Abstract
Background
Strigolactones (SLs) are a diverse class of butenolide-bearing phytohormones derived from the catabolism of carotenoids. They are associated with an increasing number of emerging regulatory roles in plant growth and development, including seed germination, root and shoot architecture patterning, nutrient acquisition, symbiotic and parasitic interactions, as well as mediation of plant responses to abiotic and biotic cues.
Scope
Here, we provide a concise overview of SL biosynthesis, signal transduction pathways and SL-mediated plant responses with a detailed discourse on the crosstalk(s) that exist between SLs/components of SL signalling and other phytohormones such as auxins, cytokinins, gibberellins, abscisic acid, ethylene, jasmonates and salicylic acid.
Conclusion
SLs elicit their control on physiological and morphological processes via a direct or indirect influence on the activities of other hormones and/or integrants of signalling cascades of other growth regulators. These, among many others, include modulation of hormone content, transport and distribution within plant tissues, interference with or complete dependence on downstream signal components of other phytohormones, as well as acting synergistically or antagonistically with other hormones to elicit plant responses. Although much has been done to evince the effects of SL interactions with other hormones at the cell and whole plant levels, research attention must be channelled towards elucidating the precise molecular events that underlie these processes. More especially in the case of abscisic acid, cytokinins, gibberellin, jasmonates and salicylic acid for which very little has been reported about their hormonal crosstalk with SLs.
Keywords: Abscisic acid, auxin, ethylene, cytokinins, D3/MAX2, D53/SMXL, gibberellins, GR24, jasmonates, salicylic acid, strigolactone signalling, strigolactone interactions
INTRODUCTION
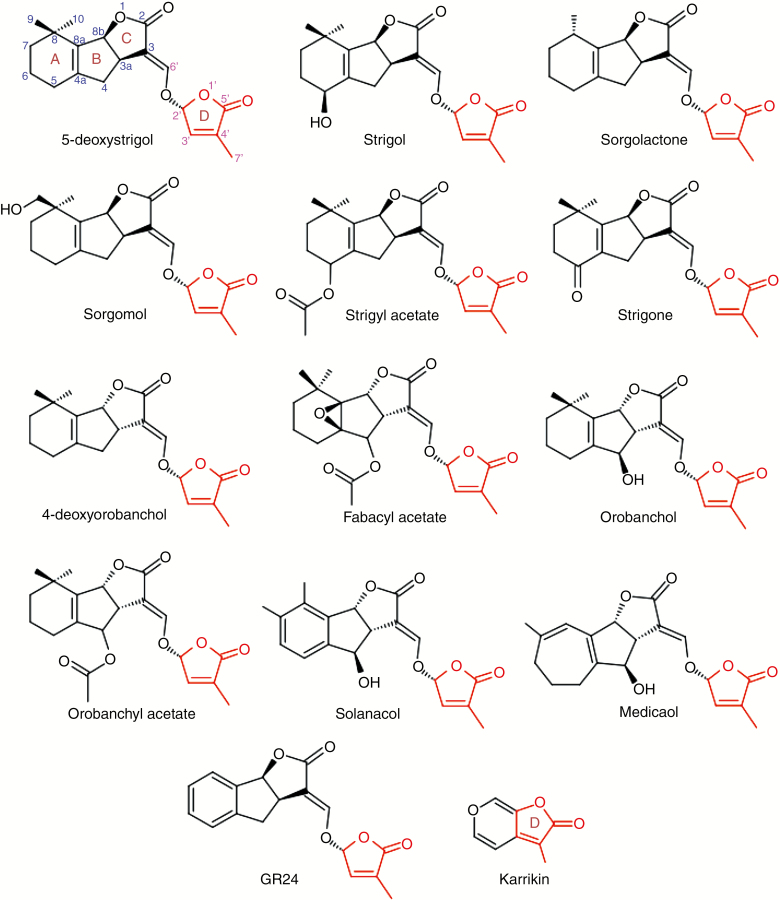
Biomolecules with butenolide moieties elicit a plethora of biological activities in plants. Their bioactivities range from acting as chemical cues or signal molecules for host–parasite/symbiont interactions (Akiyama et al., 2005, 2010; Foo and Davies, 2011; De Cuyper et al., 2015), stimulation and/or inhibition of germination in seeds (Van Staden et al., 2004; Daws et al., 2007; Soós et al., 2012), mediation of stress responses in plants (Soós et al., 2009; Cheng et al., 2017; Haider et al., 2018) to increasing seedling growth and vigour in plants (Light et al., 2010). Karrikins and strigolactones (SLs) are two groups of butenolides that have garnered huge research attention due to their influence on key aspects of plant growth and development as well as ecological significance. While SLs are largely endogenous, being synthesized by tissues in planta and transported between tissues or exuded into the rhizosphere as components of root exudates, the karrikins (Fig. 1) are components of smoke that are derived from the combustion of plant materials (Van Staden et al., 1995; Flematti et al., 2004, 2013; Kulkarni et al., 2010). Despite the marked similarities in bioactivities and signal pathways of karrikins and SLs (Nelson et al., 2011; Waters et al., 2012c), the discovery of kai2 mutants which are defective in karrikin perception but still retain their SL sensitivity and of d14 mutants lacking response to SLs but still retain their sensitivity to karrikins confirm that plants can distinguish between these two molecular cues (Waters et al., 2012b, c; Flematti et al., 2013). Against the backdrop of extant and emerging experimental evidences karrikins and SLs are largely seen as distinct chemical cues with distinct roles in the regulation of plant growth and development, although experimental data exist that allude to the sharing of roles and signal pathways by both signal molecules (Li et al., 2017).
Fig. 1.
Chemical structures of canonical strigolactones, karrikin and gr24 (a synthetic strigolactone).
Against the backdrop of experimental findings that suggest that SL bioactivities are mediated via a direct or indirect influence on the activities of other phytohormones, we provide brief highlights on the chemical nature, biosynthesis and signalling of SLs while focusing more on the interactions between SLs/elements of SL signal pathways and their influence(s) on the activities of other key phytohormones such as abscisic acid (ABA), auxins, cytokinins, ethylene, gibberellins, jasmonates and salicylic acid.
Chemical nature of strigolactones
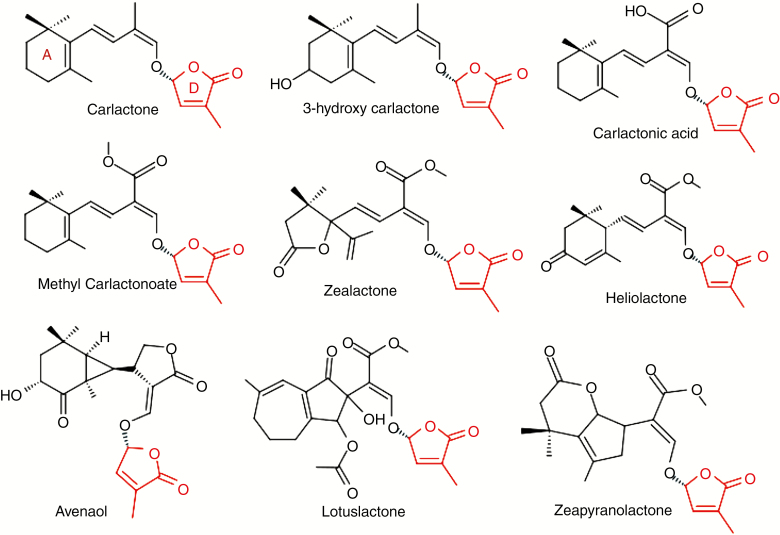
Endogenous SLs are grouped into two classes, canonical and non-canonical SLs, based on the presence or absence of a tricyclic lactone ring. Canonical SLs consist of a four-ring system designated A, B, C and D (see Fig. 1). The tricyclic lactone is linked to a butenolide moiety, the D ring, via an enol–ether bridge. As a result of functional group variability, A and B rings show maximum divergence, whereas C and D are highly conserved (Fig. 1). On the other hand, non-canonical SLs (Fig. 2) are very diverse in the structure of their ABC ring. Carlactone-type SLs possess the A and D rings as well as the enol–ether bridge but lack the B and C rings (i.e. having an unclosed ring) (Fig. 2). Among others in this group are carlactone and its derivatives such as carlactonoic acid, 3-hydroxycarlactone, methyl carlactonoate (Alder et al., 2012; Abe et al., 2014; Seto et al., 2014; Baz et al., 2018), avenaol (Kim et al., 2014) and zealactones (Charnikhova et al., 2017; Xie et al., 2019). Reports of non-canonical SLs which differ structurally from carlactone-type SLs, such as heliolactone (Ueno et al., 2014), zeapyranolactone [with 4,4-dimethyltetrahydropyran-2-one as its A ring (Charnikhova et al., 2018)] and lotuslactone (Xie et al., 2019), suggest that biomolecules with SL activity accommodate a high level of structural plasticity in their ABC rings.
Fig. 2.
Structures of non-canonical strigolactones.
With respect to the steric orientation of the BC ring junction, canonical SLs fall into either strigol or orobanchol classes. This stereochemistry has been shown to determine the functional specificity of specific SLs (Akiyama et al., 2010; Nomura et al., 2013). The C-ring in strigol-type SLs is in the β-orientation while in orobanchol-type SLs, it is in the α-orientation. Plants usually produce and transport one of either class of SLs, although both have been observed in some species (Xie et al., 2013, 2016). Several studies including those on structure–activity relationships of SLs in stimulating germination in parasitic plants and hyphal branching in arbuscular mycorrhizal fungi (AMF) have established the butenolide moiety as the essential ‘bioactiphore’ for SL activity (Akiyama et al., 2010; Kim et al., 2010; Zwanenberg and Pospísil, 2013).
Biosynthesis of strigolactones
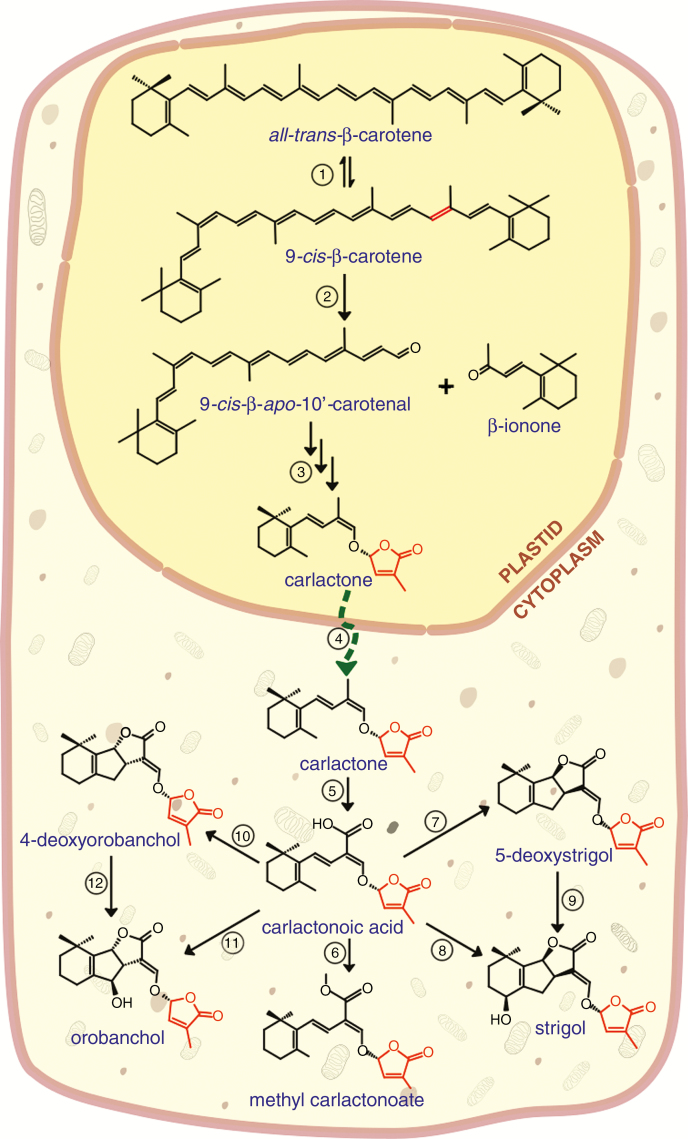
SLs are downstream products of carotenoid catabolism. SL biosynthesis occurs mainly in the root, although shoot tissues do produce SLs in lower quantities compared to the root but sufficient for shoot function in the absence of root-derived SLs (Domagalska and Leyser, 2011). For lucidity here, we mainly use rice and Arabidopsis gene nomenclature; for orthologues in other plants, see Table 1. The early dedicated steps in the biosynthesis of SLs lead to the production of carlactone, the key precursor of SLs (Seto et al., 2014; Iseki et al., 2017) (Fig. 3). Carlactone is derived from a series of reactions, including: the isomerization of all-trans-β-carotene C9–C10 double bonds by DWARF27 (D27) (Alder et al., 2012; Waters et al., 2012a; Bruno and Al-Babili, 2016) to form 9-cis-β-carotene; the stereospecific cleavage of 9-cis-β-carotene to yield 9-cis-β-apo-10′-carotenal and β-ionone catalysed by carotenoid cleavage dioxygenase 7 (CCD7) (Booker et al., 2004; Bruno et al., 2014); and the conversion of 9-cis-β-apo-10′-carotenal to carlactone by CCD8 in a combination of reactions which include oxygenation and functional group rearrangements (Alder et al., 2012; Waters et al., 2012a). The resulting carlactone is exported into the cytoplasm where it undergoes oxidation, ring closures and functional group modifications in a series of reactions catalysed by MORE AXILLARY GROWTH 1 (MAX1), a class of cytochrome P450 monooxygenases (Booker et al., 2005; Seto et al., 2014; Zhang et al., 2014). Yoneyama et al. (2018) demonstrated that MAX1 first converts carlactone to carlactonoic acid which is then used to synthesize other SLs, and this reaction is conserved among plants. In rice, OsMAX1 (Os900) serves as a carlactone oxidase which converts carlactone via carlactonoic acid to 4-deoxyorobanchol, the precursor for the orobanchol class of SLs (Zhang et al., 2014; Yoneyama et al., 2018). Just like Os900, Selaginella moellendorfii MAX1 homologues, Sm-MAX1a and Sm-MAX1b, catalyse the conversion of carlactone to 4-deoxyorobanchol (Yoneyama et al., 2018). On the other hand, MAX1 homologues of maize and rice, Zm-MAX1b and Os1400 respectively, which also oxidized carlactone to carlactonoic acid, synthesized orobanchol via hydroxylation of 4-deoxyorobanchol (Cardoso et al., 2014; Zhang et al., 2014; Yoneyama et al., 2018). However, At-MAX1 only produced carlactonoic acid and its methyl ester from carlactone (Abe et al., 2014; Yoneyama et al., 2018). Brewer et al. (2016) identified LATERAL BRANCHING OXIDOREDUCTASE (LBO), a branching gene in Arabidopsis which encodes an oxidoreductase-like enzyme of the 2-oxoglutarate and iron (II)-dependent dioxygenase superfamily. LBO acts downstream of MAX1 to produce an SL-active compound which is yet to be chemically described.
Table 1.
Orthology of genes associated with strigolactone biosynthesis and signalling
| Oryza sativa | Arabidopsis thaliana | Petunia hybrida | Medicago truncatula | Striga hermonthica | Protein encoded and function. | Reference(s) |
|---|---|---|---|---|---|---|
| Strigolactone biosynthesis | ||||||
| D27 | At-D27 | – | Mt-D27 | – | β-carotene isomerase: Converts all-trans-β-carotene to 9-cis-β-carotene. | Liu et al. (2011); Alder et al. (2012); Waters et al. (2012a); Challis et al. (2013); Seto et al. (2014); van Zeijl et al. (2015); Bruno and Al-Babili (2016) |
| Os-CCD7/D17/HTD1 | MAX3 | DAD3 | Mt-CCD7 | – | Carotenoid cleavage dioxygenase: Cleaves β-carotene to form an apo-carotenal. | Booker et al. (2004); Zou et al. (2006); Simons et al. (2007); Challis et al. (2013); Bruno et al. (2014); Lauressergues et al. (2015) |
| Os-CCD8/D10 | MAX4 | DAD1 | Mt-CCD8 | – | Carotenoid cleavage dioxygenase: Carlactone production. | Arite et al. (2007); Simons et al. (2007); Zhang et al. (2010); Challis et al. (2013); Lauressergues et al. (2015) |
| Os-MAX1 (Os900, Os1400, Os5100) | MAX1 | – | Mt-MAX1 (Medtr3g104560) | – | Cytochrome P450 cytochrome 711 monooxygenase, carlactone oxidase and orobanchol synthase: Converts carlactone to canonical SLs and carlactone-type SLs. | Booker et al. (2005); Challis et al. (2013); Seto et al. (2014); Zhang et al. (2014); Yoneyama et al. (2018) |
| Os01g0935400 | LBO | – | – | – | 2-Oxoglutarate and Fe(II)-dependent dioxygenase: Converts methyl carlactone to a yet to be identified SL active compound. | Brewer et al. (2016) |
| Strigolactone signalling | ||||||
| D3 | MAX2/ORE9 | PhMAX2a, PhMAX2b | Mt-MAX2 (MTR_4g080020) | Sh-MAX2 | F-box protein: Component of SCF ubiquitin ligase complex required for signal transduction. | Woo et al. (2001); Stirnberg et al. (2007); Challis et al. (2013); Zhao et al. (2015); Yao et al. (2017) |
| Os-D14/D88/HTD2 | AtD14 | DAD2 | Mt-D14 | Sh-HTL7 | α/β Hydrolase: SL perception and hydrolysis. | Simons et al. (2007); Gao et al. (2009); Liu et al. (2009); Hamiaux et al. (2012); Lauressergues et al. (2015); Tsuchiya et al. (2015); de Saint Germain et al. (2016); Marzec et al. (2016); Yao et al. (2017); Yao et al. (2018) |
| D53/D53-LIKE | SMXL6, SMXL7, SMXL8 | – | – | – | Class I Clp ATPase: Poly-ubiquitination and proteolytic targets of SL signalling. Transcriptional repressors of SL responses. | Jiang et al. (2013); Soundappan et al. (2015); Wang et al. (2015) |
Abbreviations: CCD, CAROTENOID CLEAVAGE DIOXYGENASE; D, DWARF; DAD, DECREASED APICAL DOMINANCE; HTD, HIGH-TILLERING DWARF; HTL, HYPOSENSITIVE TO LIGHT; LBO, LATERAL BRANCHING OXIDOREDUCTASE; MAX, MORE AXILLARY GROWTH; ORE9, ORESARA9; SMXL, SUPPRESSOR OF MAX2 1-LIKE.
Fig. 3.
Strigolactone biosynthetic pathway originating from β-carotene in plastids. ① D27 isomerise all-trans-β-carotene to form 9-cis-β-carotene; ② CCD7 cleavage of 9-cis-β-carotene to yield 9-cis-β-apo-10′-carotenal and β-ionone; ③ carlactone is produced from 9-cis-β-apo-10′-carotenal by CCD8; ④ carlactone is exported from the plastid into the cytoplasm; MAX1 catalyses the biosynthesis of ⑤ carlactonoic acid from carlactone and the conversion of carlactonoic acid to ⑥ methyl carlactonoate; ⑦ 5-deoxystrigol; ⑧ strigol directly as seen in moonseed; ⑨ strigol via 5-deoxystrigol as seen in cotton; ⑩ 4-deoxyorobanchol, ⑪ orobanchol directly in cowpea, and ⑫ orobanchol via 4-deoxyorobanchol as in rice.
Recent experimental data show that MAX1 is able to catalyse the biosynthesis of both canonical and non-canonical SLs from carlactone (Fig. 3) (Yoneyama et al., 2018); and both carlactone and carlactonoic acid serve as precursors for the biosynthesis of orobanchol- and strigol-type SLs (Iseki et al., 2017; Yoneyama et al., 2018). Furthermore, the inability of cowpea to convert 4-deoxyorobanchol despite converting carlactone and carlactonoic acid into orobanchol and alectrol, and the fact that 5-deoxystrigol was not converted to strigol in moonseed (Menispermum dauricum) are clear indications that the biosynthesis of hydroxylated SLs does not always require a deoxy SL precursor (Iseki et al., 2017).
Strigolactone transport
Aside those delegated to eliciting local actions within root tissues, there are two major destinations for root-derived SLs: the rhizosphere and the shoot. SLs released into the rhizosphere as part of the root exudate serve as cues to initiate mycorrhizae or nodule formation. They also induce the germination of seeds of root parasitic plants thus mediating such interactions. On the other hand, root-to-shoot transport of SLs serves as a major source of SLs in shoot tissues where they modulate diverse aspects of shoot growth and development. Shoot-ward directional transport of SLs and localized exudation into the rhizosphere is mediated by an ABC subtype G (ABCG) class of transporter, PLEIOTROPIC DRUG RESISTANCE 1 (PDR1) (Kretzschmar et al., 2012; Sasse et al., 2015). Thus far, the only identified SL transporter is PDR1 from Petunia axillaris and P. hybrida. Over expression of PDR1 in P. hybrida upregulates SL biosynthesis while also influencing SL-related responses such as an increase in root biomass, auxin distribution and mycorrhization (Liu et al., 2018). Petunia axillaris PDR1 (Pa-PDR1) exhibits a cell-type-specific polar and asymmetrical localization as it is localized on the apical membrane of hypodermal cells in the root apex but laterally localized on the outer membrane of hypodermal passage cells that occur above the root tip and also serve as entry point for AMF during mycorrhization (Sasse et al., 2015). In stem tissues, Ph-PDR1 expression occurs in the vasculature and nodal tissues in close proximity to the lateral axils but is absent in dormant bud (Kretzschmar et al., 2012). The expression of these transporters is upregulated by endogenous and exogenous cues that are known to elicit SL production and signalling such as auxin signals, limited inorganic phosphate (Pi) and AMF colonization (Kretzschmar et al., 2012). Furthermore, interference with Pa-PDR1 function results in phenotypes that are associated with flawed SL production and signalling (Sasse et al., 2015). The aforementioned observations from localization studies, in addition to impaired SL transport towards the shoot and exudation into the rhizosphere in Pa-pdr1 mutants (Sasse et al., 2015), as well as reduced SL levels in root exudates of Ph-pdr1 mutants (Kretzschmar et al., 2012) together establish the roles of PDR1 in SL transport.
In a bid to determine the root-to-shoot path of SL transport, Xie et al. (2015b) screened xylem saps from several plant species. SLs were not detected in any of the xylem saps although exogenous SLs applied to roots were detected in shoot tissues after 20 h of treatment. Their observations suggest a cell-to-cell movement of SLs (not via the xylem stream) from the root to the shoot. SL transport was further demonstrated to be a structure- and stereo-specific process (Xie et al., 2016). The literature on the significance of SL transport and its regulation in the growth and development of plants as well as plant–microbe interactions is quite extensive and will not be discussed in detail here. Readers are encouraged to consult excellent recent reviews on the subject by Borghi et al. (2016) and Kameoka and Kyozuka (2018).
Strigolactone perception and signal transduction
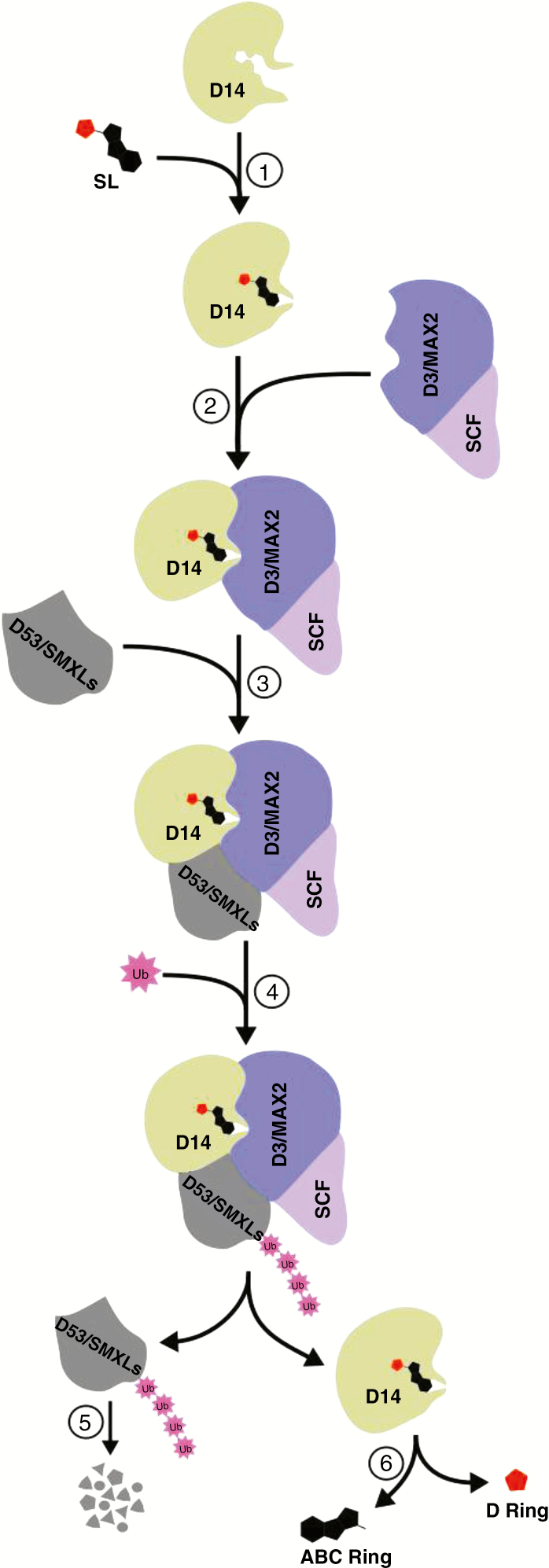
SL signalling is a hormone-activated cascade of events that culminate in the poly-ubiquitination and proteolysis of specific target proteins as well as the hydrolytic inactivation of the hormone. This process is mediated by three main components: DWARF14 (D14), D3 and a Skp1-Cullin-F-box (SCF) E3 ubiquitin ligase complex. The prevailing SL signalling model (Fig. 4) posits that D14 – the SL receptor/perception protein – is activated by the binding of an SL molecule, and the ligand (SL)-bound D14 then forms a signalling complex with other signalling partners leading to the transduction of the hormonal signal and finally the hydrolysis of the bound SL at the enol–ether bridge to deactivate the hormone (Waters et al., 2012b; Hamiaux et al., 2012; Marzec et al., 2016).
Fig. 4.
A model of strigolactone perception and signal transduction pathway. ① SL binds in the catalytic cleft of D14 and induces a destabilization/conformational change in D14; ② SL-induced destabilization of D14 facilitates D14 interaction with SCFD3; ③ SCFD3-D14 recruits a target protein (e.g. D53 or SMXLs); ④ D53/SMXLs undergo polyubiquitination; ⑤ proteasomal degradation; ⑥ D14 deactivates the bound SL molecule by hydrolytic cleavage of the enol–ether bridge and releases the ABC and D rings.
SL signal perception involves ligand (SL) docking in the catalytic cleft of D14, which results in conformational changes (open-to-closed state transition) in D14 (Yao et al., 2016, 2018; Seto et al., 2019). D14 belongs to the α/β serine hydrolase superfamily (Hamiaux et al., 2012; Nakamura et al., 2013; Tsuchiya et al., 2015) and its hydrolytic activity is mediated by a serine–histidine–aspartate catalytic triad located within its active site (Hamiaux et al., 2012; Zhao et al., 2013). The conformational changes induced in D14 by ligand binding are characteristic of a catalytically inactive state and are due to rearrangement of the four top helices that constitute a V-shaped lid domain as well as an alteration in the shape of the catalytic triad due to a shift by the loop bearing the catalytic Asp residue (Zhao et al., 2013; Yao et al., 2016; Seto et al., 2019).
Interactions/complex formation involving SL-bound D14, F-box protein and target proteins leading to the poly-ubiquitination and proteasomal degradation of target proteins are believed to underpin SL signal transduction. In its active state, D14 associates with D3 – the F-box protein component of the SCF E3 ubiquitin ligase complex (Stirnberg et al., 2007; Zhao et al., 2013, 2015) – and then a specific protein substrate is recruited for poly-ubiquitination and 26S proteasome-mediated degradation (Jiang et al., 2013; Zhao et al., 2015). The lid domain of D14 interacts with D3 (Zhao et al., 2013) via a C-terminal α-helix of a leucine-rich repeat (LRR) domain on D3, with D14 catalytic cavity facing the LRR domain of D3 (Yao et al., 2016; Shabek et al., 2018). The SL-induced D14–D3 interaction further destabilizes D14 (Zhao et al., 2015), but how this specifically bears on SL signal transduction is yet to be described in detail. According to Shabek et al. (2018), SCFD3–D14 forms a stable ternary complex with a target protein (e.g. D53) via an ATPase domain on D53. The target protein undergoes proteasome-mediated degradation after poly-ubiquitination (Jiang et al., 2013; Soundappan et al., 2015; Wang et al., 2015).
After SL signal transduction, D14 hydrolyses the bound SL molecule, thus inactivating it. The hydrolysis of SL is induced in order to restore the catalytic triad to its open state conformation (Seto et al., 2019). A nucleophilic attack on the enol–ether bridge of the docked SL molecule separates the ABC ring from its D ring. The suggestions that D14 is a single-turnover enzyme and that SL hydrolysis and a SL-derived covalently linked intermediate molecule (CLIM) were central or necessary for SL signal transduction (de Saint Germain et al., 2016; Yao et al., 2016, 2018; Saeed et al., 2017) have been subjects of serious debate. These views have been shown to be inconsistent with recent experimental observations. First, Carlsson et al. (2018) – after re-analysing structural data including electron density maps calculated from coordinates of the crystallized SL-induced At-D14-D3-ASK1 complex reported by Yao et al. (2016) – concluded that what was previously thought to be bound to the active cleft of D14 was not CLIM but probably a component of the crystallization reagent. By monitoring the timing of At-D14 activation relative to SL hydrolysis, Seto et al. (2019) demonstrated that the intact SL molecule, not CLIM nor the hydrolysis products, triggers the conformational changes in At-D14 and SL signal transduction. Similarly, functional analysis of At-D14 catalytic triad mutants with reduced hydrolase activity showed that these mutants were still capable of SL signal transduction and complementation of the At-d14 mutant phenotype despite lacking their hydrolase activity (Seto et al., 2019). In the same study, missense mutation of a highly conserved amino acid in At-D14 and Os-D14 significantly influenced SL signal transduction without affecting the hydrolytic function of D14. Together, these are clear indications that SL hydrolysis during SL signalling is not required for signal transduction but serves to deactivate the hormone.
SCFD3–D14 target proteins such as SUPPRESSOR OF MAX2 1 (SMAX1) (Stanga et al., 2013), D53 (Jiang et al., 2013; Zhou et al., 2013) and SUPPRESSOR OF MAX2-LIKE6–8 (SMXL6, SMXL7 and SMXL8) are known repressors of downstream SL responses (Soundappan et al., 2015; Wang et al., 2015). Strigolactone-D14-D3 facilitated proteasomal degradation of these repressors paves way for the activation of the hitherto repressed transcription factors (TFs), which in turn leads to the transcriptional activation of associated genes. Examples of such TFs repressed by D53/SMXLs are BRANCHED1 (BRC1) (Soundappan et al., 2015), TEOSINTE BRANCHED1 (TB1) (Liu et al., 2017), IDEAL PLANT ARCHITECTURE1 (IPA1) (Song et al., 2017) and SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3/17 (SPL3/17) (Liu et al., 2017). BRI1-EMS-SUPPRESSOR1 (BES1), a positive regulator in brassinosteroid signalling, was suggested to be targeted for degradation via SCFMAX2 (Wang et al., 2013), although a contrasting observation was recently reported by Bennett et al. (2016). In addition, D53/SMXL interaction with the TOPLESS (TPL)/TPL-RELATED (TPR) family of transcriptional repressors via Ethylene-responsive element-binding factor-associated Amphiphilic Repression (EAR) motifs also suggests a putative role for SL signalling in TPL/TPR-mediated regulation of gene expression (Jiang et al., 2013; Soundappan et al., 2015; Wang et al., 2015; Ma et al., 2017).
Major strigolactone-mediated responses in plants
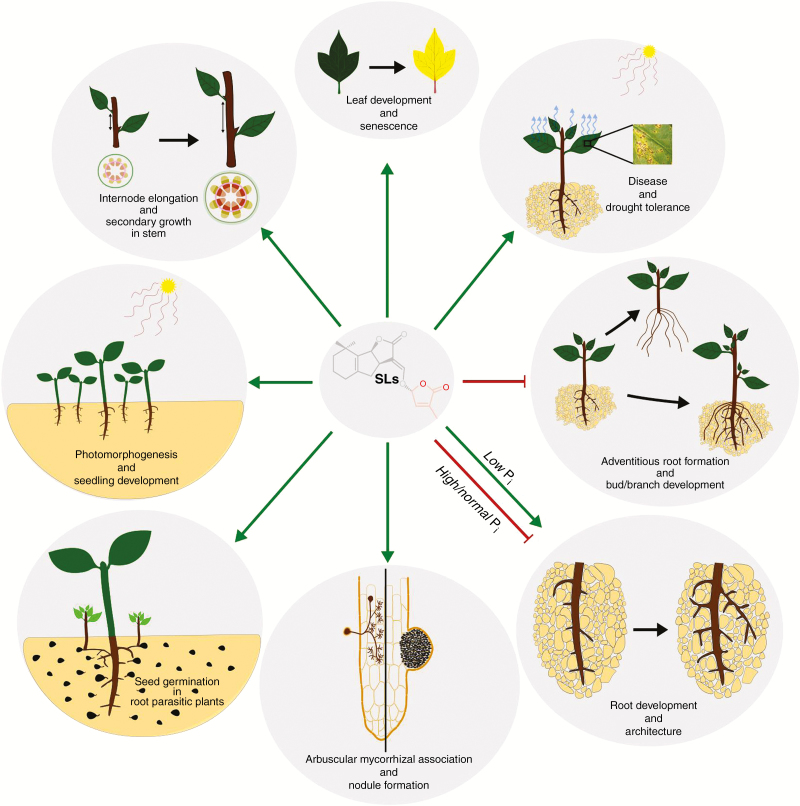
SLs mediate the regulation of a broad group of developmental processes in plants (Fig. 5). Some of the major responses in plants elicited by SLs are highlighted below.
Fig. 5.
Major growth and developmental processes mediated by strigolactones in plants.  , stimulatory effect/regulation;
, stimulatory effect/regulation;  , inhibitory effect/regulation.
, inhibitory effect/regulation.
Germination stimulants for seeds of root parasitic plants.
The first SL bioactivity to be demonstrated was the induction of seed germination in root parasitic plants. Strigol, strigyl acetate and sorgolactone act as germination stimulants for Striga species. These compounds are secreted in root exudates of cotton, maize and rice, among others (Cook et al., 1972; Hauck et al., 1992). Alectrol and orobanchol were identified as germination stimulants for Alectra vogelii (Müller et al., 1992) and Orobanche species respectively, both of which parasitize some temperate crops (Yokota et al., 1998). Brun et al. (2018) provide an extensive review on this aspect of SL bioactivity.
Strigolactones as chemical cues for plant–microbe symbiotic interactions.
Another early discovery is SL-induced hyphal branching in AMF. Akiyama et al. (2005) identified 5-deoxystrigol in root exudates of Lotus japonicus as a cue that triggered hyphal branching. Other SLs like sorgolactone, strigol, orobanchol (Akiyama et al., 2010) as well as carlactone and its derivatives (Mori et al., 2016) also induced hyphal branching. In addition to the induction of hyphal branching, the roles of SLs in AM symbiosis are evident from the following observations. First, the production of short-chain chitin oligomers, a group of mycorrhizal factors that are believed to elicit the symbiotic signals necessary for fungal root colonization in plants, is enhanced in AMF by GR24 (Genre et al., 2013). Second, fungal metabolism – evident as increased ATP production/respiration and mitochondrial division, up-regulation of the expression of mitochondrial genes as well as proteins associated with other cell components – is also induced by SLs (reviewed by Lanfranco et al., 2018). Finally, in addition to serving as molecular cues and their effects on the mycosymbiont in the stages just before fungal colonization of the root, SLs also perform endogenous roles within the root during mycorrhizal development. This is evident from the fact that SL-signalling mutants show defective mycorrhizal phenotypes. For instance, rms4 mutants (defective in SL perception but having relatively normal SL content) have significantly reduced mycorrhizal colonization compared to wild-type (WT) plants (Foo et al., 2013). In a similar manner, Sl-IAA27-silenced tomato lines with undetectable SL content showed mycorrhizal defects characterized by a decrease in infection frequency and arbuscule abundance (Guillotin et al., 2017). Furthermore, AMF-inoculated d3-1 rice mutants showed defects in the early stages of AM symbiosis and an almost abolished expression of AM-inducible genes (Yoshida et al., 2012). Despite these observations, the endogenous roles of SLs in mycorrhizal development remain largely elusive. It is of note that SL production/exudation does not translate into an ability to form AM associations as non-AM host plants also produce SLs that induce AM activities and exogenous supply of SLs does not result in AM formation in non-host plants (Mori et al., 2016).
The early stages of root nodule organogenesis are characterized by an exchange of chemical signals between the bacterial symbiont and the host plant. Chemical cues exuded into the rhizosphere by the host plant trigger the expression of bacterial nodulation genes which direct the production of signal molecules such as nodulation (Nod) factors which when perceived by the host plant elicit nodule organogenesis such as root hair curling, infection thread formation, and the attendant division of inner cortical and pericycle cells to form nodule meristem. SL-deficient mutants form fewer nodules, and treatment with GR24 increased nodule number in both mutants and WT plants (Foo and Davies, 2011; Foo et al., 2013; De Cuyper et al., 2015). Unlike in AM symbiosis, SL response mutants displayed enhanced nodulation with increased nodule number (Foo et al., 2013). Attempts to establish how SLs feature in nodulation revealed that Nod factors induce rapid expression of SL biosynthesis genes (Liu et al., 2011; van Zeijl et al., 2015) and SL actions are limited to the formation of infection threads and the expression of some genes that are induced by the Nod factor signal cascade (McAdam et al., 2017).
Regulation of photomorphogenesis.
How SLs feature in photomorphogenesis is still emerging, with conflicting reports of SL influences in this aspect of plant development. Shen et al. (2012) showed that MAX2 interacts with gibberellin and ABA signalling to mediate light-induced seed germination and CONSTITUTIVE PHOTOMORPHOGENESIS 1 (COP1) to regulate photomorphogenesis, but SLs were not involved. Similarly, seedling photomorphogenic responses were not affected by strigolactone deficiency in Arabidopsis (Nelson et al., 2011) or in pea (Urquhart et al., 2015). Despite this evidence, recent studies indicate that SLs are active in photomorphogenesis. For instance, SLs regulate hypocotyl elongation via several processes that require the actions of cryptochrome, phytochrome (Jia et al., 2014) and STH7/BBX20, a member of the B-box zinc-finger protein family, which are known downstream effectors of photomorphogenesis (Thussagunpanit et al., 2017).
Regulation of shoot branching and plant architecture.
In shaping the overall structure of plants, SLs control bud activation/branching, secondary growth and root morphology. SL-deficient and signalling mutants exhibit excessive shoot branching, and direct application of SL to axillary buds fully inhibits bud development/branching (Braun et al., 2012; Dun et al., 2013). The influence of SLs on bud development is predominantly mediated via either or both of these two mechanisms (reviewed by Waters et al., 2017). One involves SL-induced direct up-regulation of a broad group of conserved negative regulators of shoot branching such as BRC1 (Dun et al., 2013), its orthologue in rice, FINE CULM1 (FC1) (Xu et al., 2015) and IPA1 (Song et al., 2017). In addition, BES1 (a gene that encodes a positive regulator of shoot branching) undergoes SL-SCFMAX2-induced proteasomal degradation (Wang et al., 2013). The other mechanism is auxin dependent and involves SL-induced modulation of polar auxin transport (PAT)/canalization (see discussion below).
In rice, FC1 acts downstream of SLs to suppress tillering given that fc1 mutants are insensitive to high doses of GR24 when compared to WT plants with respect to bud growth and tillering. However, unlike BRC1, FC1 expression is not induced by GR24 treatment (Minakuchi et al., 2010), but rather FC1 (reported as Os-TB1) perturbs the suppression of D14 transcription by Os-MADS57, a TF of the MADS-domain family, by binding to Os-MADS57 (Guo et al., 2013). This shows that the interactions between SL signal elements and the BRC1 family of TFs is complex and requires further investigations to develop a lucid picture of how they function to regulate shoot branching.
To promote internode/stem elongation and secondary growth, SLs stimulate cell division/increase in cell number (de Saint Germain et al., 2013) and cambial activity within the stem (Agusti et al., 2011). In addition to shoot elongation, leaf margin serrations in Medicago truncatula are also controlled by SLs in a process that appears to be dependent on auxin transport (Lauressergues et al., 2015). Leaf senescence, which marks the last stage of leaf development, is also regulated by SLs (Yamada et al., 2014). Leaves of the oresara9 (ore9) mutant of Arabidopsis exhibit delayed onset of senescence. At-ORE9 is identical to At-MAX2 (Woo et al., 2001; Stirnberg et al. 2007). Leaf senescence is accelerated under phosphate limiting conditions and in the presence of exogenous SL (Yamada et al., 2014; Ueda and Kusaba, 2015; Tian et al., 2018).
SLs mediate the tuning of crucial aspects of root architecture. This is evident in: the restoration of normal primary root length in SL mutants of Arabidopsis by GR24 (Ruyter-Spira et al., 2011); GR24-induced suppression of adventitious root (AR) formation in Arabidopsis and pea (Rasmussen et al., 2012) while promoting crown root growth and AR elongation in rice (Arite et al., 2012; Sun et al., 2014); the repression of lateral root (LR) formation (Kapulnik et al., 2011a, b; Ruyter-Spira et al., 2011, De Cuyper et al., 2015); and the regulation of root hair elongation (Kapulnik et al., 2011a, b). Sun et al. (2016) and Marzec and Melzer (2018) provide detailed reviews of SLs as regulators of root development.
Proteoid (cluster) roots, a unique morphological adaptation of roots to limiting nutrient conditions, are found in certain species of families such as Casuarinacea, Fabaceae and Myricacea but are almost ubiquitous among Proteaceae (Watt and Evans, 1999; Shane and Lambers, 2005). The development of this particular root structure has been linked to limiting phosphate conditions and is driven by phosphate supply (Neumann et al., 2000; Felderer et al., 2015). Interestingly, SL biosynthesis is induced in plants under low phosphate conditions (Yoneyama et al., 2007; Koltai 2013). Despite the aforementioned, and that SLs feature prominently in the regulation of root architecture, experimental data on the role of SLs in proteoid root development are still lacking.
Mediation of plant tolerance to nutrient deficiency.
Under limited nutrient conditions, especially in phosphate- and nitrogen-deficient soils, plants increase SL biosynthesis which becomes evident as an increase in SL content of root exudates (Yoneyama et al., 2007, 2012; Koltai, 2013, 2015). This is significant because high levels of SLs facilitate root branching (Ruyter-Spira et al., 2011) while also increasing the molecular signal content of root exudates for the establishment of symbiotic associations with AMF. Under limiting Pi conditions, Mayzlish-Gati et al. (2012) found both SL-deficient (max4-1) and SL-insensitive (max2-1) mutants had reduced root hair densities. Also, treatment with GR24 increased root hair density in max4-1 to similar levels as in the WT but did not significantly alter root hair densities in max2-1. These processes promote efficient mobilization of limited nutrients in soils (Akiyama et al., 2010; Kapulnik et al., 2011a; Liu et al., 2018). SLs may also modulate Pi utilization in addition to Pi acquisition in plants under limited Pi. Czarnecki et al. (2013) suggested that SL-induced suppression of shoot branching in Arabidopsis in response to Pi deficiency may be a means to reduce Pi utilization. SLs also regulate plant responses to nitrogen supply. Both SL-deficient (d10 and d27) and SL-insensitive (d3) mutants of rice lack the root responses to low Pi and nitrogen concentrations observed in the WTs (Sun et al., 2014). In addition, GR24 treatments compensate for the reduced responses in SL-deficient mutants but not in the SL-insensitive mutants. Genetic evidence for the role of SLs in plant responses to nitrogen deficiency was also provided by Ito et al. (2016). They reported an alteration in the expression of MAX3 and MAX4 under nitrogen-deficient conditions.
Mediation of plant responses to abiotic and biotic stressors.
The role of SLs in plant responses to abiotic stress cues still requires more research attention. However, available data portray SLs as positive regulators of abiotic stress responses. Stomatal closure, a process that fosters drought tolerance by reducing water loss via transpiration, is induced by SLs (Lv et al., 2018; Zhang et al., 2018). Similarly, SL-deficient and SL-responsive mutants exhibited hypersensitivity to drought (Bu et al., 2014; Ha et al., 2014; Li et al., 2016) with shoots receiving little SL from the root behaving as if they were under mild drought stress even when supplied with enough water (Visentin et al., 2016). Furthermore, treatment with exogenous SLs rescued drought-sensitivity in SL-deficient mutants but not in signalling mutants (Ha et al., 2014) while also enhancing drought tolerance in WTs. Under salinity stress, the actions of ABA in ameliorating the deleterious effects of salinity was seen to be dependent on SL biosynthesis and signalling (Ren et al., 2018).
In establishing the roles of SLs in the regulation of plant responses to biotic stressors, Cheng et al. (2017) showed that SL-deficient tomato plants displayed increased susceptibility to Phelipanche ramosa infection and rapid development of the parasite. A report by Torres-Vera et al. (2014) also indicated that SL-deficient tomato mutants, Sl-ccd8, were susceptible to foliar fungal pathogens, Botrytis cinerea and Alternaria alternata, and this was found to be coupled to a reduction in the concentrations of defence-related hormones such as jasmonates, salicylic acid and ABA.
Strigolactone interactions with other phytohormones
Strigolactone crosstalk with auxin signalling.
Endogenous auxins act in concert with other hormones, either synergistically or antagonistically, to regulate biological processes in plants (Naseem et al., 2015; Liu et al., 2017; Leyser, 2018). Several studies have evinced links between auxin and SL signalling. Auxin influence on SL signalling is largely via modulating SL biosynthesis, which in turn may act as a second messenger in eliciting some auxin-dependent responses. The expression of CCD7- and CCD8-encoding genes are up-regulated by auxin to exert control on shoot branching (Zou et al., 2006; Arite et al., 2007). Hayward et al. (2009) found these to be an AUXIN RESISTANCE1 (AXR1)-dependent process. Experimental data are lacking on the role(s) of auxin in SL flux and transport.
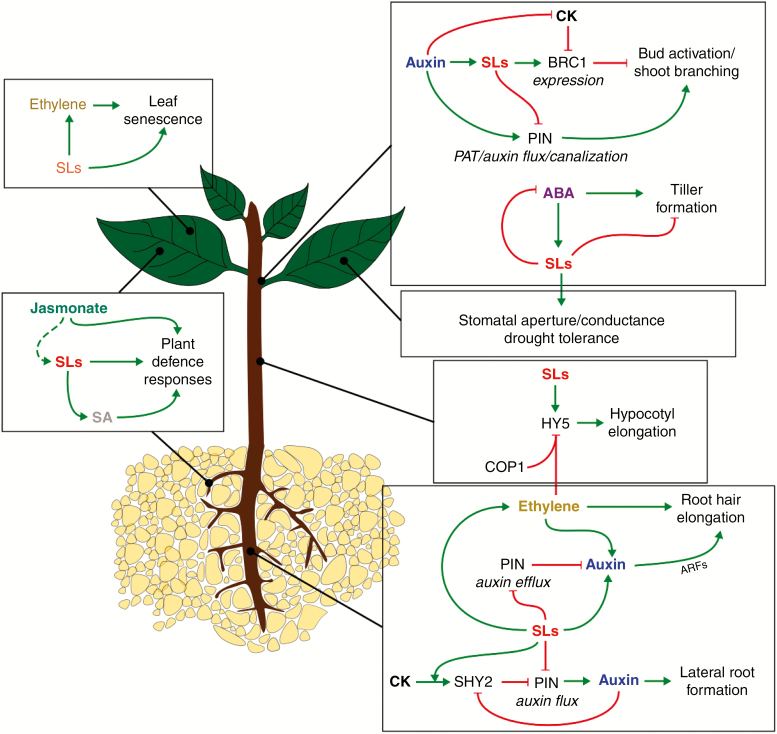
In regulating shoot branching, SLs interfere with PAT/distribution and, in effect, auxin canalization (Bennett et al., 2006; Ruyter-Spira et al., 2011; Sun et al., 2014) (Fig. 6). Auxin flux/canalization is a significant factor in bud activation and outgrowth (Prusinkiewicz et al., 2009; Balla et al., 2011, 2016). SL-mediated down-regulation of the expression of PIN-FORMED (PIN) proteins, a family of transporters responsible for auxin influx and efflux from cells, and their polarized localization on the plasma membrane (Crawford et al., 2010; Shinohara et al., 2013; Hu et al., 2018) dampens auxin sink strength of the PAT stream, thus hampering auxin export from buds and canalization, which ultimately results in the repression of bud development. However, reports that diminished auxin transport in pea had little inhibitory effects on bud outgrowth in SL-deficient mutants and the ability of SLs to inhibit bud outgrowth in pea plants with impaired auxin transport (Brewer et al., 2015) indicate that although PAT/canalization may be used in SL-mediated modulation of branching, SLs can also act independent of this mechanism.
Fig. 6.
Strigolactone interactions with abscisic acid, auxin, cytokinin, ethylene and salicylic acid. Abbreviations: ABA, abscisic acid; BRC1, BRANCHED1; CK, cytokinin; COP1, CONSTITUTIVE PHOTOMORPHOGENESIS1; HY5, ELONGATED HYPOCOTYL5; PDR1/6, PLEIOTROPIC DRUG RESISTANCE1/6; PIN, PIN-FORMED; SHY2, SHORT HYPOCOTYL2; SA, salicylic acid; SLs, strigolactones.  , stimulatory effect/regulation;
, stimulatory effect/regulation;  , possible stimulatory effect;
, possible stimulatory effect;  , inhibitory effect/regulation.
, inhibitory effect/regulation.
Experimental observations have revealed inconsistencies in the two models of SL action in bud activation. Among these are: the uncoupling of the TB1 sub-network from SL signalling in maize (Guan et al., 2012); the insensitivity of FC1 expression to GR24 (Minakuchi et al., 2010); buds lacking BRC1 expression remaining inhibited and being sensitive to inhibition by SLs, buds with high BRC1 transcripts being active (Seale et al., 2017); and conflicting reports of SL influence on auxin transport and canalization mentioned above. All these call for a rethink of these models with a view to developing one which takes these limitations into consideration. The way forward may lie in the events that occur upstream, in parallel with and/or downstream of BRC1/FC1/TB1 activities. Auxin canalization and the repression of branching factors may be necessary for bud activation/development, and some other mechanisms which may be under the influence of these two hormones may determine whether an activated bud proceeds to develop into a branch. Research efforts tailored towards investigating how nutrient partitioning affects development among competing buds will also provide progress in illuminating this aspect of plant development. For instance, sucrose features significantly in apical dominance, promoting bud release and down-regulation of BRC1 (Mason et al., 2014), so how SL and other key hormonal signals are integrated with sugar signals during bud activation should be a subject for further investigation.
The regulation of secondary growth by SLs occurs by positively modulating interfascicular cambial activity (Agusti et al., 2011). The slight induction of cambium-like cell division in max2 plants by GR24 treatment, which contrasts with the complete insensitivity observed in other processes, suggests other factors act in parallel with MAX2-dependent SL signalling to affect secondary growth (Agusti et al., 2011). In addition, observations made using max and auxin response mutants suggest SLs affect secondary growth directly, independent of auxin accumulation, and they act downstream of auxin. This then raises the question of how SLs elicit cambial activity and secondary growth if auxin-induced cambial activity is not employed.
As with shoot branching, SLs interact with auxin to control root development by modulating auxin sensitivity (Mayzlish-Gati et al., 2012), PAT from shoot to root (Sun et al., 2014) and auxin flux within root tissues (Koren et al., 2013; Kumar et al., 2015). Here, auxin signal modules act downstream of SLs. The early steps in LR formation – which includes the priming of pericycle cells in the basal meristem to specify them for LR initiation, transition from founder cells to LR initiation and primordium formation, and the development of LR primordium until LR emergence – are all driven by local auxin gradients and response maxima (reviewed by Olatunji et al., 2017). Since GR24 have been shown to affect the polarization and localization of PIN proteins as well as LR-forming potential (Ruyter-Spira et al., 2011; Pandya-Kumar et al., 2014; Kumar et al., 2015), SLs probably influence these processes by modulating local auxin flux and homeostasis that is required to establish the auxin response maxima.
Similarly, the inhibitory effects of SLs on auxin efflux via SLs modulation of PIN activities also result in the accumulation of auxin within primary root meristem cells. This promotes cell division and number, expansion of meristem and transition zone sizes and consequently primary root length (Ruyter-Spira et al., 2011). Similar crosstalk between SLs and auxin occurs in the regulation of root hair development where SLs induce an increase in auxin accumulation/levels within epidermal cells by modulating auxin efflux (Koltai et al., 2010) to promote root hair elongation. ROOT HAIR DEFECTIVE 6-LIKE 4 (RSL4) is an auxin-responsive TF which positively regulates root hair formation by controlling genes associated with root hair morphogenesis and is suggested to be an integrator of internal and external cues (Yi et al., 2010). Pi deficiency significantly enhances RSL4 synthesis and half-life (Datta et al., 2015). Against these backgrounds, it is possible that RSL4 might serve as a nexus for SL-auxin crosstalk in modulating root hair development. Experimental data suggest ethylene features prominently in SL-auxin crosstalk in the regulation of root hair growth (Kapulnik et al., 2011b) (see section on Strigolactone crosstalk with ethylene signalling).
In regulating AR formation, SLs and auxin elicit opposing effects. The role of auxin in promoting AR formation and the inhibition of the same by SLs have been shown experimentally. In a bid to unravel the nature of SL–auxin crosstalk in AR development, Rasmussen et al. (2012) discovered that GR24 can partially revert the stimulatory effects of auxin on AR formation while auxin increased the number of ARs in max mutants of Arabidopsis and pea. Of note is the fact that the inhibitory effects of SLs occurred even in the presence of elevated auxin content. These findings suggest both hormones act independently and SL suppression of AR formation might not be mediated by limiting local auxin build-up. In a rather contrasting manner, rice mutants flawed in SL biosynthesis and signalling exhibited reduced AR formation, and GR24 treatment increased AR number in SL-deficient mutants but not in signalling mutants (Sun et al., 2015). Modulation of PAT also seemed to be employed to regulate AR formation in rice. Taken together, it appears SL–auxin crosstalk in this process is a complex one and the actions of SLs in regulating AR formation might be species-dependent. Further investigations of this process in other species and the effects of SL signals on the expression and stability of downstream targets of auxin signals involved in AR development, such as ADVENTITIOUS ROOTLESS 1 (an auxin‐responsive factor involved in auxin‐mediated cell dedifferentiation and AR formation; Liu et al., 2005), will be invaluable in drawing valid conclusions on SL–auxin interactions in AR development.
Plant Pi status is critical in SLs’ influence on root development and architecture. Under normal/high Pi levels, LR development is inhibited but stimulated under Pi-limiting conditions (Ruyter-Spira et al., 2011; Mayzlish-Gati et al., 2012; Jiang et al., 2015). Although it is clear that SLs serve to translate ambient signals into growth cues in the root, the precise mechanism that enables SLs to delineate their influence under normal Pi levels from those under Pi-limiting conditions is yet to be fully described. A possible avenue for exploration is how SLs influence ethylene signalling under Pi status because ethylene is known to stall auxin-driven LR development (Lewis et al., 2011). In addition, the molecular and/or genetic events that relay Pi status cues to induce SL biosynthesis and signalling await full characterization.
The hormonal interactions between auxin and SLs during AM and rhizobial symbiosis are not well characterized. Experimental observations by Foo (2013) suggested that auxin regulates the early stages of AM symbiosis by modulating SL levels. Recently, the expression of Sl-IAA27 – a downstream component/repressor of auxin signalling – was shown to be up-regulated by AM colonization and Sl-IAA27-silencing adversely affected AM colonization (Guillotin et al., 2017). Interestingly, aside the down-regulation of genes involved in SL biosynthesis in Sl-IAA27-silenced plants, treatment with GR24 complemented the mycorrhizal defect by increasing infection frequency and arbuscule abundance. These demonstrate clearly the existence of a yet-to-be identified hormonal signal link between auxin and SLs in mycorrhizal development. The signal channel(s) by which Sl-IAA27-induced SL production and signalling influence arbuscule development also remains to be elucidated.
Computer modelling and experimental data have established the crucial role of auxin accumulation in nodule organogenesis (reviewed by Kohlen et al., 2017). Given the impact of SLs in modulating auxin transport and flux, it is possible that SLs might elicit some influence on nodule development via this channel.
Strigolactone crosstalk with cytokinin signalling.
The interactions between cytokinin and SLs seem to vary with physiological processes. They act independent of each other in adventitious rooting (Rasmussen et al., 2012), antagonistically in bud activation and shoot branching (Dun et al., 2012; Xu et al., 2015; Manandhar et al., 2018), but synergistically in the regulation of LR development (Jiang et al., 2015). Both hormones regulate expression of the other’s biosynthesis genes (Dun et al., 2012; Zhang et al., 2010). While SL-associated genes have been shown to mediate cytokinin biosynthesis and export from the root (Beveridge, 2000; Dun et al., 2012), reports on cytokinin influence on SL transport are lacking.
In mediating bud activation/shoot branching, SLs and cytokinin interact directly in buds with their actions converging at the transcriptional regulation of BRC1 in Arabidopsis and pea (Aguilar-Martinez et al., 2007; Braun et al., 2012; Dun et al., 2012) and FC1 in rice (Xu et al., 2015) (Fig. 6). Given the above, any factor that influences SLs and cytokinin homeostasis in the bud will determine bud development. This is where auxin features; its induction of SL biosynthesis but inhibition of cytokinin biosynthesis in the root ensures more SLs get to the shoot and repress branching. To shed more light on cytokinin–SL crosstalk in regulating bud development, the influence(s), if any, of SLs/SL signals on downstream cytokinin signal components/pathways involved in bud activation must be elucidated. In resolving the role of sucrose mentioned above, other crucial questions to be investigated include: does sucrose supply affect SLs and/or cytokinin homeostasis in the bud? If yes, what are the molecular mechanisms that drive this process and at what point in this cascade of events is the sucrose signal integrated?
On the other hand, cytokinin signalling components are required in GR24-induced suppression of LR development (Jiang et al., 2015). The repression of LR development by GR24 under sufficient Pi, by down-regulating the expression of PIN genes, is mediated by AHK3, ARR1 and ARR12 via SHORT HYPOCOTYL2 (SHY2). Because auxin transport is required to develop an auxin gradient, which is vital for the induction of LR initiation from pericycle founder cells, SHY2 repression of PIN activity stalls LR formation (Goh et al., 2012). A loss-of-function mutation in SHY2 results in an insensitivity to GR24 with respect to LR development (Koren et al., 2013). SHY2 is also central to the balancing of auxin and cytokinin signalling, with cytokinin promoting its expression and auxin promoting its degradation (Dello Ioio et al., 2008; Moubayidin et al., 2010). It is clear that SHY2 acts as a nexus for the integration of auxin, cytokinin and SL signalling to regulate LR development in Pi-sufficient conditions, although whether SLs use this same channel and crosstalk to promote LR development under Pi-limiting conditions is yet to be determined.
The relative rate of cell division to cell differentiation is a key driver of growth in root meristem. Growth, fostered by cell division, is repressed when the rates of cell division and differentiation reach a balance after final meristem size is attained. Cytokinin promotes cell differentiation and hence reduces meristem size (Dello Ioio et al., 2007, 2008) while GR24 promotes an increase in meristem cell number and size (Ruyter-Spira et al., 2011; Koren et al., 2013). As with LR development, SHY2 could be a converging point for the antagonistic interplay between cytokinin and SLs in regulating primary root growth. This is supported by observations such as the insensitivity of a loss-of-function mutant, shy2-31, to GR24 with respect to meristem cell number/size (Koren et al., 2013); the induction of SHY2 transcript expression by cytokinin (Dello Ioio et al., 2008; Moubayidin et al., 2010); and the reduced sensitivity of shy2-31 and max2 to cytokinin (Koren et al., 2013). However, how both hormones use a single channel to elicit the repression of LR formation but induce opposing effects during primary root growth needs further investigation.
Strigolactone crosstalk with abscisic acid signalling.
With an increase in experimental data suggesting an active role for SLs in the mediation of plant responses and resilience to abiotic and biotic stressors, it is expected that SLs might interact, either directly or indirectly, with ABA in the regulation of adaptive stress responses in plants. So far, three highpoints can be inferred from available data with regard to ABA–SL interactions. First, ABA acts upstream of SLs and SL production/signalling is required and modulated to elicit ABA-mediated responses. In support of this view are the observations that the expression of SL biosynthesis genes and SL content are significantly lowered in ABA-deficient plants (López‐ Ráez et al., 2010; Wang et al., 2018); SL-deficient and signalling mutants are hypersensitive to drought stress, are less sensitive to exogenous ABA (Ha et al., 2014; Visentin et al., 2016) and exhibit reduced stomatal sensitivity to ABA (Bu et al., 2014; Liu et al., 2015; Lv et al., 2018); an increase in stomatal aperture and water loss caused by infection-induced increases in ABA levels were not seen in SL-deficient lines (Cheng et al., 2017); and, finally, ABA up-regulated the expression of SL production and signalling genes to mediate tolerance to salt stress in Sesbania cannabina, but was only able to induce partial and transient increases in salt tolerance of plants treated with an inhibitor of SL synthesis (Ren et al., 2018). Second, SL and ABA interactions are antagonistic in regulating certain aspects such as shoot architecture and fruit ripening. ABA suppresses the expression of SL biosynthesis and signalling genes to enhance tiller formation (Wang et al., 2018). On the other hand, rather than promoting ABA action, exogenous application of GR24 markedly inhibited ABA-induced accumulation of sugars and anthocyanins in grape berries (Ferrero et al., 2018). Finally, in addition to being a requirement for ABA-mediated responses, SLs may also serve as a feedback channel in modulating ABA signalling. This is evident from reports that treatment with GR24 resulted in the reduction of endogenous ABA concentrations: GR24 inhibited the stress-induced increase in ABA levels, repressed the transcriptional activation of ABA biosynthetic genes in Lotus japonicas root (Liu et al., 2015) and up-regulated the expression of ABA catabolic genes but not biosynthetic genes (Ferrero et al., 2018). Also in support of this view is the observed high ABA content in SL-deficient and SL-insensitive mutant rice plants with greater tolerance to drought (Haider et al., 2018).
Despite all the above, some experimental observations suggest inconsistencies. For instance, SL-deficient mutants did not display the rapid water loss and hypersensitivity to ABA and drought observed in SL-signalling mutants, thus suggesting MAX2 signalling, but not SLs, is involved in drought responses (Bu et al., 2014). These allude to the existence of a complex interaction between SLs, ABA and their downstream signalling components that only further extensive investigations would unravel. Given that the expression and accumulation of ELONGATED HYPOCOTYL 5 (HY5) (a negative TF and an established integrator of light and ABA responses; Chen et al., 2008) is under the control of SLs (Jia et al., 2014), it is possible that HY5 might be one of the missing links in ABA–SL crosstalk. To further determine if in fact SLs serve as feedback modulators of ABA actions, the idea that SL signals might interfere and dampen ABA activity by influencing Type 2C protein phosphatases and SNF1-related protein kinases, key components of ABA signalling, is worth investigating.
Strigolactone crosstalk with gibberellin signalling.
Although still largely unclear, SL–gibberellin crosstalk is implied by some experimental observations. For instance, Lantzouni et al. (2017) reported an additive transcriptional change in an overlapping set of genes in response to gibberellin and GR24. Their data also suggested an antagonistic effect by gibberellin on SL-induced up-regulation of some ABC-type transporters. Furthermore, in silico analysis showed that SL biosynthesis is under gibberellin regulation (Marzec and Muszynska, 2015) which was later demonstrated experimentally to be mediated via a GID1-DELLA signal pathway (Ito et al., 2017). In the control of branching and tillering, the observations that ORYZA SATIVA HOMEOBOX 1 (OSH1) expression is up-regulated in highly branched gibberellin-deficient mutants (Lo et al., 2008) and SL-signalling mutants (Gao et al., 2009) suggests the sharing of a similar molecular mechanism by SLs and gibberellin, although this has yet to be demonstrated experimentally.
Attempts have been made to find a direct molecular link between downstream components of gibberellin signalling and those of SLs. For instance, D14 interacts with SLENDER RICE1 (SLR1) – a DELLA protein which is a downstream signal component and repressor of gibberellin signalling – in an SL-dependent manner (Nakamura et al., 2013). Despite this, SL-induced degradation of SLR1 is yet to be demonstrated experimentally and the functional relevance/downstream effect(s) of this interaction are still largely unknown. Recent attempts at finding a molecular nexus between SL and gibberellin signalling have yielded data that support a functional independence between both hormones. For instance, the stability (Bennett et al., 2016) and accumulation (Lantzouni et al., 2017) of DELLA proteins were not affected by SL signalling. Experimental data are lacking regarding the influence of SL signals on the stability and transcriptional regulation of vital components of gibberellin signalling such as GIBBERELLIN INSENSITIVE DWARF1 and 2 (GID1 and 2), GIBBERELLIC ACID INSENSITIVE (GAI), SLEEPY1 (SLY1) and SNEEZY (SNE) (Nelson and Steber, 2016). Further supporting a functional diversification for both hormones is the report that SLs stimulate the elongation of internodes independent of gibberellin by increasing cell number and not cell length (de Saint Germain et al., 2013). Against the backdrop of the above, it is likely that SL and gibberellin crosstalk is limited, or might not be mediated by direct interactions between signalling components, and inhibition of SL synthesis by gibberellin serves only to modulate SL content. Whatever the case may be, further investigations are required to reveal the true nature of SL–gibberellin crosstalk.
Strigolactone crosstalk with ethylene signalling.
Ethylene signals are involved in the control of some growth and developmental processes that are also regulated by SLs. Among these are hypocotyl growth, root hair elongation, seed germination and leaf senescence (Fig. 6). During hypocotyl development, the COP1–HY5 complex acts as an integrator of light and hormonal signalling in regulating hypocotyl growth. In light, SLs inhibit hypocotyl elongation by up-regulating HY5 expression in a MAX2-dependent process (Jia et al., 2014) while ethylene enhances COP1-mediated degradation of HY5 to promote hypocotyl elongation (Yu et al., 2013). Together, these observations suggest that an antagonistic interaction between SLs and ethylene signalling might exist in regulating hypocotyl growth. Because hypocotyl growth is rapidly arrested in light, an important question is how SLs override the promoting effects of ethylene on hypocotyl growth in light.
SL control of root hair elongation is also dependent on ethylene signalling. This is evident as ethylene signalling mutants, At-ein and At-etr, displayed reduced sensitivity to GR24 treatment and blockage of ethylene production completely eliminated SL effects on root hair elongation (Kapulnik et al., 2011b). Furthermore, GR24 treatment enhanced At-ACS2 transcription, a gene involved in ethylene biosynthesis (Kapulnik et al., 2011b). Observations from the same study also suggest SLs use ethylene, which may in turn recruit auxin signalling, thus implying the existence of a three-way hormonal crosstalk in the control of root hair development. Nonetheless, the report that a transcriptional complex – consisting of an ethylene-activated factor and a positive regulator of hair cells – co-activates a hair length-determining gene (ROOT HAIR DEFECTIVE 6-LIKE) and other root hair genes (Feng et al., 2017) shows that ethylene can act independently and does not always require auxin signalling to influence root hair development.
Further demonstrating a crosstalk between SLs and ethylene, an early report indicated that SLs induced the biosynthesis of ethylene in the seeds of Striga prior to germination (Sugimoto et al., 2003). In promoting leaf senescence, SLs also acted by activating ethylene-mediated senescence signals, although ethylene-independent pathways may be employed (Ueda and Kusaba, 2015). Taken together, in addition to suggesting that ethylene acts downstream of SLs, the above shows that SL-mediated responses that require ethylene signalling are elicited by modulating ethylene content in tissues. Also supporting the view that ethylene may act downstream of SLs is ethylene control over AR initiation sites in Arabidopsis hypocotyl, which is independent of SLs (Rasmussen et al., 2017). Regardless of these, the precise molecular channels used by SLs to elicit the transcriptional control of ethylene production remains undescribed. Because light is a potent regulator of ethylene biosynthesis – via processes that are mediated by phytochrome/phytochrome interacting factors (reviewed by Zdarska et al., 2015) – and given that some SL responses are elicited via cryptochrome and phytochrome (Jia et al., 2014), the idea that SLs might modulate ethylene signalling in a phytochrome-dependent manner is worth investigating.
Again, as with gibberellins, describing a lucid and complete model of SL–ethylene crosstalk in regulating developmental processes in plant is still beyond reach with extant experimental data. Providing answers on how D14/MAX2 signalling and D53/SMXL activity affect key ethylene signalling components and ethylene-responsive TFs and vice versa will be invaluable in understanding the role of SL–ethylene hormonal and signalling crosstalk(s) in processes such as root development, fruit ripening and senescence.
Strigolactone crosstalk with jasmonate signalling.
Jasmonates, a group of plant oxylipins, mediate a range of physiological processes during vegetative growth, secondary metabolism, plant–insect and plant–pathogen interactions, wounding, etc. The nature of SL–jasmonate crosstalk cannot be clearly described at this point due to limited experimental data. However, the report that jasmonic acid (JA) content and the expression of a jasmonate-dependent gene, PINII (a gene responsible for tomato resistance to Botrytis cinerea) were reduced in an SL-deficient tomato mutant (Sl-ccd8) (Torres-Vera et al., 2014) points to a connection between SLs and jasmonates in disease tolerance. Further alluding to this link is the fact that methyl jasmonate, a plant defence signalling molecule, exerts some influence on Nicotiana tabacum PDR6 (Nt-PDR6), which is an orthologue of the SL transporter gene, Ph-PDR1 (Xie et al., 2015a). Although strongly induced by phosphate starvation and also involved in the regulation of shoot branching (Xie et al., 2015a), Nt-PDR6 has not been reported to be involved in SL transport. A rather inconsistent observation was reported recently in which GR24 supply did not affect JA accumulation in WT plants, At-max1 and At-max2, nor did inoculation with Mucor sp. (Rozpądek et al., 2018). Together, these observations are not sufficient to draw valid conclusions on the nature of SL–jasmonate crosstalk, but the fact that both hormones elicit responses in similar developmental processes – such as mesocotyl elongation, senescence and plant–microbe interactions – offer some indications that SL–jasmonate crosstalk is likely to feature actively in these processes and cannot be completely ruled out.
Strigolactone crosstalk with salicylic acid signalling.
Salicylic acid (SA) features prominently in plant perception and defence against pathogens as well as tolerance to abiotic stressors (Herrera-Vásquez et al., 2015; Khan et al., 2015; Prodhan et al., 2018). Its control in this aspect is largely because of its influence on the reactive oxygen species (ROS) status of plants (Herrera-Vásquez et al., 2015). SA controls drought tolerance (Askari and Ehsanzadeh, 2015), senescence (Ji et al., 2016) and stomatal closure/conductance (Prodhan et al., 2018), all of which are under SL regulation. Thus far, SA–SL interactions have only been demonstrated in plant–endophytic fungus interactions where GR24 induced the accumulation of SA while an SL signalling mutant, max2, had decreased SA concentrations (Rozpądek et al., 2018). This is an indication that SLs may influence plant defence responses to infection by inducing SA signalling. Whether SLs elicit SA accumulation by a direct transcriptional control of SA biosynthetic genes or by inducing oxidative outburst/ROS (which are known to induce SA production) remains to be determined. Given the recent reports of SL actions in ABA-induced stomatal closure under drought conditions (Ha et al., 2014; Visentin et al., 2016) and senescence (Yamada et al., 2014; Ueda and Kusaba, 2015; Tian et al., 2018), exploring SL–SA crosstalk in these aspects may provide invaluable tools for breeding programmes aimed at developing drought-tolerant crops with increased fruit shelf life.
PERSPECTIVES
Among many other facts, the interactions (both directly and indirectly) between chemically diverse phytohormones in the modulation of developmental processes in plants have been consistently established by experimental observations over the years. From the sui generis physiological processes that mark the germination stages of seed development to vegetative growth, flowering and setting of seeds, maturation of fruits and senescence, hormonal interactions form an integral circuit of the molecular signals that give shape to developmental patterns of plants in response to internal and external cues. Despite the significant progress in understanding hormonal interactions at the molecular (Fig. 6), cellular and whole plant levels, much still needs to be done with respect to SL crosstalk(s) with other phytohormones.
For instance, the actions of SLs in several processes such as its influence on cell division appear to be tissue-specific given that SLs stall cell proliferation in axillary meristems via BRC1 to inhibit branching while promoting cambial activity/cell division to enhance stem elongation and growth. While much has been done to understand SL crosstalk(s) with other key hormonal players in bud activation and branching, the precise molecular channel(s) by which SLs delineate their influences on internode elongation and secondary growth from their actions during bud activation remains to be unravelled. A possible way forward is to look into selective interactions between SL signals and TFs as well as hormones associated with both processes and into how SL signalling achieves this selectivity if found to be present. Given the central role of cytokinins as regulators of cambial activity, SL–cytokinin interactions in modulating cambial activity might also hold the answer to the aforementioned. In addition, SL actions in root secondary growth also remain uncharacterized.
The pivotal role of ABA, jasmonates and SA hormonal actions in enabling plants cope with limiting and stressful ambient conditions cannot be over emphasized. In addition, with the emerging roles of SLs in this aspect of plant development, it is imperative to characterize the crosstalk between SLs and these hormones to develop a model of the circuit of hormonal actions that drive plants’ innate response and defence mechanisms against biotic and abiotic stress in their environment.
Furthermore, meaningful progress can also be achieved by studies tailored towards the identification and characterization of SL-responsive MYB class proteins and how they feature in SL signalling and responses. This group of transcriptional regulators feature prominently as downstream targets of hormonal signals and key modulators in a myriad of growth and developmental processes in plants via their active recognition and binding to specific DNA targets and the modulation of the expression of such target genes.
Finally, the impact of epigenetic cues and associated regulatory mechanisms on the interactions between SL signals and those of other phytohormones as well as their evolution during plant adaptation to new environments require much research attention. These are crucial if SL-mediated plant responses are to be fully understood in the face of dynamic ambient conditions, as is being experienced with the current change in global weather and climate patterns and the rapidly evolving microbial communities within the rhizosphere.
LITERATURE CITED
- Abe S, Sado A, Tanaka K. 2014. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proceedings of the National Academy of Sciences USA 111: 18084–18089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Martinez JA, Poza-Carrion C, Cubas P. 2007. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J, Herold S, Schwarz M, et al. . 2011. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proceedings of the National Academy of Sciences USA 108: 20242–20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Ogasawara S, Ito S, Hayashi H. 2010. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant and Cell Physiology 51: 1104–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder A, Jamil M, Marzorati M, et al. . 2012. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335: 1348–1351. [DOI] [PubMed] [Google Scholar]
- Arite T, Iwata H, Ohshima K, et al. . 2007. DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. The Plant Journal 51: 1019–1029. [DOI] [PubMed] [Google Scholar]
- Arite T, Kameoka H, Kyozuka J. 2012. Strigolactone positively controls crown root elongation in rice. Journal of Plant Growth Regulation 31: 165–172. [Google Scholar]
- Askari E, Ehsanzadeh P. 2015. Effectiveness of exogenous salicylic acid on root and shoot growth attributes, productivity, and water use efficiency of water-deprived fennel genotypes. Horticulture, Environment and Biotechnology 56: 687–696. [Google Scholar]
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S. 2011. Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. The Plant Journal 65: 571–577. [DOI] [PubMed] [Google Scholar]
- Balla J, Medvedóvá Z, Kalousek P, et al. . 2016. Auxin flow-mediated competition between axillary buds to restore apical dominance. Scientific Reports 6: 35955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz L, Mori N, Mi J, et al. . 2018. 3-Hydroxycarlactone, a novel product of the strigolactone biosynthesis core pathway. Molecular Plant 11: 1312–1314. [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. 2006. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology 16: 553–563. [DOI] [PubMed] [Google Scholar]
- Bennett T, Liang Y, Seale M, Ward S, Müller D, Leyser O. 2016. Strigolactone regulates shoot development through a core signalling pathway. Biology Open 5: 1806–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA. 2000. Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regulation 32: 193–203. [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. 2004. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Current Biology 14: 1232–1238. [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, et al. . 2005. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch inhibiting hormone. Developmental Cell 8: 443–449. [DOI] [PubMed] [Google Scholar]
- Borghi L, Liu GW, Emonet A, Kretzschmar T, Martinoia E. 2016. The importance of strigolactone transport regulation for symbiotic signaling and shoot branching. Planta 243: 1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot JP, et al. . 2012. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiology 158: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Gui R, Mason MG, Beveridge CA. 2015. Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiology 168: 1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Yoneyama K, Filardo F, et al. . 2016. LATERAL BRANCHING OXIDOREDUCTASE acts in the final stages of strigolactone biosynthesis in Arabidopsis. Proceedings of the National Academy of Sciences USA 113: 6301–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun G, Braem L, Thoiron S, Gevaert K, Goormachtig S, Delavault P. 2018. Seed germination in parasitic plants: what insights can we expect from strigolactone research? Journal of Experimental Botany 69: 2265–2280. [DOI] [PubMed] [Google Scholar]
- Bruno M, Al-Babili S. 2016. On the substrate specificity of the rice strigolactone biosynthesis enzyme DWARF27. Planta 243: 1429–1440. [DOI] [PubMed] [Google Scholar]
- Bruno M, Hofmann M, Vermathen M, Alder A, Beyer P, Al-Babili S. 2014. On the substrate- and stereospecificity of the plant carotenoid cleavage dioxygenase 7. FEBS Letters 588: 1802–1807. [DOI] [PubMed] [Google Scholar]
- Bu Q, Lv T, Shen H, et al. . 2014. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiology 164: 424–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C, Zhang Y, Jamil M, et al. . 2014. Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proceedings of the National Academy of Sciences USA 111: 2379–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson GH, Hasse D, Cardinale F, Prandi C, Andersson I. 2018. The elusive ligand complexes of the DWARF14 strigolactone receptor. Journal of Experimental Botany 69: 2345–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis RJ, Hepworth J, Mouchel C, Waites R, Leyser O. 2013. A role for MORE AXILLARY GROWTH1 (MAX1) in evolutionary diversity in strigolactone signaling upstream of MAX2. Plant Physiology 161: 1885–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnikhova TV, Gaus K, Lumbroso A, et al. . 2017. Zealactones. Novel natural strigolactones from maize. Phytochemistry 137: 123–131. [DOI] [PubMed] [Google Scholar]
- Charnikhova TV, Gaus K, Lumbroso A, et al. . 2018. Zeapyranolactone − A novel strigolactone from maize. Phytochemistry Letters 24: 172–178. [Google Scholar]
- Chen H, Zhang J, Neff MM, et al. . 2008. Integration of light and abscisic acid signaling during seed germination and early seedling development. Proceedings of the National Academy of Sciences USA 105: 4495–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Floková K, Bouwmeester H, Ruyter-Spira C. 2017. The role of endogenous strigolactones and their interaction with ABA during the infection process of the parasitic weed Phelipanche ramosa in tomato plants. Frontiers in Plant Science 8: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CE, Whichard LP, Wall ME, et al. . 1972. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). Journal of the American Chemical Society 94: 6198–6199. [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, et al. . 2010. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913. [DOI] [PubMed] [Google Scholar]
- Czarnecki O, Yang J, Weston DJ, Tuskan GA, Chen JG. 2013. A dual role of strigolactones in phosphate acquisition and utilization in plants. International Journal of Molecular Sciences 14: 7681–7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SP, Prescott H, Dolan L. 2015. Intensity of a pulse of RSL4 transcription factor synthesis determines Arabidopsis root hair cell size. Nature Plants 1: 15138. [DOI] [PubMed] [Google Scholar]
- Daws MI, Davies J, Pritchard HW, Brown NAC, Van Staden J. 2007. Butenolide from plant-derived smoke enhances germination and seedling growth of arable weed species. Plant Growth Regulation 51: 73–82. [Google Scholar]
- De Cuyper C, Fromentin J, Yocgo RE, et al. . 2015. From lateral root density to nodule number, the strigolactone analogue GR24 shapes the root architecture of Medicago truncatula. Journal of Experimental Botany 66: 137–146. [Erratum Journal of Experimental Botany66: 4091.] [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, et al. . 2007. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Current Biology 17: 678–682. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, et al. . 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384. [DOI] [PubMed] [Google Scholar]
- de Saint Germain A, Ligerot Y, Dun EA, et al. . 2013. Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiology 163: 1012–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint Germain A, Clavé G, Badet-Denisot MA, et al. . 2016. An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nature Chemical Biology 12: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O. 2011. Signal integration in the control of shoot branching. Nature Reviews Molecular Cell Biology 12: 211–221. [DOI] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. 2012. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiology 158: 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA. 2013. Dynamics of strigolactone function and shoot branching responses in Pisum sativum. Molecular Plant 6: 128–140. [DOI] [PubMed] [Google Scholar]
- Felderer B, Vontobel P, Schulin R. 2015. Cluster root allocation of white lupin (Lupinus albus L.) in soil with heterogeneous phosphorus and water distribution. Soil Science and Plant Nutrition 61: 940–950. [Google Scholar]
- Feng Y, Xu P, Li B, et al. . 2017. Ethylene promotes root hair growth through coordinated EIN3/EIL1 and RHD6/RSL1 activity in Arabidopsis. Proceedings of the National Academy of Sciences USA 114: 13834–13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero M, Pagliarani C, Novák O, et al. . 2018. Exogenous strigolactone interacts with abscisic acid-mediated accumulation of anthocyanins in grapevine berries. Journal of Experimental Botany 69: 2391–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flematti GR, Ghisalberti EL, Dixon KW, Trengove RD. 2004. A compound from smoke that promotes seed germination. Science 305: 977. [DOI] [PubMed] [Google Scholar]
- Flematti GR, Waters MT, Scaffidi A, et al. . 2013. Karrikin and cyanohydrin smoke signals provide clues to new endogenous plant signaling compounds. Molecular Plant 6: 29–37. [DOI] [PubMed] [Google Scholar]
- Foo E. 2013. Auxin influences strigolactones in pea mycorrhizal symbiosis. Journal of Plant Physiology 170: 523–528. [DOI] [PubMed] [Google Scholar]
- Foo E, Davies NW. 2011. Strigolactones promote nodulation in pea. Planta 234: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB. 2013. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Molecular Plant 6: 76–87. [DOI] [PubMed] [Google Scholar]
- Gao Z, Qian Q, Liu X, et al. . 2009. Dwarf 88, a novel putative esterase gene affecting architecture of rice plant. Plant Molecular Biology 71: 265. [DOI] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Balzergue C, et al. . 2013. Short‐chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytologist 198: 190–202. [DOI] [PubMed] [Google Scholar]
- Goh T, Kasahara H, Mimura T, Kamiya Y, Fukaki H. 2012. Multiple AUX/IAA-ARF modules regulate lateral root formation: the role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences 367: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JC, Koch KE, Suzuki M, et al. . 2012. Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching-specific subnetwork. Plant Physiology 160: 1303–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillotin B, Etemadi M, Audran C, Bouzayen M, Bécard G, Combier JP. 2017. Sl-IAA27 regulates strigolactone biosynthesis and mycorrhization in tomato (var. MicroTom). New Phytologist 213: 1124–1132. [DOI] [PubMed] [Google Scholar]
- Guo S, Xu Y, Liu H, et al. . 2013. The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nature Communication 4: 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CV, Leyva-González MA, Osakabe Y, et al. . 2014. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proceedings of the National Academy of Sciences USA 111: 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider I, Andreo-Jimenez B, Bruno M, et al. . 2018. The interaction of strigolactones with abscisic acid during the drought response in rice. Journal of Experimental Botany 69: 2403–2414. [DOI] [PubMed] [Google Scholar]
- Hamiaux C, Drummond RSM, Janssen BJ, et al. . 2012. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Current Biology 22: 2032–2036. [DOI] [PubMed] [Google Scholar]
- Hauck C, Müller S, Schildknecht H. 1992. A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. Journal of Plant Physiology 139: 474–478. [Google Scholar]
- Hayward A, Stirnberg P, Beveridge C, Leyser O. 2009. Interactions between auxin and strigolactone in shoot branching control. Plant Physiology 151: 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Vásquez A, Salinas P, Holuigue L. 2015. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Frontiers in Plant Science 6: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Zhang S, Huang B. 2018. Strigolactones and interaction with auxin regulating root elongation in tall fescue under different temperature regimes. Plant Science 271: 34–39. [DOI] [PubMed] [Google Scholar]
- Iseki M, Shida K, Kuwabara K, et al. . 2017. Evidence for species-dependent biosynthetic pathways for converting carlactone to strigolactones in plants. Journal of Experimental Botany 69: 2305–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Ito K, Abeta N, Takahashi R, Sasaki Y, Yajima S. 2016. Effects of strigolactone signaling on Arabidopsis growth under nitrogen deficient stress condition. Plant Signaling & Behavior 11: e1126031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Yamagami D, Umehara M, et al. . 2017. Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiology 174: 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Liu J, Xing D. 2016. Low concentrations of salicylic acid delay methyl jasmonate-induced leaf senescence by up-regulating nitric oxide synthase activity. Journal of Experimental Botany 67: 5233–5245. [DOI] [PubMed] [Google Scholar]
- Jia KP, Luo Q, He SB, Lu XD, Yang HQ. 2014. Strigolactone-regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis. Molecular Plant 7: 528–540. [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, et al. . 2013. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Matthys C, Marquez-Garcia B, et al. . 2015. Strigolactones spatially influence lateral root development through the cytokinin signaling network. Journal of Experimental Botany 67: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameoka H, Kyozuka J. 2018. Spatial regulation of strigolactone function. Journal of Experimental Botany 69: 2255–2264. [DOI] [PubMed] [Google Scholar]
- Kapulnik Y, Delaux PM, Resnick N, et al. . 2011. a Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233: 209–216. [DOI] [PubMed] [Google Scholar]
- Kapulnik Y, Resnick N, Mayzlish-Gati E, et al. . 2011. b Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. Journal of Experimental Botany 62: 2915–2924. [DOI] [PubMed] [Google Scholar]
- Khan MI, Fatma M, Per TS, Anjum NA, Khan NA. 2015. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Frontiers in Plant Science 6: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HI, Xie X, Kim HS, et al. . 2010. Structure–activity relationship of naturally occurring strigolactones in Orobanche minor seed germination stimulation. Journal of Pesticide Science 35: 344–347. [Google Scholar]
- Kim HI, Kisugi T, Khetkam P, et al. . 2014. Avenaol, a germination stimulant for root parasitic plants from Avena strigosa. Phytochemistry 103: 85–88. [DOI] [PubMed] [Google Scholar]
- Kohlen W, Ng JLP, Deinum EE, Mathesius U. 2017. Auxin transport, metabolism, and signalling during nodule initiation: indeterminate and determinate nodules. Journal of Experimental Botany 69: 229–244. [DOI] [PubMed] [Google Scholar]
- Koltai H. 2013. Strigolactones activate different hormonal pathways for regulation of root development in response to phosphate growth conditions. Annals of Botany 112: 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltai H. 2015. Cellular events of strigolactone signalling and their crosstalk with auxin in roots. Journal of Experimental Botany 66: 4855–4861. [DOI] [PubMed] [Google Scholar]
- Koltai H, Dor E, Hershenhorn J, et al. . 2010. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. Journal of Plant Growth Regulation 29: 129–136. [Google Scholar]
- Koren D, Resnick N, Mayzlish Gati E, et al. . 2013. Strigolactone signaling in the endodermis is sufficient to restore root responses and involves SHORT HYPOCOTYL 2 (SHY2) activity. New Phytologist 198: 866–874. [DOI] [PubMed] [Google Scholar]
- Kretzschmar T, Kohlen W, Sasse J, et al. . 2012. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 483: 341–344. [DOI] [PubMed] [Google Scholar]
- Kulkarni MG, Ascough GD, Verschaeve L, Baeten K, Arruda MP, Van Staden J. 2010. Effect of smoke-water and a smoke-isolated butenolide on the growth and genotoxicity of commercial onion. Scientia Horticulturae 124: 434–439. [Google Scholar]
- Kumar M, Pandya-Kumar N, Dam A, et al. . 2015. Arabidopsis response to low-phosphate conditions includes active changes in actin filaments and PIN2 polarization and is dependent on strigolactone signalling. Journal of Experimental Botany 66: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco L, Fiorilli V, Venice F, Bonfante P. 2018. Strigolactones cross the kingdoms: plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. Journal of Experimental Botany 69: 2175–2188. [DOI] [PubMed] [Google Scholar]
- Lantzouni O, Klermund C, Schwechheimer C. 2017. Largely additive effects of gibberellin and strigolactone on gene expression in Arabidopsis thaliana seedlings. The Plant Journal 92: 924–938. [DOI] [PubMed] [Google Scholar]
- Lauressergues D, André O, Peng J, et al. . 2015. Strigolactones contribute to shoot elongation and to the formation of leaf margin serrations in Medicago truncatula R108. Journal of Experimental Botany 66: 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK. 2011. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138: 3485–3495. [DOI] [PubMed] [Google Scholar]
- Leyser O. 2018. Auxin signaling. Plant Physiology 176: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Nguyen KH, Watanabe Y, Yamaguchi S, Tran LS. 2016. OaMAX2 of Orobanche aegyptiaca and Arabidopsis AtMAX2 share conserved functions in both development and drought responses. Biochemical and Biophysical Research Communications 478: 521–526. [DOI] [PubMed] [Google Scholar]
- Li W, Nguyen KH, Chu HD, et al. . 2017. The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genetics 13: e1007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light ME, Burger BV, Staerk D, Kohout L, Van Staden J. 2010. Butenolides from plant-derived smoke: natural plant-growth regulators with antagonistic actions on seed germination. Journal of Natural Products 73: 267–269. [DOI] [PubMed] [Google Scholar]
- Liu G, Pfeifer J, de Brito Francisco R, et al. . 2018. Changes in the allocation of endogenous strigolactone improve plant biomass production on phosphate-poor soils. New Phytologist 217: 784–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang S, Yu X, et al. . 2005. ARL1, a LOB-domain protein required for adventitious root formation in rice. The Plant Journal 43: 47–56. [DOI] [PubMed] [Google Scholar]
- Liu J, He H, Vitali M, et al. . 2015. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: exploring the interaction between strigolactones and ABA under abiotic stress. Planta 241: 1435–1451. [DOI] [PubMed] [Google Scholar]
- Cheng Liu J, Liu P, Sun J. 2017. miR156-Targeted SBP-Box transcription factors interact with DWARF53 to regulate TEOSINTE BRANCHED1 and BARREN STALK1 expression in bread wheat. Plant Physiology 174: 1931–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Moore S, Chen C, Lindsey K. 2017. Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: from experiments to systems modeling, and back again. Molecular Plant 10: 1480–1496. [DOI] [PubMed] [Google Scholar]
- Liu W, Wu C, Fu Y, et al. . 2009. Identification and characterization of HTD2: a novel gene negatively regulating tiller bud outgrowth in rice. Planta 230: 649–658 [DOI] [PubMed] [Google Scholar]
- Liu W, Kohlen W, Lillo A, et al. . 2011. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 23: 3853–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SF, Yang SY, Chen KT, et al. . 2008. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. The Plant Cell 20: 2603–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Kohlen W, Charnikhova T, et al. . 2010. Does abscisic acid affect strigolactone biosynthesis? New Phytologist 187: 343–354. [DOI] [PubMed] [Google Scholar]
- Lv S, Zhang Y, Li C, et al. . 2018. Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytologist 217: 290–304. [DOI] [PubMed] [Google Scholar]
- Ma H, Duan J, Ke J, et al. . 2017. A D53 repression motif induces oligomerization of TOPLESS corepressors and promotes assembly of a corepressor-nucleosome complex. Science Advances 3: e1601217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar S, Funnell KA, Woolley DJ, Cooney JM. 2018. Interaction between strigolactone and cytokinin on axillary and adventitious bud development in Zantedeschia. Journal of Plant Physiology & Pathology 6: 1. [Google Scholar]
- Marzec M, Melzer M. 2018. Regulation of root development and architecture by strigolactones under optimal and nutrient deficiency conditions. International Journal of Molecular Sciences 19: 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Muszynska A. 2015. In silico analysis of the genes encoding proteins that are involved in the biosynthesis of the RMS/MAX/D pathway revealed new roles of strigolactones in plants. International Journal of Molecular Sciences 16: 6757–6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Gruszka D, Tylec P, Szarejko I. 2016. Identification and functional analysis of the HvD14 gene involved in strigolactone signalling in Hordeum vulgare L. Physiologia Plantarum 158: 341–355. [DOI] [PubMed] [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA. 2014. Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences USA 111: 6092–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayzlish-Gati E, De-Cuyper C, Goormachtig S, et al. . 2012. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiology 160: 1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam EL, Hugill C, Fort S, et al. . 2017. Determining the site of action of strigolactones during nodulation. Plant Physiology 175: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi K, Kameoka H, Yasuno N, et al. . 2010. FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant and Cell Physiology 51: 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N, Nishiuma K, Sugiyama T, Hayashi H, Akiyama K. 2016. Carlactone-type strigolactones and their synthetic analogues as inducers of hyphal branching in arbuscular mycorrhizal fungi. Phytochemistry 130: 90–98. [DOI] [PubMed] [Google Scholar]
- Moubayidin L, Perilli S, Dello Ioio R, Di Mambro R, Costantino P, Sabatini S. 2010. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Current Biology 20: 1138–1143. [DOI] [PubMed] [Google Scholar]
- Müller S, Hauck C, Schildknecht H. 1992. Germination stimulants produced by Vigna unguiculata Walp cv Saunders Upright. Journal of Plant Growth Regulation 11: 77–84. [Google Scholar]
- Nakamura H, Xue YL, Miyakawa T, et al. . 2013. Molecular mechanism of strigolactone perception by DWARF14. Nature Communications 4: 2613. [DOI] [PubMed] [Google Scholar]
- Naseem M, Srivastava M, Tehseen M, Ahmed N. 2015. Auxin crosstalk to plant immune networks: a plant-pathogen interaction perspective. Current Protein & Peptide Science 16: 389–394. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Scaffidi A, Dun EA, et al. . 2011. F-box protein MAX2 has dual roles in karrikin and strigolactone signalling in Arabidopsis thaliana. Proceedings of the National Academy of Sciences USA 108: 8897–8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SK, Steber CM. 2016. Gibberellin hormone signal perception: down-regulating DELLA repressors of plant growth and development. In: Hedden P, Thomas SG, eds. Annual Plant Reviews: The Gibberellins Vol. 49. Chichester: John Wiley & Sons, 153–188. [Google Scholar]
- Neumann G, Massonneau A, Langlade N, et al. . 2000. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Annals of Botany 85: 909–919. [Google Scholar]
- Nomura S, Nakashima H, Mizutani M, Takikawa H, Sugimoto Y. 2013. Structural requirements of strigolactones for germination induction and inhibition of Striga gesnerioides seeds. Plant Cell Reports 32: 829–838. [DOI] [PubMed] [Google Scholar]
- Olatunji D, Geelen D, Verstraeten I. 2017. Control of endogenous auxin levels in plant root development. International Journal of Molecular Sciences 18: 2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya-Kumar N, Shema R, Kumar M, et al. . 2014. Strigolactone analog GR24 triggers changes in PIN2 polarity, vesicle trafficking and actin filament architecture. New Phytologist 202: 1184–1196. [DOI] [PubMed] [Google Scholar]
- Prodhan MY, Munemasa S, Nahar MN, Nakamura Y, Murata Y. 2018. Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiology 178: 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, et al. . 2009. Control of bud activation by an auxin transport switch. Proceedings of the National Academy of Sciences USA 106: 17431–17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Mason MG, De Cuyper C, et al. . 2012. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiology 158: 1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen A, Hu Y, Depaepe T, et al. . 2017. Ethylene controls adventitious root initiation sites in Arabidopsis hypocotyls independently of strigolactones. Journal of Plant Growth Regulation 36: 897–911. [Google Scholar]
- Ren CG, Kong CC, Xie ZH. 2018. Role of abscisic acid in strigolactone-induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biology 18: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozpądek P, Domka AM, Nosek M, et al. . 2018. The role of strigolactone in the cross-talk between Arabidopsis thaliana and the endophytic fungus Mucor sp. Frontiers in Microbiology 9: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, et al. . 2011. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiology 155: 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed W, Naseem S, Ali Z. 2017. Strigolactones biosynthesis and their role in abiotic stress resilience in plants: a critical review. Frontiers in Plant Science 8: 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse J, Simon S, Gübeli C, et al. . 2015. Asymmetric localizations of the ABC transporter PaPDR1 trace paths of directional strigolactone transport. Current Biology 25: 647–655. [DOI] [PubMed] [Google Scholar]
- Seale M, Bennett T, Leyser O. 2017. BRC1 expression regulates bud activation potential but is not necessary or sufficient for bud growth inhibition in Arabidopsis. Development 144: 1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto Y, Sado A, Asami K, et al. . 2014. Carlactone is an endogenous biosynthesis precursor for strigolactones. Proceedings of the National Academy of Science USA 111: 1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto Y, Yasui R, Kameoka H, et al. . 2019. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nature Communications 10: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabek N, Ticchiarelli F, Mao H, Hinds TR, Leyser O, Zheng N. 2018. Structural plasticity of D3–D14 ubiquitin ligase in strigolactone signalling. Nature 563: 652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, Lambers H. 2005. Cluster roots: a curiosity in context. Plant and Soil 274: 99–123. [Google Scholar]
- Shen H, Zhu L, Bu QY, Huq E. 2012. MAX2 affects multiple hormones to promote photomorphogenesis. Molecular Plant 5: 750–762. [DOI] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O. 2013. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biology 11: e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JL, Napoli CA, Janssen BJ, Plummer KM, Snowden KC. 2007. Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiology 143: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Lu Z, Yu H, et al. . 2017. IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Research 27: 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soós V, Sebestyén E, Juhász A, et al. . 2009. Stress-related genes define essential steps in the response of maize seedlings to smoke-water. Functional & Integrative Genomics 9: 231–242. [DOI] [PubMed] [Google Scholar]
- Soós V, Sebestyén E, Posta M, et al. . 2012. Molecular aspects of the antagonistic interaction of smoke-derived butenolides on the germination process of Grand Rapids lettuce (Lactuca sativa) achenes. The New Phytologist 196: 1060–1073. [DOI] [PubMed] [Google Scholar]
- Soundappan I, Bennett T, Morffy N, et al. . 2015. SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 27: 3143–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanga JP, Smith SM, Briggs WR, Nelson DC. 2013. SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiology 163: 318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Leyser HMO. 2007. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. The Plant Journal 50: 80–94. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Ali AM, Yabuta S, Kinoshita H, Inanaga S, Itai A. 2003. Germination strategy of Striga hermonthica involves regulation of ethylene biosynthesis. Physiologia Plantarum 119: 137–145. [Google Scholar]
- Sun H, Tao J, Liu S, et al. . 2014. Strigolactones are involved in phosphate and nitrate deficiency-induced root development and auxin transport in rice. Journal of Experimental Botany 65: 6735–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tao J, Hou M, et al. . 2015. A strigolactone signal is required for adventitious root formation in rice. Annals of Botany 115: 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tao J, Gu P, Xu G, Zhang Y. 2016. The role of strigolactones in root development. Plant Signaling & Behavior, 11: e1110662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thussagunpanit J, Nagai Y, Nagae M, et al. . 2017. Involvement of STH7 in light-adapted development in Arabidopsis thaliana promoted by both strigolactone and karrikin. Bioscience, Biotechnology, and Biochemistry 81: 292–301. [DOI] [PubMed] [Google Scholar]
- Tian MQ, Jiang K, Takahashi I, Li GD. 2018. Strigolactone-induced senescence of a bamboo leaf in the dark is alleviated by exogenous sugar. Journal of Pesticide Science 43: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vera R, García JM, Pozo MJ, López-Ráez JA. 2014. Do strigolactones contribute to plant defence? Molecular Plant Pathology 15: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Yoshimura M, Sato Y, et al. . 2015. PARASITIC PLANTS. Probing strigolactone receptors in Striga hermonthica with fluorescence. Science 349: 864–868. [DOI] [PubMed] [Google Scholar]
- Ueda H, Kusaba M. 2015. Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant Physiology 169: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Furumoto T, Umeda S, et al. . 2014. Heliolactone, a non-sesquiterpene lactone germination stimulant for root parasitic weeds from sunflower. Phytochemistry 108: 122–128. [DOI] [PubMed] [Google Scholar]
- Urquhart S, Foo E, Reid JB. 2015. The role of strigolactones in photomorphogenesis of pea is limited to adventitious rooting. Physiologia Plantarum 153: 392–402. [DOI] [PubMed] [Google Scholar]
- Van Staden J, Drewes F, Jäger A. 1995. The search for germination stimulants in plant-derived smoke extracts. South African Journal of Botany 61: 260–263. [Google Scholar]
- Van Staden J, Jäger AK, Light ME, Burger BV. 2004. Isolation of the major germination cue from plant-derived smoke. South African Journal of Botany 70: 654–659. [Google Scholar]
- van Zeijl A, Liu W, Xiao TT, et al. . 2015. The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biology 15: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin I, Vitali M, Ferrero M, et al. . 2016. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytologist 212: 954–963. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen W, Eggert K, et al. . 2018. Abscisic acid influences tillering by modulation of strigolactones in barley. Journal of Experimental Botany 69: 3883–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang B, Jiang L, et al. . 2015. Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation. Plant Cell 27: 3128–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun S, Zhu W, Jia K, Yang H, Wang X. 2013. Strigolactone/MAX2 induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Developmental Cell 27: 681–688. [DOI] [PubMed] [Google Scholar]
- Waters MT, Brewer PB, Bussell JD, Smith SM, Beveridge CA. 2012a The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiology 159: 1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Nelson DC, Scaffidi A, et al. . 2012. b Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 139: 1285–1295. [DOI] [PubMed] [Google Scholar]
- Waters MT, Scaffidi A, Flematti GR, Smith SM. 2012c Karrikins force a rethink of strigolactone mode of action. Plant Signaling & Behavior 7: 969–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Gutjahr C, Bennett T, Nelson DC. 2017. Strigolactone signalling and evolution. Annual Review of Plant Biology 68: 291–322. [DOI] [PubMed] [Google Scholar]
- Watt M, Evans JR. 1999. Proteoid roots. Physiology and development. Plant Physiology 121: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HR, Chung KM, Park JH, et al. . 2001. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell 13: 1779–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Kisugi T, et al. . 2013. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Molecular Plant 6: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wang G, Yang L, et al. . 2015. a Cloning and characterization of a novel Nicotiana tabacum ABC transporter involved in shoot branching. Physiologia Plantarum 153: 299–306. [DOI] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Kisugi T, et al. . 2015. b Strigolactones are transported from roots to shoots, although not through the xylem. Journal of Pesticide Science 40: 214–216. [Google Scholar]
- Xie X, Yoneyama K, Kisugi T, et al. . 2016. Structure- and stereospecific transport of strigolactones from roots to shoots. Journal of Pesticide Science 41: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Kisugi T, Yoneyama K. 2017. Methyl zealactonoate, a novel germination stimulant for root parasitic weeds produced by maize. Journal of Pesticide Science 42: 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Mori N, Yoneyama K, et al. . 2019. Lotuslactone, a non-canonical strigolactone from Lotus japonicus. Phytochemistry 157: 200–205. [DOI] [PubMed] [Google Scholar]
- Xu J, Zha M, Li Y, et al. . 2015. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.). Plant Cell Reports 34: 1647. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Furusawa S, Nagasaka S, Shimomura K, Yamaguchi S, Umehara M. 2014. Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 240: 399–408. [DOI] [PubMed] [Google Scholar]
- Yao R, Ming Z, Yan L, et al. . 2016. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 536: 469–473. [DOI] [PubMed] [Google Scholar]
- Yao R, Wang F, Ming Z, Du X, et al. . 2017. ShHTL7 is a non-canonical receptor for strigolactones in root parasitic weeds. Cell Research 27: 838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R, Wang L, Li Y, et al. . 2018. Rice DWARF14 acts as an unconventional hormone receptor for strigolactone. Journal of Experimental Botany 69: 2355–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L. 2010. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nature Genetics 42: 264–267. [DOI] [PubMed] [Google Scholar]
- Yokota T, Sakai H, Okuno K, Yoneyama K, Takeuchi Y. 1998. Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 49: 1967–1973. [Google Scholar]
- Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H. 2007. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225: 1031–1038. [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Kim HI, et al. . 2012. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235: 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Mori N, Sato T, et al. . 2018. Conversion of carlactone to carlactonoic acid is a conserved function of MAX1 homologs in strigolactone biosynthesis. New Phytologist 218: 1522–1533. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kameoka H, Tempo M, et al. . 2012. The D3 F‐box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytologist 196: 1208–1216. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang J, Zhang Z, et al. . 2013. Ethylene promotes hypocotyl growth and HY5 degradation by enhancing the movement of COP1 to the nucleus in the light. PLoS Genetics 9: e1004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdarska M, Dobisová T, Gelová Z, Pernisová M, Dabravolski S, Hejátko J. 2015. Illuminating light, cytokinin, and ethylene signalling crosstalk in plant development. Journal of Experimental Botany 66: 4913–4931. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li G, Fang J, et al. . 2010. The interactions among DWARF10, auxin and cytokinin underlie lateral bud outgrowth in rice. Journal of Integrative Plant Biology 52: 626–638. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lv S, Wang G. 2018. Strigolactones are common regulators in induction of stomatal closure in planta. Plant Signaling & Behavior 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, van Dijk ADJ, Scaffidi A, et al. . 2014. Rice cytochrome P450 MAX1 homologs catalyse distinct steps in strigolactone biosynthesis. Nature Chemical Biology 10: 1028–1033. [DOI] [PubMed] [Google Scholar]
- Zhao LH, Zhou XE, Wu ZS, et al. . 2013. Crystal structures of two phytohormone signal-transducing α/β hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Research 23: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LH, Zhou XE, Yi W, et al. . 2015. Destabilization of strigolactone receptor DWARF14 by binding of ligand and E3-ligase signalling effector DWARF3. Cell Research 25: 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, et al. . 2013. D14-SCFD3-dependent degradation of D53 regulates strigolactone signaling. Nature 504: 406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Zhang S, Zhang W, et al. . 2006. The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation of the outgrowth of axillary buds. The Plant Journal 48: 687–698. [DOI] [PubMed] [Google Scholar]
- Zwanenberg B, Pospísil T. 2013. Structure and activity of strigolactones: new plant hormones with a rich future. Molecular Plant 6: 38–62. [DOI] [PubMed] [Google Scholar]