Abstract
OBJECTIVE
In September 2016, the U.S. Food and Drug Administration approved the Medtronic 670G “hybrid” closed-loop system. In Auto Mode, this system automatically controls basal insulin delivery based on continuous glucose monitoring data but requires users to enter carbohydrates and blood glucose for boluses. To track real-world experience with this first commercial closed-loop device, we prospectively followed pediatric and adult patients starting the 670G system.
RESEARCH DESIGN AND METHODS
This was a 1-year prospective observational study of patients with type 1 diabetes starting the 670G system between May 2017 and May 2018 in clinic.
RESULTS
Of the total of 84 patients who received 670G and consented, 5 never returned for follow-up, with 79 (aged 9–61 years) providing data at 1 week and 3, 6, 9, and/or 12 months after Auto Mode initiation. For the 86% (68 out of 79) with 1-week data, 99% (67 out of 68) successfully started. By 3 months, at least 28% (22 out of 79) had stopped using Auto Mode; at 6 months, 34% (27 out of 79); at 9 months, 35% (28 out of 79); and by 12 months, 33% (26 out of 79). The primary reason for continuing Auto Mode was desire for increased time in range. Reasons for discontinuation included sensor issues in 62% (16 out of 26), problems obtaining supplies in 12% (3 out of 26), hypoglycemia fear in 12% (3 out of 26), multiple daily injection preference in 8% (2 out of 26), and sports in 8% (2 out of 26). At all visits, there was a significant correlation between hemoglobin A1c (HbA1c) and Auto Mode utilization.
CONCLUSIONS
While Auto Mode utilization correlates with improved glycemic control, a focus on usability and human factors is necessary to ensure use of Auto Mode. Alarms and sensor calibration are a major patient concern, which future technology should alleviate.
Introduction
Insulin replacement treatment is a significant burden, with a narrow therapeutic margin. Too much insulin results in acutely debilitating hypoglycemia, and too little over long periods results in hyperglycemia with subsequent microvascular complications and cardiovascular disease (1,2). One method of reducing disease burden is to use continuous glucose monitoring (CGM) data to alter insulin delivery from an insulin pump using a software controller. In September 2016, following years of clinical trials (3–12), the U.S. Food and Drug Administration approved the Medtronic 670G “hybrid” closed-loop system (13). In Auto Mode, this system automatically controls basal insulin delivery based on Guardian 3 CGM data, but requires users to enter carbohydrate intake for meal boluses and finger-stick glucose readings for correction boluses (as the Guardian 3 is not approved for nonadjunctive use). Prior studies have demonstrated improved time in range associated with the use of Auto Mode (14). There have been recent case series presenting clinical experience with the 670G in the pediatric setting (15). To track our real-world experience with this first commercial closed-loop device, we followed pediatric and adult patients placed on the 670G in our clinics.
Research Design and Methods
This was a 1-year prospective observational study of both pediatric and adult patients with type 1 diabetes starting the 670G system at Stanford University Medical Center between May 2017 and May 2018. Those with type 1 diabetes over the age of 7 years who had received the Medtronic 670G insulin pump and Guardian 3 CGM were eligible. This was an observational study based on a convenience sampling for those patients who were interested in starting the 670G closed-loop system. As such, there was no formal power calculation, and we report on all participants recruited over 1 year. A little over one-quarter of participants were upgrading from the prior 630G pump with Guardian 2 CGM through the priority access program and free upgrade pathway. The majority of participants had used Medtronic pumps in the past. The Stanford University Institutional Review Board approved the research protocol. The trial was registered on Clinicaltrials.gov as NCT03017482.
Observational data gathered through routine clinical care were collected on those patients providing informed consent. Study data were collected in REDCap electronic data capture tools hosted at Stanford University (16). The proposed start-up protocol included a thorough explanation of expectations, risks, and benefits of starting the 670G system. This discussion was to take place with the physician, diabetes educators, and device representatives. Participants would wear the pump and Guardian 3 sensor for 1 week with suspend before low enabled. During this same period, a 48-h Auto Mode warmup occurs, even before Auto Mode is activated. Thereafter, Auto Mode is started in clinic. Weekly CareLink downloads occur for the first month with provider calls and intervention as needed. Patients would then be seen for routine follow-up at 3, 6, 9, and 12 months.
Demographics collected included age, diabetes duration, race/ethnicity, highest education achieved by the patient or patient’s guardian, and the prior pump systems (four maximum) and CGM systems (three maximum) used by the participants. With the exception of seven participants, baseline HbA1c was available 1 month prior to Auto Mode initialization. At the time of Auto Mode initialization, participants had a minimum of 1 week of data regarding CGM usage and time in range (defined as 70–180 mg/dL) collected from Guardian 3 sensor downloads.
The data were analyzed using MATLAB (2018; MathWorks, Natick, MA), and violin plots were generated using code from Holger Hoffmann. Qualitative and quantitative measures are compared for those using Auto Mode at 1 year versus those who discontinued use of Auto Mode. We summarize quantitative measures using means and SDs with comparisons made using an unpaired statistical hypothesis test. A two-proportion z test is used to compare differences in qualitative measures. Linear regression was performed on percent time in Auto Mode and HbA1c at each time point.
Reasons for continuing and discontinuing use of the system were derived from chart review. During the observation period, sensor technology advanced considerably. Dexcom released the G6 system, which is the first CGM not requiring calibrations, resistant to acetaminophen interference, and approved for nonadjunctive use (17,18). Given these benefits over the Guardian 3, we assessed Dexcom G6 use among participants at the end of the trial.
Results
Eighty-four patients with type 1 diabetes received 670G and consented to be in the study. Five never returned for follow-up. Of the remaining 79 participants (aged 9–61 years), 26 (33%) were <18 years of age. These 79 individuals provided data for at least one visit at 1 week, 3 months, 6 months, 9 months, and/or 12 months after Auto Mode initiation. Demographic data for the group are presented in Table 1 and further subdivided by the age of the participants.
Table 1.
Baseline demographic data subdivided by age-groups
| All participants | Age <18 years | Age ≥18 years | |
|---|---|---|---|
| n (%) | 79 (100) | 26 (33) | 53 (67) |
| Age (years), mean ± SD | 27.2 ± 14.4 | 14.8 ± 2.2 | 33.3 ± 13.9 |
| Diabetes duration (years), mean ± SD | 14.6 ± 10.5 | 7.0 ± 4.3 | 18.3 ± 10.7 |
| Sex | |||
| Male | 46 (58) | 16 (62) | 30 (57) |
| Female | 32 (41) | 10 (38) | 22 (42) |
| Transfeminine | 1 (1) | 0 (0) | 1 (2) |
| Baseline HbA1c, % (mmol/mol), mean ± SD | 7.9 ± 1.4 (63 ± 15.3) | 8.3 ± 1.7 (67 ± 18.6) | 7.8 ± 1.2 (62 ± 13.1) |
| Percent time using CGM prior to Auto Mode initialization, mean ± SD | 77 ± 25 | 73 ± 28 | 80 ± 22 |
| Baseline time in range (70–180 mg/dL) (%), mean ± SD | 60 ± 17 | 58 ± 18 | 61 ± 17 |
| Number of visits (total 5 possible), mean ± SD | 4.1 ± 1.0 | 4.3 ± 0.9 | 4.0 ± 1.1 |
| Race | |||
| African | 3 (4) | 2 (8) | 1 (2) |
| Asian | 9 (11) | 5 (19) | 4 (8) |
| Caucasian | 57 (72) | 18 (69) | 39 (74) |
| Middle Eastern | 3 (4) | 0 (0) | 3 (6) |
| Native American | 1 (1) | 1 (4) | 0 (0) |
| Unknown | 3 (4) | 0 (0) | 3 (6) |
| Declined | 3 (4) | 0 (0) | 3 (6) |
| Highest education for subject or guardian | |||
| Some high school | 5 (6) | 0 (0) | 5 (9) |
| High school degree | 2 (3) | 2 (8) | 0 (0) |
| Some college | 8 (10) | 7 (27) | 1 (2) |
| College degree | 19 (24) | 13 (50) | 6 (11) |
| Graduate degree | 23 (29) | 15 (58) | 8 (15) |
| Other | 4 (5) | 4 (15) | 0 (0) |
| Declined | 18 (23) | 12 (46) | 6 (11) |
| Prior insulin delivery systems | |||
| Animas | 13 (9) | 3 (8) | 10 (10) |
| Medtronic, older | 62 (45) | 19 (49) | 43 (44) |
| Medtronic 630G | 37 (27) | 13 (33) | 24 (24) |
| Omnipod | 5 (4) | 1 (3) | 4 (4) |
| Roche | 1 (1) | 0 (0) | 1 (1) |
| Tandem | 4 (3) | 0 (0) | 4 (4) |
| Multiple daily injections | 4 (3) | 1 (3) | 3 (3) |
| Other | 11 (8) | 2 (5) | 9 (9) |
| Prior CGM | |||
| Abbott FreeStyle Libre | 3 (3) | 2 (6) | 1 (1) |
| Dexcom, G5 or older | 54 (51) | 16 (47) | 38 (54) |
| Medtronic Enlite | 35 (33) | 11 (32) | 24 (34) |
| None | 8 (8) | 3 (9) | 5 (7) |
| Other | 5 (5) | 2 (6) | 3 (4) |
Data are n (%) unless otherwise indicated.
Not every patient came to a 1-week, 3-month, 6-month, 9-month, or 12-month visit. For example, more participants had data available at 3 months than at 1 week. For the 86% (68 out of 79) with Auto Mode initiation data, 99% (67 out of 68) successfully started. Among the 79 participants providing some data, Auto Mode use was as follows:
3 months: 22 (28%) discontinued, 50 (63%) continued, and 7 (9%) provided no data
6 months: 27 (34%) discontinued, 40 (50%) continued, and 12 (15%) provided no data
9 months: 28 (35%) discontinued, 35 (44%) continued, and 16 (20%) provided no data
12 months: 26 (33%) discontinued, 30 (38%) continued, and 23 (29%) provided no data
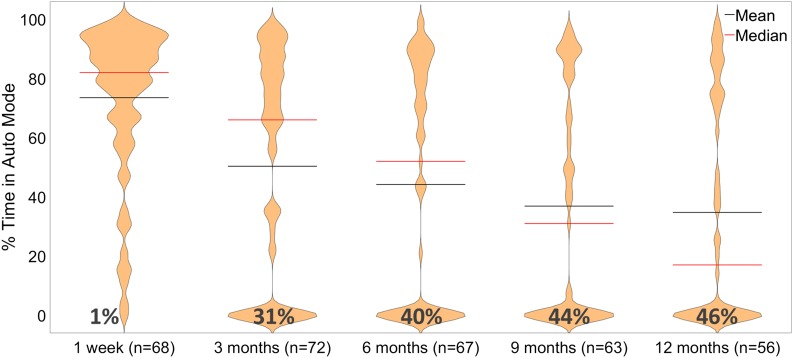
Figure 1 illustrates this decline in the use of Auto Mode over time for those with available data. By 3 months, 31% (22 out of 72) stopped using Auto Mode; at 6 months, 40% (27 out of 67); at 9 months, 44% (28 out of 63); and by 12 months, 46% (26 out of 56). Use of Auto Mode >70% of the time was 43% (31 out of 72) at 3 months, 40% (27 out of 67) at 6 months, 30% (19 out of 63) at 9 months, and 32% (18 out of 56) at 12 months.
Figure 1.
Violin plot reflecting percentage of time in Auto Mode by time of follow-up, with the number of participants with available data noted. For the available data, usage of Auto Mode diminishes over time. At 1 week, mean 74% and median 82%; at 3 months, mean 50% and median 66%; at 6 months, mean 44% and median 52%; at 9 months, mean 37% and median 31%; and at 12 months, mean 35% and median 17%.
Variations in demographics between those using Auto Mode and discontinuing at 1 year are presented in Table 2. (Further delineation by age-groups is provided in Supplementary Tables 1 and 2.) Age (P = 0.02), CGM use prior to initiating Auto Mode (P = 0.001), and self-report of college or graduate degree (P = 0.02) were significantly different between those using Auto Mode and those who discontinued after 12 months. Among participants with available data, 61% of those <18 years of age discontinued, while 39% of those ≥18 years discontinued.
Table 2.
Demographic differences among those who use and do not use Auto Mode at 1 year
| Using Auto Mode | Discontinued Auto Mode | P value | |
|---|---|---|---|
| n (%) | 30 (54) | 26 (46) | NA |
| Age (years), mean ± SD | 31.5 ± 17.8 | 22.3 ± 10.0 | 0.02* |
| Percent time using CGM prior to Auto Mode initialization, mean ± SD | 84 ± 13 | 61 ± 32 | 0.001* |
| Diabetes duration (years), mean ± SD | 17.0 ± 13.1 | 11.1 ± 9.3 | 0.06 |
| Sex, n (%) | |||
| Male | 15 (50) | 17 (65) | 0.25 |
| Female | 14 (47) | 9 (35) | 0.36 |
| Transfeminine | 1 (3) | 0 (0) | 0.35 |
| Baseline HbA1c, % (mmol/mol), mean ± SD | 7.7 ± 1.1 (61 ± 12.0) | 8.3 ± 1.7 (67 ± 18.6) | 0.13 |
| HbA1c at 1 year, % (mmol/mol), mean ± SD | 7.6 ± 0.8 (60 ± 8.7) | 8.3 ± 1.2 (67 ± 13.1) | 0.06 |
| Number of visits (total five possible), mean ± SD | 4.7 ± 0.7 | 4.6 ± 0.5 | 0.57 |
| Race | |||
| African | 0 (0) | 2 (8) | 0.12 |
| Asian | 6 (20) | 1 (4) | 0.07 |
| Caucasian | 23 (77) | 17 (65) | 0.35 |
| Middle Eastern | 0 (0) | 3 (12) | 0.06 |
| Native American | 0 (0) | 1 (4) | 0.28 |
| Unknown | 0 (0) | 1 (4) | 0.28 |
| Declined | 1 (3) | 1 (4) | 0.92 |
| Highest education for subject or guardian | |||
| Some high school | 1 (3) | 3 (12) | 0.23 |
| High school degree | 2 (7) | 0 (0) | 0.18 |
| Some college | 1 (3) | 5 (19) | 0.05 |
| College degree | 9 (30) | 6 (23) | 0.06 |
| Graduate degree | 13 (43) | 5 (19) | 0.05 |
| Other | 2 (7) | 2 (8) | 0.88 |
| Declined | 2 (7) | 5 (19) | 0.16 |
| Prior insulin delivery systems | |||
| Animas | 4 (7) | 4 (9) | 0.81 |
| Medtronic, older | 24 (44) | 20 (43) | 0.10 |
| Medtronic 630G | 15 (28) | 14 (30) | 0.77 |
| Omnipod | 2 (4) | 3 (7) | 0.52 |
| Roche | 1 (2) | 0 (0) | 0.35 |
| Tandem | 1 (2) | 1 (2) | 0.91 |
| MDI | 1 (2) | 1 (2) | 0.91 |
| Other | 6 (11) | 3 (7) | 0.42 |
| Prior CGM | |||
| Abbott FreeStyle Libre | 1 (2) | 1 (3) | 0.84 |
| Dexcom, G5 or older | 23 (55) | 16 (50) | 0.68 |
| Medtronic Enlite | 13 (31) | 11 (34) | 0.76 |
| None | 1 (2) | 4 (13) | 0.09 |
| Other | 4 (10) | 0 (0) | 0.07 |
| Concurrent Dexcom G6 use | 4 (13) | 8 (31) | 0.11 |
Data are n (%) unless otherwise indicated. MDI, multiple daily injections; NA, not applicable.
P < 0.05.
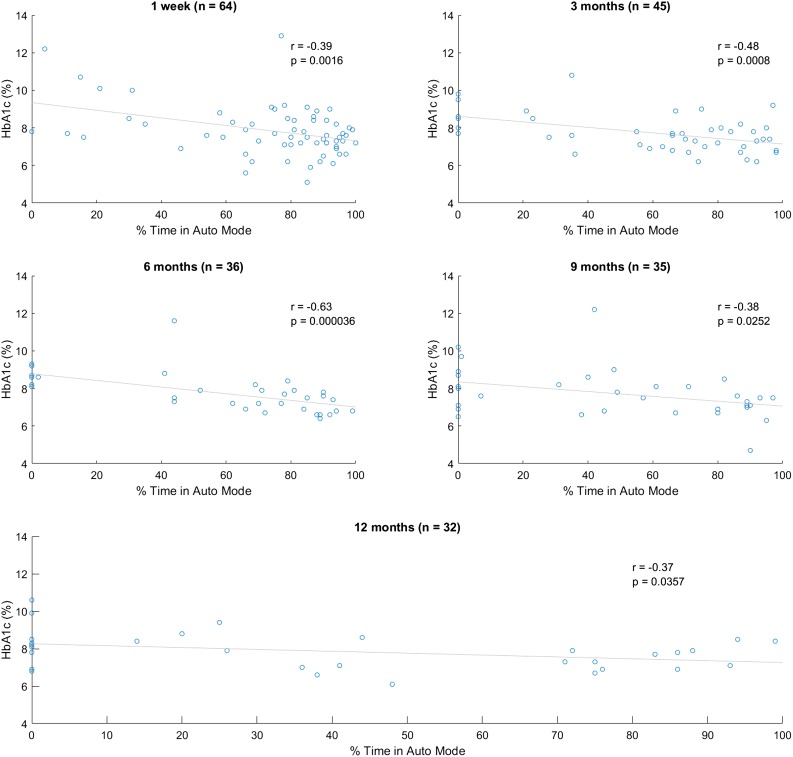
Among those for whom data were available at 12 months, we conducted chart review on all to assess for themes in those who continued and discontinued use of the system. Broadly speaking, the primary reason for continuing the system was desire for increased time in range. This includes prevention of both hypoglycemia and hyperglycemia. At all time points, there was a significant correlation between HbA1c and time in Auto Mode (Fig. 2). This held true even when comparing baseline HbA1c just prior to initiation of Auto Mode versus Auto Mode use 1 week after initialization.
Figure 2.
Scatter plots of percentage of time in Auto Mode vs. HbA1c by time of follow-up. Linear regression demonstrates significant correlation at every time point. One-week data are constructed from baseline HbA1c compared with Auto Mode use 1 week after initiation of the 670G system.
At 1 year, reasons for discontinuation included sensor issues (16 out of 26; 62%), issues obtaining supplies (3 out of 26; 12%), fear of hypoglycemia (3 out of 26; 12%), preference for multiple daily injections (2 out of 26; 8%), and incompatibility with playing sports (2 out of 26; 8%). Sensor issues reported include multiple requests for calibrations, alarms relevant to sensor use, and the system exiting Auto Mode requiring additional blood glucose checks. Eight participants who discontinued use of Auto Mode moved to the Dexcom G6. Four participants who continued Auto Mode wore a Dexcom G6 to calibrate the Guardian 3 sensor.
Conclusions
The objective of this study was to track real-world experience with the first commercial closed-loop device, the Medtronic 670G. Results indicate that at least 33% stop using Auto Mode at 1 year, and primary reasons for discontinuation were sensor issues including alarms for calibrations. Even among those continuing Auto Mode, average utilization fell over time. When continued, Auto Mode was associated with lower A1C and more time in range. These findings highlight the promise of hybrid closed-loop while illuminating significant variables related to user experience that limit its potential benefits.
Older age, increased Guardian 3 CGM use prior to Auto Mode initialization, and college education may be predictors of continued use of Auto Mode. Less than 60% Guardian 3 CGM use prior to Auto Mode initialization is a particularly early marker of Auto Mode discontinuation at 1 year and could serve as a point of critical educational and behavioral intervention. Utilization of Auto Mode correlated with improved glycemic control. Notably, baseline HbA1c just prior to Auto Mode initialization also correlated with the use of Auto Mode at 1 week. Because Auto Mode played no part in the baseline HbA1c measure, it draws causality of this relationship into question. One might hypothesize that those with lower HbA1c were able to keep themselves in Auto Mode longer and that this may contribute to the association. Additionally, the relationship between HbA1c and time in Auto Mode may simply be an expression of compliance.
The primary concern of those who stopped using Auto Mode was difficulty with the sensor. Requests for blood glucose checks coupled with alarms and exiting Auto Mode due to prolonged maximal basal insulin were common. Medtronic has recognized this problem and issued a transmitter upgrade for those experiencing significant repeated blood glucose requests (https://info.medtronicdiabetes.com/bgcheck). None of the participants in the current study had upgraded to this new transmitter version. Over time, the difficulty obtaining supplies has reduced (19). While hypoglycemia is reduced with use of Auto Mode (11,20–23), it can still occur in the context of sports and large insulin boluses. In spite of the data supporting reduction in hypoglycemia, some users who experienced lows reported hypoglycemia as a reason for discontinuing. Education and adequate preparation are crucial in setting realistic expectations for closed-loop systems (24,25). We are strong believers that technology should be offered to everyone interested. Anecdotally, those who continue to use Auto Mode report benefits. In clinical trials, Auto Mode improved glycemic control even in the poorly controlled (26). In this real-world experience, usability was a barrier to Auto Mode utilization. We must provide the same care, attention, follow-up, and discussion of risks and benefits as we do in clinical trials. Most importantly, a focus on usability and human factors is necessary to ensure patients stay on treatment (27–29). Next-generation technology must balance safety concerns with simplified device operation.
While one might expect that those using the system at 1 year would continue thereafter, this was not always the case. Among the 30 participants using Auto Mode for any percentage of time at 1 year, at least 1 moved to Decom G6 with 670G insulin pump, and another discontinued after the 1-year follow-up for Loop, a do-it-yourself system.
There are limitations inherent to this prospective observational study. While we can demonstrate correlations, we cannot establish causality, and findings are merely hypothesis generating. The design is prone to selection and volunteer bias. Especially important, only participants with insurance covering the system were included, introducing a socioeconomic discrepancy. Indeed, these same individuals may have access to alternative technologies like the Dexcom G6 or do-it-yourself systems. Because this was clinical care, patients did not attend every visit, and thus, there are gaps in the data. Additionally, themes for continuing and discontinuing 670G use were derived from analysis of notes in the electronic medical record. Despite this, the strengths of the study include a varied clinical patient population receiving training at a center, which established an educational program for initialization of 670G therapy. The data present the challenges encountered with first-generation technology, the need for setting realistic expectations, and the importance of human factors and usability in the design and clinical implementation of closed-loop insulin delivery systems.
Supplementary Material
Article Information
Acknowledgments
The authors thank Saniya Kishnani for setting up the REDCap data collection system; Ruth Wu for performing data collection; Karen Barahona and Nora Arrizon-Ruiz for recruitment; the clinical diabetes educators Barry Conrad, Jeannine Leverenz, Kristine Peterson, and Annette Chmielewski for teaching and tracking pediatric patients (all from the Division of Endocrinology, Department of Pediatrics, Stanford University School of Medicine); and the adult endocrine nurse practitioners and diabetes educators Kathleen Judge and Leticia Wilke (Division of Endocrinology, Department of Medicine, Stanford University School of Medicine). The REDCap platform services are made possible by the Stanford University School of Medicine Research Office.
Funding. The Ruth and Donald Seiler Research Fund generously supported this research. The REDCap platform services at Stanford University School of Medicine are subsidized by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant UL1-TR-001085. R.A.L. is a Stephen Bechtel Endowed Adult and Pediatric Endocrinology Fellow through the Stanford Maternal and Child Health Research Institute and is supported by a Diabetes, Endocrinology and Metabolism Training Grant from the National Institute for Diabetes and Digestive and Kidney Diseases (grants T32-DK-007217 and 1K12-DK-122550). D.M.M. received research support from NIH (including grants P30-DK-116074 and 1K12-DK-122550), JDRF, the National Science Foundation, and the Helmsley Charitable Trust.
The data content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duality of Interest. R.A.L. has consulted for GlySens Incorporated and Abbott Diabetes Care. D.M.M. has consulted for Abbott, Sanofi, and Eli Lilly and Company and has served on an advisory board for Insulet Corporation, and his institution received research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, and Roche. K.H. received research support from Dexcom for an investigator-initiated study and consulting fees from Insulet Corporation, Lilly’s Cambridge Innovation Center, and LifeScan Diabetes Institute (formerly Johnson & Johnson Diabetes Institute). B.B. is on medical advisory boards for Convatec, Medtronic, and Tolerion, Inc., and has received research support from Medtronic, Tandem, Insulet Corporation, and Dexcom. D.M.W. is on the advisory board for Tolerion, Inc., and has received research support from Dexcom.
Author Contributions. R.A.L. wrote the manuscript, performed data processing, and generated the tables and figures. M.B., D.M.M., K.H., and B.B. helped recruit participants and reviewed and edited the manuscript. R.A.L., M.B., D.M.M., K.H., B.B., and D.M.W. assisted in study design. D.M.W. wrote the original protocol, obtained institutional review board approval, wrote the manuscript, and reviewed and edited the manuscript. D.M.W. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
Clinical trial reg. no. NCT03017482, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0855/-/DC1.
References
- 1.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 3.Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care 2015;38:1205–1211 [DOI] [PubMed] [Google Scholar]
- 4.Ly TT, Keenan DB, Roy A, et al. Automated overnight closed-loop control using a proportional-integral-derivative algorithm with insulin feedback in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Technol Ther 2016;18:377–384 [DOI] [PubMed] [Google Scholar]
- 5.Ly TT, Weinzimer SA, Maahs DM, et al. Automated hybrid closed-loop control with a proportional-integral-derivative based system in adolescents and adults with type 1 diabetes: individualizing settings for optimal performance. Pediatr Diabetes 2017;18:348–355 [DOI] [PubMed] [Google Scholar]
- 6.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 7.Calhoun PM, Buckingham BA, Maahs DM, et al.; In Home Closed Loop Study Group . Efficacy of an overnight predictive low-glucose suspend system in relation to hypoglycemia risk factors in youth and adults with type 1 diabetes. J Diabetes Sci Technol 2016;10:1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckingham BA, Bailey TS, Christiansen M, et al. Evaluation of a predictive low-glucose management system in-clinic. Diabetes Technol Ther 2017;19:288–292 [DOI] [PubMed] [Google Scholar]
- 10.Adams RN, Tanenbaum ML, Hanes SJ, et al. Psychosocial and human factors during a trial of a hybrid closed loop system for type 1 diabetes management. Diabetes Technol Ther 2018;20:648–653 [DOI] [PubMed] [Google Scholar]
- 11.Wood MA, Shulman DI, Forlenza GP, et al. In-clinic evaluation of the MiniMed 670G system “suspend before low” feature in children with type 1 diabetes. Diabetes Technol Ther 2018;20:731–737 [DOI] [PubMed] [Google Scholar]
- 12.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. Safety evaluation of the MiniMed 670G system in children 7-13 years of age with type 1 diabetes. Diabetes Technol Ther 2019;21:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smalley E. Medtronic automated insulin delivery device gets FDA nod. Nat Biotechnol 2016;34:1220. [DOI] [PubMed] [Google Scholar]
- 14.Stone MP, Agrawal P, Chen X, et al. Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther 2018;20:689–692 [DOI] [PubMed] [Google Scholar]
- 15.Goodwin G, Waldman G, Lyons J, Oladunjoye A, Steil G. OR14-5 challenges in implementing hybrid closed loop insulin pump therapy (Medtronic 670g) in a ‘real world’ clinical setting. J Endocr Soc 2019;3(Suppl. 1):OR14-5 [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calhoun P, Johnson TK, Hughes J, Price D, Balo AK. Resistance to acetaminophen interference in a novel continuous glucose monitoring system. J Diabetes Sci Technol 2018;12:393–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsh JB, Zhang X, Puhr SA, et al. Performance of a factory-calibrated, real-time continuous glucose monitoring system in pediatric participants with type 1 diabetes. J Diabetes Sci Technol 2019;13:254–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallant B, Brown A. Medtronic MiniMed 670G and Guardian Sensor 3 back in stock [Internet], 2018. Available from https://diatribe.org/medtronic-minimed-670g-and-guardian-sensor-3-back-stock. Accessed 19 August 2019
- 20.Choudhary P, Olsen BS, Conget I, Welsh JB, Vorrink L, Shin JJ. Hypoglycemia prevention and user acceptance of an insulin pump system with predictive low glucose management. Diabetes Technol Ther 2016;18:288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham MB, Nicholas JA, Ly TT, et al. Safety and efficacy of the predictive low glucose management system in the prevention of hypoglycaemia: protocol for randomised controlled home trial to evaluate the Suspend before low function. BMJ Open 2016;6:e011589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong A, Choudhary P, McMahon C, et al. Effectiveness of automated insulin management features of the MiniMed® 640G sensor-augmented insulin pump. Diabetes Technol Ther 2016;18:657–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham MB, Nicholas JA, Smith GJ, et al.; PLGM Study Group . Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care 2018;41:303–310 [DOI] [PubMed] [Google Scholar]
- 24.Messer LH, Forlenza GP, Sherr JL, et al. Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care 2018;41:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aleppo G, Webb KM. Integrated insulin pump and continuous glucose monitoring technology in diabetes care today: a perspective of real-life experience with the Minimed™ 670g hybrid closed-loop system. Endocr Pract 2018;24:684–692 [DOI] [PubMed] [Google Scholar]
- 26.Tauschmann M, Thabit H, Bally L, et al.; APCam11 Consortium . Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forlenza GP, Messer LH, Berget C, Wadwa RP, Driscoll KA. Biopsychosocial factors associated with satisfaction and sustained use of artificial pancreas technology and its components: a call to the technology field. Curr Diab Rep 2018;18:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherr JL, Tauschmann M, Battelino T, et al. ISPAD clinical practice consensus guidelines 2018: diabetes technologies. Pediatr Diabetes 2018;19(Suppl. 27):302–325 [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes Association 7. Diabetes technology: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S71–S80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.