Abstract
Background and Aims
The strengths of biotic interactions such as herbivory are expected to decrease with increasing latitude for native species. To what extent this applies to invasive species and what the consequences of this variation are for competition among native and invasive species remain unexplored. Here, herbivore impacts on the invasive plant Alternanthera philoxeroides and its competition with the native congener A. sessilis were estimated across latitudes in China.
Methods
An common garden experiment spanning ten latitudinal degrees was conducted to test how herbivore impacts on A. philoxeroides and A. sessilis, and competition between them change with latitude. In addition, a field survey was conducted from 21°N to 36.8°N to test whether A. philoxeroides invasiveness changes with latitude in nature as a result of variations in herbivory.
Key Results
In the experiment, A. sessilis cover was significantly higher than A. philoxeroides cover when they competed in the absence of herbivores, but otherwise their cover was comparable at low latitude. However, A. philoxeroides cover was always higher on average than A. sessilis cover at middle latitude. At high latitude, only A. sessilis emerged in the second year. Herbivore abundance decreased with latitude and A. philoxeroides emerged earlier than A. sessilis at middle latitude. In the field survey, the ratio of A. philoxeroides to A. sessilis cover was hump shaped with latitude.
Conclusion
These results indicate that herbivory may promote A. philoxeroides invasion only at low latitude by altering the outcome of competition in favour of the invader and point to the importance of other factors, such as earlier emergence, in A. philoxeroides invasion at higher latitudes. These results suggest that the key factors promoting plant invasions might change with latitude, highlighting the importance of teasing apart the roles of multiple factors in plant invasions within a biogeographic framework.
Keywords: Alternanthera philoxeroides, Alternanthera sessilis, biotic interaction hypothesis, enemy release, herbivory, latitudinal gradient, plant invasions, plant phenology, seasonal priority
INTRODUCTION
Understanding the mechanisms that confer competitive advantage to non-native species over co-occurring native species is one of the central tasks of invasion biology (Levine et al., 2004). Competition between native and non-native plants and, consequently, the fate of non-native plants in their new ranges are determined by a diverse array of biotic (e.g. herbivores) and abiotic (e.g. climate) factors (Keane and Crawley, 2002; Petitpierre et al., 2012; Joshi et al., 2014; Mráz et al., 2014), which may change with latitude (Schemske et al., 2009; Thackeray et al., 2016; Bogdziewicz et al., 2019). Moreover, many non-native plants can invade over a wide latitudinal range and experience varying abiotic and biotic environments (Stohlgren et al., 2005; Morriën et al., 2010; Cronin et al., 2015; Liu et al., 2017; Sakata et al., 2017). Therefore, the roles of these abiotic and biotic factors in non-native plant invasions are expected to change with latitude (Bezemer et al., 2014), possibly contributing to the observed latitudinal variations in the invasiveness (i.e. the propensity of non-native species to outcompete native species) of some non-native plants (Lonsdale, 1999; Stohlgren et al., 2005; Alexander et al., 2011). To date, however, most studies exploring the causes of plant invasions have been restricted to a single community or ecosystem in one area, and biogeographic studies (e.g. along latitudinal gradients), other than comparisons between invasive species’ native and invaded regions, are limited (Cronin et al., 2015).
Herbivory, a key driver of plant community diversity and composition in terrestrial ecosystems (Borer et al., 2014; Kaarlejarvi et al., 2017), can have a profound effect on non-native plant invasions (Elton, 1958; Keane and Crawley, 2002). For example, release from coevolved specialist enemies or lower effects of enemies in introduced ranges, which confers a competitive advantage for non-native species, was proposed as a driving mechanism promoting non-native plant invasions (the Enemy Release Hypothesis) (Keane and Crawley, 2002). In contrast, biotic resistance arising from native herbivores or competitors has been proposed to explain the failure of some non-native plants in their new ranges (the Biotic Resistance Hypothesis) (Elton, 1958). Both hypotheses have been extensively tested at local scales or between plants’ native and invasive ranges, and received mixed support (see references in Maron and Vila, 2001; Levine et al., 2004; González-Browne et al., 2016), which may arise in part because of latitudinal variations in plant–herbivore interactions for native and invasive species, as shown in recent studies (Colautti and Barrett, 2013; Cronin et al., 2015; Allen et al., 2017; Bhattarai et al., 2017a; Sakata et al., 2017).
The strength of biotic interactions, such as herbivory, is expected to decrease with rising latitude (the Latitudinal Biotic Interaction Hypothesis) (Schemske et al., 2009), with important ecological and evolutionary consequences for plant–insect interactions, species range expansions and plant communities (Pennings and Silliman, 2005; Morriën et al., 2010; Moles et al., 2011). The Latitudinal Biotic Interaction hypothesis has been tested extensively for native plants and received mixed support (Schemske et al., 2009; Moles et al., 2011; Anstett et al., 2014; Zhang et al., 2016). Many studies showed higher levels of herbivory at low than at high latitudes, whereas some studies have found no such pattern (reviewed by Schemske et al., 2009; Moles et al., 2011; Zhang et al., 2016). However, whether this latitudinal pattern exists for non-native invasive plants in their new ranges has been explored much less (Cronin et al., 2015; Sakata et al., 2017). Moreover, how the impacts of herbivores on competition among native and invasive plants change with latitude remains unclear, which is critical for understanding the role of herbivory in non-native plant invasions within a biogeographic framework.
Recent studies have found that the strength of interactions with native herbivores does not change with latitude for some invasive plant species in their introduced ranges (Cronin et al., 2015; Bhattarai et al., 2017a; Endriss et al., 2018). This pattern may have arisen because non-native plants have not had sufficient time or genetic variation to evolve such latitudinal patterns, or there is weak selection in invaded ranges (Endriss et al., 2018). For example, in North America, above-ground herbivory decreased with latitude for a native Phragmites australis genotype, but this latitudinal pattern was not observed for the invasive genotype (Cronin et al., 2015; Bhattarai et al., 2017a). The non-parallel latitudinal patterns of herbivory between the native and invasive genotypes of P. australis were probably due to the novelty of the invasive genotype to the local herbivores (Bhattarai et al., 2017a), and may create latitudinal variation in enemy release or biotic resistance (Bezemer et al., 2014). Thus, testing the role of herbivores in a system with native and introduced congeners within a biogeographic framework could provide a foundation for understanding the spatial heterogeneity of plant invasions and the underlying causes.
A majority of latitudinal studies on plant–herbivore interactions only estimate the intensity of herbivory (e.g. level of defoliation) and assume that herbivory will directly translate into loss of plant fitness (Schemske et al., 2009). However, the effects of herbivory on a plant can be negative, neutral or positive depending on herbivore diversity, intensity and timing of herbivory and plant defences, as well as abiotic (e.g. light availability) factors (Strauss and Agrawal, 1999; de Vries et al., 2018). Consequently, how herbivore impacts on invasive plants, native plants and their competition change with latitude remains an open question. Addressing this question is essential for development of a more comprehensive invasion theory incorporating variations in herbivory to explain spatial heterogeneity of plant invasions.
Here, we tested how herbivore impacts on the invasive plant Alternanthera philoxeroides (Mart.) Griseb (Amaranthaceae) and its native congener A. sessilis (L.) R.Br.ex DC. change with latitude in China when growing alone or in competition. Alternanthera philoxeroides and A. sessilis co-occur and compete with each other in an area south of 36.8°N (Lu et al., 2015, 2016; Wang et al., 2019). The two plant species are mainly damaged by the same group of above-ground insects in China (Lu et al., 2015). Compared with A. sessilis, A. philoxeroides allocates more resource to below-ground tissues and thus is more tolerant to herbivory (Sun et al., 2010; Lu et al., 2015). In a previous field experiment, we found that herbivory increased competitive ability of the native species relative to the invasive species under elevated temperature, suggesting a role for herbivory in shaping competition between the two species (Lu et al., 2016). In a large-scale field survey, we found that A. sessilis defoliation caused by above-ground herbivores decreased with latitude, but this latitudinal pattern was non-existent for A. philoxeroides (Lu et al., 2015). Based on these findings, the impacts of above-ground herbivory on the susceptible native species are expected to decrease with latitude but not change for the tolerant invasive species. However, this prediction has not been tested yet, especially in a way that explicitly disentangles the individual and interactive effects of herbivory and competition.
We conducted a common garden experiment cultivating the invasive A. philoxeroides or its native congener, A. sessilis, alone and in mixture with and without herbivores in southern (25.07°N), central (30.53°N) and northern (35.00°N) China, to test how the effect of herbivores on the native and invasive plant and their competition changes with latitude. We also quantified the abundance and diversity of insects in the three gardens. In addition, we conducted a field survey across the entire latitudinal range of A. philoxeroides in continental China from 21°N to 36.8°N, to test whether the relative abundance of the invasive plant changes with latitude due to variations in herbivory. Specifically, we asked the following questions. (1) Do the diversity and abundance of insect herbivores vary with latitude? (2) If so, do the effects of herbivory on the native and invasive plants and their competition, and consequently the invasiveness of the non-native plant in natural conditions, change with latitude? We predict that: (1) the diversity and abundance of insect herbivores decrease with latitude; (2) the impacts of herbivory on the susceptible native species decrease with latitude, but not for the tolerant invasive species, and thus the positive effects of herbivory on the invasive plant when competing with the native plant decrease with latitude; and (3) in natural conditions the relative abundance of the invasive species decreases with latitude, given the importance of herbivory in A. philoxeroides invasions.
MATERIALS AND METHODS
Study species
Alternanthera philoxeroides, native to South America, was first introduced into China in the 1930s and is now found on land and in water from 21°N to 36.8°N in continental China (Lu et al., 2013). Alternanthera philoxeroides propagates solely via vegetative means in China as well as in other invaded regions, but it can produce viable seeds in its native range (South America) (Julien et al., 1995). Alternanthera sessilis, native to China, can propagate via seeds or stem buds, and its latitudinal range completely overlaps with the range of A. philoxeroides in continental China (Lu et al., 2013, 2015).
In China, A. philoxeroides and A. sessilis are mainly defoliated by the same group of above-ground insect herbivores, including the oligophagous beetle Cassida piperata Hope (Coleoptera: Cassididae, defoliator), the generalist lepidopterans Hymenia recurvalis (Fabricius) (defoliator) and Spodoptera litura (Fabricius) (defoliator), the grasshopper Atractomorpha sp. (Orthoptera, defoliator), various aphids (Hemiptera, sucking insect), stinkbugs (Hemiptera, sucking insect) and the introduced biocontrol beetle Agasicles hygrophila (Coleoptera: Chrysomelidae, defoliator) (Lin et al., 1990; Lu et al., 2015).
Common garden experiment
To test how the effects of herbivory on the native and invasive plants and their competition change with latitude, we planted the two species together and separately, and with and without herbivory in three common gardens. We recorded herbivory and several aspects of plant performance. The experiment was established at Guangxi Botanical Garden, Guilin (25.07°N, 110.31°E, hereafter referred to as low latitude); Wuhan Botanical Garden CAS, Wuhan (30.53°N, 114.41°E, hereafter referred to as middle latitude) and Henan Provincial Academy of Agricultural Sciences Field Station, Yuanyang (35.00°N, 113.71°E, hereafter referred to as high latitude). This set of experiments enabled us to examine how the timing of emergence of native and invasive plants, and in turn the invasive plant’s seasonal priority due to earlier emergence, changed with latitude under field conditions. From July 2015 to September 2016, the average and minimum temperature at the low, middle and high latitudinal gardens were 21.92 °C and 1.2 °C, 19.56 °C and –9 °C, and 17.09 °C and –13.6 °C, respectively. The total precipitation for the three gardens was 2030.7, 1560.3 and 574 mm, respectively (National Meteorological Center of China, www.nmc.cn).
All plant materials used in the experiments were collected from a single field at the middle latitude to minimize potential transgenerational environmental impacts and genetic variations on plant performance. However, choosing one population for both species may underestimate the role of rapid adaptation of plants or herbivores to local environments (e.g. interacting herbivore or plant, or climate) in shaping plant–insect interactions (Pennings and Silliman, 2005; Kalske et al., 2016; Bhattarai et al., 2017a). In China, the native plant mainly reproduces from seeds, especially at middle and high latitudes, but the invasive plant can only reproduce clonally. Therefore, we propagated the native plant from seeds and the invasive plant from cut stems collected from the same field. Then, plants were grown in top soil in a naturally lit greenhouse in May 2015 at middle latitude.
In June 2015, we selected and transported plants to the gardens and planted similar sized (about 10 cm height) native plant seedlings and invasive plant ramets in pots at each garden with the same method. In each garden, we placed 72 pots, 40 cm (length) × 50 cm (width) × 30 cm (depth), in a field (1 m apart) and then filled the pots with local top soil. We drilled 12 drainage holes (1 cm in diameter, 10 cm apart) at the bottom of each pot. We randomly assigned each experimental pot to one of six treatment combinations: (1) herbivory, A. philoxeroides; (2) herbivory, A. sessilis; (3) herbivory, A. philoxeroides + A. sessilis; (4) undamaged control, A. philoxeroides; (5) undamaged control, A. sessilis; and (6) undamaged control, A. philoxeroides + A. sessilis. According to the treatments, we then planted two individual native seedlings (native only), invasive ramets (invasive only) or one native seedling and one invasive ramet (mixture) in the centre of each pot (15 cm apart). After planting, we caged all pots with nylon cages (60 × 60 × 100 cm, 80 mesh) to prevent herbivore access. One month later, we randomly assigned half of the pots from each plot-level treatment to herbivory treatments by cutting two 50 × 50 cm windows in opposite sides of the nylon cages to allow herbivore access but left the other half of the cages intact as control treatments. We watered plants weekly (to 100 % soil water holding capacity for each time) only in the first month to promote plant survival. We intended to replicate each treatment combination 12 times but, owing to plant mortality during transport, the final number of replicates for each treatment combination within sites varied from 7 to 12 (Supplementary data Table S1).
Data collection for the common garden experiment
In late September and early November in 2015, and in late April, late June and early September in 2016, we recorded the abundance and composition of insect herbivores (grouped into orders) for each pot that received the herbivory treatment. We counted insect herbivores using the quadrat method by placing a 0.2 × 0.2 m frame with 100 cells (each 2 × 2 cm) above the canopy in the middle of each pot. We visually estimated the number of insect herbivores and summed them across cells to obtain the total number of insect herbivores in each pot. With this method, we only recorded visible insect herbivores on plant leaves at the time points of our surveys and thus reflected only a small proportion of total insect occurrence. We recorded plant defoliation levels (i.e. percentage of leaf area removed, visually estimated by one person to 5 %) in September of 2015 and 2016. We also measured plant cover for both species for each pot with the same quadrat method as in the field survey with 0.2 × 0.2 m quadrats in November of both years.
To monitor each plant’s spring emergence, we counted the number of disconnected ramets for the invasive plant, and the number of disconnected ramets (at low latitude) and seedlings (at all latitudes) for the native plant with the same quadrat method. We selected up to six ramets or seedlings from the centre of each pot, if available, and measured their height as the length of the longest stems between 25 February and 3 March (hereafter referred to as March survey) and 14 April and 21 April (hereafter referred to as April survey) in 2016.
In November 2016, when A. sessilis seeds had matured, we counted native plant fruit number with the same quadrat method and determined the number of seeds from a selection of ten fruits collected in the centre of each pot. Then, we calculated the number of native plant seeds per quadrat as the number of fruits multiplied by the average number of seeds per fruit. At the end of the experiment, we took above- and below-ground plant materials back to the lab, washed plant materials to remove soil and then we dried (80 ºC for 48 h) and weighed plant above- and below-ground mass separately. We calculated plant total mass as the sum of above- and below-ground mass.
Data analysis for the common garden experiment
We conducted mixed model analyses of variance (ANOVAs) to test the fixed factors latitude (low, middle or high), pot-level treatment (monoculture invasive, monoculture native or mixture), herbivory (herbivory vs. control), plant species nested in pot-level treatment (native vs. invasive plants), their interactions and the random factor pot nested in latitude × pot-level treatment × herbivory on plant cover (total cover divided by 2 for monocultures) at the end of both years, plant mass (total mass divided by 2 for monocultures) at the end of the experiment, and the number and average length of plants (ramets for the invasive plant, ramets and seedlings for the native plant) in March and April surveys. We conducted mixed ANOVA to test the dependence of native plant fruit and seed numbers on the fixed effects latitude, pot-level treatment, herbivory, their interactions and the random factor pot nested in latitude × pot-level treatment × herbivory for plants that received the herbivory treatment. We conducted another mixed ANOVA to test the dependence of plant defoliation levels in September of 2015 and 2016 on the fixed effects latitude, pot-level treatment, plant species nested in pot-level treatment, their interactions and the random factor pot nested in latitude × pot-level treatment × herbivory for plants that received the herbivory treatment.
We calculated the Shannon diversity index for insect herbivores. We conducted mixed ANOVAs to test the dependence of insect diversity and abundance on the fixed effects latitude, pot-level treatment, plant species nested in pot-level treatment (native vs. invasive plants), their interactions and the random factor pot nested in latitude × pot-level treatment × herbivory.
All analyses were conducted with SAS 9.4 (SAS Institute, Cary, NC, USA). Data were transformed when necessary to improve normality and homogeneity of variance. When significant interactive effects occurred, we examined differences among treatment combinations with adjusted means partial difference tests (P ≤ 0.05).
Field survey
To estimate how performance of the native and invasive Alternanthera species and the relative abundance of the invasive species change with latitude, we measured plant cover at 44 sites (at least 10 km apart from each other) ranging from 21°N to 36.8°N in terrestrial habitats (e.g. river bank and abandoned farmland) in 2013. Each site contained individuals of both species and was larger than 10 × 10 m. We randomly chose ten to fifteen 0.5 × 0.5 m quadrats (>2 m apart) along two or three 10 m transects (>3 m apart) at each site. We measured plant cover using a quadrat method by placing a 0.5 × 0.5 m quadrat (divided into one hundred 5 × 5 cm cells) above the canopy, visually estimating the percentage of cover of each species in each cell and summing the total cover for each species. The native and invasive plant species could be easily distinguished by their morphology in the field. Alternanthera sessilis has obovate and light-green leaves on irregular-shaped stems, whereas A. philoxeroides has broadly elliptic and dark-green leaves on smooth, round stems.
We calculated the ratio of invasive plant cover vs. native plant cover to reflect the outcome of competition between the two species. We then regressed cover of the native and invasive species and their ratio against latitude separately. The ratio of invasive plant cover vs. native plant cover was log transformed.
RESULTS
Common garden experiment
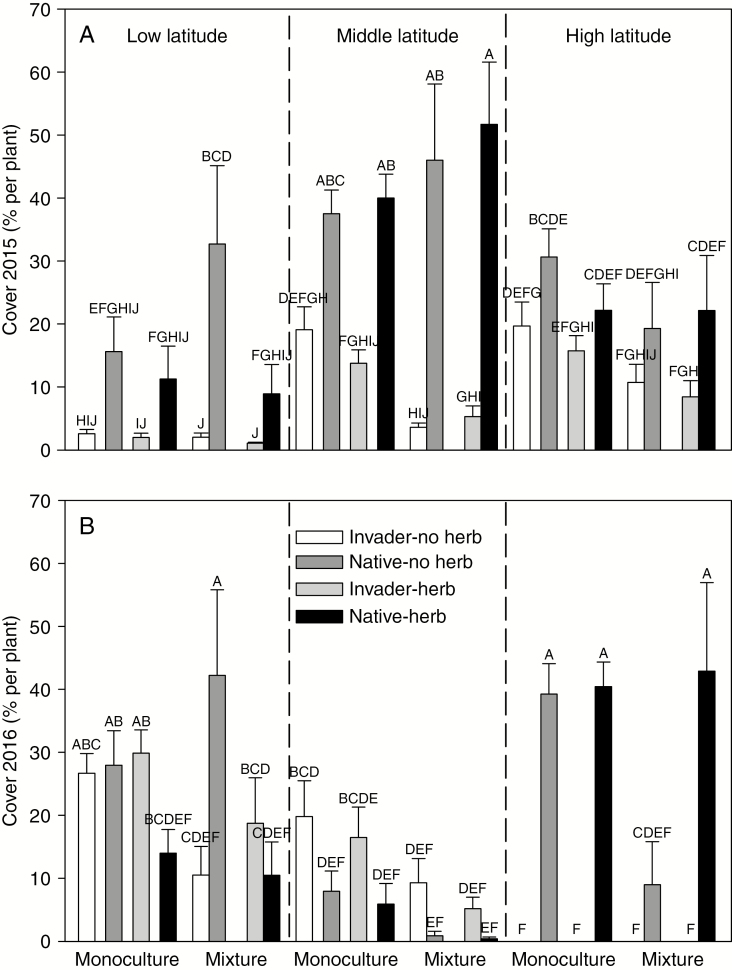
Cover in 2015 depended on latitude, pot-level treatment and species regardless of herbivory treatments (Supplementary data Table S2). At all the gardens, native plant cover on average was higher than invasive plant cover (Fig. 1A). Native plant cover on average was the highest at middle latitude (Fig. 1A). Invasive plant cover on average was the lowest at low latitude (Fig. 1A).
Fig. 1.
Plant cover at the end of 2015 (A) and 2016 (B) for the invasive (Invader) and native (Native) plants with (herb) and without (no herb) herbivores growing in monocultures (bars spaced apart) or mixtures (both species in the same pot – bars close together) in the experiment. Mean + 1 s.e.. Means with the same letters were not different in post-hoc tests, P > 0.05.
The interaction of latitude, pot-level treatment, herbivory and plant species significantly affected plant cover and biomass at the end of 2016 (Supplementary data Table S2). At low latitude, the invasive and native plants had similar cover and biomass when growing in monocultures, but when competing in mixtures the native plant had higher cover and biomass when herbivores were excluded (Fig. 1B; Supplementary data Fig. S1). At middle latitude, invasive plant cover and biomass were on average higher than that of the native plant (Fig. 1B; Supplementary data Fig. S1). At high latitude, only the native plant emerged, and native plant mass was lower in no herbivore, mixture pots (Fig. 1B; Supplementary data Fig. S1). The native plant produced the most seeds at low latitude and the least seeds at middle latitude, and herbivory only decreased native plant seed production at low latitude (Supplementary data Fig. S2; Table S3).
Latitude and plant species interactively affected the number and height of plants during both the March and April surveys (Supplementary data Table S4). In the March survey, the native plant only emerged at low latitude, and the invasive plant emerged at low and middle latitudes (Supplementary data Fig. S3A). At low latitude, native plants (i.e. ramets and seedlings) were more numerous (>6 times greater) but shorter (24 % less than the invasive plant) than the invasive plant ramets (Supplementary data Fig. S3A, C). In mid-April, we observed the native plant at all sites and only observed the invasive plant at low and middle latitudes (Supplementary data Fig. S3B). The native plants (i.e. seedlings) were more abundant than the invasive plants (i.e. ramets) only at middle latitude (Supplementary data Fig. S3B), and the native plants (i.e. seedlings and ramets), for which height decreased by 97 % along the latitudinal gradient, were shorter (31 % and 9 % of the invasive plant) than the invasive plant ramets at both low and middle latitudes (Supplementary data Fig. S3D). Herbivory decreased invasive plant length but had no effect on native plant length in either the March or April surveys (Supplementary data Table S4).
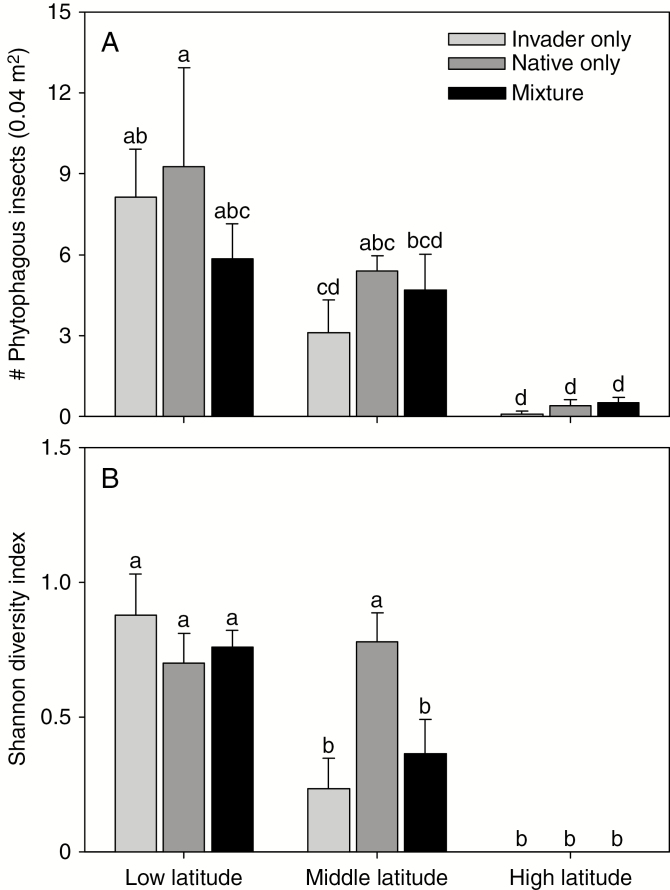
The total number of insect herbivores decreased by 96 % (averaged across pot-level treatments) along the latitudinal gradient (latitude: F2, 82 = 15.25, P < 0.001, Fig. 2A). Insect herbivore diversity was high in both monocultures and mixtures at low latitude and in native plant monocultures at middle latitude, and was low for other pot-level treatments at middle latitude and for all pot-level treatments at high latitude (two-way interaction: F4, 58 = 3.04, P = 0.024, Fig. 2B). At high latitude, we observed only Atractomorpha sp. (Supplementary data Fig. S4). The insect herbivore community was mainly composed of native species, including H. recurvalis, C. piperata, stinkbugs (e.g. Eysacoris guttiger) and grasshoppers (e.g. Locusta migratoria L. and Atractomorpha sp.). We only observed the introduced beetle A. hygrophila in three pots (one at low latitudes and two at middle latitudes and only once in each pot) throughout the experiment.
Fig. 2.
The total number (A) and diversity (B) of insect herbivores observed throughout the study at each latitude for monocultures of the invasive (Invader only) and native (Native only) plants or their mixtures (Mixture) in the experiment. Mean + 1 s.e.. Means with the same letters were not different in post-hoc tests, P > 0.05.
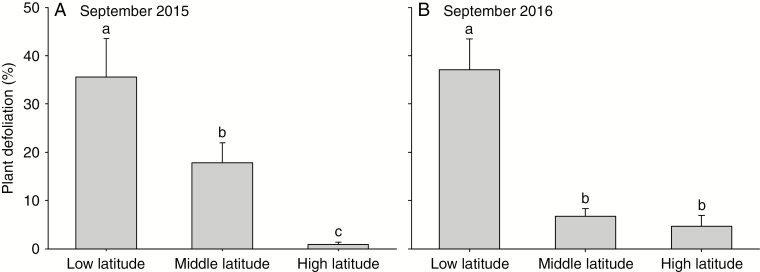
In September 2015, plant defoliation decreased with latitude, regardless of other treatments (Fig. 3A; Supplementary data Table S5). In September 2016, plant defoliation was the highest at low latitude, and plants received the same level of defoliation at middle and high latitudes (Fig. 3B; Supplementary data Table S5). In general, the native plant experienced nearly three times as much herbivory as the invasive plant, on average (11 % vs. 3 %), in September 2016.
Fig. 3.
Defoliation (i.e. percentage of leaf area that was removed) of plants in September 2015 and 2016 at each latitude in the experiment. Mean + 1 s.e. Means with the same letters were not different in post-hoc tests, P > 0.05.
Field surveys
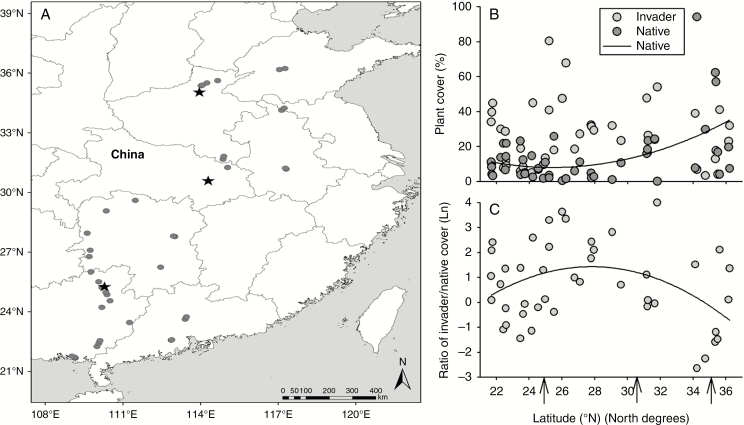
Native plant cover was a quadratic function of latitude (R2 = 0.24, P = 0.004) that first decreased then increased with latitude (Fig. 4B). Invasive plant cover was not related to latitude (P > 0.05) (Fig. 4B). The ratio of invasive plant cover vs. native plant cover was hump shaped (R2 = 0.14, P = 0.038) with a peak between 27°N and 29°N, and was <1 in the area north of 34.7°N (Fig. 4C).
Fig. 4.
Locations for the field survey (dots) and experimental gardens (stars) (A), plant cover for the invasive (Invader) and native (Native) plants (B) and their ratio (C) across latitudes in the field survey. The arrows in (C) indicate the latitudes of the experimental gardens.
DISCUSSION
Consistent with our first and second predictions, the abundance of herbivores decreased with latitude, and herbivory suppressed A. sessilis and thus facilitated A. philoxeroides at low latitude but had no impact on the two species and their competition at middle latitude in the experiment. In addition, A. philoxeroides failed to overwinter in terrestrial habitats at high latitude. However, the relative abundance of the introduced A. philoxeroides compared with the native A. sessilis in natural field conditions was hump shaped with the latitude, in contrast to our third prediction. Our results suggest that enemy release may promote A. philoxeroides invasion but only at low latitudes, and that A. philoxeroides invasion at higher latitudes is likely to be due to different factors such as earlier emergence and overwintering in aquatic habitats. Therefore, this study highlights the importance of simultaneously considering multiple factors when exploring plant invasion mechanisms within a biogeographic context.
Herbivore abundance and, in turn, the damage to plants were predicted to decrease with latitude by the Latitudinal Biotic Interaction Hypothesis (Schemske et al., 2009). In this study, herbivore abundance decreased with latitude. Plant defoliation for both species was the highest at low latitude in both years. In the first year, plant defoliation gradually decreased towards higher latitude, while plant defoliation was not significantly different between middle and high latitudes in the second year. This pattern generally supports the Latitudinal Biotic Interaction Hypothesis, but also indicates that the patterns may vary slightly between years. The interannual variation in latitudinal patterns for plant defoliation reflects the temporal fluctuations in biotic interactions or community composition across biogeographic gradients (Yasuhara et al., 2009). Therefore, it is necessary to consider temporal fluctuations in biotic interactions and their ecological implications in biogeographic studies.
Herbivore impacts on A. sessilis performance (i.e. cover and mass) shifted from negative at low latitudes to neutral or positive at high latitudes, but there was no detectable herbivore impact on A. philoxeroides across latitudes, resulting in contrasting latitudinal patterns in herbivore impacts for these plants. Herbivore abundance and plant defoliation decreased from low to middle latitudes for the native plant in both years, though there was similar herbivore diversity at both sites. This finding may explain why herbivores decreased native plant cover and mass at low latitude but had no impact at middle latitude at the end of the experiments. Herbivores may also increase plant performance (e.g. biomass) (termed plant overcompensation) by increasing soil resource availability (e.g. dead bodies or faeces) (Yang, 2004), modifying soil biota communities (Heath and Lau, 2011) or suppressing co-occurring competing plant species (apparent competition) (Lu et al., 2016). In addition, A. sessilis was only damaged by folivorous insects at high latitude, but it was damaged by both folivorous and sucking insects at other sites. A plant is more likely to fully compensate or overcompensate for a single kind of damage (Strauss and Agrawal, 1999), which could have contributed to the positive impacts of herbivory on the native plant at high latitude in the mixture treatment. The non-significant herbivore impact on A. philoxeroides across latitudes was probably due to its high tolerance to herbivory (Lu and Ding, 2010; Sun et al., 2010), and native herbivores’ preference for the native plant, corresponding to the observed higher defoliation level.
The non-parallel latitudinal patterns in above-ground herbivore impacts, as well as soil biota impacts (Lu et al., 2018), on the native and invasive plant species may have led to a decreased strength in enemy release with latitude and potential indirect biotic resistance at high latitudes for A. philoxeroides. Herbivory suppressed A. sessilis cover and mass that, in turn, increased relative competitive ability of A. philoxeroides at low latitude, as predicted by the Enemy Release Hypothesis (Keane and Crawley, 2002). Indeed, it was only when the plants were competing in mixture pots that exclusion of herbivores had a strong and clear positive effect on the native plant. However, herbivory did not impact either species or their competition at middle latitude, suggesting a negligible role for insect herbivores in A. philoxeroides invasions in this region. In contrast, herbivore exclusion decreased A. sessilis cover and mass at high latitude in mixed pots compared with A. sessilis monocultures or herbivory mixed pots, raising the possibility that in some situations herbivores modify the competition between these plant species at higher latitudes.
Latitudinal variation in biotic interactions, plant–insect interactions in particular, plays an important role in structuring plant communities and in determining species range expansions across broad ranges (Pennings and Silliman, 2005; Schemske et al., 2009) with important implications for plant invasions (Engelkes et al., 2008; Bhattarai et al., 2017a). However, so far, latitudinal variation in plant–insect interactions has only been tested for the invasive genotypes of P. australis in North America (Cronin et al., 2015; Bhattarai et al., 2017b) and the invasive plant Solidago altissima in Japan (Sakata et al., 2017). Most of these studies uncritically assume a linear relationship between the intensity of herbivory and plant fitness loss, but have rarely tested the net impacts of herbivores on plant species and their competitors (but see Bhattarai et al., 2017b). Here, we found non-parallel impacts of herbivory with latitude, yet herbivory on both species decreased with latitude in the first year, pointing to the potential importance of plant tolerance to herbivory in shaping latitudinal clines of herbivory impacts. Therefore, it is necessary to directly test herbivore impacts and fully consider the potential roles of plant defences in this kind of research.
At middle latitude, A. philoxeroides outperformed A. sessilis regardless of herbivore presence in the experiment; this finding was consistent with the results of the field surveys, suggesting that factors other than herbivory promoted A. philoxeroides invasion in this region. Compared with co-occurring native plants, some invasive plants emerge earlier, which results in a seasonal ‘priority advantage’ (Wolkovich and Cleland, 2011), allowing invasive plants to colonize ‘temporal empty niches’ and subsequently explore and pre-empt resources (Wainwright et al., 2012). Two lines of evidence suggest the potential role of seasonal priority due to earlier emergence for A. philoxeroides invasion at middle latitude in China. First, in this study, the invasive plant emerged earlier than the native plant in the spring of the second year at middle latitude. Secondly, in a previous study at middle latitude, we found that the invasive plant, which emerged earlier, outcompeted the native plant under ambient temperatures, but this competitive advantage disappeared when warmer temperature resulted in earlier native plant emergence, suggesting the role of plant phenology in determining competition between the two plant species (Lu et al., 2016). Taken together, these pieces of information suggest that seasonal priority due to earlier emergence might promote A. philoxeroides invasion at middle latitude.
The ranges of some plant species, northern limits in particular, are interactively determined by species’ thermal tolerance and the temperature experienced in a given region (Petitpierre et al., 2012). In our experiment, A. philoxeroides failed to overwinter at high latitude, but A. sessilis successfully overwintered. Our results suggest that cold winter temperature may act as an important abiotic filter restricting A. philoxeroides invasion in terrestrial habitats in northern China. The temperature increase in recent decades therefore has probably promoted the northern range expansion of A. philoxeroides in China (Lu et al., 2013). However, in the field surveys, we found that natural populations of the invasive species mainly occurred in water bodies or on riverbanks above 31.8°N, pointing to the potential role of water bodies in the transportation and overwintering of A. philoxeroides and the role of clonal integration in facilitating A. philoxeroides expansion from water bodies to land at high latitudes (Wang et al., 2009; Wang et al., 2014).
Our results show that herbivory may only promote A. philoxeroides invasion at low latitude, thus A. philoxeroides invasion at higher latitudes could be attributed to other factors, such as seasonal priority. In our study, we only used one population of each plant species, thus the results might not reflect the effects of potential adaptions of plant and herbivore to local environments (Pennings and Silliman, 2005; Kalske et al., 2016; Bhattarai et al., 2017b). Despite this limitation, our finding has important implications for invasive species management. In classical biological control programmes, co-evolved specialist enemies are introduced and released to control invasive species (Thomas and Reid, 2007). However, the effects of the introduced enemies on invasive plants may depend on many biotic and abiotic factors (Thomas and Reid, 2007; Clewley et al., 2012). Our results suggest that factors other than enemy release can underlie successful invasions and that the drivers of plant invasions may vary with latitude; therefore, the successful management of similar invasive species will probably differ from region to region (Henriksen et al., 2018). Thus, teasing apart the roles of multiple factors and identifying the key factor promoting biological invasions across latitudinal gradients or invasion stages is urgently needed to improve our ability to manage invasive species.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: the number of replications for each treatment combination at each latitude. Table S2: results of mixed ANOVAs on the fixed factors latitude, pot-level treatment, herbivory, plant species nested in pot-level treatment, their interactions and the random factor pot nested in latitude × pot-level treatment × herbivory on plant cover at the end of 2015 and 2016 and plant mass at the end of 2016 in the experiment. Table S3: results of mixed ANOVAs on the fixed factors latitude, pot-level treatment, herbivory, their interactions and the random factor pot nested in latitude × pot-level treatment × herbivory on the number of fruits and seeds of the native plant at the end of the experiment. Table S4: results of mixed ANOVAs on the fixed factors latitude, pot-level treatment, herbivory, plant species nested in pot-level treatment, their interactions and the random factor pot nested in latitude × pot-level treatment × herbivory on the number and length of plants in the March and April surveys in 2016 in the experiment. Table S5: results of mixed ANOVAs on the fixed factors latitude, pot-level treatment, plant species nested in pot-level treatment, their interactions and the random factor pot nested in latitude × pot-level treatment × herbivory on plant defoliation in September of 2015 and 2016 for plants that received the herbivory treatment in the experiment. Figure S1: plant mass at the end of 2016 for the native and invasive plants in the presence or absence of herbivores in different plot-level treatments in the experiment. Figure S2: production of native plant seeds in the presence or absence of herbivores at each latitude at the end of the experiment. Figure S3: the number and height of disconnected ramets and seedlings for the native plant and disconnected ramets for the invasive plant observed in the late February–early March survey and mid April survey in 2016 at each latitude in different plot-level treatments in the experiment. Figure S4: component of insect orders observed for monocultures of the native and invasive plant species and their mixture at each latitude throughout the experiment.
ACKNOWLEDGEMENTS
We thank Jin Miao, Chunqiang Wei, Jinwen Chen, Yanan Tang and Tong Li for field assistance, Professor Wim H. van der Putten for advice on experiment design, and Luke Flory and five anonymous referees for comments on the manuscript.
FUNDING
This work was supported by NSF-China (31570540, 31872034 and 31870364), the Natural Science Foundation of Hubei Province (2017CFA073) and the National Key Research and Development Program (2017YFC1200100).
LITERATURE CITED
- Alexander JM, Kueffer C, Daehler CC, et al. 2011. Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proceedings of the National Academy of Sciences, USA 108: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen WJ, Meyerson LA, Cummings D, Anderson J, Bhattarai GP, Cronin JT. 2017. Biogeography of a plant invasion: drivers of latitudinal variation in enemy release. Global Ecology and Biogeography 26: 435–446. [Google Scholar]
- Anstett DN, Naujokaitis-Lewis I, Johnson MTJ. 2014. Latitudinal gradients in herbivory on Oenothera biennis vary according to herbivore guild and specialization. Ecology 95: 2915–2923. [Google Scholar]
- Bezemer TM, Harvey JA, Cronin JT. 2014. Response of native insect communities to invasive plants. Annual Review of Entomology 59: 119–141. [DOI] [PubMed] [Google Scholar]
- Bhattarai GP, Meyerson LA, Anderson J, Cummings D, Allen WJ, Cronin JT. 2017a Biogeography of a plant invasion: genetic variation and plasticity in latitudinal clines for traits related to herbivory. Ecological Monographs 87: 57–75. [Google Scholar]
- Bhattarai GP, Meyerson LA, Cronin JT. 2017b Geographic variation in apparent competition between native and invasive Phragmites australis. Ecology 98: 349–358. [DOI] [PubMed] [Google Scholar]
- Bogdziewicz M, Maria Espelta J, Bonal R. 2019. Tolerance to seed predation mediated by seed size increases at lower latitudes in a Mediterranean oak. Annals of Botany 123: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer ET, Seabloom EW, Gruner DS, et al. 2014. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508: 517–520. [DOI] [PubMed] [Google Scholar]
- Clewley GD, Eschen R, Shaw RH, Wright DJ. 2012. The effectiveness of classical biological control of invasive plants. Journal of Applied Ecology 49: 1287–1295. [Google Scholar]
- Colautti RI, Barrett SCH. 2013. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342: 364–366. [DOI] [PubMed] [Google Scholar]
- Cronin JT, Bhattarai GP, Allen WJ, Meyerson LA. 2015. Biogeography of a plant invasion: plant–herbivore interactions. Ecology 96: 1115–1127. [DOI] [PubMed] [Google Scholar]
- Elton CS. 1958. The ecology of invasions by animals and plants. London: Methuen. [Google Scholar]
- Endriss SB, Alba C, Norton AP, Pyšek P, Hufbauer RA. 2018. Breakdown of a geographic cline explains high performance of introduced populations of a weedy invader. Journal of Ecology 106: 699–713. [Google Scholar]
- Engelkes T, Morriën E, Verhoeven KJF, et al. 2008. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456: 946–948. [DOI] [PubMed] [Google Scholar]
- González-Browne C, Murúa MM, Navarro L, Medel R. 2016. Does plant origin influence the fitness impact of flower damage? A meta-analysis. PLoS One 11: e0146437. doi: 10.1371/journal.pone.0146437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath KD, Lau JA. 2011. Herbivores alter the fitness benefits of a plant–rhizobium mutualism. Acta Oecologica 37: 87–92. [Google Scholar]
- Henriksen JW, Lim DS, Lu X, Ding J, Siemann E. 2018. Strong effects of hydrologic environment and weak effects of elevated CO2 on the invasive weed Alternanthera philoxeroides and the biocontrol beetle Agasicles hygrophila. Arthropod-Plant Interactions 12: 691–700. [Google Scholar]
- Joshi S, Gruntman M, Bilton M, Seifan M, Tielbörger K. 2014. A comprehensive test of evolutionarily increased competitive ability in a highly invasive plant species. Annals of Botany 114: 1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien MH, Skarratt B, Maywald GF. 1995. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. Journal of Aquatic Plant Management 33: 55–60. [Google Scholar]
- Kaarlejarvi E, Eskelinen A, Olofsson J. 2017. Herbivores rescue diversity in warming tundra by modulating trait-dependent species losses and gains. Nature Communications 8: 419. doi: 10.1038/s41467-017-00554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalske A, Leimu R, Scheepens JF, Mutikainen P. 2016. Spatiotemporal variation in local adaptation of a specialist insect herbivore to its long-lived host plant. Evolution 70: 2110–2122. [DOI] [PubMed] [Google Scholar]
- Keane RM, Crawley MJ. 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution 17: 164–170. [Google Scholar]
- Levine JM, Adler PB, Yelenik SG. 2004. A meta-analysis of biotic resistance to exotic plant invasions. Ecology Letters 7: 975–989. [Google Scholar]
- Lin G, Yang Y, Hu J. 1990. Studies on biology and control of Alternanthera philoxeroides. Journal of Jiangsu Agricultural College 11: 57–63. [Google Scholar]
- Liu W, Strong DR, Pennings SC, Zhang Y. 2017. Provenance by environment interaction of reproductive traits in the invasion of Spartina alterniflora in China. Ecology 98: 1591–1599. [DOI] [PubMed] [Google Scholar]
- Lonsdale WM. 1999. Global patterns of plant invasions and the concept of invasibility. Ecology 80: 1522–1536. [Google Scholar]
- Lu X, Ding J. 2010. Flooding compromises compensatory capacity of an invasive plant: implications for biological control. Biological Invasions 12: 179–189. [Google Scholar]
- Lu X, Siemann E, Shao X, Wei H, Ding J. 2013. Climate warming affects biological invasions by shifting interactions of plants and herbivores. Global Change Biology 19: 2339–2347. [DOI] [PubMed] [Google Scholar]
- Lu X, Siemann E, He MY, Wei H, Shao X, Ding JQ. 2015. Climate warming increases biological control agent impact on a non-target species. Ecology Letters 18: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Siemann E, He MY, Wei H, Shao X, Ding JQ. 2016. Warming benefits a native species competing with an invasive congener in the presence of a biocontrol beetle. New Phytologist 211: 1371–1381. [DOI] [PubMed] [Google Scholar]
- Lu X, He M, Ding J, Siemann E. 2018. Latitudinal variation in soil biota: testing the biotic interaction hypothesis with an invasive plant and a native congener. ISME Journal 12: 2811–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron JL, Vila M. 2001. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95: 361–373. [Google Scholar]
- Moles AT, Bonser SP, Poore AGB, Wallis IR, Foley WJ. 2011. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Functional Ecology 25: 380–388. [Google Scholar]
- Morriën E, Engelkes T, Macel M, Meisner A, Van der Putten WH. 2010. Climate change and invasion by intracontinental range-expanding exotic plants: the role of biotic interactions. Annals of Botany 105: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mráz P, Tarbush E, Müller-Schärer H. 2014. Drought tolerance and plasticity in the invasive knapweed Centaurea stoebe s.l. (Asteraceae): effect of populations stronger than those of cytotype and range. Annals of Botany 114: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings SC, Silliman BR. 2005. Linking biogeography and community ecology: latitudinal variation in plant–herbivore interaction strength. Ecology 86: 2310–2319. [Google Scholar]
- Petitpierre B, Kueffer C, Broennimann O, Randin C, Daehler C, Guisan A. 2012. Climatic niche shifts are rare among terrestrial plant invaders. Science 335: 1344–1348. [DOI] [PubMed] [Google Scholar]
- Sakata Y, Craig TP, Itami JK, Yamasaki M, Ohgushi T. 2017. Parallel environmental factors drive variation in insect density and plant resistance in the native and invaded ranges. Ecology 98: 2873–2884. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution, and Systematics 40: 245–269. [Google Scholar]
- Stohlgren TJ, Barnett D, Flather C, Kartesz J, Peterjohn B. 2005. Plant species invasions along the latitudinal gradient in the United States. Ecology 86: 2298–2309. [Google Scholar]
- Strauss SY, Agrawal AA. 1999. The ecology and evolution of plant tolerance to herbivory. Trends in Ecology & Evolution 14: 179–185. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ding J, Frye MJ. 2010. Effects of resource availability on tolerance of herbivory in the invasive Alternanthera philoxeroides and the native Alternanthera sessilis. Weed Research 50: 527–536. [Google Scholar]
- Thackeray SJ, Henrys PA, Hemming D, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535: 241–245. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Reid AM. 2007. Are exotic natural enemies an effective way of controlling invasive plants? Trends in Ecology & Evolution 22: 447–453. [DOI] [PubMed] [Google Scholar]
- de Vries J, Poelman EH, Anten N, Evers JB. 2018. Elucidating the interaction between light competition and herbivore feeding patterns using functional–structural plant modelling. Annals of Botany 121: 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright CE, Wolkovich EM, Cleland EE. 2012. Seasonal priority effects: implications for invasion and restoration in a semi-arid system. Journal of Applied Ecology 49: 234–241. [Google Scholar]
- Wang A, Luo F-L, Yu F-H, Huang L, Zhang M-X, Chen Y. 2014. Shifting effects of physiological integration on performance of a clonal plant during submergence and de-submergence. Annals of Botany 113: 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Yu FH, Li PX, et al. 2009. Clonal integration supports the expansion from terrestrial to aquatic environments of the amphibious stoloniferous herb Alternanthera philoxeroides. Plant Biology 11: 483–489. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ismail M, Huang W, Wang Y, Ding J. 2019. Population dynamics and overwintering of a biological control beetle, Agasicles hygrophila, on a nontarget plant Alternanthera sessilis, along a latitudinal gradient. Journal of Pest Science 92: 835–845. [Google Scholar]
- Wolkovich EM, Cleland EE. 2011. The phenology of plant invasions: a community ecology perspective. Frontiers in Ecology and the Environment 9: 287–294. [Google Scholar]
- Yang LH. 2004. Periodical cicadas as resource pulses in North American forests. Science 306: 1565–1567. [DOI] [PubMed] [Google Scholar]
- Yasuhara M, Hunt G, Cronin TM, Okahashi H. 2009. Temporal latitudinal-gradient dynamics and tropical instability of deep-sea species diversity. Proceedings of the National Academy of Sciences, USA 106: 21717–21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang Y, Ma K. 2016. Latitudinal variation in herbivory: hemispheric asymmetries and the role of climatic drivers. Journal of Ecology 104: 1089–1095. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.