Abstract
Diabetic retinopathy is a major complication of diabetes mellitus and a leading cause of blindness. The pathogenesis of diabetic retinopathy is accompanied by chronic low-grade inflammation. Evidence shows that the blockade of adenosine A2A receptors (A2AR) affords protection to the retina through the control of microglia-mediated neuroinflammation. Herein, we investigated the therapeutic potential of an antagonist of A2AR in a model of diabetic retinopathy. Type 1 diabetes was induced in 4–5 months old C57BL/6 J mice with a single intraperitoneal injection streptozotocin. Animals were treated one month after the onset of diabetes. The A2AR antagonist was delivered by intravitreal injection once a week for 4 weeks. Microglia reactivity and inflammatory mediators were increased in the retinas of diabetic animals. The treatment with the A2AR antagonist was able to control microglial reactivity and halt neuroinflammation. Furthermore, the A2AR antagonist rescued retinal vascular leakage, attenuated alterations in retinal thickness, decreased retinal cell death and the loss of retinal ganglion cells induced by diabetes. These results demonstrate that intravitreal injection of the A2AR antagonist controls inflammation, affords protection against cell loss and reduces vascular leakage associated with diabetes, which could be envisaged as a therapeutic approach for the early complications of diabetes in the retina.
Subject terms: Retinal diseases, Diabetes complications
Introduction
Diabetic retinopathy is a major complication of diabetes mellitus and a leading cause of blindness and vision impairment in working-age adults1. Blood-retinal-barrier (BRB) breakdown is a hallmark of the disease2. The current available treatments mainly target neovascularization through the use of anti-vascular endothelial growth factor therapies, laser treatment and surgery3. Nevertheless, diabetic retinopathy is now considered a neuro-vascular disease in which a low-grade chronic inflammatory environment contribute to BRB breakdown and retinal dysfunction1,4–8. This inflammatory state is mainly due to glial cell dysfunction and BRB breakdown, possible induced by the death of retinal cells4–7. Microglial cells were shown to impact retinal cell function in diabetic conditions by increasing the pro-inflammatory milieu9–14. Taking the role of inflammation in the pathophysiology of diabetic retinopathy, therapeutic strategies targeting the control of neuroinflammation may offer protective options to manage diabetic retinopathy, halting disease progression.
Adenosine is a neuromodulator of the central nervous system exerting its actions through the activation of four adenosine receptors, A1R, A2AR, A2BR and A3R. Several reports demonstrate that detrimental microglial cell response might be amended by A2AR antagonists in different brain and retinal diseases15–21. In the brain, A2AR was found to be involved in the control of blood-brain barrier (BBB) permeability22,23. Therefore, we hypothesized that the blockade of A2AR might confer protection to the retina by modulating microglia reactivity, thus altering the course of the effects of diabetes. To address this hypothesis, we used an animal model of type 1 diabetes, which was treated with a selective A2AR antagonist, SCH 58261, by an intravitreal injection every week, for four weeks, starting one month after the onset of diabetes. This experimental protocol allows the study of the therapeutic potential of the A2AR antagonist, since the retina is already affected by the disease when the treatment is initiated.
Results
Retinal thickness is reduced one month after diabetes induction
All the animals used in this study were monitored for weight and glycemic before diabetes induction (baseline), and 1 and 2 months after the onset of diabetes. Hemoglobin A1C (HbA1C) was measured only in a subset of animals after 2 months of diabetes induction, randomly chosen. Biochemical data and weight of the animals from this sub-set are presented in Table 1.
Table 1.
Representative data on glycemia, weight and HbA1c of the animals used in the study
| Control | Diabetic | |||||
|---|---|---|---|---|---|---|
| Baseline | 1 month | 2 months | Baseline | 1 month | 2 months | |
| Glycemia (mg/dL) | 240 ± 10.5 | 238 ± 12.0 | 246 ± 3.4 | 244 ± 11.8 | 385 ± 28.8 *** | 404 ± 29.0*** |
| Weight (g) | 26.5 ± 1.1 | 27.7 ± 1.0 | 27.6 ± 0.9 | 26.0 ± 0.9 | 25.9 ± 1.1 | 25.3 ± 0.8 |
| HbA1c (%) | — | — | 4.9 ± 0.08 | — | — | 7.0 ± 0.4 |
| n | 5–7 | 9–12 | ||||
***p < 0.001, compared with baseline, One-way ANOVA test, followed by Holm-Šídák multiple comparison test.
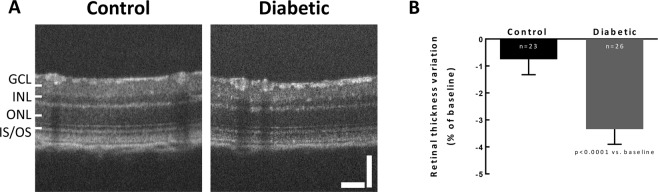
Retinal thinning has been reported as an early alteration in diabetic animals24. Four weeks after the onset of diabetes, retinal thickness significantly (p < 0.0001) decreased in diabetic animals when compared with control animals (Fig. 1), a structural indication of alterations due to diabetes. Since the aim of this work was to investigate the therapeutic potential of a selective A2AR antagonist for the treatment of diabetic retinopathy, we wanted to start the intravitreal injections after established retinal alterations. Therefore, the A2AR antagonist was injected intravitreally thereafter every week, in a total of 4 injections.
Figure 1.
Retinal thickness is reduced one month after diabetes induction. (A) Representative OCT images. (B) OCT was performed before (baseline) and 4 weeks after diabetes induction, and retinal thickness was measured in OCT images after segmentation. The results were normalized to baseline, n = 23–26 animals. Wilcoxon matched-pairs signed rank test. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer; IS-OS: photoreceptors inner and outer segments. Scale bars: 50 µm.
Treatment with A2AR antagonist decreases microglia reactivity in the retina of diabetic mice
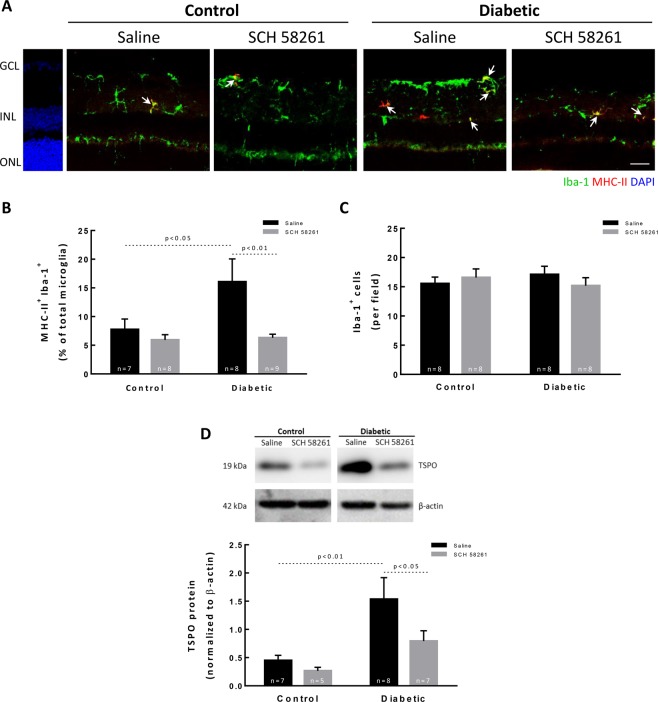
A large body of evidence demonstrate that inflammation contributes to diabetic retinopathy and microglia reactivity occurs early in the course of the disease11. Microglia were labelled with antibodies against ionized calcium-binding adaptor molecule 1 (Iba-1, a marker of microglia) and major histocompatibility complex class II (MHC-II, highly expressed in reactive microglia) (Fig. 2A). Therefore, microglial cells (Iba-1+ cells) labeled with MHC-II (MHC-II+ Iba-1+ cells) were considered reactive microglia15. As expected, microglia reactivity was increased in the retina of diabetic animals (16.1 ± 4.0% of total Iba-1+ cells; p < 0.05) when compared with the retinas of control animals (7.7 ± 1.8% of total Iba-1+ cells) (Fig. 2B). The intravitreal injection of SCH 58261, the A2AR antagonist, significantly decreased microglial reactivity in the retinas of diabetic animals (6.3 ± 0.6% of total Iba-1+ cells; p < 0.01). The administration of SCH 58261 to non-diabetic animals did not significantly change the number of reactive microglia (5.9 ± 0.9% of total Iba-1+ cells). These alterations in microglia reactivity in diabetic animals were not due to changes in the total number of microglia (Iba-1+ cells) (Fig. 2C). The Translocator protein (18 kDa) (TSPO) is constitutively expressed in retinal microglia and in inflammatory conditions TSPO expression increases25,26. Therefore, the effect of SCH 58261 on microglia reactivity was further studied by evaluating the protein levels of TSPO (Fig. 2D). Diabetes significantly increased by 3.4-fold the protein levels of TSPO in the retina of diabetic animals (p < 0.01) when compared with the control condition (saline-injected retinas from non-diabetic animals) (Fig. 2D), and this effect was abolished by the A2AR antagonist. The treatment of non-diabetic animals with the A2AR antagonist did not change TSPO protein levels (Fig. 2D).
Figure 2.
Treatment with A2AR antagonist decreases microglia reactivity in the retina of diabetic mice. (A) Retinal sections were stained with antibodies against Iba-1 (green) and MHC-II (red). Nuclei were stained with DAPI (blue). Representative images are depicted and arrows indicate some MHC-II+ Iba-1+ cells found in each condition. (B) Activated microglia (MHC-II+ Iba-1+ cells) were counted and normalized to the percentage of total microglial cells (Iba-1+ cells) from 7–9 animals. (C) The number of microglia per retinal section was counted. (D) TSPO protein levels were assessed by Western blot and the results are expressed as a ratio to β-actin from 6–9 independent experiments. Representative Western blots are presented. Full length uncropped images are presented as Supplementary Fig. 1. One-way ANOVA test, followed by Holm-Šídák multiple comparison test. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. Scale bar: 100 µm.
Overall, these results indicate that the A2AR antagonist has the potential to control diabetes-induced retinal microglia reactivity.
Treatment with A2AR antagonist impacts retinal neuroinflammation in the diabetic retinas
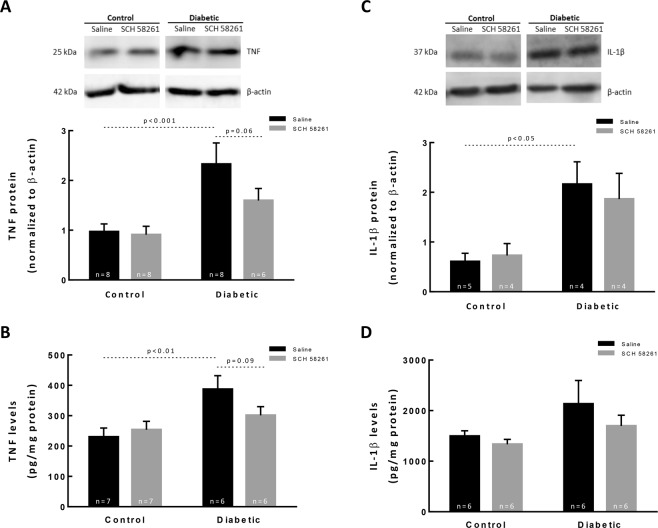
The levels of the pro-inflammatory cytokines (tumor necrosis factor) TNF and (interleukin 1 beta) IL-1β in the retina were determined by Western blot and ELISA (Fig. 3). Several cytokines have been demonstrated to play a role in diabetic retinopathy5,27, including TNF and IL-1β. The A2AR antagonist was reported to decrease the levels of these two cytokines in other experimental models, including in the retina16,18,28. Therefore, in the current work we focused on assessing the effect of SCH 58261 on the levels of TNF and IL-1β in the retinas of diabetic animals, although we do not exclude the contribution of other cytokines to the disease progression. The protein expression of TNF was significantly increased in the diabetic retinas when compared with control retinas, as assessed by Western blot (Fig. 3A, p < 0.001). The TNF levels were also quantified by ELISA (Fig. 3B), and a significant increase in the retinas of diabetic animals was found (386.8 ± 110.2 pg/mg protein, p < 0.01), when compared with control retinas (229.9 ± 78.0 pg/mg protein). While the treatment of non-diabetic animals with A2AR antagonist did not modify the protein levels of TNF, the treatment of diabetic animals slightly reduced the protein levels of TNF. The IL-1β protein levels were also increased by 3.5-fold in the retinas of diabetic animals (p < 0.05) when compared with control retinas (Fig. 3C). Diabetes significantly increased the IL-1β levels in the retinas (2130.5 ± 1140.0 pg/mg protein; p < 0.001) when compared with the control (1492.9 ± 264.2 pg/mg protein) (Fig. 3D). The treatment of diabetic animals with A2AR antagonist did not modify the levels of IL-1β.
Figure 3.
Treatment with A2AR antagonist impacts retinal neuroinflammation in the diabetic retinas. Retinal extracts were assayed for Western blot analysis of TNF (A) and IL-1β (C), and for ELISA to quantify TNF (B) and IL-1β (D). The results obtained from Western blot are expressed as the ratio to β-actin from 6–9 independent experiments. Representative Western blots are presented. Full length uncropped images are presented as Supplementary Fig. 2 (A,C). The results obtained by ELISA are presented as a ratio to the total amount of protein in the retina from 6–7 independent experiments for TNF, and from 4–5 independent experiments for IL-1β (B,D). One-way ANOVA test, followed by Holm-Šídák multiple comparison test.
Treatment with SCH 58261 decreases iNOS in the diabetic retinas
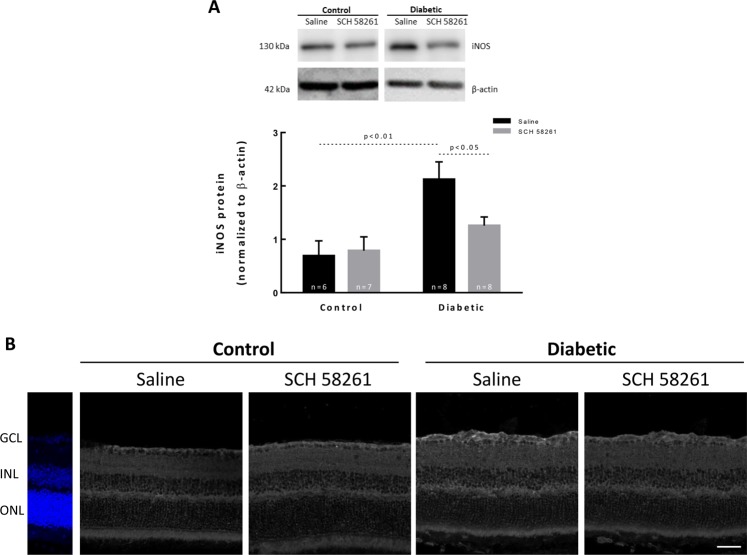
An increase in the protein levels of inducible nitric oxide synthase (iNOS) has been reported in the retina in diabetic conditions29. The protein levels of iNOS were significantly increased (3-fold change) in the retinas of diabetic animals (injected with saline) when compared with the control condition (p < 0.01, Fig. 4A). The treatment with the A2AR antagonist significantly decreased the iNOS protein levels (p < 0.05) comparatively to the diabetic retinas treated with saline. The intravitreal injection of SCH 58261 to non-diabetic animals did not change iNOS protein levels. Despite the overall increase in iNOS protein in the retinas of diabetic mice and the decrease by A2AR blockade (observed both by Western blot and by immunohistochemistry, Fig. 4), we were not able to identify a specific cell type responsible for these alterations since there were no changes in the distribution of iNOS in retinal vertical sections (Fig. 4B).
Figure 4.
Treatment with SCH 58261 decreases iNOS in the diabetic retinas. (A) The protein levels of iNOS were evaluated by Western blot. The results are presented as a ratio to β-actin, from 6–8 independent experiments. Representative Western blots are presented. Full length uncropped images are presented as Supplementary Fig. 3. (B) Retinal sections were immunolabeled for iNOS (grey). Nuclei were stained with DAPI (blue). One-way ANOVA test, followed by Holm-Šídák multiple comparison test. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. Scale bar: 100 µm.
Administration of the A2AR antagonist decreases cell death in the retina of diabetic animals
Diabetes results in the death of retinal cells30. It is widely accepted that neuroinflammation triggers cell death, including in the diabetic retina31. Retinal cell death was evaluated by TUNEL assay in retinal sections (Fig. 5). The number of TUNEL+ cells significantly increased after 8 weeks of diabetes (15.2 ± 3.9 TUNEL+ cells per field, p < 0.05) when compared with the saline injected control retinas (7.2 ± 1.5 TUNEL+ cells per field) (Fig. 5A,B). The intravitreal injection of SCH 58261 to diabetic animals significantly decreased diabetes-induced retinal cell death (5.8 ± 0.9 TUNEL+ cells per field, p < 0.05).
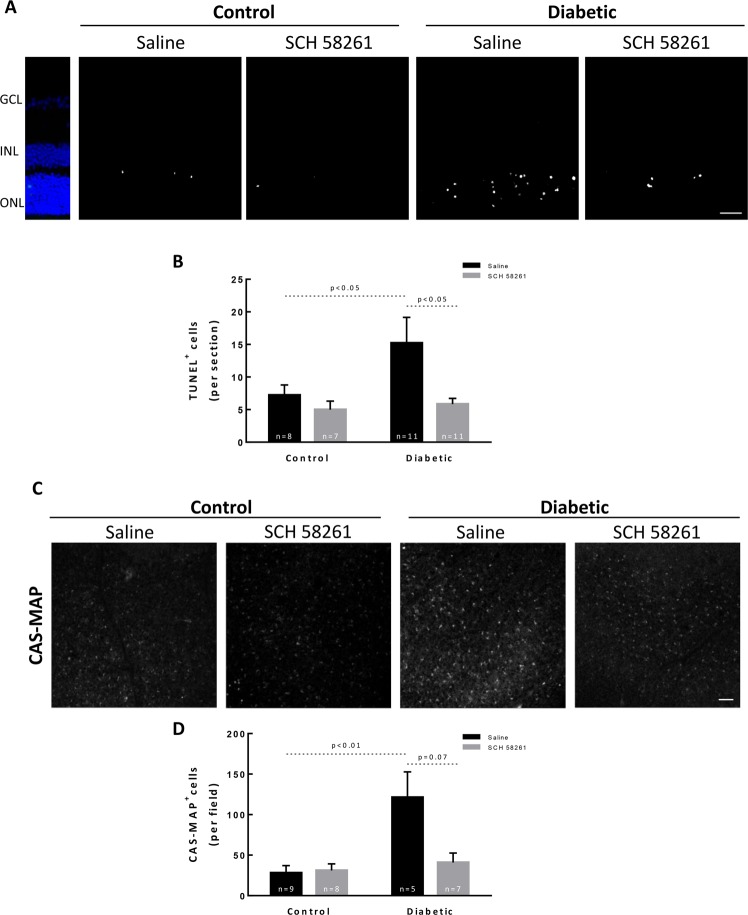
Figure 5.
Administration of the A2AR antagonist decreases cell death in the retina of diabetic animals. (A) Cell death was evaluated by TUNEL assay in vertical retinal sections. Nuclei were stained with DAPI (blue). Representative images are depicted (B). The number of TUNEL+ cells (white) was counted from 7 to 11 independent experiments. (C) The presence of active caspases in the ganglion cell layer was assessed with CAS-MAP probe (grey). Representative images are depicted. (D) The number of cells CAS-MAP+ was counted from 5–9 independent experiments. One-way ANOVA test, followed by Holm-Šídák multiple comparison test. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. Scale bar: 100 µm.
The activation of caspase-dependent signaling apoptotic mechanisms has been associated with the loss of retinal cells in diabetic retinopathy, including retinal ganglion cells (RGCs)32–34. The presence of active caspases in the ganglion cell layer was evaluated using the CAS-MAP reagent, a probe to detect active caspases 1, 2, 3, 6, 8, 9, or 10 (Fig. 5C). Diabetes significantly increased the number of apoptotic cells in the ganglion cell layer, as indicated by the increase in the number of cells labeled with CAS-MAP (121.3 ± 31.5 CAS-MAP+ cells, p < 0.01) when compared with control retinas (28.2 ± 9.0 CAS-MAP+ cells) (Fig. 5C,D). The intravitreal injection of SCH 58261 to the retinas of diabetic animals attenuated the number of retinal cells undergoing caspase-dependent cell death (41.0 ± 11.7 CAS-MAP+ cells, p = 0.07) (Fig. 5C,D). The administration of SCH 58261 to non-diabetic animals did not change the number of retinal cells with active caspases (31.1 ± 8.3 CAS-MAP+ cells).
A2AR antagonist attenuates RGC loss in a mouse model of diabetic retinopathy
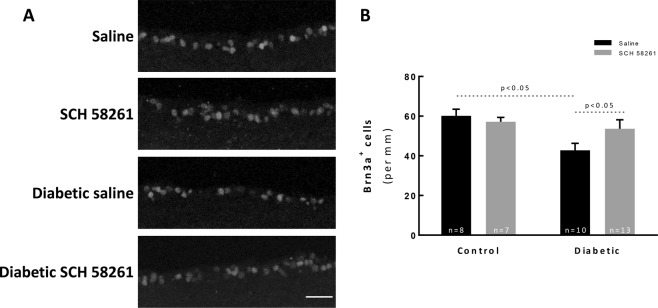
Previous findings in the STZ-induced diabetic mouse model have reported loss of RGCs30,35. RGCs were labeled with an antibody anti-Brn3a (Fig. 6), a transcription factor that is expressed by these cells36. There was a decrease in the number of RGCs after 8 weeks of diabetes (42.8 ± 3.5 Brn3a+ cells/mm, p < 0.05) when comparing with control animals (60.2 ± 3.3 Brn3a+ cells/mm, Fig. 6A,B). The A2AR antagonist did not change the number of Brn3a+ cells of non-diabetic animals (57.2 ± 2.2 Brn3a+ cells per mm). However, the treatment of diabetic animals with the A2AR antagonist promoted the survival of RGCs (53.6 ± 4.5 Brn3a+ cells/mm, p < 0.05).
Figure 6.
A2AR antagonist attenuates RGC loss in a mouse model of diabetic retinopathy. (A) The number of RGCs was evaluated in vertical sections by immunolabeling against Brn3a (grey). The nuclei were stained with DAPI (blue). Representative images are depicted. (B) The number of Brn3a+ cells were counted, and the results were normalized to the length of the section; from 7–13 animals. One-way ANOVA test, followed by Holm-Šídák multiple comparison test. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. Scale bar: 100 µm.
Intravitreal administration of SCH 58261 inhibits retinal thinning in diabetic animals
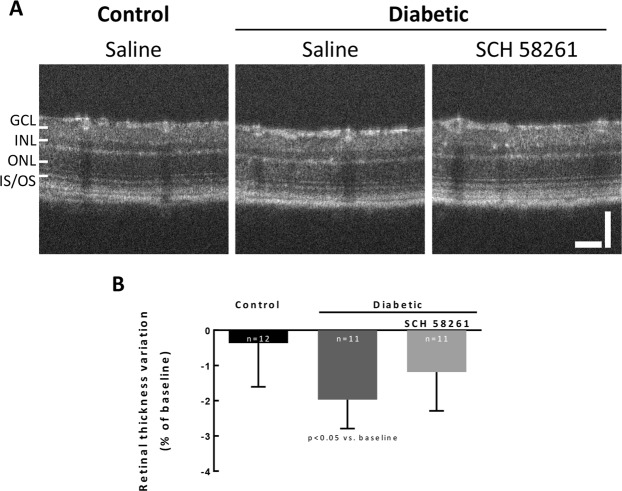
Retinal thinning can be a feature of retinal cell loss associated with diabetic retinopathy progression37. Retinal thickness was measured by OCT. Since OCT allows for longitudinal studies, retinal thickness was referred to the baseline and alterations to the baseline were documented (Fig. 7). The thickness of the retinas of control animals remained quite constant throughout the study. However, thinning was found in the retinas of diabetic (8 weeks) animals (p < 0.05 when compared with the baseline; Fig. 7A,B). The treatment of diabetic animals with SCH 58261 for 4 weeks did not statistically modify retinal thickness, when compared with the baseline values (Fig. 7A,B), which might be in accordance with a protective effect of the A2AR antagonist.
Figure 7.
Intravitreal administration of SCH 58261 inhibits retinal thinning in diabetic animals. Retinal thickness was calculated after layer segmentation in OCT images. (A) Representative OCT images at eight weeks of diabetes. (B) The results were normalized to baseline. Wilcoxon matched-pairs signed rank test. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer; IS-OS: photoreceptor inner and outer segments. Scale bars: 50 µm.
Treatment with A2AR antagonist reduces retinal vascular leakage in diabetic mice
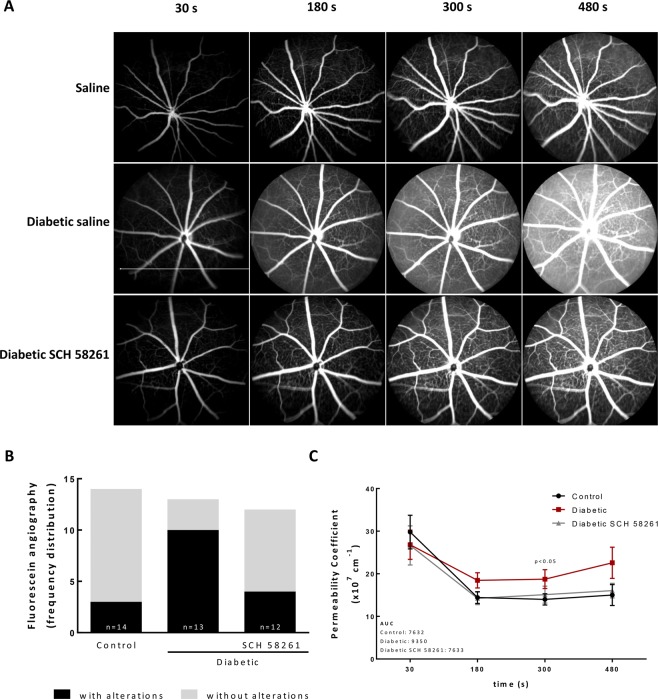
BRB dysfunction is a hallmark of diabetic retinopathy and can be assessed by fluorescein angiography. Vascular leakage, as a measurement of BRB dysfunction, was assessed in vivo by fluorescein angiography (Fig. 8). In most of non-diabetic mice (79% of animals), fluorescein was maintained within the retinal vessels, indicating absence of vascular leakage. The angiograms of diabetic animals showed regions of vascular leakage and an overall diffuse green haze, indicating extravascular fluorescein, a sign of vascular leakage. The treatment of diabetic animals with the A2AR antagonist reduced the number of animals with retinal vascular fluorescein leakage (Fig. 8A,B), suggesting that the A2AR antagonist restored the BRB integrity lost in the course of diabetes. Furthermore, the analysis of the permeability coefficient, obtained from the images of the angiograms over time, shows a tendency to an increase in permeability in the retina of diabetic animals, which was not present in the eyes of the diabetic animals treated with the A2AR antagonist (Fig. 8C). Indeed, the curve profile of the diabetic eyes treated with SCH 58261 was very similar to the control group (Fig. 8C). The area under the curve (AUC) (Fig. 8C), for each group suggested that the treatment with A2AR antagonist may attenuate the effects of diabetes on vascular leakage. Moreover, the permeability coefficient of the diabetic animals was significantly higher than the control animals and SCH 58261 was able to significantly decrease the permeability coefficient of diabetic animals (at 300 seconds, two-way ANOVA using Fisher’s LSD test, p < 0.05).
Figure 8.
Treatment with A2AR antagonist reduces retinal vascular leakage in diabetic mice. (A) Fluorescein angiography was performed after intraperitoneal injection of 5% fluorescein in control and diabetic non-treated animals or treated with the A2AR antagonist. Images were acquired with the Micron IV (Phoenix Technology) in equivalent time frames. The images represent the distribution of fluorescein across the retina, acquired 30 seconds, 180 seconds, 300 seconds and 480 seconds after injection. (B) The images were classified for the presence or absence of extravasation of fluorescein by 3 independent researchers and the frequency of retinas with and without fluorescein leakage was expressed in percentage of total animals. (C) Permeability coefficient obtained by the quantification of the fluorescence between a major vessel and the interstitial space over time. The area under the curve (AUC) of the permeability coefficient is presented in the graph insert for the different experimental conditions. Permeability coefficient was analyzed with two-way ANOVA using Fisher’s LSD test.
Discussion
In this work, we demonstrate the therapeutic potential of an A2AR antagonist for the treatment of the retinal complications in diabetes. Importantly, the treatment of STZ-induced type I diabetic mice with the A2AR antagonist by intravitreal injection was initiated 4 weeks after the onset of diabetes, when the retina is already compromised6,29,38.
Diabetes and elevated glucose concentration increase the expression of A2AR in retinal cell cultures and in animal models of diabetes39,40. Retinal microglia are endowed with A2AR16,28,41, but other cells in the retina may also express the receptor (Supplementary Fig. 4 and42). We and others demonstrated that the blockade of A2AR affords protection to the retina and brain against noxious conditions involving microglia-mediated neuroinflammation control15–20,28,43,44. The intravitreal administration of SCH 58261 in diabetic animals decreased retinal microglial reactivity. Previously, we demonstrated that blocking A2AR in microglia prevents the reactivity of these cells and retinal neural cell loss28, suggesting that the effects observed herein occur through the modulation of microglia phenotype. One limitation of this study is the use of only one drug to infer on the therapeutic potential of A2AR for the treatment of diabetic retinopathy. In previous works, we demonstrated the beneficial effects of oral administration of KW6002 (selective A2AR antagonist) and caffeine (non-selective adenosine receptor antagonist) to the retina, through the control of microglia-mediated neuroinflammation15,45. Since caffeine is a non-selective adenosine receptor antagonist it would not be helpful when elucidating the role of A2AR in the diabetic retina. Moreover, the oral administration of KW6002 or caffeine, as performed in past works, could interfere with other signaling pathways given the systemic nature of diabetes. Another issue is the lack of the pharmacological evidence of the role played by A2AR, using the A2AR selective agonist. These issues are critically important since the effects mediated by A2AR are bidirectional and depend on the target cell46. The global A2AR knockout mouse would not be relevant to study the therapeutic potential of A2AR blockade since this receptor interferes with the development of retinal vasculature, neuronal wiring and glial cell function, and impacts visual function47–50. Others demonstrated that the systemic delivery of an A2AR agonist decreases retinal cell death in diabetic animals41. The apparent contradictory findings might be explained by the different routes of drug administration, doses used and stage of the disease.
Diabetic retinopathy is a low-grade chronic inflammatory disease and a large body of evidence supports the role of inflammation in BRB dysfunction1,4,51,52. Diabetes increased the retinal levels of TNF and IL-1β, but the treatment with SCH 58261 only slightly attenuated the expression of TNF. The differential modulation of the levels of proinflammatory cytokines by the A2AR antagonist might be dependent on the pathological conditions or the animal species being evaluated. In the current work, the treatment with the A2AR antagonist was initiated four weeks after the onset of diabetes. This may explain the lack of effects of SCH 58261 on reducing the levels of TNF and IL-1β since at four weeks of diabetes the inflammatory environment is already established29. In a previous work, we reported that a single intravitreal injection of SCH 58261 prevents the increase in the expression of IL-1β induced by transient retinal ischemia in Wistar rats, without altering the levels of TNF16. In contrary, the mouse model of transient retinal ischemia promotes an increase in TNF without interfering with IL-1β retinal levels15.
Also, microglial reactivity and neuroinflammation were shown to contribute to retinal degeneration in diabetic retinopathy9–13. Caspases are family of cysteine proteases known to be involved in the initiation and execution of apoptosis and have been implicated in the death of RGCs34, including in diabetic retinopathy32,53. Our results show an increase in the number of cells with active caspases within the GCL. The GCL is composed not only by RGCs but also astrocytes and displaced amacrine cells, and it is estimated that in the rodent retina RGCs comprise 50% of the total GCL population54,55. We found a reduction in the number of RGCs, suggesting that diabetes is causing caspase-dependent RGC death. Nevertheless, displaced amacrine cells may also degenerate in diabetes56. Moreover, we do not rule out the possibility that active caspases are also present in other retinal layers, since other cells, like photoreceptors are also affected by diabetes57–60. Interestingly, TUNEL staining was mainly observed in the ONL. Several mechanisms orchestrate cell death in the retina during diabetes, and cell death does not occur at the same time window. The absence of TUNEL staining in the GCL may be due to the efficient phagocytosis by microglial cells that become reactive in the course of diabetes61 and actively phagocyte RGC debris62. The A2AR antagonist afforded protection to the retinas of diabetic animals, namely to RGCs, probably the controlling microglia reactivity, as demonstrated in other noxious conditions15,16,18,28,63.
The protein levels of iNOS were increased in the retinas of diabetic animals, as reported previously29,64,65. Once expressed, iNOS is constantly active thus producing high amounts of NO66, with detrimental effects to the retina, such as increased leukostasis and BRB breakdown29. The cells expressing iNOS were not identified but one can speculate, based on previous reports, that iNOS expression occurs mainly in microglia and astrocytes in the retina of diabetic mice13,67. In fact, the A2AR antagonist prevents iNOS increase in microglia triggered by noxious inflammatory conditions16,18. In the current work the treatment with the A2AR antagonist reduced the diabetes-induced expression of iNOS. Taking the role of iNOS to the disruption of BRB in mouse models of diabetic retinopathy29,64 and our data on the control of microglia-mediated neuroinflammation by A2AR antagonist, we might postulate that the blockade of the A2AR improves BRB properties by decreasing microglia reactivity. Nevertheless, it was demonstrated that A2AR activation mediates pathological angiogenesis in the retina by promoting HIF-1α accumulation and increased glycolysis that lead to endothelial cell proliferation and sprouting68, and therefore we cannot discard that A2AR antagonist may also be targeting endothelial cells.
Our data support the therapeutic potential of A2AR antagonists for the treatment of diabetic retinopathy. We can suggest the control of microglia-mediated neuroinflammation as the axis that will contribute to a decrease in proinflammatory mediators and nitrosative stress, maintenance of BRB integrity and neuroprotection. The A2AR emerges as a candidate to be further tested in the context of diabetic retinopathy.
Materials and Methods
Animals
C57BL/6 J mice were housed in a controlled environment (21.8 ± 0.1 °C and 67.6 ± 1.6% relative humidity, 12 hours light/12 hours dark cycle) with free access to water and standard rodent chow. All procedures were approved by the Animal Welfare Committee of the Faculty of Medicine of University of Coimbra (ORBEA: 24–2015) and conducted in accordance with the European Community directive guidelines for the use of animals in laboratory (2010/63/EU) transposed to the Portuguese law (Decreto-Lei 113/2013) and were also in agreement with the Association for Research in Vision and Ophthalmology statement for animal use.
Induction of type 1 diabetes
Four-month-old C57BL/6 J mice were randomly assigned to control or diabetic animals. Diabetes was induced with streptozotocin (STZ; 150 mg/kg, i.p.) (Sigma-Aldrich, St Louis, MO, USA) prepared immediately before injection in citrate buffer (10 mM Na-citrate, pH 4.5) (Sigma-Aldrich, St Louis, MO, USA). Diabetes was confirmed one week later by tail blood glycemic values above 250 mg/dl. STZ-injected animals with glucose levels below 250 mg/dl were excluded from the study.
Drug administration
Four weeks after the onset of diabetes, animals were treated with the A2AR selective antagonist (SCH 58261) or with the vehicle, by intravitreal injection (one injection per week, for four weeks). Animals were anesthetized with 2.5% isoflurane (IsoFlo; Abbott Laboratories, Chicago, USA) in 1 l/min O2, pupils were dilated (Tropicil Top®, 10 mg/ml) and topical anesthesia (oxybuprocaine hydrochloride, Anestocil®, 4 mg/ml) was applied. SCH 58261 (Tocris, Bristol, UK), 2 µl of 100 mM solution prepared in 0.9% NaCl, or 2 µl vehicle (0.9% NaCl) was injected into the vitreous using a 36 G needle connected to an intraocular injection kit (NanoFil™ Application Kits, World Precision Instruments, Hertfordshire, UK) coupled to a 10 µl syringe and an automated pump controlled with a footswitch (Micro4; World Precision Instruments, Hertfordshire, UK).
Hemoglobin A1c measurement
At the end of the study, blood from the tail vein was collected and hemoglobin A1c (HbA1c) values were determined using a DCA Vantage analyzer according to the manufacturer instructions (Siemens, Munich, Germany). HbA1c was reported as percentage of the total albumin in each sample: % HbA1c = (HbA1c/Total Hemoglobin) × 100.
Retinal cryosections
Animals were deeply anesthetized with ketamine (80 mg/kg; Imalgene® 1000) and xylazine (5 mg/kg; Ronpum® 2%) and transcardially perfused with phosphate-buffered saline (PBS; in mM: 137 NaCl, 2.7 KCl, 10 Na2HPO4, and 1.8 KH2PO4; pH 7.4) followed by 4% (w/v) paraformaldehyde (PFA). Eye cups were prepared as previously described15. Retinal cryosections of 14 µm thickness were cut in a cryostat (Leica CM3050 S, Leica Biosystems, Wetzlar, Germany) and mounted on Superfrost Plus glass slides (Menzel-Gläser; Thermo Scientific, Massachusetts, USA).
Immunohistochemistry
Retinal sections were immunolabeled for Iba-1 and MHC-II as previously described15 using the antibodies listed in Table 2. Retinal sections were imaged using an inverted fluorescence microscope (Zeiss Axio HXP-120, Zeiss, Oberkochen, Germany) with a 20x objective (Plan Achromat 20 × /0.8 M27). The number of cells immunoreactive to Iba-1 was counted and the number of cells immunoreactive to both Iba-1 and MHC-II (reactive microglia) was normalized to the % of total microglia.
Table 2.
List of primary and secondary antibodies used in this work.
| Supplier, Cat # | Host | Dilution | Technique | |
|---|---|---|---|---|
| Primary antibodies | ||||
| anti-Iba-1 | Wako, 019–19741 | rabbit | (1:1000) | Microscopy |
| anti-MHC-II | eBioscience, 14–5321–82 | rat | (1:500) | |
| anti-Brn3a | Santa Cruz, sc-31984 | goat | (1:500) | |
| anti-β-actin | Sicgen, AB0041-500 | goat | (1:1000) | Western blot |
| anti-TNF | Abcam, ab66579 | rabbit | (1:1000) | |
| anti-iNOS | Santa Cruz, sc-650 | rabbit | (1:500) | |
| anti-IL-1β | R&D Systems, AF-401-NA | goat | (1:1000) | |
| anti-PBR (TSPO) | Abcam, ab109497 | rabbit | (1:1000) | |
| Secondary antibodies | ||||
| Alexa Fluor® 488 Goat Anti-Rabbit IgG (H + L) | Invitrogen, A11008 | goat | (1:500) | Microscopy |
| Alexa Fluor® 568 Goat Anti-Rat IgG (H + L) | Invitrogen, A11077 | goat | (1:500) | |
| Alexa Flour® 568 Donkey Anti-Goat (H + L) | Invitrogen, A11057 | donkey | (1:500) | |
| anti-rabbit IgG (H + L), HRP | BioRad,1706515 | goat | (1:10000) | Western blot |
| HRP-coupled anti-goat IgG (H + L) | Invitrogen, LTI 611620 | rabbit | (1:10000) | |
| AP-coupled anti-goat IgG (H + L) | Invitrogen, LTI 611622 | rabbit | (1:10000) | |
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay
Cell death was determined in retinal sections by DeadEnd™ Fluorometric TUNEL assay following the manufacturer’s instructions (Promega, Madison, WI, USA). Nuclei were counterstained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) (Invitrogen, Carlsbad, CA, USA) (1:2000). The preparations were mounted with Glycergel mounting medium (DAKO, Agilent, Santa Clara, CA, USA) and were observed in an inverted fluorescence microscope (Zeiss Axio HXP-120, Zeiss, Oberkochen, DE) with a 20x objective (Plan Achromat 20×/0.8 M27). The number of TUNEL-positive cells was counted in the entire retinal section (4 sections per eye).
Caspase activity detection assay by in vivo labelling
The detection of active caspases within the GCL was performed using the pan-caspase CAS-MAP probe. Animals were anesthetized with 2.5% isoflurane (IsoFlo; Abbott Laboratories, Chicago, IL, USA) in 1 l/min O2. Following pupil dilation (Tropicil Top®, 10 mg/ml) and topical anesthesia (oxybuprocaine hydrochloride, Anestocil®, 4 mg/ml), 2 µl of 100 nM/kg of CAS-MAP probe (Vergence Bioscience, Minneapolis, MN, USA) was administered into the vitreous with a 36 G needle coupled to a Hamilton syringe. Then, 24 hours later, the animals were anesthetized with ketamine (80 mg/kg; Imalgene® 1000) and xylazine (5 mg/kg; Ronpum® 2%) and transcardially perfused with PBS and 4% (w/v) PFA. The retinas were then dissected and washed with PBS. Nuclei were stained with DAPI and the retinas were mounted with Glycergel mounting media (DAKO, Agilent, CA, USA). The samples were observed in a confocal microscope (LSM 710, Zeiss, Oberkochen, Germany) using a 20x objective (Plan-Apochromat 20 × /0.8 M27), and from each retina two images per quadrant were randomly acquired in the ganglion cell layer focusing plane. The number of CAS-MAP+ cells was counted per image.
Western blot
Preparation of retinal extracts and Western blot was performed as previously described69. In order to avoid biased matched comparisons, samples from both retinas of each animal were loaded, side by side in the gel, assuming separated membranes for control and diabetic animals. Nevertheless, membranes were developed at the same time and comparisons were made to the β-actin ratio. The membranes were incubated with the antibodies described in Table 2. Immunolabeling was detected using WesternBright Sirius™ (Advansta, Menlo Park, CA, USA) or with ECF™ (GE Healthcare Amersham™, Little Chalfont, UK), in accordance with the manufacturer’s instructions.
Enzyme-linked immunosorbent assay (ELISA)
The retinas were collected in ice-cold ELISA buffer (20 mM imidazole-HCl, 100 mM KCl, 1 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100), supplemented with phosphatase inhibitors (10 mM NaF and 1 mM Na3VO4) and complete mini protease inhibitor cocktail tablets (Roche, Sigma-Aldrich, St Louis, MO, USA). The tissue was homogenized by sonication, centrifuged at 10000 × g for 5 min at 4 °C and the supernatant was collected. Protein concentration was determined using BCA (Pierce Biotechnology, Rockford, IL, USA). The levels of TNF and IL-β were quantified in accordance to the manufacturer’s instructions (PeproTech, London, UK), using a microplate reader (Synergy HT; Biotek, Winooski, VT, USA). The results were normalized to the total amount of protein in each sample.
Optical coherence tomography (OCT)
Retinal thickness and structure were analyzed followed Spectrum Domain-OCT (coupled to a Phoenix Micron IV Retinal Imaging Microscope, Phoenix Technology, Pleasanton, CA, USA), as previously described70, with minor modifications as follows. Briefly, the animals were anesthetized using ketamine (80 mg/kg; Imalgene® 1000) and xylazine (5 mg/kg; Ronpum® 2%), the pupils were dilated using tropicamide (Tropicil Top®, 10 mg/ml) and oxybuprocaine (Anestocil®) was applied in the cornea for topical anesthesia. Corneal hydration was maintained using carmellose sodium (Celluvisc®). OCT was performed at baseline (before diabetes induction), one month after the onset of diabetes and at the end of the study (2 months after diabetes onset). Eye fundus images were obtained using the Micron IV Retinal Imaging Microscope for image guidance. The thickness of retinal layers was analyzed after OCT scan data segmentation with InSight (Phoenix Technology). For each eye, 4 B-scans were analyzed (2 images above and 2 images bellow the optic nerve). The results were normalized to the baseline, as follows:
xi represents baseline retinal thickness; x represents retinal thickness at the defined timepoint.
Fluorescein angiography
The animals were anesthetized as described for OCT and the eyes were treated equally. Animals were injected with 5% fluorescein (100 μl, i.p.), as described previously71,72. Fluorescein angiography images were acquired with the Micron IV Retinal Imaging Microscope (Phoenix Technology, Pleasanton, CA, USA) sequentially until saturation. For this experiment, only one eye per animal was analyzed (SCH 58261 treated and non-treated diabetic retinas are from different animals) in order to keep the acquisition time equivalents between conditions. The images were classified to the presence of leaky areas by three independent researchers that were masked to the experimental condition.
The permeability coefficient was estimated as previously described73,74, as follows. The fluorescence intensity of a major vessel and of the interstitial space was determined with ImageJ for 4 time points (30 seconds, 180 seconds, 300 seconds and 480 seconds after injection).
Fluorescence intensity ratio F was calculated to obtain the slope of the linear equation:
F represents the fluorescence intensity in a given time point; ΔC represents the fluorescence difference between a major vessel and the interstitial space; t is the time after injection in seconds.
The permeability coefficient P was calculated applying the formula:
slope F represents the slope obtained from the fluorescence intensity over time; ΔC represents the fluorescence difference between the major vessel and the interstitial space at a given time; A represents the area of the region of interest.
Statistical analysis
Data is presented as mean ± SEM. The results were analyzed using Graphpad Prism software version 6.01 for Windows. The OCT data was analyzed using IBM SPSS statistics software version 24 for Windows. The distribution of the data was evaluated by Shapiro-Wilk normality test. The data with normal distribution was analyzed with parametric one-way ANOVA followed by Sidak’s multiple comparisons test, and for data without Gaussian distribution the statistical significance was determined with Kruskall-Wallis test followed by Dunn’s multiple comparison test or Mann-Whitney test, as indicated in the figure legends. For the analysis of OCT data, the Wilcoxon matched-pairs signed rank test was used. Fluorescein angiography was analyzed by two-way ANOVA using Fisher’s LSD test and the area under the curve (AUC) of the permeability coefficient was calculated setting the baseline at Y = 0. Statistical significance was defined for p < 0.05.
Supplementary information
Acknowledgements
Global Ophthalmology Awards Program from Bayer 2015 (US2083156314). Foundation for Science and Technology (FCT), Portugal, and COMPETE-FEDER: PTDC/BIM-MEC/0913/2012; PTDC/NEU-OSD/3123/2014; FCOMP-01-0124-FEDER-028417; UID/NEU/04539/2013, UID/NEU/04539/2019 and POCI-01-0145-FEDER-007440); FCT PhD fellowships PD/BD/127821/2016 and PD/BD/114115/2015; Centro 2020 Regional Operational Programme (CENTRO-01-0145-FEDER-000008: BRAINHEALTH 2020). The authors declare no competing interests.
Author contributions
I.D.A., M.H.M. and R.B. performed most of the experiments and analyzed the respective data. A.C.R.N. extracted the O.C.T. data and J.M.M. performed statistical analyzes for the O.C.T. data. A.R.S., I.D.A. conceived and designed the experiments. I.D.A. and A.R.S. wrote the manuscript. M.H.M., R.B., A.C.N.R., J.M.M. and A.F.A. revised the manuscript. Data will be made available upon request. All contributing authors have read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-53627-y.
References
- 1.Santiago AR, Boia R, Aires ID, Ambrosio AF, Fernandes R. Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy. Frontiers in physiology. 2018;9:820. doi: 10.3389/fphys.2018.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartnett ME, Baehr W, Le YZ. Diabetic retinopathy, an overview. Vision research. 2017;139:1–6. doi: 10.1016/j.visres.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Marozas LM, Fort PE. Diabetic Retinopathy-Update on Prevention Techniques, Present Therapies, and New Leads. US ophthalmic review. 2014;7:54–58. doi: 10.17925/USOR.2014.07.01.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang, J. & Kern, T. S. Inflammation in diabetic retinopathy. Progress in retinal and eye research30, 343–358, doi:10.1016/j.preteyeres.2011.05.002 (2011). [DOI] [PMC free article] [PubMed]
- 5.Abcouwer, S. F. Angiogenic Factors and Cytokines in Diabetic Retinopathy. Journal of clinical & cellular immunology Suppl1, 10.4172/2155-9899 (2013). [DOI] [PMC free article] [PubMed]
- 6.Villarroel M, Ciudin A, Hernandez C, Simo R. Neurodegeneration: An early event of diabetic retinopathy. World journal of diabetes. 2010;1:57–64. doi: 10.4239/wjd.v1.i2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simo R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902–1912. doi: 10.1007/s00125-018-4692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisma JH, Dulle JE, Fort PE. Current knowledge on diabetic retinopathy from human donor tissues. World journal of diabetes. 2015;6:312–320. doi: 10.4239/wjd.v6.i2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaucher D, et al. Microglial changes occur without neural cell death in diabetic retinopathy. Vision research. 2007;47:612–623. doi: 10.1016/j.visres.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Sorrentino FS, Allkabes M, Salsini G, Bonifazzi C, Perri P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life sciences. 2016;162:54–59. doi: 10.1016/j.lfs.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Krady JK, et al. Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. 2005;54:1559–1565. doi: 10.2337/diabetes.54.5.1559. [DOI] [PubMed] [Google Scholar]
- 12.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Archives of ophthalmology. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 13.Grigsby JG, et al. The role of microglia in diabetic retinopathy. Journal of ophthalmology. 2014;2014:705783. doi: 10.1155/2014/705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arroba AI, et al. Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochimica et biophysica acta. 2016;1862:1663–1674. doi: 10.1016/j.bbadis.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Boia R, et al. Treatment with A2A receptor antagonist KW6002 and caffeine intake regulate microglia reactivity and protect retina against transient ischemic damage. Cell death & disease. 2017;8:e3065. doi: 10.1038/cddis.2017.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madeira MH, et al. Selective A2A receptor antagonist prevents microglia-mediated neuroinflammation and protects retinal ganglion cells from high intraocular pressure-induced transient ischemic injury. Translational research: the journal of laboratory and clinical medicine. 2016;169:112–128. doi: 10.1016/j.trsl.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, et al. The Effect of A2A Receptor Antagonist on Microglial Activation in Experimental Glaucoma. Investigative ophthalmology & visual science. 2016;57:776–786. doi: 10.1167/iovs.15-18024. [DOI] [PubMed] [Google Scholar]
- 18.Madeira MH, et al. Adenosine A2AR blockade prevents neuroinflammation-induced death of retinal ganglion cells caused by elevated pressure. Journal of neuroinflammation. 2015;12:115. doi: 10.1186/s12974-015-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boia R, Ambrósio AF, Santiago AR. Therapeutic Opportunities for Caffeine and A2A Receptor Antagonists in Retinal Diseases. Ophthalmic research. 2016;55:212–218. doi: 10.1159/000443893. [DOI] [PubMed] [Google Scholar]
- 20.Gyoneva S, et al. Adenosine A2A receptor antagonism reverses inflammation-induced impairment of microglial process extension in a model of Parkinson’s disease. Neurobiology of disease. 2014;67:191–202. doi: 10.1016/j.nbd.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. Journal of leukocyte biology. 2008;83:447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DG, Bynoe MS. A2A Adenosine Receptor Regulates the Human Blood-Brain Barrier Permeability. Molecular neurobiology. 2015;52:664–678. doi: 10.1007/s12035-014-8879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurtado-Alvarado G, Dominguez-Salazar E, Velazquez-Moctezuma J, Gomez-Gonzalez B. A2A Adenosine Receptor Antagonism Reverts the Blood-Brain Barrier Dysfunction Induced by Sleep Restriction. PloS one. 2016;11:e0167236. doi: 10.1371/journal.pone.0167236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson LR, Grover S, Dominguez JM, 2nd, Balaiya S, Chalam KV. Retinal thickness measurement obtained with spectral domain optical coherence tomography assisted optical biopsy accurately correlates with ex vivo histology. PloS one. 2014;9:e111203. doi: 10.1371/journal.pone.0111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlstetter M, et al. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. Journal of neuroinflammation. 2014;11:3. doi: 10.1186/1742-2094-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, et al. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. The. Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:3793–3806. doi: 10.1523/JNEUROSCI.3153-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Hwang DK, Song X, Tao Y. Association between Aqueous Cytokines and Diabetic Retinopathy Stage. Journal of ophthalmology. 2017;2017:9402198. doi: 10.1155/2017/9402198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aires, I. D. et al. Blockade of microglial adenosine A2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells. Glia, 10.1002/glia.23579 (2019). [DOI] [PMC free article] [PubMed]
- 29.Leal EC, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Investigative ophthalmology & visual science. 2007;48:5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 30.Kern TS, Barber AJ. Retinal ganglion cells in diabetes. The Journal of physiology. 2008;586:4401–4408. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadlubowska J, Malaguarnera L, Waz P, Zorena K. Neurodegeneration and Neuroinflammation in Diabetic Retinopathy: Potential Approaches to Delay Neuronal Loss. Current neuropharmacology. 2016;14:831–839. doi: 10.2174/1570159X14666160614095559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adamiec-Mroczek J, Zajac-Pytrus H, Misiuk-Hojlo M. Caspase-Dependent Apoptosis of Retinal Ganglion Cells During the Development of Diabetic Retinopathy. Advances in clinical and experimental medicine: official organ Wroclaw Medical University. 2015;24:531–535. doi: 10.17219/acem/31805. [DOI] [PubMed] [Google Scholar]
- 33.Oshitari T, Yamamoto S, Hata N, Roy S. Mitochondria- and caspase-dependent cell death pathway involved in neuronal degeneration in diabetic retinopathy. The British journal of ophthalmology. 2008;92:552–556. doi: 10.1136/bjo.2007.132308. [DOI] [PubMed] [Google Scholar]
- 34.Thomas CN, Berry M, Logan A, Blanch RJ, Ahmed Z. Caspases in retinal ganglion cell death and axon regeneration. Cell death discovery. 2017;3:17032. doi: 10.1038/cddiscovery.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivares AM, et al. Animal Models of Diabetic Retinopathy. Current diabetes reports. 2017;17:93. doi: 10.1007/s11892-017-0913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadal-Nicolas FM, et al. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investigative ophthalmology & visual science. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- 37.Lang GE. Optical coherence tomography findings in diabetic retinopathy. Developments in ophthalmology. 2007;39:31–47. doi: 10.1159/000098498. [DOI] [PubMed] [Google Scholar]
- 38.Shi R, et al. Alterations in retinal nerve fiber layer thickness in early stages of diabetic retinopathy and potential risk factors. Current eye research. 2018;43:244–253. doi: 10.1080/02713683.2017.1387669. [DOI] [PubMed] [Google Scholar]
- 39.Vindeirinho J, Costa GN, Correia MB, Cavadas C, Santos PF. Effect of diabetes/hyperglycemia on the rat retinal adenosinergic system. PloS one. 2013;8:e67499. doi: 10.1371/journal.pone.0067499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vindeirinho J, Santiago AR, Cavadas C, Ambrosio AF, Santos PF. The Adenosinergic System in Diabetic Retinopathy. Journal of diabetes research. 2016;2016:4270301. doi: 10.1155/2016/4270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibrahim AS, El-Shishtawy MM, Zhang W, Caldwell RB, Liou GI. A((2)A) adenosine receptor (A((2)A)AR) as a therapeutic target in diabetic retinopathy. The American journal of pathology. 2011;178:2136–2145. doi: 10.1016/j.ajpath.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang PC, et al. Adenosine A(2A) receptor up-regulates retinal wave frequency via starburst amacrine cells in the developing rat retina. PloS one. 2014;9:e95090. doi: 10.1371/journal.pone.0095090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochimica et biophysica acta. 2011;1808:1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madeira MH, et al. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Scientific reports. 2016;6:27532. doi: 10.1038/srep27532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai SS, Zhou YG. Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Reviews in the neurosciences. 2011;22:231–239. doi: 10.1515/RNS.2011.020. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro FF, Sebastiao AM. Adenosine A2A receptors in neuronal outgrowth: a target for nerve regeneration? Neural regeneration research. 2016;11:706–708. doi: 10.4103/1673-5374.182683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell death and differentiation. 2010;17:1071–1082. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paes-De-Carvalho R. Adenosine as a signaling molecule in the retina: biochemical and developmental aspects. Anais da Academia Brasileira de Ciencias. 2002;74:437–451. doi: 10.1590/S0001-37652002000300007. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, et al. Genetic deletion of the adenosine A2A receptor confers postnatal development of relative myopia in mice. Investigative ophthalmology & visual science. 2010;51:4362–4370. doi: 10.1167/iovs.09-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semeraro F, et al. Diabetic Retinopathy: Vascular and Inflammatory Disease. Journal of diabetes research. 2015;2015:582060. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubsam, A., Parikh, S. & Fort, P. E. Role of Inflammation in Diabetic Retinopathy. International journal of molecular sciences 19, 10.3390/ijms19040942 (2018). [DOI] [PMC free article] [PubMed]
- 53.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investigative ophthalmology & visual science. 2011;52:1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlamp CL, et al. Evaluation of the percentage of ganglion cells in the ganglion cell layer of the rodent retina. Molecular vision. 2013;19:1387–1396. [PMC free article] [PubMed] [Google Scholar]
- 55.Mead B, Tomarev S. Evaluating retinal ganglion cell loss and dysfunction. Experimental eye research. 2016;151:96–106. doi: 10.1016/j.exer.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Investigative ophthalmology & visual science. 2006;47:3143–3150. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- 57.Szabo K, et al. Histological Evaluation of Diabetic Neurodegeneration in the Retina of Zucker Diabetic Fatty (ZDF) Rats. Scientific reports. 2017;7:8891. doi: 10.1038/s41598-017-09068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park SH, et al. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260–1268. doi: 10.1007/s00125-003-1177-6. [DOI] [PubMed] [Google Scholar]
- 59.McAnany JJ, Park JC. Cone Photoreceptor Dysfunction in Early-Stage Diabetic Retinopathy: Association Between the Activation Phase of Cone Phototransduction and the Flicker Electroretinogram. Investigative ophthalmology & visual science. 2019;60:64–72. doi: 10.1167/iovs.18-25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kern TS, Berkowitz BA. Photoreceptors in diabetic retinopathy. Journal of diabetes investigation. 2015;6:371–380. doi: 10.1111/jdi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abcouwer SF. Neural inflammation and the microglial response in diabetic retinopathy. Journal of ocular biology, diseases, and informatics. 2011;4:25–33. doi: 10.1007/s12177-012-9086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadal-Nicolas FM, et al. Microglial dynamics after axotomy-induced retinal ganglion cell death. Journal of neuroinflammation. 2017;14:218. doi: 10.1186/s12974-017-0982-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madeira MH, Rashid K, Ambrosio AF, Santiago AR, Langmann T. Blockade of microglial adenosine A2A receptor impacts inflammatory mechanisms, reduces ARPE-19 cell dysfunction and prevents photoreceptor loss in vitro. Scientific reports. 2018;8:2272. doi: 10.1038/s41598-018-20733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng L, et al. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007;50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]
- 65.Mishra A, Newman EA. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia. 2010;58:1996–2004. doi: 10.1002/glia.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. European heart journal. 2012;33(829-837):837a–837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin ES, Huang Q, Gurel Z, Sorenson CM, Sheibani N. High glucose alters retinal astrocytes phenotype through increased production of inflammatory cytokines and oxidative stress. PloS one. 2014;9:e103148. doi: 10.1371/journal.pone.0103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z, et al. Endothelial adenosine A2a receptor-mediated glycolysis is essential for pathological retinal angiogenesis. Nature communications. 2017;8:584. doi: 10.1038/s41467-017-00551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baptista FI, et al. Diabetes induces changes in KIF1A, KIF5B and dynein distribution in the rat retina: implications for axonal transport. Experimental eye research. 2014;127:91–103. doi: 10.1016/j.exer.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Alves MRP, et al. Subtle thinning of retinal layers without overt vascular and inflammatory alterations in a rat model of prediabetes. Molecular vision. 2018;24:353–366. [PMC free article] [PubMed] [Google Scholar]
- 71.Lundh von Leithner P, et al. Complement factor h is critical in the maintenance of retinal perfusion. The American journal of pathology. 2009;175:412–421. doi: 10.2353/ajpath.2009.080927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao PL, et al. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic retinopathy. Scientific reports. 2017;7:44985. doi: 10.1038/srep44985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen, C., Horton, K., Malhi, N., Batson, J. & Bates, D. The SRPK1 inhibitor SPHINX31 prevents increased retinal permeability in a rodent model of diabetes. Acta Ophthalmologica95, 10.1111/j.1755-3768.2017.0F066 (2017).
- 74.Allen, C. & Bates, D. In vivo measurement of increased vascular permeability after STZ induction of diabetes in rats by fluorescence angiography using the Micron IV. Acta Ophthalmologica94, 10.1111/j.1755-3768.2016.0468 (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.