Abstract
Background:
Personal care products (PCPs), known sources of endocrine disrupting chemicals (EDCs) such as phthalates and parabens, are widely used among women of reproductive age. EDCs have been linked to pregnancy complications such as gestational diabetes (GDM), and PCP use could represent a modifiable source of exposure in this sensitive time window. Yet, to our knowledge, no study has directly evaluated the association between pregnancy use of PCP and late pregnancy glucose levels, established risk factors for complications such as GDM.
Methods:
233 women from the Environment and Reproductive Health (EARTH) Study had data available on 1st and/or 2nd trimester PCP use, assessed through self-reported use over the previous 24h, and blood glucose levels after the glucose loading test (GLT), taken at late 2nd trimester. Associations between each individual PCP and total PCP with glucose levels were evaluated in multivariable adjusted linear regression models.
Results:
Both positive and negative differences in glucose levels were observed when comparing users vs. non-users of several PCPs including 2nd trimester use of deodorant (adjusted mean difference: 12.2mg/dL, 95% CI:−0.6, 24.9); bar soap (6.9mg/dL, 95% CI:−0.9, 14.7mg/dL); and liquid soap (−13.3, 95% CI:−26.8, 0.1mg/dL), and 1st trimester use of sunscreen (−14.6mg/dL, 95% CI:−27.8, −1.5mg/dL). Total number of PCPs used in the 2nd trimester was also associated with higher glucose levels, with the largest difference of 20mg/dL when comparing individuals who used eight vs none PCPs (95% CI: 3–37).
Conclusions:
In a pregnancy cohort of women seeking care at a fertility clinic, we found the use of several PCPs to be positively or negatively associated with glucose levels in the late second trimester, which may reflect increased risk of GDM and subsequent perinatal outcomes. These results strengthen the role of product use as a potentially modifiable source of EDCs that may impact glucose levels.
Keywords: pregnancy cohort, endocrine disruptors, environmental reproductive epidemiology, sources of exposure
Graphical abstract
1. Introduction
The use of personal care products (PCPs) has consistently increased over the last decades,(1) with women between the ages of 18–34 having the highest use.(1) Most PCPs such as soaps and cosmetics are unregulated in the market,(2) and have been identified as potential sources of endocrine disrupting chemicals (EDCs) such as phthalates, parabens, and benzophenone-3.(3–5) These chemicals have been associated with a variety of adverse health outcomes because of their ability to perturb the normal functioning of the endocrine system.(6–8) Widespread use of PCPs makes women between the ages of 18–34 a population at high risk of exposure to EDCs. This age group is also the primary age of pregnancy, which could be a sensitive window of exposure to these chemicals with respect to maternal and child health outcomes.(9–12)
Exposure to EDCs has been extensively studied in pregnancy cohorts, and associations have been observed with several reproductive outcomes.(13,14) One important complication that affects approximately 7% of all pregnancies is gestational diabetes (GDM). Studies conducted in ongoing prospective pregnancy cohort such as the Environment and Reproductive Health (EARTH) Study, have found associations between body burden of certain phthalate metabolites and phenols—chemicals commonly used in personal care products—and pregnancy glucose levels, impaired glucose tolerance, and other risk factors of GDM.(12,15–17) EDCs are thought to impact glucose metabolism through estrogen-dependent signaling that could alter normal beta cell functioning, increasing the risk of GDM.(12) Yet, no study, to our knowledge, has evaluated PCP use as an important source of EDCs to determine whether these EDCs could impact pregnancy glucose levels, with implications for GDM risk, later-life health, as well as child health outcomes.(18)
Few population-based studies have evaluated PCP use as an independent risk factor of pregnancy complications,(19,20) an approach that could have relevant implications for public health interventions and recommendations. Therefore, we used a population-based cohort of pregnant women from a fertility clinic to evaluate associations between self-reported PCP use in the first and second trimesters of pregnancy, and blood glucose levels assessed in the late second trimester.
2. Materials and methods
2.1. Study population
We included women enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort established in 2004 including women between 18 and 45 years attending the Massachusetts General Hospital (MGH) Fertility Center (Boston, MA). For this study, we used data on women enrolled between 2005 and 2015 who had completed at least one in vitro fertilization (IVF) cycle. We a priori excluded IVF cycles for which women used an egg donor (n=18) and cryo-thaw cycles (n=34). For the purposes of this study, we also excluded women who had missing data on all assessed PCPs (n=8). In total, 233 women with pregnancies that resulted in live births were included in this study. Only the first pregnancy was evaluated if participating women had more than one pregnancy during the study period. For each woman, we used data from two medical visits, one occurring during the first trimester of pregnancy (median 7 gestation weeks) and the second occurring during the early second trimester (median 21 gestation weeks). The Human Studies Institutional Review Boards of the MGH, Harvard T.H. Chan School of Public Health approved this study.
2.2. PCP use assessment
PCP use was assessed by using a self-administered questionnaire.(21) The questionnaire asked if participants had used several PCPs within the previous 24 hours. Assessed PCPs were: deodorant, shampoo, conditioner/crème rinse, hairspray/hair gel, other hair care products (e.g., mousse, hair bleach, relaxer, perm), shaving cream, cologne/perfume, bar soap, liquid soap/body wash, face moisturizer, hand/body lotion, colored cosmetics (hair dye, foundation, blush, eye shadow, eye liner, or mascara), sunscreen lotion, nail polish, nail polish remover, hand sanitizer, toothpaste, mouthwash, and any other toiletry product.
We conducted our main analyses by focusing on the use of each specific PCP as an independent binary exposure (yes/no) for use in the past 24 hours. We also evaluated, in secondary analysis, the total number of PCPs used in that same time period, calculated by summing the number of individual items from the questionnaire. Because some of the items included in the questionnaire asked categories of products that could contain several products (e.g. colored cosmetics, other toiletry products, other hair products), the aggregated measure can only be seen as a proxy of the total number of products actually used in the previous 24 hours, and should be interpreted as a total number of categories of products. . Out of all the PCPs, five (liquid soap, face moisturizer, nail polish remover, aftershave, other toiletry products) were added to the questionnaire later in the study, and have a lower rate of response. To minimize missingness, these PCPs were only evaluated in the independent primary analysis. Out of the 233 women included in this analysis, 202 provided information on PCP use in the first trimester, and 193 in the second trimester.
2.3. Primary and secondary outcome
The primary outcome investigated in this study was glucose, assessed from blood samples collected at MGH in the late 2nd trimester of pregnancy (median: 27 weeks gestation) during a 1-h non-fasting, 50-gram glucose loading test (GLT), which is used as the first step in the screening procedure for GDM.(22) In a secondary analysis, we also evaluated blood glucose levels from the GLT as a binary outcome, defining women with glucose levels ≥140 mg/dL as having abnormal GLT; this cut-off confers additional screening for GDM using the two-step method applied at MGH for diagnosis of this pregnancy complication. As secondary outcomes, we evaluated BMI at the first prenatal visit, calculated by dividing weight (kg) over squared height (m2), and total gestational weight gain (GWG, assessed by taking the difference, in kg, between the weight at the first medical visit, and the weight at delivery). All analyses evaluating glucose as the outcome used exposure measurements at both time points, while the association between total PCP and first trimester BMI, or total GWG, used only first trimester product use as the exposure variables. For all individuals in the study, outcome assessment (median 27 weeks gestation) was conducted after the second exposure assessment (median 21 weeks gestation). The median time period between the completion of the second questionnaire and the GLT was 6 weeks.
2.4. Covariates
We selected a priori several potential confounders of the associations of interest. Specifically, we adjusted all statistical models for maternal age (continuous, years), pre-pregnancy BMI (continuous, only analyses on glucose), total physical activity (continuous, h/week), race (binary, white/non-white), infertility diagnosis (female factor, male factor, or unexplained), number of fetuses (binary, one/more).. Information on sociodemographic factors, family medical history, and lifestyle factors were obtained from a brief questionnaire administrated by the study staff at enrollment and from a detailed take-home questionnaire. Infertility diagnosis by a physician was assigned to each patient based on the Society for Assisted Reproductive Technology classifications. The percent of covariates with missing data was limited (< 15%), and all adjusted models were therefore conducted as complete-case analyses. Race, BMI, and infertility diagnosis, were also evaluated as potential effect modifiers by means of stratified analyses.
3. Calculation
We first evaluated the baseline characteristics in the entire study population, as well as stratifying by abnormal GLT (glucose < vs ≥140 mg/dL). We also assessed, in the overall population and stratifying by abnormal GLT, the prevalence of use of each of the investigated PCPs. All analyses presented for glucose were conducted in two separate sets of analyses, by focusing respectively on first and second trimester measurements of PCP use.
Our primary analyses focused on evaluating individual products as independent binary exposures. PCPs with an overall usage of >99% (toothpaste) or <1% (aftershave) were not evaluated in this analysis. We used linear regression models to estimate glucose levels as a function of each PCP (self-reported use vs no use in the past 24 hours), while adjusting for maternal age, BMI, smoking, race, education, infertility diagnosis and number of fetuses. Several sensitivity analysis were conducted: i) we evaluated glucose as a binary outcome using logistic regression models, ii) we evaluated racial differences in products use, and replicated the main analysis by stratifying by race (white vs non-white), and iii) we stratified our main analysis by other potential effect modifiers such as BMI (normal weight, overweight, obese) and type of infertility.
As a secondary analysis, we focused on the total number of PCPs as it related to late second trimester glucose levels. We evaluated the number of total PCPs, as a continuous exposure, using restricted cubic splines to relax any linearity assumption in the dose-response association. These analyses were evaluated using multiple regression models adjusted for all potential confounders mentioned above.
Finally, we also investigated first trimester BMI (i.e. assessed at the first MGH visit) and total GWG as independent outcomes. We both evaluated differences in BMI and GWG over individual products, as well as investigated the association between first trimester total number of products and BMI or GWG, using linear regression models with restricted cubic splines to model the continuous exposures. All analyses were conducted using the statistical software Stata (version 15).
4. Results
Table 1 presents the baseline characteristics of the 233 women included in the study, overall and by levels of late second trimester glucose. Women with abnormal GLT had lower levels of physical activity, were more likely to be former or current smokers, more likely Asian, and more often had infertility diagnosis primarily because of female factors.
Table 1.
Baseline characteristics of 233 women included in the analysis, overall, and by impaired glucose tolerance status (glucose from GLT ≥140 mg/dL versus <140 mg/dL)
| Characteristics | Total (n=233) | Glucose <140 (n=192) | Glucose≥140 (n=41) |
|---|---|---|---|
| Maternal age, years (sd) | 35.4 (3.9) | 35.4 (3.9) | 35.8 (3.7) |
| BMI, kg/m2 (sd) | 24.2 (4.8) | 24.0 (4.6) | 25.2 (5.8) |
| Total physical activity, h/week (sd) | 6.5 (6.8) | 6.7 (7.2) | 5.3 (4.8) |
| Smoking, n (%) | |||
| Never | 175 (75) | 147 (77) | 28 (68) |
| Former | 52 (22) | 41 (21) | 11 (27) |
| Current | 6 (3) | 4 (2) | 2 (5) |
| Race, n (%) | |||
| White | 203 (87) | 171 (89) | 32 (79) |
| Black/African American | 5 (2) | 4 (2) | 1 (2) |
| Asian | 17 (7) | 10 (5) | 7 (17) |
| Other | 8 (4) | 7 (4) | 1 (2) |
| Education, n (%) | |||
| High school graduate or less | 28 (12) | 23 (12) | 5 (12) |
| College graduate or higher | 205 (88) | 169 (88) | 36 (88) |
| Infertility diagnosis, n (%) | |||
| Male factor | 76 (33) | 64 (33) | 12 (29) |
| Female factor | 65 (28) | 46 (24) | 19 (46) |
| Unexplained | 91 (39) | 82 (43) | 10 (25) |
| More than one fetus, n (%) | 46 (19) | 35 (18) | 9 (22) |
The prevalence of using each of the 20 PCPs overall and by glucose levels is provided in Table 2, for second trimester, and in Supplementary table S1 for first trimester. A greater proportion of women with abnormal glucose tolerance tests (GLT≥140mg/dL) had higher self-reported use of shampoo (second trimester only), , bar soap (second trimester only), hand/body lotion (both trimester), and other toiletry products (both trimesters). On the other hand, more women using hand sanitizer had glucose levels <140 mg/dL, with the negative association between hand sanitizer and GLT at the second trimester being the only comparison to reach the conventional threshold of statistical significance (p<0.05). Liquid soap and nail polish remover were positively associated with GLT at the first trimester, and negatively associated at the second trimester. Table S2 presents the number (%) of women who reported having used each specific product during both trimesters (either use/use or not use/not use), showing that most women (68 to 100%) did not change products usage between the first and second trimester.
Table 2.
Second trimester self-reported personal care product use among women in EARTH overall and by IGT in the late second trimester of pregnancy
| Overall | GLT<140 | GLT ≥140 | |||
|---|---|---|---|---|---|
| n | % of usage | % of usage | % of usage | p-value | |
| Deodorant | 185 | 89 | 89 | 91 | 0.77 |
| Shampoo | 184 | 69 | 67 | 78 | 0.22 |
| Conditioner/crème rinse | 185 | 64 | 63 | 69 | 0.52 |
| Hair spray/hair gel | 185 | 32 | 31 | 38 | 0.50 |
| Other hair products | 184 | 25 | 26 | 19 | 0.37 |
| Shaving cream | 185 | 11 | 11 | 9 | 0.77 |
| Cologne/perfume | 185 | 34 | 33 | 38 | 0.65 |
| Bar soap | 182 | 60 | 57 | 72 | 0.13 |
| Liquid soap/body wash | 82 | 73 | 76 | 50 | 0.08 |
| Face moisturizer/lotion | 82 | 84 | 85 | 80 | 0.70 |
| Hand/body lotion | 185 | 77 | 76 | 84 | 0.29 |
| Colored cosmetics | 185 | 68 | 69 | 66 | 0.74 |
| Suntan/sunblock lotion | 185 | 15 | 16 | 13 | 0.65 |
| Nail polish | 184 | 4 | 4 | 6 | 0.56 |
| Nail polish remover | 82 | 6 | 5 | 1 | 0.73 |
| Hand sanitizer | 155 | 28 | 32 | 9 | 0.02 |
| Mouthwash | 178 | 17 | 18 | 13 | 0.57 |
| Other toiletry product | 68 | 66 | 63 | 89 | 0.12 |
P-values obtained using chi square test
Table 3 presents adjusted mean differences in glucose levels between users and non-users of each specific product at both first and second trimesters, evaluated in separate statistical models (one linear model for each product), with 95% confidence intervals (CIs). Compared to non consumers, higher glucose levels were observed for women reporting 2nd trimester use of deodorant (2nd trimester adjusted mean difference:12.2mg/dL, 95% CI: −0.6, 24.9), shampoo (2nd trimester adjusted mean difference:7.2mg/dL, 95% CI: −1.2, 15.6mg/dL), bar soap (2nd trimester adjusted mean difference:6.9mg/dL, 95% CI: −0.9, 14.7mg/dL). Lower levels were observed among users of other hair products (1st trimester adjusted mean difference:−9mg/dL, 95% CI: −19.3, 1.2; 2nd trimester adjusted mean difference:−6.7mg/dL, 95% CI: −15.4, 1.9mgdL), suntan/sunblock (1st trimester adjusted mean difference:−14.6mg/dL, 95% CI: −27.8, −1.5mg/dL), liquid soap (2nd trimester adjusted mean difference:−13.3, 95% CI: −26.8, 0.1mg/dL).
Table 3.
Differences in glucose levels between self-reported PCP users and non-users of each specific products, estimated from multivariable-adjusteda linear regression models
| First trimester | Second trimester | |||
|---|---|---|---|---|
| Nb | Difference in glucose, mg/dL (95%CI) | N | Difference in glucose, mg/dL (95%CI) | |
| Deodorant | 176 | 3.9 (−9.5, 17.3) | 184 | 12.2 0(−.6, 24.9) |
| Shampoo | 176 | −0.8 (−10.4, 8.9) | 183 | 7.2 (−1.2, 15.6) |
| Conditioner/crème rinse | 177 | −3.1 (−12.4, 6.1) | 184 | 5.5 (−2.6, 13.5) |
| Hair spray/hair gel | 175 | 2.3 (−6.7, 11.3) | 184 | 6.3 (−2.1, 14.7) |
| Other hair products | 172 | −9.0 (−19.3, 1.2) | 183 | −6.7 (−15.4, 1.9) |
| Shaving cream | 177 | −2.3 (−14.1, 9.4) | 184 | −1.3 (−13.4, 10.8) |
| Cologne/perfume | 177 | 4.0 (−5.1, 13.2) | 184 | 0.3 (−8.1, 8.7) |
| Bar soap | 177 | 2.6 (−6.0, 11.1) | 181 | 6.9 (−0.9, 14.7) |
| Liquid soap/body wash | 69 | 2.0 (−14.2, 18.1) | 81 | −13.3 (−26.8, 0.1) |
| Face moisturizer/lotion | 69 | −4.6 (−26.0, 16.8) | 81 | 5.5 (−11.4, 22.5) |
| Hand/body lotion | 176 | 2.6 (−6.2, 11.3) | 184 | 2.6 (−6.6, 11.9) |
| Colored cosmetics | 176 | 2.8 (−6.1, 11.8) | 184 | −0.1 (−8.2, 8.1) |
| Suntan/sunblock lotion | 177 | −14.6 (−27.8, −1.5) | 184 | −8.4 (−19.0, 2.3) |
| Nail polish | 177 | −8.01 (−25.3, 9.2) | 183 | −6.6 (−25.5, 12.2) |
| Nail polish remover | 68 | 16.7 (−4.6, 38.1) | 81 | −8.8 (−30.7, 13.2) |
| Hand sanitizer | 149 | −4.0 (−13.9, 6.0) | 154 | −4.0 (−13.6, 5.5) |
| Mouthwash | 171 | −2.0 (−13.8, 9.9) | 177 | −0.2 (−10.8, 10.4) |
| Other toiletry product | 57 | 7.8 (−8.4, 23.9) | 67 | −0.6 (−14.1, 15.4) |
Adjusted for maternal age, smoking, race, education, infertility diagnosis, n of fetuses
Number of individuals included in the model (with data on product and all covariates)
Results were similar when evaluating glucose as a binary outcome (data not shown) using logistic regression. When stratifying by race (white vs non-white), we observed substantial differences in the pattern of product use, with non-white women reporting significantly lower usage than white women of deodorant (46% vs 81%), shampoo (46% vs 68%), conditioner (32% vs 63%), and hair spray gel (14% vs 37%) (Table 4). Nevertheless, no differences were observed in the association between PCP and glucose when stratifying by race, possibly due to the low number of non-white participants in our cohort (data not shown). Finally, negligible differences were observed when evaluating the association between PCP and glucose over levels of BMI and type of infertility (data not shown), as compared to the ones observed in the overall population.
Table 4.
First trimester use of specific products by race-ethnicity, (%)
| White (n=230) | Non-White (n=30) | p-valuea | |
|---|---|---|---|
| Deodorant | 81 | 46 | <0.001 |
| Shampoo | 68 | 46 | 0.06 |
| Conditioner/crème rinse | 63 | 32 | <0.001 |
| Hair spray/hair gel | 37 | 14 | 0.06 |
| Other hair products | 17 | 14 | 0.51 |
| Shaving cream | 13 | 11 | 0.87 |
| Cologne/perfume | 29 | 29 | 0.91 |
| Bar soap | 53 | 57 | 0.75 |
| Liquid soap/body wash | 25 | 18 | 0.59 |
| Face moisturizer/lotion | 29 | 25 | 0.88 |
| Hand/body lotion | 54 | 71 | 0.11 |
| Colored cosmetics | 60 | 46 | 0.36 |
| Suntan/sunblock lotion | 10 | 11 | 0.90 |
| Nail polish | 7 | 0 | 0.18 |
| Nail polish remover | 15 | 0 | 0.21 |
| Hand sanitizer | 28 | 27 | 0.93 |
| Mouthwash | 16 | 25 | 0.29 |
| Other toiletry product | 60 | 50 | 0.65 |
P-values obtained using chi square test
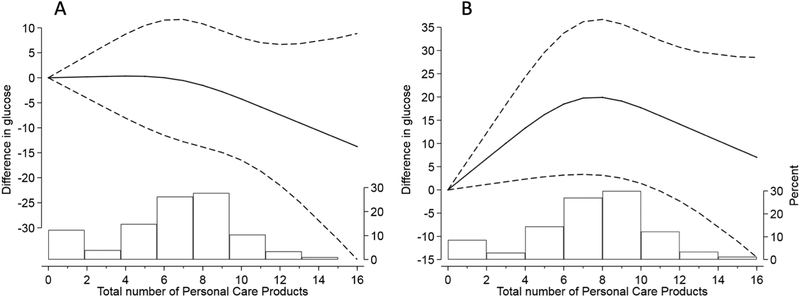
We next investigated the total number of products as a continuous exposure, flexibly modeled with restricted cubic splines, as it relates to glucose levels in late second trimester. Dose-response curves are reported in Figure 1. Significant changes in glucose at higher number of products were observed at the second trimesters (panel B). For instance, individuals with a total use of 8 PCPs had, on average, higher glucose of 20 mg/dL (95% CI: 3–37) as compared to individuals with no use of PCPs.
Figure 1.
Differences in mean glucose levels at increased total number of products used at the first (A) and second trimester (B). Exposure modeled as continuous with restricted cubic splines. Adjusted for maternal age, smoking, race, education, infertility diagnosis, n of fetuses. The histogram represents the PCP distribution in the population.
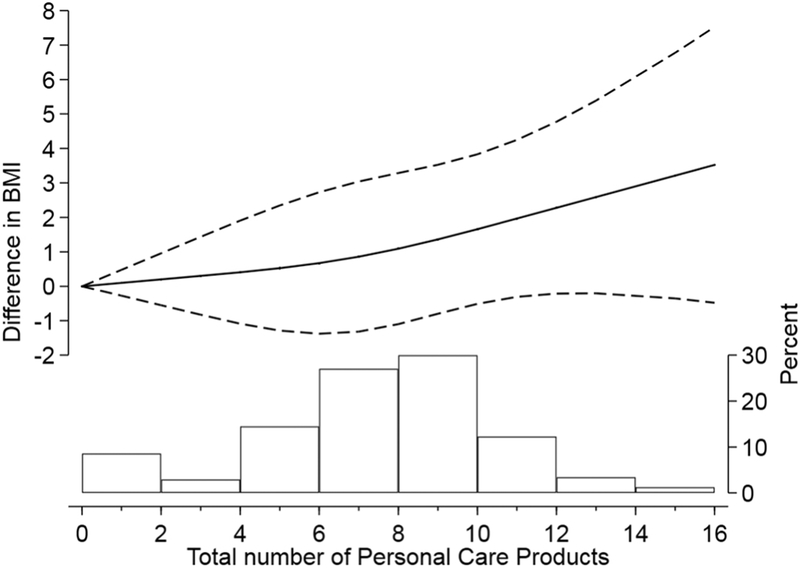
Finally, we evaluated first trimester BMI and GWG as independent secondary outcomes. We did not observe any significant difference in BMI or GWG by use of individual products (data not shown). On the other hand, when investigating the cross-sectional association of BMI with total number of products reported during the 1st trimester, evaluated as a continuous covariate modeled with restricted cubic splines, we observed higher BMI at increasingly higher numbers of products used (Figure 2).
Figure 2.
Differences in baseline BMI at increased total number of products used at the first trimester. Exposure modeled as continuous with restricted cubic splines. Adjusted for maternal age, smoking, race, education, infertility diagnosis, n of fetuses. The histogram represents the PCP distribution in the population.
5. Discussion
In a pregnancy cohort of women seeking care at a fertility clinic, we found the use of several personal care products to be associated with glucose levels in the late second trimester, an established risk factor for gestational diabetes and subsequent perinatal outcomes. We also documented racial differences in patterns of products use. Further, we found that greater use of products was associated with higher first trimester BMI. These findings warrant further examination into whether chemicals in consumer products may alter metabolic function, with impact on obesity and dysglycemia risk.
Use of PCPs is widespread, especially among women of reproductive age.(1) Previous studies among women from the same study cohort (the EARTH study) as well as in other pregnancy and general population cohorts, found PCP use to be primary sources of hundreds of chemicals, including EDCs such as phthalates, parabens, and benzophenone-3.(3–5,10,23,24) Researchers have suggested that EDCs are potential risk factors for several reproductive and cardiometabolic outcomes,(6,7) including gestational diabetes, an increasing condition affecting around 7% of pregnant women.(12) In the context of GDM, lifestyle factors, such as diet and physical activity have been one of the major risk factors and intervention targets. Yet, only 50% of GDM is thought to be attributed to these established lifestyle factors.(25) With recent data suggesting that EDCs could also be an important risk factor of GDM and its risk factors, considering the sources of EDCs, including PCPs, could help to identify targets for developing interventions and recommendations that could decrease GDM risk.(26)
Previous studies have generally focused on establishing a relationship between PCPs used on a daily basis (e.g. shampoo, cosmetics, soaps) and urinary concentrations of phthalates and parabens,(4,9–11,27–30) and few studies have investigated PCP as a primary exposure with respect to a given health outcome.(19,20) We found several products to be suggestively associated with higher glucose levels, with common products such as shampoo, bar soap and deodorant consistently associated in both adjusted and unadjusted analyses. While deodorant, lotion, and bar soap were correlated with phthalates and parabens in our data and in other studies, shampoo was not.(4,5,24,31) Of interest, our results show higher usage of lotion among women who developed abnormal GLT, which based on previous reports from this data has moderate to high correlation with monoethyl phthalate and butyl paraben, which have been shown to be associated with higher glucose levels.(16,17) At the same time, we did not observe an association between other established sources of phthalates, such as cologne/perfume, and glucose. We also observed the use of some products to be associated with lower glucose levels. In particular, we observed significantly lower glucose levels among users of sunscreen, a common source of parabens and benzophenone-3.(31) This result is in line with previous results observed in the same cohort, where some paraben compounds were associated with lower glucose levels.(17) In this study, we also observed substantial differences in product use between white and non-white participants, even though associations between PCP and glucose did not differ when stratifying by race. While the predominantly white population in our cohort does not allow for to provide any substantial conclusion, further work is needed to determine whether differences in patterns of PCP use impacting exposure to EDC could contribute to racial-ethnic disparities in perinatal and reproductive outcomes.(32,33) Another interesting finding is that several associations differed when focusing on either the first or second trimester of pregnancy. While a chance component can not be excluded, and different levels of measurement errors could occur between the two time points, it is important to note that these results are in line with other studies in which we observed the relationship between EDC concentrations and pregnancy glucose levels to vary over pregnancy. (16,17) Future studies should further investigate the time-varying association between exposure to EDC and GDM, assessing whether specific time windows of susceptibility to exposure exist during pregnancy.
This study is subject to several limitations. First, the evaluation of PCP use was self-reported and only consisted in asking the use of several products in the previous 24 hours, without any additional questions about regular on-going use, or questions on products brand. Moreover, assessed items included categories such as “colored cosmetics” that could include specific items such as hair dye, foundation, blush, which are different in terms of chemical composition. In addition to self-reported product use, other techniques (e.g. product scanning) could better quantify type and ingredients of PCPs. Also, we did not have information on the amount or type of application (e.g. on skin, spray), with varying routes of exposure possibly impacting health differentially.(2) Second, because of a relatively low number of GDM cases we could only evaluate glucose levels from the 50-gram GLT as a binary and continuous variable. The GLT is standardly given to all women as a part of the GDM screening test in late 2nd trimester in this study population. Our findings focus on continuous glucose levels as a primary outcome, given that results for the binary outcome may be subject to instability due to the relatively low number of events in multivariable-adjusted logistic models. Even in linear regression models and univariate chi-square tests, however, we reported a high instability in our results, with large confidence intervals and high p-values, and only few results reached the conventional threshold of statistical significance (p-value<0.05). Because of this high variability, and the presence of multiple comparisons of interest, our results should be interpreted with caution, and we warrant replication in larger pregnancy cohorts. Moreover, results from the non-fasting GDM screening could be influenced by the timing or content of the last meal, information that was unavailable in our dataset. Finally, all our results are based on a population of predominantly non-Hispanic white women seeking care at a fertility clinic. As such, results may not be generalizable to women who conceived naturally or to subgroups of the population underrepresented in our sample (e.g. non-white). We adjusted for type of infertility only accounting for either female or male factor based on first assigned diagnosis. With this approach, however, results do not account for type of infertility attributed to both male and female factors.
This study also has several strengths. First, it is among the first studies to evaluate sources of environmental chemicals such as PCP as an independent exposure for adverse health outcomes. This is a crucial step in identifying the potential effects of public health interventions, which would target the sources of exposure rather than the biomarker themselves. Future studies with larger samples should further integrate product use and concentrations of chemicals in the same analysis, to evaluate the extent to which EDCs are responsible for the observed associations.(26) Moreover, the prospective design of the study, together with the assessment of exposures at both 1st and 2nd trimesters, allowed us to evaluate a potentially sensitive time window of pregnancy. The prospective nature of the study also strengthens the interpretation of our results by reducing the risk of reverse causation.
6. Conclusions
In conclusion, in a pregnancy cohort of women attending a fertility clinic we observed associations between use of PCPs and glucose levels in the late second trimester. These results strengthen the role of product use as a potentially modifiable source of EDCs, while also highlighting the need for future studies to better capture exposure levels and its association with chemicals and health outcomes.
Supplementary Material
Highlights.
Assessing sources of environmental exposures is crucial for environmental policies
Personal care products (PCP) are potential sources of endocrine disrupting chemicals
Women of reproductive age have the highest use of PCP, with implications for health
In a pregnancy cohort, the use of PCP was suggestively associated with glucose levels
PCP use may represent a target for reducing EDCs exposure and the associated risks
Acknowledgments
Funding: This work was supported by National Institutes of Health Grants R01ES026166, R01ES022955, R01ES009718, R01ES000002, and P30ES000002 from the National Institute of Environmental Health Sciences.
Abbreviations.
- BMI
body mass index
- EDC
endocrine disrupting chemicals
- GDM
gestational diabetes
- GWG
gestational weight gain
- PCP
personal care products
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: nothing to declare
References
- 1.US Bureau of Labor Statistics. Consumer expenditures in 2017. https://www.bls.gov/opub/reports/consumer-expenditures/2017/home.htm [Google Scholar]
- 2.Steinemann AC. Fragranced consumer products and undisclosed ingredients. Environ Impact Assess Rev. 2009. January 1;29(1):32–8. [Google Scholar]
- 3.Dodson Robin E, Nishioka Marcia, Standley Laurel J., Perovich Laura J., Brody Julia Green, Rudel Ruthann A. Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products. Environ Health Perspect. 2012. July 1;120(7):935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parlett LE, Calafat AM, Swan SH. Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. 2013. March;23(2):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel JA, Mounessa JS, Dellavalle RP, Dunnick CA. Comparison of Contact Allergens in Bar Soaps and Liquid Body Washes. Dermatitis. 2018. February;29(1):51. [DOI] [PubMed] [Google Scholar]
- 6.Hauser R, Calafat AM. Phthalates and Human Health. Occup Environ Med. 2005. November 1;62(11):806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabir ER, Rahman MS, Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol. 2015. July 1;40(1):241–58. [DOI] [PubMed] [Google Scholar]
- 8.Crinnion WJ. Toxic effects of the easily avoidable phthalates and parabens. Altern Med Rev. 2010;15(3):190–197. [PubMed] [Google Scholar]
- 9.Lang C, Fisher M, Neisa A, MacKinnon L, Kuchta S, MacPherson S, et al. Personal Care Product Use in Pregnancy and the Postpartum Period: Implications for Exposure Assessment. Int J Environ Res Public Health. 2016. January 6;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polinski KJ, Dabelea D, Hamman RF, Adgate JL, Calafat AM, Ye X, et al. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res. 2018. April;162:308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE, et al. Consumer product exposures associated with urinary phthalate levels in pregnant women. J Expo Sci Environ Epidemiol. 2012. September;22(5):468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich S, Lambers D, Baccarelli A, Khoury J, Macaluso M, Ho S-M. Endocrine Disruptors: A Potential Risk Factor for Gestational Diabetes Mellitus. Am J Perinatol. 2016. November;33(13):1313–8. [DOI] [PubMed] [Google Scholar]
- 13.Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to Phthalates and Phenols during Pregnancy and Offspring Size at Birth. Environ Health Perspect. 2012. March;120(3):464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014. January 1;168(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, et al. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ Int. 2016. November 1;96(Supplement C):118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James-Todd TM, Chiu Y-H, Messerlian C, Mínguez-Alarcón L, Ford JB, Keller M, et al. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ Health. 2018. June 14;17(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellavia A, Chiu Y-H, Brown FM, Mínguez-Alarcón L, Ford JB, Keller M, et al. Urinary concentrations of parabens mixture and pregnancy glucose levels among women from a fertility clinic. Environ Res. 2019. January;168:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TR, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care. 2012;35(3):574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James-Todd T, Terry MB, Rich-Edwards J, Deierlein A, Senie R. Childhood hair product use and earlier age at menarche in a racially diverse study population: a pilot study. Ann Epidemiol. 2011. June;21(6):461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghazarian AA, Trabert B, Robien K, Graubard BI, McGlynn KA. Maternal use of personal care products during pregnancy and risk of testicular germ cell tumors in sons. Environ Res. 2018. July;164:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messerlian C, Williams PL, Ford JB, Chavarro JE, Mínguez-Alarcón L, Dadd R, et al. The Environment and Reproductive Health (EARTH) Study: A Prospective Preconception Cohort. Hum Reprod Open. 2018. February;2018(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773. [DOI] [PubMed] [Google Scholar]
- 23.Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, et al. Reducing Phthalate, Paraben, and Phenol Exposure from Personal Care Products in Adolescent Girls: Findings from the HERMOSA Intervention Study. Environ Health Perspect. 2016. October;124(10):1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, Tobias DK, Chavarro JE, Bao W, Wang D, Ley SH, et al. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: prospective cohort study. Bmj. 2014;349:g5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellavia A, Zota AR, Valeri L, James-Todd T. Multiple mediators approach to study environmental chemicals as determinants of health disparities. Environ Epidemiol. 2018. June;2(2):e015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh C-J, Chang Y-H, Hu A, Chen M-L, Sun C-W, Situmorang RF, et al. Personal care products use and phthalate exposure levels among pregnant women. Sci Total Environ. 2019. January 15;648:135–43. [DOI] [PubMed] [Google Scholar]
- 28.Arbuckle TE, Fisher M, MacPherson S, Lang C, Provencher G, LeBlanc A, et al. Maternal and early life exposure to phthalates: The Plastics and Personal-care Products use in Pregnancy (P4) study. Sci Total Environ. 2016. May 1;551–552:344–56. [DOI] [PubMed] [Google Scholar]
- 29.Just AC, Adibi JJ, Rundle AG, Calafat AM, Camann DE, Hauser R, et al. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. J Expo Sci Environ Epidemiol. 2010. November;20(7):625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, et al. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere. 2018. February;193:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassan FL, Coull BA, Gaskins AJ, Williams MA, Skakkebaek NE, Ford JB, et al. Personal Care Product Use in Men and Urinary Concentrations of Select Phthalate Metabolites and Parabens: Results from the Environment And Reproductive Health (EARTH) Study. Environ Health Perspect. 2017. 18;125(8):087012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James-Todd TM, Chiu Y-H, Zota AR. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep. 2016;3(2):161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zota AR, Shamasunder B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol. 2017;217(4):418–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.