Abstract
Background
Women with dense breasts may have less-accurate preoperative evaluation of extent of disease, potentially affecting the achievement of negative margins. The goal of this study was to examine the association between breast density and re-excision rates in women having breast conserving surgery for invasive breast cancer.
Methods
Women with stage I/II invasive breast cancer treated with breast-conserving surgery between 1/1/2014–10/31/2014 were included. Breast density was assessed by two radiologists. The association between breast density and re-excision was examined using logistic regression.
Results
701 women were included. Overall, 106 (15.1%) women had at least one re-excision. Younger age at diagnosis was associated with increased breast density (p < 0.001). On univariable analysis, increased breast density was associated with significantly increased odds of re-excision (odds ratio [OR] 1.38, 95% confidence interval [CI] 1.04–1.83), as was multifocal disease, HER2 positive status, and extensive intraductal component (EIC)(all p < 0.05). On multivariable analysis, breast density remained significantly associated with increased odds of re-excision (OR 1.37, 95% CI 1.00–1.86), as did multifocality and EIC. HER2 positive status was not significantly associated with re-excision on multivariable analysis.
Conclusions
Women with dense breasts are more likely to need additional surgery (re-excision after breast-conserving surgery), but increased breast density did not adversely affect disease-free survival in our study. Our findings support the need for further study in developing techniques that can help decrease re-excisions for women with dense breasts who undergo breast-conserving surgery.
Keywords: breast-conservation therapy, breast cancer, breast density, re-excision, margins
Synopsis:
In this examination of the association between breast density and re-excision rates in women undergoing breast-conserving surgery, we find that increased breast density was significantly associated with increased odds of re-excision on multivariable analysis.
INTRODUCTION
Increased breast density has been shown to be associated with a higher risk of the development of breast cancer.1–3 There is also evidence that mammography is less sensitive in denser breasts, leading to increased rates of interval cancers in patients with higher categories of breast density.4–6 Mammographic density has also been suggested as a predictor of local recurrence.7
However, studies have yielded conflicting evidence regarding the association between breast density and rate of re-excision, with some studies showing higher rates of re-excision in those with dense breasts, and others showing no significant difference.8–10 Traditionally, it has been hypothesized that the diagnostic challenges posed by increased density may lead to inaccurate preoperative sizing, making clear margins more difficult to achieve. Recent advances in imaging, and changes in the definition of acceptable margins, may have reduced this challenge.
“No ink on tumor” is now widely recognized as an adequate pathological margin for invasive carcinoma, as research has shown that this results in acceptable in-breast recurrence rates.11 Additionally, consensus guidelines have advocated a wider margin (2 mm) for in situ disease.12 Chagpar et al. reported that the use of cavity shave margins at the time of lumpectomy decreased the rate of positive margins from 34% to 19% (p = 0.01) using the definition of a positive margin as invasive carcinoma touching the ink, or 1 mm for ductal carcinoma in situ.13
The purpose of our study is to examine the association between breast density and re-excision rate after the adoption of the SSO-ASTRO consensus guidelines on margins for women with invasive breast cancer having breast-conserving surgery and radiation.
METHODS
After receiving study approval by the Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Board, we performed a retrospective review of prospective institutional databases and electronic medical records to identify all women who had breast-conserving surgery (BCS) for stage I or stage II invasive breast cancer between January 1, 2014 and October 31, 2014, including those who then went on to have re-excision or mastectomy. Women who received neoadjuvant chemotherapy, had bilateral invasive breast cancers, a personal history of prior breast cancer, or who had their index operation at another institution were excluded.
Clinical, pathologic factors and treatment variables were collected. During the study period, all surgeons at our institution routinely utilized a seed-localization technique for non-palpable lesions and practiced a cavity shave margin technique.14 This technique does not orient the primary lumpectomy specimen, but marks the new margin of each cavity shave margin, which is inked separately. If tumor is identified in this separately submitted margin, then a measurement is provided to the inked margin. Margins were defined as positive if tumor was present at the inked margin.
All mammograms were reviewed by two study authors (radiologists)—SB with 12 years of experience, and LLZ with 5 years of experience in breast imaging—and density categorized using the BIRADS (Breast Imaging-Reporting and Data System) classification of breast density,15 where a score of 1 indicates fatty, 2 indicates scattered, 3 indicates heterogeneous, and 4 indicates extreme density. Breast density was treated as an ordinal variable and dichotomized by score as 1/2 versus 3/4. Inter-reader agreement was assessed using data on all study subjects, and intra-reader agreement was assessed on a random sample of 121 study subjects. Inter- and intra-reader agreement were assessed using a weighted kappa statistic with squared weights. Confidence intervals were based on percentiles from 1000 bootstrap samples.
Demographic as well as operative, detailed pathologic, and outcome data were summarized by breast density using the median and range for continuous variables, and the frequency and percentage for categorical variables. Associations with breast density were assessed using the Wilcoxon rank-sum test for continuous variables, and Fisher’s exact test for categorical variables. Logistic regression was used to model the association between breast density and re-excision. Likelihood ratio tests were used to test for interaction between breast density and time period on re-excision rates. Multivariable logistic regression adjusted for patient and disease characteristics associated with re-excision on univariable logistic regression analysis.
The minimum value of breast density according to the two readers was used for the primary analysis because we expected increased breast density to be associated with increased re-excision rates; this represents a conservative approach as a result. Sensitivity analyses examined associations between breast density and re-excision using the maximum value according to the two readers, and using the breast density as measured by each reader separately.
A p-value < 0.05 was considered statistically significant. All analyses were conducted using R software version 3.2.5 (R Core Development Team, Vienna, Austria).
RESULTS
Patient Characteristics
A total of 701 women were included in this analysis, of whom 106 (15.1%) had at least one re-excision. Median age was 58 years (range 29–90). Median tumor size was 1.2 cm (range 0.1–4.5 cm). Multifocal disease was diagnosed in 139 (19.8%). Mammogram was the mode of diagnosis in 665 (94.9%), 27 (3.9%) were diagnosed by MRI or ultrasound. Preoperative MRI was performed at MSK on 103 (14.7%). The majority (82.6%) of the cohort had invasive ductal carcinoma. Of the remainder, 76 (10.8%) had invasive lobular carcinoma and 25 (3.6%) had mixed lobular and ductal subtype. DCIS was also seen in 566 (80.7%). Extensive intraductal component (EIC) was reported in 52 (7.4%). With regards to receptor status, 620 (88.4%) were ER positive, 552 (78.7%) were PR positive, and 56 (8%) were HER2 positive. 101 (14.4%) had positive sentinel lymph nodes. BMI for the entire cohort was 27 kg/m2 (range 15.5–54.1 kg/m2). (Table 1)
TABLE 1.
Density and Clinical Characteristics
| Minimum Density | ||||
|---|---|---|---|---|
| Overall | 1/2 | 3/4 | p-value | |
| (n = 701) | (n = 487) | (n = 214) | ||
| Age, mean (range) | 58 (28–90) | 61 (29–90) | 52 (30–9) | <.001 |
| Tumor size | 0.699 | |||
| Mean (range) | 1.2 cm (0.1–4.5) | 1.2 cm (0.1–4.5) | 1.2 cm (0.1–4) | |
| Missing | 1 | 1 | 0 | |
| Race | 0.027 | |||
| Asian | 46 (6.6%) | 24 (4.9%) | 22 (10.3%) | |
| Black | 70 (10%) | 55 (11.3%) | 15 (7%) | |
| Other | 12 (1.7%) | 8 (1.6%) | 4 (1.9%) | |
| White | 528 (75.3%) | 367 (75.4%) | 161 (75.2%) | |
| Unknown | 45 (6.4%) | 33 (6.8%) | 12 (5.6%) | |
| Multifocal | 1 | |||
| No | 560 (79.9%) | 388 (79.7%) | 172 (80.4%) | |
| Yes | 139 (19.8%) | 97 (19.9%) | 42 (19.6%) | |
| Missing | (0.3%) | 2 (0.4%) | 0 (0) | |
| Mode of diagnosis | 0.002 | |||
| Mammogram detected | 665 (94.9%) | 475 (97.5%) | 190 (88.8%) | |
| MRI/Ultrasound detected | 27 (3.9%) | 11 (2.3%) | 16 (7.5%) | |
| Missing | 9 (1.3%) | 1 (0.2%) | 8 (3.7%) | |
| MRI | < 0.001 | |||
| No | 598 (85.3%) | 443 (91.0%) | 155 (72.4%) | |
| Yes | 103 (14.7%) | 44 (9.0%) | 59 (27.6%) | |
| pT | 0.592 | |||
| T1/0 | 572 (81.6%) | 395 (81.1%) | 177 (82.7%) | |
| T2 | 125 (17.8%) | 90 (18.5%) | 35 (16.4%) | |
| Missing | 4 (0.6%) | 2 (0.4%) | 2 (0.9%) | |
| Histological subtype | 0.636 | |||
| IDC | 579 (82.6%) | 405 (83.2%) | 174 (81.3%) | |
| ILC | 76 (10.8%) | 53 (10.9%) | 23 (10.7%) | |
| IDC and ILC | 25 (3.6%) | 17 (3.5%) | 8 (3.7%) | |
| Other | 21 (3%) | 12 (2.5%) | 9 (4.2%) | |
| Tumor Type | 0.602 | |||
| Invasive carcinoma only | 134 (19.1%) | 96 (19.7%) | 38 (17.8%) | |
| With DCIS | 566 (80.7%) | 390 (80.1%) | 176 (82.2%) | |
| Missing | 1 (0.1%) | 1 (0.2%) | 0 (0) | |
| Number of sentinel nodes | ||||
| Mean (range) | 3 (0–13) | 2 (0–13) | 3 (1–11) | 0.008 |
| Missing | 20 | 17 | 3 | |
| SLN | 0.103 | |||
| Negative | 580 (82.7%) | 393 (80.7%) | 187 (87.4%) | |
| Positive | 101 (14.4%) | 77 (15.8%) | 24 (11.2%) | |
| Missing | 20 (2.9%) | 17 (3.5%) | 3 (1.4%) | |
| EIC | 0.212 | |||
| NO | 649 (92.6%) | 455 (93.4%) | 194 (90.7%) | |
| Yes | 52 (7.4%) | 32 (6.6%) | 20 (9.3%) | |
| ER | 0.12 | |||
| Negative | 65 (9.3%) | 51 (10.5%) | 14 (6.5%) | |
| Positive | 620 (88.4%) | 428 (87.9%) | 192 (89.7%) | |
| Missing | 16 (2.3%) | 8 (1.6%) | 8 (3.7%) | |
| PR | 0.204 | |||
| Negative | 131 (18.7%) | 98 (20.1%) | 33 (15.4%) | |
| Positive | 552 (78.7%) | 380 (78%) | 172 (80.4%) | |
| Missing | 18 (2.6%) | 9 (1.8%) | 9 (4.2%) | |
| HER2 | 0.761 | |||
| Negative | 622 (88.7%) | 437 (89.7%) | 185 (86.4%) | |
| Positive | 56 (8%) | 38 (7.8%) | 18 (8.4%) | |
| Missing | 23 (3.3%) | 12 (2.5%) | 11 (5.1%) | |
| BMI | ||||
| Median (range) | 27 (15.5–54.1) | 28.6 (17.2–54.1) | 23.6 (15.5–46.9) | < 0.001 |
| Missing | 11 | 5 | ||
pT pathologic tumor stage, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, DCIS ductal carcinoma in situ, SLN sentinel lymph node, EIC extensive intraductal component, ER estrogen receptor, PR progesterone receptor
Mammographic Density Interpretation
The weighted kappa for inter-reader agreement on the full sample was 0.633 (95% confidence interval [CI] 0.604–0.663). On the random sub-sample of 121 women, the weighted kappa for intra-reader agreement was 0.755 (95% CI 0.663–0.834). These values indicate moderate to substantial agreement.
The minimum breast density according to the two readers was recorded according to BIRADS classification: 1 (fatty) in 129 (18.4%); 2 (scattered) in 358 (51.1%); 3 (heterogenous) in 197 (28.1%); and 4 (extremely dense) in 17 (2.4%).
Table 2 shows a cross tabulation between the two readers and level of agreement at each density category. Greater reader agreement was noted at higher density categories, i.e., concordance was noted as 83% for density 3, and 71% for density 4. The reported pattern of results held in sensitivity analyses separately by reader, and using the maximum value of breast density according to the two readers.
TABLE 2.
Cross Tabulation of Agreement on Density Category Between Readers
| Density | Overall (n = 1208) | 1 (n = 213) | 2 (n = 620) | 3 (n = 327) | 4 (n = 48) |
|---|---|---|---|---|---|
| 1 | 67 (5.5%) | 60 (28.2%) | 7 (1.1%) | 0 (0%) | 0 (0%) |
| 2 | 498 (41.2%) | 149 (70%) | 336 (54.2%) | 11 (3.4%) | 2 (4.2%) |
| 3 | 562 (46.5%) | 3 (1.4%) | 275 (44.4%) | 272 (83.2%) | 12 (25%) |
| 4 | 81 (6.7%) | 1 (0.5%) | 2 (0.3%) | 44 (13.5%) | 34 (70.8%) |
Breast Density and Cohort Characteristics
Increased breast density was associated with younger age at diagnosis (median age 61 years in those with breast density scores of 1/2 versus 52 years in those with breast density scores of 3/4, p < 0.001), and lower BMI (p < 0.001). There were significant differences in breast density according to race (p = 0.027) such that Asians more frequently had density scores of 3/4 (10.3%) versus density scores of 1/2 (4.9%), and Black women less frequently had density scores of 3/4 (7.0%) versus density scores of 1/2 (11.3%). Those with denser breasts were more frequently diagnosed by ultrasound or MRI than those with less-dense breasts (p = 0.002). MRI was more frequently utilized in women with denser breasts (27.6% versus 9%, p < 0.001). Breast density was not associated with multifocality, histological subtype, presence of DCIS, EIC, receptor status, or nodal status (Table 1). Women with denser breasts had more sentinel lymph nodes removed (median 3 versus 2, p = 0.008).
Re-excision
Negative margins (no tumor on ink) were achieved at the time of the first surgery in 595 (84.9%) of patients. One re-excision was required in 98 (14%), in order to achieve clear margins. 6 (0.9%) underwent 2 re-excisions and 2 (0.3%) had 3 or more re-excisions. Mastectomy was ultimately performed in 6 (0.9%) patients.
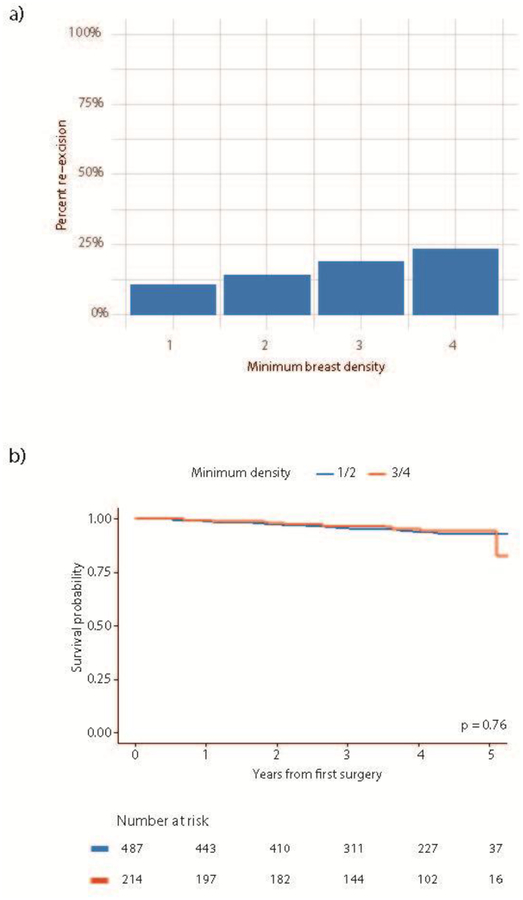
Increased breast density was significantly associated with increased odds of re-excision on univariable analysis (odds ratio [OR] 1.38, 95% CI 1.04–1.83, p = 0.026). Among the patients with fatty breasts, only 10.9% underwent re-excision, as compared to 14.2% with scattered density, 18.9% with heterogenous density, and 23.5% of those deemed to have extremely dense breasts (Fig. 1). Patients with multifocal disease (OR 4.34, 95% CI 2.79–6.76, p < 0.001), extensive intraductal component (EIC)(OR 4.49, 95% CI 2.47–8.18, p < 0.001), and HER2 positive tumors (OR 2.05, 95% CI 1.07–3.91, p = 0.029) also had significantly increased odds of re-excision. Presence of ductal carcinoma in situ (DCIS), age at surgery, BMI, tumor size, histological subtype, ER status, and PR status, and use of MRI were not significantly associated with odds of re-excision (Table 3).
Fig. 1.
(a) Percentage of re-excision according to minimum breast density; (b) breast density and recurrence-free survival
TABLE 3.
Univariable and Multivariable Logistic Regression Results for Associations with Re-Excision
| Univariable Analysis OR (95% CI), | p-value | Multivariable Analysis OR (95% CI) | p-value | |
|---|---|---|---|---|
| Minimum Breast Density | 1.38 (1.04–1.83) | 0.026 | 1.37 (1–1.86) | 0.049 |
| Age | 0.99 (0.97–1) | 0.138 | ||
| Race (White) | 1.01 (0.59–1.81) | 0.967 | ||
| BMI | 0.96 (0.93–1) | 0.052 | ||
| Mammogram/US detected | 0.97 (0.52–1.57) | 0.958 | ||
| MRI Performed | 0.71 (0.37–1.34) | 0.289 | ||
| Tumor size (cm) | 0.9 (0.69–1.16) | 0.424 | ||
| SLN positive | 0.78 (0.4–1.42) | 0.443 | ||
| Multifocal Disease | 4.34 (2.78–6.76) | <0.001 | 4.24 (2.65–6.76) | <.001 |
| pT2 versus pT1 | 0.93 (0.52–1.57) | 0.781 | ||
| Other | 0.28 (0.02–1.36) | |||
| Associated DCIS | 1.83 (1.02–3.53) | 0.054 | ||
| EIC present | 4.49 (2.44–8.14) | <0.001 | 3.5 (1.8–6.84) | <.001 |
| ER positive | 0.96 (0.49–2.06) | 0.906 | ||
| PR positive | 1.04 (0.62–1.83) | 0.878 | ||
| HER2 positive | 2.05 (1.04–3.83) | 0.029 | 1.52 (0.74–3.1) | 0.251 |
OR odds ratio, CI confidence interval, US ultrasound, SLN sentinel lymph node, pT pathologic tumor stage, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, DCIS ductal carcinoma in situ, EIC extensive intraductal component, ER estrogen receptor, PR progesterone receptor
On multivariable analysis, breast density (p = 0.049), tumor multifocality (p < 0.001), and presence of EIC (p < 0.001) remained significantly associated with increased odds of re-excision. HER2 status was not significantly independently associated with re-excision (Table 3).
We found that although density was associated with positive margins for the invasive cancer component, it was not significantly associated with positive or close DCIS margins, increased number of re-excisions, or mastectomy rate (Table 4).
TABLE 4.
Outcome Data Per Density Grouping
| Factor/Outcome | Overall (n = 701) | Density 1/2 (n = 487) | Density 3/4 (n = 214) | p-value |
|---|---|---|---|---|
| Margin width, invasive* | 0.009 | |||
| Negative (> 1 mm) | 622 (88.7%) | 444 (91.2%) | 178 (83.2%) | |
| Close (≤ 1 mm) | 46 (6.6%) | 25 (5.1%) | 21 (9.8%) | |
| Positive | 33 (4.7%) | 18 (3.7%) | 15 (7%) | |
| Margin width, DCIS | 0.31 | |||
| Negative (> 1 mm)† | 570 (81.3%) | 397 (81.5%) | 173 (80.8%) | |
| Close (≤ 1 mm) | 106 (15.1%) | 76 (15.6%) | 30 (14%) | |
| Positive | 25 (3.6%) | 14 (2.9%) | 11 (5.1%) | |
| Number of Re-excisions | 0.161 | |||
| 0 | 595 (84.9%) | 422 (86.7%) | 173 (80.8%) | |
| 1 | 98 (14%) | 60 (12.3%) | 38 (17.8%) | |
| 2 | 6 (0.9%) | 4 (0.8%) | 2 (0.9%) | |
| 3 | 2 (0.3%) | 1 (0.2%) | 1 (0.5%) | |
| Mastectomy performed‡ | 6 (0.9%) | 3 (0.6%) | 3 (1.4%) | 0.376 |
DCIS ductal carcinoma in situ
Width of closest margin, invasive component only
Width of closest margin, DCIS component only
Mastectomy performed following attempted breast-conserving surgery
Outcomes
Median follow-up was 4 years (range 0–5.3 years). During follow-up, there were 35 recurrences. There was no significant association between breast density and disease-free survival (Fig. 1).
DISCUSSION
In our study, we found women with increased breast density had increased odds of undergoing re-excision of margins. Several other studies have shown that women with denser breasts more frequently undergo re-excision of margins following BCS.8 Proposed reasons for this include inaccurate preoperative sizing and intraoperative difficulty in palpating the abnormal area in the presence of dense breast tissue. Intuitively, this should mean that clear margins are more difficult to achieve in these patients. In contrast, Kapoor et al. reported that although initial BCS was less likely amongst women with extremely dense breasts, density did not significantly correlate with margin status or subsequent mastectomy.10 Edwards et al. found that patients with denser breasts were more likely to undergo initial mastectomy (40% versus 30.5%, p = 0.0118)9, but they reported no significant correlation between either traditional BIRADS classification or automated volumetric density and the likelihood of requiring additional surgery for positive margins.
Although young age has been reported a significant predictor for re-excision16, we did not identify this in our study. We did identify EIC was independently related to higher rate of re-excision. Other studies have also shown this, and that EIC is a predictor of residual disease in re-excised margins.16–18 In our study, 33.8% of patients with multifocal disease underwent re-excision of margins, supporting the previously reported association between multifocal breast cancer and higher risk of re-excision.19,20
In our study, 22 of 46 (47.8%) Asian women included had heterogeneously or extremely dense breasts, a finding that has been previously reported in Asian women.21,22 We did not find an association between race and odds of re-excision.
Additionally, elevated BMI has been reported to be associated with less-dense breasts23, as we also found. Lower BMI has previously been associated with surgical margin involvement, but we did not replicate this finding in our cohort.24
An interesting finding is the association of HER2+ disease and positive margins22, but in our study on multivariable analysis, we did not identify this as an independent risk factor.
Increased breast density poses several problems for radiologists and breast surgeons. It has been shown that extremely dense breast tissue is associated with an increased risk of breast cancer and increased risk of interval cancers.25–30 A study from Copenhagen, Denmark found that women with mixed/dense breasts were more likely to develop an interval cancer (OR 1.62, 95% CI 1.14–2.3).31 It is now federally mandated to discuss breast density status and offer women with dense breasts additional screening modalities, such as ultrasonography. As a result, physicians are increasingly required to discuss breast density and its implications for breast cancer detection with women across all states in the U.S.
It has been hypothesized that mammographic density likely corresponds with the number of epithelial cells at risk of malignant transformation, and with the amount of stromal cells in the breast tissue.1 Boyd et al32 found that increases in breast density associated with hormone use were greater in patients who developed breast cancer than they were with cancer-free controls, suggesting that the effect of hormones on breast density may be a predictor of future cancer risk. This suggests that the increased risk of breast cancer in these women could be hormonally driven. However, we found no association between breast density and hormone receptor status. Kerlikowske et al. also found no association between breast density and hormone receptor status.33 However, Holm et al. showed that interval cancers in women with denser breasts were more likely to be ER positive than interval cancers diagnosed in women with lower breast density.34
Differences on the effect of breast density on disease free survival have been reported. A recent case-control study reported women with breast cancer with > 75% mammographic breast density had a greater risk of loco-regional recurrence.7 Conversely, other studies have failed to show an association between mammographic density and local recurrence in women undergoing BCS for DCIS.35,36 With a median follow-up of 4 years, we did not identify any difference in disease-free survival based on breast density.
Several limitations are present in this retrospective study. First, breast density categorization is a subjective analysis by a radiologist. However, our study minimized the subjectivity of mammographic breast density by re-interpreting the mammograms by two independent radiologists, and by assessing inter- and intra-observer variability. Advancements in mammographic technology have led to the development of tools to produce automated assessment of breast density, with promising results.37–39 This may reduce variability in estimation of breast density in the future. Secondly, we were only able to collect data on the MRIs that were performed at MSK; these data may be under-representative of the use of MRI in the entire study population.
Conclusions
Women with dense breasts are more likely to need additional surgery (re-excision after breast-conserving surgery), but increased breast density did not adversely affect disease-free survival in our study. Our findings support the need for further study in developing techniques that can help decrease re-excisions for women with dense breasts who undergo breast-conserving surgery.
ACKNOWLEDGEMENTS
The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in poster format at the 40th Annual San Antonio Breast Cancer Symposium, December 5–9, 2017, San Antonio, TX. The authors have no conflict of interest disclosures to report.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102(16):1224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerlikowske K, Ichikawa L, Miglioretti DL, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst. 2007;99(5):386–95. [DOI] [PubMed] [Google Scholar]
- 3.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. [DOI] [PubMed] [Google Scholar]
- 4.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92(13):1081–7. [DOI] [PubMed] [Google Scholar]
- 5.Wanders JO, Holland K, Veldhuis WB, et al. Volumetric breast density affects performance of digital screening mammography. Breast Cancer Res Treat. 2017;162(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park CC, Rembert J, Chew K, Moore D, Kerlikowske K. High mammographic breast density is independent predictor of local but not distant recurrence after lumpectomy and radiotherapy for invasive breast cancer. Int J Radiat Oncol Biol Phys. 2009;73(1):75–9. [DOI] [PubMed] [Google Scholar]
- 8.Bani MR, Lux MP, Heusinger K, et al. Factors correlating with reexcision after breast-conserving therapy. Eur J Surg Oncol. 2009;35(1):32–7. [DOI] [PubMed] [Google Scholar]
- 9.Edwards BL, Guidry CA, Larson KN, Novicoff WM, Harvey JA, Schroen AT. Does Mammographic Density have an Impact on the Margin Re-excision Rate After Breast-Conserving Surgery? Ann Surg Oncol. 2016;23(3):782–8. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor NS, Eaton A, King TA, et al. Should breast density influence patient selection for breast-conserving surgery? Ann Surg Oncol. 2013;20(2):600–6. [DOI] [PubMed] [Google Scholar]
- 11.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32(14):1507–15. [DOI] [PubMed] [Google Scholar]
- 12.Pilewskie M, Zabor EC, Mamtani A, Barrio AV, Stempel M, Morrow M. The Optimal Treatment Plan to Avoid Axillary Lymph Node Dissection in Early-Stage Breast Cancer Patients Differs by Surgical Strategy and Tumor Subtype. Ann Surg Oncol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chagpar AB, Killelea BK, Tsangaris TN, et al. A Randomized, Controlled Trial of Cavity Shave Margins in Breast Cancer. N Engl J Med. 2015;373(6):503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberger LH, Mamtani A, Fuzesi S, et al. Early Adoption of the SSO-ASTRO Consensus Guidelines on Margins for Breast-Conserving Surgery with Whole-Breast Irradiation in Stage I and II Invasive Breast Cancer: Initial Experience from Memorial Sloan Kettering Cancer Center. Ann Surg Oncol. 2016;23(10):3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sickles E, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS® Mammography 2013.
- 16.Torabi R, Hsu CH, Patel PN, Dave H, Bouton ME, Komenaka IK. Predictors of margin status after breast-conserving operations in an underscreened population. Langenbecks Arch Surg. 2013;398(3):455–62. [DOI] [PubMed] [Google Scholar]
- 17.Alrahbi S, Chan PM, Ho BC, Seah MD, Chen JJ, Tan EY. Extent of margin involvement, lymphovascular invasion, and extensive intraductal component predict for residual disease after wide local excision for breast cancer. Clin Breast Cancer. 2015;15(3):219–26. [DOI] [PubMed] [Google Scholar]
- 18.Kitchen PR, Cawson JN, Moore SE, et al. Margins and outcome of screen-detected breast cancer with extensive in situ component. ANZ J Surg. 2006;76(7):591–5. [DOI] [PubMed] [Google Scholar]
- 19.Agostinho JL, Zhao X, Sun W, et al. Prediction of positive margins following breast conserving surgery. Breast. 2015;24(1):46–50. [DOI] [PubMed] [Google Scholar]
- 20.Reedijk M, Hodgson N, Gohla G, et al. A prospective study of tumor and technical factors associated with positive margins in breast-conservation therapy for nonpalpable malignancy. Am J Surg. 2012;204(3):263–8. [DOI] [PubMed] [Google Scholar]
- 21.del Carmen MG, Hughes KS, Halpern E, et al. Racial differences in mammographic breast density. Cancer. 2003;98(3):590–6. [DOI] [PubMed] [Google Scholar]
- 22.El-Bastawissi AY, White E, Mandelson MT, Taplin S. Variation in mammographic breast density by race. Ann Epidemiol. 2001;11(4):257–63. [DOI] [PubMed] [Google Scholar]
- 23.Gillman J, Chun J, Schwartz S, Schnabel F, Moy L. The relationship of obesity, mammographic breast density, and magnetic resonance imaging in patients with breast cancer. Clin Imaging. 2016;40(6):1167–72. [DOI] [PubMed] [Google Scholar]
- 24.Lai HW, Huang RH, Wu YT, et al. Clinicopathologic factors related to surgical margin involvement, reoperation, and residual cancer in primary operable breast cancer - An analysis of 2050 patients. Eur J Surg Oncol. 2018;44(11):1725–35. [DOI] [PubMed] [Google Scholar]
- 25.Boyd NF, Huszti E, Melnichouk O, et al. Mammographic features associated with interval breast cancers in screening programs. Breast Cancer Res. 2014;16(4):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162(10):673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BK, Choi YH, Nguyen TL, et al. Mammographic density and risk of breast cancer in Korean women. Eur J Cancer Prev. 2015;24(5):422–9. [DOI] [PubMed] [Google Scholar]
- 28.Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta-analysis. Ann Intern Med. 2012;156(9):635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson A, Graff RE, Ursin G, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaghjyan L, Colditz GA, Rosner B, Tamimi RM. Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to the time since the mammogram. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen AH, Bihrmann K, Jensen MB, Vejborg I, Lynge E. Breast density and outcome of mammography screening: a cohort study. Br J Cancer. 2009;100(7):1205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyd NF, Melnichouk O, Martin LJ, et al. Mammographic density, response to hormones, and breast cancer risk. J Clin Oncol. 2011;29(22):2985–92. [DOI] [PubMed] [Google Scholar]
- 33.Kerlikowske K, Gard CC, Tice JA, Ziv E, Cummings SR, Miglioretti DL. Risk Factors That Increase Risk of Estrogen Receptor-Positive and -Negative Breast Cancer. J Natl Cancer Inst. 2017;109(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holm J, Humphreys K, Li J, et al. Risk factors and tumor characteristics of interval cancers by mammographic density. J Clin Oncol. 2015;33(9):1030–7. [DOI] [PubMed] [Google Scholar]
- 35.Habel LA, Capra AM, Achacoso NS, et al. Mammographic density and risk of second breast cancer after ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2488–95. [DOI] [PubMed] [Google Scholar]
- 36.Hwang ES, Miglioretti DL, Ballard-Barbash R, Weaver DL, Kerlikowske K. Association between breast density and subsequent breast cancer following treatment for ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2587–93. [DOI] [PubMed] [Google Scholar]
- 37.Brand JS, Czene K, Shepherd JA, et al. Automated measurement of volumetric mammographic density: a tool for widespread breast cancer risk assessment. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1764–72. [DOI] [PubMed] [Google Scholar]
- 38.Nickson C, Arzhaeva Y, Aitken Z, et al. AutoDensity: an automated method to measure mammographic breast density that predicts breast cancer risk and screening outcomes. Breast Cancer Res. 2013;15(5):R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pertuz S, McDonald ES, Weinstein SP, Conant EF, Kontos D. Fully Automated Quantitative Estimation of Volumetric Breast Density from Digital Breast Tomosynthesis Images: Preliminary Results and Comparison with Digital Mammography and MR Imaging. Radiology. 2016;279(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]