Abstract
Background and Aims
Through careful field examination of the growth habit of the gametophytes and sporophytes of Hymenasplenium volubile across an ontogenetic series, we aim to understand better the evolution of epiphytism in this poorly understood group of ferns
Methods
We made field observations of H. volubile sporophytes and gametophytes, and brought specimens back to the lab for microscopic analysis. In the field, sporophytes at each ontogenetic stage were photographed to document the species’ growth habit. We used an existing phylogeny to optimize growth form of New World Hymenasplenium.
Key Results
Young sporophytes were at first fully epiphytic and produced one or two long feeding roots that extend to the soil where they branch profusely. The feeding roots remain in contact with the soil throughout the life of the plant. Thus, H. volubile is a hemiepiphyte. While immature, gametophytes are appressed to the tree trunk, but, as their gametangia mature, their lower margin lifts upward, imparting a shelf-like appearance to the thallus. The thallus attaches to the substrate by branched rhizoids produced along the margin of the thallus in contact with the substrate.
Conclusions
Hemiepiphytes are a key link in the evolution of epiphytic ferns and may act as a bridge between the forest floor and the canopy. Our finding is the first report of hemiepiphytism in Aspleniaceae, a large lineage with many epiphytic and terrestrial taxa. This work serves as an important model to understand the evolution of epiphytism in this group specifically and in ferns in general. The majority of our understanding of fern gametophyte biology is derived from laboratory studies. Our efforts represent a fundamental contribution to understanding fern gametophyte ecology in a field setting.
Keywords: Ferns, growth habits, systematics, ecology, gametophytes, hemiepiphyte, Aspleniaceae, Hymenasplenium, Hymenasplenium volubile
INTRODUCTION
The Aspleniaceae is a cosmopolitan lineage that contains >750 species (PPG, 2016). Its two main clades are well defined and currently recognized as genera. The first is Asplenium L., with about 700 species, and the second is Hymenasplenium Hayata, with about 60 species (Murakami, 1995; Xu et al., 2018). The latter genus, which is the subject of this study, has been strongly supported by molecular phylogenetic studies (Murakami and Schaal, 1994; Murakami, 1995; Schneider et al., 2004; Ohlsen et al., 2014; Loriga et al., 2017; Sessa et al., 2018). At an organismal level, it differs from Asplenium by creeping rhizomes, dorsiventral steles, a unique rachis–costae structure and a chromosome base numbers of x = 38 or 39 (Hayata, 1927, 1928; Iwatsuki, 1975a, b; Murakami, 1992; Murakami and Moran, 1993; Murakami and Schaal, 1994). Hymenasplenium is pantropical, and most of its approx. 60 species are Asian (Xu et al., 2018). However, 11 of the species (and three hybrids) occur in the Neotropics (Murakami and Moran, 1993; Moran and Sundue, 2004). These Neotropical species form a clade sister to the Paleotropical species (Xu et al., 2018).
The Neotropical species display several growth forms. A few are terrestrial and grow on wet, shaded forest floors, such as H. basicopicum (R. C. Moran & Sundue) L. Regalado & Prada, H. delitescens (Maxon) L. Regalado & Prad and H. purpurascens (Mett. ex Kuhn) L. Regalado & Prada. Most are associated with rocky habitats, typically growing on wet rocks or boulders in or along stream beds, such as H. hoffmannii (Hieron.) L. Regalado & Prada and H. triquetrum (N. Murak. & R. C. Moran) L. Regalado & Prada. One epipetric species, H. obtusifolium (L.) L. Regalado & Prada, is notable because it also thrives near or under the constant spray of waterfalls (Murakami and Moran, 1993). Yet another species, H. riparium (Liebm.) L. Regalado & Prada, is a rheophyte, growing on rocks in streams that are flooded periodically by fast-flowing water (the authors, pers. obs.). Perhaps the most distinctive growth form found among the Neotropical species is that of H. repandulum (Kunze) L. Regalado & Prada and H. volubile (N. Murak. & R. C. Moran) L. Regalado & Prada. These species have the longest creeping rhizomes in the genus (Murakami, 1992). They are climbing and were described as ‘epiphytic’ by Murakami and Moran (1993), who monographed the Neotropical species of the genus.
During fieldwork in Costa Rica, we had the opportunity to observe a population of H. volubile. We found both its gametophytes and sporophytes. Our observations were prompted by scepticism that H. volubile was really an epiphyte as stated by Murakami and Moran (1993). Instead, we suspected it might be a hemiepiphyte; i.e. a plant fully epiphytic for part of its life but eventually contacting the soil by means of roots, and maintaining that connection for the rest of its life (Zotz, 2013a, 2016). This life form in ferns has been either overlooked in species that are true hemiepiphytes or attributed incorrectly to species that are terrestrial root climbers (Canestraro et al., 2014). Only recently has hemiepiphytism in ferns been documented by fieldwork. These studies dealt with species from a diverse array of families of ferns, such as Vandenboschia collariata (Bosch) Ebihara & K. Iwats. in the Hymenophyllaceae (Nitta and Epps, 2009), Elaphoglossum amygdalifolium (Mett. ex Kuhn) Christ in the Dryopteridaceae (Lagomarsino et al., 2012) and Colysis ampla Copel. in the Polypodiaceae (Testo and Sundue, 2014). Hemiepiphytism also occurs in most species of Lomariopsis in the Lomariopsidaceae (the authors, pers. obs.). Here we study the growth habit of H. volubile to determine whether it is an epiphyte, hemiepiphyte or terrestrial root climber. We also describe its gametophyes and, for the first time, report their unusual changes in growth habit as they mature.
MATERIALS AND METHODS
We studied H. volubile at the Las Cruces Biological Station near the town of San Vito, Costa Rica in Puntarenas. The station is located in the southern Pacific Highlands close to the border with Panama (8°47′7’’N, 82°57′32’’W). The station harbours 365 ha of largely pre-montane forest. At the station, H. volubile grows along the Melissa Trail, at about 1000 m elevation, where the trail is crossed by a small stream. The fern occurred in a shaded, mesic habitat along the banks of this stream. The sporophyte population consisted of many large fertile plants on the north side of the stream bank, and it stretched about 200 m upstream from where the stream crossed the trail.
The gametophytes were identified by finding small sporophytes attached and tracing these to successively larger ones that could be readily assigned as H. volubile (Fig. 1). We observed about 20 sporophyte individuals, and several hundred gametophytes on about 30 tree trunks. Of the gametophytes, 28 were examined in the ‘shelf stage’ of development with mature gametangia. Gametophytes and sporophytes were photographed in the field, and gametophytes were brought back to the lab (permit nos. No PI-R-072-2018 to J. E. Watkins Jr) for microscopic examination and photographic documentation.
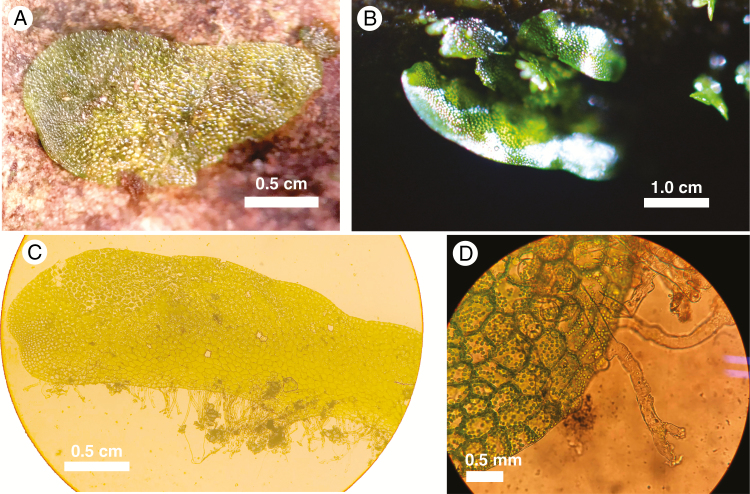
Fig. 1.
Hymenasplenium volubile gametophytes. (A) Immature (without gametangia), appressed to bark of the host tree. (B) Mature (with gametangia), with one edge elevated from the substrate, termed the ‘shelf stage’. (C) Gametophyte with pluricellular meristem and ventral rhizoids. (D) Gametophyte with branched rhizomes. Scale bars are approximate.
We compared the growth form of H. volubile with that of H. repandulum and H. riparium. We focused on H. repandulum because it has been previously described as ‘epiphytic’ by Murakami and Moran (1993), and H. riparium because it is a common rheophyte in Costa Rica. Specimens were examined at NY (New York Botanical Garden) and images were consulted on the online database https://www.gbif.org/ (GBIF.org, 2019). From the latter, we downloaded images of all three species and processed them in Photoshop to prepare black and white silhouettes for morphological comparison. Additional images of H. riparium and H. volubile may be found at www.plantsystematics.org. For phylogenetic inference, we redrew the majority rule consensus tree from Murakami and Schaal (1994) and reconstructed the ancestral growth habit under maximum likelihood using the R package ‘phytools’. The reconstruction was carried out under a continuous-time Markov chain model with an equal rate character state transition matrix (Lewis, 2001) using the ‘ace function of the R package ‘ape’ (Paradis et al., 2004) and the ‘lik.anc’ function from ‘phytools’ (Revell, 2012).
RESULTS
Gametophytes
All gametophytes of Hymenasplenium volubile were found growing epiphytically; none was on the soil. They occurred on small diameter angiosperm trees [<10 cm diameter at breast height (DBH)] that represented several unidentified species. Most of the gametophytes were situated <1 m above the soil.
Morphologically, the gametophytes were strap-shaped and unbranched, with a pluricellular meristem located in a well-defined apical notch (Fig. 1A–C). The thalli were oriented horizontally or nearly so. Gemma and hairs were absent. Rhizoids were produced only along the upper margin of the thallus (i.e. nearest the substrate), and they were frequently branched at the tip (Fig. 1D). The thalli of H. volubile correspond to the Type II gametophyte type of Farrar et al. (2008).
Immature gametophytes were appressed to the tree bark (Fig. 1A). When larger, their lower margins lifted upward so that the thallus assumed a shelf-like appearance. The side against the substrate was flattened and attached to the bark by the rhizoids, and the distal side extended horizontally (Fig. 1A). Of the gametophytes examined in the ‘shelf stage’, most were sexually mature and several were found with archegonia (but no antheridia) just below the apical notch. The archegonia were typical of other leptosporangiate species, being composed of four rows of neck cells. We microscopically examined dozens of gametophytes that had not reached the shelf stage, and all were sexually immature. When present, the first few sporeling leaves were produced at or near the apical meristem of thalli, and only those thalli producing the shelf stage were observed to make sporophytes. No polyembryony was observed; only one sporeling was produced per gametophyte. The sporeling leaves emerged between the substrate and the gametophyte, not on the side of the uplifted margin of the thallus.
Sporophytes
In their earliest stages, the sporelings are fully epiphytic because they were produced from epiphytic gametophytes. While the first few leaves emerge, the sporeling produces long, delicate, unbranched roots that extend toward the forest floor. Upon contact with the soil, the roots branch (Fig. 2D). Root proliferations (young plantlets vegetatively produced from the roots) were not observed.
Fig. 2.
Hymenasplenium volubile sporophytes. (A) Climbing habit. (B) Creeping rhizome with several dark clasping roots faintly visible, about 1 m from the ground on a small diameter tree trunk. (C) Specimen removed from tree trunk (at right), showing long feeding roots attached to the mineral soil. (D) Habit of plant detached from the host and soil. Arrows indicate where roots branch when in contact with the mineral soil. Scale bars are approximate.
After the roots from the sporeling touch the soil, the rhizome begins to climb (Fig. 2A–C). It produces two kinds of roots. The first are long unbranched feeding roots – the same kind as produced by the sporelings. On large mature plants, several feeding roots are typically seen on the proximal portions of the climbing rhizomes (Fig. 2C, D). The roots extend to the soil, where they branch profusely. The second kind of roots are clasping roots. These anchor the rhizome to the host. They are generally 1–2 cm long, tightly appressed to the substrate and oriented more or less perpendicularly to the rhizome (Fig. 2B). Both types of roots – feeding and clasping – persist for the life of the plant.
All climbing rhizomes started their growth at ≥0.25 above the soil. The apex of the highest climbing rhizome was about 1.2 m above the soil.
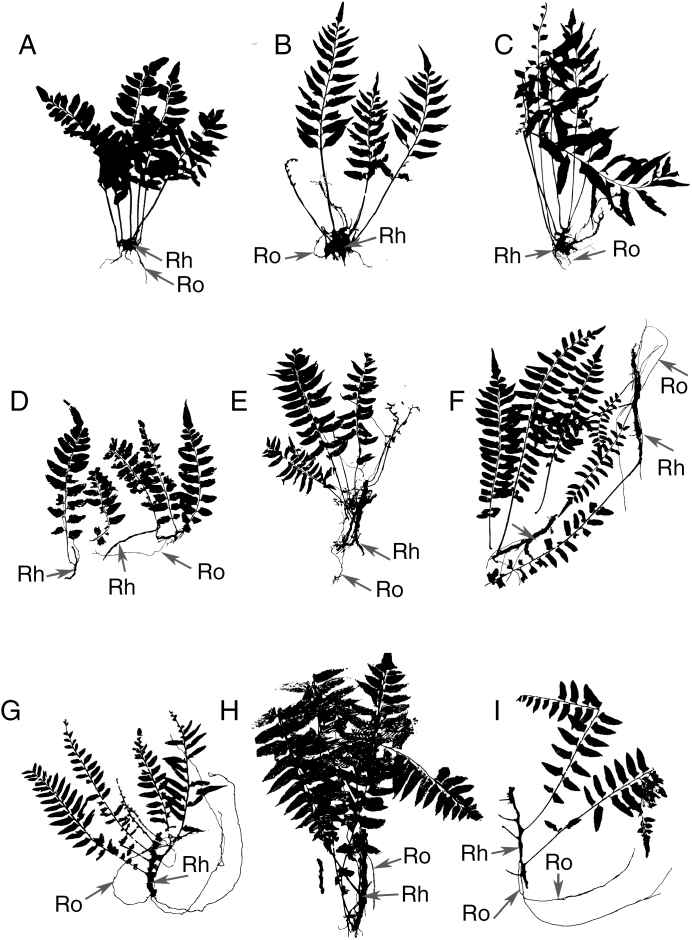
Many herbarium sheets of H. repandulum have long creeping rhizomes and long, unbranched feeding roots like those seen during fieldwork on H. volubile (Fig. 3G–I). Hymenasplenium riparium has shorter creeping rhizomes and shorter roots compared with those of H. repandulum and H. volubile.
Fig. 3.
Comparison of three Neotropical species of Hymenasplenium. (A–C) Hymenasplenium riparium. (D–F) Hymenasplenium volubile. (G–I) Hymenasplenium repandulum. Rh, rhizome, Ro, root. Top row: (H. riparium) A (left), Costa Rica, J. D. Smith 6885 (USA). B (middle), Mexico, D. E. Breedlove 35410 (MO). C (right), Mexico, D. E. Breedlove 26082 (F). Middle row: (H. volubile) D (left), Costa Rica, Gómez et al. 19195 (CR). E (middle), Colombia, Cuatrecasas 8607 (US). F (right), Cuatrecasas 8607 (US). Bottom row: (H. repandulum) G (left), Ecuador, Grubb et al. 1502 (NY). H (middle), Peru, J. D. Smith 5168 (MO). I (right), Ecuador, Ayala 57 (QCA).
DISCUSSION
Growth habit
Our field studies show that Hymenasplenium volubile is a hemiepiphyte sensuZotz (2013a, 2016). The young sporophytes (being borne on epiphytic gametophytes) are at first fully epiphytic. They emit long unbranched roots – the feeding roots – that then grow straight downward into the soil, thus establishing a connection with the ground. This connection is maintained throughout the life of the plant. This is the first report of the hemiepiphytic growth habit in the Aspleniaceae and adds to our growing understanding of the occurrence of this markedly rare growth form in ferns.
Hemiepiphytes are a special category of plants that do not fit the definition of either terrestrial or epiphytic plants. Instead, they fit into their own category because they share characteristics of both terrestrial and epiphytic plants during different stages of their development. Historically, hemiepiphytes have been divided into two groups: primary hemiepiphytes, those that initiate as epiphytes and extend roots down to the terrestrial soil (Benzing, 1990; Kress, 1989); and secondary hemiepiphytes, those that start on the soil, climb into the canopy and eventually lose their terrestrial connections (Holbrook and Putz, 1996). Hymenasplenium volubile fits clearly within the concept of the former. A great deal of attention has been focused on the concept of secondary hemiepiphytes. Zotz (2013a, 2016) considered this growth habit unlikely. In an unpublished survey of hundreds of aroids previously described as secondary hemiepiphytes, Zotz (2016) found that all maintained their connections to the soil after climbing; none lost the connection. What advantage would there be to a terrestrially rooted climbing plant to later lose its connection to the soil and its store of water and nutrients? While Zotz (2016) did not challenge the existence of this growth form, he proposed that such plants, if they exist, would not be homologous to hemiepiphytes, and prefers the term nomadic vine sensuMoffett (2000). We found no evidence that H. volubile lost connection to the soil.
We know of no fern species that have been demonstrated to be secondary hemiepiphytes or nomadic vines; however, we know of many that have been documented as terrestrial root climbers, such as Mickelia (Gay, 1993; Hebantmauri and Gay, 1993; Moran et al., 2010) and Polybotrya (Young and León, 1991; Canestraro et al., 2014). Terrestrial root climbers have frequently been mistaken for hemiepiphytes. In both, gametophytes typically recruit on rotting logs or rocks in damp understorey habitats. Following sporophyte formation, the species grow down from these substrates and produce elongated rhizomes that grow across the forest floor until they contact a trunk (Canestraro et al., 2014). They then grow up the trunk and, similarly to H. volubile, produce clasping roots that attach to the host. In these two genera, contact with the soil is always maintained. These observations are only possible by applying a detailed life cycle perspective in the field.
In ferns, both hemiepiphytes and terrestrial root climbers display dimorphic roots, which are differentiated as either feeding or clasping. Presumably, the feeding roots transport water and mineral nutrients from the soil, but it is less certain whether the clasping roots absorb anything. Their function might be purely for attachment of the rhizome. Root dimorphy has been documented in other ferns, such as the hemiepiphytes Elaphoglossum amygdalifolium (Dryopteridaceae; Lagomarsino et al., 2012) and Vandenboschia collariata (Hymenophyllaceae; Nitta and Epps, 2009), and the terrestrial root climbers Mickelia (the authors, pers. obs.), Polybotrya (Canestraro et al., 2014) and Colysis ampla (Testo and Sundue, 2014). We did not notice vegetative proliferations or plantlets from the roots, regardless of whether the roots were in the soil or on the trunks. This does not support the claim by Murakami and Moran (1993) that all the New World species of Hymenasplenium have proliferous roots, a character thought to distinguish them from the Old World species.
Gametophytes
The gametophytes of H. volubile exhibit a behaviour only recently recorded in ferns (Watkins and Moran, 2019). The thalli are at first appressed to the substrate, but, as their gametangia mature, the lower edge of the thallus lifts upward, away from the substrate, creating a shelf-like form. This pattern of gametophyte development has been reported in Lomariopsis and Dracoglossum (Lomariopsidaceae; Watkins and Moran, 2019). Notably, Lomariopsis is also a hemiepiphyte. Laterally attached shelf-like gametophytes are common in epiphytic ferns with elongate thalli (Atkinson and Stokey, 1964; Dassler and Farrar, 1997; Farrar et al., 2008). What is unusual is the shift from an appressed form to the shelf form, which apparently accompanies sexual maturity. This shift might facilitate access to the archegonia by the sperm or may be a phototrophic response, but these ideas have not been evaluated.
Our work represents only the second study to examine gametophyte morphology in Hymenasplenium. Regalado and Prada (2011) cultivated gametophytes in agarose cultures from the spores of H. delitescens collected in Cuba. Similarly to H. volubile, they report that the gametophytes of this species are elongate, glabrous, and without gemmae. They do not report the occurrence of the shelf form in sexually mature thalli, yet such a form is unlikely to develop in agarose culture. Unlike our field collections of H. volubile that were either asexual or archegoniate, they found that some of the gametophytes in culture became hermaphroditic, producing antheridia and archegonia mixed along the central cushion. In spite of the presence of mixed gametangia, their cultures failed to produce sporophytes after 2 years of cultivation. This suggests that the species may be an obligate outcrosser and carries sufficient genetic load to prevent gametophytic and/or sporophytic selfing (Haufler et al., 2016). Given the success of their cultures, it is unlikely that culture conditions hindered sporophyte production. It remains unclear as to how gametophyte breeding systems vary between in situ culture and field conditions. Limited, unpublished evidence (Ranker and Houston, 2002; C. Riedel and D. Farrar, pers. com.) suggests that gametophyte sexuality is similar in the field and lab, but more work needs to be done in this area.
A consistent pattern observed for hemiepiphytic ferns is that their epiphytic gametophytes typically occur within 2 m of the forest floor (J. E. Watkins and R. C. Moran, unpubl. data). We found a similar pattern in H. volubile, whose gametophytes were situated ≥1 m above the soil. This might be related to higher humidity closer to the ground, which would decrease chances of drying. Fern gametophytes, however, have been found to be more resistant to drought and desiccation than previously thought (Watkins et al., 2007; Watkins and Cardelús, 2012; Pittermann et al., 2013). The gametophytes of epiphytic ferns can be particularly desiccation tolerant (Watkins et al., 2007). Nothing is known about the desiccation tolerance of H. volubile gametophytes, but most species in the genus occur along humid stream beds and on rocks in streams. This suggests that the gametophytes of this group are more sensitive to drought than other hemiepiphytic taxa. An issue worthy of more study is the degree of tolerance in the early prothallial stages of gametophyte development. While large mature gametophytes can sometimes be tolerant, it is not clear how younger stages respond to stress. It is possible that these stages are particularly sensitive, yet studies that examine stage-based tolerance in gametophytes are lacking.
Hymenasplenium repandulum and H. volubile were cited by Murakami and Moran (1993) as epiphytes, and this descriptor is often seen on herbarium labels. Many herbarium specimens of H. repandulum (e.g. Guiana, Boudrie et al. 4319, NY; Peru, Monteagudo et al. 14918, NY) have long feeding roots (Fig. 3G–I). Both species have creeping rhizomes, and both have been documented to have the longest internodes of any Neotropical Hymenasplenium (Murakami, 1992). Judging from herbarium specimens, H. repandulum is probably a hemiepiphyte just like H. volubile. The two species have been resolved as sister in the molecular phylogenetic analysis of Murakami and Schaal (1994). In turn, they were sister to H. riparium, and all three of these species were sister to H. obtusifolium (Fig. 4). Because H. riparium and H. obtusifolium, the two closest outgroup species, are associated with rocky habitats in or along streams, it is most parsimonious to assume that the hemiepiphytic habit of H. repandulum and H. volubile was derived once from an epipetric habit (Murakami and Schaal, 1994).
Fig. 4.
Phylogeny of American Hymenasplenium showing life forms optimized on the branches. The tree, and the character states mapped onto it, were scored from Murakami and Schaal (1994).
Tracing the evolution of epiphytism has long been a goal of many botanists (Kress, 1989; Benzing, 1990; Wikstrom et al., 1999; Gravendeel et al., 2004; Tsutsumi and Kato, 2006; Watkins and Cardelús, 2012; Zotz, 2013b; Sosa et al., 2016). Much of this work has been driven by attempts to understand mechanisms of radiation into such radically different environments. The epiphytic environment differs greatly from the terrestrial one. The former has reduced nutrient pools (Nadkarni et al., 2002, 2004; Cardelús, 2010), less water availability and decreased humidity, and at the same time an increase in temperature and light levels (Cardelús and Chazdon, 2005; Watkins and Cardelús, 2009). These differences have driven remarkable functional adaptations in epiphytic plants: from extreme drought and desiccation tolerance (Watkins and Cardelús, 2012; Gaff and Oliver, 2013; Pittermann et al., 2013) to morphological and physiological adaptations affording protection from excess light (Tausz et al., 2001; Watkins et al., 2006), and a suite of traits linked with nutrient and water uptake and use efficiency (Benzing, 1990; Pierce et al., 2002; Winkler and Zotz, 2010; Watkins and Cardelús, 2012). Collectively, these studies demonstrate that life in the epiphytic habitat requires unique and complex adaptations. While there are dozens of hemiepiphytes described in angiosperms (Zotz, 2013a, 2016), with our current study taken into account, there are only a small number of fern species properly documented as hemiepiphytes (Nitta and Epps, 2009; Lagomarsino et al., 2012; Testo and Sundue, 2014; Watkins and Moran, 2019). Such rarity of hemiepiphytes in ferns is probably explained by the complex requirements of gametophytic and sporophytic adaptations to both the epiphytic and terrestrial habitats. Given their complex biology, what are the pathways to epiphytism and hemiepiphytism? Hymenasplenium volubile and its relatives provide an interesting group to study this question. This group displays a transition from terrestrial to epipetric to hemiepiphytic forms (Fig. 4). It might be that the rupestral ecology acted as a bridge into the hemiepiphytic form. The hemiepiphytic form may thus be a bridge into the epiphytic habit for some species (Watkins and Cardelús, 2012). Careful field work that examines ferns from a life cycle perspective as demonstrated by this study are critical to elucidating organismal ecology that will be required to answer such questions.
ACKNOWLEDGEMENTS
We are particularly indebted to Weston Testo for his very careful review of this manuscript and for his help in the reconstruction of the ancestral growth habit in Fig. 4. We also thank Emily Sessa for early advice on this manuscript, and the Costa Rican government agencies SINAC (Sistema Nacional de Áreas de Conservación) and MINAE (Ministerio de Ambiente y Energía) for permission to study plants in Costa Rica (Permit numbers SINAC-ACC-PI-R: 036-2017; 072-2018 and 133–2018).
LITERATURE CITED
- Atkinson LR, Stokey AG. 1964. Comparative morphology of the gametophytes of homosporous ferns. Phytomorphology 14: 51–70. [Google Scholar]
- Benzing DH. 1990. Vascular epiphytes: general biology and related biota. Cambridge: Cambridge University Press. [Google Scholar]
- Canestraro BK, Moran RC, Watkins JE Jr. 2014. Reproductive and physiological ecology of climbing and terrestrial Polybotrya (Dryopteridaceae) at the La Selva Biological Station, Costa Rica. International Journal of Plant Sciences 175: 432–441. [Google Scholar]
- Cardelús CL. 2010. Litter decomposition within the canopy and forest floor of three tree species in a tropical lowland rain forest, Costa Rica. Biotropica 42: 300–308. [Google Scholar]
- Cardelús CL, Chazdon RL. 2005. Inner-crown microenvironments of two emergent tree species in a lowland wet forest. Biotropica 37: 238–244. [Google Scholar]
- Dassler CL, Farrar DR. 1997. Significance of form in fern gametophytes: clonal, gemmiferous gametophytes of Callistopteris baueriana (Hymenophyllaceae). International Journal of Plant Sciences 158: 622–639. [Google Scholar]
- Farrar DR, Dassler C, Watkins JE Jr, Skelton C. 2008. Gametophyte ecology. In: Ranker TA, Haufler CH, eds. Biology and evolution of ferns and lycophytes. Cambridge: Cambridge University Press, 222N256. [Google Scholar]
- Gaff DF, Oliver M. 2013. The evolution of desiccation tolerance in angiosperm plants: a rare yet common phenomenon. Functional Plant Biology 40: 315–328. [DOI] [PubMed] [Google Scholar]
- Gay H. 1993. The architecture of a dimorphic clonal fern, Lomagramma guianensis (Aublet) Ching (Dryopteridaceae). Botanical Journal of the Linnean Society 111: 343–358. [Google Scholar]
- GBIF.org 2019. GBIF home page Available from: https://www.gbif.org [12 February 2018].
- Gravendeel B, Smithson A, Slik F, Schuiteman A. 2004. Epiphytism and pollinator specialization: drivers for orchid diversity? Philosophical Transactions of the Royal Society B: Biological Sciences 359: 1523–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler C, Pryer K, Schuettpelz E, et al. . 2016. Sex and the single gametophyte: revising the homosporous vascular plant life cycle in light of contemporary research. Bioscience 66: 928–937. [Google Scholar]
- Hayata B. 1927. On the systematic importance of the stelar system in the Filicales, I. Botanical Magazine 41: 697–718. [Google Scholar]
- Hayata B. 1928. On the systematic importance of the stelar system in the Filicales. III. Botanical Magazine 42: 334–348. [Google Scholar]
- Hebantmauri R, Gay H. 1993. Morphogenesis and its relation to architecture in the dimorphic clonal fern Lomagramma guianensis (Aublet) Ching (Dryopteridaceae). Botanical Journal of the Linnean Society 112: 257–276. [Google Scholar]
- Holbrook NM, Putz FE. 1996. Of tropical vines and hemiepiphytes: plants that climb up and plants that climb down. In: Mulkey SS, Chazdon RL, Smith AP, eds. Tropical forest plant ecophysiology. New York: Chapman Hall, 363–394. [Google Scholar]
- Iwatsuki K. 1975a Stelar structure of Asplenium unilaterale and its allied species. Kalikasan 4: 165–174. [Google Scholar]
- Iwatsuki K. 1975b Taxonomic studies of Pteridophyta X. Acta Phytotaxonomica et Geobotanica 27: 39–55. [Google Scholar]
- Kress W. 1989. The systematic distribution of vascular epiphytes. In: Lüttge U, ed. Vascular plants as epiphytes. Ecological Studies (Analysis and Synthesis), vol. 76 Berlin, Heidelberg: Springer, 234–261. [Google Scholar]
- Lagomarsino LP, Grusz AL, Moran RC. 2012. Primary hemiepiphytism and gametophyte morphology in Elaphoglossum amygdalifolium (Dryopteridaceae). Brittonia 64: 226–235. [Google Scholar]
- Lewis PO. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology 50: 913–925. [DOI] [PubMed] [Google Scholar]
- Loriga J, Regalado L, Prada C, Schneider H, Heinrichs J. 2017. Phylogenetic relationships of two Cuban spleenworts with unusual morphology: Asplenium (Schaffneria) nigripes and Asplenium pumilum (Aspleniaceae, leptosporangiate ferns). Plant Systematics and Evolution 303: 165–176. [Google Scholar]
- Moffett MW. 2000. What’s ‘up’? A critical look at the basic terms of canopy biology. Biotropica 32: 569–596. [Google Scholar]
- Moran R, Sundue M. 2004. Asplenium (sect. Hymenasplenium) basiscopicum, a new species from Bolivia. Brittonia 56: 124–127. [Google Scholar]
- Moran RC, Labiak PH, Sundue M. 2010. Synopsis of Mickelia, a newly recognized genus of bolbitidoid ferns (Dryopteridaceae). Brittonia 62: 337–356. [Google Scholar]
- Murakami N. 1992. Stelar structure of Asplenium obtusifolium and its allied species in the New World tropics, with comparison to the Asian members of Asplenium sect. Hymenasplenium. Botanical Magazine 105: 135–147. [Google Scholar]
- Murakami N. 1995. Systematics and evolutionary biology of the fern genus Hymenasplenium (Aspleniaceae). Journal of Plant Research 108: 257–268. [Google Scholar]
- Murakami N, Moran R. 1993. Monograph of the neotropical species of Asplenium sect Hymenasplenium (Aspleniaceae). Annals of the Missouri Botanical Garden 80: 1–38. [Google Scholar]
- Murakami N, Schaal B. 1994. Chloroplast DNA variation in the phylogeny of Asplenium sect. Hymenasplenium (Aspleniaceae) in the New World tropics. Journal of Plant Research 107: 245–251. [Google Scholar]
- Nadkarni NM, Schaefer D, Matelson TJ, Solano R. 2002. Comparison of arboreal and terrestrial soil characteristics in a lower montane forest, Monteverde, Costa Rica. Pedobiologia 46: 24–33. [Google Scholar]
- Nadkarni NM, Schaefer D, Matelson TJ, Solano R. 2004. Biomass and nutrient pools of canopy and terrestrial components in a primary and a secondary montane cloud forest, Costa Rica. Forest Ecology and Management 198: 223–236. [Google Scholar]
- Nitta JH, Epps MJ. 2009. Hemi-epiphytism in Vandenboschia collariata (Hymenophyllaceae). Brittonia 61: 392–397. [Google Scholar]
- Ohlsen D, Perrie L, Shepherd L, Brownsey P, Bayly M. 2014. Phylogeny of the fern family Aspleniaceae in Australasia and the south-western Pacific. Australian Systematic Botany 27: 355–371. [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Pierce S, Winter K, Griffiths H. 2002. The role of CAM in high rainfall cloud forests: an in situ comparison of photosynthetic pathways in Bromeliaceae. Plant, Cell & Environment 25: 1181–1189. [Google Scholar]
- Pittermann J, Brodersen C, Watkins J. 2013. The physiological resilience of fern sporophytes and gametophytes: advances in water relations offer new insights into an old lineage. Frontiers in Plant Science 4: 285. doi.org/10.3389/fpls.2013.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PPG 2016. A community-derived classification for extant lycophytes and ferns. Journal of Systematics and Evolution 54: 563–603. [Google Scholar]
- Ranker TA, Houston HA. 2002. Is gametophyte sexuality in the laboratory a good predictor of sexuality in nature? American Fern Journal 92: 112–119. [Google Scholar]
- Regalado L, Prada C. 2011. The genus Hymenasplenium (Aspleniaceae) in Cuba, including new combinations for the neotropical species. American Fern Journal 101: 265–281. [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Schneider H, Russell S, Cox C, et al. . 2004. Chloroplast phylogeny of asplenioid ferns based on rbcL and trnL-F spacer sequences (Polypodiidae, aspleniaceae) and its implications for biogeography. Systematic Botany 29: 260–274. [Google Scholar]
- Sessa E, Vicent M, Chambers S, Galan J. 2018. Evolution and reciprocal origins in Mediterranean ferns: the Asplenium obovatum and Adiantum nigrum complexes. Annals of the Missouri Botanical Garden 103: 175–187. [Google Scholar]
- Sosa V, Cameron K, Angulo D, Hernandez-Hernandez T. 2016. Life form evolution in epidendroid orchids: ecological consequences of the shift from epiphytism to terrestrial habit in Hexalectris. Taxon 65: 235–248. [Google Scholar]
- Tausz M, Hietz P, Briones O. 2001. The significance of carotenoids and tocopherols in photoprotection of seven epiphytic fern species of a Mexican cloud forest. Australian Journal of Plant Physiology 28: 775–783. [Google Scholar]
- Testo W, Sundue M. 2014. Primary hemiepiphytism in Colysis ampla (Polypodiaceae) provides new insight into the evolution of growth habit in ferns. International Journal of Plant Sciences 175: 526–536. [Google Scholar]
- Tsutsumi C, Kato M. 2006. Evolution of epiphytes in Davalliaceae and related ferns. Botanical Journal of the Linnean Society 151: 495–510. [Google Scholar]
- Watkins JE Jr, Cardelús C. 2009. Habitat differentiation of ferns in a lowland tropical rain forest. American Fern Journal 99: 162–175. [Google Scholar]
- Watkins JE Jr, Cardelús CL. 2012. Ferns in an angiosperm world: cretaceous radiation into the epiphytic niche and diversification on the forest floor. International Journal of Plant Sciences 173: 695–710. [Google Scholar]
- Watkins JE Jr, Moran RC. 2019. Gametophytes of the fern genera Dracoglossum and Lomariopsis (lomariopsidaceae) and their phylogenetic significance. International Journal of Plant Sciences. [Google Scholar]
- Watkins JE Jr, Kawahara A, Leicht S, Auld J, Bicksler A, Kaiser K. 2006. Fern laminar scales protect against photoinhibition from excess light. American Fern Journal 96: 83–92. [Google Scholar]
- Watkins JE Jr, Mack M, Sinclair T, Mulkey S. 2007. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytologist 176: 708–717. [DOI] [PubMed] [Google Scholar]
- Wikstrom N, Kenrick P, Chase M. 1999. Epiphytism and terrestrialization in tropical Huperzia (Lycopodiaceae). Plant Systematics and Evolution 218: 221–243. [Google Scholar]
- Winkler U, Zotz G. 2010. ‘And then there were three’: highly efficient uptake of potassium by foliar trichomes of epiphytic bromeliads. Annals of Botany 106: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zhou X, Yin Q, et al. . 2018. A global plastid phylogeny uncovers extensive cryptic speciation in the fern genus Hymenasplenium (Aspleniaceae). Molecular Phylogenetics and Evolution 127: 203–216. [DOI] [PubMed] [Google Scholar]
- Young K, León B. 1991. Observations on the understory climbing fern, Polybotrya pubens (Dryopteridaceae) in a Peruvian rain-forest. American Fern Journal 81: 63–67. [Google Scholar]
- Zotz G. 2013a Hemiepiphyte: a confusing term and its history. Annals of Botany 111: 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G. 2013b The systematic distribution of vascular epiphytes – a critical update. Botanical Journal of the Linnean Society 171: 453–481. [Google Scholar]
- Zotz G. 2016. Plants on plants – the biology of vascular epiphytes. Berlin: Springer. [Google Scholar]