Abstract
OBJECTIVE
To better understand potential facilitators of individual engagement in type 1 diabetes natural history and prevention studies through analysis of enrollment data in the TrialNet Pathway to Prevention (PTP) study.
RESEARCH DESIGN AND METHODS
We used multivariable logistic regression models to examine continued engagement of eligible participants at two time points: 1) the return visit after screening to confirm an initial autoantibody-positive (Ab+) test result and 2) the initial oral glucose tolerance test (OGTT) for enrollment into the monitoring protocol.
RESULTS
Of 5,387 subjects who screened positive for a single autoantibody (Ab), 4,204 (78%) returned for confirmatory Ab testing. Younger age was associated with increased odds of returning for Ab confirmation (age <12 years vs. >18 years: odds ratio [OR] 2.12, P < 0.0001). Racial and ethnic minorities were less likely to return for confirmation, particularly nonwhite non-Hispanic (OR 0.50, P < 0.0001) and Hispanic (OR 0.69, P = 0.0001) relative to non-Hispanic white subjects. Of 8,234 subjects, 5,442 (66%) were identified as eligible to be enrolled in PTP OGTT monitoring. Here, younger age and identification as multiple Ab+ were associated with increased odds of returning for OGTT monitoring (age <12 years vs. >18 years: OR 1.43, P < 0.0001; multiple Ab+: OR 1.36, P < 0.0001). Parents were less likely to enroll into monitoring than other relatives (OR 0.78, P = 0.004). Site-specific factors, including site volume and U.S. site versus international site, were also associated with differences in rates of return for Ab+ confirmation and enrollment into monitoring.
CONCLUSIONS
These data confirm clear differences between successfully enrolled populations and those lost to follow-up, which can serve to identify strategies to increase ongoing participation.
Introduction
The incidence of type 1 diabetes is increasing at an alarming rate worldwide (1–3). This chronic disease has substantial impact on morbidity and mortality of affected individuals and generates enormous health care costs (4). Classically defined as autoimmune destruction of insulin-producing pancreatic β-cells, genetic predisposition to type 1 diabetes can be conferred via high-risk HLA loci and >50 additional single nucleotide polymorphisms (SNPs) (5,6). However, genetic factors only account for a percentage of diabetes risk (7). Recent data have also identified β-cell intrinsic and environmental risk factors as potential contributors to ultimate diabetes development (5,8–10). Given that type 1 diabetes pathophysiology is still incompletely defined, prospective natural history studies of disease susceptibility and evolution remain a crucial component in understanding disease development.
Type 1 Diabetes TrialNet is an ongoing international clinical trial network of researchers aimed at understanding the natural history of type 1 diabetes and preventing or delaying disease. In the TrialNet Pathway to Prevention (PTP) study, first-, second-, or third-degree relatives without diabetes of individuals with type 1 diabetes, who are positive for at least one islet autoantibody (Ab+), are monitored longitudinally for the development of additional islet autoantibodies (Abs), dysglycemia, and diabetes (11). At the time of this analysis, >150,000 individuals had been screened since the inception of the PTP study. Because only ∼5% of screened individuals are Ab+ at the initial screening, enormous efforts and costs are associated with identifying eligible, Ab+ individuals (11). Attrition of known Ab+ individuals not only results in increased cost but also could introduce bias into natural history studies if certain groups of individuals consistently elude enrollment. Furthermore, because this population serves as an important source of subjects in type 1 diabetes prevention studies, a clear understanding of factors associated with successful enrollment versus loss to follow-up is critical for efficient and effective efforts moving forward in type 1 diabetes prevention and treatment.
To understand factors associated with enrollment in the PTP study, we analyzed two stages of the study. First, we compared subjects who were found to be single Ab+ at initial screening who did or did not return per protocol for a blood draw to confirm Ab positivity. Second, we compared subjects eligible for the oral glucose tolerance test (OGTT) monitoring stage of the PTP protocol (confirmed single Ab+ or multiple Ab+ at initial screening) who did or did not obtain an OGTT. We identified factors that were independently associated with continued participation versus attrition in PTP for both outcomes of interest.
Research Design and Methods
Subjects
Data from the TrialNet PTP (also called TN01; ClinicalTrials.gov reg. no. NCT00097292) as of 31 July 2017 were used. In PTP, serum from 1- to 45-year-old first-, second-, or third-degree blood relatives without diabetes of individuals with type 1 diabetes is obtained and then analyzed for pancreatic Abs, including those against glutamic acid decarboxylase antibody (GADA), insulin (mIAA), IA-2 antigen (IA-2A), zinc transporter 8 (ZnT8A), and islet cell Ab (ICA) (11). Subjects are recruited in a variety of settings, including clinics and settings where family members of individuals with type 1 diabetes gather (e.g., diabetes camps, fundraising walks, diabetes conferences) in multiple countries, including the U.S., Canada, the U.K., Germany, Italy, Sweden, Finland, Australia, and New Zealand. Parental consent is obtained for subjects <18 years of age, with assent for children >7–8 years of age, depending on local regulatory requirements.
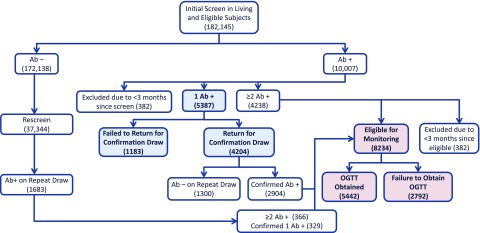
Individuals <18 years old that screen negative for Abs were eligible for a yearly rescreen to detect Abs until the age of 18. Enrolled individuals who screen positive for a single pancreatic Ab are instructed to return for a second blood draw to confirm Ab positivity. Subjects with a confirmed single positive Ab or multiple positive Abs on the initial draw are asked to return for further metabolic and genetic screening, including an OGTT, and then enter serial monitoring for development of additional Abs, dysglycemia, and diabetes. A flowchart of the PTP protocol for screening and confirmatory testing, then enrollment into monitoring, is depicted in Fig. 1.
Figure 1.
Flowchart of TrialNet PTP protocol for islet Ab screening and confirmatory testing and then monitoring with OGTT. Number of subjects identified for each category as of 31 July 2017 are indicated. We evaluated retention of subjects for Ab confirmation (shown in blue) and for return for OGTT monitoring (shown in pink).
Because the steps of obtaining a blood draw to confirm the initial test result compared with obtaining a monitoring OGTT involve participants at different levels of diabetes risk, as well as different levels of commitment/effort to participate, we evaluated the subsets of participants at each of these steps separately. We only evaluated subjects who were at least 3 months out from their initial assessment to permit enough time to return for follow-up testing. This period was chosen based on TrialNet’s defined target window for the initial evaluation. Participants who did not enroll because they received an outside diagnosis of type 1 diabetes within 3 months of initial screening or entered a type 1 diabetes prevention trial after screening were excluded.
Statistical Analyses
Differences in demographic and clinical characteristics as well as site characteristics were summarized and compared for participants who did versus those who did not 1) return for Ab+ confirmation or 2) enroll in active monitoring. Two-sample t tests were used to compare continuous measures, and χ2 tests were used to compare categorical factors between the two response groups (e.g., return vs. not). Univariable logistic regression models were used to initially identify significant factors, and a modified all-subjects regression based on the leaps-and-bounds method was used in the multivariable analysis for variable selection for each of the outcomes of interest (12). Variables evaluated included age at initial Ab screening, sex, self-reported race, ethnicity, relationship to proband (parent, sibling, offspring, or other), number of positive Abs, and site location (U.S. vs. international). Site volume was analyzed by number of subjects screened annually at the screening site. Age and number of subjects screened annually were analyzed as continuous and as categorical variables. P values <0.05 were considered statistically significant.
Results
Of the 182,145 subjects screened in the PTP study as of 31 July 2017, 11,690 (6.4%) ultimately tested Ab+, either at the initial screening (n = 10,007) or during a subsequent rescreen encounter (n = 1,683) (Fig. 1). Of those who were initially found to exhibit single Ab positivity (n = 5,387), 4,204 subjects (78%) returned for subsequent confirmatory testing. Of the 8,234 subjects with either a confirmed single Ab+ or multiple Ab+ who were eligible for OGTT monitoring, 5,442 subjects (66%) returned for an initial OGTT and were enrolled into the monitoring stage.
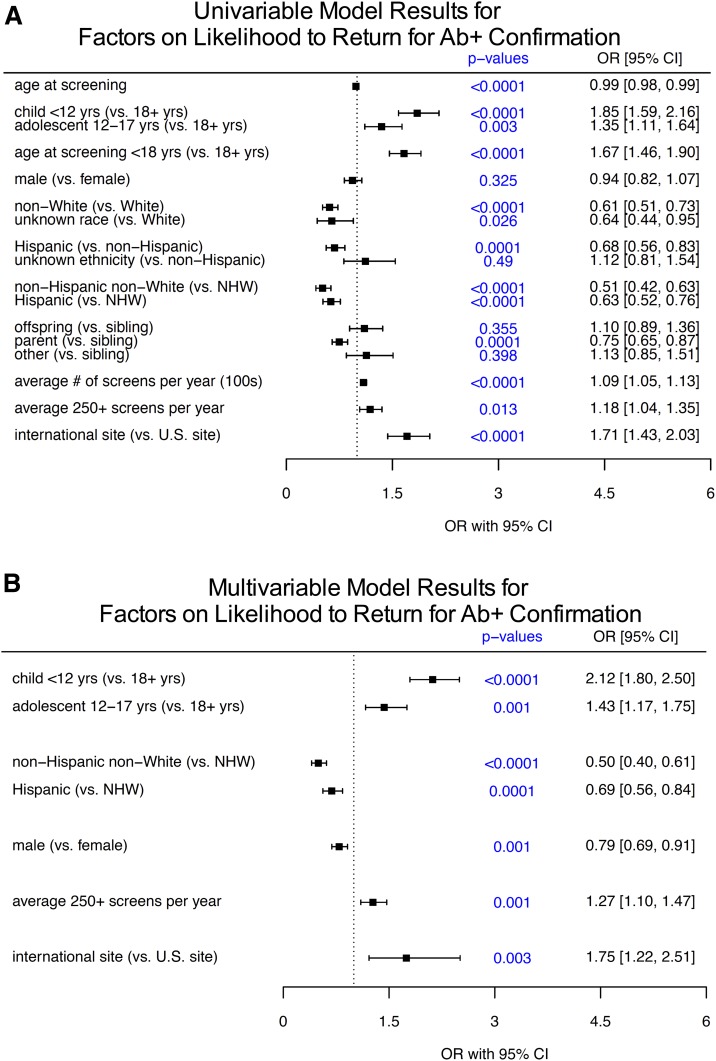
Several demographic and clinical factors were significantly associated with single Ab+ subjects who returned for Ab status confirmation (forest plot in Fig. 2A, with absolute values in Table 1). Age at the time of initial screening was a significant factor, with children <12 years (odds ratio [OR] 1.85, 95% CI 1.59–2.16, P < 0.0001) and adolescents 12–17 years (OR 1.35, 95% CI 1.11–1.64, P = 0.003) significantly more likely to obtain the requested confirmation blood draw compared with adults. Because of small sample sizes for some groups, race and ethnicity were combined for regression analyses. Here, people who self-identified as members of racial and ethnic minority groups were less likely to return for confirmation: non-Hispanic nonwhite (NHNW; OR 0.51, 95% CI 0.42–0.63, P < 0.0001) and Hispanic (OR 0.63, 95% CI 0.52–0.76, P < 0.0001) subjects were significantly less likely to return than non-Hispanic white (NHW) subjects. Parents of persons with type 1 diabetes showed lower rates of return for Ab confirmation compared with other relatives (OR 0.75, 95% CI 0.65–0.87, compared with siblings, P = 0.0001). Subject sex was not a significant factor on return rates for Ab confirmation in the univariable analysis (OR 0.94, P = 0.325).
Figure 2.
Analysis of factors associated with return for a confirmation blood draw after positive screening for Abs. Of 5,387 subjects, 4,204 (78%) screened positive for one Ab (single Ab+) and returned for confirmatory Ab testing. Forest plots represent ORs and 95% CIs for univariable analysis (A) and multivariable analysis (B).
Table 1.
Variables associated with return for confirmation of Ab+ status
| Characteristic | All participants (n = 5,387) | Participants who did not return for Ab+ confirmation (n = 1,183) | Participants who did return for Ab+ confirmation (n = 4,204) | P value* |
|---|---|---|---|---|
| Age at screening | <0.0001 | |||
| <12 years old | 1,738 | 282 (16.2) | 1,456 (83.8) | |
| 12–17 years old | 663 | 129 (19.5) | 534 (80.5) | |
| ≥18 years old | 2,986 | 772 (25.9) | 2,214 (74.2) | |
| Not reported | 13 | 9 | 4 | |
| Sex | 0.33 | |||
| Female | 3,180 | 679 (21.4) | 2,501 (78.7) | |
| Male | 2,175 | 489 (22.5) | 1,686 (77.5) | |
| Not reported | 32 | 15 | 17 | |
| Race | <0.0001 | |||
| White | 4,541 | 933 (20.6) | 3,608 (79.5) | |
| Black/African American | 279 | 113 (40.5) | 166 (59.5) | |
| Asian | 99 | 19 (19.2) | 80 (80.8) | |
| Other | 339 | 81 (23.9) | 258 (76.1) | |
| Unknown/refused | 129 | 37 (28.7) | 92 (71.3) | |
| Ethnicity | 0.0001 | |||
| Hispanic or Latino | 589 | 167 (28.4) | 422 (71.7) | |
| Not Hispanic or Latino | 4,546 | 967 (21.3) | 3,579 (78.7) | |
| Unknown/refused | 252 | 49 (19.4) | 203 (80.6) | |
| Race/ethnicity group | <0.0001 | |||
| NHW | 4,062 | 809 (19.9) | 3,253 (80.1) | |
| NHNW | 484 | 158 (32.6) | 326 (67.4) | |
| Hispanic | 589 | 167 (28.4) | 422 (71.6) | |
| Unknown/refused | 252 | 49 (19.4) | 203 (80.6) | |
| Relation to proband | 0.0001 | |||
| Parent | 2,072 | 522 (25.2) | 1,550 (74.8) | |
| Sibling | 2,104 | 424 (20.2) | 1,680 (79.9) | |
| Offspring | 774 | 144 (18.6) | 630 (81.4) | |
| Other | 362 | 66 (18.2) | 296 (81.8) | |
| Not reported | 75 | 27 | 48 | |
| Subjects screened by site/year, average n | ||||
| Median (range) | 164 (1–1,455) | 163 (1–1,455) | 164 (1–669) | <0.0001 |
| ≤250 | 3,219 | 744 (23.1) | 2,475 (76.9) | <0.0001 |
| 251–500 | 1,878 | 408 (21.7) | 1,470 (78.3) | |
| >500 | 290 | 31 (10.7) | 259 (89.3) | |
| <400 | 4,775 | 1,112 (23.3) | 3,663 (76.7) | <0.0001 |
| ≥400 | 612 | 71 (11.6) | 541 (88.4) | |
| <250 | 3,219 | 744 (23.1) | 2,475 (76.9) | 0.0128 |
| ≥250 | 2,168 | 439 (20.3) | 1,729 (79.8) | |
| Location of screening | 0.0001 | |||
| U.S. | 4,210 | 1,001 (23.78) | 3,209 (76.2) | |
| International site | 1,177 | 182 (15.5) | 995 (84.5) |
Data are presented as n, n (%), or as indicated.
P values reflect comparisons between groups using χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
Site characteristics were also associated with differences in return rates. Site annual screening volume was analyzed as a surrogate measure of site experience, infrastructure, and research support. As expected, higher site screening volume was associated with increased odds of subjects returning for Ab confirmation. Those sites screening ≥250 subjects a year were significantly more successful in having single Ab+ subjects return for confirmation of Ab+ status (OR 1.18, 95% CI 1.04–1.35, P = 0.013). Since TrialNet is an international network, we also examined whether return rates differed between U.S. and international sites. Although total numbers of screened and confirmed participants were much higher among U.S. sites (>10-fold difference), once screened, participants at international sites showed higher rates of returning for Ab confirmation (OR 1.71, 95% CI 1.43–2.03, P < 0.0001).
Multivariable analyses and variable selection (Fig. 2B) identified that younger age (children <12 years vs. adults: OR 2.12, 95% CI 1.80–2.50, P < 0.0001; children 12–17 years vs. adults: OR 1.43, 95% CI 1.17–1.75, P = 0.001), racial or ethnic minority status (NHNW vs. NHW: OR 0.50, 95% CI 0.40–0.61, P < 0.0001; Hispanic vs. NHW: OR 0.69, 95% CI 0.56–0.84, P = 0.0001), and site screening volume (sites with >250 screens per year: OR 1.27, 95% CI 1.10–1.47, P = 0.001) remained significant factors on confirmatory test return rates. Associations of non-U.S. versus U.S. sites with increased or decreased confirmatory testing return rates also remained significant (OR 1.75, 95% CI 1.22–2.51, P = 0.003). However, the observed effect of parental relationship to type 1 diabetes proband was not identified as a significant factor in the final model, likely being reflected through the subject age variable. In the multivariable setting, we observed an interaction between age at screening group and male sex, where male sex was only significant in the univariate (OR 0.72, 95% CI 0.56–0.94, P = 0.016) and multivariable (OR 0.68, 95% CI 0.52–0.89, P = 0.005) settings for those who were <12 years at time of screening. For subjects who were age ≥12 years, male sex was not a significant factor in the univariate or the multivariable models.
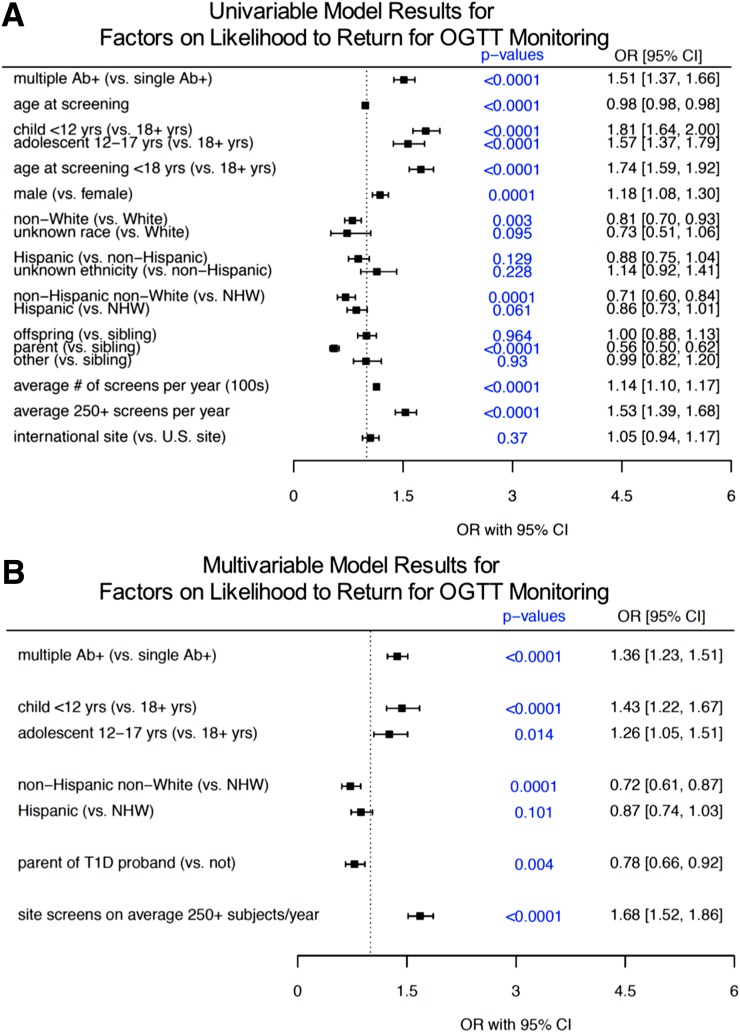
We then evaluated demographic and clinical subject factors as well as screening site-specific factors on whether Ab+ subjects identified as eligible returned for OGTT monitoring. Univariable analyses identified several factors significantly associated with enrollment into monitoring (forest plot in Fig. 3A, with absolute values in Table 2). Here, consistent with factors associated with return for Ab+ confirmation, pediatric participants were more likely to return for an OGTT, where children <12 years (OR 1.81, 95% CI 1.64–2.00, P < 0.0001), along with adolescents 12–17 years (OR 1.57, 95% CI 1.37–1.79, P < 0.0001) were more likely to return for OGTT compared with adults. Race and ethnicity subgroups were also significantly associated with monitoring enrollment likelihood, with NHNW subjects less likely to enroll into monitoring than NHW subjects (for NWNH: OR 0.71, 95% CI 0.60–0.84, P = 0.0001). Hispanic subjects also tended to be less likely than NHW to enroll in OGTT monitoring, although this did not reach statistical significance (for Hispanic: OR 0.86, 95% CI 0.73–1.01, P = 0.061). Relationship to proband was also a factor at this stage, with parents again emerging as significantly less likely to obtain an OGTT than other family members of probands with type 1 diabetes (OR 0.56, 95% CI 0.50–0.62, P < 0.0001). Unlike our evaluation of Ab+ confirmation, sex was a significant factor in the univariable setting for likelihood of enrollment in OGTT monitoring, with male subjects more likely to participate than female subjects (OR 1.18, 95% CI 1.08–1.30, P = 0.0001). Not surprisingly, the odds of subjects testing positive for multiple Abs returning for OGTT monitoring were increased compared with those testing positive for a single Ab (OR 1.51, 95% CI 1.37–1.66, P < 0.0001).
Figure 3.
Analysis of factors associated with return for monitoring with OGTT. Of 8,234 subjects identified as eligible, 5,442 (66%) enrolled in PTP OGTT monitoring. Forest plots represent ORs and 95% CIs for univariable analysis (A) and multivariable analysis (B). T1D, type 1 diabetes.
Table 2.
Variables associated with return for monitoring via OGTT
| Characteristic | All participants (n = 8,234) | Participants who did not return for OGTT monitoring (n = 2,792) | Participants who did return for OGTT monitoring (n = 5,442) | P value* |
|---|---|---|---|---|
| Ab+ status at screening | <0.0001 | |||
| Single confirmed Ab+ | 2,885 | 1,154 (40.0) | 1,731 (60.0) | |
| Multiple Ab+ | 5,349 | 1,638 (30.6) | 3,711 (69.4) | |
| Age at screening | <0.0001 | |||
| <12 years old | 4,066 | 1,175 (28.9) | 2,891 (71.1) | |
| 12–17 years old | 1,264 | 408 (32.3) | 856 (67.7) | |
| ≥18 years old | 2,874 | 1,201 (41.8) | 1,673 (58.2) | |
| Not reported | 30 | 8 | 22 | |
| Sex | 0.0001 | |||
| Female | 4,370 | 1,558 (35.7) | 2,812 (64.4) | |
| Male | 3,836 | 1,223 (31.9) | 2,613 (68.1) | |
| Not reported | 28 | 11 | 17 | |
| Race | <0.0001 | |||
| White | 7,192 | 2,391 (33.3) | 4,801 (66.8) | |
| Black/African American | 321 | 151 (47.0) | 170 (53.0) | |
| Asian | 118 | 39 (33.1) | 79 (66.9) | |
| Other | 482 | 162 (33.6) | 320 (66.4) | |
| Unknown/refused | 121 | 49 (40.5) | 72 (59.5) | |
| Ethnicity | 0.13 | |||
| Hispanic or Latino | 718 | 263 (36.6) | 455 (63.4) | |
| Not Hispanic or Latino | 7,099 | 2,400 (33.8) | 4,699 (66.2) | |
| Unknown/refused | 417 | 129 (30.9) | 288 (69.1) | |
| Race/ethnicity group | 0.00017 | |||
| NHW | 6,515 | 2,160 (33.2) | 4,355 (66.8) | |
| NHNW | 584 | 240 (41.1) | 344 (58.9) | |
| Hispanic | 718 | 263 (36.6) | 455 (63.4) | |
| Unknown/refused | 417 | 129 (30.9) | 288 (69.1) | |
| Relation to proband | <0.0001 | |||
| Parent | 1,867 | 826 (44.2) | 1,041 (55.8) | |
| Sibling | 4,238 | 1,298 (30.6) | 2,940 (69.4) | |
| Offspring | 1,463 | 449 (30.7) | 1,014 (69.3) | |
| Other | 555 | 171 (30.8) | 384 (69.2) | |
| Not reported | 111 | 48 | 63 | |
| Subjects screened by site/year, average n | ||||
| Median (range) | 180 (1–1,455) | 151 (1–1,455) | 217 (4–1,455) | <0.0001 |
| ≤250 | 4,854 | 1,834 (37.8) | 3,020 (62.2) | <0.0001 |
| 251–500 | 2,890 | 832 (28.8) | 2,058 (71.2) | |
| >500 | 490 | 126 (25.7) | 364 (74.3) | |
| <400 | 7,226 | 2,516 (34.8) | 4,710 (65.2) | <0.0001 |
| ≥400 | 1,008 | 276 (27.4) | 732 (72.6) | |
| Location of screening | 0.0001 | |||
| U.S. | 6,259 | 2,137 (34.1) | 4,122 (65.9) | |
| International site | 1,961 | 648 (33.0) | 1,313 (67.0) |
Data are presented as n, n (%), or as indicated.
P values reflect comparisons between groups using χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
Similar to likelihood of Ab+ confirmation, sites screening high volumes (≥250) of subjects per year had increased odds of Ab+ subjects returning for an OGTT (OR 1.53, 95% CI 1.39–1.68, P < 0.0001). However, by contrast to rates of Ab+ confirmation, rates of OGTT monitoring enrollment were comparable between U.S. and international sites overall (OR 1.05, 95% CI 0.94–1.17, P = 0.37).
The best predictive multivariable model identified several factors affecting entry into OGTT monitoring (Fig. 3B). Specifically, significant factors for higher likelihood were multiple Ab+ status (OR 1.36, 95% CI 1.23–1.51; P < 0.0001) and younger subject age (children <12 years: OR 1.43, 95% CI 1.22–1.67, P < 0.0001; adolescents aged 12–17 years: OR 1.26, 95% CI 1.05–1.51, P = 0.014). Here, parental relationship to type 1 diabetes proband was associated with significantly reduced odds of returning for an OGTT (OR 0.78, 95% CI 0.66–0.92, P = 0.004). Sex and ethnicity were no longer associated with monitoring enrollment in the multivariable analysis. In addition to these demographic and clinical factors, high site screening volumes were also significantly associated with a subject’s likelihood to return for OGTT monitoring (>250 subjects per year: OR 1.68, 95% CI 1.52–1.86, P < 0.0001).
Conclusions
Our findings reveal demographic- and center-related factors associated with engagement at two stages of the TrialNet PTP observational longitudinal cohort study. Approximately three-fourths of subjects screening positive for a single Ab obtained a confirmatory blood draw, and approximately two-thirds of subjects eligible for longitudinal monitoring returned for an OGTT. We identified multiple factors associated with higher or lower rates of enrollment, which will be useful for comparison as TrialNet implements continued efforts to improve enrollment in the PTP and can inform other clinical studies of individuals at risk for type 1 diabetes or comparable cohorts.
Comparison of enrollment rates for at-risk individuals among other type 1 diabetes studies is challenging given the unique nature of the PTP study. The study that is most directly comparable, the Diabetes Prevention Trial–Type 1 (DPT-1), which was the precursor study to TrialNet, screened first- and second-degree relatives of individuals with type 1 diabetes and reported that of 3,483 patients who tested positive for islet Abs, 2,523 (72%) obtained an initial intravenous glucose tolerance test (IVGTT) to determine eligibility for randomization (13). This enrollment rate is somewhat higher than the 66% enrollment rate we found in the active monitoring phase of the PTP protocol. However, the purpose of the IVGTT in DPT-1 was to determine eligibility for a trial, not for further observation. This enrollment into a clinical trial with the possibility of therapeutic benefit likely provided increased motivation for recruited subjects to undergo the IVGTT. Although participants in the PTP may be identified and recruited for future prevention studies, enrollment only guarantees participation in monitoring. Indeed, TrialNet has an extraordinarily high retention rate for individuals randomized into clinical trials. For example, 550 of 560 of randomized individuals (98%) completed the recent TrialNet oral insulin study despite a median 2.7-year trial duration, and 96–97% of randomized patients completed recent new-onset β-cell preservation trials (14–16).
We found that children under age 18 were significantly more likely to obtain Ab+ confirmation and return for OGTT monitoring than adults (Ab+ confirmation: 82.9% vs. 74.2%; OGTT: 70.4% vs. 58.2%) (see Tables 1 and 2). These findings are likely related to parental involvement in study follow-up, because parental consent is required for subjects <18 years of age. Since it is widely recognized that the rate of progression is influenced by age (17), lower follow-up rates in adults could also be related to differences in risk perception, which have been linked to study withdrawal in The Environmental Determinants of Diabetes in the Young (TEDDY) natural history cohort, and so may also affect enrollment (18–20). Adults may see themselves at lower risk because they have reached adulthood without getting the disease. Indeed, our results suggest that level of participant risk affected follow-up in this reported population. PTP participants identified as multiple Ab+ (who have higher risk of type 1 diabetes development) were more likely to return for an OGTT compared with single Ab+ subjects. In fact, 69% of multiple Ab+ participants returned for an OGTT, a percentage similar to the 72% of DPT-1 participants who obtained an initial IVGTT.
Relationship to a proband with type 1 diabetes was also significantly associated with returning for OGTT monitoring and significant on univariable analysis for confirmation of Ab status. Within this group, parents of probands with type 1 diabetes were the least likely relatives to return for subsequent testing. This may be related to interpreted lower risk, associated with lower prevalence of multiple Ab+ in adults, and because the individual had reached adulthood without development of type 1 diabetes. Even if type 1 diabetes risk is lower in adults, this population may be very relevant for further analysis, as subjects may have evaded type 1 diabetes via protective mechanisms that are important clues in prevention strategies. Of note, these data reflect enrollment rates since the original inception of the PTP. Given the recent establishment of the staging system of type 1 diabetes and recent implementation of this system within TrialNet, with modification of communication regarding participant risk, follow-up analyses will be informative (21).
Another feature of our results was the impact of race/ethnicity on enrollment. Hispanic/Latino and NHNW participants with a single Ab were less likely to return for a confirmatory draw. Although lower than rates for NHW participants, both of these groups exhibited high return rates overall, and ethnicity did not significantly impact enrollment into OGTT monitoring. Individuals identifying as Hispanic/Latino or black/African American comprised only 10.9% and 5.2% of the total screened population, respectively (Table 1). This finding is consistent with other studies in the type 1 diabetes population and is likely related in part to the lower incidence of disease among these groups compared with the NHW population (22,23). Enrollment of racial and ethnic minorities is important to understand clinical differences in disease phenotypes and in responses to therapy. Appropriate culture-specific engagement will be key to increased enrollment of these populations in future recruitment efforts. Along these lines, to facilitate communication with potential recruits speaking primarily Spanish, TrialNet has developed Spanish-speaking research teams and participant information material in Spanish. Work studying the TEDDY cohort as well as other longitudinal cohorts has also pointed to social variables and life stressors that may be more common in minority populations as independent predictors of failure to follow-up in a study; however, information on social factors was not collected in TrialNet at the time of these analyses (18,19,22,24). Regardless of the etiology, targeted efforts to encourage members of racial and ethnic minority groups to undergo initial and confirmatory testing are warranted.
Subjects at sites screening >250 subjects yearly were significantly more likely to obtain confirmation for Ab status and to return for OGTT monitoring. Within the TrialNet network, larger-volume sites reflect teams with increased financial support for well-developed and consistent clinical research infrastructure. These findings underscore the importance of clinical trial centers of excellence for efficient and effective implementation of clinical trials (25). These findings also suggest that analysis of differences in practices between more experienced clinical centers and smaller or less established sites could provide possible ways to narrow these gaps in retention success.
Given that TrialNet PTP is an international, multicenter study with recruiting site characteristics playing an important role in retention success, observed differences in confirmation and enrollment rates between countries are not surprising. These data are consistent with reports from TEDDY, which have identified site country as a significant factor associated with enrollment and withdrawal rates (26,27). Country-specific attitudes toward research participation and practices of study coordinators and personnel contacting and interacting with subjects at each center likely varied, potentially impacting retention success in ways we were unable to measure. TrialNet has recently developed new tools to ensure cross-site consistency on processes and messaging to address this possibility.
There were several limitations to our study. Because this work was performed retrospectively, we only had access to information already obtained about subjects who failed to return. Future work reaching out to these subjects to obtain more social/demographic information, including parent education and household income, subject travel distance, and reported reasons for failure to continue with testing, will help to further understand our findings. We chose to code subjects as lost to follow-up who did not obtain confirmatory testing or OGTT within a 3-month window of previous testing. This criterion likely resulted in a small number of subjects who waited >3 months to obtain subsequent testing being counted as failing to return. We chose this relatively aggressive window because identified subjects are at increased risk for type 1 diabetes, and quick follow-up and enrollment into monitoring are crucial to catch subjects before diabetes progression or development. However, to address this issue, we also performed our logistic regression analyses using a 6-month window, and significant factors associated with failure to return were unchanged (data not shown).
In addition to identifying potential gaps in retention of population groups, understanding characteristics and identifying modifiable factors leading to retention failures may allow for specific targeting efforts toward subjects identified as being high-risk. Because a certain percentage of subjects failing to return have changed contact information and cannot be reached, early identification of groups at risk could lead to changes in initial contact information obtained (i.e., multiple numbers, numbers of relatives, etc.) (24). The TEDDY Study Group reported that use of a cumulative risk model to identify highest-risk subjects for attrition, with increased engagement and consistency of interaction with identified subjects, dramatically reduced withdrawal rates within this group (28). Such tactics could also potentially be applied toward enrollment. Over the past few years, the TrialNet study group has applied a series of step-wise changes to address factors impacting successful enrollment of eligible individuals. In addition to implementation of the modified staging system, this includes identification and targeting of subjects that are at high risk of conversion to diabetes for increased enrollment efforts, amending aspects of the PTP study protocol, implementation of online screening and capillary blood testing as a screening modality, and developing consistent branding and a social media presence. Follow-up of our findings will be needed to better understand the effects of these changes on rates of successful enrollment in the PTP.
In conclusion, this work has identified areas for improvement in subject enrollment at two time points within the TrialNet PTP cohort study. By identifying factors significantly associated with failure for those with a single Ab to return for a confirmatory visit and for those with multiple Abs to enroll into monitoring, we can better understand which groups to target for more intensive recruitment efforts.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the support of the Type 1 Diabetes TrialNet Study Group, which identified study participants and provided samples and follow-up data for this study.
Funding. E.K.S. receives funding support from National Institute of Diabetes and Digestive and Kidney Diseases K08-DK-103983 and the National Institutes of Health Loan Repayment Program. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health through the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Diabetes and Digestive and Kidney Diseases, through the cooperative agreements U01-DK-061010, U01-DK-061034, U01-DK-061042, U01-DK-061058, U01-DK-085453, U01-DK-085461, U01-DK-085465, U01-DK-085466, U01-DK-085476, U01-DK-085499, U01-DK-085504, U01-DK-085509, U01-DK-103153, U01-DK-103180, U01-DK-103266, U01-DK-103282, U01-DK-106984, U01-DK-106994, U01-DK-107013, U01-DK-107014, UC4-DK-097835, and UC4 DK-106993 and support from JDRF and the American Diabetes Association.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.K.S. planned analyses, interpreted data, and wrote the manuscript. S.G. planned analyses, evaluated and interpreted data, and edited the manuscript. S.B.J., I.L., L.M.J., D.B., L.E.R., D.M. , M.A.A., H.R., M.S., H.E.L., D.K.W., C.J.G., and J.K. interpreted data and edited the manuscript. L.A.D. planned analyses, interpreted data, and wrote and edited the manuscript. All authors read and approved the final version. E.K.S. and L.A.D. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A complete list of the Type 1 Diabetes TrialNet Study Group can be found in the Supplementary Data.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0593/-/DC1.
References
- 1.DIAMOND Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Bell RA, D’Agostino RB Jr., et al.; Writing Group for the SEARCH for Diabetes in Youth Study Group . Incidence of diabetes in youth in the United States. JAMA 2007;297:2716–2724 [DOI] [PubMed] [Google Scholar]
- 3.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G; EURODIAB Study Group . Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 4.Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS One 2010;5:e11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson MA, Bluestone JA, Eisenbarth GS, et al. How does type 1 diabetes develop? The notion of homicide or β-cell suicide revisited. Diabetes 2011;60:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erlich H, Valdes AM, Noble J, et al.; Type 1 Diabetes Genetics Consortium . HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 2008;57:1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olmos P, A’Hern R, Heaton DA, et al. The significance of the concordance rate for type 1 (insulin-dependent) diabetes in identical twins. Diabetologia 1988;31:747–750 [DOI] [PubMed] [Google Scholar]
- 8.Soleimanpour SA, Stoffers DA. The pancreatic β cell and type 1 diabetes: innocent bystander or active participant? Trends Endocrinol Metab 2013;24:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forlenza GP, Rewers M. The epidemic of type 1 diabetes: what is it telling us? Curr Opin Endocrinol Diabetes Obes 2011;18:248–251 [DOI] [PubMed] [Google Scholar]
- 10.Sims EK, Chaudhry Z, Watkins R, et al. Elevations in the fasting serum proinsulin-to-C-peptide ratio precede the onset of type 1 diabetes. Diabetes Care 2016;39:1519–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al.; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group . The TrialNet natural history study of the development of type 1 diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 12.Furnival GM, Wilson RW. Regressions by leaps and bounds. Technometrics 1974;16:499–511 [Google Scholar]
- 13.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial--Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 14.Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group; Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 2017;318:1891–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller MJ, Schatz DA, Skyler JS, et al.; Type 1 Diabetes TrialNet ATG-GCSF Study Group . Low-dose anti-thymocyte globulin (ATG) preserves β-cell function and improves HbA1c in new-onset type 1 diabetes. Diabetes Care 2018;41:1917–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moran A, Bundy B, Becker DJ, et al.; Type 1 Diabetes TrialNet Canakinumab Study Group; AIDA Study Group . Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 2013;381:1905–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wherrett DK, Chiang JL, Delamater AM, et al.; Type 1 Diabetes TrialNet Study Group . Defining pathways for development of disease-modifying therapies in children with type 1 diabetes: a consensus report. Diabetes Care 2015;38:1975–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson SB, Lee HS, Baxter J, Lernmark B, Roth R, Simell T; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY) study: predictors of early study withdrawal among participants with no family history of type 1 diabetes. Pediatr Diabetes 2011;12:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson SB, Lynch KF, Baxter J, et al.; TEDDY Study Group . Predicting later study withdrawal in participants active in a longitudinal birth cohort study for 1 year: the TEDDY study. J Pediatr Psychol 2016;41:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth R, Lynch K, Lernmark B, et al.; TEDDY Study Group . Maternal anxiety about a child’s diabetes risk in the TEDDY study: the potential role of life stress, postpartum depression, and risk perception. Pediatr Diabetes 2015;16:287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baxter J, Vehik K, Johnson SB, Lernmark B, Roth R, Simell T; TEDDY Study Group . Differences in recruitment and early retention among ethnic minority participants in a large pediatric cohort: the TEDDY Study. Contemp Clin Trials 2012;33:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al.; SEARCH for Diabetes in Youth Study . Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young AF, Powers JR, Bell SL. Attrition in longitudinal studies: who do you lose? Aust N Z J Public Health 2006;30:353–361 [DOI] [PubMed] [Google Scholar]
- 25.IOM (Institute of Medicine) Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020: Workshop Summary, Washington, DC, National Academies Press, 2012 [PubMed] [Google Scholar]
- 26.Lernmark B, Johnson SB, Vehik K, et al. Enrollment experiences in a pediatric longitudinal observational study: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Contemp Clin Trials 2011;32:517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lernmark B, Lynch K, Baxter J, et al.; Teddy Study Group . Participant experiences in The Environmental Determinants of Diabetes in the Young study: common reasons for withdrawing. J Diabetes Res 2016;2016:2720650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson SB, Lynch KF, Lee HS, et al.; TEDDY Study Group . At high risk for early withdrawal: using a cumulative risk model to increase retention in the first year of the TEDDY study. J Clin Epidemiol 2014;67:609–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.