maximum hypertriglyceridemia grade.
Maximum hypertriglyceridemia grade indicates the highest grade at any time point for each patient. For categorical risk factors, the direction of the relationship with increasing maximum hypertriglyceridemia grade is indicated. Significant covariates in univariate (uni) analyses were included in a multivariate (multi) proportional odds model. Effect size with 95% confidence interval (CI) shown for univariate and multivariate analyses. Minimum serum albumin indicates the lowest observed measurement during continuation. Anti-ASP refers to anti-Elspar in TXV and anti-Oncaspar in TXVI (positive patients had detectable antibody at or before week 7). Patients with allergic reaction to front-line asparaginase formulation were considered ASP-RXN positive. Only patients with at least two triglyceride measurements were included in analysis (n=925).
| Risk Factor | Grade of hypertriglyceridemia | Effect Size (uni) | p-value (uni) | Effect Size (multi) | p-value (multi) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 0 (n=369) | Grade 1 (n=235) | Grade 2 (n=137) | Grade 3 (n=105) | Grade 4 (n=79) | ||||||
| Age at diagnosis | Median (interdecile range), years | 4.2 (1.7–14.4) | 5.0 (1.7–15.4) | 6.9 (1.7–17.0) | 9.5 (2.0–18.2) | 11.2 (2.9–17.0) | 1.1 (1.08–1.12) | <1×10−15 | 1.06 (1.03–1.08) | 5×10−9 |
| Gender | Male, n (%) | 199 (54%) | 128 (54%) | 82 (60%) | 61 (58%) | 52 (66%) | 1.16 (0.99–1.37) (male) | 0.06 | ||
| Female, n (%) | 170 (46%) | 107 (46%) | 55 (40%) | 44 (42%) | 27 (34%) | |||||
| Race | White, n (%) | 236 (64%) | 172 (73%) | 108 (79%) | 66 (63%) | 55 (70%) | 1.17 (0.98–1.41) (white) | 0.08 | ||
| Black, n (%) | 68 (18%) | 30 (13%) | 7 (5%) | 17 (16%) | 9 (11%) | |||||
| Hispanic, n (%) | 28 (8%) | 24 (10%) | 13 (9%) | 10 (10%) | 9 (11%) | |||||
| Other, n (%) | 37 (10%) | 9 (4%) | 9 (7%) | 12 (11%) | 6 (8%) | |||||
| BMI | Median (interdecile range), z-score | 0.59 (−1.63–2.14) | 0.75 (−1.61–2.71) | 0.63 (−1.50–2.33) | 0.77 (−1.41–2.48) | 0.65 (−0.90–2.01) | 1.05 (0.98–1.12) | 0.2 | ||
| Protocol | TXV, n (%) | 168 (46%) | 99 (42%) | 41 (30%) | 34 (32%) | 20 (25%) | 1.44 (1.21–1.69) (TXVI) | 3×10−5 | 1.39 (1.15–1.67) | 0.008 |
| TXVI, n (%) | 201 (54%) | 136 (58%) | 96 (70%) | 71 (68%) | 59 (75%) | |||||
| Treatment risk arm | LR, n (%) | 277 (75%) | 120 (51%) | 36 (26%) | 11 (10%) | 0 (0%) | 4.59 (3.80–5.54) (SHR) | <1×10−15 | 2.46 (1.89–3.18) | 8×10−12 |
| SHR, n (%) | 92 (25%) | 115 (49%) | 101 (74%) | 94 (90%) | 79 (100%) | |||||
| Minimum serum albumin | Median (interdecile range), mg/dL | 3.9 (2.6–4.3) | 3.5 (2.3–4.3) | 3.1 (2.4–4.1) | 2.9 (2.4–4.0) | 2.8 (2.3–3.5) | 0.34 (0.29–0.40) | <1×10−15 | 0.61 (0.50–0.74) | 4×10−6 |
| Anti-ASP Antibody | Negative, n (%) | 222 (60%) | 154 (66%) | 100 (73%) | 82 (78%) | 67 (85%) | 0.64 (0.58–0.69) | 4×10−7 | 0.84 (0.75–0.95 | 0.16 |
| Positive, n (%) | 147 (40%) | 81 (34%) | 37 (27%) | 23 (22%) | 12 (15%) | |||||
| ASP-RXN | RXN−, n (%) | 257 (70%) | 171 (73%) | 111 (81%) | 88 (84%) | 74 (94%) | 0.62 (0.51–0.75) | 1×10−6 | 0.84 (0.74–0.95) | 0.16 |
| RXN+, n (%) | 112 (30%) | 64 (27%) | 26 (19%) | 17 (16%) | 5 (6%) | |||||
Longitudinal triglyceride clustering
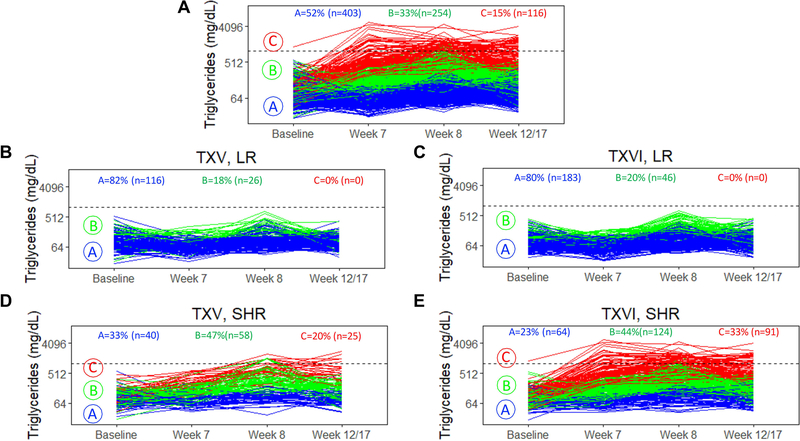
Longitudinal triglyceride clustering identified three unique groups of patients (Fig. 3A). Fifty-two percent (403/773) of patients were in cluster A, herein referred to as “low-risk cluster A” as it was defined by overall lower triglycerides and minor triglyceride changes with therapy; 33% (254/773) of patients were in cluster B, or “intermediate-risk cluster B”, as patients only had therapy-induced triglyceride changes between weeks 7 to 8; and 15% (116/773) of patients were in cluster C, defined by persistently elevated triglycerides, herein referred to as “high-risk cluster C” (Fig. 3A).
Figure 3: Longitudinal triglyceride cluster analysis identified three unique groups of patients.
A. K-means for longitudinal data (KML) was used to cluster patients (n=773, TXV+TXVI) with similar triglyceride trends over the observed course of therapy. B. TXV, LR patients (n=142). C. TXVI, LR patients (n=229). D. TXV, SHR patients (n=123). E. TXVI, SHR patients (n=279). NOTE: 84% (61/73) of patients with maximum hypertriglyceridemia grade 4 (>1000 mg/dL dotted line on graph) were in high-risk cluster C; 16% (12/73) were in intermediate-risk cluster B. Only patients with four triglyceride measurements were included (n= 773).
Over 80% of patients on LR therapy, regardless of protocol, fell into low-risk cluster A (Figs. 3B and 3C). From each protocol, a similar percentage of patients were in intermediate-risk cluster B by risk-arm: in LR patients, 18% (26/142) from TXV versus 20% (46/229) from TXVI; and in SHR patients 47% (58/123) were on TXV versus 44% (124/279) on TXVI. In contrast, only SHR patients fell into the high-risk cluster C, with 78% (91/116) of cluster C being from the TXVI cohort (Figs. 3D and 3E, Supporting Information Table S7). In a multivariate proportional odds model, older age (p=4×10−6), SHR therapy (p=2×10−14), and lower serum albumin (p=0.0005), were associated with falling in high-risk cluster C (Supporting Information Table S7).
Influence of genetics on triglycerides during ALL therapy
Using traditional genome-wide association study (GWAS), there were no genome-wide significant SNPs (p<5×10−8) to explain maximum triglycerides or longitudinal triglyceride cluster, with protocol, risk-arm, genetic race, gender, and age as covariates (n=925).
It has been previously proposed that hypertriglyceridemia is polygenic38,39. To this end, we utilized a set of 16 previously discovered SNPs used to develop a triglyceride polygenic risk score (triglyceride-PRS) associated with elevated triglycerides in the general European population (Supporting Information Table S2)38,39 and calculated a triglyceride-PRS for European ancestry patients (>90% Northern European genetic ancestry) in TXV and TXVI (n=642).
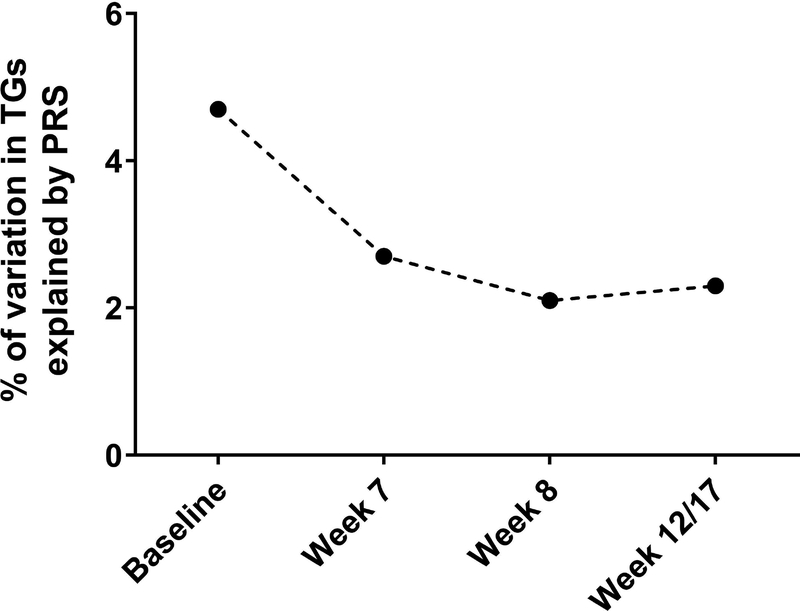
The distribution of triglyceride-PRS was 0.66–1.93; scores were not different between protocols (p=0.99; Supporting Information Figure S5). Though the triglyceride-PRS was significantly associated with triglycerides (in multivariate analyses) at all time points, the percent variability in triglycerides explained by the PRS fell from 4.7% of the variation at baseline to only 2.1–2.7% at post-therapy measurements (Fig. 4).
Figure 4: Percent of variation in triglycerides explained by the triglyceride-PRS at four time-points in white patients on TXV and TXVI.
Triglycerides (TG) were treated as a continuous variable at each time point. P-values were generated using linear regression models with TG measurement as the independent variable with covariates including age, sex, protocol, and risk-arm. Baseline, p=3×10−8; week 7, p= 1×10−6; week 8, p=1×10−6; week 12/17, p=1×10−5. N=642.
Of the 16 SNPs included in the triglyceride-PRS and those in the top 20 for GWAS (Supporting Information Table S8) of maximum triglyceride in our European ancestry patients, rs964184 in APOA1 was the only SNP to appear in both sets.
Adverse Events
We have previously shown an association between lower serum albumin and symptomatic osteonecrosis29. Herein, lower minimum serum albumin was significantly associated with symptomatic osteonecrosis (p=7×10−13) and thrombosis (p=0.002), but not pancreatitis (p=0.1), in univariate analyses (Supporting Information Figure S6). However, albumin only remained significantly associated with symptomatic osteonecrosis, and not thrombosis, when adjusting for other known risk factors including age, race, gender, risk-arm, and protocol (Supporting Information Figure S6).
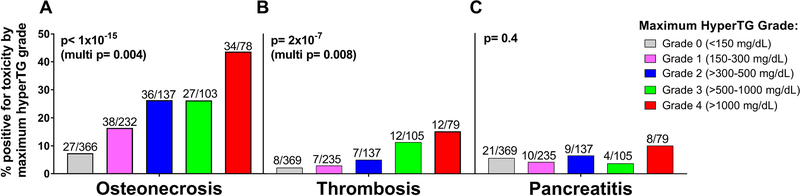
There was a step-wise increase in both symptomatic osteonecrosis (p<1×10−15) and thrombosis (p=2×10−7) by maximum hypertriglyceridemia grade, which remained significant in multivariate analyses (osteonecrosis: p=0.004, Fig. 5A; thrombosis: p=0.008, Fig. 5B). A similar pattern was observed when patients were grouped into their longitudinal triglyceride cluster, with patients in high-risk cluster C having the highest frequency of symptomatic osteonecrosis and/or thrombosis (Supporting Information Figure S7A and S7B).
Figure 5: Osteonecrosis and thrombosis, but not pancreatitis, were significantly associated with maximum hypertriglyceridemia grade.
Adverse events were A symptomatic osteonecrosis grade 2–4. B. thrombosis grade 3–4. C. pancreatitis grade 3–4 (TXV) or grade 2–4 (TXVI). Multivariate p-values from proportional odds models included age, gender, race, minimum serum albumin, risk-arm, and protocol as covariates. Ratios at top of each bar indicate the number of patients positive for the specific toxicity in each maximum hypertriglyceridemia (hyperTG) grade.
There was no association between maximum hypertriglyceridemia grade (univariate, p=0.4; Fig. 5C) or longitudinal triglyceride cluster (univariate, p=0.6; Supporting Information Figure S7C) and pancreatitis.
Discussion:
Though the relationship between acute hypertriglyceridemia and pediatric ALL therapy has been known for over 25 years4,5, it is still of relevance due to unclear associations with long-term toxicities and limited serial data, particularly with modern PEG-asparaginase containing regimens. Limitations of past studies include small sample size, potential underrepresentation of hypertriglyceridemia, and inconsistent definition of hypertriglyceridemia.
We analyzed triglycerides at predefined timepoints that were chosen in relation to dexamethasone and asparaginase exposure and were not biased by any clinical suspicions of hypertriglyceridemia. Triglycerides actually decreased in LR patients following dexamethasone monotherapy between baseline and week 7 (Figs. 1A and 1B); when combined with asparaginase (SHR patients), triglycerides increased over this same time period (Table S3), showing a greater lipemic effect of asparaginase plus dexamethasone compared to dexamethasone alone. The identical dexamethasone regimen between protocols allowed front-line asparaginase formulation to be evaluated with respect to triglycerides. Our data clearly show a greater influence of PEG- versus L-asparaginase on elevation of triglycerides, when given in combination with glucocorticoid therapy. The magnitude of increase in triglycerides from baseline to week 8 in TXVI (PEG-asparaginase) SHR patients was nearly double that of TXV (L-asparaginase) SHR patients (Figs. 1C and 1D). Dexamethasone treatment was reduced from 12 mg/m2/day to 8 mg/m2/day in SHR patients at week 7 (beginning of reinduction I) in both protocols, implicating high dose PEG-asparaginase as the driver in the development of hypertriglyceridemia. As has been shown previously in a small subset of TXVI6, no patients on Erwinia asparaginase developed grade 4 hypertriglyceridemia. Patients on SHR therapy maintained a persistent increase in triglycerides as compared to LR patients. Only SHR patients developed hypertriglyceridemia, with more patients on TXVI therapy developing grade 4 hypertriglyceridemia compared to TXV (Fig. 2). Only patients on TXVI SHR therapy had multiple episodes of grade 4 hypertriglyceridemia (Supporting Information Table S4).
As was reported in an interim analysis of TXVI6, older age and SHR therapy (which are highly correlated) were associated with hypertriglyceridemia. Here, we demonstrated this relationship through analysis of maximum hypertriglyceridemia grade and through longitudinal triglyceride clustering. Serum albumin was used as a surrogate for serum asparaginase activity: hypoalbuminemia is associated with increased asparaginase as well as increased dexamethasone exposure26–29. Asparaginase allergic reaction and/or presence of anti-asparaginase antibodies is associated with lower exposure to asparaginase23,41,42. Patients with a higher maximum hypertriglyceridemia grade (Table 1) and/or who had higher triglyceride trends (high-risk cluster C; Supporting Information Table S7) likely had higher exposure to asparaginase, as suggested by lower serum albumin, undetectable anti-asparaginase antibodies and absence of asparaginase allergy.
Consistent with a strong drug effect outweighing any genetic predisposition, we found no single SNPs of genome-wide significance to explain maximum triglyceride measurement or longitudinal triglyceride clustering, although our power to detect novel genetic predisposition is limited by relatively small sample sizes. As hypertriglyceridemia is a highly-studied polygenic phenotype, we applied a previously developed triglyceride-PRS39 and confirmed that this PRS was a significant risk factor for hypertriglyceridemia at all time-points, particularly at baseline, in European ancestry patients. However, our observation that the triglyceride-PRS explained less of the variation in triglycerides after treatment suggests that the hypertriglyceridemia phenotype was driven by therapy more than genetic factors.
We showed both symptomatic osteonecrosis and post-induction thrombosis were associated with hypertriglyceridemia in multivariate analyses (Figs. 5A and 5B; Supporting Information Figures S7A and S7B). These associations were true if either maximum hypertriglyceridemia grade (Fig. 5) or longitudinal triglyceride patterns (Supporting Information Figure S7) were considered.
Whether treatment with agents such as omega-3, nicotinic acid derivatives, or fibrates to lower triglycerides11,12,43,44 would have altered the risk of osteonecrosis or thrombosis is unknown. Though patients were encouraged to maintain a low-fat diet during asparaginase therapy, we do not have data on compliance to this recommendation. Like past studies, there was no association between triglycerides > 1000 mg/dL and pancreatitis5,6,17,45.
Both TXV and TXVI protocols, as well as Children’s Oncology Group and other NCI-approved protocols, use NCI’s CTCAE grading scales. Alternative scales, such as those suggested by the Ponte di Legno consortium working group (PTWG) have also been proposed46. For reference, in CTCAE v4.0, grades 1–3 hypertriglyceridemia would be classified as mild by PTWG [<10 times upper normal limit (UNL)] and grade 4 by CTCAE would be classified as moderate (10–20 times UNL) to severe (>20 times UNL) by PTWG.
In this study, we found that triglycerides were affected more by PEG-asparaginase than native L-asparaginase, by asparaginase more than dexamethasone, and by drug effects more than genetic effects. It is not clear whether triglycerides cause thrombosis and osteonecrosis or are simply covariates of the toxicities and/or biomarkers of increased drug exposure. Further study is required to determine if lipid-lowering interventions attenuate these toxicities.
Supplementary Material
Acknowledgements:
This work was supported by National Institutes of Health GM115279, CA142665, CA21765, and the American Lebanese Syrian Associated Charities. The authors thank the patients and parents who participated in the clinical protocols included in this study, and the participating clinicians and research staff, including Katherine M. Robinson and Jitesh D. Kawedia.
Abbreviations:
- ALL
acute lymphoblastic leukemia
- ASP-RXN
asparaginase-reaction
- BMI
body mass index
- CTCAE
Common Terminology Criteria for Adverse Events
- DVT
deep vein thrombosis
- GWAS
genome-wide association study
- IM
intramuscular
- KML
K-means for longitudinal data
- L-asparaginase
native E. Coli L-asparaginase
- LR
Low Risk treatment arm
- MRI
magnetic resonance imaging
- NCI
National Cancer Institute
- PEG-asparaginase
pegylated E.Coli L-asparaginase
- PO
Per os (by mouth)
- PTWG
Ponte di Legno consortium working group
- SHR
Standard/High Risk treatment arm
- SJCRH
St. Jude Children’s Research Hospital
- SNP
single nucleotide polymorphism
- triglyceride-PRS
triglyceride polygenic risk score
- TXV
Total Therapy TXV study
- TXVI
Total Therapy TXVI study
- UNL
upper normal limit
Footnotes
Conflict of interest disclosure: Dr. Mary Relling and St. Jude Children’s Research Hospital receive investigator-initiated research funding from Servier Pharmaceuticals.
References:
- 1.Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50(3):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. [DOI] [PubMed] [Google Scholar]
- 3.Silverman LB. Balancing cure and long-term risks in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2014;2014(1):190–197. [DOI] [PubMed] [Google Scholar]
- 4.Steinherz PG. Transient, severe hyperlipidemia in patients with acute lymphoblastic leukemia treated with prednisone and asparaginase. Cancer. 1994;74(12):3234–3239. [DOI] [PubMed] [Google Scholar]
- 5.Parsons SK, Skapek SX, Neufeld EJ, et al. Asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Blood. 1997;89(6):1886–1895. [PubMed] [Google Scholar]
- 6.Bhojwani D, Darbandi R, Pei D, et al. Severe hypertriglyceridaemia during therapy for childhood acute lymphoblastic leukaemia. Eur J Cancer. 2014;50(15):2685–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60(11):1500–1510. [DOI] [PubMed] [Google Scholar]
- 8.Hoogerbrugge N, Jansen H, Hoogerbrugge PM. Transient hyperlipidemia during treatment of ALL with L-asparaginase is related to decreased lipoprotein lipase activity. Leukemia. 1997;11(8):1377–1379. [DOI] [PubMed] [Google Scholar]
- 9.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16(16):1677–1690. [DOI] [PubMed] [Google Scholar]
- 10.Christian JB, Juneja MX, Meadowcroft AM, Borden S, Lowe KA. Prevalence, characteristics, and risk factors of elevated triglyceride levels in US children. Clin Pediatr (Phila). 2011;50(12):1103–1109. [DOI] [PubMed] [Google Scholar]
- 11.Cohen H, Bielorai B, Harats D, Toren A, Pinhas-Hamiel O. Conservative treatment of L-asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54(5):703–706. [DOI] [PubMed] [Google Scholar]
- 12.Salvador C, Entenmann A, Salvador R, Niederwanger A, Crazzolara R, Kropshofer G. Combination therapy of omega-3 fatty acids and acipimox for children with hypertriglyceridemia and acute lymphoblastic leukemia. J Clin Lipidol. 2018;12(5):1260–1266. [DOI] [PubMed] [Google Scholar]
- 13.Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raja RA, Schmiegelow K, Frandsen TL. Asparaginase-associated pancreatitis in children. Br J Haematol. 2012;159(1):18–27. [DOI] [PubMed] [Google Scholar]
- 15.Mogensen SS, Schmiegelow K, Grell K, et al. Hyperlipidemia is a risk factor for osteonecrosis in children and young adults with acute lymphoblastic leukemia. Haematologica. 2017;102(5):e175–e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raja RA, Schmiegelow K, Sorensen DN, Frandsen TL. Asparaginase-associated pancreatitis is not predicted by hypertriglyceridemia or pancreatic enzyme levels in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2017;64(1):32–38. [DOI] [PubMed] [Google Scholar]
- 17.Tong WH, Pieters R, de Groot-Kruseman HA, et al. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica. 2014;99(11):1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toskes PP. Hyperlipidemic pancreatitis. Gastroenterol Clin North Am. 1990;19(4):783–791. [PubMed] [Google Scholar]
- 19.Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48(3):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan R, Jehangir W, Regeti K, Yousif A. Hypertriglyceridemia-Induced Pancreatitis: Choice of Treatment. Gastroenterology Res. 2015;8(3–4):234–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeha SPD, Cho J , Cheng C , Sandlund JT , Coustan-Smith E , Campana D , Inaba H , Rubnitz Jeffrey E , Ribeiro Raul C , Gruber Tanya A , Raimondi Susana C, Khan Raja B , Yang Jun J , Mullighan Charles G , Downing James R , Evans WE , Relling MV , Pui CH. Improved central-nervous-system control of childhood acute lymphoblastic leukemia without cranial irradiation: St. Jude Total Therapy Study 16. Journal Clin Oncol, In Press. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Smith CA, Panetta JC, et al. Antibodies Predict Pegaspargase Allergic Reactions and Failure of Rechallenge. J Clin Oncol. 2019;37(23):2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Data Vogel M. and Methods Around Reference Values in Pediatrics, : https://cran.r-project.org/web/packages/childsds/childsds.pdf. 2019.
- 25.Pui CH CD, Sandlund JT, Bhojwani D, Evans WE, Relling MV, Jeha S. . Treatment of childhood acute lymphoblastic leukemia without cranial irradiation https://slideheaven.com/acute-leukemias-xiii.html. Ann Hematol 2011;90(90):S25–S76. [Google Scholar]
- 26.Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26(12):1932–1939. [DOI] [PubMed] [Google Scholar]
- 27.Petros WP, Rodman JH, Relling MV, et al. Variability in teniposide plasma protein binding is correlated with serum albumin concentrations. Pharmacotherapy. 1992;12(4):273–277. [PubMed] [Google Scholar]
- 28.Hak LJ, Relling MV, Cheng C, et al. Asparaginase pharmacodynamics differ by formulation among children with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2004;18(6):1072–1077. [DOI] [PubMed] [Google Scholar]
- 29.Kawedia JD, Kaste SC, Pei D, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117(8):2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute Common Terminology Criteria for Adverse Events, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40. June 14, 2010.
- 31.Schmiegelow K, Muller K, Mogensen SS, et al. Non-infectious chemotherapy-associated acute toxicities during childhood acute lymphoblastic leukemia therapy. F1000Res. 2017;6:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genolini C K-means for longitudinal data Version: 2.4.1, https://cran.r-project.org/web/packages/kml/kml.pdf. 2016.
- 33.National Cancer Institute Common Terminology Criteria for Adverse Events Version: 2.0, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf. 1999.
- 34.National Cancer Institute Common Terminology Criteria for Adverse Events Version: 3.0, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. 2006.
- 35.Fernandez CA, Smith C, Yang W, et al. Genome-wide analysis links NFATC2 with asparaginase hypersensitivity. Blood. 2015;126(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43(3):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Fernandez CA, Smith C, et al. Genome-Wide Study Links PNPLA3 Variant With Elevated Hepatic Transaminase After Acute Lymphoblastic Leukemia Therapy. Clin Pharmacol Ther. 2017;102(1):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dron JS, Wang J, Cao H, et al. Severe hypertriglyceridemia is primarily polygenic. J Clin Lipidol. 2019;13(1):80–88. [DOI] [PubMed] [Google Scholar]
- 40.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://wwwR-projectorg/. 2018. [Google Scholar]
- 41.Pieters R, Hunger SP, Boos J, et al. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117(2):238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathod S, Ramsey M, Relling MV, Finkelman FD, Fernandez CA. Hypersensitivity reactions to asparaginase in mice are mediated by anti-asparaginase IgE and IgG and the immunoglobulin receptors FcepsilonRI and FcgammaRIII. Haematologica. 2019;104(2):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvador C, Meister B, Crazzolara R, Kropshofer G. Management of hypertriglyceridemia in children with acute lymphoblastic leukemia under persistent therapy with glucocorticoids and L-asparaginase during induction chemotherapy. Pediatr Blood Cancer. 2012;59(4):771. [DOI] [PubMed] [Google Scholar]
- 44.Bostrom B Successful management of extreme hypertriglyceridemia from pegaspargase with omega-3. Pediatr Blood Cancer. 2012;59(2):350. [DOI] [PubMed] [Google Scholar]
- 45.Halton JM, Nazir DJ, McQueen MJ, Barr RD. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer. 1998;83(2):379–384. [PubMed] [Google Scholar]
- 46.Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17(6):e231–e239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.