Abstract
OBJECTIVE
To examine racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI category.
RESEARCH DESIGN AND METHODS
In a consortium of three U.S. integrated health care systems, 4,906,238 individuals aged ≥20 years during 2012–2013 were included. Diabetes and prediabetes were ascertained by diagnosis and laboratory results; antihyperglycemic medications were also included for diabetes ascertainment.
RESULTS
The age-standardized diabetes and prediabetes prevalence estimates were 15.9% and 33.4%, respectively. Diabetes but not prediabetes prevalence increased across BMI categories among all racial/ethnic groups (P for trend < 0.001). Racial/ethnic minorities reached a given diabetes prevalence at lower BMIs than whites; Hawaiians/Pacific Islanders and Asians had a diabetes prevalence of 24.6% (95% CI 24.1–25.2%) in overweight and 26.5% (26.3–26.8%) in obese class 1, whereas whites had a prevalence of 23.7% (23.5–23.8%) in obese class 2. The age-standardized prediabetes prevalence estimates in overweight among Hispanics (35.6% [35.4–35.7%]), Asians (38.1% [38.0–38.3%]), and Hawaiians/Pacific Islanders (37.5% [36.9–38.2%]) were similar to those in obese class 4 among whites (35.3% [34.5–36.0%]), blacks (36.8% [35.5–38.2%]), and American Indians/Alaskan Natives (34.2% [29.6–38.8%]). In adjusted models, the strength of association between BMI and diabetes was highest among whites (relative risk comparing obese class 4 with normal weight 7.64 [95% CI 7.50–7.79]) and lowest among blacks (3.16 [3.05–3.27]). The association between BMI and prediabetes was less pronounced.
CONCLUSIONS
Racial/ethnic minorities had a higher burden of diabetes and prediabetes at lower BMIs than whites, suggesting the role of factors other than obesity in racial/ethnic disparities in diabetes and prediabetes risk and highlighting the need for tailored screening and prevention strategies.
Introduction
More than 30 million adults had diagnosed diabetes in 2015, accounting for 12% of the U.S. population (1). As the seventh leading cause of mortality, diabetes has imposed tremendous economic and societal burden (2). Racial/ethnic disparities in diabetes remain a pervasive public health issue in the U.S. and have been linked to elevated prevalence of diabetes complications and higher mortality rates among racial/ethnic minority groups (3). The Disparities Action Plan by the U.S. Department of Human and Health Services has urged the elimination of racial/ethnic differences in chronic disease burdens, including diabetes (4). However, racial/ethnic disparities in diabetes remain substantial, although data on Hawaiians/Pacific Islanders and American Indians/Alaskan Natives are scant (5,6). According to the Centers for Disease Control and Prevention, 84 million (34%) Americans had prediabetes, including almost one in two adults aged ≥65 years (1). Nonetheless, patterns of racial/ethnic disparities in prediabetes remain elusive because of the scarcity of data.
Obesity and race/ethnicity are two major independent risk factors for diabetes. However, the racial/ethnic distribution of obesity (highest among non-Hispanic blacks and lowest among Asians) does not mirror that of diabetes (Asians are among the highest and whites among the lowest) (6,7). Limited national data are available on how obesity and race/ethnicity may interact with and influence disparities in risk of diabetes and prediabetes. Previous national data on diabetes prevalence by obesity focused more on racial/ethnic subgroups other than Asians and are as-yet absent on Hawaiians/Pacific Islanders and American Indians/Alaskan Natives (8–11); furthermore, national data on prediabetes by race/ethnicity and obesity are lacking. Understanding the relative contribution of obesity to the racial/ethnic disparities in diabetes and prediabetes may help to inform clinical and public health intervention strategies to mitigate disparities in diabetes and prediabetes and reduce associated complications and mortality. In addition, it is important to note that socioeconomic and environmental disadvantages, especially disparate access to health care and preventive care services, may also contribute to substantial racial/ethnic disparities (12). Therefore, it is particularly meaningful to examine the impact of varying BMI levels on racial/ethnic disparities in diabetes and prediabetes among a population with universal access to health care. Moreover, the residual contribution of socioeconomic status to the racial/ethnic disparities beyond BMI remains to be elucidated.
To address these critical evidence gaps, we aimed to examine the racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI category before and after controlling for socioeconomic factors in a large racially/ethnically and geographically diverse cohort of 4.9 million adults aged ≥20 years who were members of three integrated health care systems across 10 sites in the U.S. in 2012–2013.
Research Design and Methods
Study Population and Design
Data for this study were from the Patient Outcomes Research To Advance Learning (PORTAL) Network, one of the 13 Clinical Data Research Networks in the National Patient-Centered Clinical Research Network (13). The PORTAL Network combines 10 sites across three integrated health care systems serving >12 million members, ∼1 of every 30 people in the U.S. The network includes all Kaiser Permanente regions (Hawaii, Northwest [Northern Oregon and Southwest Washington], Northern California, Southern California, Colorado, Mid-Atlantic states [Maryland, Virginia, and District of Columbia], Georgia [through 2015], and Washington), HealthPartners (Minnesota), and Denver Health (Denver, CO) (Supplementary Fig. 1). Racial/ethnic and socioeconomic diversity is large and generally representative of the underlying populations of the health care service regions (14).
Inclusion criteria consisted of health plan members with at least 12 months of continuous membership between 1 January 2012 and 31 December 2013 who were aged at least 18 years as of 31 December 2013, had a weight recorded during 2012 or 2013 and a height recorded in the electronic health record (EHR), and were not pregnant during 2012–2013 (15). For Denver Health, continuous enrollment did not apply because it is a safety-net organization without an associated health plan for enrolling members; therefore, the initial eligibility criteria included all adults who had a primary care encounter during 2012–2013. Data were extracted from the Health Care Systems Research Network Virtual Data Warehouse, a standardized and federated database where all data reside at each health system behind each site’s firewall (16). The Kaiser Permanente Southern California Institutional Review Board approved the research and granted a waiver for written informed consent. The institutional review boards at the other sites reviewed the protocol and subsequently ceded review.
In the PORTAL Network, we identified >10 million individuals who had continuous membership in 2012–2013. After excluding those who were aged <18 years (n = 2,309,558), did not have a height and weight recorded in 2012–2013 (n = 1,715,657), were pregnant during 2012–2013 (n = 181,129), and had biologically implausible height, weight, or BMI measurements (height <1.2 or >2.4 m, weight <22 or >454 kg, BMI <5 or >90 kg/m2) (n = 6,954), a total of 6,218,734 adults remained (Supplementary Fig. 2). We further excluded individuals aged <20 years or with missing data on outcome ascertainment or sex (n = 1,312,496), rendering a cohort of 4,906,238 individuals as the analytical sample.
Diabetes and Prediabetes
Diabetes was defined using the methodology developed for Surveillance, Prevention, and Management of Diabetes Mellitus (SUPREME-DM), a large multisite observational consortium, without differentiation between type 1 and type 2 diabetes (17). The definition was adapted from the 2010 American Diabetes Association (ADA) criteria (18). Briefly, the definition included one inpatient diagnosis of diabetes on the basis of ICD-9 codes (250.x, 357.2, 366.41, and 362.01–362.07) or any combination of two other events: fasting plasma glucose ≥126 mg/dL (≥7.0 mmol/L), random plasma glucose ≥200 mg/dL (≥11.1 mmol/L), HbA1c ≥6.5%, outpatient diagnosis (the same as inpatient codes), or dispensation of an antihyperglycemic medication.
Prediabetes was defined on the basis of the 2010 ADA criteria (18) and the work of Schmittdiel et al. (19) as at least one from the following during the study period: 1) fasting plasma glucose measurement between 100 and 125 mg/dL (5.6–6.9 mmol/L); 2) 2-h postchallenge plasma glucose between 140 and 199 mg/dL (7.8–11.0 mmol/L); 3) HbA1c between 5.7% and 6.4%; or 4) outpatient ICD-9 codes of 790.2, 790.29, 790.21, or 790.22 (19). These laboratory and diagnosis criteria qualified for prediabetes only if they were not superseded by the criteria for diabetes.
Race/Ethnicity
Race/ethnicity was obtained from health plan administrative records, documentation during a health care encounter, or birth certificates. Individuals had the option to identify themselves as white, black or African American, Hispanic, Asian, Hawaiian/Pacific Islander, American Indian/Alaskan Native, or multiracial/other/unknown. If identified as Hispanic, the individual was placed in that category regardless of race. Given the small percentage of participants in the multiracial/other/unknown category (n = 211,178 [4% of the cohort], mostly unknown), these individuals were not included in the analyses for prevalence and risk estimates of diabetes or prediabetes.
BMI
Weight is routinely measured during outpatient clinic visits, while height is generally considered static for adults and less frequently assessed. BMI was calculated as weight (kg) divided by height squared (m2). If more than one weight, height, or BMI was available in the EHR in 2012–2013, the most recent value was used. According to World Health Organization recommendations on racial/ethnic-specific BMI cutoffs (20), we categorized non-Asian individuals as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obese class 1 (30.0–34.9 kg/m2), obese class 2 (35.0–39.9 kg/m2), obese class 3 (40.0–49.9 kg/m2), and obese class 4 (≥50.0 kg/m2) (21,22); and Asians as underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23.0–27.4 kg/m2), obese class 1 (27.5–32.4 kg/m2), obese class 2 (32.5–37.4 kg/m2), obese class 3 (37.5–47.4 kg/m2), and obese class 4 (≥47.5 kg/m2).
Neighborhood Poverty
Neighborhood poverty was estimated using geospatial entity object codes that linked addresses to 2010 U.S. census data and served as a proxy for socioeconomic status. Using census data at the block group level with addresses as listed in the EHR, each individual was assigned the probability of falling below the poverty threshold levels on the basis of the percentage below the poverty level in his/her neighborhood block group. These probabilities were divided into four categories: <5% (lowest poverty level), 5–9%, 10–19%, and ≥20% (highest poverty level) of households in the neighborhood below the poverty level.
Neighborhood Education
Using a similar approach, each individual was assigned the probability of having a high school or lower education on the basis of the percentage with high school or lower education levels in his/her neighborhood block group. These probabilities were divided into quartiles on the basis of the study population distribution: <23% high school or lower (highest education level), 23–33%, 34–48%, and >48% (lowest education level).
Statistical Analysis
Sociodemographic and clinical characteristics were summarized as numbers and proportions in the entire cohort and by race/ethnicity. The overall age-standardized prevalence of diabetes and prediabetes was derived in the entire cohort and among all racial/ethnic groups. To evaluate the racial/ethnic-specific associations of BMI category with prevalence of diabetes or prediabetes, age-standardized prevalence was derived by race/ethnicity and BMI categories using marginal standardization of predicted probabilities from Poisson regression models, and 95% CIs were obtained using 200 bootstrapped estimates. To explore potential sex-specific patterns, we further stratified estimates of age-standardized prevalence of diabetes or prediabetes by sex within each racial/ethnic group across BMI categories.
Poisson regression models with robust SEs were used to estimate relative risks (RRs) and 95% CIs of diabetes or prediabetes in association with BMI categories by race/ethnicity. We adjusted for age, sex, neighborhood poverty, neighborhood education, and data-contributing site to account for potential residual confounding as a result of socioeconomic and/or environmental disparities. The P value for interaction was obtained by the likelihood ratio test. Tests for linear trend were obtained by the Mantel-Haenszel χ2 test. Post hoc multiple comparison adjustment for P values was performed using the Benjamini-Hochberg false discovery rate (FDR) controlling method (23). To test the robustness of our findings, we further conducted sensitivity analyses by additionally including participants with missing data on outcome ascertainment in the analytic sample. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria) statistical software.
Results
The cohort of 4,695,060 eligible participants in the PORTAL Network was racially/ethnically diverse, with 50.0% white, 21.6% Hispanic, 12.7% Asian, 9.5% black, 1.4% Hawaiian/Pacific Islander, 0.5% American Indian/Alaskan Native, and 4.3% multiracial/other/unknown (Table 1). The cohort comprised slightly more women (55.7%) than men. The mean BMI was 28.8 kg/m2 (SD 6.5 kg/m2) for the entire cohort, with Asians having the lowest and blacks the highest BMI (25.6 [4.7] and 31.0 [7.3] kg/m2, respectively). Compared with whites, Asians were more likely to be overweight (47.1%) and less likely to be in obese classes 2–4 on the basis of racial/ethnic-specific BMI cutoffs, whereas blacks, Hispanics, Hawaiians/Pacific Islanders, and American Indians/Alaskan Natives were more likely to be in obese classes 2–4 and less likely to be normal weight. Neighborhood poverty levels varied by race/ethnicity, with 31.7% of blacks and 32.5% of Hispanics living in neighborhoods with ≥20% of households below the poverty level, ∼2.5-fold higher than whites (12.9%). Neighborhood education levels also varied by race/ethnicity, with 32–51% of blacks, Hispanics, and Hawaiians/Pacific Islanders living in neighborhoods with the lowest education level (>48% with a high school or lower education) compared with 15–23% among other racial/ethnic groups.
Table 1.
Participant characteristics by race/ethnicity in the PORTAL Network, 2012–2013
| White | Black | Hispanic | Asian | Hawaiian/Pacific Islander | American Indian/Alaskan Native | Multiracial/other/unknown | All | |
|---|---|---|---|---|---|---|---|---|
| n (%) | 2,454,388 (50.0) | 467,994 (9.5) | 1,058,351(21.6) | 620,813 (12.7) | 67,190 (1.4) | 26,324 (0.5) | 211,178 (4.3) | 4,906,238 (100) |
| Sex | ||||||||
| Male | 45.0 | 39.9 | 43.5 | 42.7 | 4.9 | 41.6 | 44.5 | 44.3 |
| Female | 55.0 | 60.1 | 56.5 | 57.3 | 5.9 | 58.4 | 55.5 | 55.7 |
| Age (years) | ||||||||
| 20–29 | 7.7 | 11.5 | 14.3 | 9.6 | 11.1 | 9.2 | 14.2 | 10.1 |
| 30–39 | 10.0 | 12.3 | 19 | 16.8 | 16.3 | 12.6 | 18.7 | 13.5 |
| 40–49 | 15.1 | 19.9 | 23.1 | 21.2 | 21.3 | 18.8 | 22 | 18.4 |
| 50–59 | 22.4 | 23.5 | 20.5 | 21.4 | 22.3 | 24.6 | 23 | 22.0 |
| 60–69 | 22.7 | 17.7 | 12.9 | 17.5 | 16.6 | 20.6 | 15 | 19.0 |
| 70–79 | 13.4 | 10.2 | 6.8 | 9 | 8.7 | 9.9 | 5.2 | 10.7 |
| ≥80 | 8.7 | 4.8 | 3.3 | 4.4 | 3.7 | 4.2 | 2 | 6.2 |
| BMI (kg/m2), mean (SD)‡ | 28.7 (6.4) | 31.0 (7.3) | 30.0 (6.2) | 25.6 (4.7) | 30.2 (7.5) | 30.4 (7.2) | 28.6 (6.1) | 28.8 (6.5) |
| Underweight | 1.4 | 1.0 | 0.6 | 2.6 | 1.1 | 1.1 | 1.1 | 1.3 |
| Normal weight | 29.4 | 18.9 | 19.9 | 22.7 | 24.4 | 22.6 | 28.6 | 25.3 |
| Overweight | 34.7 | 31.6 | 36.7 | 47.1 | 32.3 | 31.6 | 37.8 | 36.5 |
| Obese class 1 | 19.9 | 24.4 | 25.1 | 20.2 | 20.9 | 22.9 | 19.7 | 21.5 |
| Obese class 2 | 8.7 | 13.1 | 10.9 | 5.4 | 11.4 | 12.2 | 7.9 | 9.2 |
| Obese class 3 | 5.0 | 9.0 | 5.9 | 1.9 | 7.9 | 7.8 | 4.3 | 5.2 |
| Obese class 4 | 0.9 | 2.0 | 0.9 | 0.2 | 2.0 | 1.8 | 0.7 | 0.9 |
| Neighborhood poverty level (% below threshold) | ||||||||
| <5 | 28.6 | 17.3 | 13.6 | 29.8 | 22.2 | 22.2 | 27.1 | 24.3 |
| 5–9 | 30.7 | 22.0 | 21.5 | 30.2 | 29.3 | 27.8 | 28.0 | 27.7 |
| 10–19 | 27.7 | 29.0 | 32.5 | 26.3 | 30.7 | 30.0 | 27.5 | 28.7 |
| ≥20 | 12.9 | 31.7 | 32.5 | 13.8 | 17.8 | 20.0 | 17.4 | 19.3 |
| Neighborhood education (% of high school or lower) | ||||||||
| >48% (lowest level) | 14.5 | 35.9 | 50.7 | 19.9 | 32.4 | 22.7 | 21.8 | 25.6 |
| 34–48% | 25.0 | 29.0 | 23.0 | 23.7 | 31.8 | 29.9 | 23.9 | 24.8 |
| 23–33% | 28.0 | 20.8 | 15.8 | 26.0 | 22.1 | 25.7 | 24.3 | 24.2 |
| <23% (highest level) | 32.5 | 14.4 | 10.5 | 30.5 | 13.7 | 21.6 | 29.9 | 25.3 |
Data are % unless otherwise indicated.
Non-Asians were categorized as underweight (BMI <18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese class 1–4 (30.0–34.9, 35.0–39.9, 40.0–49.9, ≥50.0 kg/m2), and Asians were categorized as underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23.0–27.4 kg/m2), and obese class 1–4 (27.5–32.4, 32.5–37.4, 37.5–47.4, ≥47.5 kg/m2).
There was substantial racial/ethnic variation in the prevalence of diabetes and prediabetes (Table 2). The overall age-standardized prevalence of diabetes was 15.9% (95% CI 15.8–16.0%). Across racial/ethnic groups, the age-standardized diabetes prevalence was 27.7% (27.4–28.0%) in Hawaiians/Pacific Islanders, which was higher than that in Hispanics (22.2% [21.1–22.3%]), blacks (21.4% [21.3–21.6%]), American Indians/Alaskan Natives (19.6% [19.1–20.0%]), and Asians (19.3% [19.2–19.4%]) and more than double that in whites (12.2% [12.1–12.3%]). The overall age-standardized prevalence of prediabetes was 33.4% (33.3–33.5%), with the highest estimate observed in Asians (37.1% [37.0–37.2%]) followed by Hawaiians/Pacific Islanders (36.7% [36.4–37.1%]), Hispanics (35.3% [35.2–35.4%]), blacks (32.0% [31.9–32.1%]), American Indians/Alaskan Natives (31.1% [30.6–31.7%]), and whites (31.0% [30.9–31.1%]).
Table 2.
Age-standardized prevalence of diabetes and prediabetes by race/ethnicity and BMI category per 100 PORTAL Network cohort members: 2012–2013
| White | Black | Hispanic | Asian | Hawaiian/Pacific Islander | American Indian/Alaskan Native | All | |
|---|---|---|---|---|---|---|---|
| No. | 2,454,388 | 467,994 | 1,058,351 | 620,813 | 67,190 | 26,324 | 4,906,238 |
| Diabetes prevalence | |||||||
| Overall | 12.2 (12.1–12.3) | 21.4 (21.3–21.6) | 22.2 (22.1–22.3) | 19.3 (19.2–19.4) | 27.7 (27.4–28.0) | 19.6 (19.1–20.0) | 15.9 (15.8–16.0) |
| BMI* | |||||||
| Underweight | 3.5 (3.3–3.7) | 9.9 (8.9–11.0) | 8.8 (7.8–9.7) | 7.3 (6.9–7.7) | 10.2 (7.7–12.7) | 7.4 (4.2–10.6) | 5.2 (5–5.4) |
| Normal weight | 5.0 (4.9–5.1) | 13.5 (13.2–13.7) | 13.0 (12.9–13.2) | 10.1 (9.9–10.2) | 18.0 (17.5–18.6) | 9.6 (8.9–10.4) | 7.3 (7.3–7.4) |
| Overweight | 9.4 (9.4–9.5) | 17.8 (17.6–18.0) | 18.9 (18.7–19.0) | 18.3 (18.2–18.5) | 24.6 (24.1–25.2) | 15.6 (14.8–16.3) | 13.4 (13.4–13.5) |
| Obese class 1 | 16.4 (16.3–16.5) | 23.6 (23.4–23.9) | 25.6 (25.4–25.7) | 26.5 (26.3–26.8) | 32.2 (31.4–33.0) | 22.9 (21.9–24.0) | 20.5 (20.4–20.6) |
| Obese class 2 | 23.7 (23.5–23.8) | 28.6 (28.3–29.0) | 32.7 (32.3–33.0) | 35.9 (35.4–36.5) | 38.9 (37.7–40.2) | 29.7 (28.1–31.3) | 27.3 (27.2–27.5) |
| Obese class 3 | 30.5 (30.2–30.8) | 32.7 (32.2–33.2) | 39.1 (38.6–39.6) | 44.0 (42.9–45.0) | 43.6 (41.8–45.4) | 33.5 (31.0–36.0) | 33.4 (33.2–33.6) |
| Obese class 4 | 37.4 (36.6–38.1) | 36.6 (35.3–38.0) | 47.0 (45.5–48.5) | 49.1 (45.0–53.3) | 44.8 (39.6–50.0) | 41.4 (33.8–48.9) | 39.3 (38.7–39.9) |
| P for trend† | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Prediabetes prevalence | |||||||
| Overall | 31.0 (30.9–31.1) | 32.0 (31.9–32.1) | 35.3 (35.2–35.4) | 37.1 (37.0–37.2) | 36.7 (36.4–37.1) | 31.1 (30.6–31.7) | 33.4 (33.3–33.5) |
| BMI* | |||||||
| Underweight | 21.1 (20.6–21.5) | 23.9 (22.4–25.4) | 23.8 (22.4–25.2) | 29.5 (28.8–30.3) | 29.5 (25.7–33.4) | 17.5 (12.6–22.4) | 23.9 (23.5–24.3) |
| Normal weight | 24.4 (24.3–24.5) | 26.9 (26.6–27.2) | 29.3 (29.0–29.5) | 33.0 (32.7–33.2) | 33.7 (33.0–34.4) | 26.3 (25.2–27.4) | 26.8 (26.7–26.9) |
| Overweight | 31.9 (31.8–32.0) | 31.2 (31.0–31.5) | 35.6 (35.4–35.7) | 38.1 (38.0–38.3) | 37.5 (36.9–38.2) | 31.4 (30.5–32.4) | 34.3 (34.2–34.3) |
| Obese class 1 | 35.0 (34.9–35.1) | 33.3 (33.0–33.6) | 37.5 (37.3–37.7) | 39.3 (39.0–39.6) | 37.1 (36.3–37.9) | 32.3 (31.1–33.5) | 36.7 (36.6–36.8) |
| Obese class 2 | 35.4 (35.2–35.6) | 34.0 (33.6–34.4) | 36.8 (36.5–37.1) | 37.2 (36.6–37.7) | 35.1 (33.9–36.4) | 33.9 (32.1–35.6) | 36.4 (36.3–36.5) |
| Obese class 3 | 35.1 (34.8–35.4) | 34.8 (34.3–35.3) | 35.6 (35.1–36.1) | 34.1 (33.1–35.2) | 34.9 (33.2–36.7) | 34.3 (31.6–37.0) | 35.8 (35.5–36.0) |
| Obese class 4 | 35.3 (34.5–36.0) | 36.8 (35.5–38.2) | 33.9 (32.5–35.3) | 30.2 (26.4–33.9) | 37.7 (32.5–43.0) | 34.2 (29.6–38.8) | 35.9 (35.3–36.5) |
| P for trend† | 0.016 | 0.039 | 0.102 | 0.896 | 0.377 | 0.016 | 0.039 |
Data are % (95% CI).
Non-Asians were categorized as underweight (BMI <18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese class 1–4 (30.0–34.9, 35.0–39.9, 40.0–49.9, ≥50.0 kg/m2), and Asians were categorized as underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23.0–27.4 kg/m2), and obese class 1–4 (27.5–32.4, 32.5–37.4, 37.5–47.4, ≥47.5 kg/m2).
P for trend across BMI categories was adjusted for multiple comparisons using the FDR method.
The age-standardized prevalence of diabetes monotonically increased across BMI categories among all racial/ethnic groups (all FDR P for trend < 0.001) (Table 2) and increased with age up to 70–79 years among all racial/ethnic groups (all FDR P for trend < 0.001) (Supplementary Fig. 3). Furthermore, compared with whites, all other racial/ethnic groups had a higher prevalence of diabetes at a given BMI, with the difference being more pronounced at lower BMI levels (i.e., underweight, normal weight, overweight). For instance, among individuals with a normal weight, the prevalence of diabetes was 5.0% (95% CI 4.9–5.1%) in whites, approximately one-half of that in Asians (10.1% [9.9–10.2%]) and American Indians/Alaskan Natives (9.6% [8.9–10.4%]) and almost one-third of that in Hispanics (13.0% [12.9–13.2%]), blacks (13.5% [13.2–13.7%]), and Hawaiians/Pacific Islanders (18.0% [17.5–18.6%]). When further stratified by sex, men overall had a higher age-standardized prevalence of diabetes (18.4% [18.3–18.5%]) than women (13.9% [13.8–14.0%]); this sex-specific pattern persisted across BMI categories (Supplementary Table 1). Among all racial/ethnic groups, a similar, increasing trend was observed for diabetes prevalence across BMI categories among both men and women as in the entire cohort (all FDR P for trend < 0.001).
The prediabetes prevalence increased with age until reaching a plateau at 60–69 years across racial/ethnic groups (Supplementary Fig. 3), whereas the pattern of prediabetes prevalence across BMI categories differed by race/ethnicity (Table 2). An overall rising trend in the age-standardized prevalence of prediabetes across BMI categories was observed among whites, blacks, and American Indians/Alaskan Natives (all FDR P for trend < 0.05) but not among Hispanics, Asians, or Hawaiians/Pacific Islanders. The latter three racial/ethnic groups tended to have a higher prevalence of prediabetes than the former three groups at lower BMI levels (i.e., from underweight through obese class 1). For instance, the age-standardized prevalence of prediabetes among those who were overweight was 38.1% (95% CI 38.0–38.3%) in Asians, 37.5% (36.9–38.2%) in Hawaiians/Pacific Islanders, and 35.6% (35.4–35.7%) in Hispanics, similar to or even higher than the prevalence observed in obese class 4 among whites (35.3% [34.5–36.0%]), blacks (36.8% [35.5–38.2%]), and American Indians/Alaskan Natives (34.2% [29.6–38.8%]). Similar to the sex-specific pattern of diabetes prevalence, men had an overall higher age-standardized prevalence of prediabetes than women (36.5% [36.4–36.7%] vs. 31.0% [30.9–31.1%]) (Supplementary Table 2). Nonetheless, the association between BMI and prediabetes within racial/ethnic groups varied by sex. The racial/ethnic-specific prevalence of prediabetes was higher across BMI categories only among white, black, and American Indian/Alaskan Native women (all FDR P for trend < 0.01) but not among men.
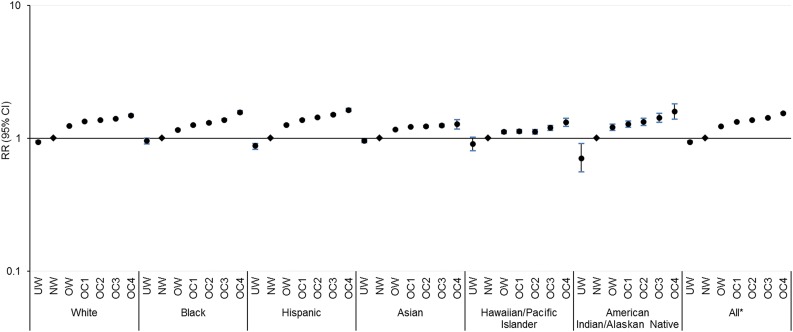
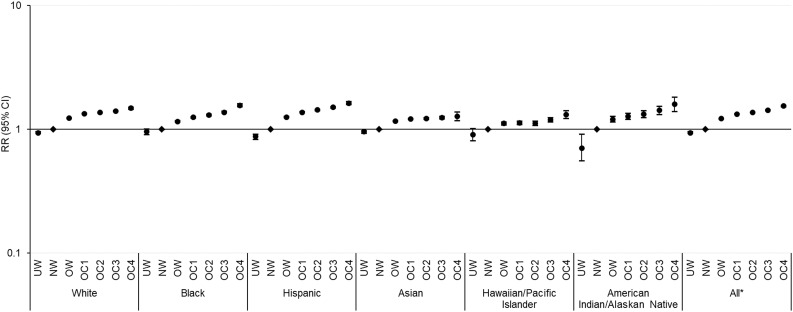
Figures 1 and 2 present the multivariable RR (95% CI) for diabetes and prediabetes for each BMI category compared with normal weight counterparts by race/ethnicity, after adjusting for covariates. Across all racial/ethnic groups, whites had the steepest BMI gradient for diabetes risk, with an adjusted RR comparing obese class 4 with normal weight of 7.64 (95% CI 7.50–7.79), followed by Asians (6.26 [5.83–6.72]), American Indians/Alaskan Natives (4.85 [4.24–5.55]), Hispanics (4.53 [4.41–4.67]), Hawaiians/Pacific Islanders (3.19 [2.95–3.45]), and blacks (3.16 [3.05–3.27]; all FDR P for trend < 0.001) (Fig. 1 and Supplementary Table 3). The BMI gradient for prediabetes was overall less pronounced, with the steepest gradient observed among Hispanics (1.63 [1.59–1.68]) followed by American Indians/Alaskan Natives (1.60 [1.40–1.82]), blacks (1.57 [1.53–1.62]), whites (1.49 [1.46–1.52]), Hawaiians/Pacific Islanders (1.32 [1.23–1.42]), and Asians (1.28 [1.18–1.39]; all FDR P for trend < 0.001) (Fig. 2 and Supplementary Table 4). In sensitivity analysis, we also included participants with missing data on outcome ascertainment who were relatively younger and more likely to be normal weight but did not vary in other sociodemographic variables compared with the analytical sample. Results were materially unchanged, and similar racial/ethnic-specific trends in BMI gradients for risk of diabetes and prediabetes were observed (data not shown).
Figure 1.
Adjusted RRs (95% CIs) of diabetes by race/ethnicity and BMI categories in a logarithmic scale. Non-Asians were categorized as underweight (UW) (BMI <18.5 kg/m2), normal weight (NW) (18.5–24.9 kg/m2), overweight (OW) (25.0–29.9 kg/m2), and obese class (OC) 1–4 (30.0–34.9, 35.0–39.9, 40.0–49.9, ≥50.0 kg/m2), and Asians were categorized as UW (<18.5 kg/m2), NW (18.5–22.9 kg/m2), OW (23.0–27.4 kg/m2), and OC 1–4 (27.5–32.4, 32.5–37.4, 37.5–47.4, ≥47.5 kg/m2). Risk estimates were adjusted for age, sex, neighborhood poverty, neighborhood education, and site. Across BMI categories, P for trend < 0.001 for all groups after FDR adjustment for multiple comparisons. *P for interaction < 0.001 between race/ethnicity and BMI categories.
Figure 2.
Adjusted RRs (95% CIs) of prediabetes by race/ethnicity and BMI categories in a logarithmic scale. Non-Asians were categorized as underweight (UW) (BMI <18.5 kg/m2), normal weight (NW) (18.5–24.9 kg/m2), overweight (OW) (25.0–29.9 kg/m2), and obese class (OC) 1–4 (30.0–34.9, 35.0–39.9, 40.0–49.9, ≥50.0 kg/m2), and Asians were categorized as UW (<18.5 kg/m2), NW (18.5–22.9 kg/m2), OW (23.0–27.4 kg/m2), and OC 1–4 (27.5–32.4, 32.5–37.4, 37.5–47.4, ≥47.5 kg/m2). Risk estimates were adjusted for age, sex, neighborhood poverty, neighborhood education, and site. Across BMI categories, P for trend < 0.001 for all groups after FDR adjustment for multiple comparisons. *P for interaction < 0.001 between race/ethnicity and BMI categories.
Conclusions
Among 4.9 million adults aged ≥20 years across 10 sites in the PORTAL Network (2012–2013), we observed a positive linear trend in age-standardized prevalence of diabetes across BMI categories among all racial/ethnic groups. However, racial/ethnic minorities reached a given prevalence at a much lower BMI than whites, whereas whites had the steepest BMI gradient for diabetes risk after adjusting for covariates, suggesting that factors other than BMI may play more important roles in the risk of diabetes among racial/ethnic minorities. On the other hand, the racial/ethnic differences in the BMI gradient for prediabetes risk were less pronounced, with the highest and lowest slope observed among Hispanics and Asians, respectively. Of note, we observed a considerably high prevalence of diabetes ranging from 4% to 18% and prediabetes ranging from 18% to 34% among underweight and overweight adults. Given that 25–30% of the U.S. population are underweight or normal weight (24), our findings call for diabetes prevention strategies among these understudied subpopulations.
The observed patterns in racial/ethnic disparities in the PORTAL cohort were similar to those observed previously (5,6,25,26), including in data from the National Health and Nutrition Examination Survey (NHANES) (5,6) (Supplementary Table 5). The racial/ethnic-specific diabetes prevalence estimates observed in PORTAL 2012–2013 were overall more similar to those observed in NHANES 2011–2012 than those in NHANES 2013–2016, except for the prevalence in men and non-Hispanic whites. This can be attributed to varied definitions, with NHANES 2011–2012 defined by fasting plasma glucose, HbA1c, self-report of physician diagnosis, plus 2-h plasma glucose (not included in NHANES 2013–2016), and ours being more inclusive by fasting glucose, HbA1c, physician diagnosis, plus random plasma glucose and antihyperglycemic medications. Furthermore, compared with NHANES, our study population was based on people with health care encounters and included high-risk racial/ethnic subgroups of Hawaiians/Pacific Islanders and American Indians/Alaskan Natives.
Although racial/ethnic disparities in obesity may lead to racial/ethnic disparities in diabetes prevalence, little is known about the relative contribution of varied obesity levels, as indicated by BMI categories, to racial/ethnic disparities in diabetes and prediabetes. In this regard, we provide unprecedented data on comprehensive prevalence estimates of diabetes and prediabetes by race/ethnicity and detailed BMI categories among U.S. adults. Our findings illustrate a higher prevalence of diabetes among racial/ethnic minority groups than among whites at a given BMI, especially among people who are normal weight and overweight. Existing data on racial/ethnic disparities in prediabetes prevalence are scarce, with inconsistent observations. Using the 2005–2008 NHANES data, Sentell et al. (27) found that racial/ethnic disparities in prediabetes varied considerably by diagnostic criteria, highlighting the importance of applying a consistent definition to ensure comparability across studies. By applying the 2010 ADA criteria supplemented with an outpatient diagnosis, we observed that prediabetes prevalence was similar in whites, blacks, and American Indians/Alaskan Natives (range 31.0–32.0%), lower than that in Asians, Hawaiians/Pacific Islanders, and Hispanics (range 36.0–38.9%).
Prevalence estimates of prediabetes were high across racial/ethnic groups and exceeded those of diabetes, implying the presence of a large number of individuals who could subsequently progress to overt diabetes. Of note, age-standardized prevalence of prediabetes among Hispanics, Asians, and Hawaiians/Pacific Islanders increased with BMI levels but plateaued at obesity class 1, suggesting progression to overt diabetes at higher BMI levels among these subgroups. Furthermore, despite the overall higher prediabetes prevalence among Asians and Hawaiians/Pacific Islanders compared with other groups, the BMI gradient for prediabetes risk was least pronounced among Asians and Hawaiians/Pacific Islanders after adjusting for covariates. These findings are of particular importance given that Asians and Hawaiians/Pacific Islanders have been shown to have suboptimal glycemic control and progress faster through the prediabetes stage compared with their white counterparts (28–30). Collectively, these findings highlight the particular importance and necessity of close surveillance and prompt intervention to mitigate the risk of prediabetes and its progression to diabetes among these high-risk subgroups across BMI levels.
The underlying mechanisms whereby Asians, Hawaiians/Pacific Islanders, and Hispanics had a disproportionately higher burden of diabetes and prediabetes at lower BMI levels remain to be elucidated. Of note, compared with other racial/ethnic groups, Asians at a given BMI have a higher percentage of body fat and visceral fat (31), which in turn have been linked to insulin resistance and an increased risk of diabetes (32). Furthermore, Asians may experience innate susceptibility to impaired insulin secretion early in the development of diabetes (33). Indeed, impaired β-cell function compared with adiposity-induced insulin resistance may play a more pronounced role in the pathogenesis of type 2 diabetes, especially in less obese individuals of Asian origin (34,35). Future mechanistic investigations on heterogenous phenotypes of diabetes by race/ethnicity, including the relatively well-studied phenotype of impaired insulin sensitivity and the less-studied phenotype of impaired β-cell function, are warranted (36,37).
Our study has several notable strengths. The cohort of >4.9 million adults of wide racial/ethnic and socioeconomic diversity is uniquely well suited to examine associations between detailed BMI levels and risk of diabetes or prediabetes across racial/ethnic categories. We extend the literature by reporting the most extensive data to our knowledge on racial/ethnic and BMI category-specific disparities in diabetes and the far lesser studied prediabetes in U.S. adults, especially among the understudied Hawaiians/Pacific Islanders and American Indians/Alaskan Natives. The cohort comprised individuals who were health plan members during the study period, thus reducing confounding as a result of disparate access to health care. Furthermore, diabetes ascertainment is based on the presence of an inpatient diagnosis or any two or more of the following events: abnormal fasting plasma glucose, random plasma glucose, or HbA1c values; outpatient diagnosis; and antihyperglycemic medication. This confirmatory approach of using a second test to diagnose diabetes may help to decrease false-positive results as demonstrated previously (38).
Certain limitations of our study merit discussion. Despite the large sample size and wide geographical coverage, our cohort of health care members may not be generalizable to the general U.S. population. However, previous studies indicate that members of integrated health care systems are representative of and reflect diversity of the underlying population in the served geographic area (14). Because of the cross-sectional nature of the study, BMI levels could have changed after diabetes diagnosis as a result of lifestyle modification or medication treatment, which, however, could have resulted in an underestimated slope of the obesity-diabetes association. In addition, the differences in time between BMI measure and diabetes or prediabetes ascertainment were nondifferential across BMI categories (data not shown). The BMI values were based on weight and height extracted from EHR. Nonetheless, prior studies have demonstrated the validity of EHR-derived BMI data against research-quality data (39). Similarly, the ascertainment of diabetes and prediabetes were based on EHR data. Although we cannot entirely rule out the possibility of disease misclassification, the quality of diagnosis codes coupled with laboratory results and medications is relatively high in managed health care systems and has been validated for many health conditions, including diabetes (40). Furthermore, the inclusion of integrated health care system members reduced the potential racial/ethnic variation in disease ascertainment as a result of disparate access to health care. If certain racial/ethnic minority members remain less likely to be screened within the health care systems, this would have resulted in an underestimated slope of the BMI-diabetes association. We did not have direct measures of adiposity or visceral fat, which may be more strongly associated with insulin resistance and diabetes risk. We also lacked data on lifestyle factors, such as smoking, diet, and physical activity. Future studies examining the role of these potential intermediate factors are warranted to further explain the racial/ethnic disparities in diabetes or prediabetes across BMI levels. In addition, Asian ethnic categories were not consistently available across all sites; thus, future investigations across Asian ethnic categories are warranted. Finally, our methods did not allow us to distinguish between type 1 and type 2 diabetes; however, the majority was type 2 on the basis of limiting our study population to adults aged ≥20 years.
In conclusion, we documented substantial racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI category among 4.9 million adults in the U.S. from 2012 to 2013. Age-standardized prevalence estimates of both diabetes and prediabetes were higher among racial/ethnic minority groups than among whites, especially at lower BMI values, suggesting the role of factors other than obesity in the disproportionate burden of diabetes and prediabetes among racial/ethnic minorities. Clinicians should be aware that the BMI cutoffs for increased risk of diabetes and prediabetes vary by race/ethnicity and that the risk is even high at relatively low BMI levels in racial/ethnic minority groups. The findings of a disproportionately higher risk of diabetes and prediabetes at lower BMI levels among racial/ethnic minority groups highlight the importance of tailored screening, prevention, and intervention strategies to mitigate the risk of diabetes and prediabetes. Future investigations to explore the relative contributions of nonobesity- and obesity-driven factors in racial/ethnic disparities in diabetes and prediabetes burden are warranted.
Supplementary Material
Article Information
Acknowledgments. The authors thank the PORTAL Network staff and participants for valuable contributions.
Funding. This study used the infrastructure developed by the PORTAL Network, a consortium of three integrated delivery systems (Kaiser Permanente, HealthPartners, and Denver Health) and their affiliated research centers. Research reported in this publication was funded through a Patient-Centered Outcomes Research Institute award (CDRN-1306-04681 Phase II). Y.Z. was supported by the National Institutes of Health Building Interdisciplinary Research Careers in Women’s Health Program grant 5K12-HD-05216 and National Institute of Diabetes and Digestive and Kidney Diseases grant K01-DK-120807.
The views in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors, or its Methodology Committee.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.Z., D.R.Y., and A.F. contributed to the study concept and design. M.A.S. contributed to the data management and statistical analysis. Y.Z. drafted the manuscript. D.A., M.F.D., J.D., S.L.F., M.A.H., C.K., E.M., C.O., D.R.Y., and A.F. contributed to the interpretation of the results and revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript. Y.Z. and A.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
See accompanying article, p. 2164.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0532/-/DC1.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. Atlanta, GA, Department of Health and Human Services, Centers for Disease Control and Prevention, 2017 [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care 2005;28:2280–2288 [DOI] [PubMed] [Google Scholar]
- 4.Koh HK, Graham G, Glied SA. Reducing racial and ethnic disparities: the action plan from the department of health and human services. Health Aff (Millwood) 2011;30:1822–1829 [DOI] [PubMed] [Google Scholar]
- 5.Mendola ND, Chen TC, Gu Q, Eberhardt MS, Saydah S. Prevalence of total, diagnosed, and undiagnosed diabetes among adults: United States, 2013-2016. NCHS Data Brief 2018:1–8 [PubMed] [Google Scholar]
- 6.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 7.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Wang Y, Huang ES. Changes in racial/ethnic disparities in the prevalence of type 2 diabetes by obesity level among US adults. Ethn Health 2009;14:439–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oza-Frank R, Ali MK, Vaccarino V, Narayan KMV. Asian Americans: diabetes prevalence across U.S. and World Health Organization weight classifications. Diabetes Care 2009;32:1644–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maskarinec G, Grandinetti A, Matsuura G, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis 2009;19:49–55 [PMC free article] [PubMed] [Google Scholar]
- 11.Gujral UP, Mohan V, Pradeepa R, Deepa M, Anjana RM, Narayan KM. Ethnic differences in the prevalence of diabetes in underweight and normal weight individuals: the CARRS and NHANES studies. Diabetes Res Clin Pract 2018;146:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson AR, Stith AY, Smedley BD. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (Full Printed Version). Washington, DC, National Academies Press, 2002 [PubMed] [Google Scholar]
- 13.McGlynn EA, Lieu TA, Durham ML, et al. Developing a data infrastructure for a learning health system: the PORTAL network. J Am Med Inform Assoc 2014;21:596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 2012;16:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young DR, Waitzfelder BA, Arterburn D, et al. The Patient Outcomes Research To Advance Learning (PORTAL) network adult overweight and obesity cohort: development and description. JMIR Res Protoc 2016;5:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross TR, Ng D, Brown JS, et al. The HMO Research Network Virtual Data Warehouse: a public data model to support collaboration. EGEMS (Wash DC) 2014;2:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols GA, Schroeder EB, Karter AJ, et al.; SUPREME-DM Study Group . Trends in diabetes incidence among 7 million insured adults, 2006-2011: the SUPREME-DM project. Am J Epidemiol 2015;181:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmittdiel JA, Adams SR, Segal J, et al. Novel use and utility of integrated electronic health records to assess rates of prediabetes recognition and treatment: brief report from an integrated electronic health records pilot study. Diabetes Care 2014;37:565–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland, World Health Organization, 2000 [PubMed] [Google Scholar]
- 22.Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health 2007;121:492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300 [Google Scholar]
- 24.Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007-2012. JAMA Intern Med 2015;175:1412–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 27.Sentell TL, He G, Gregg EW, Schillinger D. Racial/ethnic variation in prevalence estimates for United States prediabetes under alternative 2010 American Diabetes Association criteria: 1988-2008. Ethn Dis 2012;22:451–458 [PMC free article] [PubMed] [Google Scholar]
- 28.Rawshani A, Svensson A-M, Rosengren A, Zethelius B, Eliasson B, Gudbjörnsdottir S. Impact of ethnicity on progress of glycaemic control in 131,935 newly diagnosed patients with type 2 diabetes: a nationwide observational study from the Swedish National Diabetes Register. BMJ Open 2015;5:e007599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anjana RM, Rani CSS, Deepa M, et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES). Diabetes Care 2015;38:1441–1448 [DOI] [PubMed] [Google Scholar]
- 30.Hsu WC, Boyko EJ, Fujimoto WY, et al. Pathophysiologic differences among Asians, native Hawaiians, and other Pacific Islanders and treatment implications. Diabetes Care 2012;35:1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 2007;86:353–359 [DOI] [PubMed] [Google Scholar]
- 32.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496–506 [DOI] [PubMed] [Google Scholar]
- 33.Gujral UP, Narayan KM, Kahn SE, Kanaya AM. The relative associations of β-cell function and insulin sensitivity with glycemic status and incident glycemic progression in migrant Asian Indians in the United States: the MASALA study. J Diabetes Complications 2014;28:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Zhang S, Liu H, et al. Different associations of diabetes with β-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care 2014;37:2533–2539 [DOI] [PubMed] [Google Scholar]
- 35.Gujral UP, Mohan V, Pradeepa R, et al. Ethnic variations in diabetes and prediabetes prevalence and the roles of insulin resistance and β-cell function: the CARRS and NHANES studies. J Clin Transl Endocrinol 2016;4:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan KM. Type 2 diabetes: why we are winning the battle but losing the war? 2015 Kelly West Award Lecture. Diabetes Care 2016;39:653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013;1281:64–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selvin E, Wang D, Lee AK, Bergenstal RM, Coresh J. Identifying trends in undiagnosed diabetes in U.S. adults by using a confirmatory definition: a cross-sectional study. Ann Intern Med 2017;167:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arterburn D, Ichikawa L, Ludman EJ, et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract 2008;2:277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA 2002;287:2519–2527 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.