Abstract
Background and Aims
The patterns of niche occupation in functional trait space have been widely studied to understand the processes of community assembly, but are rarely linked to environmental conditions (here, stress and disturbance). In this study, we investigate (1) how the pattern of functional niche occupation, incorporating intraspecific trait variation and covariation, varies along experimental gradients of stress and disturbance, (2) whether habitat filtering and/or limiting similarity modify the pattern, and (3) whether their strength varies as a function of species richness or levels of stress and disturbance.
Methods
We constructed an experimental system consisting of 24 herbaceous plant mesocosms under different levels of stress and disturbance, and measured ten traits on five individuals for each species in each mesocosm. We quantified the total functional niche volume occupied by an entire mesocosm, the functional niche overlap among species within a mesocosm and the average functional niche volume occupied per species, and investigated how these metrics varied from species-poor to species-rich mesocosms along gradients of stress and disturbance.
Key Results
Species richness and functional niche overlap correlated positively to disturbance at low and medium levels of stress, but peaked at the intermediate level of disturbance when stress was high. The total functional niche volume and average functional niche volume did not change significantly along these gradients. Compared to null models, each mesocosm occupied a smaller total functional niche volume (habitat filtering) and the species within each mesocosm overlapped less and were more functionally specialized (limiting similarity). Moreover, the standardized metrics (to the null expectations) did not change significantly under different levels of stress and disturbance.
Conclusions
This experimental evidence shows that both habitat filtering and limiting similarity determine the patterns of functional niche occupation and species richness, but their strength does not change along environmental gradients of stress and disturbance.
Keywords: Functional niche occupation, community assembly, total functional niche volume, functional niche overlap, average functional niche volume, stress, disturbance, mesocosm
INTRODUCTION
How do species within a local community occupy and partition niche space? How does this pattern of niche occupation vary from species-poor to species-rich communities and how does it vary along different environmental gradients? These questions are fundamental and remain unresolved in community ecology but their answers can provide insights into the processes of community assembly and the mechanisms of species coexistence (MacArthur and Levins, 1967; Hutchinson, 1978; Giller, 1984; Ricklefs, 2012; Swenson and Weiser, 2014).
Investigating the patterns of niche occupation became possible after Hutchinson (1957) proposed a quantitative niche concept – an n-dimensional hypervolume – and the notions of niche volume and niche overlap. Early research on niche occupation mainly focused on conceptual descriptions or theoretical deductions (MacArthur and Levins, 1967; Rogers, 1977; Hutchinson, 1978; Rappoldt and Hogeweg, 1979), providing general ideas of niche occupation and motivating later empirical studies on this topic. However, due to the difficulties of measuring resource axes of Hutchinsonian niche space for a large number of species in natural communities, ecologists advocated an alternative approach by investigating niche occupation in functional trait space (Ricklefs and Travis, 1980; Ricklefs and Miles, 1994; Ricklefs and Marquis, 2012; Lamanna et al., 2014; Swenson and Weiser, 2014). The approach is founded on the premise that the adaptations of organisms reflect their ecological relationships, specifically that locations in functional space reflect locations in Hutchinsonian niche space (Hespenheide, 1973; Rosenfeld, 2002).
Recently, Li et al. (2018) defined three new fundamental metrics to better describe the pattern of functional niche occupation and infer the processes of community assembly by incorporating intraspecific trait variation and covariation, which are necessary for accurately estimating how a species occupies the functional niche space (Violle et al., 2012; Laughlin and Messier, 2015). The three metrics were: (1) the total functional niche volume occupied by an entire community (T, the community functional diversity), (2) the amount of functional niche overlap among species (O, the degree of interspecific functional redundancy), (3) and the average functional niche volume occupied per species (A, average intraspecific trait variation and covariation quantifying the degree of species’ functional specialization). Different responses of these metrics from species-poor to species rich communities may reveal the relative importance of different community assembly processes. Habitat filtering excludes species with inappropriate trait combinations for given abiotic and biotic conditions (Keddy, 1992; Diaz et al., 1998; Kunstler et al., 2012), and thus constrains the total functional niche volume accompanied by greater functional niche overlap and/or smaller average functional niche volume (Li et al., 2018). In contrast, limiting similarity reduces the likelihood of co-occurrence of species that overlap too much in niche space (MacArthur and Levins, 1967), and thus limits the degree of functional niche overlap accompanied by greater total functional niche volume and/or smaller average functional niche volume (Li et al., 2018). Note that the functional niche occupation is described at a small scale using only traits of individuals occurring in the same local community to estimate species’ realized functional niche.
Studies investigating functional niche occupation from species-poor to species-rich communities rarely link it to measured environmental gradients (but see Le Bagousse-Pinguet et al., 2014; Loranger et al., 2016), although such environmental variation could potentially cause changes in both species richness and functional niche occupation structure. Stress and disturbance are claimed as two primary environmental drivers of both species richness and trait variation by Tilman’s (1988) and Grime’s (2001) theories. Stress is any external abiotic constraint (e.g. soil fertility) that limits biomass production, and disturbance is any event (e.g. grazing) causing partial or total live biomass destruction (Grime, 2001). The effects of stress and disturbance on species richness and the underlying mechanisms (deterministic or stochastic) have been studied for decades (Grime, 1973; Connell, 1978; Huston, 1979, 1994; Ronen and Yuval, 2006), and are still under debate (Adler et al., 2011; Fox, 2013; Huston, 2014). Because species richness is the end product of both niche-based assembly processes and historically contingent ones including demographic stochasticity (Fukami et al., 2005; Li and Shipley, 2018), empirical relationships between species richness and stress/disturbance alone cannot distinguish between these different processes (Ronen and Yuval, 2006). However, the pattern of functional niche occupation (i.e. the values of T, O and A in relation to species richness) is expected to differ under different processes of community assembly even when the community contains an equal number of species (Li et al., 2018). Therefore, investigating such patterns along gradients of stress and disturbance might provide a promising approach to address this complication.
In this study, we constructed an experimental system consisting of 24 mesocosms (1 m2) experiencing different experimentally imposed levels of stress and disturbance. Each mesocosm consisted of naturally sorted species compositions having different levels of species richness following initial seed sowing from a common species pool. With different traits measured on multiple individuals per species per mesocosm, we aim to address the following three questions. (1) How does the pattern of functional niche occupation (the three fundamental metrics: T, O and A) vary from species-poor to species-rich mesocosms along the gradients of stress and disturbance? (2) Does habitat filtering and/or limiting similarity modify the pattern of functional niche occupation? (3) If so, does their strength vary as a function of species richness or levels of stress and disturbance?
MATERIALS AND METHODS
Experimental design
The experimental system consisted of 24 mesocosms (112.5 × 90 × 36 cm) maintained outside at the University of Sherbrooke, Quebec, Canada. Each mesocosm was 0.5 m distant from its neighbour and each was conceptually divided into 80 cells (10 × 10 cm) plus a 10-cm border. Three levels of stress and four levels of disturbance, with two replicates of each treatment combination, were randomly assigned to the 24 mesocosms. The three levels of stress (low, medium and high) were created by 3 : 1, 1 : 1 and 1 : 3 mixtures of fertile garden soil and sand plus a fixed proportion of clay soil. As expected, the soil fertility (organic matter, total C, N, P and K as well as N mineralization) and above-ground biomass production significantly declined with increasing levels of stress (Li and Shipley, 2017). Seed mixtures of 30 herbaceous species (see species selection and composition in Li and Shipley, 2017) were evenly broadcast over the soil surface of each mesocosm in 2009. After broadcasting, we allowed natural colonization from the common soil seed bank (seeds from the clay soil) and the surroundings. The four levels of disturbance (D04, D08, D27, D54) were created by disturbing four, eight, 27 or 54 (out of 80) randomly selected cells per mesocosm per year. The disturbance was applied to a cell by: (1) cutting the vegetation to ground level with the cut biomass being distributed throughout the mesocosm, (2) cutting the soil to a depth of 10 cm along the edge of the cell to sever any shallow rhizomes, and (3) lightly raking the soil surface. More details of the experimental design are given in Li and Shipley (2017).
Trait measurements
After 5 years of plant succession, we recorded 34 species over the 24 mesocosms, varying from 5 to 16 species per mesocosm. During this period, 12 of the originally seeded species disappeared while 19 new species appeared (Li and Shipley, 2017). In 2014, we measured the following plant traits during July and August (the peak of biomass production) on five randomly selected adults of each species in each mesocosm. Vegetative height (distance from the ground to the highest leaf, VH) was first measured on each selected individual, and the individual was then cut at ground level and stored in a cooler with the stem base in water. The cooler was then stored in the dark (~15 h) to allow the leaves to completely rehydrate and burn off accumulated non-structural carbohydrates so that dry mass represented structural components (Garnier et al., 2001). One mature and healthy leaf was collected from the individual the next day to measure fresh mass and leaf area (LA). This leaf was then dried at 60 °C to obtain leaf dry mass for leaf dry matter content (the ratio of leaf dry mass to leaf fresh mass, LDMC) and specific leaf area (the ratio of leaf dry mass to leaf area, SLA). The extra leaves from each sampled individual were collected and dried to determine leaf carbon concentration (LCC) and leaf nitrogen concentration (LNC) using an Elementar VarioMacro CN analyser. Finally, a section of stem was cut to measure specific stem density (the ratio of stem section dry mass to stem section volume, SSD). For plants without a prominent above-ground stem (i.e. rosette plants and some grasses), we cut a little below the ground level to include the short condensed stem, to which the leaves are attached (Pérez-Harguindeguy et al., 2013).
Quantification of observed functional niche occupation
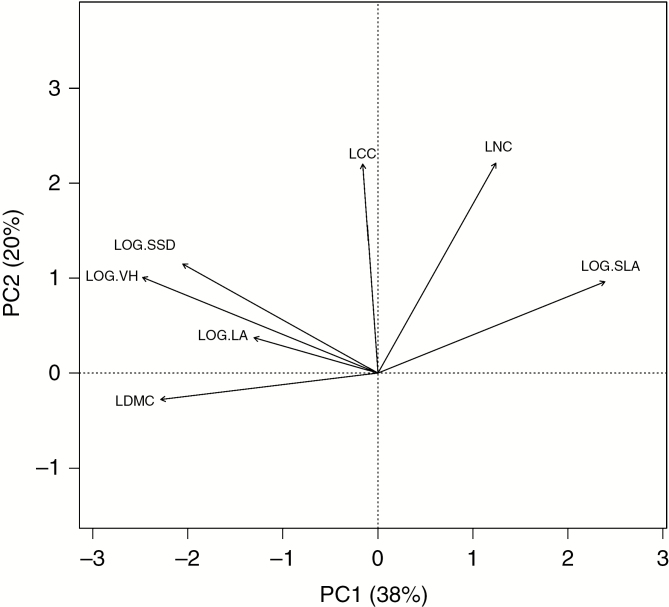
First, the realized functional niche volume of each species in each mesocosm was estimated using the five trait measurements via a hypervolume method (Blonder et al., 2014, 2018). The hypervolume was estimated using a Gaussian kernel density function (Blonder et al., 2018) and a fixed kernel bandwidth of 0.5 s.d. (Lamanna et al., 2014; Li et al., 2018). Although the hypervolume method can be used with any number of observations regardless of dimensionality, its accuracy with few observations per dimension is sensitive to the choice of bandwidth. Simulation analyses found that the hypervolume method with a fixed bandwidth of 0.5 s.d. produced a relatively better estimate than the convex hull and smaller fixed bandwidths (0.3 s.d. and 0.1 s.d.) for number of observations between three and 20 in 2-D space (appendix S2 in Li et al., 2018). We therefore performed principal component analysis on the correlation matrix of the measured traits and limited our analyses in this 2-D composite trait space defined by the first two principal axes (Fig. 1) and use a fixed bandwidth of 0.5 s.d. The three functional niche occupation metrics (total functional niche volume, functional niche overlap and average functional niche volume) were calculated for each mesocosm following the method developed by Li et al. (2018). Total functional niche volume was quantified as the union of all individual functional niche volumes, functional niche overlap was quantified as the sum of the intersections among the individual functional niche volumes weighted by the number of species involved in the intersection, and the average functional niche volume per species was calculated as the mean functional niche volume of all species. After the functional niche occupation metrics were calculated for the 24 mesocosms, we investigated the relationship between each functional niche occupation metric and species richness with linear regressions. Finally, we conducted two-way ANOVAs to determine the effect of stress and disturbance as well as their interaction on the functional niche occupation metrics and on species richness. The ANOVA for species richness was based on a generalized linear model with a ‘quasipoisson’ distribution and a log link function because species richness is a count variable with an overdispersion of the residuals (Ver Hoef and Boveng, 2007).
Fig. 1.
Principal components analysis performed on a correlation matrix of the measured traits: vegetative height (VH), leaf area (LA), leaf dry matter content (LDMC), specific leaf area (SLA), leaf carbon concentration (LCC), leaf nitrogen concentration (LNC) and specific stem density (SSD). VH, LA, SLA and SSD were log-transformed for improved normality before PCA. The first component (PC1) and second component (PC2) explained 38 % and 20 % of the total trait variation, respectively.
Null model analyses
The mathematical identity between the functional niche occupation metrics and species richness (S), S = (T + O)/A, holds for any community by definition, irrespective of its assembly process (Li et al., 2018). We accounted for this dependency by constructing null models to test the difference between the functional niche occupation metrics of observed communities and of randomly assembled (i.e. null) communities containing an equal number of species. The null model analysis was conducted with the following steps. (1) Randomization of species composition: given a mesocosm containing S species, randomly choose S species from the species pool consisting of all 34 species found in the 24 mesocosms. (2) Randomization of individuals within a species: for each species randomly chosen in step 1 and for each mesocosm, randomly choose five individuals (each with its measured trait values) belonging to this species, irrespective of the mesocosm in which this individual is found. (3) Estimate the functional niche volume of each selected species and calculate the functional niche occupation metrics of the randomly assembled community. (4) Repeat steps step 1–3 1000 times to obtain the mean (Inull) and standard deviation (σ null) of the null distribution for each niche occupation metric. (5) For a given mesocosm and a given metric, calculate the standardized effect size (SES) as
Where Iob is the observed metric (Gotelli and McCabe, 2002). A positive (or negative) SES value indicates that the observed metric is higher (or lower) than the null expectation and their significance over the 24 mesocosms is given by a Wilcoxon signed-rank test. Like the observed functional niche occupation metrics, we also investigated how the SES values of each metric varied as a function of species richness (linear regression) and environmental gradients (two-way ANOVA). All analyses were conducted in R 3.4.4 (R Core Team, 2018).
RESULTS
Variation of functional niche occupation with increasing species richness
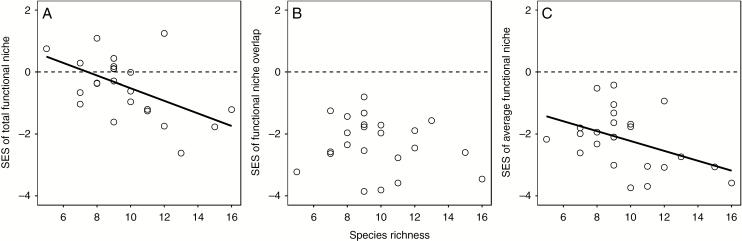
Increasing species richness (from five to 16) across the 24 mesocosms was associated with a small increase in the total functional niche volume (Fig. 2A) and a much larger increase in the functional niche overlap (Fig. 2B), while the average functional niche volume did not change significantly (Fig. 2C). Null model analyses showed that the standardized effect sizes of total functional niche volume (Fig. 3A), functional niche overlap (Fig. 3B) and average functional niche volume (Fig. 3C) were all significantly negative across the 24 mesocosms. Moreover, the standardized effect sizes of total functional niche (Fig. 3A) were negatively correlated with species richness, while the standard effect size of functional niche overlap (Fig. 3B) and average functional niche volume (Fig. 3C) did not change significantly. The significant relationship between species richness and the standard effect sizes of average functional niche volume (Fig. 3C) do not exist when two mesocosms with the largest species richness were removed.
Fig. 2.
The relationships between species richness and the total functional niche volume (A), functional niche overlap (B) or average functional niche volume (C) over the 24 mesocosms. Regression lines were added (solid line for P < 0.05 and dashed line for P < 0.1).
Fig. 3.
The relationships between species richness and the standard effect sizes (SES) of total functional niche volume (A), functional niche overlap (B) and average functional niche volume (C) over the 24 mesocosms. Regression lines were added if significant. The significant relationship between species richness and the standard effect sizes of average functional niche volume (C) do not exist when two mesocosms with the largest species richness are removed. The three standardized metrics were all significantly less than zero across the 24 mesocosms (P < 0.05, Wilcoxon signed-rank test).
Variation of functional niche occupation and species richness along environmental gradients
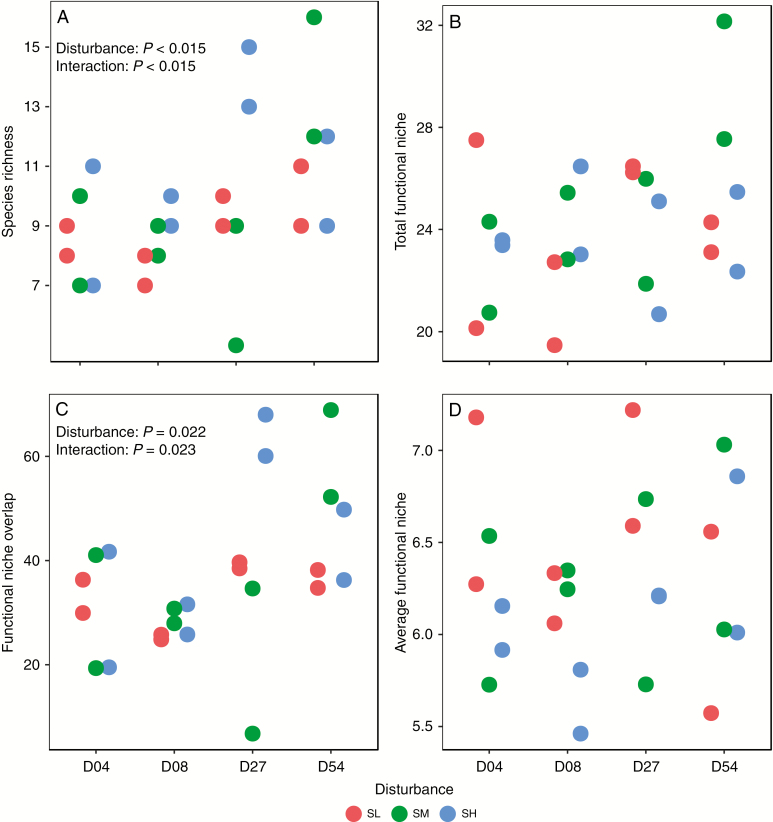
Two-way ANOVA showed that disturbance and interaction between stress and disturbance had significant effects on species richness (Fig. 4A). Specifically, species richness increased with increasing levels of disturbance at low and medium levels of stress, but peaked at the intermediate level of disturbance (D27) when stress was high (Fig. 4A). The pattern of variation in functional niche overlap along the gradients of stress and disturbance had essentially the same trend as that shown by species richness (Fig. 4C). On the other hand, total functional niche volume (Fig. 4B) and average functional niche volume (Fig. 4D) did not change significantly with respect to either levels of stress or disturbance. After standardizing to null expectations, none of the functional niche occupation metrics varied significantly along the gradients of stress and disturbance, either (Fig. 5).
Fig. 4.
Variation of species richness (A), total functional niche volume (B), functional niche overlap (C) and average functional niche volume (D) of each mesocosm along gradients of stress and disturbance over the 24 mesocosms. D04, D08, D027 and D54 represent four, eight, 27 and 54 out of 80 cells disturbed per mesocosm per year, respectively. SL, SM and SH represent low, medium and high levels of stress, respectively. The effects of stress and disturbance as well as their interactions on species richness and functional niche occupation were analysed by two-way ANOVAs, with significant effects indicated by the P-values.
Fig. 5.
Variation of the standard effect sizes (SES) of total functional niche volume (A), functional niche overlap (B) and average functional niche volume (C) along gradients of stress and disturbance over the 24 mesocosms. D04, D08, D027 and D54 represent four, eight, 27 and 54 out of 80 cells disturbed per mesocosm per year, respectively. SL, SM and SH represent low, medium and high levels of stress, respectively. The effects of stress and disturbance as well as their interactions on the SES of functional niche occupation metrics were analysed by two-way ANOVAs. None of the effects is significant.
DISCUSSION
As species richness increases, communities are both more functionally diverse (increase in total functional niche volume, Fig. 2A) and more functionally redundant (increase in functional niche overlap, Fig. 2B), but species are not more functionally specialized (no decline in average functional niche volume, Fig. 2C). This provides a clear answer to part of our first question: how does the pattern of functional niche occupation vary from species-poor to species-rich mesocosms and vary along the gradients of stress and disturbance? Such observed patterns in our experimental system are the same as those found at global scales (Li et al., 2018). Therefore, local and global studies together provide a more comprehensive and robust picture of functional niche occupation from species-poor to species-rich communities. By experimentally controlling the levels of stress and disturbance for each mesocosm, we further determine how functional niche occupation metrics and species richness vary along these environmental gradients. The relationship between species richness and disturbance is positive at low and medium levels of stress but unimodal at high level of stress (Fig. 4A). As species richness changed as a function of stress and disturbance, these experimental factors must also induce changes in some combination of the three niche metrics because species richness is completely determined by them (Li et al., 2018). In fact, only functional niche overlap showed significant differences across the levels of stress and disturbance, and these changes were almost identical to those involving species richness (Fig. 4C). Because we had only two replicate mesocosms per combination of stress and disturbance, it is possible that the lack of significance with respect to the other two niche metrics is due to low statistical power.
However, the above results do not, in themselves, imply any non-random biological process. For instance, the observed relationships between species richness and stress/disturbance can be predicted either by the dynamic equilibrium model assuming the trade-off between competitive ability and tolerance to stress/disturbance (Huston, 1979, 1994), or by a neutral model assuming functional equivalence of species (Ronen and Yuval, 2006). Evidence for non-random processes determining species richness and occupation of the functional niche space came from our null model analysis, providing an answer to our second question: does habitat filtering and/or limiting similarity modify the pattern of functional niche occupation? Although the plants in more species-rich mesocosms occupied a moderately larger volume of the functional space, they occupied less of this volume than expected from a purely neutral process (Fig. 3A). We interpret this as implying that the range of functional trait values in a given mesocosm is constrained by habitat filtering, in which individuals (or species) whose trait values are poorly adapted to the local conditions (biotic and/or biotic) are excluded. This is consistent with previous studies in forests (Lamanna et al., 2014; Swenson and Weiser, 2014) and other ecosystems (Li et al., 2018). On the other hand, the observed increase in the functional niche overlap was also constrained by limiting similarity (MacArthur and Levins, 1967), indicated by less functional niche overlap than expected (Fig. 3B). Because species were being squeezed into a constrained functional space and the amount of overlap between them was also constrained, each species must also, on average, occupy a smaller volume of functional niche than expected (Fig. 3C). Therefore, species were more functionally specialized rather than functionally redundant, contrasting to the findings at global scales (Li et al., 2018). Niche differentiation resulting from limiting similarity in plants requires competition between neighbours, so the spatial scale of a species assemblage must be fine enough to detect such interactions (de Bello et al., 2013). In our experiments, we expect species grown in the same 1-m2 mesocosm to interact with each other, thereby limiting similarity.
Because we found evidence for both habitat filtering and limiting similarity in determining functional niche occupation, we further explored our third question: does their strength vary as a function of species richness or levels of stress and disturbance? From species-poor to species-rich mesocosms, habitat filtering was more important, as indicated by the negative correlation between the standardized effect size of total functional niche and to species richness (Fig. 3A), while the strength of limiting similarity did not change because of no significant trend of standardized effect size of functional niche overlap and average functional niche volume (Fig. 3B, C). Moreover, habitat filtering and limiting similarity appear to be equally important under different environmental conditions because none of the standardized effect sizes of three niche metrics changed significantly along gradients of stress and disturbance (Fig. 5). However, as stated above, the statistical power may be too low to detect such effects because there were only two mesocosm replicates per treatment combination. It is also possible that our experimental gradients of stress and disturbance are too narrow to detect such changes. To clarify this problem, we need a general and comparable measure of ‘stress’ and ‘disturbance’ (Shipley et al., 2016; Daou and Shipley, 2019) so that these levels can be compared between studies. Despite the limitation of replication that is inherent in most mesocosm experiments, our study does provide an experimental basis for the underlying processes of habitat filtering and limiting similarity structuring community functional niches along environmental gradients of stress and disturbance.
FUNDING
The research was funded by an NSERC Discovery grant to B.S. and by a CSC (China Scholarship Council) scholarship to Y.L.
ACKNOWLEDGEMENTS
We thank for Stéphanie Blanchette and Alexandra Ares Bruneau for their help with trait measurements.
LITERATURE CITED
- Adler PB, Seabloom EW, Borer ET, et al. . 2011. Productivity is a poor predictor of plant species richness. Science 333: 1750–1753. [DOI] [PubMed] [Google Scholar]
- Blonder B, Lamanna C, Violle C, Enquist BJ. 2014. The n-dimensional hypervolume. Global Ecology and Biogeography 23: 595–609. [Google Scholar]
- Blonder B, Morrow CB, Maitner B, et al. . 2018. New approaches for delineating n‐dimensional hypervolumes. Methods in Ecology and Evolution 9: 305–319. [Google Scholar]
- Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science 199: 1302–1310. [DOI] [PubMed] [Google Scholar]
- Daou L, Shipley B. 2019. The measurement and quantification of generalized gradients of soil fertility relevant to plant community ecology. Ecology 100: e02549. [DOI] [PubMed] [Google Scholar]
- de Bello F, Vandewalle M, Reitalu T, et al. . 2013. Evidence for scale- and disturbance-dependent trait assembly patterns in dry semi-natural grasslands. Journal of Ecology 101: 1237–1244. [Google Scholar]
- Diaz S, Cabido M, Casanoves F. 1998. Plant functional traits and environmental filters at a regional scale. Journal of Vegetation Science 9: 113–122. [Google Scholar]
- Fox JW. 2013. The intermediate disturbance hypothesis should be abandoned. Trends in Ecology & Evolution 28: 86–92. [DOI] [PubMed] [Google Scholar]
- Fukami T, Martijn Bezemer T, Mortimer SR, Putten WH. 2005. Species divergence and trait convergence in experimental plant community assembly. Ecology Letters 8: 1283–1290. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. 2001. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology 15: 688–695. [Google Scholar]
- Giller P. 1984. Community structure and the niche. Dordrecht:Springer. [Google Scholar]
- Gotelli NJ, McCabe DJ. 2002. Species co-occurrence: a meta-analysis of J. M. Diamond’s assembly rules model. Ecology 83: 2091–2096. [Google Scholar]
- Grime JP. 1973. Competitive exclusion in herbaceous vegetation. Nature 242: 344–347. [Google Scholar]
- Grime JP. 2001. Plant strategies, vegetation processes, and ecosystem properties. Chichester: Wiley. [Google Scholar]
- Hespenheide HA. 1973. Ecological inferences from morphological data. Annual Review of Ecology and Systematics 4: 213–229. [Google Scholar]
- Huston MA. 1979. A general hypothesis of species diversity. The American Naturalist 113: 81–101. [Google Scholar]
- Huston MA. 1994. Biological diversity: the coexistence of species. Cambridge: Cambridge University Press. [Google Scholar]
- Huston MA. 2014. Disturbance, productivity, and species diversity: empiricism vs. logic in ecological theory. Ecology 95: 2382–2396. [Google Scholar]
- Hutchinson GE. 1957. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415–427. [Google Scholar]
- Hutchinson GE. 1978. An introduction to population ecology. New Haven: Yale University Press. [Google Scholar]
- Keddy PA. 1992. Assembly and response rules: two goals for predictive community ecology. Journal of Vegetation Science 3: 157–164. [Google Scholar]
- Kunstler G, Lavergne S, Courbaud B, et al. . 2012. Competitive interactions between forest trees are driven by species’ trait hierarchy, not phylogenetic or functional similarity: implications for forest community assembly. Ecology Letters 15: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamanna C, Blonder B, Violle C, et al. . 2014. Functional trait space and the latitudinal diversity gradient. Proceedings of the National Academy of Sciences USA 111: 13745–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin DC, Messier J. 2015. Fitness of multidimensional phenotypes in dynamic adaptive landscapes. Trends in Ecology & Evolution 30: 487–496. [DOI] [PubMed] [Google Scholar]
- Le Bagousse-Pinguet Y, de Bello F, Vandewalle M, Leps J, Sykes MT, Wardle D. 2014. Species richness of limestone grasslands increases with trait overlap: evidence from within- and between-species functional diversity partitioning. Journal of Ecology 102: 466–474. [Google Scholar]
- Li Y, Shipley B. 2017. An experimental test of CSR theory using a globally calibrated ordination method. PLoS One 12: e0175404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shipley B. 2018. Community divergence and convergence along experimental gradients of stress and disturbance. Ecology 99: 775–781. [DOI] [PubMed] [Google Scholar]
- Li Y, Shipley B, Price JN, et al. . 2018. Habitat filtering determines the functional niche occupancy of plant communities worldwide. Journal of Ecology 106: 1001–1009. [Google Scholar]
- Loranger J, Violle C, Shipley B, et al. . 2016. Recasting the dynamic equilibrium model through a functional lens: the interplay of trait-based community assembly and climate. Journal of Ecology 104: 781–791. [Google Scholar]
- MacArthur R, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist 101: 377–385. [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. . 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Rappoldt C, Hogeweg P. 1979. Niche packing and number of species. The American Naturalist 116: 480–492. [Google Scholar]
- Ricklefs RE. 2012. Species richness and morphological diversity of passerine birds. Proceedings of the National Academy of Sciences USA 109: 14482–14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE, Marquis RJ. 2012. Species richness and niche space for temperate and tropical folivores. Oecologia 168: 213–220. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Miles DB. 1994. Ecological and evolutionary inferences from morphology: an ecological perspective. In: Wainwright PC, Reilly SM, eds. Ecological morphology: integrative organismal biology. Chicago: University of Chicago Press, 13–41. [Google Scholar]
- Ricklefs RE, Travis J. 1980. A morphological approach to the study of avian community organization. The Auk 97: 321–338. [Google Scholar]
- Rogers T. 1977. Ecological capacity and species packing. Bulletin of Mathematical Biology 39: 99–107. [DOI] [PubMed] [Google Scholar]
- Ronen K, Yuval B. 2006. Effects of productivity and disturbance on species richness: a neutral model. The American Naturalist 167: 939–946. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JS. 2002. Functional redundancy in ecology and conservation. Oikos 98: 156–162. [Google Scholar]
- Shipley B, De Bello F, Cornelissen JHC, Laliberté E, Laughlin DC, Reich PB. 2016. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 180: 923–931. [DOI] [PubMed] [Google Scholar]
- Swenson NG, Weiser MD. 2014. On the packing and filling of functional space in eastern North American tree assemblages. Ecography 37: 1056–1062. [Google Scholar]
- R Core Team 2018. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Tilman D. 1988. Plant strategies and the dynamics and structure of plant communities. Princeton: Princeton University Press. [Google Scholar]
- Ver Hoef JM, Boveng PL. 2007. Quasi-poisson vs. negative binomial regression: how should we model overdispered count data? Ecology 88: 2766–2772. [DOI] [PubMed] [Google Scholar]
- Violle C, Enquist BJ, McGill BJ, et al. . 2012. The return of the variance: intraspecific variability in community ecology. Trends in Ecology & Evolution, 27: 244–252. [DOI] [PubMed] [Google Scholar]