Abstract
Background
Between 2016 and 2018, San Diego County experienced a hepatitis A outbreak with a historically high mortality rate (3.4%) that highlighted the need for early recognition of those at risk of developing acute liver failure (ALF).
Methods
A retrospective case series of adult hospitalized patients with acute hepatitis A.
Results
One hundred six patients with hepatitis A were studied, of whom 11 (10.4%) developed ALF, of whom 7 (6.6%) died. A history of alcohol abuse, hyperbilirubinemia, hypoalbuminemia, hyponatremia, and anemia were associated with increased odds of developing ALF. Initial Maddrey’s and Model of End-Stage Liver Disease Sodium (MELD-Na) scores were also associated with the development of ALF. Multivariable analysis showed that a higher initial MELD-Na score (odds ratio [OR], 1.205; 95% confidence interval [CI], 1.018–1.427) and a lower initial serum albumin concentration (OR, 9.35; 95% CI, 1.15–76.9) were associated with increased odds of developing ALF. Combining serum albumin and MELD-Na (SAM; C-statistic, 0.8878; 95% CI, 0.756–0.988) yielded a model that was not better than either serum albumin (C-statistic, 0.852; 95% CI, 0.675–0.976) or MELD-Na (C-statistic, 0.891; 95% CI, 0.784–0.968; P = .841). Finally, positive blood cultures were more common among patients with ALF compared with those without ALF (63.6% vs 4.3%; P < .00001).
Conclusions
Hypoalbuminemia was associated with an increased risk of ALF in patients with acute hepatitis A. Positive blood cultures and septic shock as a cause of death were common among patients with ALF. Providers caring for patients with acute hepatitis A should monitor for early signs of sepsis and consider empiric antibiotics, especially in patients presenting with hypoalbuminemia.
Keywords: acute liver failure, hepatitis A, MELD-Na score, serum albumin
The San Diego hepatitis A outbreak of 2017-18 was one of the largest person-to-person outbreaks in the US since 1996. Our analysis of 109 patients from this outbreak identified hypoalbuminemia as the single best predictor of acute liver failure.
Until recently, hepatitis A infection in the United States was relatively uncommon, with 2007 recorded cases in 2016, most of which were related to contaminated food sources [1]. Between 2016 and 2018, San Diego County endured one of the largest person-to-person hepatitis A outbreaks in the United States since the hepatitis A vaccine was introduced in 1996, with a total of 591 cases and 20 deaths [1–3]. Hepatitis A–associated mortality is largely a function of acute liver failure (ALF) and its associated complications of coagulopathy, encephalopathy, and secondary infection. In case series, 31% to 60% of patients with ALF secondary to hepatitis A either died or underwent liver transplant [3–5]. Early identification of risk factors for ALF is desirable, as this information would aid in the timely triage of these patients to higher-level care and possibly guide empiric therapies. To date, there are only a few reports that describe risk factors for the development of ALF in the setting of acute hepatitis A, and none of these studies involve patients from any of the recent US hepatitis A outbreaks [6–8]. The aim of this study was to identify patient characteristics—available at the time of initial presentation to a health care provider—that could be useful in predicting whether a patient with hepatitis A was at increased risk of developing ALF.
METHODS
Data Collection
We performed a retrospective cohort study using patient data collected from the electronic medical records of all patients hospitalized at the University of California San Diego Hillcrest Hospital from November 1, 2016, to October 10, 2017. Patients were included if they had a positive hepatitis A IgM antibody identified during this time period. Patients were excluded if they were younger than 18 years of age, incarcerated, or pregnant.
Patient data that were available at the time of presentation were collected, including age, gender, domicile status, history of substance use (methamphetamine, opioids, and cocaine via chart review and urine toxicology reports), HIV status, hepatitis B surface antigen positivity, hepatitis C viral load, history of cirrhosis or other previous abnormal liver imaging, initial vital signs, initial complete blood counts, initial complete metabolic panels, and initial coagulation profile. Alcohol abuse was determined based on chart review searching for provider documentation of alcohol abuse disorder. MELD-Na score [MELD Score - Na - 0.025 * MELD * (140 - Na) + 140] and Maddrey’s Discriminant Function (4.6*(PT – Control PT) + Total Bilirubin) were calculated using laboratory results on presentation to the emergency department [9, 10]. At presentation or at any time during hospitalization, ALF was defined as an International Normalized Ratio (INR) >1.5, hepatic encephalopathy, and acute liver injury developing within 26 weeks of hepatitis A infection in an individual without any known previous liver disease. Subsequent inpatient data were also collected, including presence of blood culture results, need for intensive care therapies, N-acetylcysteine (NAC) treatment, and—when a patient had expired—a cause of death.
Statistical Analysis
Bivariable comparisons between patients with and without ALF were made using the Fisher exact test and the chi-square test for categorical variables and the Mann-Whitney and independent t tests for continuous variables in order to determine independent predictors of ALF. Multivariable logistic regression analysis was used to analyze variables that were found to be significantly associated with ALF. The parameters of the final model were used to weigh the contribution of each variable in determining prognostic accuracy. Receiver operating characteristic (ROC) curves and area under the ROC curves were used to assess the accuracy of MELD-Na and SAM, a measure that incorporated both the patient’s serum albumin concentration and MELD-Na score. ROC analysis with leave-1-out cross-validation was applied to evaluate model performance. DeLong’s test was used to compare ROC curves. P values for C-statistics were calculated with the use of the bootstrap method (10 000 times bootstrap resampling). Statistical significance was considered within a 95% confidence interval. All statistical analyses were performed with R statistical software, version 3.5.1 (http://www.r-project.org).
RESULTS
Presenting Characteristics of Acute Hepatitis A Patients With and Without ALF
During the study period, 106 individuals were hospitalized with acute hepatitis A, including 11 patients who developed ALF, of whom 7 died. Table 1 shows the baseline characteristics and clinical data available at the time of presentation to the emergency department of all patients admitted with acute hepatitis A and compares the subsets of patients with and without ALF. The mean patient age was 46 ± 11.9 years, with the majority being homeless men with a history of substance use. Bivariable analysis revealed that patients with hepatitis A infection who progressed to ALF compared with patients who did not develop ALF were more likely to have a history of alcohol use disorder (90.9% vs 39.1%; P = .002), a lower serum albumin (2.3 vs 3.1; P = .0006), a lower hemoglobin (11.7 vs 13.3; P = .04), a lower serum sodium (127.5 vs 134.6; P = .0002), a higher Maddrey’s Discriminant Function (63.9 vs 21.1; P = .03), and a higher MELD-Na score (29.5 vs 19.6; P < .0001). Alternatively, patient age, history of hepatitis C co-infection (18.2% vs 5.6%; P = .28), initial vital signs, initial white blood cell count (10.5 vs 7.8; P = .26), and initial creatinine (1.4 vs 0.8; P = .15) were not significantly associated with the development of ALF among patients presenting with acute hepatitis A. Prior serum albumin levels (within the previous 17 months) were documented in 7 of 11 cases (data not shown). Only 1 patient had a prior normal serum albumin concentration (3.6 g/dL, normal defined as 3.4–5.4 g/dL).
Table 1.
Bivariable Comparison of Clinical Parameters and Patient Characteristics Between Hepatitis A Patients With and Without Acute Liver Failure
| All Patients (n = 106) | No-ALF (n = 95, 89.6%) | ALF (n = 11, 10.4%) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 46 ± 11.9 | 45.3 ± 11.8 | 52.1 ±11.8 | .09 |
| Female | 31 (29.2) | 28 (29.5) | 3 (27.3) | 1 |
| Ethnicity | .3 | |||
| Caucasian | 72 (67.9) | 62 (65.3) | 10 (90.9) | |
| African American | 17 (16) | 17 (17.9) | 0 (0) | |
| Hispanic | 17 (16) | 16 (16.8) | 1 (9.1) | |
| Homeless | 73 (68.9) | 63 (66.3) | 10 (90.9) | .17 |
| Comorbidities | ||||
| History of cirrhosis | 3 (2.8) | 3 (3.2) | 0 (0) | .55 |
| Alcohol use disorder history | 46 (44.7) | 36 (39.1) | 10 (90.9) | .002 |
| Hepatitis B positive | 3 (2.8) | 3 (3.2) | 0 (0) | .55 |
| Hepatitis C positive | 7 (6.9) | 5 (5.6) | 2 (18.2) | .28 |
| Initial vital signs | ||||
| Heart rate, BPM | 93.1 ± 16.5 | 92.9 ± 14.6 | 93.8 ± 29.7 | .93 |
| Systolic BP, mmHg | 121.5 ± 19.8 | 122.7 ± 19.7 | 111.1 ± 18.1 | .07 |
| Diastolic BP, mmHg | 75 ± 13.4 | 75.8 ± 13.4 | 68.5 ± 12.2 | .09 |
| Mean arterial pressure, mmhg | 90.5 ± 14.3 | 91.41 ± 14.2 | 82.7 ± 12.8 | .06 |
| Temperature, ºF | 98.6 ± 1.3 | 98.6 ± 1.2 | 98.6 ± 1.9 | .93 |
| Respiratory rate, RPM | 17.5 ± 2.1 | 17.5 ± 1.9 | 18 ± 2.7 | .54 |
| SaO2, % | 97.9 ± 0 1.7 | 97.9 ± 1.6 | 97.5 ± 2.5 | .63 |
| Initial labs | ||||
| AST, U/L | 1003 (25–7000) | 994 (25–7000) | 2573 (137–7000) | .08 |
| ALT, U/L | 1371 (13–7000) | 1461 (13–6681) | 991 (91–7000) | .37 |
| Albumin, g/dL | 3.0 ± 0.5 | 3.1 ± 0.5 | 2.3 ± 0.5 | .0006 |
| Bilirubin, mg/dL | 6.5 (0.2–44.6) | 6.1 (0.2–44.6) | 14.4 (0.6–27.8) | .04 |
| Alkaline phosphatase, U/L | 206.5 (59–868) | 209 (59–868) | 201 (83–666) | .97 |
| WBC, 1000/mm3 | 8 ± 12.8 | 7.8 ± 13.3 | 10.5 ± 6.2 | .26 |
| Hb, gm/dL | 13. 1 ± 2.2 | 13.3 ± 2.1 | 11.7 ± 2.1 | .04 |
| Creatinine, mg/dL | 0.9 ± 0.5 | 0.8 ± 0.3 | 1.4 ± 1.2 | .15 |
| Platelet, 1000/mm3 | 234.2 ± 80.4 | 235 ± 77.8 | 227.3 ± 104.2 | .82 |
| BUN, mg/dL | 13.6 ± 9.5 | 12.5 ± 6.9 | 23 ± 12.3 | .1 |
| Na, mmol/L | 133.8 ± 4.8 | 134.6 ± 0 4.3 | 127.5 ± 4.4 | .0002 |
| Phosphate, mg/dL | 2.9 ± 1.2 | 2.9 ± 0.7 | 3.1 ± 2.9 | .84 |
| Lactate, mmol/L | 2.9 ± 3.8 | 2.1 ± 1.3 | 5.9 ± 7.4 | .14 |
| Bicarb, mmol/L | 24.1 ± 3.9 | 24.3 ± 3.1 | 22.4 ± 8.5 | .47 |
| Initial prognostic scores | ||||
| Maddrey’s Discriminant Score | 25.7 ± 31 | 21.1 ± 22.7 | 63.9 ± 57.7 | .03 |
| MELD-Na | 20.7 ± 6 | 19.6 ± 5.0 | 29.5 ± 6.9 | <.0001 |
All variables are presented as No. (%), mean ± SD, or median (range).
Abbreviations: ALF, acute liver failure; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferace; BP, blood pressure; BPM, beats per minute; BUN, Blood Urea Nitrogen; MELD-NA, Model of End-Stage Liver Disease Sodium; RPM, Respirations Per Minute; SAM, serum albumin and MELD-Na; WBC, white blood cell count.
Multivariable Analysis of Presenting Characteristics of Patients Who Developed Acute Liver Failure
Multivariate analysis identified every 1-g/dL decrease of initial serum albumin from 4 g/dL (odds ratio [OR], 9.35; 95% confidence interval [CI], 1.15–76.9) and a higher MELD-Na score (OR, 1.205; 95% CI, 1.018–1.427) as the only 2 variables available at the time of presentation that independently predicted ALF in patients with acute hepatitis A (Table 2).
Table 2.
Multvariate Logistic Regression Analysis of Factors Associated With Acute Liver Failure
| Factor | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Initial MELD-Na | 1.205 | 1.018–1.427 | .031 |
| Initial serum albumin (every decrease of 1 g/dL from 4 g/dL) | 9.346 | 1.151–76.923 | .037 |
Abbreviations: CI, confidence interval; MELD-NA, Model of End-Stage Liver Disease Sodium.
Serum Albumin, MELD-Na, and SAM as Predictors of ALF Due to Hepatitis A
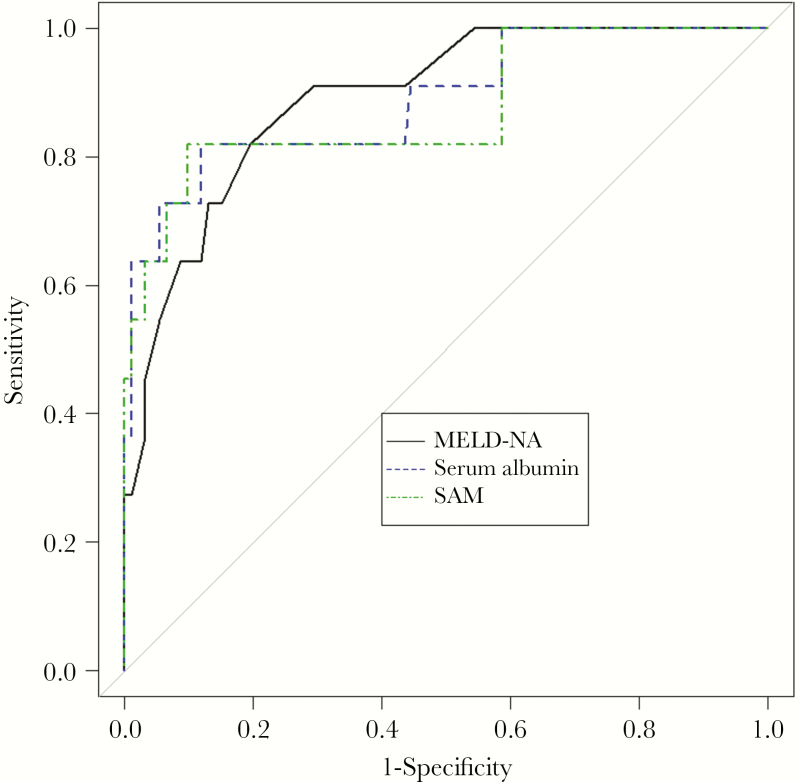
ROC curves predicting ALF were constructed for serum albumin, MELD-Na, and SAM (Figure 1). Of the patients who developed ALF, 82% (9 of 11) had an albumin <2.45, whereas in the non-ALF group, only 9.5% (9 of 95) had an albumin level <2.45. SAM was calculated using the following equation: SAM = MELD-Na + 11.99 * (4—Initial Albumin). The associated sensitivity, specificity, best cutoff value, and Youden Index for each curve are shown in Table 3. As INR is a component of the possible predictors MELD-Na and SAM and also a component of the outcome of interest, ALF, ROC analysis was also performed using SAM and MELD-Na without INR (data not shown), which resulted in a similar C-statistic (0.8745 for SAM without INR vs 0.8878 for SAM; P = .841). Serum albumin, MELD-Na SAM, and MELD-Na ROC curves did not differ statistically in terms of predicting ALF (P = .841).
Figure 1.
Receiver operating characteristic curves comparing MELD-Na, serum albumin, and SAM as predictors of acute liver failure. Abbreviations: MELD-NA, Model of End-Stage Liver Disease Sodium; SAM, serum albumin and MELD-Na.
Table 3.
AUC for MELD-NA, Initial Albumin, SAM, and the Best Cutoff Values to Predict Liver Failure
| Factor | AUC | Cutoff Value | Sensitivity, % | Specificity, % | Youden’s Index, % |
|---|---|---|---|---|---|
| Initial MELD-Na | 0.891 (0.784–0.968) | 23.99 | 81.8 | 80.4 | 62.2 |
| Initial albumin | 0.852 (0.675–0.976) | 2.45 | 81.8 | 90.2 | 72.0 |
| SAM | 0.888 (0.756–0.988) | 46.4 | 81.8 | 88.0 | 69.8 |
Abbreviations: AUC, area under the curve; MELD-NA, Model of End-Stage Liver Disease Sodium; SAM, serum albumin and MELD-Na.
As all 3 measures were not dissimilar in terms of predicting ALF, further analysis focused on serum albumin, the easiest of the 3 variables to measure. Internal validation analysis was performed using both bootstrap analysis (C-statistic, 0.8000) and leave-1-out cross-validation to evaluate model performance and showed a prediction error for serum albumin of 0.06416 (bias-corrected cross-validation error, 0.06413).
Hospital Course and Outcomes of Hepatitis A Patients With ALF
All patients with hepatitis A and ALF were evaluated for liver transplant, although none were deemed candidates due to homelessness, comorbid conditions, or active substance abuse. Five of 11 ALF patients were admitted directly to the ICU (Table 4), whereas 5 other patients did not have ALF on presentation but were diagnosed during their hospital stay and needed ICU transfer. Seven of 11 patients with ALF had positive blood cultures (including 4 patients with positive blood cultures within 24 hours of admission), compared with 2 of 47 (only 47 out of 95 non-ALF patients received blood cultures) hepatitis A patients without ALF (63.6% vs 4.3%; P < .00001). In the majority of cases, blood culture positivity occurred in 1 of 2 sets and was due to a variety of organisms including methicillin-resistant Staphylococcus aureus (n = 1), Staphylococcus epidermidis (n = 2), Staphylococcus hominis (n = 2), and Escherichia coli (n = 2) (Table 4). Analysis of a potential clinical benefit of empiric antibiotics in the emergency department was not feasible because of the broad variation in antibiotic choice, dose, and timing of administration. The most common cause of death among patients who developed ALF was septic shock. Patients with ALF were more likely to be treated with NAC within the first 24 hours of presentation (45.5% vs 18.8%; P = .05); however, multivariable analysis did not detect a mortality benefit (data not shown). Within this case series, 18 of the 95 non-ALF patients and 5 of the 11 ALF patients received NAC therapy, with 1 ALF survivor having received NAC and 4 of the 7 deceased patients having received NAC. ALF patients, compared with non-ALF patients, also required more intensive care therapies, including mechanical ventilation (27.3% vs 3.2%), renal replacement therapy (36.4% vs 2.1%), and vasopressor therapy (45.5% vs 3.2%; P < .005 for all).
Table 4.
Patients With Acute Liver Failure and Hepatitis A: Triage, Blood Culture Data, Prior Albumin Concentration, and Cause of Death
| Patient | Initial Ward | Bacteremia and Blood Culture Sets (+/Total) | Hospital Day of Culture Positivity | In-Hospital Mortality | Cause of Death |
|---|---|---|---|---|---|
| 1 | General | — | — | Y | Hemorrhagic shock |
| 2 | ICU | S. epidermidis (1/2) | 1 | Y | Septic shock |
| S. hominis (1/2) | 12 | ||||
| E. coli (1/2) | 18 | ||||
| 3 | General | — | — | Y | Cerebral edema |
| 4 | General | S. epidermidis (2/2) | 0 | Y | Septic shock |
| 5 | ICU | S. hominis (1/4) | 2 | Y | Septic shock |
| 6 | ICU | B. pumilus R. mucilaginosa S. oralis C. bifermentans (1/2) | 0 | Y | Septic shock |
| 7 | General | MRSA (4/4) | 0 | Y | Septic shock |
| 8 | General | E. coli (1/2) | 10 | N | — |
| 9 | ICU | — | — | N | — |
| 10 | ICU | — | — | N | — |
| 11 | General | S. hominis (1/4) | 3 | N | — |
Abbreviations: ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus.
Discussion
Emergency medicine providers in the United States are likely to face increasing numbers of patients with acute hepatitis A, including patients at risk for ALF. Besides California, numerous other states have experienced recent outbreaks, including Arizona, Indiana, Kentucky, Michigan, Missouri, Ohio, Tennessee, Utah, and West Virginia [1]. Centers for Disease Control and Prevention analyses of these outbreaks have identified homelessness and substance use disorder as risk factors associated with patients infected with hepatitis A. However, the risk factors available at the time of presentation to a medical provider that are associated with the development of subsequent acute liver failure are not known [1]. Although most children aged <6 years infected with hepatitis A are asymptomatic, the majority of adults with acute hepatitis A experience a severe clinical course [11]. As the hepatitis A vaccine has been administered to American children since 1996, adults now represent the population at greatest risk for hepatitis A in the United States.
Our analysis of a large subset of patients from the 2016–2018 hepatitis A outbreak in San Diego (106 of the 592 total cases including 8 of the 20 patients who died) showed that initial serum albumin and MELD-Na scores were independently associated with the development of ALF patients with hepatitis A. The model combining serum albumin and MELD-Na (SAM) did not prove to be superior statistically to either serum albumin or MELD-Na. Considering the ease of measuring serum albumin compared with calculating MELD-Na or SAM, serum albumin has better clinical potential to be used as a tool for identifying patients with acute hepatitis A who are at increased risk for developing ALF.
Serum albumin is involved in a number of physiologic functions: regulation of plasma oncotic pressure (providing up to 65%–75% of normal colloid oncotic pressure) and capillary permeability, compound transport, and free radical scavenging [12, 13]. In critical illness, hypoalbuminemia is a function of loss due to vascular permeability, dilution with fluid resuscitation, and decreased albumin synthesis. Proinflammatory mediators such as interleukin-6 and tumor necrosis factor–α, for example, reduce albumin mRNA transcription. Hypoalbuminemia is a predictor of increased mortality across a variety patient populations and medical conditions, including sepsis, end-stage renal disease on hemodialysis, and even femoral neck fracture [14].
The physiological basis for serum albumin and MELD-Na score being associated with ALF in hepatitis A is not obviously apparent. Fulminant liver injury in hepatitis A infection is believed to be the result of excessive and unregulated inflammatory response, as the virus is not considered cytopathic to hepatocytes [15]. Serum albumin, a negative acute phase reactant, was notably reduced in patients with ALF, a finding that is consistent with the theory that excessive inflammation causes hepatitis-related liver failure. Contrary to prior studies [11], we did not find that increased leukocytosis was associated with ALF in acute hepatitis A patients. The decreased serum albumin may also have been a marker of reduced synthetic function at baseline. Of note, bivariable analysis revealed that INR, another marker of synthetic function, was also elevated among ALF patients (data not shown), but because of concerns for mathematical coupling, INR was excluded from our analysis. To discern whether the observed hypoalbuminemia was due to preexisting liver disease or a function of acute hepatitis A, patient charts were reviewed, and prior serum albumin levels (within the previous 17 months) were documented in 7 of 11 cases (data not shown). Only 1 patient had a prior normal serum albumin concentration (3.6 g/dL, normal defined as 3.4–5.4 g/dL); the significance of this finding is limited, as these serum albumin measures were made in the setting of prior emergency department visits in which the patients were presumably unwell. Thus, it is not clear in this case series whether a relatively low serum albumin concentration represents baseline liver disease or whether it is a consequence of hepatitis A infection. The MELD-Na score, on the other hand, is designed to predict the mortality of patients with cirrhosis and has not been previously applied to patients with acute liver failure to our knowledge. It was interesting to note that neither MELD nor serum sodium was individually predictive of ALF in hepatitis A.
Comparing the results of this study with prior international investigations of acute hepatitis revealed both similar and disparate findings. In a case series of patients from Guatemala and Mexico, a serum albumin level of <2.5 mg/dL was associated with mortality, which was consistent with our ROC best cutoff of <2.45 mg/dL for predicting ALF [11]. On the other hand, our study differed from prior studies that suggested that serum creatinine, advanced age, alcohol use disorder, and chronic hepatitis B infection portended increased ALF incidence in acute hepatitis A [8, 11, 16]. Each of these variables was evaluated and was not significantly associated with ALF in our study.
The high mortality rate associated with the 2016–2018 San Diego hepatitis A outbreak is of particular interest. Prior hepatitis A outbreaks involving patients >40 years of age were characterized by a mortality rate of 1.1%, whereas the San Diego outbreak had a mortality rate of 3.4% [17, 18]. Socioeconomic factors presumably contributed to this mortality rate, as most patients affected by the outbreak had limited access to health care and likely presented later in their disease course. Contrary to prior studies, we did not find patient age or coinfection with hepatitis B or C to be associated with hepatitis A–associated ALF, although it should be noted that this study included few patients with other active hepatidites (3 patients with hepatitis B and 7 patients with hepatitis C) [8, 16]. Also, only 1 patient was infected with HIV. Although the study population had a high prevalence of substance use disorder (59%, of whom 55% were methamphetamine users) and homelessness (69%), these factors were not associated with the development of ALF. The viral genotype was considered a potential risk factor for ALF. Genotype 1B—common in the Mediterranean region and South Africa—historically accounts for only 2% of hepatitis A in the United States, yet it was exclusively identified in all reported cases from the San Diego outbreak [19]. Although some studies have suggested that there is no difference in clinical presentation or severity of disease based on genotype, other studies have shown that genotype 1b is more often associated with acute liver failure [20, 21]. Given that genotype 1b is relatively rare in the United States and potentially associated with more severe disease in outbreak settings, it is possible that this was a contributing factor to the high morbidity and mortality observed in the San Diego outbreak.
A potentially important finding of this study was the high rate of positive blood cultures among patients with hepatitis A and ALF. It is tempting to dismiss these results as contaminants based on the organism and the fact that many positive blood cultures were only 1 of 2 sets. Nonetheless, the difference in positive blood cultures between ALF and non-ALF patients (63.6% vs 4.3%; P < .00001), along with the high rate of septic shock as the cause of death (5/7 patients with ALF who died), is striking. It has been theorized that ALF induces an acquired immunodeficiency due to overactivation of the systemic inflammatory response and Kupffer cell dysfunction [22]. Whether the higher rates of positive blood cultures among patients with ALF represent contaminants, true infection, or simply a marker of immunodeficiency is not clear. Unfortunately, we were not able to draw any conclusions regarding empiric antibiotic use in this patient cohort. Previous studies have looked at prophylactic antibiotics in all types of ALF and found no mortality benefit; the frequency of positive blood cultures and septic shock as a cause of death among patients with hepatitis and ALF in this study would suggest that providers should monitor closely for early signs of sepsis and have a low threshold for starting empiric antibiotics, especially in patients with concomitant hypoalbuminemia [23].
Whether empiric NAC is a beneficial therapy for patients with acute hepatitis A could not be determined in this study. NAC therapy, using dosing similar to prior studies, was started within the first 24 hours in 86% of our patients, the majority of whom were given NAC for at least 24 hours [24, 25]. Multivariable analysis did not reveal a mortality benefit in ALF patients treated empirically with NAC; however, our analysis was limited by the small sample size and the fact that NAC was prescribed at the discretion of the consulting hepatologist, which was a large confounding factor. Considering its limited side effect profile and its potential to be beneficial in ALF, early empiric NAC therapy before development of ALF, possibly guided by the patient’s serum albumin concentration, is worthy of investigation in future hepatitis A outbreaks for both preventing ALF and reducing mortality.
The ability to identify acute hepatitis A patients at risk for ALF would improve triage. Currently, such decisions are challenging. Many of the traditional data points used for triaging patients (heart or respiratory rate, Mean Arterial Pressure (MAP)) were not different at presentation between patients who did or did not develop ALF. In this study, 5 of the 11 ALF patients did not have ALF on presentation; however, all 5 patients eventually developed ALF and required transfer to the ICU. One of the 11 patients had ALF on admission and was inappropriately triaged to the step-down unit and required ICU transfer later in the hospital course. Although none of the patients in this study with ALF were deemed liver transplantation candidates due to poor social support and substance abuse, it would be ideal to be able to identify patients at risk for ALF in the event that the admitting hospital system is not a liver transplant center.
Several strengths of this study should be noted. The San Diego hepatitis A outbreak represented a unique opportunity to study a large sample of patients with hepatitis A, including patients with concomitant ALF. The high prevalence of homelessness and polysubstance abuse among patients and the predominant genotype, IB, are consistent with other recent and ongoing hepatitis A outbreaks in the United States. The finding of hypoalbuminemia being associated with ALF agreed with results from another international study, which was reassuring and suggested a degree of external validity. The limitations of this study must also be noted. Many of these patients did not have previous health care exposure before presentation, so it was difficult to rule out the presence of prior liver disease including cirrhosis; thus, this study may have overestimated the incidence of ALF. The small sample size and the rarity of ALF are also limitations to the external validity of this study, which we tried to ameliorate by using internal cross-validation techniques. Also, heterogenous prescribing patterns made it difficult to investigate the effect of NAC and empiric antibiotics on preventing ALF. Ideally, future studies would validate the predictive value of hypoalbuminemia using data obtained from multiple hepatitis A outbreaks.
Conclusions
Person-to-person hepatitis A outbreaks in association with homeless and substance use are increasingly common events in the United States. There are potential advantages to the early identification of hepatitis A patients at increased risk for ALF. In this retrospective study, initial hypoalbuminemia was predictive of ALF in a large case series of patients from the 2016–2018 San Diego hepatitis A outbreak. Positive blood cultures and septic shock as a cause of death were common among patients with ALF. Providers caring for patients with acute hepatitis A should monitor for early signs of sepsis and consider empiric antibiotics, especially in patients presenting with hypoalbuminemia.
Acknowledgments
Financial support. None.
Potential conflicts of interest. The authors declare that they have no conflicts of interest. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. CDC. Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless Available at: https://emergency.cdc.gov/han/han00412.asp. Accessed 4 August 2018.
- 2. Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology 2006; 43:S164–72. [DOI] [PubMed] [Google Scholar]
- 3. Rezende G, Roque-Afonso AM, Samuel D, et al. . Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology 2003; 38:613–8. [DOI] [PubMed] [Google Scholar]
- 4. Schiodt FV, Atillasoy E, Shakil AO, et al. . Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg 1999; 5:29–34. [DOI] [PubMed] [Google Scholar]
- 5. Schiødt FV, Davern TJ, Shakil AO, et al. . Viral hepatitis-related acute liver failure. Am J Gastroenterol 2003; 98:448–53. [DOI] [PubMed] [Google Scholar]
- 6. Kamath PS, Heimbach J, Wiesner RH. Acute liver failure prognostic scores: is good enough good enough? Clin Gastroenterol Hepatol 2016; 14:621–3. [DOI] [PubMed] [Google Scholar]
- 7. Kim JI, Kim YS, Jung YK, et al. . Factors influencing the severity of acute viral hepatitis A. Korean J Hepatol 2010; 16:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin HS, Kim SP, Han SH, et al. . Prognostic indicators for acute liver failure development and mortality in patients with hepatitis A: consecutive case analysis. Yonsei Med J 2014; 55:953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim WR, Biggins SW, Kremers WK, et al. . Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008; 359:1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maddrey WC, Boitnott JK, Bedine MS, et al. . Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–9. [PubMed] [Google Scholar]
- 11. Mackinney-Novelo I, Barahona-Garrido J, Castillo-Albarran F, et al. . Clinical course and management of acute hepatitis A infection in adults. Ann Hepatol 2012; 11:652–7. [PubMed] [Google Scholar]
- 12. Pulimood TB, Park GR. Debate: albumin administration should be avoided in the critically ill. Crit Care 2000; 4:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takegawa R, Kabata D, Shimizu K, et al. . Serum albumin as a risk factor for death in patients with prolonged sepsis: an observational study. J Crit Care 2019; 51:139–44. [DOI] [PubMed] [Google Scholar]
- 14. Niccolai F, Parchi PD, Vigorito A, et al. . The correlation between preoperative levels of albumin and tlc and mortality in patients with femoral neck fracture. J Biol Regul Homeost Agents 2016; 30:187–91. [PubMed] [Google Scholar]
- 15. Phan C, Hollinger FB. Hepatitis A: natural history, immunopathogenesis, and outcome. Clin Liver Dis (Hoboken) 2013; 2:231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keeffe EB. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol 1995; 90:201–5. [PubMed] [Google Scholar]
- 17. Bianco E, Stroffolini T, Spada E, et al. . Case fatality rate of acute viral hepatitis in Italy: 1995–2000. An update. Dig Liver Dis 2003; 35:404–8. [DOI] [PubMed] [Google Scholar]
- 18. Vogt TM, Wise ME, Bell BP, Finelli L. Declining hepatitis A mortality in the United States during the era of hepatitis A vaccination. J Infect Dis 2008; 197:1282–8. [DOI] [PubMed] [Google Scholar]
- 19. Hosseini M, Ding A. 2189. Hepatitis A outbreak in San Diego County, 2016–2017: a morphologic and epidemiologic review. Open Forum Infect Dis 2018; 5(Suppl_1): S646–S. [Google Scholar]
- 20. Ajmera V, Xia G, Vaughan G, et al. ; Acute Liver Failure Study Group What factors determine the severity of hepatitis A-related acute liver failure? J Viral Hepat 2011; 18:e167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collier MG, Khudyakov YE, Selvage D, et al. ; Hepatitis A Outbreak Investigation Team Outbreak of hepatitis A in the USA associated with frozen pomegranate arils imported from Turkey: an epidemiological case study. Lancet Infect Dis 2014; 14:976–81. [DOI] [PubMed] [Google Scholar]
- 22. Rolando N, Wade J, Davalos M, et al. . The systemic inflammatory response syndrome in acute liver failure. Hepatology 2000; 32:734–9. [DOI] [PubMed] [Google Scholar]
- 23. Rolando N, Gimson A, Wade J, et al. . Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatology 1993; 17:196–201. [PubMed] [Google Scholar]
- 24. Lee WM, Hynan LS, Rossaro L, et al. . Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 2009; 137:856–64, 64 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sales I, Dzierba AL, Smithburger PL, et al. . Use of acetylcysteine for non-acetaminophen-induced acute liver failure. Ann Hepatol 2013; 12:6–10. [PubMed] [Google Scholar]