Abstract
Background
Conducted in Dayton, Ohio, the study aims to characterize user knowledge and experiences with non-pharmaceutical fentanyl-type drugs (NPFs) and compare self-reports with urine toxicology for NPFs and heroin.
Methods
Between May 2017- January 2018, 60 individuals who self-reported heroin/NPF use were interviewed using structured questionnaire on sociodemographics, NPF and other drug use practices. Unobserved urine samples were collected and analyzed using: 1) liquid-chromatography-tandem mass spectrometry (LC-MS/MS)-based method (Toxicology lab) to identify 34 fentanyl analogues, metabolites, and other synthetic opioids; 2) immunoassay-based method to screen for opiates (heroin). Sensitivity, specificity and Cohen’s kappa were calculated to assess agreement between self-reports and urine toxicology.
Results
The sample was 52% female, and over 90% white. Almost 60% reported preference for heroin, and 40% for NPF. Participants endorsed a number of ways of distinguishing heroin from NPF, including appearance (88.3%), effects (76.7%), taste (55%), and information provided by dealers (53.3%). Almost 80% felt confident they could distinguish heroin from NPF, but knowledge about fentanyl analogues was limited. LC-MS/MS testing identified 8 types of NPFs and/or metabolites. Over 88% tested positive for NPFs, including 86% fentanyl, 48% carfentanil, 42% acetyl fentanyl. About 47% screened positive for opiates/heroin, and all of them were also positive for NPFs. When comparing self-reported use of NPF to urine toxicology, sensitivity and specificity were relatively high (84% and 83.3%, accordingly), while Cohen’s Kappa was 0.445, indicating fair agreement. Sensitivity and specificity were lower for heroin (77.8% and 50.0%, accordingly), and Cohen’s Kappa was 0.296, indicating low agreement between self-reports of heroin use and urine toxicology.
Discussion
Nearly 90% of the study participants tested positive for NPF-type drugs. Participants were more likely to over-report heroin use and underreport NPF use. The majority had little knowledge about fentanyl analogues. Study findings will inform development of novel harm reduction approaches to reduce overdose mortality.
Keywords: fentanyl, non-pharmaceutical fentanyl, fentanyl analogues, novel synthetic opioids, heroin, urine toxicology
BACKGROUND
The United States is experiencing unprecedented increases in opioid-related overdose mortality (Hedegaard, Miniño, & Warner, 2018; Rudd, Aleshire, Zibbell, & Gladden, 2016). Since 2013, these increases were primarily driven by rampant adulteration of heroin supplies with non-pharmaceutical fentanyl and related drugs (NPFs) (Ciccarone, 2017; Jones, Einstein, & Compton, 2018; Macmadu, Carroll, Hadland, Green, & Marshall, 2017). NPF-type drugs include non-pharmaceutical fentanyl, which is structurally identical to pharmaceutical fentanyl but is produced in clandestine laboratories, and a broad range of fentanyl analogues (Centers for Disease Control and Prevention, 2015; Suzuki & El-Haddad, 2017). Fentanyl is approximately 35-50 times more potent than heroin (Ciccarone, Ondocsin, & Mars, 2017; Suzuki & El-Haddad, 2017), and fentanyl analogues display great variation in potency, which makes them even more dangerous in terms of overdose risks (Suzuki & El-Haddad, 2017).
Ohio is one of the high burden states in terms of NPF-related overdose mortality (Gladden, Martinez, & Seth, 2016; Rudd, Seth, David, & Scholl, 2016). Unintetional overdose deaths in Ohio nearly doubled from 2,110 in 2013 to 4,050 in 2016 (Ohio Department of Health, 2017). These increases were even more notable in Montgomery County which displays the highest unitentional overdose death rates in the state (Ohio Department of Health, 2017). In 2017, 566 people died from unintentional drug overdoses in Montgomery county, reaching unpresedented unadjusted death rate of 106.3 per 100,000 population (Public Health Dayton & Montgomery County, 2018). Over 90% of all unitentional overdose fatalities analyzed at the Montgomery County Coroners Toxicology Laboratory in 2017 tested positive for NPFs, including a broad range of fentanyl analogues, such as acryl fentanyl, furanyl fentanyl, carfentanil and others (Daniulaityte, Juhascik, et al., 2017; R. Daniulaityte, et al., 2019).
More research is needed to better understand how people who use opioids (PWUO) view and experience evolving saturation of local drug markets with NPF-type drugs. Most prior studies were conducted in the Northeastern states and reported mixed results regarding local preferences and experiences with NPFs (Carroll, Marshall, Rich, & Green, 2017; Macmadu, et al., 2017; Mars, Ondocsin, & Ciccarone, 2018; Somerville, et al., 2017). A study conducted in Massachusetts (MA) in 2016 identified a range of opinions among PWUO regarding their preferences and their ability to distinguish heroin from fentanyl; the study reported that the dealers were most commonly selling their drug as “heroin,” regardless of the presence of NPFs, and thus user choices were dependent on their skill and ability to identify NPF contamination in their heroin supply (Ciccarone, et al., 2017).
Most prior studies on NPF use practices were based on user self-reports, and very few have included confirmatory drug testing for NPFs. A study conducted in MA with 30 emergency department patients treated after heroin overdose compared user self-identification of NPF exposure with confirmatory urine drug testing for fentanyl, acetyl fentanyl and U-47700. The study found that only 55% of persons accurately identified the presence of fentanyl in heroin they used prior to overdose (Griswold, et al., 2017). Another study conducted in MA with 231 patients seeking substance use treatment found that about 87% tested positive for fentanyl, although the study did not test for fentanyl analogues. Out of 49 individuals who believed that their tests would be negative, over 71% tested positive for fentanyl (Kenney, Anderson, Conti, Bailey, & Stein, 2018). Overall, fentanyl analogues have not been a part of routine toxicology testing across the country, and there is a lack of consistent toxicological information on NPF-related exposures (Cicero, Ellis, & Kasper, 2017; Slavova, et al., 2017), in particular among community-recruited samples of PWUO.
This study aims to fill existing research gaps in relation to PWUO experiences and toxicological data on fentanyl and fentanyl analogue use. This study is based on collaboration between the Wright State University (WSU) and the Montgomery County Coroner’s Office/Miami Valley Regional Crime Lab (MCCO/MVRCL). The study uses data collected from 60 individuals who self-reported heroin/NPF use and were recruited in the Dayton, Ohio (Montgomery County) area. The key goals of the study are to: 1) characterize PWUO experiences, preferences and self-reported ability to differentiate between NPFs and heroin; 2) conduct urine toxicology to identify heroin, fentanyl, and fentanyl analogues in urine samples collected from PWUO, and 3) compare self-reported use of suspected NPFs and heroin with urine drug testing for NPFs and heroin.
METHODS
Participant recruitment
Interviews were conducted with 60 individuals between May 2017 and January 2018. To be eligible for the study, participants had to be 18 years of age or older, reside in the Dayton area, and self-report use of heroin and/or suspected non-pharmaceutical fentanyl (NPF) in the past 30 days. Participants were recruited though Craigslist ads, flyers in the community, and referrals by other participants. Interviewees were compensated $30 for their participation. Interviews were conducted after administering informed consent. The study was approved by the Wright State University’s Institutional Review Board.
Interviews
Interviews were conducted by the first and second authors in a field-site office, located in downtown Dayton. Interview protocols included structured and semi-structured sections. Interview questions were developed based on prior qualitative interviews on NPF trends in the community Daniulaityte, Lamy, et al. (2017). Structured questions included 1) socio-demographic characteristics (age, sex, race, education, and employment); 2) history of chronic pain and illicit pharmaceutical opioid use; 3) drug use practices; 4) experiences with, and attitudes about, fentanyl: a) what helps identify fentanyl vs. heroin (“In the past 6 months, what helped you distinguish heroin from street fentanyl, if anything?”); b) confidence in ability to identify fentanyl vs. heroin (“Thinking about the past 6 months, how confident have you been in your ability to tell apart heroin from street fentanyl and vice versa?”); c) availability of fentanyl (“How easy would it be for you to get street fentanyl (NOT HEROIN) if you wanted some?”); d) availability of heroin (“How easy would it be for you to get heroin (NOT FENTANYL) if you wanted some?”); e) preference for fentanyl vs. heroin (“If you had to choose between using heroin and using street fentanyl, which one would you prefer?”); and f) drug overdoses (“How many times in your life have you experienced an unintentional drug-related overdose?”); (“How many people are you personally acquainted with who have died from an unintentional drug overdose?”). Open-ended, semi-structured sections included questions that asked participants to explain and elaborate history of opioid use, preferences, reasons and practices of NPF use, and knowledge about different types of fentanyl analogues. The qualitative data will be reported in another study.
Urine Drug Testing
All participants were asked to provide an unsupervised urine specimen. Out of 60 respondents, 59 provided urine specimens, and one declined to provide a specimen. All specimen cups were labeled with a number that linked the urine specimen to the survey responses. No names or other identifiable information was placed on the specimen cups.
All urine specimens were first analyzed using One Step Drug Screen Test Card (Redwood Toxicology Laboratory), an immunoassay-based method, to identify cases testing positive for opiates (i.e., heroin, hydrocodone, hydromorphone, morphine). The One Step Test Card yields a positive result for opiates (OPI) when a concentration of morphine exceeds 300 ng/mL cut-off level. Individuals who showed positive results for opiates were labeled as “heroin-positives.” Two individuals who self-reported use of other types of opiates (hydrocodone) in the past 3 days were excluded when analyzing results to identify “heroin-positives.”
Next, urine specimens were stored onsite in a refrigerator until transportation to the Montgomery County Coroner’s Toxicology laboratory. At the MCCO laboratory, all specimens were analyzed using the liquid-chromatography-tandem mass spectrometry (LC-MS/MS)-based method (Strayer, Antonides, Juhascik, Daniulaityte, & Sizemore, 2018) to identify 34 fentanyl analogues, metabolites, and other synthetic opioids in biological matrices at sub ng mL−1 concentrations: 1) 3-Methylfentanyl; 2) 4-ANNP (Despropionyl fentanyl); 3) Acetyl fentanyl; 4) Acetyl fentanyl 4-methylphenethyl; 5) Acryl fentanyl; 6) Alfentanil; 7) Butyryl fentanyl; 8) Isobutyryl fentanyl; 9) Butyryl norfentanyl; 10) Carfentanil; 11) Despropionyl para-fluorofentanyl; 12) Fentanyl; 13) Furanyl fentanyl; 14) Furanyl norfentanyl; 15) Norfentanyl; 16) para-Fluorobutyryl; 17) fluoroisobutyryl fentanyl; 18) para-Methoxyfentanyl; 19) Remifentanil; 20) Remifentanil metabolite; 21) Sufentanil; 22) Valeryl fentanyl; 23) AH-7921; and 24) U-47700. Detection methods for these 24 analogues/metabolites is described in (Strayer, et al., 2018). Methods to test for an additional 10 analogues were developed at the MCCO Toxicology laboratory, but are not described in (Strayer, et al., 2018). These included: 25) beta-Hydroxythiofentanyl; 26) para-Fluorofentanyl; 27) Cyclopropyl; 28) Crotonyl Fentanyl, 29) Tetrahydrofuran Fentanyl, 30) Methoxyacetylfentanyl, 31) Benzyl fentanyl, 33) Benzyl carfentanil, and 34) U-49900. The isomeric forms butyryl fentanyl/isobutyryl fentanyl and para-fluorobutyryl/fluoroisobutyryl fentanyl could not be differentiated. AH-7921, U-47700 and U-49900 are synthetic opioids not structurally related to fentanyl.
Data Analysis
Quantitative data were analyzed using SPSS. Descriptive statistics were used to characterize the sample in terms of demographic and drug use characteristics. The following measures of concordance of self-report with urine samples were computed: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and Cohen’s kappa. Sensitivity is calculated as the proportion of individuals with positive urine tests for a drug who correctly self-reported use of that drug. Specificity is calculated as a proportion of individuals with negative urine test results who correctly reported no use of that drug. Positive predictive value reflects the proportion of positive self-reports that are true positives, and negative predictive value reflects the proportion of negative self-reports that are true negatives. Cohen’s kappa statistic is based on the difference between observed agreement and agreement expected to be present by chance. Its value ranges from −1 to 1, with higher values indicating a higher level of agreement between self-report and testing results (Viera & Garrett, 2005). Self-report measures of heroin and suspected fentanyl use were based on a past 3-day cut off point for self-reported use of these drugs (Griswold, et al., 2017; Hawks, Chiang, & National Institute on Drug Abuse, 1986).
RESULTS
Participant Characteristics
Out of 60 participants, almost 52% were female, and over 90% were white. Almost 70% had only high school education or less, and the majority (75%) were unemployed. The mean age was 39 years. More than half of participants said that they had been diagnosed with chronic pain (Table 1).
Table 1.
Demographic and drug use characteristics of individuals who use illicit opioids (N=60), recruited in the Dayton, Ohio, area.
| Characteristics | Number | % |
|---|---|---|
| Demographics Characteristics | ||
| Sex | ||
| Male | 29 | 48.3% |
| Female | 31 | 51.7% |
| Age | ||
| Years (Mean, Standard Dev.) | 39 | 9.5 |
| Race | ||
| White | 55 | 91.7% |
| African American | 4 | 6.7% |
| Other | 1 | 1.7% |
| Education | ||
| Less than HS | 19 | 31.7% |
| HS or GED | 22 | 36.7% |
| Some college or more | 19 | 31.7% |
| Employment | ||
| Employed, full or part time | 15 | 25.0% |
| Unemployed | 45 | 75.0% |
| Pain and History of Illicit Pain Pill Use | ||
| Ever diagnosed with chronic pain | 33 | 55.0% |
| Used illicit pain pills before heroin | 52 | 86.7% |
| Addicted to pain pills before heroin | 41 | 68.3% |
| Heroin/Fentanyl Use | ||
| Years Since first heroin use (Mean, Standard Dev.) | 10.6 | 9.2 |
| Years since first fentanyl use (Mean, Standard Dev.) | 3.9 | 5.6 |
| Heroin/Fentanyl administration (past 6 months): | ||
| Heroin/Fentanyl Injection | 47 | 78.7% |
| Intranasal | 11 | 18.3% |
| Other Substance Use (past 6 months) | ||
| Alcohol | 25 | 41.7% |
| Marijuana | 40 | 66.7% |
| Cocaine | 48 | 80.0% |
| Methamphetamine | 26 | 43.3% |
| Diverted Pain Pills | 26 | 43.3% |
| Diverted Benzodiazepines | 33 | 55.0% |
| Diverted Gabapentin | 16 | 26.7% |
| Diverted Buprenorphine | 26 | 43.3% |
| Overdose-Related Experiences | ||
| Number of overdose experiences, lifetime (Mean, Std. Dev.) | 2.8 | (3.9) |
| Number of friends/family who died from an OD(Mean, Std. Dev.) | 9.7 | (10.0) |
| Perceived Personal Risk of OD in the past 30 days: | ||
| None | 9 | 15.0% |
| Little | 17 | 38.3% |
| Moderate | 20 | 33.3% |
| High | 7 | 11.7% |
| Self-Reported Use1 | ||
| Street Fentanyl (NPF) | 44 | 73.3% |
| Heroin | 35 | 58.3% |
| Groups by Self-reported Heroin and/or NPF Use/Non-Use: | ||
| Street Fentanyl ONLY, NO Heroin | 14 | 23.3% |
| Street Fentanyl AND Heroin | 29 | 48.3% |
| Heroin ONLY, NO Street Fentanyl | 6 | 10.0% |
| NO Heroin, NO Street Fentanyl | 7 | 11.7% |
Out of all 60 cases, data about self-reported street fentanyl use in the past 3 days were missing for 3 individuals, data about heroin use were missing for 4 individuals.
Mean number of years since first heroin use was 10.6 (Std. Dev. 9.2). Almost 80% of the study participants reported injection as the primary mode of administration in the past 6 months. About 87% reported use of illicit pharmaceutical opioids before initiating heroin use, and nearly 70% reported being dependent on illicit pharmaceutical opioids before their transition to heroin. Use of other drugs was common. About 67% self-reported using marijuana, 80% cocaine, over 50% diverted benzodiazepines, and about 43% methamphetamine in the past 6 months prior to the interview. Participants reported a mean of 2.8 unintentional drug-related overdoses in their lifetime. They reported knowing an average of almost 10 people who had died from an unintentional drug overdose (Table 1). Only 11.7% believed their risk of overdose was high, and about 33% believed their risk was moderate.
Knowledge and Experiences Related to Non-Pharmaceutical Fentanyl
The majority of the participants reported first experiences with non-pharmaceutical or street fentanyl (NPF) in the past few years, coinciding with the timeline of the current outbreak of NPF in the Dayton, Ohio area in 2013. Mean number of years since first experience of using street fentanyl was 3.9 (Table 1). A few individuals reported occasional availability of street fentanyl well before the current outbreak, but those were viewed as short-lived occurrences.
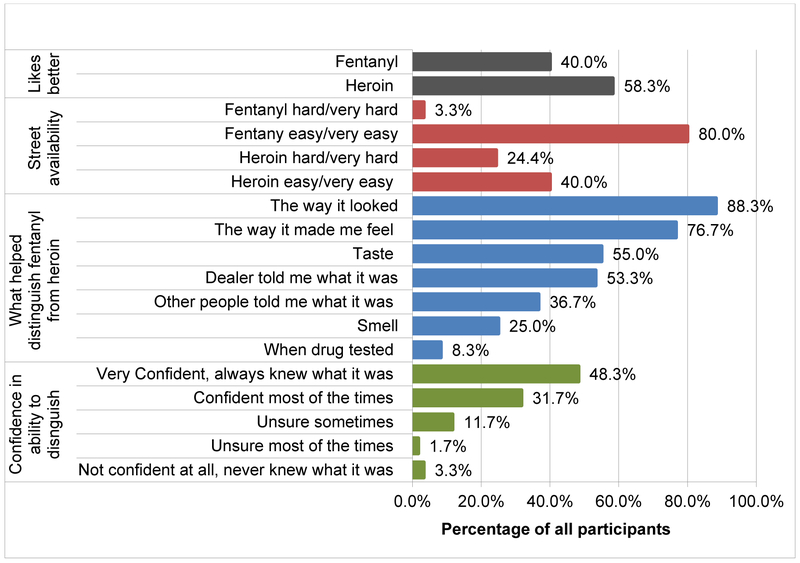
More individuals (58.3%) reported preference for heroin rather than for street fentanyl (40%). The majority viewed fentanyl to be easier to access compared to heroin—80% reported that street fentanyl is easy or very easy to obtain, and only 40% reported easy availability of heroin. Similarly, nearly 30% reported that heroin is hard or very hard to find, and only 3% believed that fentanyl was hard to find (Figure 1).
Figure 1.
Preferences, perceived availability and identification of street fentanyl versus heroin (N=60).
Participants reported relying on a number of strategies to distinguish street fentanyl from heroin. Nearly 90% mentioned that appearance was a way to identify fentanyl. Reportedly, heroin had a brownish/tan color, while fentanyl was generally white. Nearly 80% reported that they could identify fentanyl from the way it made them feel, and many reported that they could judge from the taste and smell (heroin bitter, vinegar-like smell and taste, while NPFs “sweet” taste and pharmaceutical-like smell). More than half reported that dealers informed them that the substance they bought was street fentanyl (Figure 1). When asked how confident they had been in their ability to tell apart fentanyl from heroin, nearly half reported that there were very confident (Figure 1). However, participants had very limited knowledge about fentanyl analogues. Although some had heard about carfentanil, and a few reported using it, the majority cited recent media reports as the sources of their information about carfentanil. Although many individuals reported seeing and using fentanyl-type drugs that varied in color (e.g., yellow or pink hue) and in the way it made them feel, the majority had no knowledge about other types of fentanyl analogues.
Self-Reported Use of Street Fentanyl
A greater number of study participants (44, 73.3%) self-reported suspected use of street fentanyl than heroin (35, 58%) in the past 3 days (Table 1). About 23% self-reported using suspected street fentanyl only, no heroin in the past 3 days, and nearly 50% self-reported use of both suspected street fentanyl and heroin. There were 7 individuals who self-reported no use of heroin and/or suspected street fentanyl in the past 3 days prior to the interview (Table 1).
Results of Toxicological Testing
Onsite testing using One Step Test Cards found that almost 47% screened positive for opiates (Table 2). Toxicological analyses using the LC-MS/MS-based method found that over 88% of participants tested positive for fentanyl and/or fentanyl analogues/metabolites (Table 2). Fentanyl was identified in 86.4% of all tested cases, almost 48% tested positive for carfentanil and about 42% for acetyl fentanyl. Overall, our testing results showed the presence of 8 types of fentanyl/fentanyl analogues (fentanyl, carfentanil, acetyl fentanyl, fluorobutyryl fentanyl, cyclopropyl fentanyl, furanyl fentanyl, benzyl fentanyl, acryl fentanyl, excluding separate counts of metabolites norfentanyl, furanyl norfentanyl and despopionyl fentanyl, which is precursor chemical, impurity and metabolite of NPFs) in the urine samples obtained from 59 participant. The majority of NPF-positives contained multiple NPF-type drugs. Over 80% tested positive for 2 or more NPF-type drugs, and about 47% testing positive for at least 3 NPFs. Among NPF positives, a mean number of 2.6 NPF-type substances were identified (Table 2).
Table 2.
Urine testing toxicology results for heroin and street fentanyl/fentanyl analogs (NPFs) among individuals who use illicit opioids (N=59), recruited in the Dayton, Ohio, area.
| Drug | All Cases, N=59 | Positive for any NPF, N=52 |
||
|---|---|---|---|---|
| N | % | N | % | |
| Any NPF (LC-MC testing) | 52 | 88.1% | 52 | 100% |
| Fentanyl1 | 51 | 86.4% | 51 | 96.2% |
| Carfentanil | 28 | 47.5% | 28 | 53.8% |
| Acetyl Fentanyl | 25 | 42.4% | 25 | 48.1% |
| Despropionyl Fentanyl2 | 22 | 37.3% | 22 | 42.3% |
| Para-Fluorobutyryl | 14 | 23.7% | 14 | 26.9% |
| Cyclopropyl Fentanyl | 7 | 11.9% | 7 | 13.5% |
| Furanyl Fentanyl3 | 5 | 8.3% | 5 | 9.6% |
| Benzyl Fentanyl | 4 | 6.8% | 4 | 7.7% |
| Acryl Fentanyl | 2 | 3.4% | 2 | 3.8% |
| Number of NPF-type drugs identified per case: | ||||
| 1 type of NPF | 10 | 19.2% | ||
| 2 types of NPFs | 23 | 44.2% | ||
| 3 types of NPFs | 6 | 11.5% | ||
| 4 types of NPFs | 5 | 9.6% | ||
| 5 types of NPFs | 6 | 11.5% | ||
| 5 types of NPFs | 2 | 3.8% | ||
| Average number of NPFs per case (Mean, SD) | 2.6 | 1.4% | ||
| Heroin4 (Onsite One Step Drug Screen Test Card, OPI, 300) | 27 | 46.6% | 27 | 54.0% |
| Groups by Heroin and NPF Positive/Negative Testing Results: | ||||
| Positive for any NPF, negative for heroin | 23 | 39.7% | ||
| Positive for any NPF and heroin | 27 | 46.6% | ||
| Negative for any NPF, positive for heroin | 0 | 0 | ||
| Negative for any NPFs and heroin | 7 | 12.1% | ||
Includes fentanyl and/or its metabolite norfentanyl positive cases.
Despropionyl Fentanyl (or 4ANNP) is a precursor chemical, impurity and metabolite of NPFs.
Includes furanyl fentanyl and/or its metabolite furanyl norfentanyl positive cases.
2 individuals who self-reported hydrocodone use in the past 3 days but no heroin use were excluded from reporting heroin (OPI 300) testing results.
Comparing Testing and Self-Reports
Out of 43 individuals who self-reported suspected street fentanyl use in the past 3 days and submitted urine samples for analyses, 42 tested positive for NPFs, which results in positive predictive value of 97.7%. However, negative predictive value was low (38.5%), indicating that many of the individuals who self-reported no use of street fentanyl in the past 3 days actually tested positive for NPFs. As shown in Table 4, sensitivity and specificity were relatively high for NPFs, 84% and 83.3%, accordingly. Cohen’s Kappa index value was 0.445, which indicates fair agreement between self-reported use of suspected street fentanyl and testing results.
Positive predictive value for heroin was lower—only 61.8% of those who self-reported heroin use in the past 3 days tested positive for heroin/morphine. Negative predictive value for heroin was higher (68.4%), compared to NPFs (38.5%). Compared to NPFs, sensitivity and specificity were lower for heroin as well, 77.8% and 50.0% accordingly. Cohen’s Kappa value was 0.296, indicating low level of agreement between self-reports of heroin use and testing results (Table 4).
DISCUSSION
The study is one of the first to explore user experiences with NPFs in the Midwestern U.S. and to conduct toxicological analyses to test for a broad range of fentanyl analogues in a community-recruited sample of individuals who use illicit opioids. Participants described local “street dope” market being over-run with NPF-type drugs, and noted decreased availability of heroin. Not surprisingly, nearly 90% of the study participants tested positive for NPF-type drugs, and less that 50% tested positive for heroin. Similar rate of NPF positives (although limited to fentanyl only) was found by a study conducted in 2017 in Massachusetts among individuals seeking opioid withdrawal management (Kenney, et al., 2018). More importantly, our data demonstrate exposure to a large variability of NPF-type drugs. Besides fentanyl, there were 7 types of fentanyl analogues identified in the tested samples, and almost 80% of NPF-positive cases tested positive for more than one type of NPF, including acetyl fentanyl, carfetanyl, furanyl fentanyl and other. Compared to the local overdose fatality data from the same time period, a greater proportion of our study participants tested positive for heroin (46.6% vs. 9%) and acetyl fentanyl (42.4% vs. 4%) (Public Health Dayton & Montgomery County, 2018). Acetyl fentanyl and heroin are less potent than fentanyl and some other analogues (European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), 2017; Lozier, et al., 2015; Stogner, 2014) and thus is it is not surprising to see their greater prevalence in toxicology reports of living individuals than in overdose fatalities. These data highlight the complexity and unpredictable nature of synthetic opioid market in the US and emphasize the need for expanded NPF testing to include not just fatality data but also community-recruited samples of PWUO to assess evolving risk environments of NPF outbreaks.
High prevalence of carfentanil positives in our sample (47.5%) is remarkable but consistent with other data sources. In 2017, Ohio had the highest numbers of carfentanil drug seizures in the country (US Drug Enforcement Administration & Office of Diversion Control, 2019), and over 60% of all unintentional overdose death cases that occurred in Montgomery County in 2017 tested positive for carfentanil (Public Health Dayton & Montgomery County, 2018). With an exception of a few recent studies that reported identification of carfentanil-positives in non-fatal impaired driving cases in the U.S. (Sofalvi, et al., 2017; Tiscione & Alford, 2018), most prior data on carfentanil-related trends were based on overdose fatality cases (Daniulaityte, Juhascik, et al., 2017; Raminta Daniulaityte, et al., 2019; J. O'Donnell, Gladden, Mattson, & Kariisa, 2018). Identification of carfentanil positives in living persons emphasize the need to better understand the complexity of drug-related (e.g., concentration of carfentanil, along with other NPFs and/or non-psychoactive adulterants in drug samples), situational, as well as individual (e.g., tolerance, metabolic) and behavioral characteristics that may protect some individuals from unintentional overdose risks, even when they are exposed to such highly potent synthetic opioids as carfentanil (Armenian, Vo, Barr-Walker, & Lynch, 2017; Suzuki & El-Haddad, 2017). More research is needed to better understand how exposure to such potent opioids as carfentanil and other NPFs may impac effectiveness of the established medication-assisted treatment protocols for individuals with opioid use disorders (Bisaga, 2019).
Comparison of self-reports to urine toxicology indicates that individuals were mostly correct in their identification of drugs that they believe contained fentanyl-type substances; lack of concordance stemmed from misidentification of drugs PWUO believed to contain heroin. Overall, our study results show greater level of sensitivity (84%) in relation to street fentanyl self-reporting, compared to prior studies. A study conducted with PWUO recruited in Canada in 2015 found sensitivity at 26% (Amlani, et al., 2015), while among overdose survivors surveyed in MA, sensitivity was 55% (Griswold, et al., 2017). Greater level of accuracy in self-identification of fentanyl-type drugs could be related to the fact that our study was conducted at a later stage of NPF epidemic with PWUO gaining greater knowledge and experience with NPF-type drugs. Moreover, with increased availability and associated increased frequency of NPF-type drug use, sensitivity is also likely to increase (Donovan, et al., 2012).
Further, it is important to emphasize that concordance measures examined NPFs as a general class of fentanyl-type drugs. It is not possible for PWUO to differentiate between different types of analogues present in their street fentanyl samples. Many participants generally had little knowledge about the availability of different types of fentanyl analogues or their range of potency levels. Our study findings are highly relevant when considering development of novel overdose prevention programs. There is an increased interest in promotion of testing technologies to be used by PWUO to identify fentanyl in their drug supplies (Ciccarone, 2017; Krieger, et al., 2018; McGowan, Harris, Platt, Hope, & Rhodes, 2018; Park, Weir, Allen, Chaulk, & Sherman, 2018; Tupper, McCrae, Garber, Lysyshyn, & Wood, 2018). For example, a recent study conducted in North Carolina gave BTNX’ Rapid Response Fentanyl Test Strips and instructions to people who had injected illicit opioids in the past 24 hours. The study found that of out 125 people assessed, 43% reported some changes in their drug use behavior, and 77% believed the fentanyl test strips increased their overdose safety (Peiper, et al., 2018). Fentanyl Testing Strips provide qualitative identification of fentanyl and some analogues, but cannot differentiate between different types of analogues (McGowan, et al., 2018). Our findings indicate that unless such technologies can be reliable and specific in identifying emerging fentanyl analogues and other novel synthetic opioids, they can be of limited value in the context of current NPF epidemic with increased presence and diversification of fentanyl-type drugs and other novel opioids.
Although overall concordance measures between urine toxicology and self-reported use were relatively low, the majority of PWUO felt confident in their ability to identify NPFs vs. heroin. Similar to prior studies (Carroll, et al., 2017; Ciccarone, et al., 2017), our participants reported relying primarily on the appearance and effects to identify fentanyl-containing drugs. However, over 50% also reported reliance on information provided by their dealers regarding presence of fentanyl-type drugs in their “dope.” This contrasts with some of the earlier studies reporting a general pattern of NPFs being sold as heroin to unsuspecting individuals seeking to purchase heroin (Griswold, et al., 2017; Mars, et al., 2018; Stogner, 2014). Increased reports about user reliance on dealer information might indicate regional variations and/or changes over time associated with increased saturation of illicit drug markets with NPF-type drugs. These findings support increased public health interest in development of intervention strategies that engage drug dealers in targeted communication of harm reduction messages to PWUO (Bardwell, Boyd, Arredondo, McNeil, & Kerr, 2019).
About 40% of our participants expressed preference for fentanyl, while about 60% preferred heroin. In comparison, a qualitative study among heroin injectors in Rhode Island in 2016 found that most PWUO had a strong dislike for fentanyl (Carroll, et al., 2017), while a study conducted in North Carolina has found that about 31% expressed preference for fentanyl (Peiper, et al., 2018). This variation might be linked to a growing availability of NPF-type drugs in local drug markets and associated evolution of user preferences and attitudes about NPFs. Further, increased exposure to NPF-type drugs may result in greater tolerance among PWUO, which inevitably may contribute to shifting preferences for more potent opioids (Peiper, et al., 2018).
Limitations of this study include recall bias when asking PWUO about their substance use in the past 3 days, and individual metabolic and drug use factors that may contribute to variations in detection window for selected drugs by urine toxicology (Donovan, et al., 2012). Another limitation is related to the fact that urine sample collection was unsupervised. We also acknowledge that the One Step Urine Test Card provides only a qualitative, preliminary analytical result in contrast to much more sensitive results on NPFs through LC-MS/MS testing. Due to time and funding constraints, the LC-MS/MS method was not used to test for heroin and other drugs. In addition, the testing cannot distinguish between pharmaceutical and non-pharmaceutical fentanyl, however interview data indicate that participants did not report recent use of diverted pharmaceutical fentanyl products. Other data sources also indicate that availability and use of diverted pharmaceutical fentanyl products (e.g.., Duragesic) are low in the Dayton area (Marinetti & Ehlers, 2014; Ohio Substance Abuse Monitoring Network, 2017). Co-occurrence of NPFs, with or without heroin, identified in our sample could be due to several factors— multiple episodes of illicit opioid use in the past 3 days, involving different batches of drugs from different sources/dealers, and/or a combination of different analogues from a single source/dealer. The underlying reasons for combinations of multiple NPF analogues (and heroin) are unknown.
Finally, our sample was relatively small and not randomly recruited, so the results cannot be generalized to a larger population in Ohio or elsewhere. Research with larger samples of PWUO is needed to track user experiences and behaviors related to NPF use. However, the sample resembles demographic profiles of PWUO identified by other epidemiological studies (J. K. O’Donnell, Halpin, Mattson, Goldberger, & Gladden, 2017; Public Health Dayton & Montgomery County, 2018; Shiels, Freedman, Thomas, & Berrington de Gonzalez, 2018). Similar to prior research findings, our participants reported a wide range of polysubstance use (Bobashev, Tebbe, Peiper, & Hoffer, 2018), and almost 70% of the sample reported dependence on non-prescribed pharmaceutical opioids prior to initiating heroin use (Cicero, Ellis, Surratt, & Kurtz, 2014; Lankenau, et al., 2012; Mars, Bourgois, Karandinos, Montero, & Ciccarone, 2014; Siegal, Carlson, Kenne, & Swora, 2003).
Our research findings demonstrate the erratic complexity of the current “street dope” market and highlight the need for robust surveillance efforts to track NPF-type substances not just in forensic toxicology but also in community-recruited samples of PWUO. Along with the continuing expansion of access to naloxone, it is crucial for the community overdose prevention programs to develop rapid dissemination of up-to-date information to PWUO about changes in availability of specific NPF products and empower PWUO to engage in safer practices (Fairbairn, Coffin, & Walley, 2017; Peiper, et al., 2018) such as using testing doses to assess potency and effects of the drug, switching from intravenous use to snorting, and using in settings where immediate assistance from other individuals is available.
Table 3.
Concordance measures between self-reports and urine toxicology for street fentanyl (NPF) and heroin among individuals who use illicit opioids, recruited in the Dayton, Ohio, area.
| Concordance Measures | NPFs | Heroin |
|---|---|---|
|
Sensitivity The proportion of users with positive urine testing who correctly self-reported use of that drug. |
84.0% | 77.8% |
|
Specificity The proportion of users with negative urine testing who correctly reported no use of that drug. |
83.3% | 50.0% |
|
Positive predictive value The proportion of positive self-reports that tested positive for that drug. |
97.7% | 61.8% |
|
Negative predictive value The proportion of negative self-reports that tested negative for that drug. |
38.5% | 68.4% |
| Kappa Statistic | 0.429 | 0.245 |
Acknowledgements:
We want to thank Montgomery County Coroner’s office toxicology laboratory, in particular Dr. Kent E. Harshbarger, and Heather M. Antonides for their advice, support, and help with the project.
Funding Source:
This work was supported by the National Institutes of Health/National Institute on Drug Abuse: 1R21DA042757 (Daniulaityte, PI) and R01 DA040811 (Daniulaityte, PI). The funding source had no further role in the study design, in the collection, analysis and interpretation of the data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amlani A, McKee G, Khamis N, Raghukumar G, Tsang E, & Buxton JA (2015). Why the FUSS (Fentanyl Urine Screen Study)? A cross-sectional survey to characterize an emerging threat to people who use drugs in British Columbia, Canada. Harm reduction journal, 12, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenian P, Vo KT, Barr-Walker J, & Lynch KL (2017). Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology. [DOI] [PubMed] [Google Scholar]
- Bardwell G, Boyd J, Arredondo J, McNeil R, & Kerr T (2019). Trusting the source: The potential role of drug dealers in reducing drug-related harms via drug checking. Drug Alcohol Depend, 198, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A (2019). What should clinicians do as fentanyl replaces heroin? Addiction. [DOI] [PubMed] [Google Scholar]
- Bobashev G, Tebbe K, Peiper N, & Hoffer L (2018). Polydrug use among heroin users in Cleveland, OH. Drug Alcohol Depend, 192, 80–87. [DOI] [PubMed] [Google Scholar]
- Carroll JJ, Marshall BDL, Rich JD, & Green TC (2017). Exposure to fentanyl-contaminated heroin and overdose risk among illicit opioid users in Rhode Island: A mixed methods study. Int J Drug Policy, 46, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2015). Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. CDC; 2015 . In HAN Health Advisory. Atlanta, GA: US Department of Health and Human Services. [Google Scholar]
- Ciccarone D (2017). Fentanyl in the US heroin supply: A rapidly changing risk environment. Int J Drug Policy, 46, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone D, Ondocsin J, & Mars SG (2017). Heroin uncertainties: Exploring users’ perceptions of fentanyl-adulterated and -substituted ‘heroin’. Int J Drug Policy, 46, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, & Kasper ZA (2017). Increases in self- reported fentanyl use among a population entering drug treatment: The need for systematic surveillance of illicitly manufactured opioids. Drug Alcohol Depend, 177, 101–103. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, & Kurtz SP (2014). The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA psychiatry, 71, 821–826. [DOI] [PubMed] [Google Scholar]
- Daniulaityte R, Juhascik MP, Strayer KE, Sizemore IE, Harshbarger KE, Antonides HM, & Carlson RR (2017). Overdose Deaths Related to Fentanyl and Its Analogs - Ohio, January-February 2017. MMWR Morb Mortal Wkly Rep, 66, 904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Juhascik MP, Strayer KE, Sizemore IE, Zatreh M, Nahhas RW, Harshbarger KE, Antonides HM, Martins SS, & Carlson RG (2019). Trends in fentanyl and fentanyl analogue-related overdose deaths - Montgomery County, Ohio, 2015– 2017. Drug Alcohol Depend, 198, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Juhascik MP, Strayer KE, Sizemore IE, Zatreh M, Nahhas RW, Harshbarger KE, Antonides HM, Martins SS, & Carlson RR (2019). Trends in Fentanyl and Fentanyl Analogue-Related Overdose Deaths - Montgomery County, Ohio, 2015–2017. Drug and alcohol dependence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniulaityte R, Lamy FR, Juhascik M, Sizemore IE, Zatreh M, Strayer KE, & Carlson R (2017). “That fentanyl dope is way worse”: Characterizing fentanyl outbreaks in the Dayton (Ohio) area In College on Problems of Drug Dependence. Montreal, Canada. [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen J, Gardin JG, Hamilton JA, Huestis MA, Hughes JR, Lindblad R, Marlatt GA, Preston KL, Selzer JA, Somoza EC, Wakim PG, & Wells EA (2012). Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction (Abingdon, England), 107, 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). (2017). Risk assessment report on a new psychoactive substance: N- (1- phenethylpiperidin-4-yl)-N-phenylacrylamide (acryloylfentanyl). In. Brussels: Council of the European Union. [Google Scholar]
- Fairbairn N, Coffin PO, & Walley AY (2017). Naloxone for heroin, prescription opioid, and illicitly made fentanyl overdoses: Challenges and innovations responding to a dynamic epidemic. Int J Drug Policy, 46, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden RM, Martinez P, & Seth P (2016). Fentanyl Law Enforcement Submissions and Increases in Synthetic Opioid-Involved Overdose Deaths - 27 States, 2013–2014. MMWR Morb Mortal Wkly Rep, 65, 837–843. [DOI] [PubMed] [Google Scholar]
- Griswold MK, Chai PR, Krotulski AJ, Friscia M, Chapman B, Boyer EW, Logan BK, & Babu KM (2017). Self-identification of non-pharmaceutical fentanyl exposure following heroin overdose. Clin Toxicol (Phila), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks RL, Chiang CN, & National Institute on Drug Abuse. (1986). Urine testing for drugs of abuse. [Google Scholar]
- Hedegaard H, Miniño AM, & Warner M (2018). Drug overdose deaths in the United States, 1999–2017 In NCHS data brief (Vol. 329). Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Jones CM, Einstein EB, & Compton WM (2018). Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010–2016. Jama, 319, 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney SR, Anderson BJ, Conti MT, Bailey GL, & Stein MD (2018). Expected and actual fentanyl exposure among persons seeking opioid withdrawal management. J Subst Abuse Treat, 86, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger MS, Goedel WC, Buxton JA, Lysyshyn M, Bernstein E, Sherman SG, Rich JD, Hadland SE, Green TC, & Marshall DL (2018). Use of rapid fentanyl test strips among young adults who use drugs. Int J Drug Policy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau SE, Teti M, Silva K, Bloom JJ, Harocopos A, & Treese M (2012). Initiation into prescription opioid misuse amongst young injection drug users. The International journal on drug policy, 23, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier MJ, Boyd M, Stanley C, Ogilvie L, King E, Martin C, & Lewis L (2015). Acetyl Fentanyl, a Novel Fentanyl Analog, Causes 14 Overdose Deaths in Rhode Island, March-May 2013. J Med Toxicol, 11, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmadu A, Carroll JJ, Hadland SE, Green TC, & Marshall BD (2017). Prevalence and correlates of fentanyl-contaminated heroin exposure among young adults who use prescription opioids non- medically. Addict Behav, 68, 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti LJ, & Ehlers BJ (2014). A series of forensic toxicology and drug seizure cases involving illicit fentanyl alone and in combination with heroin, cocaine or heroin and cocaine. J Anal Toxicol, 38, 592598. [DOI] [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, & Ciccarone D (2014). “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy, 25, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars SG, Ondocsin J, & Ciccarone D (2018). Sold as Heroin: Perceptions and Use of an Evolving Drug in Baltimore, MD. J Psychoactive Drugs, 50, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CR, Harris M, Platt L, Hope V, & Rhodes T (2018). Fentanyl self-testing outside supervised injection settings to prevent opioid overdose: Do we know enough to promote it? Int J Drug Policy, 58, 31–36. [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Gladden RM, Mattson CL, & Kariisa M (2018). Notes from the Field: Overdose Deaths with Carfentanil and Other Fentanyl Analogs Detected - 10 States, July 2016-June 2017. MMWR Morb Mortal Wkly Rep, 67, 767–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell JK, Halpin J, Mattson CL, Goldberger BA, & Gladden RM (2017). Deaths Involving Fentanyl, Fentanyl Analogs, and U-47700 – 10 States, July-December 2016. MMWR Morb Mortal Wkly Rep, 66, 1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohio Department of Health. (2017). 2016 Ohio Drug Overdose Data: General Finindgs In. Columbus, OH: ODH. [Google Scholar]
- Ohio Substance Abuse Monitoring Network. (2017). Surveillance of Drug Abuse Trends in the State of Ohio, June 2016-January 2017. In: Ohio Department of Mental Health and Addiction Services Office of Quality, Planning and Research. [Google Scholar]
- Park JN, Weir BW, Allen ST, Chaulk P, & Sherman SG (2018). Fentanyl-contaminated drugs and non-fatal overdose among people who inject drugs in Baltimore, MD. Harm Reduct J, 15, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper NC, Clarke SD, Vincent LB, Ciccarone D, Kral AH, & Zibbell JE (2018). Fentanyl test strips as an opioid overdose prevention strategy: Findings from a syringe services program in the Southeastern United States. Int J Drug Policy. [DOI] [PubMed] [Google Scholar]
- Public Health Dayton & Montgomery County. (2018). Accidental Overdose Death Totals, Mongomery County, Ohio, 2017. In. [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, & Gladden RM (2016). Increases in Drug and Opioid Overdose Deaths - United States, 2000–2014. MMWR Morb Mortal Wkly Rep, 64, 1378–1382. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, & Scholl L (2016). Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep, 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Freedman ND, Thomas D, & Berrington de Gonzalez A (2018). Trends in U.S. Drug Overdose Deaths in Non-Hispanic Black, Hispanic, and Non-Hispanic White Persons, 2000–2015. Ann Intern Med, 168, 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal HA, Carlson RG, Kenne DR, & Swora MG (2003). Probable relationship between opioid abuse and heroin use. American Family Physician, 67, 942, 945. [PubMed] [Google Scholar]
- Slavova S, Costich JF, Bunn TL, Luu H, Singleton M, Hargrove SL, Triplett JS, Quesinberry D, Ralston W, & Ingram V (2017). Heroin and fentanyl overdoses in Kentucky: Epidemiology and surveillance. Int J Drug Policy, 46, 120–129. [DOI] [PubMed] [Google Scholar]
- Sofalvi S, Schueler HE, Lavins ES, Kaspar CK, Brooker IT, Mazzola CD, Dolinak D, Gilson TP, & Perch S (2017). An LC-MS-MS Method for the Analysis of Carfentanil, 3-Methylfentanyl, 2-Furanyl Fentanyl, Acetyl Fentanyl, Fentanyl and Norfentanyl in Postmortem and Impaired-Driving Cases. J Anal Toxicol, 41, 473–483. [DOI] [PubMed] [Google Scholar]
- Somerville NJ, O’Donnell J, Gladden RM, Zibbell JE, Green T, Younkin M, Ruiz S, Babakhanlou-Chase H, Chan M, Callis BP, Kuramoto-Crawford J, Nields HM, & Walley AY (2017). Characteristics of Fentanyl Overdose - Massachusetts, 2014–2016. MMWR Morb Mortal Wkly Rep, 66, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogner JM (2014). The potential threat of acetyl fentanyl: legal issues, contaminated heroin, and acetyl fentanyl “disguised” as other opioids. Ann Emerg Med, 64, 637–639. [DOI] [PubMed] [Google Scholar]
- Strayer KE, Antonides HM, Juhascik MP, Daniulaityte R, & Sizemore IE (2018). LC-MS/MS-Based Method for the Multiplex Detection of 24 Fentanyl Analogues and Metabolites in Whole Blood at Sub ng mL(−1) Concentrations. ACS Omega, 3, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, & El-Haddad S (2017). A review: Fentanyl and non-pharmaceutical fentanyls. Drug Alcohol Depend, 171, 107–116. [DOI] [PubMed] [Google Scholar]
- Tiscione NB, & Alford I (2018). Carfentanil in Impaired Driving Cases and the Importance of Drug Seizure Data. J Anal Toxicol, 42, 476–484. [DOI] [PubMed] [Google Scholar]
- Tupper KW, McCrae K, Garber I, Lysyshyn M, & Wood E (2018). Initial results of a drug checking pilot program to detect fentanyl adulteration in a Canadian setting. Drug Alcohol Depend, 190, 242–245. [DOI] [PubMed] [Google Scholar]
- US Drug Enforcement Administration, & Office of Diversion Control. (2019). NFLIS Report: Tracking Fentanyl and Fentanyl-Related Substances Reported in NFLIS-Drug by State, 2016–2017; Special Maps Release. In. Springfield, VA. [Google Scholar]
- Viera AJ, & Garrett JM (2005). Understanding interobserver agreement: the kappa statistic. Fam Med, 37, 360–363. [PubMed] [Google Scholar]