Abstract
Background and Aims
Bat-pollination is an important system in terms of occurrence and distribution, although it remains little studied. Thus, the role of particular flower traits in this interaction remains uncertain. Flower height along the shoot axis, associated with flower exposure, has often been deemed a key trait in this system, but its effect on fitness has not previously been assessed. We aimed to test its role and propose that taller flowers attain higher fitness due to a higher degree of accessibility and conspicuity to foraging bats.
Methods
We assessed the effect of floral height on bat visiting rates to individual flowers of Crescentia cujete (Bignoniaceae), a cauliflorous model bat-pollinated species with a marked gradient in flower height along the shoot axis. Additionally, we tested the effect of this variable on seed/ovule ratio measurements from seven other species from different families along a herb–tree gradient. Hypotheses were tested through mixed-effect linear models.
Key Results
Bat visiting rates varied positively as a function of flower height in C. cujete, but significance was found only for the subset of flowers located on the trunk, closer to the ground. Similarly, seed/ovule ratios were positively correlated with flower height only for the three species with the shortest statures along the height gradient and shortest average floral heights. These results suggest that proximity to the ground, associated with herbaceous or bushy surrounding vegetation, may be an obstacle to the foraging of nectar-feeding bats, which in turn should explain the morphological convergence of inflorescence length and exposure strategies of short-statured bat-pollinated plants.
Conclusions
Flower height has a species-specific effect on plant fitness. This study provides a novel numerical perspective to the role of an unexplored trait in bat-pollination, and has elucidated some aspects of the adaptive importance of flower height based on limitations imposed by ecologically complex pollinators.
Keywords: Acanthaceae, bat-pollination, Capparaceae, cauliflory, Cleomaceae, Crescentia cujete, Fabaceae, fitness, floral height, Malvaceae, styliflory
INTRODUCTION
Bat-pollination is among the most distinctive interactions within the reproductive strategies of flowering plants, despite being a relatively recent ecological relationship with its origins in the early Eocene, in comparison to basal systems such as insect-pollination (mid-Cretaceous) (Hu et al., 2008; Fleming et al., 2009). Chiropterophily has a broad pantropical occurrence, encompassing at least 67 plant families and more than 250 phylogenetically diverse genera, and revolves around a morphological- and ecologically derived pollinator guild (Dobat and Peikert-Holle, 1985; Fleming et al., 2009).
In the Neotropics, the subfamilies Glossophaginae and Lonchophyllinae (Chiroptera: Phyllostomidae) comprise species specialized to nectar consumption (Datzmann et al., 2010). Their relatively large body area available for pollen carryover and long-distance, trapline foraging result in overall higher outcrossing rates for plants, and they are thus considered to be particularly efficient pollinators (Muchhala, 2006; Fleming et al., 2009). Such potential has triggered a quick irradiation of tropical and subtropical plants with a series of floral traits adapted to the interaction with fast-flying, energetically demanding vertebrates, which employ olfactive cues and echolocation to define their foraging strategies (Heithaus et al., 1975; von Helversen, 1993; Knudsen and Tollsten, 1995; von Helversen et al., 2003; von Helversen and Winter, 2005).
Bat-pollinated flowers have a series of diagnostic traits, among which the most reliable are nocturnal or crepuscular anthesis, relatively large, echo-reflecting corollas with generally white or inconspicuous shades, a strong and musty odour, and copious nectar and pollen production (Faegri and Van Der Pijl, 1979; Willmer, 2011). However, given that this system has only recently begun to be thoroughly studied, there are still traits whose role in the interaction with nectarivorous bats remains uncertain. Flower height along the shoot axis is one such trait.
Flower position in chiropterophilous species is frequently discussed as one of the variables related to the interaction with bats and is associated with cases of cauliflory (emission of flowers by the trunk) and flagelliflory (inflorescences on branches or peduncles hanging away from the crown foliage) (Baker, 1961; von Helversen, 1993; Muchhala, 2003). In terrestrial herbs and shrubs in particular, flowers located well above the vegetation and positioned on long peduncles or stalks are a recurring phenomenon (Dobat and Peikert-Holle, 1985; von Helversen, 1993). In response to the lack of a term to categorize the latter strategy, we propose styliflory (lat. stylus: ‘column’, ‘pillar’) to refer to it. Indeed, Fleming et al. (2009) suggested flower exposure away from surrounding vegetation as a unifying feature of bat-pollination. The effect of flower height on the fitness of chiropterophilous plants, however, has not previously been explored in detail or tested in a quantitative way. There are some assumptions related to consistent morphological patterns observed among bat-pollinated plants, and to known idiosyncrasies and requirements of nectar-feeding bats while foraging, which combined support floral height along the shoot axis as a key trait in this system.
First, the habit assumption, which derives from the fact that bat-pollinated plants generally occur as trees and less frequently as bushes, while herbaceous species are normally epiphytes (Dobat and Peikert-Holle, 1985; Sazima et al., 1999; Machado and Vogel, 2004). Terrestrial bat-pollinated herbs are rare and are associated more frequently with open habitats such as mountainous fields or clearings, as noted by Machado et al. (1998) when describing how challenging it was for glossophagine bats to visit the flowers of the delicate herb Irlbachia alata (Gentianaceae) while flying close to the ground. Machado and Vogel (2004) reported a similar situation during their observations of Adenocalymma dichilum (Bignoniaceae). The rarity of such cases is exemplified by the pioneering work of Baker (1961) in unifying the typical characters of bat-pollinated flowers, in which he highlighted the high frequency of overall plant robustness and arboreal habit in this system.
This trend is probably related to the optimal contact assumption: pollinating bats have a large body area and wide wingspan, and require a relatively clutter-free environment to perform hovering visits to flowers, a convergent behaviour among New World nectar-feeding bats (von Helversen and Winter, 2005). Baker (1961) summarizes this limitation by pointing to the need of free space for wing beats during flight as a prominent factor in determining flower exposure strategies in tropical bat-pollinated trees. This assumption also tackles the effect of acoustic masking of resources (i.e. flowers) in narrow environments by high levels of clutter from surrounding vegetation (Schnitzler et al., 2003). In canopy trees, traits such as cauliflory and flagelliflory may be regarded as measures to avoid acoustic clutter from surrounding crowns and to improve flower signalling. For low-stature, terrestrial plants, however, individual flower height might become a limiting factor to bat navigation in areas covered by dense shrubby vegetation, such as in tropical dry forests, which may impair the overall accessibility and signalling potential of flowers close to the ground.
Finally, the energetical assumption relates to the metabolical limitation of phytophagous bats while foraging as a function of a diet based on simple exogenous carbohydrates (Voigt et al., 2006; Voigt and Speakman, 2007). Because of the need for a steady resource intake, these animals may avoid foraging routes that do not fall in the optimal linear trajectory such as clutter-rich environments (Voigt et al., 2017). This is a particular concern for specialized nectar-feeding bats that employ hovering flight during their visits to plants, which is an energy-demanding technique (Welch and Suarez, 2008), and require strategies to optimize foraging in order to maintain a stable metabolical balance (Howell and Hartl, 1980). As such, increasing floral height may play a role both in distancing flowers from obstacles (e.g. branches from nearby bushes or from the crown interior) and in lifting flowers to a flight-friendly zone, which would result in a more direct pathway towards and between flowers, leading bats to select these profitable feeding routes over those composed of lower flowers that are difficult to access.
Evidence for optimal foraging has been reported for other guilds of pollinators. Flower height, along with flower display and spatial orientation, has a strong effect on the attraction, establishment of flight routes or flower visiting rates of beetles (Dafni and Potts, 2004), bees (Larson and Larson, 1990; Donnelly et al., 1998; O’Connell and Johnston, 1998) and wasps (Peakall and Handel, 1993). These findings agree with the ‘effective pollination’ hypothesis proposed by Aarssen (1995), in which plants favoured by apical dominance are more attractive to pollinators and attain higher rates of outcrossing by providing a foraging zone with the highest net fitness for these animals (e.g. Lortie and Aarssen, 1999). These studies, however, have focused largely on insects, and details of preferences on flower height of less typical pollinators such as bats remain scarce.
With these premises in mind, we propose the hypothesis that taller flowers from bat-pollinated plants, located farther away from the ground and vegetation clutter, attain a higher fitness than shorter flowers due to access and signalling advantages. We provide, for the first time, an assessment of the effect of flower height along the shoot axis on different levels of floral fitness of chiropterophilous plants in order to understand the key traits of the system and to discuss its ecological and evolutionary implications. We focus our study on Crescentia cujete (Bignoniaceae), a model species within this system. We then expand our experiment to seven other bat-pollinated species of varying habits and families, to build a representative picture of the overall importance of this trait.
METHODS
Sampling species and sites
The study was conducted from August 2016 to December 2018. Samplings were carried out in three localities of Pernambuco state, north-eastern Brazil: (1) the Caatinga seasonally dry deciduous forest [Parque Nacional (PARNA) do Catimbau – Buíque], (2) the Atlantic Rainforest (Recife) and (3) the Agreste ecotone (Caruaru) (Table 1).
Table 1.
Habit, sampling locations and references indicating chiropterophily for the selected model species
| Family | Species | Habit | Inflorescence positioning strategy | Sampling location† | Reference |
|---|---|---|---|---|---|
| Acanthaceae | Harpochilus nessianus Mart. | Bush | Styliflory‡ | PARNA Catimbau (−8.562027; −37.259009) | Vogel et al. (2004) |
| Bignoniaceae | Crescentia cujete L.* | Bush/tree | Cauliflory | Recife (−8.050998; −34.949470) | Diniz et al. (2019) |
| Capparaceae | Crateva tapia L. | Tree | Styliflory | Recife (−8.048097; −34.948772) | Baker (2010) |
| Neocalyptrocalyx longifolium (Mart.) Cornejo & Iltis | Bush | Styliflory | PARNA Catimbau (−8.551986; −37.302564) | Machado and Lopes (2004) | |
| Cleomaceae | Tarenaya spinosa (Jacq.) Raf. | Herb | Styliflory | Caruaru (−8.289585; −35.948260) | Machado et al. (2006) |
| Fabaceae | Bauhinia pentandra (Bong.) D.Dietr. | Bush | NA | PARNA Catimbau (−8.576802; −37.252224) | Santos et al. (2012) |
| Mimosa lewisii Barneby | Bush | Styliflory | PARNA Catimbau (−8.509898; −37.278142) | Vogel et al. (2005) | |
| Malvaceae | Pachira aquatica Aubl.* | Tree | NA | Recife (−8.051820; −34.952926) | Hernández-Montero and Sosa (2016) |
* Exotic species.
† WGS 84 (EPSG: 4326) coordinate reference system.
‡ As proposed here for species whose flowers are exposed above the plant and positioned on long peduncles or stalks.
NA, not applicable.
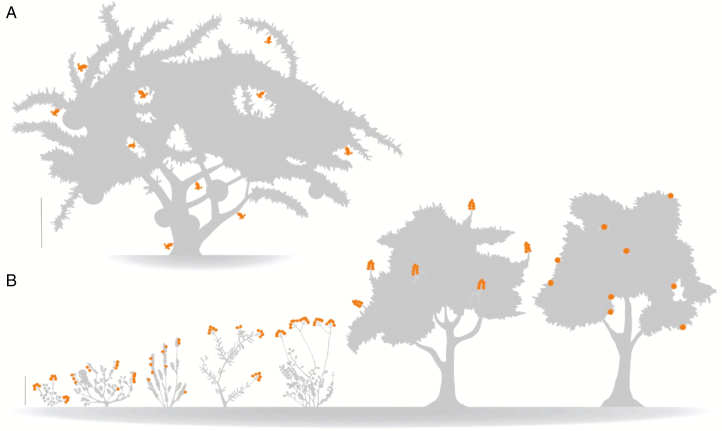
We focused our efforts on the bat-pollinated Crescentia cujete L. (Bignoniaceae), a species native to Central America and introduced into Brazil as an ornamental. It is fully dependent on bats for pollen dispersal (Diniz et al., 2019), and its floral traits, which are highly correlated to pollination by these animals, have led it to be used to validate the role of echo-reflecting morphologies, scent and nectar secretion (Lemke, 1984; Knudsen and Tollsten, 1995; von Helversen et al., 2003). Crescentia cujete is cauliflorous (sensuWeberling, 1992), and thus its flowers are organized along the entire length of the shoot axis, from the base of the trunk up to superior branches (Fig. 1). This trait, added to its intermediate growth habit between a bush and a tree, makes it an excellent model for testing patterns associated with the floral height gradient.
Fig. 1.
A schematic illustration of our selected bat-pollinated species, with flowers displayed in orange: (A) the cauliflorous Crescentia cujete; (B) species composing the herb–tree gradient, from left to right: Tarenaya spinosa, Bauhinia pentandra, Harpochilus nessianus, Neocalyptrocalyx longifolium, Mimosa lewisii, Crataeva tapia and Pachira aquatica. Vertical bars = 1 m.
We expanded our experimental design within the syndrome: we selected seven other representative bat-pollinated species belonging to different clades (Fig. 1, Table 1), from families in which bat-pollination is rare (e.g. Acanthaceae) to those in which chiropterophily is common (e.g. Capparaceae) (Vogel et al., 2004; Fleming et al., 2009). The study species fell within the herb–bush–tree continuum, including both short-stature plants naturally occurring in clusters in semi-arid habitats and urban landscapes such as Tarenaya spinosa (Cleomaceae) and large wetland trees such as Pachira aquatica (Malvaceae). Moreover, the different inflorescence growth strategies between species (Fig. 1) enabled us to gain a general picture of the importance of flower height among these bat-pollination systems.
Quantifying floral fitness
We used flower height above ground as an explanatory variable for the fitness of individual flowers of C. cujete, which we estimated from the number of pollination events (Bigio et al., 2016), i.e. bat visiting rates. This fitness measure is a proxy for the attractiveness, accessibility and quality of resources of individual flowers or floral displays, and is a reliable predictor of seed-set (Vaughton and Ramsey, 1998; Lobo et al., 2016; Lindström et al., 2018). We used a comparative approach while sampling fitness measures, which were always taken from flowers in simultaneous anthesis during censuses. We made direct field observations of focal individuals to quantify the visiting intensity to flowers covering a height gradient. For inter-census analyses, we calculated the relative visitation rate for each flower as a ratio of the absolute number of bat visits to the flower in relation to the total number of bat visits in its respective census. Only three individuals were used for data sampling, given the sparse distribution of the species and that only these three plants emitted, per night, a consistently large number of flowers during the sampling period, enough to allow intra-individual comparisons. One individual had two to five flowers marked per census, which were observed simultaneously for bat visits. All censuses had the same duration (90 min) and occurred at the peak of bat activity (1800–2100 h).
The flowers of C. cujete comprise two conspicuous groups with regard to their location: flowers emitted from the base of the trunk, exposed from the foliage and close to the ground, and flowers emitted within the crown, taller and surrounded by foliage (Fig. 1). We analysed the effect of flower height along the shoot axis on bat visiting rates for all the flowers pooled as a single population, followed by an independent analysis for both groups (see Data Analysis below for more details).
For the seven additional species of varying habits, we quantified the female fitness of individual flowers by estimating seed/ovule ratios (Holland and Chamberlain, 2007), an indirect measure of pollinator visiting rates and a proxy for the quality of the interaction with pollinators that considers the interindividual variation of ovules per flower. This measure was logistically viable for our multi-individual sampling nested to a multi-species scale. Crescentia cujete was excluded from this fitness sampling because its fruits are systematically removed by the local community due to its ornamental potential, disrupting any relevant height gradient for this structure.
We calculated seed:/ovule ratios by collecting three random ovaries from each individual of the study populations to estimate the average quantity of ovules per ovary. From the same individuals, we collected fruits set at varying heights for seed counts. The seed/ovule ratio of each fruit was regarded as a measure of fitness, and varies from zero (no ovules fertilized) to one (all ovules fertilized). Because this estimate derives from an average number of ovules per ovary, values above one may occur sporadically. Up to 30 individuals were sampled per species, depending on their rarity. Thirty individuals were sampled only from the abundant species T. spinosa and Harpochilus nessianus, while samples decreased consistently according to rarity (Bauhinia pentandra = 29, Mimosa lewisii = 18, Neocalyptrocalyx longifolium = 10, Crataeva tapia = 7, P. aquatica = 4). Sampled individuals had fruits collected according to a height gradient of the shoot axis. Most species had two fruits collected per individual, specifically the shortest and tallest fruit. Exceptions included N. longifolium, Crataeva tapia and P. aquatica, due to their especially small populations (three to ten fruits per individual). All species underwent a single sampling event each, so no temporal variation in ovule counts occurred.
Data analysis
We used mixed-effect linear models fitted by maximum likelihood to assess the relationship between the variables of interest (McLean et al., 1991; Luke, 2017). Flower height along the shoot axis was used as the fixed explanatory variable, and fitness measurements – bat visiting rates and seed/ovule ratios – as the response variables. For bat visiting rates in C. cujete, observations were nested into focal individuals, as well as into sampling censuses, which were specified as the two levels of random effects. Data from flowers of both location groups (below and within crown) were pooled together to test for an entire-axis effect of flower height. Simultaneously, an independent model was fitted for each group. Additionally, we carried a Welch’s t-test to compare the average flower fitness of the two groups. For the multi-species samplings of seed/ovule ratio, independent models were fitted for each species. Observations were nested within different subject individuals, which were specified as random predictors for all models. Similarly to C. cujete, we fitted a general model for all species pooled together with ‘individual’ and ‘species’ as the two levels of random effects.
The covariance matrices were kept as unstructured across all models. We specified a maximum random effect structure whenever possible (Barr et al., 2013), which was the case for the seed/ovule ratio models of H. nessianus, Crataeva tapia and P. aquatica. For the other species, the low number of observations per individual (or per census in the case of C. cujete) would cause random slopes to become confounded with residual variation, and those models were fitted with random intercepts only instead. All statistical analyses were carried out in the R software (version 3.3.2), lme4 package (R Development Core Team, 2013; Bates et al., 2015; Wickham, 2016). Satterthwaite’s method of approximation for degrees of freedom was used to estimate significance (lmerTest package) (Luke, 2017; Kuznetsova et al., 2017). All models were checked a priori for residual normality.
RESULTS
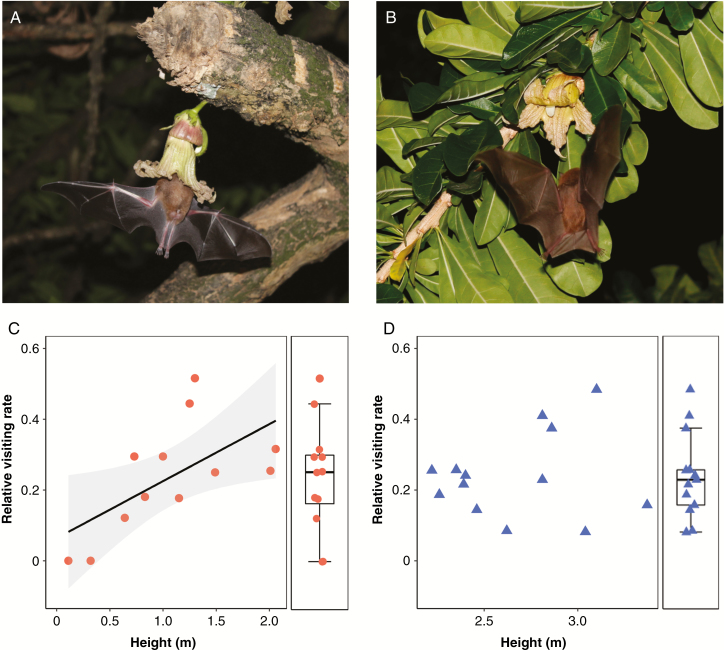
A total of 1800 min of field observations of focal individuals of C. cujete (n = 3) were carried out within Recife, and 949 bat visits were recorded, at an average of 37.96 ± 20.27 visits per flower (n = 25). Relative bat visitation rates varied positively as a function of absolute flower height along the shoot axis (Table 2). Visitation rates, however, showed no significant difference between flowers located below the crown (n = 12) and within the crown (n = 13) (d.f. = 20.97; t = 0.547; P = 0.59). By fitting independent models for each group, a significant, strong correlation of fitness measures with flower height was found only in flowers emitted by the trunk, closer to the ground, indicating that this group was responsible for the significance of the general model (Table 2; Fig. 2)
Table 2.
Summary of the measurements for the two flower groups of Crescentia cujete analysed, along with the parameters reporting the effect of the fixed variable on individual flower fitness
| Group | n | Flower height (m) ± s.d. | Fitness ± s.d. | β 0 | β 1 | s.e. | d.f. | t | P |
|---|---|---|---|---|---|---|---|---|---|
| Below crown | 12 | 1.07 ± 0.6 | 0.25 ± 0.16 | 0.030 | 0.201 | 0.047 | 6.39 | 4.26 | 0.005 |
| Within crown | 13 | 2.67 ± 0.36 | 0.22 ± 0.13 | 0.488 | −0.069 | 0.051 | 7.13 | −1.346 | 0.219 |
| Pooled | 25 | 1.9 ± 0.94 | 0.24 ± 0.14 | 0.172 | 0.049 | 0.023 | 19.65 | 2.149 | 0.042 |
n = flowers observed; β 0 = intercept estimate; β 1 = slope estimate, s.e. = standard error. Significant P-values (<0.05) are in bold type.
Fig. 2.
Effect of flower height along the shoot axis on relative bat visiting rates to Crescentia cujete flowers. (A) A Pallas long-tongued bat Glossophaga soricina visiting a below-crown flower and (B) a within-crown flower, followed by the models fitted for each respective group (C: below crown; D: within crown). The 95% confidence interval is displayed in grey and the regression line at the population level is shown only where it is significant.
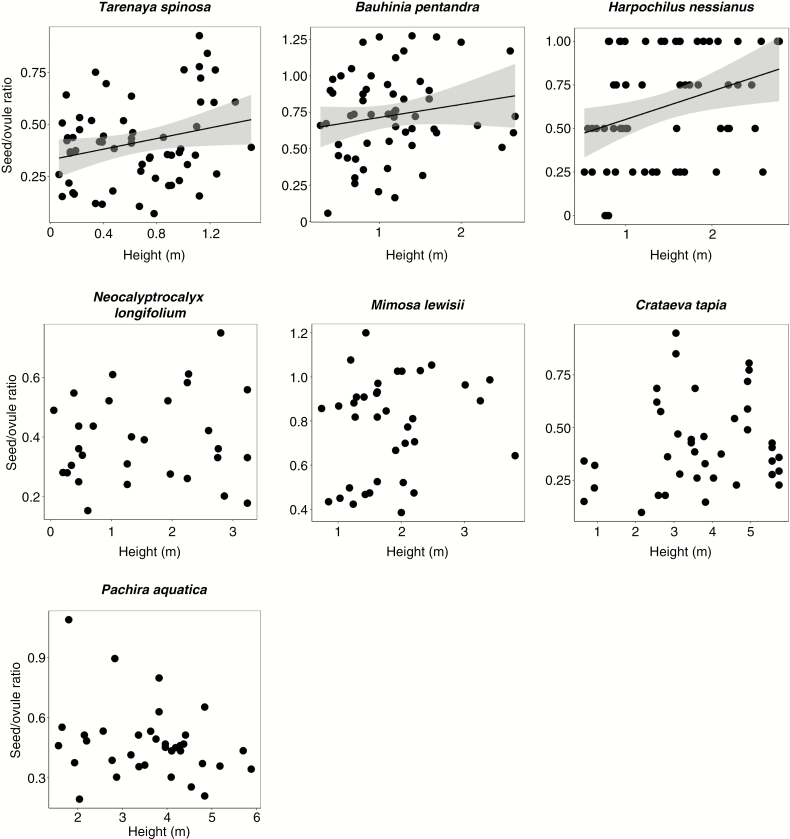
We quantified 318 seed/ovule ratios from the seven species along the herb–tree gradient (Fig. 3; Table 3). Pooling all fitness values, the effect of flower height on fitness was positive and significant, although minor. The fitting of species-specific models revealed a significant and positive height–fitness relationship for Tarenaya spinosa, Bauhinia pentandra and Harpochilus nessianus, the three shortest species along the stature spectrum with the shortest average fruit heights (Table 3; Fig. 3).
Fig. 3.
Relationship between flower height along the shoot axis and seed/ovule ratio for the seven species analysed independently. Regression lines for the fitted models at the population level are displayed only where they are significant (P < 0.05). The 95% confidence intervals are displayed in grey.
Table 3.
Summary of the sampling results of seed/ovule ratios for seven bat-pollinated species: averages and standard deviations for the height of collected fruits and fitness measurements, as well as the reported estimates for the effect of the fixed variable; species are listed from shortest to tallest
| Species | n | f | Fruit height (m) ± s.d. | Seed/ovule ratio ± s.d. | β 0 | β 1 | s.e. | d.f. | t | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Tarenaya spinosa | 30 | 60 | 0.66 ± 0.4 | 0.41 ± 0.2 | 0.332 | 0.125 | 0.061 | 47.401 | 2.028 | 0.048 |
| Bauhinia pentandra | 29 | 58 | 1.14 ± 0.59 | 0.73 ± 0.30 | 0.582 | 0.127 | 0.049 | 37.754 | 2.569 | 0.014 |
| Harpochilus nessianus | 30 | 60 | 1.45 ± 0.63 | 0.63 ± 0.32 | 0.39 | 0.162 | 0.061 | 60 | 2.632 | 0.011 |
| Neocalyptrocalyx longifolium | 10 | 30 | 1.53 ± 1.1 | 0.39 ± 0.14 | 0.361 | 0.022 | 0.024 | 12.306 | 0.922 | 0.374 |
| Mimosa lewisii | 18 | 36 | 1.82 ± 0.7 | 0.78 ± 0.22 | 0.828 | −0.028 | 0.052 | 34.453 | −0.543 | 0.591 |
| Crataeva tapia | 4 | 39 | 3.7 ± 1.44 | 0.42 ± 0.21 | 0.354 | 0.018 | 0.02 | 3.753 | 0.901 | 0.421 |
| Pachira aquatica | 7 | 35 | 3.61 ± 1.14 | 0.47 ± 0.18 | 0.508 | −0.018 | 0.028 | 11.353 | −0.634 | 0.538 |
| Pooled | 128 | 318 | 1.81 ± 1.37 | 0.56 ± 0.28 | 0.475 | 0.036 | 0.014 | 232.410 | 2.608 | 0.010 |
n = individuals sampled, f = fruits collected, β 0 = intercept estimates; β 1 = slope estimates, s.e. = standard error. Significant P-values (<0.05) are in bold type.
DISCUSSION
Our results indicate that flower height along the shoot axis has a significant effect on individual flower fitness. Our hypothesis, however, was only partially confirmed, as the role of the trait seems to differ among the studied species. A significant correlation between flower height and fitness arose only in groups of flowers closer to the ground, a zone with a higher potential richness of obstacles to bat foraging. Below we address this pattern while discussing the morphological diversity of the shoot axis and inflorescences of bat-pollinated plants and the trade-offs related to investment in primary growth.
The height–fitness relationship
Our findings of pollination events in C. cujete indicate that the relationship between flower height along the shoot axis and fitness is more complex than initially proposed. We found null fitness values from flowers very close to the ground, and these increase consistently as flowers approach the crown level (Fig. 3B). This pattern suggests the existence of a zone of limited access imposed by the ground, which is followed by a foraging-friendly zone composed of flowers exposed below the crown foliage. Within the crown, the concentration of relatively tall flowers, much like typical tree species, appears to result in no significant gradient of proximity to the ground. Thus, fitness (which included seemingly random low and high values alike) is probably influenced by other factors such as random clutter levels caused by leaves surrounding individual flowers, which may hamper echo perception (Schnitzler et al., 2003) in a less deterministic fashion.
The fitness–height relationship for the other species making up the herb–tree gradient showed similar trends. Tarenaya spinosa, Bauhinia pentandra and Harpochilus nessianus, the shortest-statured species that generally occur in clusters and emit flowers mostly below 2 m, were the only ones that showed a significant relationship between flower height and fitness. The absence of any observable effect for taller species, which have a consistently increasing proportion of flowers located above 2 m, was analogous to that recorded for the within-crown flowers of C. cujete. For these species, the effect of proximity to the ground on shorter flowers was probably masked by the increasing independence of the fitness of taller flowers on this factor.
The tendency for increasing fitness only among overall shorter flowers suggests that flower height may be a key trait only when it brings a significant improvement in accessibility amidst the clutter-rich environment normally exploited by nectarivorous bats (Schnitzler and Kalko, 1998). Such improvement would be achieved by distancing flowers from surrounding low-statured plants in sub-canopy areas or from dense, bushy vegetation, thus reducing the energetically expensive manoeuvres required from bats to reach flowers (Voigt et al., 2010). Above a certain threshold, distance from the ground appears to bring no significant contribution to the accessibility gradient and does not cause the directional selection of flowers by bats due to the lack of relatively more profitable routes to energy saving.
Nonetheless, it is equally likely that ecological factors other than foraging efficiency determine the selective behaviour of bats. Hopkins and Hopkins (1982) reported the predation by a snake on a bat during a visit to flowers of Parkia nitida (Fabaceae), while other bats foraging nearby showed signs of noticing the predator and avoiding it. Fruit-eating bats, which must also glean towards the resource to consume it, are similarly vulnerable (França and Lima, 2012; Carvalho et al., 2019). Given that bats undergo low natural predation rates due to their vagility (Mikula et al., 2016), foraging too close to the ground or in the cluttered space within tree crowns may expose them to opportunistic ground-based and branch-based predators. Therefore, aversion to predators may be an additional factor in determining the preference of bats to taller or more exposed flowers.
Thus, in summary, flower height along the shoot axis has a determining effect on the fitness of short-statured and terrestrial bat-pollinated plants. Nonetheless, these conclusions are conservative given the limited assemblage of model species evaluated, specifically the low number of sampled tree individuals. As a consequence of the low density of these tall-statured species at the sampling sites, multiple measurements from few individuals may deliver a certain degree of dependence to the data because visits could possibly have been carried out by a few specific bats. This constraint does not preclude interpretations to be made regarding the foraging behaviour of bats, but the effect estimates for these species must be interpreted with caution.
The pattern reported, however, is clear. Pollen deposition, as for other ecological processes at the interaction level, is affected by degrees of stochasticity because of the erratic behaviour of pollinators and highly variable pollen loads delivered during visits (Morris et al., 1995; Skogsmyr and Lankinen, 1999), which probably accounted for the high residual values of most of the fitted models. An observable trend, in spite of such quality, emphasizes the validity of the explanation proposed for the proposed ecological question.
Flower height and positioning in bat-pollination systems
The marked effect of flower height observed for low-statured species may bring light to some structural strategies employed by chiropterophilous plants to improve floral fitness. Stylifloric inflorescences, i.e. located on long, vertical peduncles or stalks, generally accompanied by a robust plant axis in cases of pollination by non-hovering bats, are recurring traits in non-arboreal species (Fleming et al., 2009). They can be observed in tropical herbs such as the arborescent Phenakospermum guyannense (Strelitziaceae) (Kress and Stone, 1993), typical herbs such as Irlbachia alata (Gentianaceae) (Machado et al., 1998) and Tarenaya spinosa (Cleomaceae) (Machado et al., 2006) (Fig. 1) and many epiphytic and terrestrial bromeliads, such as members of the genera Vriesea and Werauhia, and Encholirium, respectively (Sazima et al., 1989, 1999; Tschapka and von Helversen, 2007; Queiroz et al., 2016).
In bushes, styliflory is an expressive trait among Caatinga-dwelling species, such as H. nessianus (Vogel et al., 2004), M. lewisii (Vogel et al., 2005) and N. longifolium (Machado and Lopes, 2004) (Fig. 1). The frequency of styliflory in the Brazilian Caatinga possibly reflects the high frequency of predominantly bushy vegetation on its crystalline bedrock formations (Andrade-Lima, 1981), which results in an obstacle-rich and low-accessibility zone at strata closer to the ground. In fact, chiropterophilous trees may also present a functionally stylifloric strategy, such as the pteropodid-pollinated palaeotropical Oroxylum indicum (Bignoniaceae), which towers above 15 m and emits inflorescences on long peduncles exposed above the canopy (Srithongchuay et al., 2008). Although constraints related to echolocation do not apply to pteropodid bats, the accessibility issue is probably even more severe in this group due to their larger body size and wingspan, along with non-specialized, clinging visits to flowers (Fleming and Muchhala, 2008).
Epiphytic herbs that occur at or close to canopy level, on the other hand, to which proximity to the ground is not a limiting factor, present flagelliflory as a common structural strategy, possibly providing a functional outcome similar to styliflory by distancing flowers from surrounding crowns. This type of inflorescence has been recorded, for example, for epiphytes of the genera Mucuna (Fabaceae) (Baker, 1970; Grünmeier, 1993), Weberocereus (Cactaceae) (Tschapka et al., 1999) and Marcgravia (Marcgraviaceae) (Sazima and Sazima, 1980; Tschapka and von Helversen, 1999), and also for some canopy trees such as Parkia pendula (Fabaceae) (Piechowski et al., 2010) and Kigelia africana (Bignoniaceae) (Baker, 1961). These traits agree with the proposed importance of accessibility and conspicuity for bats that forage within the understorey, which, in turn, are attained regardless of absolute flower height, unlike short terrestrial species.
The cost of growing tall
The base-architecture of arboreal species, which represent most of the richness of bat-pollinated species, appears to be intrinsically more adjusted to the accessibility requirements of bats, in contrast to herbs and shrubs to which an extra investment in morphology to provide exposure seems to be necessary to improve flower conspicuity (as detailed by Dobat and Peikert-Holle, 1985). In this case, the evolution of bat-pollination in trees may provide an example of exaptation, because a prior investment in height may have proved secondarily advantageous during the transition to chiropterophily. The investment in primary growth, its benefits, consequences and trade-offs are basic topics in plant ecology (Falster and Westoby, 2003; Moles et al., 2009). A strong apical dominance is deemed to be a competition-orientated strategy because it enhances light capture to the detriment of close neighbours and yields a higher relative fitness (Adams et al., 2007; Gruntman et al., 2017). However, the magnitude of this investment is known to be calibrated by some physical constraints such as hydraulic and mechanical restrictions, which require physiological costs to be overcome (Chiariello et al., 1989; Niklas, 2007). Because plants are inherently limited by resource availability (Herms and Mattson, 1992), the biomass investment in vertical growth may thus exist only when justified by a positive fitness balance, which also takes reproductive function into account.
Nectar-feeding bats are considered to be very efficient pollinators and it is not surprising that, despite its recent appearance in the Miocene, this mutualistic interaction has already resulted in highly derived floral traits (Heithaus et al., 1975; Marshall, 1983; Gribel et al., 1999; Tripp and Manos, 2008; Muchhala and Thompson, 2010). However, it is unlikely that such efficiency has driven drastic structural modifications such as the development and intensification of the arboreal habit, especially because our data indicate that flower height affects fitness only until a certain threshold of proximity to the ground. The net positive fitness given by these animals was probably sufficient, on the other hand, to determine the structural evolution of short-statured plants to some extent, such as the convergence of traits such as robustness and stylifloric inflorescences (Fleming et al., 2009). Nonetheless, despite presenting different adaptation profiles, the differences in form and variability in bat-pollinated plants undoubtedly represent a unifying attempt to attend to the constraints imposed by a guild of ecologically complex pollinators.
FUNDING
This work was supported by the National Council of Scientific and Technological Development (CNPq) through a scholarship granted to U.M.D. [157052/2017-6], and through a research grant [311021/2014-0] and partial financial support [459485/2014–8] to I.C.M. This work was also supported by Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) via a scholarship awarded to A.D.M. (IBPG-0550-2.03/14) and APQ 0808-2.03/16 to I.C.M. This study was also funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance Code 001.
ACKNOWLEDGEMENTS
We thank Sinzinando L. Albuquerque (photograph credits), Ana B. Silva and Mateus C. Silva for their contribution to data collection on the field, and the PELD Catimbau for accommodation and resources during fieldwork. We also thank Dr Artur M. Wanderley and Dr Ana Carolina G. Costa for their critical review of early drafts of the manuscript, and the two anonymous referees for their helpful suggestions during the revision process.
LITERATURE CITED
- Aarssen LW. 1995. Hypotheses for the evolution of apical dominance in plants: implications for the interpretation of overcompensation. Oikos 74: 149. [Google Scholar]
- Adams T, Purves D, Pacala S. 2007. Understanding height-structured competition in forests: is there an R* for light? Proceedings of the Royal Society B: Biological Sciences 275: 3039–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Lima D. 1981. The Caatinga dominium. Revista Brasileira de Botânica 4: 149–163. [Google Scholar]
- Baker HG. 1961. The adaptation of flowering plants to nocturnal and crepuscular pollinators. The Quarterly Review of Biology 36: 64–73. [Google Scholar]
- Baker HG. 1970. Two cases of bat pollination in Central America. Revista de Biología Tropical 17: 187–197. [Google Scholar]
- Baker HG. 2010. Chemical aspects of the pollination biology of woody plants in the tropics. In: Tomlinson PB, Zimmerman M, eds. Tropical trees as living systems. Cambridge: Cambridge University Press, 57–82. [Google Scholar]
- Barr DJ, Levy R, Scheepers C, Tily HJ. 2013. Random effects structure for confirmatory hypothesis testing: keep it maximal. Journal of Memory and Language 68: 255–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bigio L, Lebel M, Sapir Y. 2016. Do different measures of maternal fitness affect estimation of natural selection on floral traits? A lesson from Linum pubescens (Linaceae). Journal of Plant Ecology 10: 406–413. [Google Scholar]
- Carvalho WDD, Silvestre SM, Mustin K, Hilario RR, Toledo JJD. 2019. Predation of an American fruit-eating bat (Artibeus sp.) by an Amazon tree boa (Corallus hortulanus) in the northern Brazilian Amazon. Acta Amazonica 49: 24–27. [Google Scholar]
- Chiariello NR, Mooney HA, Williams K. 1989. Growth, carbon allocation and cost of plant tissues. In: Pearcy RWet al. , eds. Plant physiological ecology. Dordrecht: Springer, 327–365. [Google Scholar]
- Dafni A, Potts SG. 2004. The role of flower inclination, depth, and height in the preferences of a pollinating beetle (Coleoptera: Glaphyridae). Journal of Insect Behavior 17: 823–834. [Google Scholar]
- Datzmann T, von Helversen O, Mayer F. 2010. Evolution of nectarivory in phyllostomid bats (Phyllostomidae Gray, 1825, Chiroptera: Mammalia). BMC Evolutionary Biology 10: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz UM, Lima SA, Machado IC. 2019. Short-distance pollen dispersal by bats in an urban setting: monitoring the movement of a vertebrate pollinator through fluorescent dyes. Urban Ecosystems 22: 281–291. [Google Scholar]
- Dobat K, Peikert-Holle T. 1985. Blüten und Fledermäuse. Bestäubung durch Fledermäuse und Flughunde (Chiropterophilie), 1st edn. Frankfurt am Main: Verlag Waldemar Kramer. [Google Scholar]
- Donnelly SE, Lortie CJ, Aarssen LW. 1998. Pollination in Verbascum thapsus (Scrophulariaceae): the advantage of being tall. American Journal of Botany 85: 1618–1625. [PubMed] [Google Scholar]
- Faegri K, Van Der Pijl L. 1979. The principles of pollination ecology, 3rd edn. Oxford: Pergamon Press. [Google Scholar]
- Falster DS, Westoby M. 2003. Plant height and evolutionary games. Trends in Ecology & Evolution 18: 337–343. [Google Scholar]
- Fleming TH, Muchhala N. 2008. Nectar‐feeding bird and bat niches in two worlds: pantropical comparisons of vertebrate pollination systems. Journal of Biogeography 35: 764–780. [Google Scholar]
- Fleming TH, Geiselman C, Kress WJ. 2009. The evolution of bat pollination: a phylogenetic perspective. Annals of Botany 104: 1017–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França FGR, Lima RA. 2012. First record of predation on the bat Carollia perspicillata by the false coral snake Oxyrhopus petolarius in the Atlantic Rainforest. Biotemas 25: 307–309. [Google Scholar]
- Gribel R, Gibbs PE, Queiróz A. 1999. Flowering phenology and pollination biology of Ceiba pentandra (Bombacaceae) in Central Amazonia. Journal of Tropical Ecology 15: 247–263. [Google Scholar]
- Grünmeier R. 1993. Bestäubung der Fabaceae Mucuna flagellipes durch Flughunde in Kamerun. In: Barthlott W, Naumann CM, Schmidt-Loske K, Schuchmann KL, eds. Animal–plant interactions in tropical environments. Bonn: Museum A. Koenig, 29–39. [Google Scholar]
- Gruntman M, Groß D, Májeková M, Tielbörger K. 2017. Decision-making in plants under competition. Nature Communications 8: 2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heithaus ER, Fleming TH, Opler PA. 1975. Foraging patterns and resource utilization in seven species of bats in a seasonal tropical forest. Ecology 56: 841–854. [Google Scholar]
- Herms DA, Mattson WJ. 1992. The dilemma of plants: to grow or defend. The Quarterly Review of Biology 67: 283–335. [Google Scholar]
- Hernández‐Montero JR, Sosa VJ. 2016. Reproductive biology of Pachira aquatica Aubl. (Malvaceae: Bombacoideae): a tropical tree pollinated by bats, sphingid moths and honeybees. Plant Species Biology 31: 125–134. [Google Scholar]
- Holland JN, Chamberlain SA. 2007. Ecological and evolutionary mechanisms for low seed: ovule ratios: need for a pluralistic approach?. Ecology 88: 706–715. [DOI] [PubMed] [Google Scholar]
- Hopkins HC, Hopkins MJ. 1982. Predation by a snake of a flower-visiting bat at Parkia nitida (Leguminosae: Mimosoideae). Brittonia 34: 225–227. [Google Scholar]
- Howell DJ, Hartl DL. 1980. Optimal foraging in glossophagine bats: when to give up. The American Naturalist 115: 696–704. [Google Scholar]
- Hu S, Dilcher DL, Jarzen DM, Taylor DW. 2008. Early steps of angiosperm–pollinator coevolution. Proceedings of the National Academy of Sciences 105: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen JT, Tollsten L. 1995. Floral scent in bat‐pollinated plants: a case of convergent evolution. Botanical Journal of the Linnean Society 119: 45–57. [Google Scholar]
- Kress WJ, Stone DE. 1993. Morphology and floral biology of Phenakospermum (Strelitziaceae), an arborescent herb of the Neotropics. Biotropica 25: 290–300. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. Journal of Statistical Software 82: 1–26. [Google Scholar]
- Larson KS, Larson RJ. 1990. Lure of the locks: showiest ladies-tresses orchids, Spiranthes romanzoffiana, affect bumblebee, Bombus spp., foraging behavior. Canadian Field-Naturalist 104: 519–525. [Google Scholar]
- Lemke TO. 1984. Foraging ecology of the long‐nosed bat, Glossophaga soricina, with respect to resource availability. Ecology 65: 538–548. [Google Scholar]
- Lindström SA, Klatt BK, Smith HG, Bommarco R. 2018. Crop management affects pollinator attractiveness and visitation in oilseed rape. Basic and Applied Ecology 26: 82–88. [Google Scholar]
- Lobo JA, Ramos DL, Braga AC. 2016. Visitation rate of pollinators and nectar robbers to the flowers and inflorescences of Tabebuia aurea (Bignoniaceae): effects of floral display size and habitat fragmentation. Botanical Journal of the Linnean Society 181: 667–681. [Google Scholar]
- Lortie CJ, Aarssen LW. 1999. The advantage of being tall: higher flowers receive more pollen in Verbascum thapsus L. (Scrophulariaceae). Ecoscience 6: 68–71. [Google Scholar]
- Luke SG. 2017. Evaluating significance in linear mixed-effects models in R. Behavior Research Methods 49: 1494–1502. [DOI] [PubMed] [Google Scholar]
- Machado IC, Lopes AV. 2004. Floral traits and pollination systems in the Caatinga, a Brazilian tropical dry forest. Annals of Botany 94: 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado IC, Vogel S. 2004. The north‐east‐Brazilian liana, Adenocalymna dichilum (Bignoniaceae) pollinated by bats. Annals of Botany 93: 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado ICS, Sazima I, Sazima M. 1998. Bat pollination of the terrestrial herb Irlbachia alata (Gentianaceae) in northeastern Brazil. Plant Systematics and Evolution 209: 231–237. [Google Scholar]
- Machado IC, Lopes AV, Leite AV, de Brito Neves C. 2006. Cleome spinosa (Capparaceae): Polygamodioecy and pollination by bats in urban and Caatinga areas, northeastern Brazil. Botanische Jahrbücher 127: 69–82. [Google Scholar]
- Marshall AG. 1983. Bats, flowers and fruit: evolutionary relationships in the Old World. Biological Journal of the Linnean Society 20: 115–135. [Google Scholar]
- McLean RA, Sanders WL, Stroup WW. 1991. A unified approach to mixed linear models. The American Statistician 45: 54–64. [Google Scholar]
- Mikula P, Morelli F, Lučan RK, Jones DN, Tryjanowski P. 2016. Bats as prey of diurnal birds: a global perspective. Mammal Review 46: 160–174. [Google Scholar]
- Moles AT, Warton DI, Warman L, et al. 2009. Global patterns in plant height. Journal of Ecology 97: 923–932. [Google Scholar]
- Morris WF, Mangel M, Adler FR. 1995. Mechanisms of pollen deposition by insect pollinators. Evolutionary Ecology 9: 304–317. [Google Scholar]
- Muchhala N. 2003. Exploring the boundary between pollination syndromes: bats and hummingbirds as pollinators of Burmeistera cyclostigmata and B. tenuiflora (Campanulaceae). Oecologia 134: 373–380. [DOI] [PubMed] [Google Scholar]
- Muchhala N. 2006. The pollination biology of Burmeistera (Campanulaceae): specialization and syndromes. American Journal of Botany 93: 1081–1089. [DOI] [PubMed] [Google Scholar]
- Muchhala N, Thomson JD. 2010. Fur versus feathers: pollen delivery by bats and hummingbirds and consequences for pollen production. The American Naturalist 175: 717–726. [DOI] [PubMed] [Google Scholar]
- Niklas K. 2007. Maximum plant height and the biophysical factors that limit it. Tree Physiology 27: 433–440. [DOI] [PubMed] [Google Scholar]
- O’Connell LM, Johnston MO. 1998. Male and female pollination success in a deceptive orchid, a selection study. Ecology 79: 1246–1260. [Google Scholar]
- Peakall R, Handel SN. 1993. Pollinators discriminate among floral heights of a sexually deceptive orchid: implications for selection. Evolution 47: 1681–1687. [DOI] [PubMed] [Google Scholar]
- Piechowski D, Dötterl S, Gottsberger G. 2010. Pollination biology and floral scent chemistry of the Neotropical chiropterophilous Parkia pendula. Plant Biology 12: 172–182. [DOI] [PubMed] [Google Scholar]
- Queiroz JA, Quirino ZGM, Lopes AV, Machado IC. 2016. Vertebrate mixed pollination system in Encholirium spectabile: a bromeliad pollinated by bats, opossum and hummingbirds in a tropical dry forest. Journal of Arid Environments 125: 21–30. [Google Scholar]
- R Development Core Team 2013. R: A language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing; (https://www.r-project.org/) [Google Scholar]
- Santos FDAR, Novaes DM, Queiroz LP. 2012. Pollen of Bauhinia L. and Phanera Lour. (Leguminosae-Caesalpinioideae) from the Brazilian Caatinga. American Journal of Plant Sciences 3: 909–920. [Google Scholar]
- Sazima I, Vogel S, Sazima M. 1989. Bat pollination of Encholirium glaziovii, a terrestrial bromeliad. Plant Systematics and Evolution 168: 167–179. [Google Scholar]
- Sazima M, Sazima I. 1980. Bat visits to Marcgravia myriostigma Tr. et Planch. (Marcgraviaceae) in southeastern Brazil. Flora 169: 84–88. [Google Scholar]
- Sazima M, Buzato S, Sazima I. 1999. Bat-pollinated flower assemblages and bat visitors at two Atlantic forest sites in Brazil. Annals of Botany 83: 705–712. [Google Scholar]
- Schnitzler HU, Kalko EKV. 1998. How echolocating bats search and find food. In: Kunz TH, Racey PA, eds. Bat biology and conservation. Washington, DC: Smithsonian Institution Press, 183–196. [Google Scholar]
- Schnitzler HU, Moss CF, Denzinger A. 2003. From spatial orientation to food acquisition in echolocating bats. Trends in Ecology & Evolution 18: 386–394. [Google Scholar]
- Skogsmyr I, Lankinen Å. 1999. Selection on pollen competitive ability in relation to stochastic factors influencing pollen deposition. Evolutionary Ecology Research 1: 971–985. [Google Scholar]
- Srithongchuay T, Bumrungsri S, Sripao-raya E. 2008. The pollination ecology of the late-successional tree, Oroxylum indicum (Bignoniaceae) in Thailand. Journal of Tropical Ecology 24: 477–484. [Google Scholar]
- Tripp EA, Manos PS. 2008. Is floral specialization an evolutionary dead‐end? Pollination system transitions in Ruellia (Acanthaceae). Evolution: International Journal of Organic Evolution 62: 1712–1737. [DOI] [PubMed] [Google Scholar]
- Tschapka M, von Helversen O. 1999. Pollinators of syntopic Marcgravia species in Costa Rican lowland rain forest: bats and opossums. Plant Biology 1: 382–388. [Google Scholar]
- Tschapka M, von Helversen O. 2007. Phenology, nectar production and visitation behaviour of bats on the flowers of the bromeliad Werauhia gladioliflora in a Costa Rican lowland rain forest. Journal of Tropical Ecology 23: 385–395. [Google Scholar]
- Tschapka M, von Helversen O, Barthlott W. 1999. Bat pollination of Weberocereus tunilla, an epiphytic rain forest cactus with functional flagelliflory. Plant Biology 1: 554–559. [Google Scholar]
- Vaughton G, Ramsey M. 1998. Floral display, pollinator visitation and reproductive success in the dioecious perennial herb Wurmbea dioica (Liliaceae). Oecologia 115: 93–101. [DOI] [PubMed] [Google Scholar]
- Vogel S, Machado IC, Lopes AV. 2004. Harpochilus neesianus and other novel cases of chiropterophily in neotropical Acanthaceae. Taxon 53: 55–60. [Google Scholar]
- Vogel S, Lopes AV, Machado IC. 2005. Bat pollination in the NE Brazilian endemic Mimosa lewisii: an unusual case and first report for the genus. Taxon 54: 693–700. [Google Scholar]
- Voigt CC, Speakman JR. 2007. Nectar‐feeding bats fuel their high metabolism directly with exogenous carbohydrates. Functional Ecology 21: 913–921. [Google Scholar]
- Voigt CC, Kelm DH, Visser GH. 2006. Field metabolic rates of phytophagous bats: do pollination strategies of plants make life of nectar-feeders spin faster? Journal of Comparative Physiology B 176: 213–222. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Schuller BM, Greif S, Siemers BM. 2010. Perch-hunting in insectivorous Rhinolophus bats is related to the high energy costs of manoeuvring in flight. Journal of Comparative Physiology B 180: 1079–1088. [DOI] [PubMed] [Google Scholar]
- Voigt CC, Frick WF, Holderied MW, et al. 2017. Principles and patterns of bat movements: from aerodynamics to ecology. The Quarterly Review of Biology 92: 267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Helversen D, Holderied MW, von Helversen O. 2003. Echoes of bat-pollinated bell-shaped flowers: conspicuous for nectar-feeding bats? Journal of Experimental Biology 206: 1025–1034. [DOI] [PubMed] [Google Scholar]
- von Helversen O. 1993. Adaptations of flowers to pollination by glossophagine bats. In: Barthlott W, Naumann CM, Schmidt-Loske K, Schuchmann KL, eds. Animal-plant interactions in tropical environments. Bonn: Zoologisches Forschungsinstitut und Museum Alexander König, 41–59. [Google Scholar]
- von Helversen O, Winter Y. 2005. Glossophagine bats and their flowers: Costs and benefits for plants and pollinators. In: Kunz TH, Fenton MB, eds. Bat ecology. Chicago, IL: The University of Chicago Press, 346–397. [Google Scholar]
- Weberling F. 1992. Morphology of flowers and inflorescences, 2nd edn. Cambridge: Cambridge University Press. [Google Scholar]
- Welch KC, Suarez RK. 2008. Dietary sugar as a direct fuel for flight in the nectarivorous bat Glossophaga soricina. Journal of Experimental Biology 211: 310–316. [DOI] [PubMed] [Google Scholar]
- Wickham H. 2016. ggplot2: Elegant graphics for data analysis, 2nd edn. New York, NY: Springer. [Google Scholar]
- Willmer P. 2011. Pollination and floral ecology, 1st edn. Princeton, NJ: Princeton University Press. [Google Scholar]